Abstract
Objective
To investigate dual-energy spectral CT in characterization of hepatocellular Carcinoma (HCC) in patients with chronic liver disease.
Methods
Dynamic computed tomography (CT) was performed in 3600 patients (2879 males; 721 females, mean age 50.9 ± 11.9 years) with working clinical diagnosis of liver cirrhosis for hepatocellular carcinoma screening and other clinical indications. The study was conducted over a period of 3 years. During dynamic CT scanning, spectral (monochromatic) and routine (polychromatic) CT acquisitions were obtained on a single tube, dual energy, 64 slice multi-detector CT scanner. Imaging findings were studied on routine CT. On the basis of routine CT findings, indeterminate lesions (lesions not showing characteristic hypervascularity followed by washout on dynamic routine CT scan) that were referred for biopsy or surgery were segregated. A retrospective blinded review of the lesions, acquired by the spectral CT acquisitions was done with the help of gem stone imaging (GSI) software to characterize these lesions. All the above lesions were analyzed qualitatively in the arterial phase for lesion conspicuity as well as quantitatively using the monochromatic data sets and nodule Iodine concentration on material density maps, respectively. This data was studied with respect to predictability of HCC using the spectral CT technique. Iodine density of the lesion, surrounding liver parenchyma, and lesion to liver parenchyma ratio (LLR) were derived and statistically analyzed. Histopathology of the lesion in question was treated as gold standard for analysis.
Results
It was observed via statistical analysis that the value of iodine density of the lesion on material density sets of ≥29.5 mg/dl, enabled a discriminatory power of 86.5%, sensitivity of 90.5% with 95% confidence Interval (CI) (69.2–98.8%) and specificity of 81.2% with 95% Confidence Interval (54.4–95.9%) in predicting HCC. Qualitative assessment also showed higher lesion conspicuity with spectral CT image sets as compared to routine CT data.
Conclusion
This study reveals that spectral imaging is an excellent qualitative as well as a quantitative tool for assessing and predicting hepatocellular carcinoma in cirrhotic patients.
Abbreviations: HCC, hepatocellular carcinoma; CT, computed tomography; GSI, gem stone imaging; DECT, dual energy computed tomography; LLR, lesion to liver parenchyma ratio; MMD, monochromatic material density; CI, confidence interval
Keywords: Hepatocellular carcinoma, Dual energy computed tomography, Material density images, Spectral computed tomography, Functional imaging, Iodine quantification
1. Introduction
The primary objective of our study was to determine whether spectral CT technique can improve accuracy in diagnosis of HCC in cirrhotic patients. This analysis was performed to evaluate both qualitative (lesion conspicuity) and quantitative (using material iodine density values) aspects of spectral CT respectively. Secondary end points and objectives of this study were to:
-
1.
Explore spectral CT as a functional tool in patients with HCC.
-
2.
Assess the accuracy and reliability of a new assessment parameter of measuring iodine density of enhancing liver nodules on the arterial phase of CT instead of the traditional quantification done by calculating their attenuation values in Hounsfield units.
-
3.
Assign a range of iodine density values to confirmed HCC nodules so as to be able to form an objective, quantitative, value-based classification for liver cancer nodules on a future long term basis.
The revised European association of study of liver diseases (EASL) and American association of study of liver diseases (AASLD) practice guidelines on the diagnosis and management of HCC, given in 2012 and 2010 respectively, changed the way; imaging is being performed for surveillance and detection of liver lesions in patients with chronic liver disease. [1] AASLD guidelines recommend abdominal ultrasonography every 6 months for surveillance of ‘at risk’ population of active chronic hepatitis B, cirrhotic patients and patients in the 40–50 years bracket. [1] The EASL guidelines recommend patients with chronic hepatitis C infection and advanced stage of F3 liver fibrosis, regardless of cirrhosis, to undergo surveillance with ultrasonography. [2] The practical approach to reliability of ultrasonography to detect small lesions (< 1 cm) in a diffusely nodular liver appears questionable. For this reason, dynamic, contrast enhanced, CT is recommended to further detect and characterize such lesions. [3], [4] The standard algorithm for diagnosis of HCC is the demonstration of the phenomenon of hypervascularity of a nodule or lesion in the arterial phase with subsequent hypoattenuation or “wash-out” of the lesion compared to rest of the normally enhancing liver parenchyma on the portal venous or equilibrium phase. [3], [4], [5] The challenge however; is to characterize nodules, which do not show adequate hypervascularity on the arterial phase or demonstrate inadequate “wash-out” on later phases of routine dynamic CT or MRI. These lesions are characterized as ‘indeterminate’ nodules on imaging and require biopsy with histopathology or further investigations (e.g. contrast enhanced sonography with kupffer cell specific contrast/MRI with SPIO or hepatocyte specific contrast) which may or may not be available at all centers. MRI is universally recognized as a problem solving modality and has a higher procedure cost with worldwide limited availability. These indeterminate/atypical tumors may be difficult to diagnose, even with all the above criteria put together. Biopsy is the current gold standard of diagnosis however; smaller lesions are technically difficult to visualize and may show lack of sufficient tissue, with nodule heterogeneity within the lesion itself. The fine needle aspiration or biopsy sample may not be representative of the entire nodule. To overcome the above lacunae, we have tried to explore spectral CT for its diagnostic capabilities in context to diagnosis of HCC, in patients with pre existing chronic liver disease, regardless of the underlying etiology. The advent of spectral CT is based on the principle that this technique involves scanning at distinctly different energies (most commonly used energy levels are 70 and 140 kVp) using a setup of single or dual x-ray tubes with detectors operated at two distinct energies or rapidly switching between two different energies depending on the manufacturer. [6], [7], [8], [9] This is based on the physics principle of ‘Photoelectric effect’ which, is used to obtain additional information regarding tissue composition. The photoelectric effect essentially utilizes the difference in the K-edges of two elements. This principle works best for post contrast CT scans due to the use of Iodine, which has a K-edge of (33.2 keV). This K-edge is closer to lower energies (80 kVp) compared with higher energies (140 kVp) on a spectral (essentially a dual energy) CT scan and hence Iodine containing tissue is more attenuating (better visualized) at lower energies. The higher attenuation of Iodine results in improved conspicuity of hypervascular (Iodine rich) nodules in contrast to rest of the liver parenchyma.
