Abstract
The discovery of the transforming growth factor β (TGF-β) family ligands and the realization that their bioactivities need to be tightly controlled temporally and spatially led to intensive research that has identified a multitude of extracellular modulators of TGF-β family ligands, uncovered their functions in developmental and pathophysiological processes, defined the mechanisms of their activities, and explored potential modulator-based therapeutic applications in treating human diseases. These studies revealed a diverse repertoire of extracellular and membrane-associated molecules that are capable of modulating TGF-β family signals via control of ligand availability, processing, ligand–receptor interaction, and receptor activation. These molecules include not only soluble ligand-binding proteins that were conventionally considered as agonists and antagonists of TGF-β family of growth factors, but also extracellular matrix (ECM) proteins and proteoglycans that can serve as “sink” and control storage and release of both the TGF-β family ligands and their regulators. This extensive network of soluble and ECM modulators helps to ensure dynamic and cell-specific control of TGF-β family signals. This article reviews our knowledge of extracellular modulation of TGF-β growth factors by diverse proteins and their molecular mechanisms to regulate TGF-β family signaling.
Diverse molecules modulate TGF-β family signals by controlling ligand availability and ligand–receptor functionality. These molecules include soluble regulators as well as extracellular matrix proteins and proteoglycans.
Transforming growth factor β (TGF-β) family signaling uses a large number of secreted growth factors that engage a limited number of cell-surface receptors, and regulate diverse processes, such as embryonic induction and patterning, tissue maintenance and repair, stem cell renewal and differentiation, and organism growth and regeneration. The prevalence of TGF-β family signaling in almost all metazoan cell types, the overlapping and distinct functions of many related ligands, and the strict temporal and spatial requirement for suitable signaling levels necessitate stringent control of TGF-β family signaling. A prominent strategy used by cells to regulate TGF-β family signaling is through the use of extracellular agonists and antagonists of TGF-β family ligands. An impressive array of such regulatory molecules has been identified and they associate with TGF-β family ligands directly or indirectly to modulate their processing, secretion, stability, diffusion, and presentation. Collectively, extracellular agonists and antagonists play crucial roles in determining TGF-β family signaling strength, range, timing, and duration, and serve as nodes for signal cross talk with other growth factor pathways. Here, I combine the TGF-β family modulators into two groups, soluble regulators versus extracellular matrix (ECM) residents and proteoglycans. My main emphasis is on the various modes to regulate ligand availability and activity, with omission of many additional functions because of limited space.
SECRETED REGULATORS OF TGF-β FAMILY LIGANDS
Extracellular modulation of TGF-β family signals is achieved by structurally diverse soluble TGF-β-binding proteins (Table 1). These secreted regulators have overlapping and distinct substrate specificities, bind to ligands with different affinities, and show differential interactions with ECM components or cell-surface molecules. Their combined actions are often crucial elements in defining the outcome of TGF-β family signals (Figs. 1 and 2).
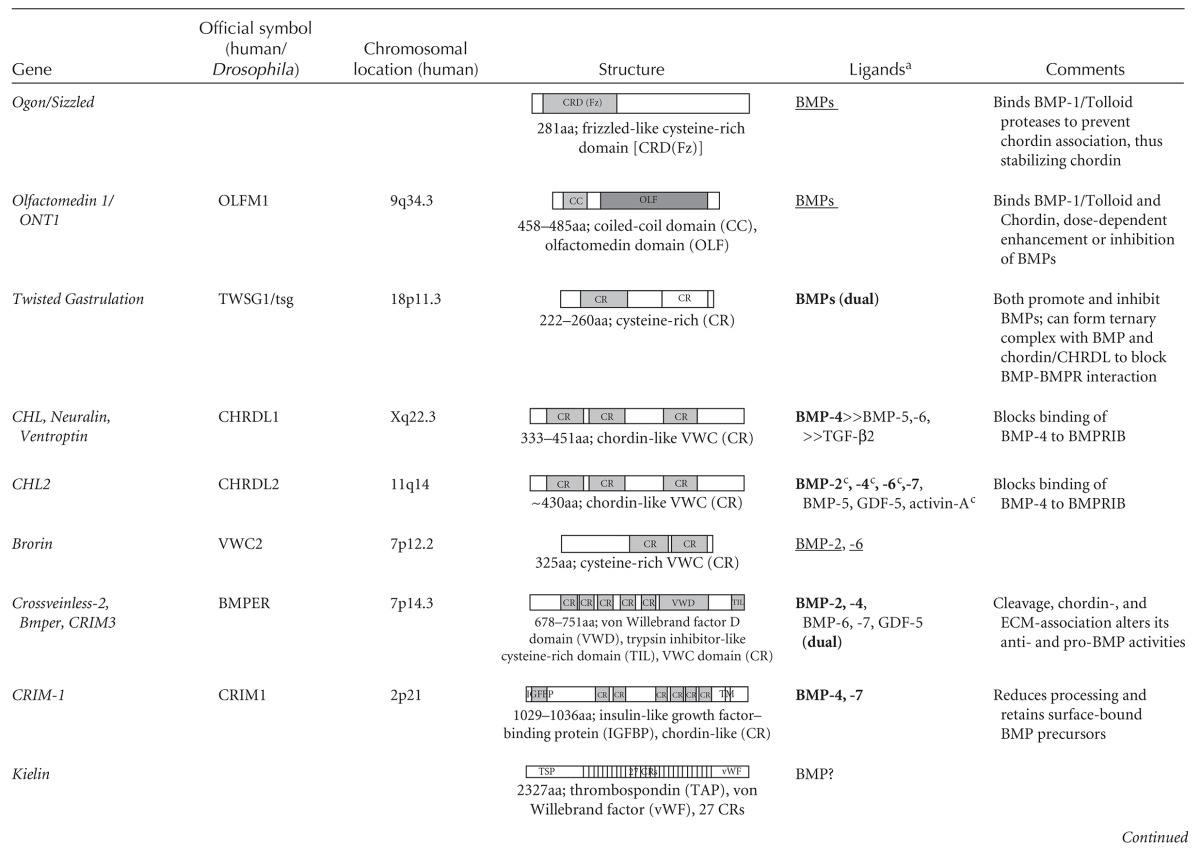
Table 1.
Agonists and antagonists of TGF-β family growth factors
Figure 1.
Regulation of transforming growth factor β (TGF-β) family signals by extracellular agonists and antagonists. Most extracellular agonists and antagonists act to facilitate or prevent binding of mature TGF-β family ligands to their receptor complexes, respectively. The secreted proteins CHRDL1, BMPER/CV-2, KCP/CRIM2, and connective tissue growth factor (CTGF) act both as agonists and antagonists depending on the particular ligands they regulate and the presence or absence of other factors in cell-type-specific microenvironments they encounter. Certain soluble modulators, including follistatin (FST), FSTL1, BMPER/CV-2, Lefty, and bone morphogenetic protein 3 (BMP-3), can also bind to type I and/or type II receptors to form a nonsignaling complex. Regulation of ligand processing, secretion, activation, and/or stability by CRIM1, SOST, GREM1, and the propeptides in the ligand-producing cells can control ligand availability. Extracellular regulation of ligand processing by Emilin1 and ligand release by Tolloid/BMP-1 family proteinases also control ligand bioactivity. Furthermore, TGF-β family ligands can form heterodimers or interact with each other, which leads to either blocking or enhancing TGF-β family signaling depending on the particular ligands involved. TGN, trans-Golgi network.
Figure 2.
Regulation of bone morphogenetic protein (BMP) signaling by chordin-dependent extracellular regulatory network. Inhibition of BMP signaling by chordin can be enhanced by formation of a ternary complex with Twsg1/Tsg. At the same time, this complex promotes transport of the BMP ligands to a distant site to allow formation of a sharp high BMP activity center. Inactivation of chordin function is achieved by Tolloid/BMP-1-dependent proteolytic processing to release the associated BMP ligands. Cleavage of chordin is prevented by Sizzled, Crescent, or Sfrp5, which titrate Tolloid/BMP-1 away from chordin. The scaffolding protein ONT1 binds both Tolloid/BMP-1 and chordin to facilitate chordin processing. The proteins containing chordin-like cysteine-rich (CR) domains, including CHRDLs and CV-2, can form a similar ternary complex with BMP and Twsg1 to have stronger BMP-inhibitory activity.
Follistatin Domain Proteins
Follistatin
The follistatin (FS) domain is defined by 10 spatially conserved cysteines in a ∼70 amino acid stretch. Follistatin (FST), the prototype protein, contains three FS modules and is a monomeric glycoprotein that binds activin with high affinity (KD 46 to 900 pm) (Nakamura et al. 1990; Sugino et al. 1993; Schneyer et al. 1994; Hashimoto et al. 2000; Sidis et al. 2001; Keutmann et al. 2004). Interaction with follistatin masks the receptor binding sites in activin, resulting in inhibition of activin signaling (de Winter et al. 1996; Thompson et al. 2005; Harrington et al. 2006). Of the three follistatin isoforms, the short form, Fst288, has a 6- to 10-fold higher affinity for activin than the long form, Fst315, and associates uniquely with cell-surface heparan sulfate proteoglycan (HSPG) to promote endocytosis and subsequent lysosomal degradation of activin in pituitary cells (Inouye et al. 1991; Nakamura et al. 1991; Sugino et al. 1993; Hashimoto et al. 1997). The antagonistic interaction between activin and follistatin modulates a variety of cellular processes in many tissues, such as gonads, pituitary gland, vasculature, and liver (reviewed by Phillips and de Kretser 1998; Bilezikjian et al. 2012).
Besides activin, follistatin also interacts functionally and biochemically with other TGF-β family members. Follistatin binds directly to bone morphogenetic protein 2 (BMP-2), BMP-4, and BMP-7, but with much lower affinities (KD ∼5.29 nm to 80 nm) than to activin (Fainsod et al. 1997; Iemura et al. 1998; Amthor et al. 2002). Follistatin does not block BMP-4 from binding to its type I BMP receptor. Instead, it forms a nonfunctional ternary complex with BMP and its receptor (Iemura et al. 1998) and inhibits BMP signaling. For example, follistatin regulates BMP activities during dorsoventral patterning of early Xenopus embryos (Hemmati-Brivanlou et al. 1994; Fainsod et al. 1997; Iemura et al. 1998; Yamamoto et al. 2000), BMP-7 function in muscle growth (Amthor et al. 2002) and blocks the growth-inhibitory activity of BMP-7 in mammalian cell culture (Yamashita et al. 1995). Different from what is expected from its in vitro measured affinity, follistatin blocks BMP-7 more efficiently than BMP-4 in functional assays (Liem et al. 1997). Follistatin also binds myostatin (growth and differentiation factor 8 [GDF-8]) with high affinity (KD 584 pm) (Amthor et al. 2004), blocks association of myostatin with its type II receptor, ActRIIB, and interferes with the function of myostatin to inhibit muscle growth (Lee and McPherron 2001; Zimmers et al. 2002; Amthor et al. 2004). Additionally, follistatin can form an inactive complex with BMP-15 and prevents it from regulating proliferation and differentiation of granulosa cells in the ovary (Otsuka et al. 2001). Furthermore, follistatin antagonizes the activities of BMP-11 and antidorsalizing morphogenetic protein (ADMP) during early Xenopus development, although biochemical interactions of follistatin with BMP-11 or ADMP have not been shown (Gamer et al. 1999; Dosch and Niehrs 2000).
FSTL1 and FSTL3
Follistatin-like 1 (FSTL1, also known as FRP, Flik, or TSC36) is a secreted glycoprotein with a single FS module. Biochemical analyses reveal some contradictory results on FSTL1 binding to multiple TGF-β family ligands, possibly because of different cell types and methods used (Tanaka et al. 2010; Geng et al. 2011; Xu et al. 2012a). FSTL1 binds to BMP-2, BMP-4 (KD 0.19 nm to 7.2 nm), TGF-β1 (KD 12 pm to 36 nm), and possibly activin A (KD 1.43 nm). FSTL1 is also reported to bind several type II receptors (BMPRII, ActR-IIB, TβRII), as well as type I BMP receptors (ALK-3/BMPRIA and ALK-6/BMPRIB), to modulate TGF-β family signaling. FSTL1 regulates many developmental processes in vertebrates, such as dorsoventral patterning, lung, skeletal, and ureter development, and altered FSTL1 levels are associated with many diseases, including inflammation, cardiac diseases, and cancer (reviewed by Sylva et al. 2013). A related protein, Follistatin-like 3 (FSTL3, also known as FSRP or FLRG), is a glycoprotein with two FS modules and binds activin A with an affinity of 40–850 pm and to BMP-2, -6, and -7 with low affinity. Like follistatin, FSTL3 inhibits the activities of activin and BMPs in cell culture (Tsuchida et al. 2000; Schneyer et al. 2001; Sidis et al. 2002, 2006). FSTL3 also forms a tight complex with myostatin in serum, and can inhibit myostatin function (Hill et al. 2002). Crystal structure reveals a unique association of an amino-terminal domain of FSTL3 with myostatin, but not activin A (Cash et al. 2012). Mice with targeted deletion of Fstl3 are viable and fertile with defects in glucose and lipid homeostasis (Mukherjee et al. 2007). Other follistatin-like proteins also exist. FSTL2, also known as IGFBP7, may act in the insulin-like growth factor pathway (Evdokimova et al. 2012), whereas the biological activities of FSTL4 and FSTL5 are not well understood.
WFIKKN1 and WFIKKN2
WFIKKN1 and WFIKKN2 (also known as GASP-2 and GASP-1 for “growth and differentiation factor-associated serum protein” -2 and -1) are secreted factors that contain a single FS module in addition to a WAP, an immunoglobulin, two Kunitz-type protease inhibitors, and an NTR domain. Both proteins bind with high affinity to mature myostatin (GDF-8, KD 33.5 nm and 286 pm, respectively) as well as GDF-11 (KD 2.25 nm and 164 pm, respectively), and they also associate with the propeptide of myostatin. Domain analysis shows that WFIKKN1 binds myostatin primarily through its FS module, whereas it binds the myostatin propeptide with its NTR domain. WFIKKN binding inhibits the activity of myostatin to regulate muscle development (Hill et al. 2003; Kondas et al. 2008; Szlama et al. 2013). WFIKKN1 and WFIKKN2 also bind TGF-β1, BMP-2, and BMP-4 with affinities in the micromolar range. However, unlike GDF-8 and GDF-11, WFIKKN binding to these growth factors does not block their signaling in reporter assays (Szlama et al. 2010).
TMEFFs
TMEFFs (or tomoregulins) are transmembrane proteins with two FS modules in their ectodomains (Eib and Martens 1996; Horie et al. 2000). One of these, TMEFF1, inhibits nodal, Vg1, and BMP signaling in Xenopus without affecting activin signaling (Chang et al. 2003). Unlike most FS domain proteins, TMEFF1 does not bind nodal. Instead, it interacts with the nodal coreceptor Cripto to prevent assembly of a signaling ligand–receptor complex. This represents a novel mechanism for regulation of TGF-β signaling by FS module–containing proteins (Harms and Chang 2003).
Other FS domain–containing proteins include ECM proteins like SPARC and agrin, but their functions in regulating TGF-β family growth factors have not been shown, although they may affect ligand expression at the transcriptional level (Schneyer et al. 2001).
Noggin
Noggin is a small soluble protein of 210–240 amino acids. It contains a carboxy-terminal cysteine-rich domain and is secreted as a homodimeric glycoprotein. Noggin was originally identified in Xenopus embryos as a dorsalizing factor (Smith and Harland 1992; Lamb et al. 1993; Smith et al. 1993). Subsequently, four noggin homologs were identified in zebrafish, Fugu, Xenopus tropicalis, Xenopus laevis, and chick, but only one noggin gene exists in mammals (Furthauer et al. 1999; Fletcher et al. 2004; Eroshkin et al. 2006). Noggin binds directly to BMP-2 and BMP-4 with high affinity (KD of 19 pm), and less efficiently to BMP-7, and prevents ligand interaction with their cognate BMP receptors (Zimmerman et al. 1996). Noggin also interacts biochemically and functionally with several other BMP ligands, including GDF-5 (Merino et al. 1999a), GDF-6 (Chang and Hemmati-Brivanlou 1999), BMP-5, and BMP-6 (Aspenberg et al. 2001; Beck et al. 2001; Haudenschild et al. 2004). Noggin2 in Xenopus has also been shown to associate with activin B, nodal/Xnrs, and Wnt8 to inhibit their signaling (Bayramov et al. 2011). The sequences of BMP-6 and GDF-5 mutants that are not inhibited by noggin identify key residues that mediate BMP inhibition by noggin (Seemann et al. 2009; Song et al. 2010). The crystal structure of noggin bound to BMP-7 reveals that the noggin dimer assumes a cystine-knot structure similar to that of BMP-7, and that a “back-to-back” arrangement of noggin and BMP-7 dimers results in masking of a hydrophobic patch in BMP-7 that is required for contact with type II receptors (Groppe et al. 2002). Besides BMPs, noggin also binds heparan sulfate and is retained at the cell surface by HSPGs. Heparan sulfate-bound noggin remains functional and binds BMP-4 at the plasma membrane. Binding of noggin to cell-surface HSPGs may limit the diffusion, and thus the action range, of this antagonist (Paine-Saunders et al. 2002), and may also contribute to noggin’s ability to promote BMP-2 internalization (Alborzinia et al. 2012). A developmentally regulated endosulfatase Qsulf1 can release noggin from HSPGs and may regulate noggin distribution (Viviano et al. 2004). Inhibition of BMP signaling by noggin plays important roles in many processes during embryogenesis and adult homeostasis, such as regulation of neural induction, patterning of the neural tube and somites, guidance of dorsal root ganglion axons, joint formation and skeletal development, fusion of cranial sutures, and hair follicle development (Brunet et al. 1998; McMahon et al. 1998; Botchkarev et al. 1999; Bachiller et al. 2000; Anderson et al. 2002; Dionne et al. 2002; Warren et al. 2003; Khokha et al. 2005). Hypomorphic mutations in the human NOGGIN gene associate with skeletal dysplasia syndromes, such as proximal symphalangism (SYM1), multiple synostoses syndrome (SYNS1), and tarsal/carpal coalition syndrome (TCC) (Balemans and Van Hul 2002), illustrating the importance of noggin in joint and skeletal development.
Besides noggin, several genes in planarians encode noggin-like proteins (nlgs) that have a sequence insertion between the fifth and sixth cysteine residues. Functional assays indicate that planarian nlg8 and a similar Xenopus Xl-nlg ventralize embryos and suppress neurogenesis, partially mimicking effects of ectopic BMP signals. However, nlg8 and Xl-nlg do not significantly affect Smad1 or 5-phosphorylation, suggesting that they may regulate patterning independent of Smad activation (Molina et al. 2009, 2011). The functions of these nlg genes are distinct from that of a noggin-like gene in Hydra, which encodes canonical BMP-inhibitory activities when expressed in Xenopus (Chandramore et al. 2010).
Chordin and Its Regulators: BMP-1/Tolloid Proteases, Ogon/Sizzled, Crescent/Frzb2-Sfrp5, Olfactomedin 1, and Twisted Gastrulation
The chordin-dependent BMP regulatory network is complex and extensive, and best characterized in Xenopus and Drosophila in the context of early dorsoventral patterning. It utilizes multiple extracellular factors (Fig. 2) to regulate the interaction between BMPs and chordin by controlling the stability of chordin, the transport of the BMP–chordin complex to a distant site, and the transcription of the network components. Together, they constitute a reaction–diffusion feedback regulatory loop to finely tune BMP signaling levels for robust embryonic patterning along the dorsoventral axis.
Chordin
Chordin was originally identified as the product of a dorsally expressed gene in Xenopus gastrulae that showed dorsalizing activities (Sasai et al. 1994, 1995). This gene encodes a secreted protein of 941 amino acids that contains four cysteine-rich (CR) repeats with a long linker sequence between the first and second CR domain. Like noggin, chordin antagonizes BMP function through direct binding to BMP-2, -4, and -7, thus preventing the ligands from interacting with the BMP receptors. The affinity of chordin for BMP-4 (KD 300 pm) is ∼10-fold lower than that of noggin (Piccolo et al. 1996), and the first and third CR domains can individually bind BMP-4 with a KD of 2 nm and inhibit BMP signals less efficiently than full-length chordin (Larrain et al. 2000). Differentially spliced variants of human chordin (CHRD) mRNA encode isoforms with different numbers of CR domains and distinct abilities to block BMP signaling (Millet et al. 2001). Besides BMPs, chordin may bind and block ADMP, albeit with conflicting results (Joubin and Stern 1999; Dosch and Niehrs 2000; Reversade and De Robertis 2005). In vertebrate development, chordin, either alone or in cooperation with noggin and follistatin, regulates early dorsal patterning, forebrain formation, mandibular outgrowth, pharyngeal development, septation of the cardiac outflow tract, chondrocyte maturation, and axial skeleton development, among other processes (Hammerschmidt et al. 1996a,b; Schulte-Merker et al. 1997; Streit et al. 1998; Streit and Stern 1999; Bachiller et al. 2000, 2003; Stottmann et al. 2001; Anderson et al. 2002; Zhang et al. 2002; Oelgeschlager et al. 2003a; Khokha et al. 2005). Some phenotypes in Chrd−/− mice resemble those in patients with DiGeorge syndrome, implying that CHRD mutations may contribute to the etiology of this disease (Bachiller et al. 2003).
The antagonism between chordin and BMPs may have arisen early during metazoan evolution (Rentzsch et al. 2007). Comparative studies in Drosophila and vertebrates yield insight into the conservation and divergence of chordin function in animals. The Drosophila homolog of chordin, short gastrulation (Sog), binds and inhibits the BMP ligands Screw (Scw), Glass-bottom boat (Gbb), and, with less efficiency, Decapentaplegic (Dpp) to regulate dorsoventral patterning of early embryos and pattern formation in imaginal discs (Francois et al. 1994; Francois and Bier 1995; Holley et al. 1995; Schmidt et al. 1995; Biehs et al. 1996; Neul and Ferguson 1998; Nguyen et al. 1998). Interestingly, although Sog blocks BMPs locally, it can enhance signaling by Dpp/Scw dimer in cells that are distant from the source of Sog production by facilitating transport and presentation of BMP ligands. This role is essential for establishing a sharp domain of peak levels of Dpp/Scw activity in the dorsal region of early fly embryos (Ashe and Levine 1999; Decotto and Ferguson 2001; Eldar et al. 2002; Shimmi and O’Connor 2003; Shimmi et al. 2005a; Wang and Ferguson 2005). Vertebrate chordin cannot replace Sog to promote Dpp signaling in Drosophila (DeCotto and Ferguson 2001), although some evidence suggests that chordin may collaborate with BMP-2b in early dorsoventral patterning of the zebrafish tail (Wagner and Mullins 2002), and help shuttle BMP ligands from the intervertebral disc to the vertebral body during mouse skeletal development (Zakin et al. 2010). It is therefore possible that vertebrate chordin maintains its ability to transport BMP in certain tissue contexts.
BMP-1/Tolloid Family of Metalloproteinases
Tolloid, a zygotic gene in the Drosophila dorsal developmental regulatory network, encodes a protein homologous to human BMP-1 in that both contain an amino-terminal zinc metalloproteinase domain (Shimell et al. 1991). Genes for a second Drosophila homolog, Tolkin/Tlr-1, and four genes encoding vertebrate BMP-1/Tolloid-like (BMP-1/TLL) proteins were subsequently identified (Maeno et al. 1993; Nguyen et al. 1994; Finelli et al. 1995; Scott et al. 1999). Biochemical, genetic, and embryological studies in Drosophila, Xenopus, and mammalian cells show that BMP-1/TLL family members cleave Sog/chordin at several sites to inactivate or attenuate this BMP antagonist, and thus promote BMP signaling (Blader et al. 1997; Marques et al. 1997; Piccolo et al. 1997; Scott et al. 1999; Serpe et al. 2005). However, some forms of partially cleaved Sog/chordin may display stronger BMP-inhibitory activity or an altered spectrum of preferred BMP ligands (Yu et al. 2000; Troilo et al. 2014, 2015). Although cleavage of Sog in Drosophila requires the presence of Dpp, vertebrate BMP-1/TLLs can process chordin in the absence of BMPs (Peluso et al. 2011). BMP-4 can bind to the CUB domain of BMP-1/TLL to inhibit its proteinase activity, and this may provide concentration-dependent feedback modulation of BMP signaling (Lee et al. 2009). A further difference between Drosophila and vertebrates lies in the number and locations of the processing sites in Sog and chordin (Marques et al. 1997; Piccolo et al. 1997; Scott et al. 1999). In addition, the four vertebrate BMP-1/TLL members have differential abilities to cleave chordin (Scott et al. 1999; Pappano et al. 2003; Berry et al. 2010). Inactivation of Sog by Tolloid in Drosophila generates a ventral-high to dorsal-low gradient of Sog protein (Srinivasan et al. 2002), and is essential to establish a reverse dorsal-high, ventral-low Dpp/Scw activity gradient in the dorsal region of early embryos (Marques et al. 1997; Shimmi and O’Connor 2003; Peluso et al. 2011). In vertebrates, BMP-1/TLLs modulate chordin distribution along the dorsoventral axis and are required for ventral tissue development in Xenopus and zebrafish (Piccolo et al. 1997; Goodman et al. 1998; Connors et al. 1999, 2006; Wardle et al. 1999; Blitz et al. 2000; Jasuja et al. 2006; Plouhinec et al. 2013). The activity of Tolloid/BMP-1 is positively modulated by the ECM proteins fibronectin and collagen IV, as these proteins bind Tolloid/BMP-1 and enhance its processing of chordin (Huang et al. 2009; Winstanley et al. 2015). Besides chordin, BMP-1 family proteases target other substrates in the extracellular milieu, including latent TGF-β-binding protein 1 (LTBP1), and this cleavage contributes to activation of TGF-β ligands (Pappano et al. 2003; Ge and Greenspan 2006a,b).
Sizzled/Ogon, Crescent, and Sfrp5
Sizzled (also known as mercedes or short tail) was first identified as the zebrafish Ogon mutant that caused ventralization of embryos similar to chordin mutants. Genetic interactions with other dorsoventral patterning factors in zebrafish suggest that the gene product is a BMP antagonist that acts upstream of BMP-2b and the type I BMP receptor ALK-8 (Miller-Bertoglio et al. 1999; Wagner and Mullins 2002). The encoded protein, Sizzled, inhibits BMP signals in Xenopus and zebrafish, and regulates dorsoventral patterning (Bradley et al. 2000; Collavin and Kirschner 2003; Martyn and Schulte-Merker 2003; Yabe et al. 2003). Inhibition of BMPs by Sizzled is chordin-dependent and uses a novel mechanism. Sizzled competes with chordin to bind BMP-1 and Tolloid-like 1, but cannot be cleaved by these metalloproteinases. In this way, Sizzled prevents BMP-1 proteins from processing chordin, and thus helps to stabilize chordin and enhance its antagonistic effect on BMP in vivo (Fig. 2) (Lee et al. 2006; Muraoka et al. 2006). Counterintuitively, Sizzled is expressed in the ventral regions and its expression is stimulated by BMP signaling. Sizzled thus acts as a negative feedback regulator of BMPs that diffuses over a long distance to finely tune the BMP signaling levels along the dorsoventral axis in a chordin- and Tolloid-dependent manner (Inomata et al. 2013). No Sizzled-like molecule has been found in Drosophila to regulate signals by the BMP-like ligands Dpp, Scw, or Gbb, implying that this mechanism arose later in evolution.
Crescent and Sfrp5 are secreted Frizzled-related proteins (sFRP’s) that are closely related to Sizzled. Similar to Sizzled, Crescent (KD ∼11 nm) and Sfrp5 bind BMP-1/TLL and block their proteinase activity. They thus protect chordin from degradation and participate in an extracellular BMP regulatory network to modulate dorsoventral patterning of early Xenopus and zebrafish embryos. Unlike Sizzled, Crescent is expressed in the dorsal region of Xenopus gastrulae, and both Xenopus Crescent and zebrafish Sfrp5 are expressed in embryonic endoderm and additionally inhibit canonical and noncanonical Wnt signals (Pera and De Robertis 2000; Schneider and Mercola 2001; Shibata et al. 2005; Ploper et al. 2011; Stuckenholz et al. 2013). Interestingly, mammalian sFRP-2 enhances, rather than inhibits, BMP-1 in processing procollagen C, but has no effect on chordin processing (Kobayashi et al. 2009). Studies of other mammalian sFRPs show that they lack the ability to modify BMP-1 proteinase activity, suggesting that specific residues in the frizzled domain may be crucial for sFRP to inhibit BMP-1/TLL proteinases (Bijakowski et al. 2012).
Olfactomedin 1 (ONT1)
ONT1, a member of olfactomedin family of secreted proteins, acts as an extracellular scaffold to facilitate association of chordin and BMP-1. ONT1 has a coiled-coil domain near its amino terminus and a conserved olfactomedin domain in its carboxy-terminal half. These domains bind BMP-1/Tolloid and chordin, respectively. By bringing the enzyme and its substrate together, ONT1 promotes chordin degradation (Fig. 2). This pro-BMP action of ONT1 is concentration-dependent, as high levels of ONT1 permit binding of chordin and BMP-1 in distinct complexes and thus separate the two proteins. ONT1 is expressed in the dorsal organizer of early Xenopus embryos; thus, its expression and activity are opposite to those of Sizzled. Both ONT1 and Sizzled are critical in the robust dorsoventral self-regulating patterning system in Xenopus (Inomata et al. 2008, 2013).
Twisted Gastrulation (TWSG1/tsg)
Twisted gastrulation (tsg) was first identified in Drosophila as a mutation that affects the development of the dorsal midline amnioserosa (Zusman and Wieschaus 1985). The gene encodes a small secreted protein with two cysteine-rich domains and is required for peak Dpp activity in early Drosophila embryos (Mason et al. 1994, 1997; Ross et al. 2001). Tsg enhances Sog to bind and inhibit Dpp in the dorsolateral region. However, at the same time Tsg promotes the transport of the Sog–Dpp complex to the dorsal midline, and facilitates the processing and alternative cleavage of Sog by Tolloid once it reaches the most dorsal region (Yu et al. 2000; Shimmi and O’Connor 2003). This Tsg–Sog–Dpp–Tolloid interaction network ensures the establishment of a sharp dorsal boundary between the peak and immediate BMP activity levels so that cells adopt either an amnioserosa or dorsal ectodermal fate (Eldar et al. 2002; Shimmi and O’Connor 2003; Shimmi et al. 2005a; Wang and Ferguson 2005). Another twisted gastrulation-like gene, shrew, performs a similar function to promote peak BMP signaling at the aminoserosa (Bonds et al. 2007). In addition, a comparable Dpp transport and release mechanism may operate during wing development in Drosophila, in which a Tsg homolog, Tsg2/Crossveinless (Cv), cooperates with Sog to create a spatially restricted Dpp/Gbb signaling center in the posterior crossvein of the developing wing (Shimmi et al. 2005b; Vilmos et al. 2005). Tsg can also alter the processing of Sog by Tolloid to create a novel Sog cleavage product, called Supersog, which has a broader spectrum of BMP inhibition than full-length Sog (Yu et al. 2000). Tsg and Tsg2/Cv therefore exert positive as well as negative effects on BMP signaling in Drosophila via regulation of Sog and Tolloid activities, and thus helps to establish a sharp high BMP signaling boundary by local inhibition and long distance enhancement of BMP signals.