We have tried to ascertain a baseline value of iodine material density in the arterial phase of histopathological proven cases of HCC which were labeled as “indeterminate” nodules on routine dynamic imaging. This is based on the hypothesis that we may be able to define a possible cut-off value at which we can predict the diagnosis of HCC using spectral CT alone. Our long term goal is to explore spectral CT as a functional tool using iodine density as a predictor of tissue composition and tumor grading. In future, this may obviate the need for biopsy, completely. We may be able to characterize HCC on a single-phase study, reducing radiation exposure, dosage of intravenous contrast as well as the need for second modality confirmation.
2. Material and methods
A total of 3600 patients (mean age 50.9 ± 11.9 years, 2879 males and 721females), who were clinically proven to have liver cirrhosis due to various etiologies were scanned by routine CT (with simultaneous acquisition of spectral CT data sets) over a period of three years at our tertiary liver care hospital. All patients with liver cirrhosis, who had indeterminate liver nodules on dynamic routine CT (irrespective of the lesion size) and were, advised a histopathological analysis on imaging were enrolled in the study group. This comprised our study group of 37 patients (mean age 51.65 ± 12.33 years, 33 males and 4 females) who underwent further histopathological assessment or surgical excision of these lesions. A blinded; retrospective, double reader, single centered analysis of the spectral CT data was conducted in a clinical framework. The workflow of the study is demonstrated in a flow chart given below. (Table 1).
Table 1.
Work flow-chart of study design and method of conducting the study.
 |
Abbreviations: HU—Hounsfield Units; HAP—Hepatic arterial Phase; LLR—Lesion to liver parenchyma iodine density ratio.
2.1. Inclusion criteria
‘Indeterminate nodule’ was defined as uncharacteristic/atypical enhancement pattern of HCC, not conforming to classical arterial hypervascularity and portal venous or equilibrium phase washout on dynamic scans.
2.2. Exclusion criteria
Patients who did not have background liver cirrhosis, patients who did not consent to evaluation with biopsy and histopathology and pregnant and lactating women. Patients in whom no possibility of imaging diagnosis of HCC was present on routine CT were also excluded from this study group. The institutional ethical committee waived off permission for retrospective analysis of data. Strict confidentiality was maintained and voluntary informed consent was taken before participation.
2.3. Spectral CT scanning technique
Routine dynamic CT was performed on a single tube, multi-detector (64 slice) spectral scanner with fast kVp switching technology (GE Healthcare, USA) scanner. All patients were scanned cranio-caudally, in the supine position using injectable nonionic contrast material (Iomeprol, 400 mg/dl Iodine, calculated as 1.5 ml per kilogram of body weight) at the rate of 4 ml/s pumped through a pressure injector. A saline bolus of 50 ml was given at the same flow rate after the contrast injection. Scan delay for dynamic phase was determined by using automated scan triggering software (Smart Prep; GE Healthcare). Scanning was simultaneously performed in the spectral imaging mode with fast tube voltage switching between 80 and 140 kVp on adjacent views during a single rotation. Other scanning parameters were as follows: collimation thickness of 0.625 mm, tube current of 600 mA, rotation speed of 0.6 s& helical pitch of 0.983. The three sub groups of image data sets which were reconstructed from the single spectral acquisition for analysis were as follows: a set of polychromatic images or the routine dynamic CT, corresponding to the conventional 140 kVp imaging (which was studied previously and used for segregating our patient study group as indeterminate lesions for a blind review through spectral imaging), monochromatic image sets with photon energies ranging from 40 to 140 keV(used for qualitative analysis) and Iodine-based material-decomposition images (used for quantitative analysis). (Fig. 1) The latter two sets of spectral CT acquisitions were analyzed with the GSI Viewer software 4.4 (GE Healthcare, Waukesha, Wisconsin), with a standard soft-tissue display window preset (WL 40 and WW 400). The monochromatic image sets were read at a low keV of 55 keV (which has been found optimal for studying liver lesions, without minimum image noise and best visualization of contrast between lesion and normal parenchyma). [10], [11] For the quantitative analysis of monochromatic sets, two circular regions-of-interest (ROI) were placed on the lesion and the liver parenchyma individually. For each ROI the mean number of pixels were 400; range, 310–560 which was placed in the maximum enhancing portion of the lesion, hepatic parenchyma surrounding the lesion and the abdominal aorta at the same axial section of the abdomen, keeping the size, shape, and position of the ROI consistent by applying the copy-and-paste function. The values of the ROI in HU were then plotted on a spectral HU graph with z axis representing 40–140 kVp spectrums and the Y axis representing the HU values (Fig. 2). This is a graphical representation of lesion conspicuity with respect to the background liver. The attenuation of Aorta was used to normalize (baseline standard reference) value of the lesion and liver for constructing the spectral curve. (Fig. 2).
Fig. 1.
55 year old male with hepatitis B related chronic liver disease on follow up CT scan with three sets of image datasets for analysis. a. Polychromatic routine dynamic arterial phase image to depict faintly enhancing lesion in segment IV of liver (bold arrow). b. Monochromatic image viewed at 55 keV from the spectrum of images derived between 40 to 140 kVp with lesion conspicuity more than previous routine image (bold arrow). c. Material decomposition image showing Iodine containing structures with rest of the components appearing suppressed, the focal lesion (bold arrow) shows iodine enhancement on the Iodine map.
Fig. 2.
55 year old male with hepatitis B related chronic liver disease on follow up CT scan showing axial section of the superior aspect of the liver in the arterial phase with spectral HU curve of enhancing lesion. a. Axial section of the superior aspect of liver showing ROI’s in the enhancing portion of the liver lesion labeled as (1), the surrounding liver parenchyma as (3) and within the aortic lumen as (2). b Spectral HU curve of the same lesion denoting the normalized aorta on the top of the graph (bold vertical arrow), the graph of the liver lesion in comparison to the normalized aorta (horizontal arrow without fill) and the normal liver curve (vertical arrow without fill).
The qualitative analysis of lesion visibility and conspicuity was graded on an ascending scale of 1–5 with Grade 1 indicating poor visualization of lesion, Grade 2: lesion visualized however arterial enhancement absent, Grade3: adequate visualization of lesion with mild arterial enhancement, Grade 4: adequate visualization of lesion with moderate arterial enhancement and Grade 5 indicative of excellent lesion visualization with bright arterial enhancement. The sets of monochromatic images described above and the Iodine-based material-decomposition datasets were taken into consideration. These were independently read by two radiologists (YV, STL) with experience in hepatobiliary CT imaging for 5 and 10 years respectively. Qualitative analysis of lesion conspicuity with the same grading system was done on the polychromatic images acquired from routine CT data and both data were compared using agreement analysis, Interclass correlation (ICC) with 95% confidence intervals and Bland-Altmann graphs.
2.4. Quantitative analysis
The Iodine-based material-decomposition set of images were used to derive iodine quantification (measured in mg/dl) of the lesion and rest of the liver, in the HAP (Fig. 3). Scatter plots were drawn after quantifying the iodine content using standard and uniform sized ROI’s within the enhancing portion of the lesion, liver parenchyma and the Aorta (for baseline enhancement and normalization of data). (Fig. 3). These parameters were tabulated and statistically analyzed. The various calculated parameters were as follows:
-
a)
On the Material decomposition image sets (Iodine concentration measured in mg/dl)
-
b)
GSI enhancement value (GSI) of Lesion = GSI lesion
-
c)
GSI enhancement value (GSI) of Liver = GSI Liver
-
d)
Lesion-to–parenchyma ratio (LLR) = Ratio of Iodine concentration in lesion/Iodine concentration in liver
-
e)
GSI enhancement value (GSI) of Aorta = GSI Aorta
Fig. 3.
45 year old male patient with a cirrhotic liver showing hypervascular lesions. Axial material decomposition images showing iodine density values and scatter plot of the lesions. a. Axial iodine density map of the material decomposition image dataset depicting iodine quantification done within the liver lesion using an ROI labeled (1), the surrounding liver parenchyma labeled as (2) and the Aorta as (3). b The values of the ROI’s measuring Iodine density in mg/ml of the liver lesion (horizontal arrow without fill) and the surrounding liver curve (vertical arrow without fill) has been plotted on the scatter plot with values normalized against the density in the Aorta ((bold vertical arrow).
2.5. Reference standards
The imaging diagnosis was made with the radiologists blinded to the end result of biopsy and histopathology which were considered as reference gold standard. The grading system of Edmondson and Steiner was used as standard for HCC grading protocol by the pathologist. [12] The investigators were also blinded to the clinical history and results of serum Alfa feto protein (AFP) level, which is used as an important screening and surveillance marker for HCC. The results were used to further sub-divide the patient groups into true positive HCC (those which were confirmed by reference gold standard to be same as imaging results of HCC), true negatives (those which were reported as non malignant by imaging and confirmed to be non malignant on biopsy), false positives (those which were diagnosed on imaging as HCC but were proven to be non-malignant on histopathology) and false negatives (those HCC’s which were missed on Imaging but were confirmed HCC on histopathology).
2.6. Calculation/statistical analysis
The data is presented as proportions; mean ± standard deviations, median with interquartile range or in frequencies (%). The statistical methods applied were proportion test and Mann Whitney U test, wherever applicable. Besides this, diagnostic tests were applied to calculate the various characteristics for diagnosis. Data were also compared using agreement analysis between the two readers using Interclass correlation (ICC) with 95% confidence intervals and Bland-Altmann graphs were plotted.
Receiver operating characteristic curves (ROC) were used to depict cut off values and calculate the area under curve. Sensitivity, specificity, positive predictive values, negative predictive values were used to diagnose and characterize lesions objectively. P value less than 0.05 were considered as significant.