Vertebrate Twisted gastrulation, abbreviated as Twsg1 and not as Tsg as in Drosophila, has also been shown to act as both agonist and antagonist of BMPs. Twsg1 binds BMP-2, -4, and -7 and forms a ternary complex with chordin and BMPs to enhance the inhibitory activity of chordin on BMP (Oelgeschlager et al. 2000; Chang et al. 2001; Larrain et al. 2001; Ross et al. 2001; Scott et al. 2001; Zakin et al. 2005). In cell culture, Twsg1, either alone or in combination with chordin, blocks BMP-mediated effects on proliferation and/or differentiation of osteoblasts, osteoclasts, and thymocytes (Graf et al. 2002; Gazzerro et al. 2005; Petryk et al. 2005; Pham et al. 2011). However, Twsg1 may also promote chordin degradation by BMP-1 family metalloproteinases, and thus promote BMP signaling (Oelgeschlager et al. 2000; Larrain et al. 2001). Twsg1 increases the rate of chordin processing by Tolloid-like 1 and alters the processing site of mouse chordin, but not zebrafish or Xenopus chordin. In Xenopus, a dominant negative Tolloid-like 1 (Xolloid) blocks the BMP stimulatory activity of Twsg1 (Larrain et al. 2001; Scott et al. 2001; Xie and Fisher 2005). In addition, Twsg1 may have a chordin-independent, BMP-enhancing activity (Oelgeschlager et al. 2003a, 2004; Little and Mullins 2004; Xie and Fisher 2005), indicating that other factors also interact with Twsg1 to modulate BMP signaling. Although it is tempting to speculate that Twsg1 may help present BMP ligands to their receptors when stimulating BMP signaling, binding of BMPs per se is not required for this stimulatory activity of Twsg1 (Oelgeschlager et al. 2003b). In osteoclast cells, mutations that abolish Twsg1 binding to BMP change its activity from BMP-inhibitory to BMP-enhancing, arguing for a ligand-binding-independent mechanism for Twsg1 to promote BMP signaling (Huntley et al. 2015).
The in vivo functions of vertebrate Twsg1, as assessed by loss-of-function studies, reveal an equally complex story. Depletion of endogenous Twsg1 expression using morpholino antisense oligonucleotides shows that Twsg1 and chordin coordinate in regulating dorsoanterior development of early Xenopus embryos (Blitz et al. 2003; Wills et al. 2006), supporting the critical role of Twsg1 as a BMP antagonist in Xenopus. Twsg1 and BMP-7, however, also act together in controlling the posteroventral mesoderm and ventral tail fin formation in X. laevis, suggesting stimulation of BMP activity by Twsg1 (Zakin et al. 2005). In zebrafish, antisense oligonucleotide-mediated knockdown experiments show that Twsg1 may promote rather than inhibit BMP signaling (Little and Mullins 2004; Xie and Fisher 2005). In mammals, Twsg1−/− mice show defects in axial skeleton, thymocyte development, and craniofacial structures (Graf et al. 2001; Nosaka et al. 2003; Petryk et al. 2004; Zakin and De Robertis 2004). Twsg1 may function as a BMP antagonist in axial skeletal and T-cell development (Nosaka et al. 2003; Ikeya et al. 2008; Zakin et al. 2008) and neutralizes the protective activity of BMP-7 in podocyte injury (Yamada et al. 2014). However, Twsg1 may enhance BMP signaling during forebrain development and postnatal mammary gland morphogenesis (Zakin and De Robertis 2004; Forsman et al. 2013). Thus, Twsg1 has context-dependent pro- or anti-BMP activities. A second twisted gastrulation gene has also been identified in Xenopus, and shows a temporal expression pattern that differs from Twsg1 expression (Oelgeschlager et al. 2004). In addition, Twsg1 may regulate TGF-β signaling in T lymphocytes by binding to TGF-β proteins (Tzachanis et al. 2007), implying a broader spectrum of TGF-β ligand regulation.
Other Modulators of Chordin
The activities of chordin are regulated by additional factors, such as proteoglycans and other cell-surface molecules. Two secreted small leucine-rich proteoglycans, biglycan and Tsukushi, can each form a ternary complex with chordin and BMP-4 and enhance the inhibitory function of chordin on BMP (Ohta et al. 2004; Moreno et al. 2005). Chordin also binds HSPGs, such as syndecans, but does not associate with the basement membrane HSPG perlecan. Binding of chordin to cell-surface HSPGs potentiates the BMP antagonistic function of chordin, facilitates chordin retention and uptake by cells, and limits diffusion of chordin over a long distance in tissues (Jasuja et al. 2004). Interaction of chordin with HSPGs hence regulates the local concentration and gradient formation of chordin and its ability to block BMP signaling. The distribution of chordin may also be affected by its interaction with integrins that act as receptors for ECM proteins. Chordin binds α3-integrin in vertebrate cells, and this binding facilitates endocytosis and uptake of chordin into cells (Larrain et al. 2003). In Drosophila, Sog interacts genetically with several integrins, including βPS, αPS1, and αPS3 (PS stands for position-specific antigen), during wing vein specification. Sog and a truncated Sog directly bind αPS1, and the function of integrins is required for Sog transport from the intervein to pro-vein regions (Araujo et al. 2003). Integrins may thus control the range and the availability of different forms of Sog and chordin proteins to regulate BMP signals.
Chordin-Like Cysteine-Rich (CR) Domain-Containing Proteins
CHL/Neuralin/Ventroptin and CHL2 (CHRDL1 and 2)
Chordin has four CR modules (also known as von Willebrand factor type C, or VWC domains) that are defined by characteristic spacing of 10 conserved cysteines within a stretch of 60 to 80 amino acids (Garcia Abreu et al. 2002). Chordin-like CR domains are found in several extracellular proteins, including matrix proteins such as procollagen (see below) as well as soluble factors. Chordin-like (CHL/CHL1, CHRDL1) is a secreted molecule with three CR modules and is also known as neuralin in the mouse, and ventroptin in the chick (Coffinier et al. 2001; Nakayama et al. 2001; Sakuta et al. 2001). It has an expression pattern complementary to that of chordin during early mouse embryogenesis. CHRDL1 binds BMP-4, -5, and -6, but not activin A, and blocks binding of BMPs to their receptors (Nakayama et al. 2001; Sakuta et al. 2001). Unlike chordin, CHRDL1 also binds TGF-β2 (Nakayama et al. 2001). When overexpressed, CHRDL1 induces secondary axis formation in early Xenopus embryos, dorsalizes zebrafish embryos, represses BMP-4 activity during dorsoventral patterning of chick retina, impairs distal digit formation in chick limbs, and promotes neuronal differentiation of adult neural stem cells (Coffinier et al. 2001; Nakayama et al. 2001; Sakuta et al. 2001; Branam et al. 2010; Allen et al. 2013; Gao et al. 2013). Interestingly, CHRDL1 enhances BMP-4 and BMP-7 signaling in several cell lines when expressed alone, but switches into a selective BMP-7 antagonist when in complex with Twsg1. The BMP-inhibitory function of Chrdl1 and Twsg1 may regulate injury repair and homeostasis of mammalian kidney (Larman et al. 2009). Mutations in CHRDL1 are associated with X-linked megalocornea disorder in human patients (Webb et al. 2012). Another closely related and secreted factor with 3 chordin-type CR domains is CHRDL2 (CHL2), which binds BMP-2, -4, -5, -6, -7, and GDF-5, but not activin A or TGF-βs. Overexpression of CHRDL2 blocks BMP-mediated differentiation of C2C12 cells (Nakayama et al. 2004), and a ternary complex with Twsg1 and BMP-2 enhances the BMP-inhibitory activity of CHRDL2 (Zhang et al. 2007). Recombinant human CHRDL2, however, interacts with activin A, but not BMP-2, -4, or -6. The functional significance of these differences in interactions is unknown (Oren et al. 2004). The discrepancy between the two studies may be because of the complex alternative splicing patterns of CHRDL2 in different tissues, as spliced variants may have different functions and specificities in the regulation of distinct TGF-β family ligands (Oren et al. 2004). Like chordin, zebrafish CHRDL can be processed by BMP-1, which blocks its dorsalizing activity (Branam et al. 2010).
Brorin/Vwc2 and Brorin-Like (Vwc2l)
Brorin (also known as Vwc2) was isolated from mouse as a brain-specific secreted protein with two chordin-like CR domains. Brorin inhibits BMP-2 and -6 activities in cultured preosteoblastic cells and promotes neurogenesis in neural precursor cells (Koike et al. 2007). A similar protein, Brorin-like (Vwc2-like or Vwc2l), was isolated from mouse, human, and zebrafish. It has an expression profile that partially overlaps with that of Brorin, but has a weaker BMP-inhibitory activity (Miwa et al. 2009).
Crossveinless-2 (BMPER/cv-2)
Crossveinless-2 (cv-2) is a gene mutation in Drosophila that leads to a loss of crossveins in the wing. The gene encodes a secreted protein with five chordin-type CR domains followed by a partial von Willebrand factor (vWF) domain. Genetic studies suggest that Crossveinless-2 enhances BMP signals in the crossveins, which require a high level of Dpp/Gbb signaling, but antagonizes BMP signaling during early embryogenesis (Conley et al. 2000; Gavin-Smyth et al. 2013). Crossveinless-2 binds the type I BMP receptor Thickveins and shows a concentration-dependent biphasic modulation toward BMPs (Serpe et al. 2008). Vertebrate Crossveinless-2, also known as Bmper, has been identified in zebrafish, chick, mouse, and human, and contains an additional carboxy-terminal trypsin-inhibitor-like cysteine-rich domain that is not found in Drosophila Crossveinless-2. Cv2/Bmper is expressed in chick and mouse at sites that require elevated BMP signals, such as the posterior primitive streak and ventral tail bud, whereas functional assays suggest BMP agonist and antagonist activities of Cv2/Bmper in vertebrates. (Coffinier et al. 2002; Coles et al. 2004; Kamimura et al. 2004). Cv2/Bmper binds BMP-2, -4, -6, -7, -9 (KD ∼1.4–3.5 nm for BMP-2, -4, -7) and GDF-5 (KD ∼ 34 nm). Structural analysis shows that Cv2/Bmper binds BMP-2 via the amino-terminal Clip segment and subdomain 1 in the first CR module (VWC1 domain), resulting in masking of the type I and type II receptor-binding interfaces in BMP-2 (Zhang et al. 2008; Fiebig et al. 2013). As a BMP inhibitor, Cv2/Bmper induces a secondary axis in Xenopus, blocks BMP-responsive gene expression in 293T cells, and interferes with BMP-dependent differentiation of embryonic stem cells into the endothelial lineage and BMP-induced chondrogenic and osteogenic differentiation. However, Cv2/Bmper also enhances Smad1 phosphorylation in COS7 cells and promotes premature neural crest cell migration in chick. The latter results are consistent with its function in elevating BMP signaling (Moser et al. 2003; Binnerts et al. 2004; Coles et al. 2004; Kamimura et al. 2004; Ambrosio et al. 2008; Yao et al. 2012). Gene silencing in zebrafish and mice suggest that Cv2/Bmper enhances BMP signaling during gastrulation, neural crest specification, nephrogenesis, cardiovascular development, and axial skeletal formation, although it may block BMP-9 signaling in vascular endothelium, whereas Cv2/Bmper overexpression shows activities that are consistent with functions in both enhancing and inhibiting BMP signaling (Ikeya et al. 2006, 2008, 2010; Rentzsch et al. 2006; Moser et al. 2007; Zakin et al. 2008; Yao et al. 2012; Reichert et al. 2013; Dyer et al. 2014). Several mechanisms, including differential activities of full-length and processed Cv2/Bmper and differential association with other BMP modulators and/or ECM components, may account for the switch between the two opposing activities of Cv2/Bmper. Full-length Cv2/Bmper is a BMP antagonist and binds to HSPG-containing ECM with high affinity. The cleaved product of Cv2/Bmper that contains only five CR domains acts as BMP agonist and does not associate efficiently with the ECM. Processing of Cv2/Bmper may thus alter its regulation of BMP signaling (Rentzsch et al. 2006). Cv2/Bmper also interacts genetically and biochemically with other BMP modulators to affect BMP signaling. Cv2 binds chordin using sequences distinct from those for BMP binding and is required for relocalization of chordin from intervertebral disc to vertebral body during axial skeletal development. Binding of chordin, rather than BMP, is essential for Cv2’s pro-BMP effect (Ambrosio et al. 2008; Zakin et al. 2010; Zhang et al. 2010). Cv2 also complexes with Twsg1 and BMP and genetically acts with Twsg1 to regulate axial skeleton and embryonic nephron development (Ambrosio et al. 2008; Ikeya et al. 2008, 2010; Zakin et al. 2008). Furthermore, Cv2/Bmper binds LRP1 (low density lipoprotein receptor-related protein 1) and may contribute to endocytic regulation of BMP availability and signaling (Kelley et al. 2009; Pi et al. 2012). Cross talk with various BMP modulators, ECM components, and cell-surface proteins may thus provide a basis for the activities of Cv2/Bmper as agonist or antagonist of BMP ligands. Mutations in BMPER have been linked to an autosomal recessive perinatal lethal skeletal disorder, diaphanospondylodysostosis, in humans (Funari et al. 2010; Ben-Neriah et al. 2011; Zong et al. 2015).
CRIM1
CRIM1 (cysteine-rich motor neuron 1) is a glycosylated type I transmembrane protein with six chordin-type CR repeats and an amino-terminal insulin-like growth factor–binding protein (IGFBP)-like motif in its extracellular domain. CRIM1 can be cleaved to release a soluble ectodomain. Although soluble CRIM1 does not bind BMPs in solution, CRIM1 interacts with BMP-4 and BMP-7 in the Golgi compartments when coexpressed in the same cell. This association is mediated by the CR domains and can lead to reduced processing and secretion of BMPs (Fig. 1) (Wilkinson et al. 2003). CRIM1 thus antagonizes BMP activity using a unique mechanism in that it regulates the production and release of mature BMPs cell-autonomously in BMP-expressing cells. Consistent with this idea, the Drosophila CRIM1 homolog, Crimpy, inhibits the BMP ligand Gbb cell-autonomously in motorneurons (James and Briohier 2011). However, the Caenorhabditis elegans CRIM1 homolog, Crm-1, facilitates BMP presentation and promotes BMP signaling to control body size (Fung et al. 2007). In vertebrates, CRIM1 is expressed in the notochord, somites, limb, floor plate, motor neurons, and sensory organs, but CRIM1 overexpression in chick does not affect neural patterning in the spinal cord. Instead, loss-of-function studies in chick, frog, zebrafish, and mouse suggest that CRIM1 modulates the formation of renal vasculature, neural tube morphogenesis, limb patterning, and eye development (Kolle et al. 2000, 2003; Glienke et al. 2002; Kinna et al. 2006; Pennisi et al. 2007; Ponferrada et al. 2012; Fan et al. 2014). CRIM1 may control some of these processes by acting through extracellular signals other than BMPs such as vascular endothelial growth factor (VEGF)-A and cadherins (Ponferrada et al. 2012; Fan et al. 2014).
Kielin and KCP (CRIM2)
Kielin is a 2327 amino acid protein, containing 27 chordin-like CR modules, an amino-terminal thrombospondin homology region and a carboxy-terminal vWF type D domain-like sequence. Kielin was identified by screening for secreted molecules in Xenopus and is expressed in dorsal midline structures during early frog development. Overexpression of Kielin dorsalizes ventral mesodermal explants; however, Kielin is not sufficient to induce secondary axis formation or neural induction in Xenopus, suggesting that, unlike noggin or chordin, Kielin may not efficiently inhibit BMP signaling (Matsui et al. 2000). No interaction of Kielin with TGF-β ligands has been reported and it is unclear whether Kielin regulates TGF-β signals directly or through other signaling pathways. A kielin/chordin-like protein, KCP (CRIM2), has also been isolated in the mouse, and contains 18 CR motifs and a carboxy-terminal vWF type D domain. KCP binds BMP-7, but intriguingly increases BMP-7 binding to BMPRIA/ALK-3 instead of blocking this interaction, and may form a ternary complex with them. KCP enhances Smad1 activation and BMP-responsive gene expression, and promotes BMP signaling to attenuate renal interstitial fibrosis (Lin et al. 2005). KCP also binds activin A and TGF-β1, and blocks Smad2/3 activation and Smad2/3-mediated transcription (Lin et al. 2006). KCP hence functions in opposite ways to regulate activin/TGF-β and BMP signals. In adult mouse kidney, KCP attenuates acute and chronic renal injury (Soofi et al. 2013).
Amnionless
Amnionless was discovered as a recessive insertional gene mutation in the mouse that interferes with the development of the primitive streak that gives rise to trunk mesoderm (Wang et al. 1996). The gene encodes a type I transmembrane protein with a single CR module in its extracellular region and is therefore thought to regulate BMP signals (Kalantry et al. 2001). However, Amnionless acts with cubilin, a multiligand scavenger receptor, to regulate vitamin B12 uptake and absorption of low molecular weight proteins in visceral endoderm and embryonic kidney (Strope et al. 2004; Pedersen et al. 2010; Zhang et al. 2013). Therefore, although Amnionless contains a CR domain homologous to chordin, it may not participate in the regulation of BMP signals.
DAN/Cerberus/Gremlin Family Members
The DAN/Cerberus/Gremlin family includes several small soluble proteins with a characteristic cysteine-rich domain (CAN domain) with the consensus sequence CX6QX6CX6NX2-CXGXCXSX3PX(8–13)CX2CXPX8TLXCX(15–18)-CXC (Avsian-Kretchmer and Hsueh 2004). All family members are secreted as glycosylated monomers or dimers that bind and inhibit BMPs.
DAN/NBL1
DAN (differential screening-selected gene aberrative in neuroblastoma, also known as NBL1) is the founding member of the family, and was first identified as the product of a gene that is down-regulated in oncogene-transformed rat fibroblasts (Ozaki and Sakiyama 1993). Dan encodes a secreted molecule that forms a noncovalent homodimer, and has dynamic expression patterns in mouse, chick, and frog (Hsu et al. 1998; Stanley et al. 1998; Pearce et al. 1999; Eimon and Harland 2001; Ogita et al. 2001; Gerlach-Bank et al. 2002; Kattamuri et al. 2012; Nolan et al. 2015). Dan binds and antagonizes BMP-2, BMP-4, BMP-7, and GDF-5, but does not block nodal-like signaling in Xenopus or chick (Hsu et al. 1998; Stanley et al. 1998; Dionne et al. 2001; Katsu et al. 2012). In the chick, DAN controls left–right patterning and inner ear development (Yamanishi et al. 2007; Katsu et al. 2012). In the mouse, Dan is expressed in the somites, cranial and facial mesenchyme, and axonal processes. However, Dan−/− mice do not display obvious abnormalities, suggesting compensation for loss-of-function in Dan activities (Dionne et al. 2001).
Cerberus (CER1)
Cerberus/Cer1 was originally isolated in Xenopus by screening for dorsally enriched genes (Bouwmeester et al. 1996). It encodes a small secreted protein localized in the anterior organizer of Xenopus gastrulae. Cerberus binds Xnr1, Wnt8, and BMP-4 through distinct domains and blocks signal transduction of all three pathways. By simultaneously inhibiting these signals, Cerberus promotes the head structure formation in Xenopus (Glinka et al. 1997; Hsu et al. 1998; Piccolo et al. 1999). Loss-of-function studies indicate that Cerberus regulates head and heart development in Xenopus (Schneider and Mercola 1999; Silva et al. 2003; Foley et al. 2007). In zebrafish, Cerberus, also known as Charon, inhibits nodal activity and is required for left–right patterning of the body axis (Hashimoto et al. 2004). In chick, Cerberus (i.e., Caronte) binds BMP-4, -7, and nodal, but not BMP-5, GDF-5, or activin. Cerberus is expressed in the left lateral plate mesoderm to antagonize BMP signals on the left side, and participates in left–right axis determination (Rodriguez Esteban et al. 1999; Yokouchi et al. 1999; Zhu et al. 1999a; Tavares et al. 2007). Chick Cerberus is also expressed in the hypoblast cell layer in early embryos and regulates formation of the primitive streak by antagonizing nodal signaling (Bertocchini and Stern 2002; Chapman et al. 2002).
In mouse, Cerberus (also known as Cerberus-like/Cer-l, Cerberus-related gene/Cerr1) is expressed during early gastrulation in anterior visceral endoderm, a region equivalent to the Xenopus Cerberus expression domain in the deep anterior endoderm of the organizer (Belo et al. 1997; Biben et al. 1998; Shawlot et al. 1998; Pearce et al. 1999). Like its Xenopus homolog, mouse Cerberus binds and inhibits BMP-4 and Xnr1, but unlike the Xenopus gene, mouse Cerberus does not block Wnt signaling and does not induce a secondary head in Xenopus (Belo et al. 1997, 2000; Pearce et al. 1999). Mice with targeted disruption of Cerberus/Cer1 do not show morphological or molecular defects in the head or other structures, suggesting compensation for loss of Cerberus (Simpson et al. 1999; Belo et al. 2000; Shawlot et al. 2000; Stanley et al. 2000). Indeed, compound mutants lacking functional expression of Cerl and Lefty1 develop ectopic primitive streaks and patterning defects of the streaks, which are rescued by eliminating one copy of the Nodal gene. The results thus suggest that Cerberus plays a role in spatially restricting primitive streak formation by antagonizing nodal signaling in mouse (Perea-Gomez et al. 2002). Human Cerberus also binds and inhibits nodal to suppress aggressive phenotypes of breast cancer cells (Aykul et al. 2015).
Coco/Dante/Cerl-2
Coco is a Xenopus homolog of Cerberus, and, like Cerberus, binds Xnr1 and BMP-4, blocks signaling by Xnr, BMP, and Wnt, and induces neural markers directly in ectodermal explants. However, Coco is not expressed in the organizer, but shows maternal expression in an animal-to-vegetal gradient with the highest level in ectodermal cells, and may regulate cell fate specification and competence before onset of gastrulation (Bell et al. 2003). A second gene encoding Cerberus-like 2 (Cerl-2, previously known as Dante) was shown to modulate mouse left–right laterality. Cerl-2 is first expressed in a symmetric pattern around the mouse node, but its expression level on the left side gradually diminishes, whereas the right side expression remains strong (Pearce et al. 1999; Marques et al. 2004). This positions Cerl-2 as a unique patterning molecule in left–right axis formation. Cerl2 knockout mice show laterality defects as well as left ventricular cardiac hyperplasia (Araujo et al. 2014). Like other members of the Cerberus family, Cerl-2 binds and inhibits Xnr1 and BMP-4, and balanced regulation of the activities of these TGF-β ligands may explain its function in left–right axis specification (Marques et al. 2004).
Drm/Gremlin (GREM1)
Drm (down-regulated in mos-transformed cells) was first identified in the rat as the product of a gene down-regulated in oncogene-transformed fibroblasts. Like Dan, Drm is a secreted glycoprotein with growth-inhibitory activities in cultured cells (Topol et al. 1997, 2000). The Xenopus Drm homolog, Gremlin (Grem1), was identified in a screen for genes with dorsalizing ability (Hsu et al. 1998). Gremlin binds and inhibits BMP-2, -4, and -7, but does not block nodal, Vg1, or activin (Hsu et al. 1998; Church et al. 2015). Although Gremlin prevents BMPs from interacting with their receptors, an intracellular inhibitory mechanism may also operate, whereby Gremlin binds the BMP-4 precursor and blocks processing and secretion of mature BMP-4 (Fig. 1) (Sun et al. 2006). Gremlin is expressed in posterior limb mesenchyme in vertebrate embryos, and is the principal BMP antagonist in the developing limb bud. It acts to maintain a Sonic hedgehog (Shh)/fibroblast growth factor (FGF) feedback regulatory loop to control limb outgrowth (Capdevila et al. 1999; Merino et al. 1999b; Zuniga et al. 1999; Khokha et al. 2003; Verheyden and Sun 2008). Gremlin also regulates myogenesis in the head, lung morphogenesis, kidney development, angiogenesis, and bone formation during vertebrate embryogenesis (Lu et al. 2001; Tzahor et al. 2003; Michos et al. 2004, 2007; Gazzerro et al. 2007; Stabile et al. 2007; Stafford et al. 2011; Canalis et al. 2012). The Gremlin–BMP axis is involved in pathogenesis of several diseases, including diabetic nephropathy, pulmonary hypertension, and cancer progression (Zhang and Zhang 2009; Cahill et al. 2012; Karagiannis et al. 2015). Besides BMPs, Gremlin interacts with and activates VEGF receptor 2 to stimulate angiogenesis (Mitola et al. 2010), and binds and inhibits macrophage migration inhibitory factor (MIF) to attenuate atherosclerosis (Muller et al. 2013).
PRDC (GREM2)
PRDC (protein related to DAN and Cerberus) was first identified by gene trapping in embryonic stem cells (Minabe-Saegusa et al. 1998). It encodes a small secreted glycoprotein that forms noncovalent, hydrogen-bonded homodimers that assume a TGF-β-like two-finger-wrist conformation (Kattamuri et al. 2012; Nolan et al. 2013). PRDC binds and inhibits BMP-2 and BMP-4 efficiently. PRDC and Gremlin also weakly inhibit BMP-6 and BMP-7 signaling, but do not affect the activities of activin, TGF-β, GDF-5, or GDF-9 (Sudo et al. 2004). Heparin binding by PRDC interferes with its ability to block BMP signaling (Nolan et al. 2013). PRDC is widely distributed, with high-level expression in ovary, brain, and spleen. PRDC regulates BMP signaling to control placode neurogenesis during cranial nerve formation in chick and to modulate follicle development in ovary (Sudo et al. 2004; Kriebitz et al. 2009). PRDC also influences osteoblast differentiation during osteogenesis (Ideno et al. 2009). Mice deficient for the Prdc gene have smaller incisors, implying a role of the gene in tooth morphogenesis (Vogel et al. 2015).
Sclerostin (SOST)
Sclerostin/SOST was first identified as the product of a gene whose mutations are responsible for sclerostosis, a recessive autosomal sclerosing bone dysplasia characterized by progressive skeletal overgrowth (Balemans et al. 2001; Brunkow et al. 2001). Sclerostin is a small secreted factor with a cystine-knot structure similar to that in Dan, Cerberus, and Gremlin, and was therefore proposed to be a BMP antagonist. Sclerostin binds BMP-5, -6, and -7, but not TGF-βs, and prevents binding of BMPs to their cognate receptors. It is expressed in osteocytes and inhibits BMP-5- or -6-stimulated bone differentiation, but has less effect on BMP-2 and -4 (Kusu et al. 2003; Winkler et al. 2003; van Bezooijen et al. 2004). In contrast to the loss-of-function phenotype leading to skeletal overgrowth, transgenic mice overexpressing sclerostin show reduced bone mass and bone strength (Winkler et al. 2003). Subsequently, sclerostin was shown to inhibit BMP-7 activity only in a cell-autonomous fashion, when coexpressed, because of direct binding of sclerostin to both the pro- and mature peptides of BMP-7 in ligand-expressing cells, thus promoting intracellular retention and proteasomal degradation of BMP-7 (Fig. 1) (Krause et al. 2010). The catabolic effect of sclerostin on bone growth is now mainly attributed to its ability to inhibit Wnt signaling by direct binding to the Wnt coreceptor LRP5/LRP6 (Li et al. 2005; Semenov et al. 2005; van Benzooijen et al. 2007; Kamiya et al. 2008).
Ectodin/Wise/USAG-1 (SOSTDC1)
SOSTDC1 was isolated as USAG-1 (uterine sensitization-associated gene 1) from sensitized endometrium of rat uterus, as ectodin from mouse, and Wise (Wnt modulator in surface ectoderm) from Xenopus (Simmons and Kennedy 2002; Itasaki et al. 2003; Laurikkala et al. 2003). It is a small secreted factor that is closely related to sclerostin, and binds and inhibits BMP-2, -4, -6, and -7 (Laurikkala et al. 2003; Yanagita et al. 2004). Functional studies with SOSTDC1-deficient mice reveal its role in regulation of BMP-7 signaling during renal injury as well as in teeth patterning during development (Kassai et al. 2005; Yanagita et al. 2006; Murashima-Suginami et al. 2008; Kiso et al. 2014). SOSTDC1/Wise also selectively inhibits BMP-7, but not BMP-2 or Wnt-3a, in breast cancer cells (Clausen et al. 2011), and suppresses both BMP-7 and Wnt-3a in renal cancer cells (Blish et al. 2008). Similarly to sclerostin, SOSTDC1 binds LRP6 to modulate Wnt signaling, and regulates early Xenopus patterning and development of bone, teeth, mammary, and skin appendage placodes in mice (Itasaki et al. 2003; Lintern et al. 2009; Ahn et al. 2010, 2013; Ellies et al. 2014).
The CCN Family Proteins
The CCN family of growth factors includes six members, including CYR61 (CCN1), connective tissue growth factor (CTGF, CCN2), nephroblastoma overexpressed (NOV, CCN3), and WISP1, 2, and 3 (CCN4, 5, 6). These proteins show similar sequence configurations with four conserved domains, an amino-terminal domain similar to that in IGFBP, a CR module found in chordin and vWF, a thrombospondin type I repeat (TSP-1)-like sequence, and a carboxy-terminal motif with the cystine-knot structure (reviewed in Brigstock 2003; Perbal 2004; Katsube et al. 2009). Several CCN members regulate TGF-β signals. CTGF binds BMP-2 (KD 0.77 nm), -4, -7 (KD 14 nm) and TGF-β1 through its CR domain. By doing so, CTGF inhibits BMP-4 binding to its type I receptor but enhances TGF-β1 binding to its receptor complex. CTGF thus blocks BMP and promotes TGF-β signaling in cultured cells (Abreu et al. 2002; Nguyen et al. 2008; Maeda et al. 2009). Disruption of its gene in mouse reveals that CTGF is required for coordination of chondrogenesis and angiogenesis during skeletal development (Ivkovic et al. 2003), and this may depend on the activity of CTGF to modulate BMP signaling during chondrocyte differentiation (Maeda et al. 2009; Mundy et al. 2014). CTGF also inhibits BMP-7 in the diabetic kidney and contributes to diabetic nephropathy (Nguyen et al. 2008). CCN3 blocks BMP-2 function during osteoblast differentiation and modulates bone regeneration as an inhibitor of BMP-induced Smad signaling (Minamizato et al. 2007; Rydziel et al. 2007; Matsushita et al. 2013). Targeted disruption of the Ccn3 gene results in abnormal skeletal and cardiac development (Heath et al. 2008). In contrast, CCN4 binds BMP-2 and enhances its signaling during osteogenic differentiation (Ono et al. 2011). Besides TGF-β family ligands, CCN proteins also interact with many other signaling proteins, including integrins, LRP, Wnt, VEGF-A, and Notch, to control multiple developmental and physiological processes (Babic et al. 1999; Segarini et al. 2001; Mercurio et al. 2004; Perbal 2013).
Insulin-Like Growth Factor–Binding Protein 3 (IGFBP3)
IGFBP3 was originally identified as the main carrier of IGF1 in serum, and acts as reservoir and modulator of IGF1 (Baxter 2014). However, IGFBP3 directly binds and antagonizes BMP-2 and -4 in zebrafish, and induces chordin expression to further inhibit BMP signaling (Zhong et al. 2011). IGFBP3 thus exerts an IGF1-independent function in modulating early zebrafish development. In mammals, IGFBP3 also enhances TGF-β1 activity and opposes BMP-7 signaling in glomerular podocytes to control cell survival or apoptosis, although direct association of IGFBP3 with these TGF-β family ligands has not been reported in this context (Peters et al. 2006).
Norrie (NDP)
Norrie is a secreted cysteine-rich protein encoded by the NDP gene whose mutations are associated with Norrie disease and familial exudative vitreoretinopathy (FEVR), two X-linked recessive disorders that affect neuroretina degeneration or result in incomplete development of retinal vasculature (Berger et al. 1992; Chen et al. 1993). Norrie stimulates the canonical Wnt-β-catenin pathway to control retinal vasculature development (Xu et al. 2004). However, Norrie also binds directly to nodal-related growth factors and BMP-4, and inhibits their signaling to promote anterior neural patterning in Xenopus. Norrie blocks BMP-2 and BMP-4 signaling in reporter assays in mammalian cells. Some Norrie mutants responsible for FEVR and Norrie disease show normal Wnt-enhancing ability, but impaired BMP-inhibitory function, suggesting that control of BMP signaling contributes to the activity of Norrie in eye development (Xu et al. 2012b; Deng et al. 2013).
Propeptides of TGF-β Family Ligands
Proteolytic cleavage to release TGF-β family proteins from their precursors is mediated by subtilisin/kexin-like proprotein convertases (PCs) (reviewed by Nakayama 1997; Taylor et al. 2003; Constam 2014). Although most of these enzymes are located in the trans-Golgi network and act in this compartment to process ligands, secreted convertases may additionally function in a non-cell-autonomous fashion to cleave soluble precursors in vivo (Beck et al. 2002; Birsoy et al. 2005). With the exception of the nodal-related Xnr2, which has diminished signaling capacity as precursor (Beck et al. 2002; Eimon and Harland 2002), other TGF-β family proteins are not active when the cleavage sites are mutated, and many such mutants act as dominant-negative ligands (Hawley et al. 1995; Joseph and Melton 1998; Sun et al. 1999; Eimon and Harland 2002). Regulation of ligand processing, by intracellular PCs or secreted factors, thus constitutes one of the first steps in the regulation of TGF-β family signaling (Constam 2014). Subsequent to ligand processing, cleaved propeptide products regulate the folding, sorting, stability, and/or activity of the mature TGF-β family proteins.
The classical example is TGF-β, which is in a latent state by noncovalent association with its precursor polypeptide latency-associated peptide (LAP) (reviewed in Harrison et al. 2011). Similarly, myostatin (GDF-8), its close relative GDF-11 (BMP-11), and BMP-10 all form latent, noncovalent complexes with their respective propeptides. Activation of these ligands is achieved by cleavage of the propeptides by BMP-1/Tolloid metalloproteinases, and/or by integrin-mediated interaction with the latent complex, leading to its conformational changes and release of the mature ligands (Hill et al. 2002; Wolfman et al. 2003; Ge et al. 2005; Sengle et al. 2011). The prodomain of the BMP-like ligand dorsalin also associates with the mature protein, although the functional consequence of this interaction is unclear (Constam and Robertson 1999). Besides regulating the bioactivity of mature ligands, propeptides also control the deposition of the ligands in the ECM, and stability and secretion of TGF-β family proteins. The prodomain of BMP-4, for example, associates with mature BMP-4 and directs it to lysosome- and proteasome-mediated degradation. A second cleavage inside the prodomain is required to release mature BMP-4, thereby stabilizing it, enhancing its activation, and allowing long-range signaling by this growth factor (Cui et al. 2001; Degnin et al. 2004). The prodomain of BMP-7 may similarly regulate the secretion and stability of mature BMP-7, because a single amino acid mutation in the proregion leads to reduced BMP-7 activity in zebrafish without affecting its processing (Dick et al. 2000). The prodomain of nodal facilitates degradation of mature nodal after cleavage (Constam and Robertson 1999), which may promote autocrine signaling and restrict its signaling range. Because uncleaved nodal precursor is detected in cell culture medium, long-range nodal signaling may be regulated by nodal transport in its precursor form to desired sites before processing into mature growth factor for signaling (Le Good et al. 2005). The proregions of Xnr3 and Xnr5, two nodal-related ligands in Xenopus, also bind and inhibit mature BMP-4, providing a mechanism for propeptides to regulate TGF-β family signaling in trans (Haramoto et al. 2004).
TGF-β Family Ligands as Agonists/Antagonists of TGF-β Family Signaling
TGF-β family signaling is not only regulated by soluble agonists and antagonists, but often modulated by heterodimerization or interaction with other TGF-β family polypeptides. An example is the inhibition of activin homodimer signaling by heterodimeric inhibin (reviewed by Bilezikjian et al. 2012). Other ligands that also modulate TGF-β family signaling include lefty, Xnr3, GDF-3, and BMP-3.
Lefty
Lefty (also known as antivin) is a divergent TGF-β family member that is asymmetrically expressed along the left–right axis (Meno et al. 1996). It lacks the cysteine involved in disulfide bond formation between dimer subunits, and thus exists as a monomer. Two lefty genes are found in vertebrates and play important roles in negative feedback regulation of left-sided nodal signaling during left–right axis determination (Meno et al. 1997, 1998, 1999; Cheng et al. 2000; Ishimaru et al. 2000). In addition, lefty expression during gastrulation limits nodal signaling in mesendoderm formation (Bisgrove et al. 1999; Thisse and Thisse 1999; Tanegashima et al. 2000; Agathon et al. 2001; Branford and Yost 2002; Chen and Schier 2002; Feldman et al. 2002; Cha et al. 2006). Besides nodal, lefty also antagonizes Vg1, GDF-1, and GDF-3, but has no effect on activin or TGF-β1 signaling. Lefty binds to the Cripto/Cryptic coreceptor for nodal and Vg1, GDF-1 and GDF-3, and blocks the formation of a functional ligand–receptor complex (Chen and Shen 2004; Cheng et al. 2004; Tanegashima et al. 2004). Lefty also binds directly to mature nodal and GDF-3 and prevents them from activating activin receptors (Chen and Shen 2004; Chen et al. 2006). In addition, lefty has been implicated in interference with BMP and Wnt signaling; however, the mechanisms involved in the regulation of these signals have not been defined (Meno et al. 1997; Branford et al. 2000; Ulloa and Tabibzadeh 2001; Branford and Yost 2002). In zebrafish and Xenopus embryos, it is proposed that nodal-lefty forms a reaction–diffusion system to pattern embryonic tissues, as lefty is induced by nodal and diffuses faster than nodal (Marjoram and Wright 2011; Muller et al. 2012). However, this view is challenged by the observation that nodal may only signal over a short range, and the gradient of nodal signaling readout may rely on differential temporal regulation of nodal and lefty expression (van Boxtel et al. 2015).
Xnr3
Xnr3 is a divergent nodal-related factor in Xenopus that lacks the carboxy-terminal cysteine (Cys-7) that is conserved among all other TGF-β family members, and functions as a monomer (Smith et al. 1995; Haramoto et al. 2007). Unlike other nodal-related proteins, Xnr3 does not specify mesendodermal cell fate; instead, it induces neural marker expression and blocks mesodermal induction by BMP-4 and activin, but not by Xnr2, in Xenopus (Hansen et al. 1997; Haramoto et al. 2004). Studies of Xnr2 and Xnr3 chimeras suggest that amino- and carboxy-terminal segments of mature Xnr3 are required for neural inducing activity, whereas the central region can be replaced with that of mature Xnr2 to induce dorsal mesoderm (Ezal et al. 2000). The prodomain of Xnr3 alone binds and inhibits BMP-4 and is both necessary and sufficient for Xnr3 to block BMP-4 activities (Haramoto et al. 2004, 2006). A role of Xnr3 in blocking ligand–receptor assembly has also been proposed (Ezal et al. 2000). Loss-of-function studies reveal that Xnr3 regulates convergent extension movements during gastrulation in Xenopus, and may do so by interacting with the divergent Cripto family protein FRL1, which binds and activates the FGF receptor 1. The prodomain of Xnr3 may be important for activation of FGF signaling in this process (Glinka et al. 1996; Yokota et al. 2003).
GDF-3
GDF-3 is closely related to Vg1, but lacks the conserved cysteine used in dimer formation of TGF-β family ligands (McPherron and Lee 1993). GDF-3 signals through the nodal pathway, using ALK-4 and ALK-7 receptors and Cripto coreceptor, during early mouse development (Chen et al. 2006; Andersson et al. 2007, 2008). In addition, GDF-3, both as precursor and as mature peptide, inhibits BMP signaling through direct interaction with mature BMPs (Levine and Brivanlou 2006; Levine et al. 2009). GDF-3 expression is high in pluripotent cell types, including human embryonic stem cells, and may regulate their maintenance and differentiation (Levine and Brivanlou 2006; Peerani et al. 2007).
BMP-3
BMP-3 and Xenopus BMP-3b are closely related BMP-like ligands. Unlike BMPs, however, BMP-3 and BMP-3b dorsalize Xenopus embryos, implying that they can act as BMP antagonists (Hino et al. 2003; Gamer et al. 2005). BMP-3 and BMP-3b block BMP-2 and ADMP signaling in Xenopus ectodermal explants. BMP-3 also inhibits activin but not Xnr1 or a nodal-like ligand Derriere, whereas BMP-3b blocks both Xnr1 and Derriere. In addition, BMP-3 inhibits BMP-2-induced differentiation of osteoprogenitor cells and opposes TGF-β1 actions in bone marrow stromal cells (Faucheux et al. 1997; Daluiski et al. 2001). BMP-3 and BMP-3b form heterodimers with BMP-2, ADMP, and Derriere, suggesting that BMP-3 or BMP-3b may exert its effects through formation of inactive ligand dimers (Hino et al. 2003). BMP-3b also binds Xnr1 noncovalently and may prevent Xnr1 from associating with its receptors (Hino et al. 2003). BMP-3 further interacts with the type II receptor ActRIIB without inducing Smad activation, and this provides another means for the ligand to inhibit activin and BMP signaling (Gamer et al. 2005; Kokabu et al. 2012). In myoblastic C2C12 cells, however, BMP-3b can stimulate Smad2/3 signaling and activin-responsive reporter expression. It also inhibits BMP-2-induced osteoblastic differentiation in this system, apparently through competing for the common Smad4 by activated Smad2/3 (Matsumoto et al. 2012).
Heterodimers
Formation of heterodimers among TGF-β family proteins can regulate the activities of the ligands (Guo and Wu 2012). As mentioned, BMP-3 and BMP-3b can form heterodimers with BMP-2, ADMP, and Derriere, and inhibit the activities of these factors (Hino et al. 2003). Nodal also forms heterodimers with BMPs to antagonize BMP signaling (Yeo and Whitman 2001). Nodal heterodimers with GDF-1, in contrast, have higher activity than nodal homodimers (Fuerer et al. 2014). Similarly, heterodimers of BMP-2 and -7 or BMP-4 and -7 are more active in mesoderm induction or patterning in Xenopus and zebrafish and in inducing bone formation in osteoprogenitor cells than their homodimers (Aono et al. 1995; Israel et al. 1996; Suzuki et al. 1997; Nishimatsu and Thomsen 1998; Little and Mullins 2009). Heterodimers, but not homodimers, of BMP-2 and BMP-7 engage simultaneously two different type I BMP receptors (i.e., ALK-3 or ALK-6 and ALK-2 or ALK-8) in the receptor complex to activate BMP signaling in zebrafish (Little and Mullins 2009). BMP-7 and GDF-7 also form heterodimers in vitro that have a higher axon-orienting activity than BMP-7 or GDF-7 homodimers (Butler and Dodd 2003). Heterodimers of GDF-9 and BMP-15 are more potent regulators of ovarian granulosa and cumulus cells than their corresponding homodimers (Peng et al. 2013; Mottershead et al. 2015). BMP-2 and BMP-6 heterodimers are also more effective than the corresponding homodimers to induce differentiation of human embryonic stem cells (Valera et al. 2010). The formation of heterodimers between Drosophila Dpp and Scw also facilitates transport of the ligands in early Drosophila embryos and promotes threshold readout of the BMP morphogen gradient (Shimmi et al. 2005a).
PROTEOGLYCANS AND EXTRACELLULAR MATRIX PROTEINS
Secreted TGF-β family ligands encounter not only soluble regulatory factors, as discussed, but also various ECM proteins. The complex interplay between TGF-β family proteins, their soluble regulators and ECM proteins controls the diffusion of the ligands among target cells, and ligand availability for receptor binding and activation. The ECM-associated proteins are often not dedicated TGF-β modulators. The HSPG glypicans, for example, regulate signals from FGF, Wingless/Wnt, and hedgehog family proteins in addition to TGF-β/BMPs (Lin and Perrimon 2000). Many ECM molecules can also act as both agonists and antagonists. A balance in actions between sequestration, storage, and presentation of ligands may account for the dual functions of ECM proteins (Fig. 3).
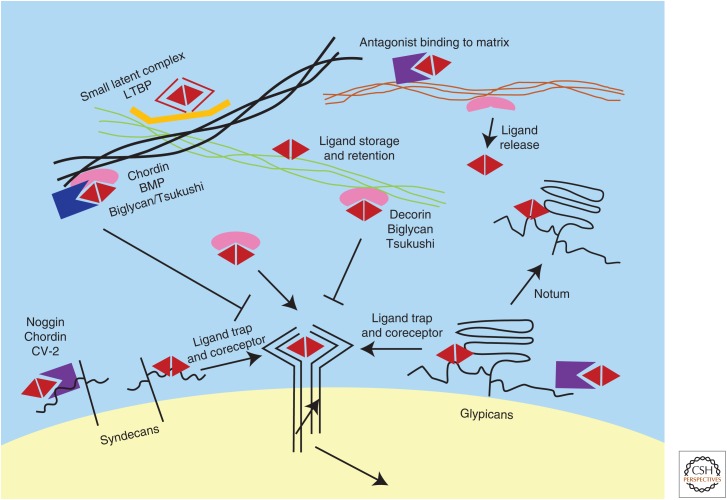
Figure 3.
Regulation of transforming growth factor β (TGF-β) family signals by proteoglycans and extracellular matrix proteins. Small leucine-rich proteoglycans, such as decorin, biglycan, and Tsukushi, interact with both TGF-β family ligands and extracellular matrix (ECM) proteins. Depending on the composition of ECM proteins, they may tether the ligands in the ECM to prevent signaling, or release the ligands to create a local pool of cytokines to enhance signaling. Biglycan and Tsukushi also form a ternary complex with chordin and bone morphogenetic protein (BMP) to block BMP signaling. Cell-surface heparan sulfate proteoglycans (HSPGs), including glypicans and syndecans, interact with both TGF-β family proteins and their secreted modulators. They can serve as ligand coreceptors to enhance TGF-β family signaling, but they can also trap and facilitate internalization of the ligands and their soluble regulators to modulate signal duration and range. They, therefore, have cell-context-dependent positive or negative roles in regulation of TGF-β signals. The Drosophila protein Notum facilitates release of glypicans from the cell surface and can convert glypicans from ligand-presenting factors to ligand-binding antagonists. Several ECM proteins bind TGF-β family ligands directly and influence storage, activation, and diffusion of these proteins to control signaling strength and range. Secreted TGF-β family regulators can also associate with ECM proteins to affect ligand availability. LTBPs (latent TGF-β-binding proteins) interact with ECM proteins to control TGF-β activation from the small latent complex (SLC).
Small Leucine-Rich Proteoglycans (SLRPs)
The SLRP family consists of 17 members with similar domain organization (reviewed in Hocking et al. 1998; Iozzo 1999; Young et al. 2003; Schaefer and Iozzo 2008). The core proteins contain six to twelve 24-amino-acid leucine-rich repeats and a characteristic amino-terminal cysteine-rich region. Chondroitin/dermatan sulfate and keratin sulfate glycosaminoglycan (GAG) chains are attached to the core proteins. Several SLRP proteins are shown to regulate TGF-β signaling.
Decorin (DCN)
Decorin is a small ECM proteoglycan that is expressed in connective tissues. It binds to mature, but not latent, TGF-β1, -β2, and -β3 through its polypeptide core, with GAG modifications reducing this binding. Decorin reduces the interaction of TGF-β1 with its type I and type III receptors, and may sequester the ligand in the ECM (Hildebrand et al. 1994). Cells deficient in the Dcn gene show enhanced TGF-β binding to its receptors (Droguett et al. 2006). Expression of decorin in CHO cells blocks TGF-β-induced cell proliferation (Yamaguchi et al. 1990), and administration of decorin in a mouse kidney disease model can attenuate the disease manifestation, similarly to TGF-β neutralization with antibody (Border et al. 1992). Although these data suggest that decorin inhibits TGF-β function, other studies imply that decorin enhances the bioactivity of TGF-β. Addition of decorin promotes TGF-β1 binding to the TGF-β receptors and betaglycan, and strengthens the inhibitory effect of TGF-β1 on osteoblast cell proliferation, whereas depletion of decorin reduces myoblast responsiveness to TGF-β-mediated inhibition of skeletal muscle differentiation (Takeuchi et al. 1994; Riquelme et al. 2001). Several explanations may account for the dual functions of decorin in regulating TGF-β activities. Decorin binds TGF-β1 at two interfaces with high and low affinity, respectively (Hildebrand et al. 1994; Takeuchi et al. 1994). Association of TGF-β1 with different sites may affect the conformation and/or stability of decorin-TGF-β1 complex, thus affecting retention of TGF-β1 inside the ECM or ligand presentation to its receptors. In addition, decorin interacts with many ECM components, such as collagens and fibronectin. Differential association of decorin with distinct ECM proteins may control the function of decorin (Kresse and Schonherr 2001). ECM-immobilized decorin sequesters TGF-β and prevents it from signaling, whereas soluble decorin does not control TGF-β activity (Markmann et al. 2000). In addition, dermatopontin, a small ECM component, can interact with both decorin and TGF-β1. Whereas free dermatopontin competes with decorin for TGF-β1 and decreases the formation of the decorin–TGF-β1 complex, a dermatopontin–decorin complex has enhanced binding to TGF-β1. Thus, dermatopontin may differentially influence the activity of decorin depending on whether it forms a ternary complex with decorin and TGF-β1, or interacts with the two proteins separately (Okamoto et al. 1999). Besides TGF-βs, decorin binds myostatin and reverses its inhibition of myoblast proliferation in vitro (Miura et al. 2006). It also associates with activin C and may modulate its activity in stimulating cell growth and migration of colorectal cancer cells (Bi et al. 2015).
Biglycan (BGN)
Biglycan is closely related to decorin in structure and has similar and distinct functions in binding and regulating TGF-β ligands (Hildebrand et al. 1994; Droguett et al. 2006; Wu et al. 2014). One distinct activity of biglycan is its role in modulating BMP signals. Targeted disruption of the Bgn gene leads to age-related osteoporosis (Xu et al. 1998), and reduced osteoblast responsiveness to BMP-4-induced differentiation may at least partially account for this defect (Chen et al. 2004b). Although the GAG chains enhance biglycan’s ability to promote BMP-4 signaling in osteoblasts, they reduce BMP-2 signaling in myogenic C2C12 cells (Miguez et al. 2011; Ye et al. 2012). The conflicting results may be because of cell-type-specific ECM proteins that can modify the activity of biglycan. Although these findings indicate a role of biglycan in promoting BMP signaling in skeletal development, biglycan can inhibit BMPs in early Xenopus development. Biglycan binds BMP-4 directly and blocks its function upstream of the BMP receptors. It can also form a ternary complex with BMP and chordin, and enhance the BMP-inhibitory activity of chordin (Moreno et al. 2005). Similarly to decorin, the dual activities of biglycan may depend on the microenvironment and the presence or absence of other factors, such as chordin, BMP-1/Tolloid metalloproteinases, or other ECM proteins (Wadhwa et al. 2004).
Tsukushi (TSK)
Tsukushi, a unique SLRP protein, was first discovered in the chick, and vertebrate homologs were identified in Xenopus, zebrafish, mouse, and human. Although structurally different from biglycan (e.g., Tsukushi has 12 leucine-rich repeats instead of 10 in biglycan), Tsukushi has similar activities as biglycan in that it binds directly to BMP-4 and blocks BMP signaling in chick and frog. Tsukushi also forms a ternary complex with BMP-4 and chordin and enhances the BMP-inhibitory activity of chordin (Ohta et al. 2004). Differently from biglycan, Tsukushi associates with Xnr-2 and Vg1, and enhances nodal-like signaling during germ layer and primitive streak formation in Xenopus and chick, respectively (Ohta et al. 2006; Morris et al. 2007). Besides TGF-β family ligands, Tsukushi can bind and inhibit FGF-8b, interact with Delta to regulate Notch signaling, and bind Frizzled 4 (Fzd4) to antagonize Wnt signaling (Kuriyama et al. 2006; Morris et al. 2007; Ohta et al. 2011). Loss-of-function studies indicate that Tsukushi regulates neural crest formation during early Xenopus development and controls anterior commissure formation in mouse brain (Kuriyama et al. 2006; Ito et al. 2010).
Fibromodulin (FMOD) and Lumican (LUM)
Fibromodulin and lumican are both SLRPs with keratin sulfate GAG chains, in contrast to chondroitin/dermatan sulfate found in decorin and biglycan. Like decorin and biglycan, fibromodulin binds TGF-β1 and may inhibit its signaling through ligand sequestration in the ECM (Hildebrand et al. 1994; Embree et al. 2010). Physical interaction between lumican and TGF-β ligands has not been reported, but lumican may repress TGF-β2 signaling in osteosarcoma cells (Nikitovic et al. 2011). Lumican also binds to the type I TGF-β receptor TβRI/ALK-5 to regulate epithelial wound healing (Yamanaka et al. 2013).
Heparan Sulfate Proteoglycans
HSPGs are proteins with heparan sulfate glycosaminoglycan chains attached. They regulate diverse cellular processes ranging from adhesion and migration to proliferation, differentiation, and morphogenesis (Bernfield et al. 1999). Two families of surface HSPGs exist, glypicans, which are linked to the plasma membrane via glycosylphosphatidylinositol (GPI) anchor, and syndecans, which are transmembrane HSPGs. Four syndecans and six glypicans exist in mammals, whereas a single syndecan and two glypicans are found in Drosophila. Several HSPG family proteins modulate TGF-β signaling.
Glypicans
The two Drosophila glypican homologs, Dally and Dally-like, do not regulate signaling from Dpp/BMP during early embryogenesis. However, they are critically required at the pupal stages when imaginal discs, such as eye, antenna, genitalia, and wing discs, develop (Jackson et al. 1997; Tsuda et al. 1999; Fujise et al. 2003; Belenkaya et al. 2004). Mosaic mutant clone studies indicate that Dally and Dally-like control cell-autonomous responses to Dpp as well as cell-non-autonomous movement of Dpp to form a Dpp morphogen gradient in the wing disc. In addition, Dally can act as both positive and negative regulator of Dpp. Low doses of Dally are required for Dpp signaling, whereas high doses of Dally reduce Dpp responses. Dally may thus serve as a coreceptor for Dpp to enhance its signaling but, at the same time, limits Dpp diffusion to restrict its distribution (Fujise et al. 2003; Belenkaya et al. 2004). Another secreted molecule, Pentagon, interacts with Dally to facilitate long-range transport of Dpp in the wing disc (Vuilleumier et al. 2010). Dally-like can be modified by the secreted enzyme Notum, which facilitates its cleavage at the GPI anchor. Notum thus converts this glypican from a membrane-tethered coreceptor to a soluble, ligand-binding antagonist (Kreuger et al. 2004). Interestingly, in addition to acting as a coreceptor for Dpp, Dally can also promote Dpp signaling in trans, in neighboring cells in the Drosophila ovary, to maintain germline stem cells. Stabilization of Dpp at the cell surface may contribute to this cell-contact-dependent, trans-Dpp stimulatory function of Dally (Akiyama et al. 2008; Guo and Wang 2009; Hayashi et al. 2009; Dejima et al. 2011). A role of heparan sulfate in glypican-dependent modulation of Dpp is implied by the observation that mutations in heparan sulfate biosynthesis enzymes in Drosophila attenuate Dpp signals in the wing imaginal disc (Bornemann et al. 2004; Han et al. 2004; Takei et al. 2004). In C. elegans, the glypican LON-2 binds BMP-2 to inhibit its signaling in body length control (Gumienny et al. 2007; Taneja-Bageshwar and Gumienny 2012). In mammals, glypican-3 enhances BMP-4 signaling during limb patterning and skeletal development (Paine-Saunders et al. 2000), and participates in BMP-regulated renal branching morphogenesis (Grisaru et al. 2001). However, increased glypican-3 expression in hepatocellular carcinoma inhibits BMP-7 signaling (Midorikawa et al. 2003). Glypican-1 is required for optimal TGF-β1 signaling in pancreatic cancer cells (Li et al. 2004), but inhibits BMP-4 signaling in lymphoblastoid cells (O’Connell et al. 2007). Both glypican-1 and -3 also bind BMP-2 and block BMP-2 signaling in cranial osteogenesis (Dwivedi et al. 2013). In zebrafish, glypican-4 attenuates BMP signaling to regulate cardiac development (Strate et al. 2015). As glypicans also regulate Wnt, hedgehog, and FGF signaling (Bernfield et al. 1999; Lin and Perrimon 2000; Fico et al. 2011), the six vertebrate glypicans may differentially modulate overlapping and distinct signaling pathways during vertebrate embryogenesis and homeostasis.
Syndecans
The role of syndecan in signal transduction of TGF-β family ligands is less understood. Among four vertebrate homologs, syndecan-2 binds TGF-β1 and Vg1, and less efficiently activin, through its ectodomain polypeptide backbone (Kramer and Yost 2002; Chen et al. 2004a). A mutant syndecan-2 inhibits signaling by Vg1, but not activin or nodal, in Xenopus ectodermal explants. Syndecan-2 may act as a coreceptor for Vg1 during left–right patterning in Xenopus (Kramer and Yost 2002). Syndecan-2 also increases TGF-β1-induced fibronectin expression in renal papillary fibroblasts (Chen et al. 2004a) and promotes TGF-β2-enhanced fibrosarcoma cell adhesion (Mytilinaiou et al. 2013). Syndecan-4 also binds TGF-β1, but does so through its heparan sulfate chains instead of the core polypeptide. Syndecan-4-deficient mice show impaired inhibition of interleukin-1β production by TGF-β1 in macrophages (Ishiguro et al. 2001) and reduced induction of dermal fibroblast contraction by TGF-β1 (Chen et al. 2005). Enhanced expression of syndecan-4 in cutaneous T-cell lymphomas traps TGF-β1 at the cell surface and facilitates its inhibition of T-cell activation (Chung et al. 2011). Unlike syndecan-2 and -4, syndecan-3 may block, rather than promote, BMP-2 signaling during cartilage differentiation of limb mesenchyme, although no interaction of syndecan-3 with BMP-2 or BMP receptors has been shown (Fisher et al. 2006). Syndecan-1 has a biphasic effect on BMP signaling. It enhances BMP signaling at low doses and inhibits it at high doses. Endogenous syndecan-1 regulates BMP signaling during dorsoventral patterning of embryonic ectoderm in Xenopus (Olivares et al. 2009).
In addition to binding TGF-β family ligands and thus directly regulating their activities, HSPGs also associate with soluble BMP regulators such as noggin, chordin, and Crossveinless-2 (Paine-Saunders et al. 2002; Jasuja et al. 2004; Viviano et al. 2004; Rentzsch et al. 2006). These interactions may restrict their diffusion and help define their activities (see above). HSPGs thus regulate TGF-β family signaling by controlling the activities of both the TGF-β family ligands and their soluble modulators.
Extracellular Matrix Proteins
α2-Macroglobulin
α2-Macroglobulin (α2M) is a large homotetrameric plasma glycoprotein that functions as an extracellular inhibitor of all four classes of proteases, that is, serine, cysteine, aspartate, and metalloproteases. Cleavage of α2M by bound proteases results in a conformational change that traps and inhibits the proteases in nondissociable complexes. Protease-activated α2-macroglobulin (α2M*) then loses protease-inhibitory activity and is recognized by the cell-surface receptor LRP. The role of α2M in controlling TGF-β activity was first revealed by the observation that TGF-β forms an inactive complex with α2M in serum (O’Connor-McCourt and Wakefield 1987; Huang et al. 1988). In contrast, latent TGF-β does not bind α2M (Wakefield et al. 1988). α2M binds TGF-β2 with higher affinity (KD ∼14 nm) than TGF-β1 (KD 320 nm), and, accordingly, inhibits TGF-β2 more efficiently (Danielpour and Sporn 1990; Webb et al. 1996). Protease activation modulates the affinity of α2M for TGF-βs, as trypsin and thrombin decrease and plasmin and methylamine enhance α2M* binding to TGF-β (LaMarre et al. 1991; Webb et al. 1996). Methylamine-α2M* promotes TGF-β1-induced smooth muscle proliferation using the LRP receptor (Stouffer et al. 1993). The interfaces that mediate TGF-β interaction with α2M are mapped to hydrophobic regions in both proteins, and may mimic the hydrophobic interface between TGF-β and TβRII, which may explain α2M’s ability to block TGF-β binding to TβRII (Liu et al. 2001; Arandjelovic et al. 2003). Heparin and heparan sulfate reverse the inhibition of TGF-β by α2M by promoting the dissociation of TGF-β from α2M, and does so more efficiently for TGF-β1 than TGF-β2 (McCaffrey et al. 1989; Lyon et al. 1997). α2M may function with other tissue-specific ECM components to control TGF-β ligand availability.
Collagens
Collagens have long been shown to bind TGF-β family ligands, including TGF-βs, BMPs, and activin (Paralkar et al. 1992), and are used as collagen sponges to protect and deliver BMPs during interventions aimed at bone and cartilage repair (Geiger et al. 2003). Type IV collagen directly controls BMP signaling in Drosophila (Wang et al. 2008; Bunt et al. 2010; Sawala et al. 2012). The carboxy-terminal region of collagen IV binds the BMP ligand Dpp, but not Scw or Gbb, and serves as a scaffold to facilitate formation of the Dpp shuttling complex with Sog/chordin and Tsg, thus augmenting long-range Dpp signaling by promoting Dpp gradient formation. Collagen IV may use a similar mechanism to enhance BMP signaling in Drosophila renal tubule morphogenesis (Bunt et al. 2010). However, collagen IV sequesters Dpp and restricts its signaling range in the Drosophila ovary (Wang et al. 2008), suggesting that the function of collagen IV in BMP signal regulation depends on cellular context.
Type II procollagen also modulates BMP signaling. Type IIA procollagen contains an amino-terminal 69-amino acid CR sequence, which is spliced out in the type IIB isoform that is expressed in mature chondrocytes. The CR domain is homologous to that in chordin and mediates type IIA procollagen binding to BMP-2 and -4 and TGF-β1. This domain does not bind activin or epidermal growth factor (EGF), and the type IIB form that lacks this domain does not interact with BMP or TGF-β (Zhu et al. 1999b; Larrain et al. 2000). Binding of procollagen IIA to BMPs can inhibit BMP function in Xenopus and induce a secondary axis (Larrain et al. 2000). Procollagen IIA may control storage and release of BMPs and TGF-βs and, thus, the availability of active TGF-β family ligands for cell differentiation and tissue homeostasis.
Matrilin-3 (MATN3)
Matrilins are a family of four ECM proteins that share structural features that consist of vWF A (vWFA) domains, EGF-like repeats, and a carboxy-terminal coiled-coil module. Matrilins form oligomers and can bind to other ECM components such as collagens and aggrecan (Deak et al. 1999). Both matrilin (MATN)-1 and -3 regulate cartilage homeostasis. MATN3, but not MATN1, inhibits BMP-dependent collagen X expression in hypertrophic chondrocytes via its EGF repeats and coiled-coil domain. MATN3 directly binds BMP-2 with a KD of 217 nm, suggesting that binding of BMP ligands may explain its role in blocking BMP signaling in chondrocytes (Yang et al. 2014). Mutations in MATN3 result in several skeletal diseases, such as multiple epiphyseal dysplasia and age-related osteoarthritis (Mostert et al. 2003; Stefansson et al. 2003).