3. Results
A total of 37 patients (mean age 23.5 ± 10.7 years) 22 males and 39 females, were analyzed within the study group. Of these, 37 patients; 21 were diagnosed to have HCC vs 16 patients who were found to have non HCC lesions on histopathology (Table 2). Histopathological tumor grading evaluation of HCC lesions showed that 4/21 (19%) tumors were Grade 3 and 17/21 (81%) lesions were Grade 2 HCC. Mean age of the HCC vs Non HCC groups was 52.67 ± 12.14 Vs 50.3 ± 12.5 years (p = 0.57), gender distribution: 54.5 males vs 25.6% females (p = 0.77) respectively. The distribution of lesions in HCC vs non HCC group (p = 0.30) with respect to segment of liver has been depicted in (Table 3). Median lesion size of HCC vs non HCC groups was found to be 2 (IQR 0–16) vs 4 (IQR 1–9) cms, (p = 0.77).
Table 2.
Histopathology results of Non Hepatocellular lesions found indeterminate on Routine CT.
| Non HCC histopathology | Number of particular lesions (Of a total of 16) | Percentage of the lesion (in%) |
|---|---|---|
| Regenerative Nodule | 4 | 25 |
| Hepatocellular −Cholangiocarcinoma (mixed tumor) | 3 | 18.75 |
| Secondaries from unknown occult primary tumor | 2 | 12.5 |
| No tumor cells, normal hepatocytes (Likely arterio-portal shunts) | 3 | 18.75 |
| Fibrolamelllar Carcinoma | 1 | 6.25 |
| Mesenchymal tumor | 6.25 | |
| Cholangiocarcinoma | 1 | 6.25 |
| Hemangioendothelioma | 1 | 6.25 |
Table 3.
Anatomical CT distribution of various lesions in the segments of liver.
| Location of the lesions in segment of liver | HCC lesions | Non HCC Lesion (n = 21 lesions) | Total lesions (n = 16lesions) |
|---|---|---|---|
| Segment 2 | 3 (14.3%) | 1 (6.2%) | 4 (10.8%) |
| Segment 3 | 3 (14.3%) | 0 (0%) | 3 (8.1%) |
| Segment 4 | 3 (14.3%) | 5 (31.2%) | 8(21.6%) |
| Segment 5 | 2(9.5%0 | 2(9.5%0 | 4(10.8%) |
| Segment 6 | 3(14.35) | 3(18.8%) | 6 (16.2%) |
| Segment 7 | 2(9.5%) | 4(25.0%) | 6(16.2%) |
| Segment 8 | 5(23.8%) | 1(6.2%) | 6(16.2%) |
The median grade (qualitative analysis) for visualization of HCC on routine CT was 2 (IQR 1–4) vs 2.5 (IQR 1–4) for non HCC lesions, (p = 0.59). The median grade for visualization of HCC on spectral CT was 4 (IQR 3–5) vs 5 (IQR 3–5) for non HCC lesions, (p = 0.30).
The agreement between two observers was seen using Intra class correlation (ICC). The ICC for routine CT was found to be 91.6% with 95% CI (81–96%). 2.7% cases were not in agreement with the two observers. (Fig. 4) Similarly the agreement between the two observers on spectral CT was found to be 88.9%, with 95% CI (77–94). 2.7% cases were not in agreement with the two observers. (Fig. 5).
Fig. 4.
Bland and Altmann graph depicting 2 observer agreement analysis on routine CT observations of qualitative lesion conspicuity.
Fig. 5.
Bland and Altmann graph depicting 2 observer agreement analysis on spectral CT observations of qualitative lesion conspicuity.
The parameters that were considered for quantitative analysis were subgroups of iodine density GSI lesion, GSI aorta, GSI liver and ratio of GSI lesion to surrounding liver parenchyma (LLR). The two groups for summary statistics were divided as per the biopsy results into biopsy proven HCC and non HCC as shown in Table 4. There were total of 37 cases, out of which 21 were HCC and 16 were non HCC cases on histopathology. On spectral CT, there were 13 (35.1%) true positive and 12 (32.4%) true negatives. The number of false negative (missed on spectral CT) cases were 8 in number (21.6%) and the false positive cases (incorrectly classified as HCC on spectral CT were 4 (10.8%).
Table 4.
Comparison of spectral CT parameters between HCC and non HCC groups.
| Parameter calculated on spectral CT | HCC lesions N = 21 Mean ± SD | Non HCC lesions N = 16 Mean ± SD | p value (Student ‘t’- test) |
|---|---|---|---|
| GSI lesion (mg/dl) | 67.7 ± 29.1 | 23.2 ± 10.8 | <0.001 |
| GSI Aorta (mg/dl) | 152.3 ± 24.2 | 144.4 ± 26.3 | 0.802 |
| GSI liver (mg/dl) | 60.6 ± 13.9 | 62.0 ± 20.4 | 0.349 |
| GSI LLR ratioa | 1.2 (0.7–1.4) | 0.3 (0.2–0.6) | <0001# |
# Mann-Whitney U test.
Median with Inter-quartile range.