Cartilage Oligomeric Matrix Protein (COMP)
COMP (also known as thrombospondin-5, or TSP-5) is a large pentameric extracellular glycoprotein that interacts with many ECM proteins, such as fibronectin, collagens, matrilins, and aggrecan, and plays an important role in ECM assembly (Acharya et al. 2014). Each COMP monomer consists of an amino-terminal coiled-coil domain, four EGF repeats, eight calcium-binding type III TSP repeats, and a carboxy-terminal globular domain. COMP binds TGF-β1, BMP-2, -4, and -7 using its carboxy-terminal domain. This binding enhances TGF-β1 and BMP-2 signaling in human bone mesenchymal stem cells (Haudenschild et al. 2011; Ishida et al. 2013), but inhibits BMP-2 signaling in vascular smooth muscle cells by preventing BMP-2 binding to its receptors (Du et al. 2011). COMP thus regulates TGF-β signaling in a cell-type-specific fashion. Mutations in human COMP are linked to the skeletal diseases pseudoachondroplasia (PSACH) and multiple epiphyseal dysplasia (MED) (Acharya et al. 2014).
Tenascin-X
Tenascin-X (TNX) is a member of tenascin family of ECM glycoproteins. Like other tenascin proteins, it has many EGF and fibronectin modules and a carboxy-terminal fibrinogen-like (FBG) domain. It binds several ECM components, such as collagen and decorin, and regulates the three-dimensional ECM organization. TNX binds the small latent TGF-β complex through its FBG domain and activates TGF-β ligands, most likely by inducing a conformational change in the complex. This activity requires the cell-surface integrin α11β1, and may enable TNX to promote epithelial-to-mesenchymal transition in mammary epithelial cells (Alcaraz et al. 2014).
Matrix GLA Protein (MGP)
MGP is a small (10 kDa) ECM-associated protein that binds BMP-2 and -4, and regulates osteogenesis, vascular endothelial cell proliferation and migration, and sympathetic neuron growth (Bostrom et al. 2001, 2004; Moon and Birren 2008; Yao et al. 2010). The interaction of MGP with BMP enhances or attenuates BMP signaling, which may relate to the opposite effects of the amino- and the carboxy-terminal domains of MGP on BMP signaling (Bostrom et al. 2001; Zebboudj et al. 2002). MGP also enhances TGF-β1 activity in cultured endothelial cells (Bostrom et al. 2004). Mice deficient in MGP show arteriovenous malformations in lungs and kidneys and vascular calcification (Yao et al. 2010, 2011).
Emilin1
Emilin1 was first isolated from chick aorta as the secreted glycoprotein gp115 (Bressan et al. 1983) and contains an amino-terminal CR domain, followed by coiled-coil, collagen, and C1q domains (Doliana et al. 1999). Emilin1 binds pro-TGF-β1, but not mature TGF-β1 or LAP, through its CR motif. In this way, it blocks pro-TGF-β1 processing by furin-like proprotein convertases in the extracellular space and inhibits TGF-β signaling. This represents a unique mechanism for an ECM protein to modulate TGF-β availability. Emilin1 also inhibits Xnr1 and TGF-β3 activities. Lack of emilin1 expression in mice leads to elastic fiber defects in aorta and skin and increased blood pressure as a result of elevated TGF-β signaling in the vascular wall (Zanetti et al. 2004; Zacchigna et al. 2006).
Fibulins (FBLNs)
The fibulin family of extracellular glycoproteins contains seven members with multiple calcium-binding EGF motifs and a carboxy-terminal fibulin-type module. They bind diverse ECM proteins and proteoglycans and regulate tissue structures during development and in disease processes (Timpl et al. 2003; de Vega et al. 2009). Fibulin-1 (Fbln1) binds BMP-2 and is required for BMP-2-mediated induction of Osterix expression during bone formation. Fbln1-deficient mice show defects in membranous and endochondral bone formation in the skull (Cooley et al. 2014). Fibulin-3 inhibits TGF-β signaling in breast cancer and endothelial cells to prevent cancer progression. Interestingly, fibulin-3 does not bind TGF-β1 appreciably, but instead interacts with the type I receptor TβRI/ALK-5 and blocks formation of a functional ligand–receptor complex (Tian et al. 2015). Other fibulins may also regulate TGF-β signaling (Renard et al. 2010; Radice et al. 2015), but the underlying molecular mechanism has not been elucidated.
Latent TGF-β-Binding Proteins (LTBPs) and Fibrillins (FBNs)
The LTBPs and FBNs are related ECM proteins with multiple calcium-binding EGF domains and characteristic eight-cysteine-containing TGF-β-binding (TB) modules (reviewed in Olivieri et al. 2010; Davis and Summers 2012; Robertson et al. 2015). LTBPs form a covalent bond with the TGF-β prodomain in the latent TGF-β complex and facilitate secretion and folding of TGF-β ligands, target latent TGF-β complexes to ECM, and control activation of TGF-βs. LTBPs bind the amino-terminal region of fibrillins and target the large latent TGF-β complex to ECM microfibrils to control TGF-β availability (Massam-Wu et al. 2010). Fbn-1, best known as encoded by the gene whose mutations lead to type I Marfan syndrome, and Fbn-2 both regulate TGF-β signaling through LTBP-mediated sequestration (Neptune et al. 2003; Chaudhry et al. 2007; Nistala et al. 2010). Fibrillins also interact through their amino-terminal regions with the propeptide of BMP-2, -4, -5, -7, -10, and GDF-5 and target these cytokines to the ECM. Fbn1–, but not Fbn2–, deficient mice show enhanced BMP signaling (Gregory et al. 2005; Sengle et al. 2008, 2011; Nistala et al. 2010). LTBPs and fibrillins thus serve both as structural components of ECM to maintain tissue architecture and to control the bioavailability of TGF-β family ligands.
SUMMARY AND PERSPECTIVES
Since the discovery of TGF-β ligands in latent forms and the realization that extracellular factors regulate the bioactivity of TGF-βs, an escalating number of secreted modulators of TGF-β family proteins has been identified, and the mechanisms underlying their functions are increasingly clear. One insight gained from the studies is that agonists and antagonists originate very early during evolution, probably around the time that TGF-β family ligands arose. Noggin, for example, is found in sponges, the most basal metazoans (phylum Porifera) (Muller et al. 2003); and, although lost in some clades (e.g., Ctenophore) (Pang et al. 2011), noggin is expressed in a wide range of metazoans, including Hydra (Cnidaria) (Chandramore et al. 2010), Planaria (Platyhelminthes) (Molina et al. 2009, 2011), sea urchin (Echinodermata) (Lapraz et al. 2006), and all vertebrates. Similarly, chordin is expressed in Hydra and sea anemone (Cnidaria) (Matus et al. 2006; Rentzsch et al. 2007), sea urchin (Echinodermata) (Lapraz et al. 2006), and ascidians (Urochordata) (Darras and Nishida 2001). The appearance of multiple agonists and antagonists in metazoans is accompanied by extensively overlapping functions in development. This is shown, for instance, by the absence of a severe phenotype in mice with inactivated expression of individual regulators (e.g., Dan, Cerberus), and the dramatic disruption of embryonic axes and tissue development only when multiple regulators are silenced (De Robertis and Kuroda 2004; Kuroda et al. 2004; Khokha et al. 2005; Vonica and Brivanlou 2006; Stafford et al. 2011). Conversely, proteins with similar biochemical properties and expression patterns can have distinct activities. This is evident by differential effects of noggin and chordin in mesodermal patterning and somite formation during early chick development (Streit and Stern 1999). It is also increasingly appreciated that endogenous agonists and antagonists interact extensively with other extracellular molecules. Tissue-specific cellular landscapes can influence the actions of soluble modulators of TGF-β family ligands, resulting in distinct signal outcomes. Thus, in contemplating how agonists and antagonists control TGF-β family signaling, one needs to determine not only which regulators are present in which isoforms at which places, but also their specificities and affinities toward ligands, and to also integrate information on the ECM proteins and cell-surface molecules in their environments. The following points should be considered in building a holistic picture (Figs. 1–3).
First, although most abundant agonists and antagonists are soluble or membrane-associated ligand-binding factors, some do not directly interact with ligands and nonetheless control TGF-β family signals by modifying ligand-binding proteins. Examples include BMP-1/Tolloid metalloproteinases, Sizzled, and the glypican-modifying enzyme Notum. Such non-dedicated regulators can both promote and inhibit TGF-β family signaling depending on the cellular environment.
Second, although most agonists and antagonists enhance or block TGF-β family signals by regulating ligand–receptor interactions, others control secretion, storage, maturation, stabilization, transport, and release of TGF-β family ligands in a spatiotemporal manner. The actions of CRIM1 and emilin1 illustrate this point nicely. These modulators help shape the patterns of ligand distribution in tissues and determine the range and the duration of TGF-β family signals.
Third, agonists and antagonists can interact with each other in competitive or cooperative modes, and collectively modulate the outcome of TGF-β family signaling in different contexts. The BMP antagonists noggin and sclerostin, for example, show reduced BMP-inhibitory activity when associated with each other (Winkler et al. 2004). In contrast, Twsg, biglycan, and Tsukushi all interact with chordin to enhance inhibition of BMPs. Association of soluble agonists and antagonists with ECM proteins and cell-surface proteoglycans can also change the properties of these modifiers and the tissue responses to TGF-β family signals. An example is the influence of HSPGs on retention, diffusion and activities of the BMP regulators noggin, chordin, and Crossveinless-2.
Fourth, the functions of agonists and antagonists are not absolute. Depending on cellular localization (soluble or membrane-associated), and presence or absence of interacting factors in the tissue, an agonist may be converted into an antagonist, and vice versa. Proteins that act as both agonists and antagonists in different cellular contexts include decorin, biglycan, glypican, betaglycan, Crossveinless-2, Twsg, chordin/Sog, and COMP. A regulator can also act as agonist of one ligand and antagonist of another; CTGF is one example. The detailed mechanisms responsible for functional switch of these molecules may vary, and all of them are not equally understood. These examples highlight the importance of integrating all tissue-specific extracellular components to fully understand the actions of agonists and antagonists.
Fifth, agonists and antagonists often act in feedback control through transcriptional regulation by TGF-β family proteins. TGF-β modulates the expression of its regulators decorin and α2-macroglobulin, whereas BMP-2 induces the expression of its antagonists noggin and Gremlin in osteoblasts (Shi et al. 1990; Heimer et al. 1995; Gazzerro et al. 1998; Pereira et al. 2000). Noggin expression is also induced by BMP-4 in chick somites, and GDF-11 induces follistatin expression in chick limb bud (Amthor et al. 1999; Gamer et al. 2001). Stimulation of TGF-β antagonists by ligands often serves to restrict signaling range, strength, and duration. A consequence of such negative feedback regulation is the generation of stable patterns during development (Turing 1952; Meinhardt and Gierer 2000). Nodal-lefty feedback regulation establishes a nodal activity gradient in zebrafish (Chen and Schier 2001, 2002), whereas BMP-Sizzled and noggin/chordin-ADMP feedback loops regulate dorsoventral patterning in Xenopus (Collavin and Kirschner 2003; Reversade and De Robertis 2005; De Robertis 2009; Inomata et al. 2013).
Sixth, agonists and antagonists of TGF-β family proteins can be regulated by other signaling pathways and often cross-modulate other signals as well. This is illustrated with the regulation of Wnt signal transduction by CTGF, sclerostin, and ectodin/Wise/USAG-1. Cerberus coregulates nodal, BMP, and Wnt signals by associating with these ligands. Tsukushi modulates the Delta-Notch pathway in addition to BMPs, and IGFBP3 controls IGF1 signaling. Cross-regulation among signals is a general rule for cells to integrate different stimuli, and agonists and antagonists of TGF-β ligands represent a node for integrating the ever-expanding extracellular signaling networks.
In summary, although our knowledge on individual agonists and antagonists of TGF-β family ligands has increased dramatically, further studies need to reveal dynamic interactions of these molecules with tissue-specific extracellular components. It is reasonable to expect that we will continue to uncover new agonists and antagonists of TGF-β family ligands, novel activities of these regulators in development and diseases, and unsuspected links of these proteins with other signaling pathways. Additional directions will likely include detailed characterization of biochemical interactions of these factors with relevant ligands, other modulators, ECM components, and secreted protein modification enzymes. The research will not only investigate the strength, kinetics, and feedback regulation of physical associations among these molecules, but also analyze the roles of these interactions in protein localization, stability, processing, bioavailability, and local and long-range signaling. These studies will lead to more in-depth understanding of the mechanisms used by agonists and antagonists of TGF-β family ligands to control TGF-β signaling in various tissue contexts in normal and diseased conditions.
ACKNOWLEDGMENTS
This work is supported by National Institutes of Health (NIH) Grant R01 GM098566. I apologize to the authors who have made important contributions in these areas, but whose work was not covered because of the limited space and scope. I thank Drs. Rik Derynck and Kohei Miyazono for their help with the revision of the manuscript.
Footnotes
Editors: Rik Derynck and Kohei Miyazono
Additional Perspectives on The Biology of the TGF-β Family available at www.cshperspectives.org
REFERENCES
- Abreu JG, Ketpura NI, Reversade B, De Robertis EM. 2002. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-β. Nat Cell Biol 4: 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya C, Yik JHN, Kishore A, Van Dinh V, Di Cesare PE, Haudenschild DR. 2014. Cartilage oligomeric matrix protein and its binding partners in the cartilage extracellular matrix: Interaction, regulation and role in chondrogenesis. Matrix Biol 37: 102–111. [DOI] [PubMed] [Google Scholar]
- Agathon A, Thisse B, Thisse C. 2001. Morpholino knock-down of antivin1 and antivin 2 upregulates nodal signaling. Genesis 30: 178–182. [DOI] [PubMed] [Google Scholar]
- Ahn Y, Sanderson BW, Klein OD, Krumlauf R. 2010. Inhibition of Wnt signaling by Wise (Sostdc1) and negative feedback from Shh controls tooth number and patterning. Development 137: 3221–3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn Y, Sims C, Logue JM, Weatherbee SD, Krumlauf R. 2013. Lrp4 and Wise interplay controls the formation and patterning of mammary and other skin appendage placodes by modulating Wnt signaling. Development 140: 583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Kamimura K, Firkus C, Takeo S, Shimmi O, Nakato H. 2008. Dally regulates Dpp morphogen gradient formation by stabilizing Dpp on the cell surface. Dev Biol 313: 408–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alborzinia H, Schmidt-Glenewinkel H, Ilkavets I, Breitkopf-Heinlein K, Cheng X, Hortschansky P, Dooley S, Wolfl S. 2012. Quantitative kinetics analysis of BMP2 uptake into cells and its modulation by BMP antagonists. J Cell Sci 126: 117–127. [DOI] [PubMed] [Google Scholar]
- Alcaraz LB, Exposito JY, Chuvin N, Pommier RM, Cluzel C, Martel S, Sentis S, Bartholin L, Lethias C, Valcourt U. 2014. Tenascin-X promotes epithelial-to-mesenchymal transition by activating latent TGF-β. J Cell Biol 205: 409–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JM, McGlinn E, Hill A, Warman ML. 2013. Autopodial development is selectively impaired by misexpression of chordin-like 1 in the chick limb. Dev Biol 381: 159–169. [DOI] [PubMed] [Google Scholar]
- Ambrosio AL, Taelman VF, Lee HX, Metzinger CA, Coffinier C, De Robertis EM. 2008. Crossveinless-2 is a BMP feedback inhibitor that binds Chordin/BMP to regulate Xenopus embryonic patterning. Dev Cell 15: 248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amthor H, Christ B, Patel K. 1999. A molecular mechanism enabling continuous embryonic growth: A balance between proliferation and differentiation. Development 126: 1041–1053. [DOI] [PubMed] [Google Scholar]
- Amthor H, Christ B, Rashid-Doubell F, Kemp CF, Lang E, Patel K. 2002. Follistatin regulates bone morphogenetic protein-7 (BMP-7) activity to stimulate embryonic muscle growth. Dev Biol 243: 115–127. [DOI] [PubMed] [Google Scholar]
- Amthor H, Nicholas G, McKinnell I, Kemp CF, Sharma M, Kambadur R, Patel K. 2004. Follistatin complexes Myostatin and antagonizes Myostatin-mediated inhibition of myogenesis. Dev Biol 270: 19–30. [DOI] [PubMed] [Google Scholar]
- Anderson RM, Lawrence AR, Stottmann RW, Bachiller D, Klingensmith J. 2002. Chordin and noggin promote organizing centers of forebrain development in the mouse. Development 129: 4975–4987. [DOI] [PubMed] [Google Scholar]
- Andersson O, Bertolino P, Ibanez CF. 2007. Distinct and cooperative roles of mammalian Vg1 homologs GDF1 and GDF3 during early embryonic development. Dev Biol 311: 500–511. [DOI] [PubMed] [Google Scholar]
- Andersson O, Korach-Andre M, Reissmann E, Ibanez CF, Bertolino P. 2008. Growth/differentiation factor 3 signals through ALK7 and regulates accumulation of adipose tissue and diet-induced obesity. Proc Natl Acad Sci 105: 7252–7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aono A, Hazama M, Notoya K, Taketomi S, Yamasaki H, Tsukuda R, Sasaki S, Fujisawa Y. 1995. Potent ectopic bone-inducing activity of bone morphogenetic protein-4/7 heterodimer. Biochem Biophys Res Commun 210: 670–677. [DOI] [PubMed] [Google Scholar]
- Arandjelovic S, Freed TA, Gonias SL. 2003. Growth factor-binding sequence in human α2-macroglobulin targets the receptor-binding site in transforming growth factor-β. Biochemistry 42: 6121–6127. [DOI] [PubMed] [Google Scholar]
- Araujo H, Negreiros E, Bier E. 2003. Integrins modulate Sog activity in the Drosophila wing. Development 130: 3851–3864. [DOI] [PubMed] [Google Scholar]
- Araujo AC, Marques S, Belo JA. 2014. Targeted inactivation of Cerberus like-2 leads to left ventricular cardiac hyperplasia and systolic dysfunction in the mouse. PLoS ONE 9: e102716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashe HL, Levine M. 1999. Local inhibition and long-range enhancement of Dpp signal transduction by Sog. Nature 398: 427–431. [DOI] [PubMed] [Google Scholar]
- Aspenberg P, Jeppsson C, Economides AN. 2001. The bone morphogenetic proteins antagonist noggin inhibits membranous ossification. J Bone Miner Res 16: 497–500. [DOI] [PubMed] [Google Scholar]
- Avsian-Kretchmer O, Hsueh AJW. 2004. Comparative genomic analysis of the eight-membered ring cystine knot-containing bone morphogenetic protein antagonists. Mol Endocrin 18: 1–12. [DOI] [PubMed] [Google Scholar]
- Aykul S, Ni W, Mutatu W, Martinez-Hackert E. 2015. Human Cerberus prevents nodal-receptor binding, inhibits nodal signaling, and suppresses nodal-mediated phenotypes. PLoS ONE 10: e0114954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babic AM, Chen CC, Lau LF. 1999. Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin αvβ3, promotes endothelial cell survival, and induces angiogenesis in vivo. Mol Cell Biol 19: 2958–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachiller D, Klingensmith J, Kemp C, Belo JA, Anderson RM, May SR, McMahon JA, McMahon AP, Harland RM, Rossant J, et al. 2000. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature 403: 658–661. [DOI] [PubMed] [Google Scholar]
- Bachiller D, Klingensmith J, Shneyder N, Tran U, Anderson R, Rossant J, De Robertis EM. 2003. The role of chordin/Bmp signals in mammalian pharyngeal development and DiGeorge syndrome. Development 130: 3567–3578. [DOI] [PubMed] [Google Scholar]
- Balemans W, Van Hul W. 2002. Extracellular regulation of BMP signaling in vertebrates: A cocktail of modulators. Dev Biol 250: 231–250. [PubMed] [Google Scholar]
- Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, Lacza C, Wuyts W, Van Den Ende J, Willems P, et al. 2001. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet 10: 537–543. [DOI] [PubMed] [Google Scholar]
- Baxter RC. 2014. IGF binding proteins in cancer: Mechanistic and clinical insights. Nat Rev Cancer 14: 329–341. [DOI] [PubMed] [Google Scholar]
- Bayramov AV, Eroshkin FM, Martynova NY, Ermakova GV, Solovieva EA, Zaraisky AG. 2011. Novel functions of Noggin proteins: Inhibition of Activin/Nodal and Wnt signaling. Development 138: 5345–5356. [DOI] [PubMed] [Google Scholar]
- Beck HN, Drahushuk K, Jacoby DB, Higgins D, Lein PJ. 2001. Bone morphogenetic protein-5 (BMP-5) promotes dendritic growth in cultured sympathetic neurons. BMC Neurosci 2: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S, Le Good JA, Guzman M, Haim NB, Roy K, Beermann F, Constam DB. 2002. Extraembryonic proteases regulate nodal signaling during gastrulation. Nat Cell Biol 25: 981–985. [DOI] [PubMed] [Google Scholar]
- Belenkaya T, Han C, Yan D, Opoka RJ, Khodoun M, Liu H, Lin X. 2004. Drosophila Dpp morphogen movement is independent of dynamin-mediated endocytosis but regulated by the glypican members of heparan sulfate proteoglycans. Cell 119: 231–244. [DOI] [PubMed] [Google Scholar]
- Bell E, Munoz-Sanjuan I, Altmann CR, Vonica A, Brivanlou AH. 2003. Cell fate specification and competence by Coco, a maternal BMP, TGFβ and Wnt inhibitor. Development 130: 1381–1389. [DOI] [PubMed] [Google Scholar]
- Belo JA, Bouwmeester T, Leyns L, Kertesz N, Gallo M, Follettie M, De Robertis EM. 1997. Cerberus-like is a secreted factor with neutralizing activity expressed in the anterior primitive endoderm of the mouse gastrula. Mech Dev 68: 45–57. [DOI] [PubMed] [Google Scholar]
- Belo JA, Bachiller D, Agius E, Kemp C, Borges AC, Marques S, Piccolo S, De Robertis EM. 2000. Cerberus-like is a secreted BMP and nodal antagonist not essential for mouse development. Genesis 26: 265–270. [PubMed] [Google Scholar]
- Ben-Neriah Z, Michaelson-Cohen R, Inbar-Feigenberg M, Nadjari M, Zeligson S, Shaag A, Zenvirt S, Elpeleg O, Levy-Lahad E. 2011. A deleterious founder mutation in the BMPER gene causes diaphanospondylodysostosis (DSD). Am J Med Genet A 155A: 2801–2806. [DOI] [PubMed] [Google Scholar]
- Berger W, Meindl A, van de Pol TJR, Cremers FPM, Ropers HH, Doerner C, Monaco A, Bergen AAB, Lebo R, Warburg M, et al. 1992. Isolation of a candidate gene for Norrie disease by positional cloning. Nat Genet 1: 199–203. [DOI] [PubMed] [Google Scholar]
- Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. 1999. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem 68: 729–777. [DOI] [PubMed] [Google Scholar]
- Berry R, Jowitt TA, Garrigue-Antar L, Kadler KE, Baldock C. 2010. Structural and functional evidence for a substrate exclusion mechanism in mammalian tolloid like-1 (TLL-1) proteinase. FEBS Lett 584: 657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertocchini F, Stern CD. 2002. The hypoblast of the chick embryo positions the primitive streak by antagonizing nodal signaling. Dev Cell 3: 735–744. [DOI] [PubMed] [Google Scholar]
- Bi X, Xia X, Fan D, Mu T, Zhang Q, Iozzo RD, Yang W. 2015. Oncogenic activin C interacts with decorin in colorectal cancer in vivo and in vitro. Mol Carcinog 10.1002/mc.22427. [DOI] [PubMed] [Google Scholar]
- Biben C, Stanley E, Fabri L, Kotecha S, Rhinn M, Drinkwater C, Lah M, Wang CC, Nash A, Hilton D, 1998, et al. Murine cerberus homologue mCer-1: A candidate anterior patterning molecule. Dev Biol 194: 135–151. [DOI] [PubMed] [Google Scholar]
- Biehs B, Francois V, Bier E. 1996. The Drosophila short gastrulation gene prevents Dpp from autoactivating and suppressing neurogenesis in the neuroectoderm. Genes Dev 10: 2922–2934. [DOI] [PubMed] [Google Scholar]
- Bijakowski C, Vadon-Le Goff S, Delolme F, Bourhis JM, Lecorche P, Ruggiero F, Becker-Pauly C, Yiallouros I, Stocker W, Dive C, et al. 2012. Sizzled is unique among secreted frizzled-related proteins for its ability to specifically inhibit bone morphogenetic protein-1 (BMP-1)/tolloid-like proteinases. J Biol Chem 287: 33581–33593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilezikjian LM, Justice NJ, Blackler AN, Wiater E, Vale WW. 2012. Cell-type specific modulation of pituitary cells by activin, inhibin and follistatin. Mol Cell Endocrinol 359: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binnerts ME, Wen X, Cante-Barrett K, Bright J, Chen HT, Asundi V, Sattari P, Tang T, Boyle B, Funk W, et al. 2004. Human Crossveinless-2 is a novel inhibitor of bone morphogenetic proteins. Biochem Biophys Res Commun 315: 272–280. [DOI] [PubMed] [Google Scholar]
- Birsoy B, Berg L, Williams PH, Smith JC, Wylie CC, Christian JL, Heasman J. 2005. XPACE4 is a localized pro-protein convertase required for mesoderm induction and the cleavage of specific TGFβ proteins in Xenopus development. Development 132: 591–602. [DOI] [PubMed] [Google Scholar]
- Bisgrove BW, Essner JJ, Yost HJ. 1999. Regulation of midline development by antagonism of lefty and nodal signaling. Development 126: 3253–3262. [DOI] [PubMed] [Google Scholar]
- Blader P, Rastegar S, Fischer N, Strahle U. 1997. Cleavage of the BMP-4 antagonist chordin by zebrafish tolloid. Science 278: 1937–1940. [DOI] [PubMed] [Google Scholar]
- Blish KR, Wang W, Willingham MC, Du W, Birse CE, Krishnan SR, Brown JC, Hawkins GA, Garvin AJ, D’Agostino RB Jr, et al. 2008. A human bone morphogenetic protein antagonist is down-regulated in renal cancer. Mol Biol Cell 19: 457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz IL, Shimmi O, Wunnenberg-Stapleton K, O’Connor MB, Cho KWY. 2000. Is chordin a long-range- or short-range-acting factor? Roles for BMP1-related metalloproteases in chordin and BMP4 autofeedback loop regulation. Dev Biol 223: 120–138. [DOI] [PubMed] [Google Scholar]
- Blitz IL, Cho KWY, Chang C. 2003. Twisted gastrulation loss-of-function analyses support its role as a BMP inhibitor during early Xenopus embryogenesis. Development 130: 4975–4988. [DOI] [PubMed] [Google Scholar]
- Bonds M, Sands J, Poulson W, Harvey C, Von Ohlen T. 2007. Genetic screen for regulators of ind expression identifies shrew as encoding a novel twisted gastrulation-like protein involved in Dpp signaling. Dev Dyn 236: 3524–3531. [DOI] [PubMed] [Google Scholar]
- Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi Y, Pierschbacher MD, Ruoslahti E. 1992. Natural inhibitor of transforming growth factor-β protects against scarring in experimental kidney disease. Nature 360: 361–364. [DOI] [PubMed] [Google Scholar]
- Bornemann DJ, Duncan JE, Staatz W, Selleck S, Warrior R. 2004. Abrogation of heparan sulfate synthesis in Drosophila disrupts the Wingless, Hedgehog and Decapentaplegic signaling pathways. Development 131: 1927–1938. [DOI] [PubMed] [Google Scholar]
- Bostrom K, Tsao D, Shen S, Wang Y, Demer LL. 2001. Matrix GLA protein modulates differentiation induced by bone morphogenetic protein-2 in C3H10T1/2 cells. J Biol Chem 276: 14044–14052. [DOI] [PubMed] [Google Scholar]
- Bostrom K, Zebboudj AF, Yao Y, Lin TS, Torres A. 2004. Matrix GLA protein stimulates VGEF expression through increased transforming growth factor-β activity in endothelial cells. J Biol Chem 279: 52904–52913. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Botchkareva NV, Roth W, Nakamura M, Chen LH, Herzog W, Lindner G, McMahon JA, Peters C, Lauster R, et al. 1999. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat Cell Biol 1: 158–164. [DOI] [PubMed] [Google Scholar]
- Bouwmeester T, Kim SH, Sasai Y, Lu B, De Robertis EM. 1996. Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann’s organizer. Nature 382: 595–601. [DOI] [PubMed] [Google Scholar]
- Bradley L, Sun B, Collins-Racie L, LaVallie E, McCoy J, Sive H. 2000. Different activities of the frizzled-related proteins frzb2 and sizzled2 during Xenopus anteroposterior patterning. Dev Biol 227: 118–132. [DOI] [PubMed] [Google Scholar]
- Branam AM, Hoffman GG, Pelegri F, Greenspan DS. 2010. Zebrafish chordin-like and chordin are functionally redundant in regulating patterning of the dorsoventral axis. Dev Biol 341: 444–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branford WW, Yost HJ. 2002. Lefty-dependent inhibition of nodal- and Wnt-responsive organizer gene expression is essential for normal gastrulation. Curr Biol 12: 2136–2141. [DOI] [PubMed] [Google Scholar]
- Branford WW, Essner JJ, Yost HJ. 2000. Regulation of gut and heart left–right asymmetry by context-dependent interactions between Xenopus lefty and BMP4 signaling. Dev Biol 223: 291–306. [DOI] [PubMed] [Google Scholar]
- Bressan GM, Castellani I, Colombatti A, Volpin D. 1983. Isolation and characterization of a 115,000-dalton matrix-associated glycoprotein from chick aorta. J Biol Chem 258: 13262–13267. [PubMed] [Google Scholar]
- Brigstock DR. 2003. The CCN family: A new stimulus package. J Endocrin 178: 169–175. [DOI] [PubMed] [Google Scholar]
- Brunet LJ, McMahon JA, McMahon AP, Harland RM. 1998. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science 280: 1455–1457. [DOI] [PubMed] [Google Scholar]
- Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR, Proll S, Skonier JE, Zhao L, Sabo PJ, Fu Y, et al. 2001. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet 68: 577–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunt S, Hooley C, Hu N, Scahill C, Weavers H, Skare H. 2010. Hemocyte-secreted type IC collagen enhances BMP signaling to guide renal tubule morphogenesis in Drosophila. Dev Cell 19: 296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SJ, Dodd J. 2003. A role for BMP heterodimers in roof plate-mediated repulsion of commissural axons. Neuron 38: 389–401. [DOI] [PubMed] [Google Scholar]
- Cahill E, Costello CM, Rowan SC, Harkin S, Howell K, Leonard MO, Southwood M, Cummins EP, Fitzpatrick SF, Taylor CT, et al. 2012. Gremlin plays a key role in the pathogenesis of pulmonary hypertension. Circulation 125: 920–930. [DOI] [PubMed] [Google Scholar]
- Canalis E, Parker K, Zanotti S. 2012. Gremlin1 is required for skeletal development and postnatal skeletal homeostasis. J Cell Physiol 227: 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdevila J, Tsukui T, Rodriquez Esteban C, Zappavigna V, Izpisua Belmonte JC. 1999. Control of vertebrate limb outgrowth by the proximal factor Meis2 and distal antagonism of BMPs by Gremlin. Mol Cell 4: 839–849. [DOI] [PubMed] [Google Scholar]
- Cash JN, Angerman EB, Kattamuri C, Nolan K, Zhao H, Sidis Y, Keutmann HT, Thompson TB. 2012. Structure of Myostatin • Follistain-like 3: N-terminal domains of follistatin-type molecules exhibit alternative modes of binding. J Biol Chem 287: 1043–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha YR, Takahashi S, Wright CV. 2006. Cooperative non-cell and cell autonomous regulation of Nodal gene expression and signaling by Lefty/Antivin and Brachyury in Xenopus. Dev Biol 290: 246–264. [DOI] [PubMed] [Google Scholar]
- Chandramore K, Ito Y, Takahashi S, Asashima M, Ghaskadbi S. 2010. Cloning of noggin gene from hydra and analysis of its functional conservation using Xenopus laevis embryos. Evol Dev 12: 267–274. [DOI] [PubMed] [Google Scholar]
- Chang C, Hemmati-Brivanlou. 1999. Xenopus GDF6, a new antagonist of noggin and a partner of BMPs. Development 126: 3347–3357. [DOI] [PubMed] [Google Scholar]
- Chang C, Holtzman DA, Chau S, Chickering T, Woolf EA, Holmgren LM, Bodorova J, Gearing DP, Holmes WE, Brivanlou AH. 2001. Twisted gastrulation can function as a BMP antagonist. Nature 410: 483–487. [DOI] [PubMed] [Google Scholar]
- Chang C, Eggen BJL, Weinstein DC, Brivanlou AH. 2003. Regulation of nodal and BMP signaling by tomoregulin-1 (X7365) through novel mechanisms. Dev Biol 255: 1–11. [DOI] [PubMed] [Google Scholar]
- Chapman SC, Schubert FR, Schoenwolf GC, Lumsden A. 2002. Analysis of spatial and temporal gene expression patterns in blastula and gastrula stage chick embryos. Dev Biol 245: 187–199. [DOI] [PubMed] [Google Scholar]
- Chaudhry SS, Cain SA, Morgan A, Dallas SL, Shuttleworth CA, Kielty CM. 2007. Fibrillin-1 regulates the bioavailability of TGFβ1. J Cell Biol 176: 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Schier AF. 2001. The zebrafish Nodal signal Squint functions as a morphogen. Nature 411: 607–610. [DOI] [PubMed] [Google Scholar]
- Chen Y, Schier AF. 2002. Lefty proteins are long-range inhibitors of Squint-mediated Nodal signaling. Curr Biol 12: 2124–2128. [DOI] [PubMed] [Google Scholar]
- Chen C, Shen MM. 2004. Two modes by which Lefty proteins inhibit nodal signaling. Curr Biol 14: 618–624. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Battinelli EM, Fielder A, Bundey S, Sims K, Breakefield XO, Craig IW. 1993. A mutation in the Norrie disease gene (NDP) associated with X-linked familial exudative vitreoretinopathy. Nat Genet 5: 180–183. [DOI] [PubMed] [Google Scholar]
- Chen L, Klass C, Woods A. 2004a. Syndecan-2 regulates transforming growth factor-β signaling. J Biol Chem 279: 15715–15718. [DOI] [PubMed] [Google Scholar]
- Chen XD, Fisher LW, Robey PG, Young MF. 2004b. The small leucine-rich proteoglycan biglycan modulates BMP-4-induced osteoblast differentiation. FASEB J 18: 948–958. [DOI] [PubMed] [Google Scholar]
- Chen Y, Shi-Wen X, van Beek J, Kennedy L, McLeod M, Renzoni EA, Bou-Gharios G, Wilcox-Adelman S, Goetinck PF, Eastwood M, et al. 2005. Matrix contraction by dermal fibroblasts requires transforming growth factor-β/activin-linked kinase 5, heparan sulfate-containing proteoglycans, and MEK/ERK: Insights into pathological scarring in chronic fibrotic disease. Am J Pathol 167: 1699–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Ware SM, Sato A, Houston-Hawkins DE, Habas R, Matzuk MM, Shen MM, Brown CW. 2006. The Vg1-related protein Gdf3 acts in a Nodal signaling pathway in the pre-gastrulation mouse embryo. Development 133: 319–329. [DOI] [PubMed] [Google Scholar]
- Cheng AM, Thisse B, Thisse C, Wright CV. 2000. The lefty-related factor Xatv acts as a feedback inhibitor of nodal signaling in mesoderm induction and L-R axis development in Xenopus. Development 127: 1049–1061. [DOI] [PubMed] [Google Scholar]
- Cheng SK, Olale F, Brivanlou AH, Schier AF. 2004. Lefty blocks a subset of TGFβ signals by antagonizing EGF–CFC coreceptors. PLoS Biol 2: 215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JS, Shiue LH, Duvic M, Pandya A, Cruz PD Jr, Ariizumi K. 2011. Sezary syndrome cells overexpress syndecan-4 bearing distinct heparan sulfate moieties that suppress T-cell activation by binding DC-HIL and trapping TGF-β on the cell surface. Blood 117: 3382–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church RH, Krishnakumar A, Urbanek A, Geschwindner S, Meneely J, Bianchi A, Basta B, Monaghan S, Elliot C, Stromstedt M, et al. 2015. Gremlins preferentially binds to bone morphogenetic protein-2 (BMP-2) and BMP-4 over BMP-7. Biochem J 466: 55–68. [DOI] [PubMed] [Google Scholar]
- Clausen KA, Blish KR, Birse CE, Triplette MA, Kute TE, Russell GB, D’Agostino RB Jr, Miller LD, Torti FM, Torti SV. 2011. SOSTDC1 differentially modulates Smad and β-catenin activation and is down-regulated in breast cancer. Breast Cancer Res Treat 129: 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffinier C, Tran U, Larrain J, De Robertis EM. 2001. Neuralin-1 is a novel Chordin-related molecule expressed in the mouse neural plate. Mech Dev 100: 119–122. [DOI] [PubMed] [Google Scholar]
- Coffinier C, Ketpura N, Tran U, Geissert D, De Robertis EM. 2002. Mouse Crossveinless-2 is the vertebrate homolog of a Drosophila extracellular regulator of BMP signaling. Mech Dev 119S: S179–S184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles E, Christiansen J, Economou A, Bronner-Fraser M, Wilkinson DG. 2004. A vertebrate crossveinless 2 homologue modulates BMP activity and neural crest cell migration. Development 131: 5309–5317. [DOI] [PubMed] [Google Scholar]
- Collavin L, Kirschner MW. 2003. The secreted Frizzled-related protein Sizzled functions as a negative feedback regulator of extreme ventral mesoderm. Development 130: 805–816. [DOI] [PubMed] [Google Scholar]
- Conley CA, Silburn R, Singer MA, Ralston A, Rohwer-Nutter D, Olson DJ, Gelbart W, Blair SS. 2000. Crossveinless 2 contains cysteine-rich domains and is required for high levels of BMP-like activity during the formation of the cross veins in Drosophila. Development 127: 3947–3959. [DOI] [PubMed] [Google Scholar]
- Connors SA, Trout J, Ekker M, Mullins MC. 1999. The role of tolloid/mini fin in dorsoventral pattern formation of the zebrafish embryo. Development 126: 3119–3130. [DOI] [PubMed] [Google Scholar]
- Connors SA, Tucker JA, Mullins MC. 2006. Temporal and spatial action of tolloid (mini fin) and chordin to pattern tail tissues. Dev Biol 293: 191–202. [DOI] [PubMed] [Google Scholar]
- Constam DB. 2014. Regulation of TGFβ and related signals by precursor processing. Semin Cell Dev Biol 32: 85–97. [DOI] [PubMed] [Google Scholar]
- Constam DB, Robertson EJ. 1999. Regulation of bone morphogenetic protein activity by pro domain and proprotein convertases. J Cell Biol 144: 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley MA, Harikrishnan K, Oppel JA, Miler SF, Barth JL, Haycraft CJ, Reddy SV, Argraves WS. 2014. Fibulin-1 is required for bone formation and Bmp-2-medidated induction of Osterix. Bone 69: 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Hackenmiller R, Berg L, Jean F, Nakayama T, Thomas G, Christian JL. 2001. The activity and signaling range of mature BMP-4 is regulated by sequential cleavage at two sites within the prodomain of the precursor. Genes Dev 15: 2797–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daluiski A, Engstrand T, Bahamonde ME, Gamer LW, Agius E, Stevenson SL, Cox K, Rosen V, Lyons KM. 2001. Bone morphogenetic protein-3 is a negative regulator of bone density. Nat Genet 27: 84–88. [DOI] [PubMed] [Google Scholar]
- Danielpour D, Sporn MB. 1990. Differential inhibition of transforming growth factor β1 and β2 activity by α2-macroglobulin. J Biol Chem 265: 6973–6977. [PubMed] [Google Scholar]
- Darras S, Nishida H. 2001. The BMP/CHORDIN antagonism controls sensory pigment cell specification and differentiation in the ascidian embryo. Dev Biol 236: 271–288. [DOI] [PubMed] [Google Scholar]
- Davis MR, Summers KM. 2012. Structure and function of the mammalian fibrillin gene family: Implications for human connective tissue diseases. Mol Genet Metab 107: 635–647. [DOI] [PubMed] [Google Scholar]
- Deak F, Wagener R, Kiss I, Paulsson M. 1999. The matrilins: A novel family of oligomeric extracellular matrix proteins. Matrix Biol 18: 55–64. [DOI] [PubMed] [Google Scholar]
- Decotto E, Ferguson EL. 2001. A positive role for Short gastrulation in modulating BMP signaling during dorsoventral patterning in the Drosophila embryo. Development 128: 3831–3841. [DOI] [PubMed] [Google Scholar]
- Degnin C, Jean F, Thomas G, Christian JL. 2004. Cleavage within the prodomain direct intracellular trafficking and degradation of mature bone morphogenetic protein-4. Mol Biol Cell 15: 5012–5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejima K, Kanai MI, Akiyama T, Levings DC, Nakato H. 2011. Novel contact-dependent bone morphogenetic protein (BMP) signaling mediated by heparan sulfate proteoglycans. J Biol Chem 286: 17103–17111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C, Reddy P, Cheng Y, Luo CW, Hsiao CL, Hsueh AJW. 2013. Multi-functional norrie is a ligand for the LGR4 receptor. J Cell Sci 126: 2060–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EM. 2009. Spemann’s organizer and the self-regulation of embryonic fields. Mech Dev 126: 925–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EM, Kuroda H. 2004. Dorsal–ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol 20: 285–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vega D, Iwamoto T, Yamada Y. 2009. Fibulins: Multiple roles in matrix structures and tissue functions. Cell Mol Life Sci 66: 1890–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Winter JP, ten Dijke P, de Vries CJM, van Achterberg TAE, Sugino H, de Waele P, Huylebroeck D, Verschueren K, van den Eijnden-van Raaij AJM. 1996. Follistatins neutralize activin bioactivity by inhibition of activin binding to its type II receptors. Mol Cell Endocrinol 116: 105–114. [DOI] [PubMed] [Google Scholar]
- Dick A, Hild M, Bauer H, Imai Y, Maifeld H, Schier AF, Talbot WS, Bouwmeester T, Hammerschmidt M. 2000. Essential role of Bmp7 (snailhouse) and its prodomain in dorsoventral patterning of the zebrafish embryo. Development 127: 343–354. [DOI] [PubMed] [Google Scholar]
- Dionne MS, Skarnes WC, Harland RM. 2001. Mutation and analysis of Dan, the founding member of the Dan family of transforming growth factor β antagonists. Mol Cell Biol 21: 636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne MS, Brunet LJ, Eimon PM, Harland RM. 2002. Noggin is required for correct guidance of dorsal root ganglion axons. Dev Biol 251: 283–293. [DOI] [PubMed] [Google Scholar]
- Doliana R, Mongiat M, Bucciotti F, Giacomello E, Deutzmann R, Volpin D, Bressan GM, Colombatti A. 1999. EMILIN, a component of the elastic fiber and a new member of the C1q/tumor necrosis factor superfamily of proteins. J Biol Chem 274: 16773–16781. [DOI] [PubMed] [Google Scholar]
- Dosch R, Niehrs C. 2000. Requirement for anti-dorsalizing morphogenetic protein organizer patterning. Mech Dev 90: 195–203. [DOI] [PubMed] [Google Scholar]
- Droguett R, Cabello-Verrugio C, Requelme C, Brandan E. 2006. Extracellular proteoglycans modify TGF-β bio-availability attenuating its signaling during skeletal muscle differentiation. Matrix Biol 25: 332–341. [DOI] [PubMed] [Google Scholar]
- Du Y, Wang Y, Wang L, Liu B, Tian Q, Liu CJ, Zhang T, Xu Q, Zhu Y, Ake O, et al. 2011. Cartilage oligomeric matrix protein inhibits vascular smooth muscle calcification by interacting with bone morphogenetic protein-2. Circ Res 108: 917–928. [DOI] [PubMed] [Google Scholar]
- Dwivedi PP, Grose RH, Filmus J, Hii CS, Xian CJ, Anderson PJ, Powell BC. 2013. Regulation of bone morphogenetic protein signalling and cranial osteogenesis by Gpc1 and Gpc3. Bone 55: 367–376. [DOI] [PubMed] [Google Scholar]
- Dyer L, Wu Y, Moser M, Patterson C. 2014. BMPER-induced BMP signaling promotes coronary artery remodeling. Dev Biol 386: 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eib DW, Martens GJM. 1996. A novel transmembrane protein with epidermal growth factor and follistatin domains expressed in the hypothalamo-hypophysial axis of Xenopus laevis. J Neurochem 67: 1047–1055. [DOI] [PubMed] [Google Scholar]
- Eimon PM, Harland RM. 2001. Xenopus Dan, a member of the Dan gene family of BMP antagonists, is expressed in derivatives of the cranial and trunk neural crest. Mech Dev 107: 187–189. [DOI] [PubMed] [Google Scholar]
- Eimon PM, Harland RM. 2002. Effects of heterodimerization and proteolytic processing on Derriere and nodal activity: Implications for mesoderm induction in Xenopus. Development 129: 3089–3103. [DOI] [PubMed] [Google Scholar]
- Eldar A, Dorfman R, Weiss D, Ashe H, Shilo BZ, Barkai N. 2002. Robustness of the BMP morphogen gradient in Drosophila embryonic patterning. Nature 419: 304–308. [DOI] [PubMed] [Google Scholar]
- Ellies DL, Economou A, Viviano B, Rey JP, Paine-Saunders S, Krumlauf R, Saunders S. 2014. Wise regulates bone deposition through genetic interactions with Lrp5. PLoS ONE 9: e96257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embree MC, Kilts TM, Ono M, Inkson CA, Syed-Picard F, Karsdal MA, Oldberg A, Bi Y, Young MF. 2010. Biglycan and fibromodulin have essential roles in regulating chondrogenesis and extracellular matrix turnover in temporomandibular joint osteoarthritis. Am J Pathol 176: 812–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroshkin FM, Ermakova GV, Bayramov AV, Zaraisky AG. 2006. Multiple noggins in vertebrate genome: Cloning and expression of noggin2 and noggin4 in Xenopus laevis. Gene Expr Patterns 6: 180–186. [DOI] [PubMed] [Google Scholar]
- Evdokimova V, Tognon CE, Benatar T, Yang W, Krutikov K, Pollak M, Sorensen PHB, Seth A. 2012. IGFBP7 binds to the IGF-1 receptor and blocks its activation by insulin-like growth factors. Sci Signal 5: ra92. [DOI] [PubMed] [Google Scholar]
- Ezal CH, Marion CD, Smith WC. 2000. Primary structure requirements for Xenopus nodal-related 3 and a comparison with regions required by Xenopus nodal-related 2. J Biol Chem 275: 14124–14131. [DOI] [PubMed] [Google Scholar]
- Fainsod A, Deiβler K, Yelin R, Marom K, Epstein M, Pillemer G, Steinbeisser H, Blum M. 1997. The dorsalizing and neural inducing gene follistatin is an antagonist of BMP-4. Mech Dev 63: 39–50. [DOI] [PubMed] [Google Scholar]
- Fan J, Ponferrada VG, Sato T, Vemaraju S, Fruttiger M, Gerhardt H, Ferrara N, Lang RA. 2014. Crim1 maintains renal vascular stability during development by regulating endothelial cell Vegfa autocrine signaling. Development 141: 448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucheux C, Ulysse F, Bareille R, Reddi AH, Amedee J. 1997. Opposing actions of BMP3 and TGFβ1 in human bone marrow stromal cell growth and differentiation. Biochem Biophys Res Commun 241: 787–793. [DOI] [PubMed] [Google Scholar]
- Feldman B, Concha ML, Saude L, Parsons MJ, Adams RJ, Wilson SW, Stemple DL. 2002. Lefty antagonism of Squint is essential for normal gastrulation. Curr Biol 12: 2129–2135. [DOI] [PubMed] [Google Scholar]
- Fico A, Maina F, Dono R. 2011. Fine-tuning of cell signaling by glypicans. Cell Mol Life Sci 68: 923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebig JE, Weidauer SE, Qiu LY, Bauer M, Schmieder P, Beerbaum M, Zhang JL, Oschkinat H, Sablad W, Mueller TD. 2013. The clip-segment of the von Willebrand domain 1 of the BMP modulator protein Crossveinless 2 is preformed. Molecules 18: 11658–11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finelli AL, Xie T, Bossie CA, Blackman RK, Padgett RW. 1995. The tolkin gene is a tolloid/BMP-1 homologue that is essential for Drosophila development. Genetics 141: 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MC, Li Y, Seghatoleslami MR, Dealy CN, Kosher RA. 2006. Heparan sulfate proteoglycans including syndecan-3 modulate BMP activity during limb cartilage differentiation. Matrix Biol 25: 27–39. [DOI] [PubMed] [Google Scholar]
- Fletcher RB, Watson AL, Harland RM. 2004. Expression of Xenopus tropicalis noggin1 and noggin2 in early development: Two noggin genes in a tetrapod. Gene Expr Patterns 5: 225–230. [DOI] [PubMed] [Google Scholar]
- Foley AC, Korol O, Timmer AM, Mercola M. 2007. Multiple functions of Cerberus cooperate to induce heart downstream of Nodal. Dev Biol 303: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsman CL, Ng BC, Heinze RK, Kuo C, Sergi C, Gopalakrishnan R, Yee D, Graf D, Schwertfeger KL, Petryk A. 2013. BMO-binding protein twisted gastrulation is required in mammary gland epithelium for normal ductal elongation and myoepithelial compartmentalization. Dev Biol 373: 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois V, Bier E. 1995. Xenopus chordin and Drosophila short gastrulation genes encode homologous proteins functioning in dorsal–ventral axis formation. Cell 80: 19–20. [DOI] [PubMed] [Google Scholar]
- Francois V, Solloway M, O’Neill JW, Emery J, Bier E. 1994. Dorsal-ventral patterning of the Drosophila embryo depends on a putative negative growth factor encoded by the short gastrulation gene. Genes Dev 8: 2602–2616. [DOI] [PubMed] [Google Scholar]
- Fuerer C, Nostro MC, Constam DB. 2014. Nodal • Gdf1 heterodimers with bound prodomains enable serum-independent Nodal signaling and endoderm differentiation. J Biol Chem 289: 17854–17871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujise M, Takeo S, Kamimura K, Matsuo T, Aigaki T, Izumi S, Nakato H. 2003. Dally regulates Dpp morphogen gradient formation in the Drosophila wing. Development 130: 1515–1522. [DOI] [PubMed] [Google Scholar]
- Funari VA, Krakow D, Nevarez L, Chen Z, Funari TL, Vatanavicharn N, Wilcox WR, Rimoin DL, Nelson SF, Cohn DH. 2010. BMPER mutation in diaphanospondylodysostosis identified by ancestral autozygosity mapping and targeted high-throughput sequencing. Am J Hum Genet 87: 532–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung WY, Fat KF, Eng CK, Lau CK. 2007. crm-1 facilitates BMP signaling to control body size in Caenorhabditis elegans. Dev Biol 311: 95–105. [DOI] [PubMed] [Google Scholar]
- Furthauer M, Thisse B, Thisse C. 1999. Three different noggin genes antagonize the activity of bone morphogenetic proteins in the zebrafish embryo. Dev Biol 214: 181–196. [DOI] [PubMed] [Google Scholar]
- Gamer LW, Wolfman NM, Celeste AJ, Hattersley G, Hewick R, Rosen V. 1999. A novel BMP expressed in developing mouse limb, spinal cord, and tail bud is a potent mesoderm inducer in Xenopus embryos. Dev Biol 208: 222–232. [DOI] [PubMed] [Google Scholar]
- Gamer LW, Cox KA, Small C, Rosen V. 2001. Gdf11 is a negative regulator of chondrogenesis and myogenesis in the developing chick limb. Dev Biol 229: 407–420. [DOI] [PubMed] [Google Scholar]
- Gamer LW, Nove J, Levin M, Rosen V. 2005. BMP-3 is a novel inhibitor of both activin and BMP-4 signaling in Xenopus embryos. Dev Biol 285: 156–168. [DOI] [PubMed] [Google Scholar]
- Gao WL, Zhang SQ, Zhang H, Wan B, Yin ZS. 2013. Chordin-like protein 1 promotes neuronal differentiation by inhibiting bone morphogenetic protein-4 in neural stem cells. Mol Med Rep 7: 1143–1148. [DOI] [PubMed] [Google Scholar]
- Garcia Abreu J, Coffinier C, Larrain J, Oelgeschlager M, De Robertis EM. 2002. Chordin-like CR domains and the regulation of evolutionarily conserved extracellular signaling systems. Gene 287: 39–47. [DOI] [PubMed] [Google Scholar]
- Gavin-Smyth J, Wang YC, Butler I, Ferguson EL. 2013. A genetic network conferring canalization to a bistable patterning system in Drosophila. Curr Biol 23: 2296–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzerro E, Gangji V, Canalis E. 1998. Bone morphogenetic proteins induce the expression of noggin, which limits their activity in cultured rat osteoblasts. J Clin Invest 102: 2106–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzerro E, Deregowski V, Vaira S, Canalis E. 2005. Overexpression of Twisted Gastrulation inhibits bone morphogenetic protein action and prevents osteoblast cell differentiation in vitro. Endocrinology 146: 3875–3882. [DOI] [PubMed] [Google Scholar]
- Gazzerro E, Smerdel-Ramoya A, Zanotti S, Stadmeyer L, Durant D, Economides AN, Canalis E. 2007. Conditional deletion of gremlin causes a transient increase in bone formation bone mass. J Biol Chem 282: 31549–31557. [DOI] [PubMed] [Google Scholar]
- Ge G, Greenspan DS. 2006a. BMP1 controls TGFβ1 activation via cleavage of latent TGFβ-binding protein. J Cell Biol 175: 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge G, Greenspan DS. 2006b. Developmental roles of the BMP1/TLD metalloproteinases. Birth Defects Res C Embryo Today 78: 47–68. [DOI] [PubMed] [Google Scholar]
- Ge G, Hopkins DR, Ho WB, Greenspan DS. 2005. GDF11 forms a bone morphogenetic protein 1- activated latent complex that can modulate nerve growth factor-induced differentiation of PC12 cells. Mol Cell Biol 25: 5846–5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger M, Li RH, Friess W. 2003. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev 55: 1613–1629. [DOI] [PubMed] [Google Scholar]
- Geng Y, Dong Y, Yu M, Zhang L, Yan X, Sun J, Qiao L, Geng H, Nakajima M, Furuichi T, et al. 2011. Follistatin-like 1 (Fstl1) is a bone morphogenetic protein (BMP) 4 signaling antagonist in controlling mouse lung development. Proc Natl Acad Sci 108: 7058–7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach-Bank LM, Ellis AD, Noonen B, Barald KF. 2002. Cloning and expression analysis of the chick DAN gene, an antagonist of the BMP family of growth factors. Dev Dyn 224: 109–115. [DOI] [PubMed] [Google Scholar]
- Glienke J, Sturz A, Menrad A, Thierauch KH. 2002. CRIM1 is involved in endothelial cell capillary formation in vitro and is expressed in blood vessels in vivo. Mech Dev 119: 165–175. [DOI] [PubMed] [Google Scholar]
- Glinka A, Delius H, Blumenstock C, Niehrs C. 1996. Combinatorial signalling by Xwnt-11 and Xnr3 in the organizer epithelium. Mech Dev 60: 221–231. [DOI] [PubMed] [Google Scholar]
- Glinka A, Wu W, Onichtchock D, Blumenstock C, Niehrs C. 1997. Head induction by simultaneous repression of Bmp and Wnt signalling in Xenopus. Nature 389: 517–519. [DOI] [PubMed] [Google Scholar]
- Goodman SA, Albano R, Wardle FC, Matthews G, Tannahill D, Dale L. 1998. BMP1-related metalloproteases promote the development of ventral mesoderm in early Xenopus embryos. Dev Biol 195: 144–157. [DOI] [PubMed] [Google Scholar]
- Graf D, Timmons PM, Hitchins M, Episkopou V, Moore G, Ito T, Fujiyama A, Fisher AG, Merkenschlager M. 2001. Evolutionary conservation, developmental expression, and genomic mapping of mammalian Twisted gastrulation. Mamm Genome 12: 554–560. [DOI] [PubMed] [Google Scholar]
- Graf D, Nethisinghe S, Palmer DB, Fisher AG, Merkenschlager M. 2002. The developmentally regulated expression of Twisted gastrulation reveals a role for bone morphogenetic proteins in the control of T cell development. J Exp Med 196: 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory KE, Ono RN, Charbonneau NL, Kuo CL, Keene DR, Bachinger HP, Sakai LY. 2005. The prodomain of BMP-7 targets the BMP-7 complex to the extracellular matrix. J Biol Chem 280: 27970–27980. [DOI] [PubMed] [Google Scholar]
- Grisaru S, Cano-Gauci D, Tee J, Filmus J, Rosenblum ND. 2001. Glypican-3 modulates BMP- and FGF-mediated effects during renal branching morphogenesis. Dev Biol 231: 31–46. [DOI] [PubMed] [Google Scholar]
- Groppe J, Greenwald J, Wiater E, Rodriguez-Leon J, Economides AN, Kwiatkowski W, Affolter M, Vale WW, Belmonte JCI, Choe S. 2002. Structural basis of BMP signalling inhibition by the cystine knot protein Noggin. Nature 420: 636–642. [DOI] [PubMed] [Google Scholar]
- Gumienny TL, MacNeil LT, Wang H, de Bono M, Wrana JL, Padgett RW. 2007. Glypican LON-2 is a conserved negative regulator of BMP-like signaling in Caenorhabditis elegans. Curr Biol 17: 159–164. [DOI] [PubMed] [Google Scholar]
- Guo Z, Wang Z. 2009. The glypican Dally is required in the niche for the maintenance of germline stem cells and short-range BMP signaling in the Drosophila ovary. Development 136: 3627–3635. [DOI] [PubMed] [Google Scholar]
- Guo J, Wu G. 2012. The signaling and functions of heterodimeric bone morphogenetic proteins. Cytokine Growth Factor Rev 23: 61–67. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Serbedzija GN, McMahon AP. 1996a. Genetic analysis of dorsoventral pattern formation in the zebrafish: Requirement of a BMP-like ventralizing activity and its dorsal repressor. Genes Dev 10: 2452–2461. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Pelegri F, Mullins MC, Kane DA, van Eeden FJ, Granato M, Brand M, Furutani-Seiki M, Haffter P, Heisenberg CP, et al. 1996b. dino and mercedes, two genes regulating dorsal development in the zebrafish embryo. Development 123: 95–102. [DOI] [PubMed] [Google Scholar]
- Han C, Belenkaya TY, Khodoun M, Tauchi M, Lin X, Lin X. 2004. Distinct and collaborative roles of Drosophila EXT family proteins in morphogen signaling and gradient formation. Development 131: 1563–1575. [DOI] [PubMed] [Google Scholar]
- Hansen CS, Marion CD, Steele K, George S, Smith WC. 1997. Direct neural induction and selective inhibition of mesoderm and epidermis inducers by Xnr3. Development 124: 483–492. [DOI] [PubMed] [Google Scholar]
- Haramoto Y, Tanegashima K, Onuma Y, Takahashi S, Sekizaki H, Asashima M. 2004. Xenopus tropicalis nodal-related gene 3 regulates BMP signaling: An essential role for the pro-region. Dev Biol 265: 155–168. [DOI] [PubMed] [Google Scholar]
- Haramoto Y, Takahashi S, Asashima M. 2006. Two distinct domains in pro-region of Nodal-related 3 are essential for BMP inhibition. Biochem Biophys Res Commun 346: 470–478. [DOI] [PubMed] [Google Scholar]
- Haramoto Y, Takahashi S, Asashima M. 2007. Monomeric mature protein of Nodal-related 3 activates Xbra expression. Dev Genes Evol 217: 29–37. [DOI] [PubMed] [Google Scholar]
- Harms PW, Chang C. 2003. Tomoregulin-1 (TMEFF1) inhibits nodal signaling through direct binding to the nodal coreceptor Cripto. Genes Dev 17: 2624–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington AE, Morris-Triggs SA, Ruotolo BT, Robinson CV, Ohnuma S, Hyvonen M. 2006. Structural basis for the inhibition of activin signalling by follistatin. EMBO J 25: 1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison CA, Al-Musawi SL, Walton KL. 2011. Prodomains regulate the synthesis, extracellular localisation and activity of TGF-β superfamily ligands. Growth Factors 29: 174–186. [DOI] [PubMed] [Google Scholar]
- Hashimoto O, Nakamura T, Shoji H, Shimasaki S, Hayashi Y, Sugino H. 1997. A novel role of follistatin, an activin-binding protein, in the inhibition of activin action in rat pituitary cells. Endocytotic degradation of activin and its acceleration by follistatin associated with cell-surface heparan sulfate. J Biol Chem 272: 13835–13842. [DOI] [PubMed] [Google Scholar]
- Hashimoto O, Kawasaki N, Tsuchida K, Shimasaki S, Hayakawa T, Sugino H. 2000. Difference between follistatin isoforms in the inhibition of activin signalling: Activin neutralizing activity of follistatin isoforms is dependent on their affinity for activin. Cell Signal 12: 565–571. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Rebagliati M, Ahmad N, Muraoka O, Kurokawa T, Hibi M, Suzuki T. 2004. The Cerberus/Dan-family protein Charon is a negative regulator of Nodal signaling during left–right patterning in zebrafish. Development 131: 1741–1753. [DOI] [PubMed] [Google Scholar]