The average GSI lesion value for the HCC group was 67.7 ± 29.1 mg/dl and for non HCC group was 23.2 ± 10.8 mg/dl and showed statistical significance (<0.001) between the two groups. The average value for the GSI iodine density of Aorta in the arterial phase of HCC group was 152.3 ± 24.2 mg/dl. The GSI iodine density of Aorta in the non HCC group was 144.4 ± 26.3 mg/dl. The mean GSI density of liver surrounding parenchyma in the HCC group was 60.6 ± 13.9 mg/dl and in the non HCC group was 62.0 ± 20.4 mg/dl. The ratio of lesion iodine density to the surrounding liver parenchyma iodine density (LLR) showed an average value of 1.2 (0.7–1.4) in the HCC group and 0.3 (0.2–0.6) in the control group. This was found to be significant (p < 0.001) (calculated using Mann Whitney U test). These results enabled us to differentiate HCC from other lesions on spectral CT. We further analyzed the data to distinguish the HCC group on the basis of a cut off value. These are explained in Table 5 given below.
Table 5.
Diagnostic characteristics of spectral CT in HCC.
| Parameter | Cut off value | Area under curve (95% CI) | P value | Sensitivity | Specificity | PPV | NPV | Correctly classified |
|---|---|---|---|---|---|---|---|---|
| GSI Lesion | ≥29.5 | 0.94 (0.87–1.00) | <0.001 | 90.5 (69.2–98.8) | 81.2 (54.4–95.9) | 86.4 (65.1–97.1) | 86.7 (59.5–98.3) | 86.5% |
It has been shown in Table 5 that, if the value of GSI lesion is >29.5 mg/dl of iodine, then the discriminatory power is 86.5% with a sensitivity of 90.5% CI (69.2–98.8%) and specificity of 81.2% with 95% CI (54.4–95.9%). (ROC curve, Fig. 6) Hence GSI lesion values can be used for discriminating the indeterminate cases performed on spectral CT.
Fig. 6.
ROC curve for GSI lesion in HCC group.
The comparison between biopsy results and spectral CT (GSI parameters in this case) technique shows that usage of spectral dual energy CT with objective criteria of material density in the form of iodine density of suspected lesions was able to determine at-least two more cases which could not be diagnosed on the initial qualitative assessment of lesions on the spectral HU curve analysis and monochromatic images.
4. Discussion
Surveillance and screening of HCC is an important factor in decreasing the overall cost of its treatment in patients with known CLD as well as in improving the survival of diseased patients. The aim of surveillance is to diagnose a malignant nodule at its earliest stage, so that the treatment has the highest possible likelihood of cure with the existing treatment options. Majority (almost 80%) of HCC’s are seen in patients with cirrhosis due to viral infections. [13] A prospective controlled study showed that the annual incidence of HCC in hepatitis B carriers was 2.5%. [14], [15] The annual incidence increases with age. The risk of HCC in patients with chronic hepatitis C is highest among patients who have established cirrhosis, which is between 2%-8% per year. [16], [17], [18] Our study was conducted in an endemic belt for chronic hepatitis B and C infected patients. Imaging of the cirrhotic liver is a diagnostic challenge for radiologists when it comes to characterization and differentiation of regenerative, dysplastic nodules as well as early HCC nodules. These nodules are superimposed on a background of morphologically altered and chronically diseased liver parenchyma. Regenerative nodules are essentially normal hepatocyte containing tissue that shows nodular proliferation in a damaged liver. They have no malignant potential and are usually isoattenuating to the liver parenchyma but may appear hypoattenuating to the liver. MRI is utilized as the standard problem solving modality for indeterminate lesions, for follow up of known malignancies and post radiofrequency ablation or chemoembolization. However; because of its higher cost and limited availability at all centers, it is the need of the hour today to look for alternate options within the existing technology. Although biopsy is considered as a gold standard for evaluation of indeterminate lesions, it has its own technical issues, in assessing nodules of small size, lack of sufficient tissue obtained and ill visualization of the nodule in a cirrhotic liver. Moreover, histopathology of a heterogeneous nodule, with variable amounts of hepatocytes, dysplastic tissue, malignant cells or fat within a single lesion, may not be representative of the entire nodule. Similar issues may be encountered on imaging such as on routine CT, which may be unable to differentiate the low attenuation of fat area within a steatohepatic variant of HCC from fluid. We were able to demonstrate fat within a heterogeneous lesion with the help of spectral curve values, at low (55) keV. (Fig. 7, Fig. 8, Fig. 9)
Fig. 7.
55 year old male with hepatitis B related chronic liver disease on follow up CT scan. Comparison of an enhancing heterogeneous lesion in segment VIII of liver on routine versus low keV spectral CT. a. Enhancing component (open arrow) on routine CT. b. The enhancing lesion (bold arrow) is better visualized on low (55) keV spectral CT monochromatic image. c. The low attenuation (central ROI within it) showing fluid attenuation of 5HU on the routine CT.
Fig. 8.
55 year old male with hepatitis B related chronic liver disease on follow up CT scan. Spectral HU curve plot and GSI scatter plots to depict the attenuation and iodine density values of the low density portion of the nodule in segment VIII, seen in Fig. 7c. a. Spectral HU curve depicting the negative attenuation (green tracing on the graph) suggestive of fat component with the arrow showing the approximate values compared to the lesion (royal blue color trace) and the aorta enhancement (light blue trace). The fatty attenuation of the area marked (*). b. GSI scatter plot showing the iodine density of the same three lesions as depicted in Fig. 8a.
Fig. 9.
Gross specimen post hepatectomy showing the nodule (dotted circle) − steatohepatic variety of HCC) containing fat (*) within it.
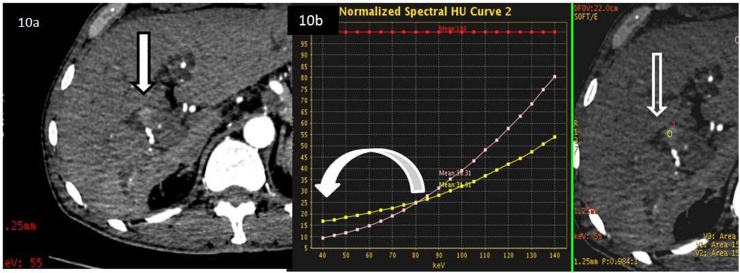
The target of differentiating malignant lesions on imaging can only be actualized by using newer and more advanced technology like spectral CT scanners. With the help of the above, characterization of the benign and malignant liver lesions with accuracy is bound to affect the prognosis and treatment methods in patients with liver cirrhosis. Accurate diagnosis and characterization of malignant nodules, on a background of a diffusely cirrhotic liver, is the responsibility of the radiologist, since clinical suspicion and tumor markers may not be elevated in all malignant or dysplastic nodules. [19], [20] Attenuation difference observed with routine computed tomography (CT) has poor sensitivity in characterization of the nature of liver nodules. [21], [22] HCC is characterized on dynamic CT by the classical pattern of arterial hypervascularity and washout on equilibrium phases; however, hypo-vascular or atypical tumors may be difficult to diagnose with these criteria. [23], [24] Compared to conventional multi-detector CT scanners, the advanced technique of spectral CT allows differentiation of material and tissue composition, based on differences in iodine and water densities [25], [26] The use of advanced spectral CT has revealed that lower (80 kVp) images demonstrate a better image contrast than higher (140) kVp images which allow relatively hypovascular lesions to be easily visualized on the low keV monochromatic datasets. [8,15, 27 and 28] An example of this has been demonstrated in (Fig. 10). This lesion was found to be relatively hypovascular on routine CT and was virtually indistinguishable on it. Application of low keV spectral CT, made the lesion conspicuous and the spectral HU curve values were able to show that the lesion was actually hypervascular compared to the liver parenchyma. (Fig. 10) Histopathology found HCC invading the bile duct with tumor thrombus within it. (Fig. 11)
Fig. 10.
63 year old cirrhotic male patient presented with suspicious blush at the porta, evaluated on a dynamic CT followed by analysis on spectral CT. a. Low keV (55kev) CT shows a definite focal (arrow, black outline) area of blush. b. Spectral HU curve shows that lesion (open white arrow in iodine density map) is visualized (white curved arrow pointing at attenuation difference) only at low keV as analyzed (yellow line tracing of the lesion).
Fig. 11.
Gross specimen of the mass (white arrow with black outlines) (moderately differentiated Grade 2 HCC on histopathology) with bile duct tumor thrombus, small vessels invasion, embolization and the bile duct, seen separate from mass.
Low keV spectral studies and MMD images appear to be useful for the evaluation of enhanced liver lesions in the arterial phase in HCC. In the liver, spectral CT may be used to increase conspicuity of hypervascular lesions; to distinguish hyperattenuating cysts from enhancing focal liver lesions and to characterize masses, hypervascular metastases, as well as to differentiate between normal variant areas of hypervascularity such as transient hepatic attenuation defects (THAD) and abnormal parenchyma. [29] An example of this was demonstrated by a patient from our study group, who showed a hypervascular mass in the left lobe, with an attending area of suspicious blush seen around it, which could not be differentiated from the mass itself. On application of spectral CT technique, we were able to differentiate the area of THAD from the actual lesion, which allowed us to make accurate decisions on treatment therapy since the lesion size could be adequately assessed with the help of the iodine density sets. It has been found on comparison of 80 and 140 kVp images that 80 kVp images with a high mA enabled the visualization of a greater number of lesions compared to 140 kVp images to detect hypervascular lesions in vitro and in vivo. [28], [30] A range of keV value has been recommended in studies published in literature to study data sets for the best contrast-to-noise ratio (CNR) between liver lesions and underlying liver parenchyma as well as for lesion conspicuity. [7], [8] 80 kVp images were also documented to be superior for the distinction of metastases in normal liver tissue. [27] Low keV spectral CT and iodine density images are able to delineate diffuse infiltrative masses which may be virtually indistinguishable on a routine CT, especially since these tumors are apparently hypovascular on routine CT and hence may not show adequate washout.
The functional aspect of spectral CT has been proposed by demonstrating how increasing arterial vascularity and decreasing portal flow to nodules is predictive of transition from borderline lesions to malignant nodules over a period of time. [21], [22] This aspect of spectral CT and quantitative analysis of iodine density within a liver nodule, which can predict its vascularity, forms the basic concept of our study. Similar study models have been utilized in the mediastinum and chest to differentiate between benign and malignant lesions within the mediastinum [31], [32].
Our study has been able to distinguish approximately 86% of the lesions which were primarily categorized as indeterminate nodules/masses on routine dynamic CT, to be malignant. This is significant in a population which underwent biopsy/invasive surgery to determine the final outcome of the nodules in question.
Our study, had a few limitations such as: sample bias (included only cirrhotic patient population), sample size (since number of patients found suitable for biopsy and surgery is limited in patients with HCC) and the need for further comparison within groups of benign (regenerative, siderotic) and malignant (dysplastic, well differentiated and poorly differentiated lesions) nodules in patients with cirrhosis. All hypervascular masses, besides HCC, which may present in a cirrhotic liver, such as hypervascular metastases or mesenchymal tumors of the liver like angiosacroma’s may be difficult to differentiate till further analysis to compare the various sub-types of hypervascular tumors is made.