- Haudenschild DR, Palmer SM, Moseley TA, You Z, Reddi AH. 2004. Bone morphogenetic protein (BMP)-6 signaling and BMP antagonist noggin in prostate cancer. Cancer Res 64: 8276–8284. [DOI] [PubMed] [Google Scholar]
- Haudenschild DR, Hong E, Yik JHN, Chromy B, Morgelin M, Snow KD, Acharya C, Takada Y, Di Cesare PE. 2011. Enhanced activity of transforming growth factor β1 (TGF-β1) bound to cartilage oligomeric matrix protein. J Biol Chem 286: 43250–43258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SH, Wunnenberg-Stapleton K, Hashimoto C, Laurent MN, Watabe T, Blumberg BW, Cho KW. 1995. Disruption of BMP signals in embryonic Xenopus ectoderm leads to direct neural induction. Genes Dev 9: 2923–2935. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Kobayashi S, Nakato H. 2009. Drosophila glypicans regulate the germline stem cell niche. J Cell Biol 187: 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath E, Tahri D, Andermarcher E, Schofield P, Fleming S, Boulter CA. 2008. Abnormal skeletal and cardiac development, cardiomyopathy, muscle atrophy and cataracts in mice with a targeted disruption of the Nov (Ccn3) gene. BMC Dev Biol 8: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer R, Bashey RI, Kyle J, Jimenez SA. 1995. TGF-β modulates the synthesis of proteoglycans by myocardial fibroblasts in culture. J Mol Cell Cardiol 27: 2191–2198. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Kelley OG, Melton DA. 1994. Follistatin, an antagonist of activin, is expressed in the Spemann organizer and displays direct neuralizing activity. Cell 77: 283–295. [DOI] [PubMed] [Google Scholar]
- Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA, Ruoslahti E. 1994. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor-β. Biochem J 302: 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JJ, Davies MV, Pearson AA, Wang JH, Hewick RM, Wolfman NM, Qiu Y. 2002. The myostatin propeptide and the follistatin-related gene are inhibitory binding proteins of myosattin in normal serum. J Biol Chem 277: 40735–40741. [DOI] [PubMed] [Google Scholar]
- Hill JJ, Qiu Y, Hewick RM, Wolfman NM. 2003. Regulation of myostatin in vivo by growth and differentiation factor-associated serum protein-1: A novel protein with protease inhibitor and follistatin domains. Mol Endocrinol 17: 1144–1154. [DOI] [PubMed] [Google Scholar]
- Hino J, Nishimatsu S, Nagai T, Matsuo H, Kangawa K, Nohno T. 2003. Coordination of BMP-3b and cerberus is required for head formation of Xenopus embryos. Dev Biol 260: 138–157. [DOI] [PubMed] [Google Scholar]
- Hocking AM, Shinomura T, McQuillan DJ. 1998. Leucine-rich repeat glycoproteins of the extracellular matrix. Matrix Biol 17: 1–19. [DOI] [PubMed] [Google Scholar]
- Holley SA, Jackson PD, Sasai Y, Lu B, De Robertis EM, Hoffmann FM, Ferguson EL. 1995. A conserved system for dorsal-ventral patterning in insects and vertebrates involving sog and chordin. Nature 376: 249–253. [DOI] [PubMed] [Google Scholar]
- Horie M, Mitsumoto Y, Kyushiki H, Kanemoto N, Watanabe A, Taniguchi Y, Nishino N, Okamoto T, Kondo M, Mori T, et al. 2000. Identification and characterization of TMEFF2, a novel survival factor for hippocampal and mesencephalic neurons. Genomics 67: 146–152. [DOI] [PubMed] [Google Scholar]
- Hsu DR, Economides AN, Wang X, Eimon PM, Harland RM. 1998. The Xenopus dorsalizing factor Gremlin identifies a novel family of secreted proteins that antagonize BMP activities. Mol Cell 1: 673–683. [DOI] [PubMed] [Google Scholar]
- Huang SS, O’Grady P, Huang JS. 1988. Human transforming growth factor-β: α2 macroglobulin complex is a latent form of transforming growth factor-β. J Biol Chem 263: 1535–1541. [PubMed] [Google Scholar]
- Huang G, Zhang Y, Kim B, Ge G, Annis DS, Mosher DF, Greenspan DS. 2009. Fibronectin binds and enhances the activity of bone morphogenetic protein 1. J Biol Chem 284: 25879–25888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley R, Davydova J, Petryk A, Billington CJ Jr, Jensen ED, Mansky KC, Gopalakrishnan R. 2015. The function of Twisted Gastrulation in regulating osteoclast differentiation is dependent on BMP binding. J Cell Biochem 116: 2239–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ideno H, Takanabe R, Shimada A, Imaizumi K, Araki R, Abe M, Nifuji A. 2009. Protein related to DAN and Cerberus (PRDC) inhibits osteoblastic differentiation and its suppression promotes osteogenesis in vitro. Exp Cell Res 315: 474–484. [DOI] [PubMed] [Google Scholar]
- Iemura S, Yamamoto TS, Takagi C, Uchiyama H, Natsume T, Shimasaki S, Sugino H, Ueno N. 1998. Direct binding of follistatin to a complex of bone-morphogenetic protein and its receptor inhibits ventral and epidermal cell fates in early Xenopus embryos. Proc Natl Acad Sci 95: 9337–9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeya M, Kawada M, Kiyonari H, Sasai N, Nakao K, Furuta Y, Sasai Y. 2006. Essential pro-Bmp roles of crossveinless 2 in mouse organogenesis. Development 133: 4463–4473. [DOI] [PubMed] [Google Scholar]
- Ikeya M, Nosaka T, Fukushima K, Kawada M, Furuta Y, Kitamura T, Sasai T. 2008. Twisted gastrulation mutation suppresses skeletal defect phenotypes in Crossveinless 2 mutant mice. Mech Dev 125: 832–842. [DOI] [PubMed] [Google Scholar]
- Ikeya M, Fukushima K, Kawada M, Onishi S, Furuta Y, Yonemura S, Kitamura T, Nosaka T, Sasai Y. 2010. Cv2, functioning as a pro-BMP factor via twisted gastrulation, is required for early development of nephron precursors. Dev Biol 337: 405–414. [DOI] [PubMed] [Google Scholar]
- Inomata H, Haraguchi T, Sasai Y. 2008. Robust stability of the embryonic axial pattern requires a secreted scaffold for chordin degradation. Cell 134: 854–865. [DOI] [PubMed] [Google Scholar]
- Inomata H, Shibata T, Haraguchi T, Sasai Y. 2013. Scaling of dorsal-ventral patterning by embryo size-dependent degradation of Spemann’s organizer signals. Cell 153: 1296–1311. [DOI] [PubMed] [Google Scholar]
- Inouye S, Guo Y, DePaolo L, Shimonaka M, Ling N, Shimasaki S. 1991. Recombinant expression of human follistatin with 315 and 288 amino acids: Chemical and biological comparison with native porcine follistatin. Endocrinology 129: 815–822. [DOI] [PubMed] [Google Scholar]
- Iozzo R. 1999. The biology of the small leucine-rich proteoglycans. J Biol Chem 274: 18843–18846. [DOI] [PubMed] [Google Scholar]
- Ishida K, Acharya C, Christiansen BA, Yik JHN, DiCesare PE, Haudenschild DR. 2013. Cartilage oligomeric matrix protein enhances osteogenesis by directly binding and activating bone morphogenetic protein-2. Bone 55: 23–35. [DOI] [PubMed] [Google Scholar]
- Ishiguro K, Kadomatsu K, Kojima T, Muramatsu H, Iwase M, Yoshikai Y, Yanada M, Yamamoto K, Matsushita T, Nishimura M, et al. 2001. Syndecan-4 deficiency leads to high mortality of lipopolysaccharide-injected mice. J Biol Chem 276: 47483–47488. [DOI] [PubMed] [Google Scholar]
- Ishimaru Y, Yoshioka H, Tao H, Thisse B, Thisse C, Wright CVE, Hamada H, Ohuchi H, Noji S. 2000. Asymmetric expression of antivin/lefty1 in the early chick embryo. Mech Dev 90: 115–118. [DOI] [PubMed] [Google Scholar]
- Israel DI, Nove J, Kerns KM, Kaufman RJ, Rosen V, Cox KA, Wozney JM. 1996. Heterodimeric bone morphogenetic proteins show enhanced activity in vitro and in vivo. Growth Factors 13: 291–300. [DOI] [PubMed] [Google Scholar]
- Itasaki N, Jones CM, Mercurio S, Rowe A, Domingos PM, Smith JC, Krumlauf R. 2003. Wise, a context-dependent activator and inhibitor of Wnt signaling. Development 130: 4295–4305. [DOI] [PubMed] [Google Scholar]
- Ito A, Shinmyo Y, Abe T, Oshima N, Tanaka H, Ohta K. 2010. Tsukushi is required for anterior commissure formation in mouse brain. Biochem Biophys Res Commun 402: 813–818. [DOI] [PubMed] [Google Scholar]
- Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, Daluiski A, Lyons KM. 2003. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development 130: 2779–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SM, Nakato H, Sugiura M, Jannuzi A, Oakes R, Kaluza V, Golden C, Selleck SB. 1997. dally, a Drosophila glypican, controls cellular responses to the TGF-β-related morphogen, Dpp. Development 124: 4113–4120. [DOI] [PubMed] [Google Scholar]
- James RE, Briohier HT. 2011. Crimpy inhibits the BMP homolog Gbb in motoneurons to enable proper growth control at the Drosophila neuromuscular junction. Development 138: 3273–3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasuja R, Allen BL, Pappano WN, Rapraeger AC, Greenspan DS. 2004. Cell-surface heparan sulfate proteoglycan potentiate chordin antagonism of bone morphogenetic protein signaling and are necessary for cellular uptake of chordin. J Biol Chem 279: 51289–51297. [DOI] [PubMed] [Google Scholar]
- Jasuja R, Voss N, Ge G, Hoffman GG, Lyman-Gingerich J, Pelegri F, Greenspan DS. 2006. bmp1 and mini fin are functional redundant in regulating formation of the zebrafish dorsoventral axis. Mech Dev 123: 548–558. [DOI] [PubMed] [Google Scholar]
- Joseph EM, Melton DA. 1998. Mutant Vg1 ligands disrupt endoderm and mesoderm formation in Xenopus embryos. Development 125: 2677–2685. [DOI] [PubMed] [Google Scholar]
- Joubin K, Stern CD. 1999. Molecular interactions continuously define the organizer during the cell movements of gastrulation. Cell 98: 559–571. [DOI] [PubMed] [Google Scholar]
- Kalantry S, Manning S, Haub O, Tomihara-Newberger C, Lee HG, Fangman J, Disteche CM, Manova K, Lacy E. 2001. The amnionless gene, essential for mouse gastrulation, encodes a visceral-endoderm-specific protein with an extracellular cysteine-rich domain. Nat Genet 27: 412–416. [DOI] [PubMed] [Google Scholar]
- Kamimura M, Matsumoto K, Koshiba-Takeuchi K, Ogura T. 2004. Vertebrate crossveinless 2 is secreted and acts as an extracellular modulator of the BMP signaling cascade. Dev Dyn 230: 434–445. [DOI] [PubMed] [Google Scholar]
- Kamiya N, Ye L, Kobayashi T, Mochida Y, Yamauchi M, Kronenberg HM, Feng JQ, Mishina Y. 2008. BMP signaling negatively regulates bone mass through sclerostin by inhibiting the canonical Wnt pathway. Development 135: 3801–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannis GS, Musrap N, Saraon P, Treacy A, Schaeffer DF, Kirsch R, Riddell RH, Diamandis EP. 2015. Bone morphogenetic protein antagonist gremlin-1 regulates colon cancer progression. Biol Chem 396: 163–183. [DOI] [PubMed] [Google Scholar]
- Kassai Y, Munne P, Hotta Y, Penttila E, Kavanagh K, Ohbayashi N, Takada S, Thesleff I, Jernvall J, Itoh N. 2005. Regulation of mammalian tooth cusp patterning by ectodin. Science 309: 2067–2070. [DOI] [PubMed] [Google Scholar]
- Katsu K, Tokumori D, Tatsumi N, Suzuki A, Yokouchi Y. 2012. BMP inhibition by DAN in Hensen’s node is a critical step for the establishment of left–right asymmetry in the chick embryo. Dev Biol 363: 15–26. [DOI] [PubMed] [Google Scholar]
- Katsube K, Sakamoto K, Tamamura Y, Yamaguchi A. 2009. Role of CCN, a vertebrate specific gene family, in development. Dev Growth Differ 51: 55–67. [DOI] [PubMed] [Google Scholar]
- Kattamuri C, Luedeke DM, Nolan K, Rankin SA, Greis KD, Zorn AM, Thompson TB. 2012. Members of the DAN family are BMP antagonists that form highly stable noncovalent dimers. J Mol Biol 424: 313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley R, Ren R, Pi X, Wu Y, Moreno I, Willis M, Moser M, Ross M, Podkowa M, Attisano L, et al. 2009. A concentration-dependent endocytic trap and sink mechanism converts Bmper from an activator to an inhibitor of Bmp signaling. J Cell Biol 184: 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keutmann HT, Schneyer AL, Sidis Y. 2004. The role of follistatin domains in follistatin biological action. Mol Endocrinol 18: 228–240. [DOI] [PubMed] [Google Scholar]
- Khokha MK, Hsu D, Brunet LJ, Dionne MS, Harland RM. 2003. Gremlin is the BMP antagonist required for maintenance of Shh and Fgf signals during limb patterning. Nat Genet 34: 303–307. [DOI] [PubMed] [Google Scholar]
- Khokha MK, Yeh J, Grammer TC, Harland RM. 2005. Depletion of three BMP antagonists from Spemann’s organizer leads to a catastrophic loss of dorsal structures. Dev Cell 8: 401–411. [DOI] [PubMed] [Google Scholar]
- Kinna G, Kolle G, Carter A, Key B, Lieschke GJ, Perkins A, Little MH. 2006. Knockdown of zebrafish crim1 results in a bent tail phenotype with defects in somite and vascular development. Mech Dev 123: 277–287. [DOI] [PubMed] [Google Scholar]
- Kiso H, Takahashi K, Saito K, Togo Y, Tsukamoto H, Huang B, Sugai M, Shimizu A, Tabata Y, Economides AN, et al. 2014. Interactions between BMP-7 and USAG-1 (uterine sensitization-associated gene 1) regulate supernumerary organ formations. PLoS ONE 9: e96938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Luo M, Zhang Y, Wilkes DC, Ge G, Grieskamp T, Yamada C, Liu TC, Huang G, Basson CT, et al. 2009. Secreted Frizzled-related protein 2 is a procollagen C proteinase enhancer with a role in fibrosis associated with myocardial infarction. Nat Cell Biol 11: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike N, Kassai Y, Kouta Y, Miwa H, Konishi M, Itoh N. 2007. Brorin, a novel secreted bone morphogenetic protein antagonist, promotes neurogenesis in mouse neural precursor cells. J Biol Chem 282: 15843–15850. [DOI] [PubMed] [Google Scholar]
- Kokabu S, Gamer L, Cox K, Lowery J, Tsuji K, Raz R, Economides A, Katagiri T, Rosen V. 2012. BMP3 suppresses osteoblast differentiation of bone marrow stromal cells via interaction with Acvr2b. Mol Endocrinol 26: 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolle G, Georgas K, Holmes GP, Little MH, Yamada T. 2000. CRIM1, a novel gene encoding a cysteine-rich repeat protein, is developmentally regulated and implicated in vertebrate CNS development and organogenesis. Mech Dev 90: 181–193. [DOI] [PubMed] [Google Scholar]
- Kolle G, Jansen A, Yamada T, Little M. 2003. In ovo electroporation of Crim1 in the developing chick spinal cord. Dev Dyn 226: 107–111. [DOI] [PubMed] [Google Scholar]
- Kondas K, Szlama G, Trexler M, Patthy L. 2008. Both WFIKKN1 and WFIKKN2 have high affinity for growth and differentiation factors 8 and 11. J Biol Chem 283: 23677–23684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer KL, Yost HJ. 2002. Ectodermal syndecan-2 mediates left–right axis formation in migrating mesoderm as a cell-nonautonomous Vg1 cofactor. Dev Cell 2: 115–124. [DOI] [PubMed] [Google Scholar]
- Krause C, Korchynskyi O, de Rooij K, Weidauer SE, de Gorter DJ, van Benzooijen RL, Hatsell S, Economides AN, Mueller TD, Lowik CW, et al. 2010. Distinct modes of inhibition by sclerostin on bone morphogenetic protein and Wnt signaling pathways. J Biol Chem 285: 41614–41626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kresse H, Schonherr E. 2001. Proteoglycans of the extracellular matrix and growth control. J Cell Physiol 189: 266–274. [DOI] [PubMed] [Google Scholar]
- Kreuger J, Perez L, Giraldez AJ, Cohen SM. 2004. Opposing activities of Dally-like glypican at high and low levels of wingless morphogen activity. Dev Cell 7: 503–512. [DOI] [PubMed] [Google Scholar]
- Kriebitz NN, Kiecker C, McCormick L, Lumsden A, Graham A, Bell E. 2009. PRDC regulates placode neurogenesis in chick by modulating BMP signalling. Dev Biol 336: 280–292. [DOI] [PubMed] [Google Scholar]
- Kuriyama S, Lupo G, Ohta K, Ohnuma S, Harris WA, Tanaka H. 2006. Tsukushi controls ectodermal patterning and neural crest specification in Xenopus by direct regulation of BMP4 and X-delta-1 activity. Development 133: 75–88. [DOI] [PubMed] [Google Scholar]
- Kuroda H, Wessely O, De Robertis EM. 2004. Neural induction in Xenopus: Requirement for ectodermal and endodermal signals via chordin, noggin, β-catenin, and Cerberus. PLoS Biol 2: 623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusu N, Laurikkala J, Imanishi M, Usui H, Konishi M, Miyake A, Thesleff I, Itoh N. 2003. Sclerostin is a novel secreted osteoclast-derived bone morphogenetic protein antagonist with unique ligand specificity. J Biol Chem 278: 24113–24117. [DOI] [PubMed] [Google Scholar]
- LaMarre J, Wollenberg GK, Gonias SL, Hayes MA. 1991. Reaction of α2-macroglobulinwith plasmin increases binding of transforming growth factor-β1 and β2. Biochim Biophys Acta 1091: 197–204. [DOI] [PubMed] [Google Scholar]
- Lamb TM, Knecht AK, Smith WC, Stachel SE, Economides AN, Stahl N, Yancopolous GD, Harland RM. 1993. Neural induction by the secreted polypeptide noggin. Science 262: 713–718. [DOI] [PubMed] [Google Scholar]
- Lapraz F, Rottinger E, Duboc V, Range R, Duloquin L, Walton K, Wu SY, Bradham C, Loza MA, Hibino T, et al. 2006. RTK and TGF-β signaling pathways genes in the sea urchin genome. Dev Biol 300: 132–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larman BW, Karolak MJ, Adams DC, Oxburgh L. 2009. Chordin-like 1 and twisted gastrulation 1 regulate BMP signaling following kidney injury. J Am Soc Nephrol 20: 1020–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrain J, Bachiller D, Lu B, Agius E, Piccolo S, De Robertis EM. 2000. BMP-binding modules in chordin: A model for signalling regulation in the extracellular space. Development 127: 821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrain J, Oelgeschlager M, Ketpura NI, Reversade B, Zakin L, De Robertis EM. 2001. Proteolytic cleavage of Chordin as a switch for the dual activities of Twisted gastrulation in BMP signaling. Development 128: 4439–4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrain J, Brown C, De Robertis EM. 2003. Integrin-α3 mediates binding of Chordin to the cell surface and promotes its endocytosis. EMBO Rep 4: 813–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurikkala J, Kassai Y, Pakkasjarvi L, Thesleff I, Itoh N. 2003. Identification of a secreted BMP antagonist, ectodin, integrating BMP, FGF, and SHH signals from the tooth enamel knot. Dev Biol 264: 91–105. [DOI] [PubMed] [Google Scholar]
- Lee SJ, McPherron AC. 2001. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci 98: 9306–9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HX, Ambrosio AL, Reversade B, De Robertis EM. 2006. Embryonic dorsal-ventral signaling: Secreted Frizzled-related proteins as inhibitors of Tolloid proteinases. Cell 124: 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HX, Mendes FA, Plouhinec JL, De Robertis EM. 2009. Enzymatic regulation of pattern: BMP4 binds CUB domains of Ttolloids and inhibits proteinase activity. Genes Dev 23: 2551–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Good JA, Joubin K, Giraldez AJ, Ben-Haim N, Beck S, Chen Y, Schier AF, Constam DB. 2005. Nodal stability determines signaling range. Curr Biol 15: 31–36. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Brivanlou AH. 2006. GDF3, a BMP inhibitor, regulates cell fate in stem cells and early embryos. Development 133: 209–216. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Levine ZJ, Brivanlou AH. 2009. GDF3 is a BMP inhibitor that can activate Nodal signaling only at very high doses. Dev Biol 325: 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Kleeff J, Kayed H, Felix K, Penzel R, Buchler MW, Korc M, Friess H. 2004. Glypican-1 antisense transfection modulates TGF-β-dependent signaling in Colo-357 pancreatic cancer cells. Biochem Biophys Res Commun 320: 1148–1155. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D. 2005. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem 280: 19883–19887. [DOI] [PubMed] [Google Scholar]
- Liem KF, Tremml G, Jessell TM. 1997. A role for the roof plate and its resident TGFβ-related proteins in neuronal patterning in the dorsal spinal cord. Cell 91: 127–138. [DOI] [PubMed] [Google Scholar]
- Lin X, Perrimon N. 2000. Role of heparin sulfate proteoglycans in cell–cell signaling in Drosophila. Matrix Biol 19: 303–307. [DOI] [PubMed] [Google Scholar]
- Lin J, Patel SR, Cheng X, Cho EA, Levitan I, Ullenbruch M, Phan SH, Park JM, Dressler GR. 2005. Kielin/chordin-like protein, a novel enhancer of BMP signaling, attenuates renal fibrotic disease. Nat Med 11: 387–393. [DOI] [PubMed] [Google Scholar]
- Lin J, Patel SR, Wang M, Dressler GR. 2006. The cysteine-rich domain protein KCP is a suppressor of transforming growth factor β/activin signaling in renal epithelia. Mol Cell Biol 26: 4577–4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintern KB, Guidato S, Rowe A, Saldanha JW, Itasaki N. 2009. Characterization of wise protein and its modular mechanism to interact with both Wnt and BMP signals. J Biol Chem 284: 23159–23168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little SC, Mullins MC. 2004. Twisted gastrulation promotes BMP signaling in zebrafish dorsal-ventral axial patterning. Development 131: 5825–5835. [DOI] [PubMed] [Google Scholar]
- Little SC, Mullins MC. 2009. Bone morphogenetic protein heterodimers assemble heteromeric type I receptor complexes to pattern the dorsoventral axis. Nat Cell Biol 11: 637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Ling TY, Shieh HS, Johnson FE, Huang JS, Huang SS. 2001. Identification of the high affinity binding site in transforming growth factor-β involved in complex formation with α2-macroglobulin. Implications regarding the molecular mechanisms of complex formation between α2-macroglobulin and growth factors, cytokines, and hormones. J Biol Chem 276: 46212–46218. [DOI] [PubMed] [Google Scholar]
- Lu MM, Yang H, Zhang L, Shu W, Blai DG, Morrisey EE. 2001. The bone morphogenetic protein antagonist gremlin regulates proximal-distal patterning of the lung. Dev Dyn 222: 667–680. [DOI] [PubMed] [Google Scholar]
- Lyon M, Rushton G, Gallagher JT. 1997. The interaction of the transforming growth factor-βs with heparin/heparan sulfate is isoform-specific. J Biol Chem 272: 18000–18006. [DOI] [PubMed] [Google Scholar]
- Maeda A, Nishida T, Aoyama E, Kubota S, Lyons KM, Kuboki T, Takigawa M. 2009. CCN family 2/connective tissue growth factor modulates BMP signalling as a signal conductor, which action regulates the proliferation and differentiation of chondrocytes. J Biochem 145: 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeno M, Xue Y, Wood TI, Ong RC, Kung HF. 1993. Cloning and expression of cDNA encoding Xenopus laevis bone morphogenetic protein-1 during early embryonic development. Gene 134: 257–261. [DOI] [PubMed] [Google Scholar]
- Marjoram L, Wright C. 2011. Rapid differential transport of Nodal and Lefty on sulfated proteoglycan-rich extracellular matrix regulates left–right asymmetry in Xenopus. Development 138: 475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markmann A, Hausser H, Schonherr E, Kresse H. 2000. Influence of decorin expression on transforming growth factor-β-mediated collagen gel retraction and biglycan induction. Matrix Biol 19: 631–636. [DOI] [PubMed] [Google Scholar]
- Marques G, Musacchio M, Shimell MJ, Wunnenberg-Stapleton K, Cho KWY, O’Connor MB. 1997. Production of a DPP activity gradient in the early Drosophila embryo through the opposing actions of the SOG and TLD proteins. Cell 91: 417–426. [DOI] [PubMed] [Google Scholar]
- Marques S, Borges AC, Silva AC, Freitas S, Cordenonsi M, Belo JA. 2004. The activity of the Nodal antagonist Cerl-2 in the mouse node is required for correct L/R body axis. Genes Dev 18: 2342–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyn U, Schulte-Merker S. 2003. The ventralized ogon mutant phenotype is caused by a mutation in the zebrafish homologue of Sizzled, a secreted Frizzled-related protein. Dev Biol 260: 58–67. [DOI] [PubMed] [Google Scholar]
- Mason ED, Konrad KD, Webb CD, Marsh JL. 1994. Dorsal midline fate in Drosophila embryos requires twisted gastrulation, a gene encoding a secrete protein related to human connective tissue growth factor. Genes Dev 8: 1489–1501. [DOI] [PubMed] [Google Scholar]
- Mason ED, Williams S, Grotendorst GR, Marsh JL. 1997. Combinatorial signaling by twisted gastrulation and decapentaplegic. Mech Dev 64: 61–75. [DOI] [PubMed] [Google Scholar]
- Massam-Wu T, Chiu M, Choudhury R, Chaudhry SS, Baldwin AK, McGovern A, Baldock C, Shuttleworth CA, Kielty CM. 2010. Assembly of fibrillin microfibrils governs extracellular deposition of latent TGFβ. J Cell Sci 123: 3006–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui M, Mizuseki K, Nakatani J, Nakanishi S, Sasai Y. 2000. Xenopus kielin: A dorsalizing factor containing multiple chordin-type repeats secreted from the embryonic midline. Proc Natl Acad Sci 97: 5291–5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Otsuka F, Hino J, Miyoshi T, Takano M, Miyazato M, Makino H, Kangawa K. 2012. Bone morphogentic protein-3b (BMP-3b) inhibits osteoblast differentiation via Smad2/3 pathway by counteracting Smad1/5/8 signaling. Mol Cell Endocrinol 350: 78–86. [DOI] [PubMed] [Google Scholar]
- Matsushita Y, Sakamoto K, Tamamura Y, Shibata Y, Minamizato T, Kihara T, Ito M, Katsube K, Hiraoka S, Koseki H, et al. 2013. CCN3 protein participates in bone regeneration as an inhibitory factor. J Biol Chem 288: 19973–19985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus DQ, Thomsen GH, Martindale MQ. 2006. Dorso/ventral genes are asymmetrically expressed and involved in germ-layer demarcation during cnidarian gastrulation. Curr Biol 16: 499–505. [DOI] [PubMed] [Google Scholar]
- McCaffrey TA, Falcone DJ, Brayton CF, Agarwal LA, Welt FG, Weksler BB. 1989. Transforming growth factor-β activity is potentiated by heparin via dissociation of the transforming growth factor-β/α2-macroglobulin inactive complex. J Cell Biol 109: 441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon JA, Takada S, Zimmerman LB, Fan CM, Harland RM, McMahon AP. 1998. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev 12: 1438–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherron AC, Lee SJ. 1993. GDF-3 and GDF-9: Two new members of the transforming growth factor-β superfamily containing a novel pattern of cysteines. J Biol Chem 268: 3444–3449. [PubMed] [Google Scholar]
- Meinhardt H, Gierer A. 2000. Pattern formation by local self-activation and lateral inhibition. BioEssays 22: 753–760. [DOI] [PubMed] [Google Scholar]
- Meno C, Saijoh Y, Fujii H, Ikeda M, Yokoyama T, Yokoyama M, Toyoda Y, Hamada H. 1996. Left–right asymmetric expression of the TGFβ-family member lefty in mouse embryos. Nature 381: 151–155. [DOI] [PubMed] [Google Scholar]
- Meno C, Ito Y, Saijoh Y, Matsuda Y, Tashiro K, Kuhara S, Hamada H. 1997. Two closely related left–right asymmetrically expressed genes, lefty-1 and lefty-2: Their distinct expression domains, chromosomal linkage and direct neuralizing activity in Xenopus embryos. Genes Cells 2: 513–524. [DOI] [PubMed] [Google Scholar]
- Meno C, Shimono A, Saijoh Y, Yashiro K, Mochida K, Ohishi S, Noji S, Kondoh H, Hamada H. 1998. lefty-1 is required for left–right determination as a regulator of lefty-2 and nodal. Cell 94: 287–297. [DOI] [PubMed] [Google Scholar]
- Meno C, Gritsman K, Ohishi S, Ohfuji Y, Heckscher E, Mochida K, Shimono A, Kondoh H, Talbot WS, Robertson EJ, et al. 1999. Mouse Lefty2 and zebrafish antivin are feedback inhibitors of nodal signaling during vertebrate gastrulation. Mol Cell 4: 287–298. [DOI] [PubMed] [Google Scholar]
- Mercurio S, Latinkic B, Itasaki N, Krumlauf R, Smith JC. 2004. Connective-tissue growth factor modulates WNT signalling and interacts with the WNT receptor complex. Development 131: 2137–2147. [DOI] [PubMed] [Google Scholar]
- Merino R, Macias D, Ganan Y, Economides AN, Wang X, Wu Q, Stahl N, Sampath KT, Varona P, Hurle JM. 1999a. Expression and function of Gdf-5 during digit skeletogenesis in the embryonic chick leg bud. Dev Biol 206: 33–45. [DOI] [PubMed] [Google Scholar]
- Merino R, Rodriguez-Leon J, Macias D, Ganan Y, Economides AN, Hurle JM. 1999b. The BMP antagonist Gremlin regulates outgrowth, chondrogenesis and programmed cell death in the developing limb. Development 126: 5515–5522. [DOI] [PubMed] [Google Scholar]
- Michos O, Panman L, Vintersten K, Beier K, Zeller R, Zuniga A. 2004. Gremlin-mediated BMP antagonism induces the epithelial-mesenchymal feedback signaling controlling metanephric kidney and limb organogenesis. Development 131: 3401–3410. [DOI] [PubMed] [Google Scholar]
- Michos O, Goncalves A, Lopez-Rios J, Tiecke E, Naillat F, Beier K, Galli A, Vainio S, Zeller R. 2007. Reduction of BMP4 activity by gremlin1 enables ureteric bud outgrowth and GDNF/WNT11 feedback signalling during kidney branching morphogenesis. Development 134: 2397–2405. [DOI] [PubMed] [Google Scholar]
- Midorikawa Y, Ishikawa S, Iwanari H, Imamura T, Sakamoto H, Miyazono K, Kodama T, Makuuchi M, Aburatani H. 2003. Glypican-3, overexpressed in hepatocellular carcinoma modulates FGF2 and BMP-7 signaling. Int J Cancer 103: 455–465. [DOI] [PubMed] [Google Scholar]
- Miguez PA, Terajima M, Nagaoka H, Mochida Y, Yamauchi M. 2011. Role of glycosaminoglycans of biglycan in BMP-2 signaling. Biochem Biophys Res Commun 405: 262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller-Bertoglio V, Carmany-Rampey A, Furthauer M, Gonzalez EM, Thisse C, Thisse B, Halpern ME, Solnica-Krezel L. 1999. Maternal and zygotic activity of the zebrafish ogon locus antagonizes BMP signaling. Dev Biol 214: 72–86. [DOI] [PubMed] [Google Scholar]