In future, similar studies with larger populations and in-depth study of nodule composition, may pave the path for reduced biopsy procedures. Furthermore, spectral assessment of material composition may allow tumor grading without histopathology. We, for the first time, tried to identify HCC from non HCC lesions by quantifying their enhancement using iodine density values, on multi spectral CT. To date, there are no studies using spectral CT for this purpose. The above results show that instead of biopsy we can use the cutoff point of iodine density within the lesion on the material density images as 29.5 mg/dl to diagnose HCC on spectral CT when routine CT is unable to characterize the nature of these lesions.
5. Conclusions
This study reveals that spectral imaging is an excellent tool for qualitative as well as quantitative tool for assessing indeterminate lesions in patients with chronic liver disease. It can be used to create a novel design with material (iodine) density measurements that will serve as a functional tool to derive the composition of liver nodules.
Contributor Information
S.T. Laroia, Email: thaparshalini@gmail.com.
Ajeet Singh Bhadoria, Email: ajeetsinghbhadoria@gmail.com.
Yamini Venigalla, Email: yaminipratap@gmail.com.
G.K. Chibber, Email: chibbegk@gmail.com.
Chagan Bihari, Email: drcbsharma@gmail.com.
Archana Rastogi, Email: drarchanarastogi@gmail.com.
S.K. Sarin, Email: shivsarin@gmail.com.
References
- 1.Bruix J., Sherman M. Management of hepatocellular carcinoma: an update AASLD, American Association for the Study of Liver Diseases. Hepatology. 2011;53(March (3)):1020–1022. doi: 10.1002/hep.24199. PMID: 21374666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Omata M., Lesmana L.A., Tateishi R., Chen P.J., Lin S.M., Yoshida H. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol. Int. 2010;4:439–474. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kudo M., Izumi N., Kokudo N., Matsui O., Sakamoto M., Nakashima O. HCC expert panel of Japan society of hepatology: management of hepatocellular carcinoma in japan: consensus-based clinical practice guidelines proposed by the Japan society of hepatology (JSH) 2010 updated version. Dig. Dis. 2011;29:339–364. doi: 10.1159/000327577. [DOI] [PubMed] [Google Scholar]
- 5.Forner A., Vilana R., Ayuso C., Bianchi L., Solé M., Ayuso J.R., Boix L., Sala M., Varela M., Llovet J.M., Brú C., Bruix J. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008;47(January (1)):97–104. doi: 10.1002/hep.21966. [DOI] [PubMed] [Google Scholar]
- 6.Morgan D.E. Dual-energy CT of the abdomen. Abdom. Imaging. 2014;39(February (1)):108–134. doi: 10.1007/s00261-013-0033-5. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L.J., Peng J., Wu S.Y. Liver virtual non-enhanced CT with dual-source, dual-energy CT: a preliminary study. Eur. Radiol. 2010;20:2257–2264. doi: 10.1007/s00330-010-1778-7. [DOI] [PubMed] [Google Scholar]
- 8.Cook T.S., Zimmerman S.L., Steingall S.R., Boonn W.W., Kim W.J. An algorithm for intelligent sorting of CT-related dose parameters. Digit Imag. 2012;25(February (1)):179–188. doi: 10.1007/s10278-011-9410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henzler T., Fink C., Schoenberg S.O., Schoepf U.J. Dual-energy CT: radiation dose aspects. AJR Am. J. Roentgenol. 2012;199(November (5 Suppl)):S16–S25. doi: 10.2214/AJR.12.9210. Review: Erratum in: AJR Am. J. Roentgenol., 2013, 200 March (3), 705, PMID: 23097163. [DOI] [PubMed] [Google Scholar]
- 10.Yeh B.M., Shepherd J.A., Wang Z.J., Teh H.S., Hartman R.P., Prevrhal S. Dual-energy and low-kVp CT in the abdomen. AJR Am. J. Roentgenol. 2009;193:47–54. doi: 10.2214/AJR.09.2592. PMID: 19542394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graser A., Johnson T.R., Chandarana H., Macari M. Dual energy CT: preliminary observations and potential clinical applications in the abdomen. Eur. Radiol. 2009;19:13–23. doi: 10.1007/s00330-008-1122-7. PMID: 18677487. [DOI] [PubMed] [Google Scholar]
- 12.Edmonson H.A., Steiner P.E. Primary carcinoma of the liver. Cancer. 1954;7:462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 13.Mc Glynn K.A., London W.T. Epidemiology and natural history of hepatocellular carcinoma. Best Pract. Res. Clin. Gastroenterol. 2005;19:3–23. doi: 10.1016/j.bpg.2004.10.004. PMID: 15757802. [DOI] [PubMed] [Google Scholar]
- 14.Beasley R.P., Hwang L.Y., Lin C.C., Chien C.S. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22707 men in Taiwan. Lancet. 1981;2:1129–1133. doi: 10.1016/s0140-6736(81)90585-7. PMID: 6118576. [DOI] [PubMed] [Google Scholar]
- 15.Coursey C.A., Nelson R.C., Boll D.T., Paulson E.K., Ho L.M., Neville A.M., Marin D., Gupta R.T. Dual-energy multidetector CT: how does it work, what can it tell us, and when can we use it in abdominopelvic imaging? Radiographics. 2010;30(July–August (4)):1037–1055. doi: 10.1148/rg.304095175. PMID: 20631367. [DOI] [PubMed] [Google Scholar]
- 16.Tsukuma H., Hiyama T., Tanaka S. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N. Engl. J. Med. 1995;328:1797–1801. doi: 10.1056/NEJM199306243282501. [DOI] [PubMed] [Google Scholar]
- 17.Fattovich G., Giustina G., Degos F., Tremolada F., Diodati G., Almasio P., Nevens F. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463–472. doi: 10.1053/gast.1997.v112.pm9024300. PMID: 9024300. [DOI] [PubMed] [Google Scholar]
- 18.Niederau C., Lange S., Heintges T., Erhardt A., Buschkamp M., Hurter D., Nawrocki M. Prognosis of chronic hepatitis C: results of a large, prospective cohort study. Hepatology. 1998;8:1687–1695. doi: 10.1002/hep.510280632. PMID: 9828236. [DOI] [PubMed] [Google Scholar]
- 19.Marrero J.A., Fontana R.J., Barrat A., Askari F., Conjeevaram H.S., Su G.L., Lok A.S. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005;41:707–716. doi: 10.1002/hep.20636. PMID: 15795889. [DOI] [PubMed] [Google Scholar]
- 20.Trevisani F., D’Intino P.E., Morselli-Labate A.M., Mazzella G., Accogli E., Caraceni P., Domenicali M. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J. Hepatol. 2001;34:570–575. doi: 10.1016/s0168-8278(00)00053-2. PMID: 11394657. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi M., Matsui O., Ueda K., Kawamori Y., Gabata T., Kadoya M. Progression to hypervascular hepatocellular carcinoma: correlation with intranodular blood supply evaluated with CT during intraarterial injection of contrast material. Radiology. 2002;225(October (1)):143–149. doi: 10.1148/radiol.2251011298. (PMID: 12354998. [DOI] [PubMed] [Google Scholar]
- 22.Matsui O. Imaging of multistep human hepatocarcinogenesis by CT during intra-arterial contrast injection. Intervirology. 2004;47(3–5):271–276. doi: 10.1159/000078478. PMID: 15383735. [DOI] [PubMed] [Google Scholar]
- 23.Sherman M. Screening for hepatocellular carcinoma. Baillieres Best Pract. Res. Clin. Gastroenterol. 1999;13:623–635. doi: 10.1053/bega.1999.0052. PMID: 10654924. [DOI] [PubMed] [Google Scholar]
- 24.Fattovich G., Stroffolini T., Zagni I., Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(November (5 Suppl. 1)):S35–S50. doi: 10.1053/j.gastro.2004.09.014. PMID: 15508101. [DOI] [PubMed] [Google Scholar]
- 25.Robinson E., Babb J., Chandarana H., Macari M. Dual source dual energy MDCT: comparison of 80 kVp and weighted average 120 kVp data for conspicuity of hypo-vascular liver metastases. Invest. Radiol. 2010;45:413–418. doi: 10.1097/RLI.0b013e3181dfda78. PMID: 20458250. [DOI] [PubMed] [Google Scholar]
- 26.Kim K.S., Lee J.M., Kim S.H. Image fusion in dual energy computed tomography for detection of hypervascular liver hepatocellular carcinoma: phantom and preliminary studies. Invest. Radiol. 2010;45:149–157. doi: 10.1097/RLI.0b013e3181d32119. PMID: 20142749. [DOI] [PubMed] [Google Scholar]
- 27.Johnson T.R., Krauss B., Sedlmair M. Material differentiation by dual energy CT: initial experience. Eur. Radiol. 2007;17:1510–1517. doi: 10.1007/s00330-006-0517-6. PMID: 17151859. [DOI] [PubMed] [Google Scholar]
- 28.Marin D., Nelson R.C., Samei E. Hypervascular liver tumors: low tube voltage, high tube current multidetector CT during late hepatic arterial phase for detection–initial clinical experience. Radiology. 2009;251:771–779. doi: 10.1148/radiol.2513081330. PMID: 19346514. [DOI] [PubMed] [Google Scholar]
- 29.Fletcher J.G., Takahashi N., Hartman R. Dual-energy and dual-source CT: is there a role in the abdomen and pelvis? Radiol. Clin. North Am. 2009;47:41–57. doi: 10.1016/j.rcl.2008.10.003. PMID: 19195533. [DOI] [PubMed] [Google Scholar]
- 30.Marin D., Nelson R.C., Schindera S.T. Low-tube-voltage, high-tube-current multidetector abdominal CT: improved image quality and decreased radiation dose with adaptive statistical iterative reconstruction algorithm-initial clinical experience. Radiology. 2010;254:145–153. doi: 10.1148/radiol.09090094. PMID: 20032149. [DOI] [PubMed] [Google Scholar]
- 31.De Cecco C.N., Buffa V., Fedeli S. Dual energy CT (DECT) of the liver: conventional versus virtual unenhanced images. Eur. Radiol. 2010;20(December (12)):2870–2875. doi: 10.1007/s00330-010-1874-8. [DOI] [PubMed] [Google Scholar]
- 32.Lee S.H., Hur J., Kim Y.J., Lee H.J., Hong Y.J. Choi Additional value of dual-energy CT to differentiate between benign and malignant mediastinal tumors: an initial experience. Eur. J. Radiol. 2013;82(November (11)):2043–2049. doi: 10.1016/j.ejrad.2013.05.040. Epub 2013 Jun 29. [DOI] [PubMed] [Google Scholar]