- Millet C, Lemaire P, Orsetti B, Guglielmi P, Francois V. 2001. The human chordin genge encodes several differentially expressed spliced variants with distinct BMP opposing activities. Mech Dev 106: 85–96. [DOI] [PubMed] [Google Scholar]
- Minabe-Saegusa C, Saegusa H, Tsukahara M, Noguchi S. 1998. Sequence and expression of a novel mouse gene PRDC (protein related to DAN and cerberus) identified by a gene trap approach. Dev Growth Differ 40: 343–353. [DOI] [PubMed] [Google Scholar]
- Minamizato T, Sakamoto K, Liu T, Kokubo H, Katsube K, Perbal B, Nakamura S, Yamaguchi A. 2007. CCN3/NOV inhibits BMP-2-induced osteoblast differentiation by interacting with BMP and Notch signaling pathways. Biochem Biophys Res Commun 354: 567–573. [DOI] [PubMed] [Google Scholar]
- Mitola S, Ravelli C, Moroni E, Salvi V, Leali D, Ballmer-Hofer K, Zammataro L, Presta M. 2010. Gremlin is a novel agonist of the major proangiogenic receptor VEGFR2. Blood 116: 3677–3680. [DOI] [PubMed] [Google Scholar]
- Miura T, Kishioka Y, Wakamatsu J, Hattori A, Hennebry A, Berry CJ, Sharma M, Kambadur R, Nishimura T. 2006. Decorin binds myostatin and modulates its activity to muscle cells. Biochem Biophys Res Commun 340: 675–680. [DOI] [PubMed] [Google Scholar]
- Miwa H, Miyake A, Kouta Y, Shimada A, Yamashita Y, Nakayama Y, Yamauchi H, Konishi M, Itoh N. 2009. A novel neural-specific BMP antagonist, Brorin-like, of the Chordin family. FEBS Lett 583: 3643–3648. [DOI] [PubMed] [Google Scholar]
- Molina MD, Salo E, Cebria F. 2009. Expression pattern of the expanded noggin gene family in the planarian Schmidtea mediterranea. Gene Expr Patterns 9: 246–253. [DOI] [PubMed] [Google Scholar]
- Molina MD, Neto A, Maeso I, Gomez-Skarmeta JL, Salo E, Cebria F. 2011. Noggin and noggin-like genes control dorsoventral axis regeneration in planarians. Curr Biol 21: 300–305. [DOI] [PubMed] [Google Scholar]
- Moon JI, Birren SJ. 2008. Target-dependent inhibition of sympathetic neuron growth via modulation of a BMP signaling pathway. Dev Biol 315: 404–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno M, Munoz R, Aroca F, Labarca M, Brandan E, Larrain J. 2005. Biglycan is a new extracellular component of the Chordin-BMP4 signaling pathway. EMBO J 24: 1397–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SA, Almeida AD, Tanaka H, Ohta K, Ohnuma S. 2007. Tsukushi modulates Xnr2, FGF and BMP signaling: Regulation of Xenopus germ layer formation. PLoS ONE 2: e1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M, Binder O, Wu Y, Aitsebaomo J, Ren R, Bode C, Bautch VL, Conlon FL, Patterson C. 2003. BMPER, a novel endothelial cell precursor-derived protein, antagonizes bone morphogenetic protein signaling and endothelial cell differentiation. Mol Cell Biol 23: 5664–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M, Yu Q, Bode C, Xiong JW, Patterson C. 2007. BMPER is a conserved regulator of hematopoietic and vascular development in zebrafish. J Mol Cell Cardiol 43: 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostert AK, Dijkstra PF, Jansen BRH, van Horn JR, de Graaf B, Heutink P, Lindhout D. 2003. Familial multiple epiphyseal dysplasia due to a matrilin-3 mutation: Further delineation of the phenotype including a 40 years follow up. Am J Med Genet Part A 120A: 490–497. [DOI] [PubMed] [Google Scholar]
- Mottershead DG, Sugimura S, Al-Musawi SL, Li JJ, Richani D, White MA, Martin GA, Trotta AP, Ritter LJ, Shi J, et al. 2015. Cumulin, an oocyte-secreted heterodimer of the transforming growth factor-β family, is a potent activator of granulosa cells and improve oocyte quality. J Biol Chem 290: 24007–24020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Sidis Y, Mahan A, Raher MJ, Xia Y, Rosen ED, Bloch KD, Thomas MK, Schneyer AL. 2007. FSTL3 deletion reveals roles for TGF-β family ligands in glucose and fat homeostasis in adults. Proc Natl Acad Sci 104: 1348–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WE, Korzhev M, Le Pennec G, Muller IM, Schroder HC. 2003. Origin of metazoan stem cell system in sponges: First approach to establish the model (Suberites domuncula). Biomol Eng 20: 369–379. [DOI] [PubMed] [Google Scholar]
- Muller P, Rogers KW, Jordan BM, Lee JS, Robson D, Ramanathan S, Schier AF. 2012. Differential diffusivity of Nodal and Lefty underlies a reaction-diffusion patterning system. Science 336: 721–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller I, Schonberger T, Schneider M, Borst O, Ziegler M, Seizer P, Leder C, Muller K, Lang M, Appenzeller F, et al. 2013. Gremlin-1 is an inhibitor of macrophage migration inhibitory factor and attenuates atherosclerotic plaque growth in ApoE–/– mice. J Biol Chem 288: 31635–31645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy C, Gannon M, Popoff SN. 2014. Connective tissue growth factor (CTGF/CCN2) negatively regulates BMP-2 induced osteoblast differentiation and signaling. J Cell Physiol 229: 672–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraoka O, Shimizu T, Yabe T, Nojima H, Bae YK, Hashimoto H, Hibi M. 2006. Sizzled controls dorso-ventral polarity by repressing cleavage of the Chordin protein. Nat Cell Biol 8: 329–338. [DOI] [PubMed] [Google Scholar]
- Murashima-Suginami A, Takahashi K, Sakata T, Tsukamoto H, Sugai M, Yanagita M, Shimizu A, Sakurai T, Slavkin HC, Bessho K. 2008. Enhanced BMP signaling results in supernumerary tooth formation in USAG-1 deficient mouse. Biochem Biophy Res Commun 369: 1012–1016. [DOI] [PubMed] [Google Scholar]
- Mytilinaiou M, Bano A, Nikitovic D, Berdiaki A, Voudouri K, Karamanos NK, Tzanakakis GN. 2013. Syndecan-2 is a key regulator of transforming growth factor β2/Smad2-mediated adhesion in fibrosarcoma cells. IUBMB Life 65: 134–143. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Takio K, Eto Y, Shibai H, Titani K, Sugino H. 1990. Activin-binding protein from rat ovary is follistatin. Sci 247: 836–838. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Sugino K, Titani K, Sugino H. 1991. Follistatin, an activin-binding protein, associates with heparan sulfate chains of proteoglycans on follicular granulosa cells. J Biol Chem 266: 19432–19437. [PubMed] [Google Scholar]
- Nakayama K. 1997. Furin: A mammalian subtilisin/Kex2p-like endoprotease involved in processing of a wide variety of precursor proteins. Biochem J 327: 625–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama N, Han CE, Scully S, Nishinakamura R, He C, Zeni L, Yamane H, Chang D, Yu D, Yokota T, et al. 2001. A novel chordin-like protein inhibitor for bone morphogenetic proteins expressed preferentially in mesenchymal cell lineages. Dev Biol 232: 372–387. [DOI] [PubMed] [Google Scholar]
- Nakayama N, Han CY, Cam L, Lee JI, Pretorius J, Fisher S, Rosenfeld R, Scully S, Nishinakamura R, Duryea D, et al. 2004. A novel chordin-like BMP inhibitor, CHL2, expressed preferentially in chondrocytes of developing cartilage and osteoarthritic joint cartilage. Development 131: 229–240. [DOI] [PubMed] [Google Scholar]
- Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. 2003. Dysregulation of TGF-β activation contributes to pathogenesis in Marfan syndrome. Nat Genet 33: 407–411. [DOI] [PubMed] [Google Scholar]
- Neul JL, Ferguson EL. 1998. Spatially restricted activation of the SAX receptor by SCW modulates DPP/TKV signaling in Drosophila dorsal-ventral patterning. Cell 95: 483–494. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Jamal J, Shimell MJ, Arora K, O’Connor MB. 1994. Characterization of tolloid-related-1: A BMP-1 like product that is required during larval and pupal stages of Drosophila development. Dev Biol 166: 569–586. [DOI] [PubMed] [Google Scholar]
- Nguyen M, Park S, Marques G, Arora K. 1998. Interpretation of a BMP activity gradient in Drosophila embryos depends on synergistic signaling by two type I receptors, SAX and TKV. Cell 95: 495–506. [DOI] [PubMed] [Google Scholar]
- Nguyen TQ, Roestenberg P, van Nieuwenhoven FA, Bovenschen N, Li Z, Xu L, Oliver N, Aten J, Joles JA, Vial C, et al. 2008. CTGF inhibits BMP-7 signaling in diabetic nephropathy. J Am Soc Nephrol 19: 2098–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitovic D, Chalkiadaki G, Berdidaki A, Aggelidakis J, Katonis P, Karamanos NK, Tzanakakis GN. 2011. Lumican regulates osteosarcoma cell adhesion by modulating TGFβ2 activity. Int J Biochem Cell Biol 43: 928–935. [DOI] [PubMed] [Google Scholar]
- Nishimatsu S, Thomsen GH. 1998. Ventral mesoderm induction and patterning by bone morphogenetic protein heterodimers in Xenopus embryos. Mech Dev 74: 75–88. [DOI] [PubMed] [Google Scholar]
- Nistala H, Lee-Arteaga S, Smaldone S, Siciliano G, Carta L, Ono RN, Sengle G, Arteaga-Solis E, Levasseur R, Ducy P, et al. 2010. Fibrillin-1 and -2 differentially modulate endogenous TGF-β and BMP bioavailability during bone formation. J Cell Biol 190: 1107–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan K, Kattamuri C, Luedeke DM, Deng X, Jagpal A, Zhang F, Linhardt RJ, Kenny AP, Zorn AM, Thompson TB. 2013. Structure of protein related to DAN and Cerberus: Insights into the mechanism of bone morphogenetic protein antagonism. Structure 21: 1417–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan K, Kattamuri C, Luedeke DM, Angerman EB, Rankin SA, Stevens ML, Zorn AM, Thompson TB. 2015. Structure of neuroblastoma suppressor of tumorigenicity 1 (NBL1): Insights for the functional variability across bone morphogenetic protein (BMP) antagonists. J Biol Chem 290: 4750–4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosaka T, Morita S, Kitamura H, Nakajima H, Shibata F, Morikawa Y, Kataoka Y, Ebihara Y, Kawashima T, Itoh T, et al. 2003. Mammalian twisted gastrulation is essential for skeleto-lymphogenesis. Mol Cell Biol 23: 2969–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell MP, Billings PC, Fiori JL, Deirmengian G, Roach HI, Shore EM, Kaplan FS. 2007. HSPG modulation of BMP signaling in fibrodysplasia ossificans progressiva cells. J Cell Biochem 102: 1493–1503. [DOI] [PubMed] [Google Scholar]
- O’Connor-McCourt MD, Wakefield LM. 1987. Latent transforming growth factor-β in serum. J Biol Chem 262: 14090–14099. [PubMed] [Google Scholar]
- Oelgeschlager M, Larrain J, Geissert D, De Robertis EM. 2000. The evolutionarily conserved BMP-binding protein Twisted gastrulation promotes BMP signalling. Nature 405: 757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelgeschlager M, Kuroda H, Reversade B, De Robertis EM. 2003a. Chordin is required for the Spemann organizer transplantation phenomenon in Xenopus embryos. Dev Cell 4: 219–230. [DOI] [PubMed] [Google Scholar]
- Oelgeschlager M, Reversade B, Larrain J, Little S, Mullins MC, De Robertis EM. 2003b. The pro-BMP activity of Twosted gastrulation is independent of BMP binding. Development 130: 4047–4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelgeschlager M, Tran U, Grubisic K, De Robertis EM. 2004. Identification of a second Xenopus Twisted Gastrulation gene. Int J Dev Biol 48: 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogita J, Isogai E, Sudo H, Sakiyama S, Nakagawara A, Koseki H. 2001. Expression of the Dan gene during chicken embryonic development. Mech Dev 109: 363–365. [DOI] [PubMed] [Google Scholar]
- Ohta K, Lupo G, Kuriyama S, Keynes R, Holt CE, Harris WA, Tanaka H, Ohnuma S. 2004. Tsukushi functions as an organizer inducer by inhibition of BMP activity in cooperation with chordin. Dev Cell 7: 347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta K, Kuriyama S, Okafuji T, Gejima R, Ohnuma S, Tanaka H. 2006. Tsukushi cooperates with VG1 to induce primitive streak and Hensen’s node formation in the chick embryo. Development 133: 3777–3786. [DOI] [PubMed] [Google Scholar]
- Ohta K, Ito A, Kuriyama S, Lupo G, Kosaka M, Ohnuma S, Nakagawa S, Tanaka H. 2011. Tsukushi functions as a Wnt signaling inhibitor by competing with Wnt2b for binding to transmembrane protein Frizzled 4. Proc Natl Acad Sci 108: 14962–14967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto O, Fujiwara S, Abe M, Sato Y. 1999. Dermatopontin interacts with transforming growth factor β and enhances its biological activity. Biochem J 337: 537–541. [PMC free article] [PubMed] [Google Scholar]
- Olivares GH, Carrasco H, Aroca F, Carvallo L, Segovia F, Larrain J. 2009. Syndecan-1 regulates BMP signaling and dorso-ventral patterning of the ectoderm during early Xenopus development. Dev Biol 329: 338–349. [DOI] [PubMed] [Google Scholar]
- Olivieri J, Smaldone S, Ramirez F. 2010. Fibrillin assemblies: Extracellular determinants of tissue formation and fibrosis. Fibrogenesis Tissue Repair 3: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Inkson CA, Kilts TM, Young MF. 2011. WISP-1/CCN4 regulates osteogenesis by enhancing BMP-2 activity. J Bone Miner Res 26: 193–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren A, Toporik A, Biton S, Almogy N, Eshel D, Bernstein J, Savitsky K, Rotman G. 2004. hCHL2, a novel chordin-related gene, displays differential expression and complex alternative splicing in human tissues and during myoblast and osteoblast maturation. Gene 331: 17–31. [DOI] [PubMed] [Google Scholar]
- Otsuka F, Moore RK, Iemura S, Ueno N, Shimasaki S. 2001. Follistatin inhibits the function of the oocyte-derived factor BMP-15. Biochem Biophys Res Commun 289: 961–966. [DOI] [PubMed] [Google Scholar]
- Ozaki T, Sakiyama S. 1993. Molecular cloning and characterization of a cDNA showing negative regulation in v-src-transformed 3Y1 rat fibroblasts. Proc Natl Acad Sci 90: 2593–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine-Saunders S, Viviano BL, Zupicich J, Skarnes WC, Saunders S. 2000. glypican-3 controls cellular responses to Bmp4 in limb patterning and skeletal development. Dev Biol 225: 179–187. [DOI] [PubMed] [Google Scholar]
- Paine-Saunders S, Viviano BL, Economides AN, Saunders S. 2002. Heparan sulfate proteoglycans retain Noggin at the cell surface. J Biol Chem 277: 2089–2096. [DOI] [PubMed] [Google Scholar]
- Pang K, Ryan JF, Baxevanis AD, Martindale MQ. 2011. Evolution of the TGF-β signaling pathway and its potential role in the ctenophore, Mnemiposis Leidyi. PLoS ONE 6: e24152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappano WN, Steiglitz BM, Scott IC, Keene DR, Greenspan DS. 2003. Use of Bmp1/Tll1 doubly homozygous null mice and proteomics to identify and validate in vivo substrates of bone morphogenetic protein 1/Tolloid-like metalloproteases. Mol Cell Biol 23: 4428–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paralkar VM, Weeks BS, Yu YM, Kleinman HK, Reddi AH. 1992. Recombinant human bone morphogenetic protein 2B stimulates PC12 cell differentiation: Potentiation and binding to type IV collagen. J Cell Biol 119: 1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce JJ, Penny G, Rossant J. 1999. A mouse cerberus/Dan-related gene family. Dev Biol 209: 98–110. [DOI] [PubMed] [Google Scholar]
- Pedersen GA, Charkraborty S, Steinhauser AL, Traub LM, Madsen M. 2010. AMN directs endocytosis of the intrinsic-factor vitamin B12 receptor cubam by engaging ARH or Dab2. Traffic 11: 706–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peerani R, Rao BM, Bauwens C, Yin T, Wood GA, Nagy A, Kumacheva E, Zandstra PW. 2007. Niche-mediated control of human embryonic stem cell self-renewal and differentiation. EMBO J 26: 4744–4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso CE, Umulis D, Kim YJ, O’Connor MB, Serpe M. 2011. Shaping BMP morphogen gradients through enzyme–substrate interactions. Dev Cell 21: 375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Li Q, Wigglesworth K, Rangarajan A, Kattamuri C, Peterson RT, Eppig JJ, Thompson TB, Matzuk MM. 2013. Growth differentiation factor 9:bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc Natl Acad Sci 110: E776–E785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi DJ, Wilkinson L, Kolle J, Sohaskey ML, Gillinder K, Piper MJ, McAvoy JW, Lovicu FJ, Little MH. 2007. Crim1KST264/KST264 mice display a disruption of the Crim1 gene resulting in perinatal lethality with defects in multiple organ systems. Dev Dyn 236: 502–511. [DOI] [PubMed] [Google Scholar]
- Pera E, De Robertis EM. 2000. A direct screen for secreted proteins in Xenopus embryos identifies distinct activities for the Wnt antagonists Crescent and Frzb-1. Mech Dev 96: 183–195. [DOI] [PubMed] [Google Scholar]
- Perbal B. 2004. CCN proteins: Multifunctional signalling regulators. Lancet 363: 62–64. [DOI] [PubMed] [Google Scholar]
- Perbal B. 2013. CCN proteins: A centralized communication network. J Cell Commun Signal 7: 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea-Gomez A, Vella FD, Shawlot W, Oulad-Abdelghani M, Chazaud C, Meno C, Pfister V, Chen L, Robertson E, Hamada H, et al. 2002. Nodal antagonists in the anterior visceral endoderm prevent the formation of multiple primitive streaks. Dev Cell 3: 745–756. [DOI] [PubMed] [Google Scholar]
- Pereira RC, Economides AN, Canalis E. 2000. Bone morphogenetic proteins induce gremlin, a protein that limits their activity in osteoblasts. Endocrinology 141: 4559–4563. [DOI] [PubMed] [Google Scholar]
- Peters I, Tossidou I, Achenbach J, Woroniecki R, Mengel M, Park JK, Paschy M, de Groot K, Haller H, Schiffer M. 2006. IGF-binding protein-3 modulates TGF-β/BMP-signaling in glomerular podocytes. J Am Soc Nephrol 17: 1644–1656. [DOI] [PubMed] [Google Scholar]
- Petryk A, Anderson RM, Jarcho MP, Leaf I, Carlson CS, Klingensmith J, Shawlot W, O’Connor MB. 2004. The mammalian twisted gastrulation gene functions in foregut and craniofacial development. Dev Biol 267: 374–386. [DOI] [PubMed] [Google Scholar]
- Petryk A, Shimmi O, Jia X, Carlson AE, Tervonen L, Jarcho MP, O’Connor MB, Gopalakrishnan R. 2005. Twisted gastrulation and chordin inhibit differentiation and mineralization in MC3T3-E1 osteoblast-like cells. Bone 36: 617–626. [DOI] [PubMed] [Google Scholar]
- Pham L, Beyer K, Jensen ED, Rodriguez JS, Davydova J, Yamamoto M, Petryk A, Gopalakrishnan R, Mansky KC. 2011. Bone morphogenetic protein 2 signaling in osteoclasts is negatively regulated by the BMP antagonist, twisted gastrulation. J Cell Biochem 112: 793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DJ, de Kretser DM. 1998. Follistatin: A multifunctional regulatory protein. Front Neuroendocrinol 19: 287–322. [DOI] [PubMed] [Google Scholar]
- Pi X, Schmitt CE, Xie L, Portbury AL, Wu Y, Lockyer P, Dyer LA, Moser M, Bu G, Flynn EJ III, et al. 2012. LRP1-dependent endocytic mechanism governs the signaling output of the Bmp system in endothelial cells and in angiogenesis. Circ Res 111: 564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, De Robertis EM. 1996. Dorsoventral patterning in Xenopus: Inhibition of ventral signals by direct binding of chordin to BMP-4. Cell 86: 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Lu B, Goodman S, Dale L, De Robertis EM. 1997. Cleavage of chordin by Xolloid metalloprotease suggests a role for proteolytic processing in the regulation of Spemann organizer activity. Cell 91: 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Leyns L, Bhattacharyya S, Grunz H, Bouwmeester T, De Robertis EM. 1999. The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature 397: 707–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploper D, Lee HX, De Robertis EM. 2011. Dorsal-ventral patterning: Crescent is a dorsally secreted Frizzled-related protein that competitively inhibits Tolloid proteases. Dev Biol 352: 317–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plouhinec JL, Zakin L, Moriyama Y, De Robertis EM. 2013. Chordin forms a self-organizing morphogen gradient in the extracellular space between ectoderm and mesoderm in the Xenopus embryo. Proc Natl Acad Sci 110: 20372–20379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponferrada VG, Fan J, Vallance JE, Hu S, Mamedova A, Rankin SA, Kofron M, Zorn AM, Hegde RS, Lang RA. 2012. CRIM1 complexes with β-catenin and cadherins, stabilizes cell-cell junctions and is critical for neural morphogenesis. PLoS ONE 7: e32635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radice PD, Mathieu P, Leal MC, Farias MI, Ferrari C, Puntel M, Salibe M, Chernomoretz A, Pitossi FJ. 2015. Fibulin-2 is a key mediator of the pro-neurogenic effect of TGF-β1on adult neural stem cells. Mol Cell Neurosci 67: 75–83. [DOI] [PubMed] [Google Scholar]
- Reichert S, Randall RA, Hill CS. 2013. A BMP regulatory network controls ectodermal cell fate decisions at the neural plate border. Development 140: 4435–4444. [DOI] [PubMed] [Google Scholar]
- Renard M, Holm T, Veith R, Callewaert BL, Ades LC, Baspinar O, Pickart A, Dasouki M, Hoyer J, Rauch A, et al. 2010. Altered TGFβ signaling and cardiovascular manifestations in patients with autosomal recessive cutis laxa type I caused by fibulin-4 deficiency. Eur J Hum Genet 18: 895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentzsch F, Zhang J, Kramer C, Sebald W, Hammerschmitdt M. 2006. Crossveinless 2 is an essential positive feedback regulator of Bmp signaling during zebrafish gastrulation. Development 133: 801–811. [DOI] [PubMed] [Google Scholar]
- Rentzsch F, Guder C, Vocke D, Hobmayer B, Holstein TW. 2007. An ancient chordin-like gene in organizer formation of Hydra. Proc Natl Acad Sci 104: 3249–3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reversade B, De Robertis EM. 2005. Regulation of ADMP and BMP2/4/7 at opposite embryonic poles generates a self-regulating morphogenetic field. Cell 123: 1147–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquelme C, Larrain J, Schonherr E, Henriquez JP, Kresse H, Brandan E. 2001. Antisense inhibition of decorin expression in myoblasts decreases cell responsiveness to transforming growth factor β and accelerates skeletal muscle differentiation. J Biol Chem 276: 3589–3596. [DOI] [PubMed] [Google Scholar]
- Robertson IB, Horiguchi M, Zilberberg L, Dabovic B, Hadjiolova K, Rifkin DB. 2015. Latent-TGF-β-binding proteins. Matrix Biol 47: 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Esteban C, Capdevila J, Economides AN, Pascual J, Ortiz A, Izpisua Belmonte JC. 1999. The novel Cer-like protein Caronte mediates the establishment of embryonic left–right asymmetry. Nature 401: 243–251. [DOI] [PubMed] [Google Scholar]
- Ross JJ, Shimmi O, Vilmos P, Petryk A, Kim H, Gaudenz K, Hermanson S, Ekker SC, O’Connor MB, Marsh JL. 2001. Twisted gastrulation is a conserved extracellular BMP antagonist. Nature 410: 479–483. [DOI] [PubMed] [Google Scholar]
- Rydziel S, Stadmeyer L, Zanotti S, Durant D, Smerdel-Ramoya A, Canalis E. 2007. Nephroblastoma overexpressed (Nov) inhibits osteoblastogenesis and causes osteopenia. J Biol Chem 282: 19762–19772. [DOI] [PubMed] [Google Scholar]
- Sakuta H, Suzuki R, Takahashi H, Kato A, Shintani T, Iemura S, Yamamoto TS, Ueno N, Noda M. 2001. Ventroptin: A BMP-4 antagonist expressed in a double-gradient pattern in the retina. Science 293: 111–115. [DOI] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM. 1994. Xenopus chordin: A novel dorsalizing factor activated by organizer-specific homeobox genes. Cell 79: 779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, De Robertis EM. 1995. Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature 376: 333–336. [DOI] [PubMed] [Google Scholar]
- Sawala A, Sutcliffe C, Ashe HL. 2012. Multistep molecular mechanism for Bone morphogenetic protein extracellular transport in the Drosophila embryo. Proc Natl Acad Sci 109: 11222–11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer L, Iozzo RV. 2008. Biological functions of the small leucine-rich proteoglycans: From genetics to signal transduction. J Biol Chem 283: 21305–21309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J, Francois V, Bier E, Kimelman D. 1995. Drosophila short gastrulation induces an ectopic axis in Xenopus: Evidence for conserved mechanisms of dorsal-ventral patterning. Development 121: 4319–4328. [DOI] [PubMed] [Google Scholar]
- Schneider VA, Mercola M. 1999. Spatially distinct head and heart inducers withinin the Xenopus organizer region. Curr Biol 9: 800–809. [DOI] [PubMed] [Google Scholar]
- Schneider VA, Mercola M. 2001. Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev 15: 304–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneyer AL, Rzucidlo DA, Sluss PM, Crowley WF Jr. 1994. Characterization of unique binding kinetics of follistatin and activin or inhibin in serum. Endocrinology 135: 667–674. [DOI] [PubMed] [Google Scholar]
- Schneyer A, Tortoriello D, Sidis Y, Keutmann H, Matsuzaki T, Holmes W. 2001. Follistatin-related protein (FSRP): A new member of the follistatin gene family. Mol Cell Endocrinol 180: 33–38. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Lee KJ, McMahon AP, Hammerschmidt M. 1997. The zebrafish organizer requires chordino. Nature 387: 862–863. [DOI] [PubMed] [Google Scholar]
- Scott IC, Blitz IL, Pappano WN, Imamura Y, Clark TG, Steiglitz BM, Thomas CL, Maas SA, Takahara K, Cho KWY, et al. 1999. Mammalian BMP-1/Tolloid-related metalloproteases, including novel family member mammalian Tolloid-like 1 have differential enzymatic activities and distributions of expression relevant to patterning and skeletogenesis. Dev Biol 213: 283–300. [DOI] [PubMed] [Google Scholar]
- Scott IC, Blitz IL, Pappano WN, Maas SA, Cho KWY, Greenspan DS. 2001. Homologues of Twisted gastrulation are extracellular cofactors in antagonism of BMP signalling. Nature 410: 475–478. [DOI] [PubMed] [Google Scholar]
- Seemann P, Brehm A, Konig J, Reissner C, Stricker S, Kuss P, Haupt J, Renninger S, Nickel J, Sebald W, et al. 2009. Mutations in GDF5 reveal a key residue mediating BMP inhibition by NOGGIN. PLoS Genet 5: e1000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarini PR, Nesbitt JE, Li D, Hays LG, Yates JR III, Carmichael DF. 2001. The low density lipoprotein receptor-related protein/α2-macroglobulin receptor is a receptor for connective tissue growth factor. J Biol Chem 276: 40659–40667. [DOI] [PubMed] [Google Scholar]
- Semenov M, Tamai K, He X. 2005. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem 280: 26770–26775. [DOI] [PubMed] [Google Scholar]
- Sengle G, Charbonneau NL, Ono RN, Sasaki T, Alvarez J, Keene DR, Bachinger HP, Sakai LY. 2008. Targeting of bone morphogenetic protein growth factor complexes to fibrillin. J Biol Chem 283: 13874–13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengle G, Ono RN, Sasaki T, Sakai LY. 2011. Prodomains of transforming growth factor β (TGFβ) superfamily members specify different functions: Extracellular matrix interactions and growth factor bioavailability. J Biol Chem 286: 5087–5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpe M, Ralston A, Blair SS, O’Connor MB. 2005. Matching catalytic activity to developmental function: Tolloid-related processes Sog in order to help specify the posterior crossvein in the Drosophila wing. Development 132: 2645–2656. [DOI] [PubMed] [Google Scholar]
- Serpe M, Umulis D, Ralston A, Chen J, Olson DJ, Avanesov A, Othmer H, O'Connor MB, Blair SS. 2008. The BMP-binding protein Crossveinless 2 is a short-range, concentration-dependent, biphasic modulator of BMP signaling in Drosophila. Dev Cell 14: 940–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawlot W, Deng JM, Behringer RR. 1998. Expression of the mouse cerberus-related gene, Cerr1, suggests a role in anterior neural induction and somitogenesis. Proc Natl Acad Sci 95: 6198–6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawlot W, Deng JM, Wakamiya M, Behringer RR. 2000. The cerberus-related gene, Cerr1, is not essential for mouse head formation. Genesis 26: 253–258. [PubMed] [Google Scholar]
- Shi DL, Savona C, Gagnon J, Cochet C, Chambaz EM, Feige JJ. 1990. Transforming growth factor-β stimulates the expression of α2-macroglobulin by cultured bovine adrenocortical cells. J Biol Chem 265: 2881–2887. [PubMed] [Google Scholar]
- Shibata M, Itoh M, Hikasa H, Taira S, Taira M. 2005. Role of crescent in convergent extension movements by modulating Wnt signaling in early Xenopus embryogenesis. Mech Dev 122: 1322–1339. [DOI] [PubMed] [Google Scholar]
- Shimell MJ, Ferguson EL, Childs SR, O’Connor MB. 1991. The Drosophila dorsal-ventral patterning gene tolloid is related to human bone morphogenetic protein 1. Cell 67: 469–481. [DOI] [PubMed] [Google Scholar]
- Shimmi O, O’Connor MB. 2003. Physical properties of Tld, Sog, Tsg, and Dpp protein interactions are predicted to help create a sharp boundary in Bmp signals during dorsoventral patterning of the Drosophila embryo. Development 130: 4673–4682. [DOI] [PubMed] [Google Scholar]
- Shimmi O, Umulis D, Othmer H, O’Connor MB. 2005a. Facilitated transport of a Dpp/Scw heterodimer by Sog/Tsg leads to robust patterning of the Drosophila blastoderm embryo. Cell 120: 873–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimmi O, Ralston A, Blair SS, O’Connor MB. 2005b. The crossveinless gene encodes a new member of the Twisted gastrulation family of BMP-binding proteins which, with Short gastrulation, promotes BMP signaling in the crossveins of the Drosophila wing. Dev Biol 282: 70–83. [DOI] [PubMed] [Google Scholar]
- Sidis Y, Schneyer AL, Sluss PM, Johnson LN, Keutmann HT. 2001. Follistatin: Essential role for the N-terminal domain in activin binding and neutralization. J Biol Chem 276: 17718–17726. [DOI] [PubMed] [Google Scholar]
- Sidis Y, Tortoriello DV, Holmes WE, Pan Y, Keutmann HT, Schneyer AL. 2002. Follistatin-relatd protein and follistatin differentially neutralize endogenous vs. exogenous activin. Endocrinology 143: 1613–1624. [DOI] [PubMed] [Google Scholar]
- Sidis Y, Mukherjee A, Keutmann H, Delbaere A, Sadatsuki M, Schneyer A. 2006. Biological activities of follistatin isoforms and Follistatin-like 3 is dependent on differential cell surface binding and specificity for activin, myostatin, and bone morphogenetic proteins. Endocrinology 147: 3586–3597. [DOI] [PubMed] [Google Scholar]
- Silva AC, Filipe M, Kuerner KM, Steinbeisser H, Belo JA. 2003. Endogenous Cerberus activity is required for anterior head specification in Xenopus. Development 130: 4943–4953. [DOI] [PubMed] [Google Scholar]
- Simmons DG, Kennedy TG. 2002. Uterine sensitization-associated gene-1: A novel gene induced within the rat endometrium at the time of uterine receptivity/sensitization for the decidual cell reaction. Biol Reprod 67: 1638–1645. [DOI] [PubMed] [Google Scholar]
- Simpson EH, Johnson DK, Hunsicker P, Suffolk R, Jordan SA, Jackson IJ. 1999. The mouse Cer1 (Cerberus related or homologue) gene is not required for anterior pattern formation. Dev Biol 213: 202–206. [DOI] [PubMed] [Google Scholar]
- Smith WC, Harland RM. 1992. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell 70: 829–840. [DOI] [PubMed] [Google Scholar]
- Smith WC, Knecht AK, Wu M, Harland RM. 1993. Secreted noggin protein mimics the Spemann organizer in dorsalizing Xenopus mesoderm. Nature 361: 547–549. [DOI] [PubMed] [Google Scholar]
- Smith WC, McKendry R, Ribisi S Jr, Harland RM. 1995. A nodal-related gene defines a physical and functional domain within the Spemann organizer. Cell 82: 37–46. [DOI] [PubMed] [Google Scholar]
- Song K, Krause C, Shi S, Patterson M, Suto R, Grgurevic L, Vukicevic S, van Dinther M, Falb D, ten Dijke P, et al. 2010. Identification of a key residue mediating bone morphogenetic protein (BMP)-6 resistance to noggin inhibition allows for engineered BMPs with superior agonist activity. J Biol Chem 285: 12169–12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soofi A, Zhang P, Dressler GR. 2013. Kielin/chordin-like protein attenuates both acute and chronic renal injury. J Am Soc Nephrol 24: 897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Rashka KE, Bier E. 2002. Creation of a Sog morphogen gradient in the Drosophila embryo. Dev Cell 2: 91–101. [DOI] [PubMed] [Google Scholar]
- Stabile H, Mitola S, Moroni E, Belleri M, Nicoli S, Coltrini D, Peri F, Pessi A, Orsatti L, Talamo F, et al. 2007. Bone morphogenic protein antagonist Drm/gremlin is a novel proangiogenic factor. Blood 109: 1834–1840. [DOI] [PubMed] [Google Scholar]
- Stafford DA, Brunet LJ, Khokha MK, Economides AN, Harland RM. 2011. Cooperative activity of Noggin and Gremlin 1 in axial skeletal development. Development 138: 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E, Biben C, Kotecha S, Fabri L, Tajbakhsh S, Wang CC, Hatzistavrou T, Roberts B, Drinkwater C, Lah M, et al. 1998. DAN is a secreted glycoprotein related to Xenopus cerberus. Mech Dev 77: 173–184. [DOI] [PubMed] [Google Scholar]
- Stanley EG, Biben C, Allison J, Hartley L, Wicks IP, Campbell IK, McKinley M, Barnett L, Koentgen F, Robb L, et al. 2000. Targeted insertion of a lacZ reporter gene into the mouse Cer1 locus reveals complex and dynamic expression during embryogenesis. Genesis 26: 259–264. [DOI] [PubMed] [Google Scholar]
- Stefansson SE, Jonsson H, Ingvarsson T, Manolescu I, Jonsson HH, Olafsdottir G, Palsdottir E, Stefansdottire G, Sveinbjornsdottir G, Frigge ML, et al. 2003. Genome-wide scan for hand osteoarthritis: A novel mutation in matrilin-3. Am J Hum Genet 72: 1448–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stottmann RW, Anderson RM, Klingensmith J. 2001. The BMP antagonists chordin and noggin have essential but redundant roles in mouse mandibular outgrowth. Dev Biol 240: 457–473. [DOI] [PubMed] [Google Scholar]
- Stouffer GA, LaMarre J, Gonias SL, Owens GK. 1993. Activated α2-macroglobulin and transforming growth factor-β1 induce a synergistic smooth muscle cell proliferative response. J Biol Chem 268: 18340–18344. [PubMed] [Google Scholar]
- Strate I, Tessadori F, Bakkers J. 2015. Glypican4 promotes cardiac specification and differentiation by attenuating canonical Wnt and Bmp signaling. Development 142: 1767–1776. [DOI] [PubMed] [Google Scholar]
- Streit A, Stern CD. 1999. Mesoderm patterning and somite formation during node regression: Differential effects of chordin and noggin. Mech Dev 85: 85–96. [DOI] [PubMed] [Google Scholar]
- Streit A, Lee KJ, Woo I, Roberts C, Jessell TM, Stern CD. 1998. Chordin regulates primitive streak development and the stability of induced neural cells, but is not sufficient for neural induction in the chick embryo. Development 125: 507–519. [DOI] [PubMed] [Google Scholar]
- Strope S, Rivi R, Metzger T, Manova K, Lacy E. 2004. Mouse amnionless, which is required for primitive streak assembly, mediates cell-surface localization and endocytic function of cubilin on visceral endoderm and kidney proximal tubules. Development 131: 4787–4795. [DOI] [PubMed] [Google Scholar]
- Stuckenholz C, Lu L, Thakur PC, Choi TY, Shin D, Bahary N. 2013. Sfrp5 modulates both Wnt and BMP signaling and regulates gastrointestinal organogenesis in the zebrafish, Danio rerio. PLoS ONE 8: e62470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo S, Avsian-Kretchmer O, Wang LS, Hsueh AJ. 2004. Protein related to DAN and cerberus is a bone morphogenetic protein antagonist that participates in ovarian paracrine regulation. J Biol Chem 279: 23134–23141. [DOI] [PubMed] [Google Scholar]
- Sugino K, Kurosawa N, Nakamura T, Takio K, Shimasaki S, Ling N, Titani K, Sugino H. 1993. Molecular heterogeneity of follistatin, an activin-binding protein. Higher affinity of the carboxyl-terminal truncated forms for heparan sulfate proteoglycans on the ovarian granulosa cell. J Biol Chem 268: 15579–15587. [PubMed] [Google Scholar]
- Sun BI, Bush SM, Collins-Racie LA, LaVallie ER, DiBlasio-Smith EA, Wolfman NM, McCoy JM, Sive HL. 1999. derriere: A TGF-β family member required for posterior development in Xenopus. Development 126: 1467–1482. [DOI] [PubMed] [Google Scholar]
- Sun J, Zhuang FF, Mullersman JE, Chen H, Robertson EJ, Warburton D, Liu YH, Shi W. 2006. BMP4 activation and secretion are negatively regulated by an intracellular gremlin-BMP4 interaction. J Biol Chem 281: 29349–29356. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Kaneko E, Maeda J, Ueno N. 1997. Mesoderm induction by BMP-4 and -7 heterodimers. Biochem Biophys Res Commun 232: 153–156. [DOI] [PubMed] [Google Scholar]
- Sylva M, Moorman AFM, van den Hoff MJB. 2013. Follistatin-like 1 in vertebrate development. Birth Defects Res C Embryo Today 99: 61–69. [DOI] [PubMed] [Google Scholar]
- Szlama G, Kondas K, Trexler M, Patthy L. 2010. WFIKKN1 and WFIKKN2 bind growth factors TGFβ1, BMP2 and BMP4 but do not inhibit their signalling activity. FEBS J 277: 5040–5050. [DOI] [PubMed] [Google Scholar]
- Szlama G, Trexler M, Patthy L. 2013. Latent myostatin has significant activity and this activity is controlled more efficiently by WFIKKN1 than by WFIKKN2. FEBS J 280: 3822–3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei Y, Ozawa Y, Sato M, Watanabe A, Tabata T. 2004. Three Drosophila EXT genes shape morphogen gradients through synthesis of heparan sulfate proteoglycans. Development 131: 73–82. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Kodama Y, Matsumoto T. 1994. Bone matrix decorin binds transforming growth factor-β and enhances its bioactivity. J Biol Chem 269: 32634–32638. [PubMed] [Google Scholar]
- Tanaka M, Murakami K, Ozaki S, Imura Y, Tong XP, Watanabe T, Sawaki T, Kawanami T, Kawabata D, Fujii T, et al. 2010. DIP2 disco-interacting protein 2 homolog A (Drosophila) is a candidate receptor for follistatin-related protein/follistatin-like 1—Analysis of their binding with TGF-β superfamily proteins. FEBS J 277: 4278–4289. [DOI] [PubMed] [Google Scholar]
- Tanegashima K, Yokota C, Takahashi S, Asashima M. 2000. Expression cloning of Xantivin, a Xenopus lefty/antivin-related gene, involved in the regulation of activin signaling during mesoderm induction. Mech Dev 99: 3–14. [DOI] [PubMed] [Google Scholar]
- Tanegashima K, Haramoto Y, Yokota C, Takahashi S, Asashima M. 2004. Xantivin suppresses the activity of EGF-CFC genes to regulate nodal signaling. Int J Dev Biol 48: 275–283. [DOI] [PubMed] [Google Scholar]
- Taneja-Bageshwar S, Gumienny TL. 2012. Two functional domains in C. elegans glypican LON-2 can independently inhibit BMP-like signaling. Dev Biol 371: 66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares AT, Andrade S, Silva AC, Belo JA. 2007. Cerberus is a feedback inhibitor of Nodal asymmetric signaling in the chick embryo. Development 134: 2051–2060. [DOI] [PubMed] [Google Scholar]
- Taylor NA, Van de Ven WJM, Creemers JWM. 2003. Curbing activation: Proprotein convertases in homeostasis pathology. FASEB J 17: 1215–1227. [DOI] [PubMed] [Google Scholar]
- Thisse C, Thisse B. 1999. Antivin, a novel and divergent member of the TGFβ superfamily, negatively regulates mesoderm induction. Development 126: 229–240. [DOI] [PubMed] [Google Scholar]
- Thompson TB, Lerch TF, Cook RW, Woodruff TK, Jardetzky TS. 2005. The structure of the follistatin:activin complex reveals antagonism of both type I and type II receptor binding. Dev Cell 9: 535–543. [DOI] [PubMed] [Google Scholar]
- Tian H, Liu J, Chen J, Gatza ML, Blobe GC. 2015. Fibulin-3 is a novel TGF-β pathway inhibitor in the breast cancer microenvironment. Oncogene 34: 5635–5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R, Sasaki T, Kostka G, Chu ML. 2003. Fibulins: A versatile family of extracellular matrix proteins. Nat Rev Mol Cell Biol 4: 479–489. [DOI] [PubMed] [Google Scholar]
- Topol LZ, Marx M, Laugier D, Bogdanova NN, Boubnov NV, Clausen PA, Calothy G, Blair DG. 1997. Identification of drm, a novel gene whose expression is suppressed in transformed cells and which can inhibit growth of normal but not transformed cells in culture. Mol Cell Biol 17: 4801–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topol LZ, Bardot B, Zhang Q, Resau J, Huillard E, Marx M, Calothy G, Blair DG. 2000. Biosynthesis, post-translation modification, and functional characterization of Drm/Gremlin. J Biol Chem 275: 8785–8793. [DOI] [PubMed] [Google Scholar]
- Troilo H, Zuk AV, Tunnicliffe RB, Wohl AP, Berry R, Collins RF, Jowitt TA, Sengle G, Baldock C. 2014. Nanosclae structure of the BMP antagonist chordin supports cooperative BMP binding. Proc Natl Acad Sci 111: 13063–13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troilo H, Barrett AL, Wohl AP, Jowitt TA, Collins RF, Bayley CP, Zuk AV, Sengle G, Baldock C. 2015. The role of chordin fragments generated by partial tolloid cleavage in regulating BMP activity. Biochem Soc Trans 43: 795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida K, Arai KY, Kuramoto Y, Yamakawa N, Hasegawa Y, Sugino H. 2000. Identification and characterization of a novel follistatin-like protein as a binding protein for the TGF-β family. J Biol Chem 275: 40788–40796. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Kamimura K, Nakato H, Archer M, Staatz W, Fox B, Humphrey M, Olson S, Futch T, Kaluza V, et al. 1999. The cell-surface proteoglycan Dally regulates Wingless signalling in Drosophila. Nature 400: 276–280. [DOI] [PubMed] [Google Scholar]
- Turing A. 1952. The chemical basis of morphogenesis. Phil Trans B 237: 37–72. [Google Scholar]
- Tzachanis D, Li L, Lafuente EM, Berezovskaya A, Freeman GJ, Boussiotis VA. 2007. Twisted gastrulation (Tsg) is regulated by Tob and enhances TGF-β signaling in activated T lymphocytes. Blood 109: 2944–2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzahor E, Kempf H, Mootoosamy RC, Poon AC, Abzhanov A, Tabin CJ, Dietrich S, Lassar AB. 2003. Antagonists of Wnt and BMP signaling promote the formation of vertebrate head muscle. Genes Dev 17: 3087–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa L, Tabibzadeh S. 2001. Lefty inhibits receptor-regulated Smad phosphorylation induced by the activated transforming growth factor-β receptor. J Biol Chem 276: 21397–21404. [DOI] [PubMed] [Google Scholar]
- Valera E, Isaacs MJ, Kawakami Y, Izpisua Belmonte JC, Choe S. 2010. BMP-2/6 heterodimer is more effective than BMP-2 or BMP-6 homodimers as inductor of differentiation of human embryonic stem cells. PLoS ONE 5: e11167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bezooijen RL, Roelen BA, Visser A, van der Wee-Pals L, de Wilt E, Karperien M, Hamersma H, Papapoulos SE, ten Dijke P, Lowik CW. 2004. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med 199: 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Benzooijen RL, Svensson JP, Eefting D, Visser A, van der Horst G, Karperine M, Quax PH, Vrieling H, Papapoulos SE, ten Dijke P, et al. 2007. Wnt but not BMP signaling is involved in the inhibitory action of sclerostin on BMP-stimulated bone formation. J Bone Miner Res 22: 19–28. [DOI] [PubMed] [Google Scholar]
- van Boxtel AL, Chesebro JE, Heliot C, Ramel MC, Stone RK, Hill CS. 2015. A temporal window for signal activation dictates the dimensions of a nodal signaling domain. Dev Cell 35: 175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheyden JM, Sun X. 2008. An Fgf/Gremlin inhibitory feedback loop triggers termination of limb bud outgrowth. Nature 454: 638–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilmos P, Sousa-Neves R, Lukacsovich T, Marsh JL. 2005. crossveinless defines a new family of Twisted-gastrulation-like modulators of bone morphogenetic protein signalling. EMBO Rep 6: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviano BL, Paine-Saunders S, Gasiunas N, Gallagher J, Saunders S. 2004. Domain-specific modification of heparan sulfate by Qsulf1 modulates the binding of the bone morphogenetic protein antagonist Noggin. J Biol Chem 279: 5604–5611. [DOI] [PubMed] [Google Scholar]
- Vogel P, Liu J, Platt KA, Read RW, Thiel M, Vance RB, Brommage R. 2015. Malformation of incisor teeth in Grem2–/– mice. Vet Pathol 52: 224–229. [DOI] [PubMed] [Google Scholar]
- Vonica A, Brivanlou AH. 2006. An obligatory caravanserai stop on the silk road to neural induction: Inhibition of BMP/GDF signaling. Sem Cell Dev Biol 17: 117–132. [DOI] [PubMed] [Google Scholar]
- Vuilleumier R, Springhorn A, Patterson L, Koidl S, Hammerschmidt M, Affolter M, Pyrowalakis G. 2010. Control of Dpp morphogen signalling by a secreted feedback regulator. Nat Cell Biol 12: 611–617. [DOI] [PubMed] [Google Scholar]
- Wadhwa S, Embree MC, Bi Y, Young MF. 2004. Regulation, regulatory activities, and function of biglycan. Crit Rev Eukaryot Gene Expr 14: 301–315. [DOI] [PubMed] [Google Scholar]
- Wagner DS, Mullins MC. 2002. Modulation of BMP activity in dorsal-ventral pattern formation by the chordin and ogon antagonists. Dev Biol 245: 109–123. [DOI] [PubMed] [Google Scholar]
- Wakefield LM, Smith DM, Flanders KC, Sporn MB. 1988. Latent transforming growth factor-β from human platelets. J Biol Chem 263: 7646–7654. [PubMed] [Google Scholar]
- Wang YC, Ferguson EL. 2005. Spatial bistability of Dpp-receptor interactions during Drosophila dorsal-ventral patterning. Nature 434: 229–234. [DOI] [PubMed] [Google Scholar]
- Wang X, Bornslaeger EA, Haub O, Tomihara-Newberger C, Lonberg N, Dinulos MB, Disteche CM, Copeland N, Gilbert DJ, Jenkins NA, et al. 1996. A candidate gene for the amnionless gastrulation stage mouse mutation encodes a TRAF-related protein. Dev Biol 177: 274–290. [DOI] [PubMed] [Google Scholar]
- Wang X, Harris RE, Bayston LJ, Ashe HL. 2008. Type IV collagens regulate BMP signalling in Drosophila. Nature 455: 72–77. [DOI] [PubMed] [Google Scholar]
- Wardle FC, Welch JV, Dale L. 1999. Bone morphogenetic protein 1 regulates dorsal-ventral patterning in early Xenopus embryos by degrading chordin, a BMP4 antagonist. Mech Dev 86: 75–85. [DOI] [PubMed] [Google Scholar]
- Warren SM, Brunet LJ, Harland RM, Economides AN, Longaker MT. 2003. The BMP antagonist noggin regulates cranial suture fusion. Nature 422: 625–629. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Weaver AM, Atkins-Brady TL, Gonias SL. 1996. Proteinases are isoform-specific regulators of the binding of transforming growth factor β to α2-macroglobulin. Biochem J 320: 551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb TR, Matarin M, Gardner JC, Kelberman D, Hassan H, Ang W, Michaelides M, Ruddle JB, Pennell CE, Yazar S, et al. 2012. X-linked megalocornea caused by mutations in CHRDL1 identifies an essential role for Ventroptin in anterior segment development. Am J Hum Genet 90: 247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson L, Kolle G, Wen D, Piper M, Scott J, Little M. 2003. CRIM1 regulates the rate of processing and delivery of bone morphogenetic proteins to the cell surface. J Biol Chem 278: 34181–34188. [DOI] [PubMed] [Google Scholar]
- Wills A, Harland RM, Khokha MK. 2006. Twisted gastrulation is required for forebrain specification and cooperates with Chordin to inhibit BMP signaling during X. tropicalis gastrulation. Dev Biol 289: 166–178. [DOI] [PubMed] [Google Scholar]
- Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, et al. 2003. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 22: 6267–6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler DG, Yu C, Geoghegan JC, Ojala EW, Skonier JE, Shpektor D, Sutherland MK, Latham JA. 2004. Noggin and sclerostin bone morphogenetic protein antagonists form a mutually inhibitory complex. J Biol Chem 279: 36293–36298. [DOI] [PubMed] [Google Scholar]
- Winstanley J, Sawala A, Baldock C, Ashe HL. 2015. Synthetic enzyme-substrate tethering obviates the Tolloid–ECM interaction during Drosophila BMP gradient formation. eLife 4: 05508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfman NM, McPherron AC, Nappano WN, Davies MV, Song K, Tomkinson KN, Wright JF, Zhao L, Sebald SM, Greenspan DS, et al. 2003. Activation of latent myostatin by the BMP-1/tolloid family of metalloproteases. Proc Natl Acad Sci 100: 15842–15846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Horgan CE, Carr O, Owens RT, Iozzo RV, Lechner BE. 2014. Biglycan and decorin differentially regulate signaling in the fetal membranes. Matrix Biol 35: 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Fisher S. 2005. Twisted gastrulation enhances BMP signaling through chordin dependent and independent mechanisms. Development 132: 383–391. [DOI] [PubMed] [Google Scholar]
- Xu T, Bianco P, Fisher LW, Longenecker G, Smith E, Goldstein S, Bonadio J, Boskey A, Heegaard AM, Sommer B, et al. 1998. Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nature Genet 20: 78–82. [DOI] [PubMed] [Google Scholar]
- Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, Kelley MW, Jiang L, Tasman W, Zhang K, et al. 2004. Vascular development in the retina and inner ear: Control by Norrie and frizzled-4, a high-affinity ligand-receptor pair. Cell 116: 883–895. [DOI] [PubMed] [Google Scholar]
- Xu J, Qi X, Gong J, Yu M, Zhang F, Sha H, Gao X. 2012a. Fstl1 antagonizes BMP signaling and regulates ureter development. PLoS ONE 7: e32554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Cheng F, Liang J, Wu W, Zhang J. 2012b. Maternal xNorrie, a canonical Wnt signaling agonist and a TGF-β antagonist, control early neuroectoderm specification in Xenopus. PLoS Biol 10: e1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe T, Shimizu T, Muraoka O, Bae YK, Hirata T, Nojima H, Kawakami A, Hirano T, Hibi M. 2003. Ogon/Secreted Frizzled functions as a negative feedback regulator of Bmp signaling. Development 130: 2705–2716. [DOI] [PubMed] [Google Scholar]
- Yamada S, Nakamura J, Asada M, Takase M, Matsusaka T, Iguchi T, Yamada R, Tanaka M, Higashi AY, Okuda T, et al. 2014. Twisted gastrulation, a BMP antagonist, exacerbates podocyte injury. PLoS ONE 9: e89135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Mann DM, Ruoslahti E. 1990. Negative regulation of transforming growth factor-β by the proteoglycan decorin. Nature 346: 281–284. [DOI] [PubMed] [Google Scholar]
- Yamamoto TS, Iemura S, Takagi C, Shimasaki S, Ueno N. 2000. Characterization of follistatin isoforms in early Xenopus embryogenesis. Int J Dev Biol 44: 341–348. [PubMed] [Google Scholar]
- Yamanaka O, Yuan Y, Coulson-Thomas VJ, Gesteira TF, Call MK, Zhang Y, Zhang J, Chang SH, Xie C, Liu CY, et al. 2013. Lumican binds ALK5 to promote epithelium wound healing. PLoS ONE 8: e82730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanishi T, Katsu K, Funahashi J, Yumoto E, Yokoucchi Y. 2007. Dan is required for normal morphogenesis and patterning in the developing chick inner ear. Dev Growth Differ 49: 13–26. [DOI] [PubMed] [Google Scholar]
- Yamashita H, ten Dijke P, Huylebroeck D, Sampath TK, Andries M, Smith JC, Heldin CH, Miyazono K. 1995. Osteogenic protein-1 binds to activin type II receptors and induces certain activin-like effects. J Cell Biol 130: 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagita M, Oka M, Watabe T, Iguchi H, Niida A, Takahashi S, Akiyama T, Miyazono K, Yanagisawa M, Sakurai T. 2004. USAG-1: A bone morphogenetic protein antagonist abundantly expressed in the kidney. Biochem Biophys Res Commun 316: 490–500. [DOI] [PubMed] [Google Scholar]
- Yanagita M, Okuda T, Endo S, Tanaka M, Takahashi K, Sugiyama F, Kunita S, Takahashi S, Fukatsu A, Yanagisawa M, et al. 2006. Uterine sensitization-associated gene-1 (USAG-1), a novel BMP antagonist expressed in the kidney, accelerates tubular injury. J Clin Invest 116: 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Trehan SK, Guan Y, Sun C, Moore DC, Jayasuriya CT, Chen Q. 2014. Matrilin-3 inhibits chondrocyte hypertrophy asa a bone morphogenetic protein-2 antagonist. J Biol Chem 289: 34768–34779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Bennett BJ, Wang X, Rosenfeld ME, Giachelli C, Lusis AJ, Bostrom KI. 2010. Inhibition of bone morphogenetic proteins against atherosclerosis and vascular calcification. Circ Res 107: 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Jumabay M, Wang A, Bostrom KI. 2011. Matrix Gla protein deficiency causes arteriovenous malformations in mice. J Clin Invest 121: 2993–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Jumabay M, Ly A, Radparvar M, Wang AH, Abdmaulen R, Bostrom KI. 2012. Crossveinless 2 regulates bone morphogenetic protein 9 in human and mouse vascular endothelium. Blood 119: 5037–5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Hu W, Guo F, Zhang W, Wang J, Chen A. 2012. Glycosaminoglycan chains of biglycan promote bone morphogenetic protein-4-induced osteoblast differentiation. Int J Mol Med 30: 1075–1080. [DOI] [PubMed] [Google Scholar]
- Yeo CY, Whitman M. 2001. Nodal signals to Smads through Cripto-dependent and Cripto-independent mechanisms. Mol Cell 7: 949–957. [DOI] [PubMed] [Google Scholar]
- Yokota C, Kofron M, Zuck M, Houston DW, Isaacs H, Asashima M, Wylie CC, Heasman J. 2003. A novel role for a nodal-related protein; Xnr3 regulates convergent extension movements via the FGF receptor. Development 130: 2199–2212. [DOI] [PubMed] [Google Scholar]
- Yokouchi Y, Vogan KJ, Pearse RV II, Tabin CJ. 1999. Antagonistic signaling by Caronte, a novel Cerberus-related gene, establishes left–right asymmetric gene expression. Cell 98: 573–583. [DOI] [PubMed] [Google Scholar]
- Young MF, Bi Y, Ameye L, Chen X. 2003. Biglycan knockout mice: New models for musculoskeletal diseases. Glycoconj J 19: 257–262. [DOI] [PubMed] [Google Scholar]
- Yu K, Srinivasan S, Shimmi O, Biehs B, Rashka KE, Kimelman D, O’Connor MB, Bier E. 2000. Processing of the Drosophila Sog protein creates a novel BMP inhibitory activity. Development 127: 2143–2154. [DOI] [PubMed] [Google Scholar]
- Zacchigna L, Vecchione C, Notte A, Cordenonsi M, Dupont S, Maretto S, Cifelli G, Ferrari A, Maffei A, Fabbro C, et al. 2006. Emilin1 links TGF-β maturation to blood pressue homeostasis. Cell 124: 929–942. [DOI] [PubMed] [Google Scholar]
- Zakin L, De Robertis EM. 2004. Inactivation of mouse Twisted gastrulation reveals its role in promoting Bmp4 activity during forebrain development. Development 131: 413–424. [DOI] [PubMed] [Google Scholar]
- Zakin L, Reversade B, Kuroda H, Lyons KM, De Robertis EM. 2005. Sirenomelia in Bmp7 and Tsg compound mutant mice: Requirement for Bmp signaling in the development of ventral posterior mesoderm. Development 132: 2489–2499. [DOI] [PubMed] [Google Scholar]
- Zakin L, Metzinger CA, Chang EY, Coffinier C, De Robertis EM. 2008. Development of the vertebral morphogenetic field in the mouse: Interactions between Crossveinless-2 and Twisted Gastrulation. Dev Biol 323: 6–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakin L, Chang EY, Plouhinec JL, De Robertis EM. 2010. Crossveinless-2 is required for the relocalization of Chordin protein within the vertebral field in mouse embryos. Dev Biol 347: 204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti M, Braghetta P, Sabatelli P, Mura I, Doliana R, Colombatti A, Volpin D, Bonaldo P, Bressan GM. 2004. EMILIN-1 deficiency induces elastogenesis and vascular cell defects. Mol Cell Biol 24: 638–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebboudj AF, Imura M, Bostrom K. 2002. Matrix GLA protein, a regulatory protein for bone morphogenetic protein-2. J Biol Chem 277: 4388–4394. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang Q. 2009. Bone morphogenetic protein-7 and gremlin: New emerging therapeutic targets for diabetic nephropathy. Biochem Biophys Res Commun 383: 1–3. [DOI] [PubMed] [Google Scholar]
- Zhang D, Ferguson CM, O’Keefe RJ, Puzas JE, Rosier RN, Reynolds PR. 2002. A role for the BMP antagonist chordin in endochondral ossification. J Bone Miner Res 17: 293–300. [DOI] [PubMed] [Google Scholar]
- Zhang JL, Huang Y, Qiu LY, Nickel J, Sebald W. 2007. von Willebrand factor type C domain-containing proteins regulate bone morphogenetic protein signaling through different recognition mechanisms. J Biol Chem 282: 20002–20014. [DOI] [PubMed] [Google Scholar]
- Zhang JL, Qiu LY, Kotzsch A, Weidauer S, Patterson L, Hammerschmidt M, Sebald W, Mueller TD. 2008. Crystal structure analysis reveals how the chordin family member crossveinless 2 blocks BMP-2 receptor binding. Dev Cell 14: 739–750. [DOI] [PubMed] [Google Scholar]
- Zhang JL, Patterson LJ, Qiu LY, Graziussi D, Sebald W, Hammerschmidt M. 2010. Binding between crossveinless-2 and chordin von Willebrand factor type C domains promotes BMP signaling by blocking chordin activity. PLoS ONE 5: e12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Zhao Y, Chao Y, Muir K, Han Z. 2013. Cubilin and amnionless mediate protein reabsorption in Drosophila nephrocytes. J Am Soc Nephrol 24: 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Lu L, Zhou J, Li Y, Liu Y, Clemmons DR, Duan C. 2011. IGF binding protein 3 exerts its ligand-independent action by antagonizing BMP in zebrafish embryos. J Cell Sci 124: 1925–1935. [DOI] [PubMed] [Google Scholar]
- Zhu L, Marvin MJ, Gardiner A, Lassar AB, Mercola M, Stern CD, Levin M. 1999a. Cerberus regulates left–right asymmetry of the embryonic head and heart. Curr Biol 9: 931–938. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Oganesian A, Keene DR, Sandell LJ. 1999b. Type IIA procollagen containing the cysteine-rich amino propeptide is deposited in the extracellular matrix of prechondrogenic tissue and binds to TGF-β1 and BMP-2. J Cell Biol 144: 1069–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman LB, De Jesus-Escobar JM, Harland RM. 1996. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell 86: 599–606. [DOI] [PubMed] [Google Scholar]
- Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, McPherron AC, Wolfman NM, Lee SJ. 2002. Induction of cachexia in mice by systemically administered myostatin. Science 29: 1486–1488. [DOI] [PubMed] [Google Scholar]
- Zong Z, Tees S, Miyanji F, Fauth C, Reilly C, Lopez E, Tredwell S, Goldberg YP, Delaney A, Eydoux P, et al. 2015. BMPER variants associated with a novel, attenuated subtype of diaphanospondylodysostosis. J Hum Genet 60: 743–747. [DOI] [PubMed] [Google Scholar]
- Zuniga A, Haramis AP, McMahon AP, Zeller R. 1999. Signal relay by BMP antagonism controls the SHH/FGF4 feedback loop in vertebrate limb buds. Nature 401: 598–602. [DOI] [PubMed] [Google Scholar]
- Zusman S, Wieschaus E. 1985. Requirement for zygotic gene activity during gastrulation in Drosophila melanogaster. Dev Biol 111: 359–371. [DOI] [PubMed] [Google Scholar]