Abstract
Object
In traumatic brain injury (TBI) patients, hypothermia therapy has not shown efficacy in multicenter clinical trials. With the post-hoc data from the latest clinical trial (NABIS:H II), we hypothesized that hypothermia may be beneficial in the rat acute subdural hematoma (ASDH) model by blunting the effects of ischemic/ reperfusional (I/R) injury. The major aim of our study was to test the efficacy of temperature management in reducing brain damage after ASDH.
Methods
Rats were induced with ASDH and placed into (1) Normothermia group (37°C) (2) Early hypothermia group; head and body temperature reduced to 33°C at 30 minutes prior to craniotomy (3) Late hypothermia group; temperature was lowered to 33°C at 30 minutes after decompression (4) Sham group; no ASDH and underwent only craniotomy with normothermia. To assess of neuronal and glial cell damage, we analyzed microdialysate (MD ; using 100kD probe) concentrations of: glial fibrillary acidic protein (GFAP) and ubiquitin carboxyl-terminal hydrolase-L1 (UCH-L1). Fluoro-Jade B (FJB) positive neurons and injury volume with 2,3,5-triphenyltetrazolium chloride (TTC) staining were also measured.
Results
In the early phase of reperfusion (30min- 2.5 hrs after decompression), extracellular UCH-L1 in the early hypothermia group was significantly lower than in the normothermia. (Early; 4.9±1.0 ng/dl, Late; 35.2±12.1 ng/dl, Normo; 50.20± 28.3 ng/dl, Sham; 3.1±1.3 ng/dl, Early vs Normo; p < 0.01, Sham vs Normo; p < 0.01, analyzed with ANOVA followed by a post-hoc Bonferroni’s test ). In the late phase of reperfusion (> 2.5hrs after decompression), extracellular GFAP in the early hypothermia group was also lower than in the normothermia and late hypothermia groups (Early; 5.5±2.9 ng/dl, Late; 7.4±3.4 ng/dl, Normo; 15.3±8.4 ng/dl, Sham; 3.3±1.0 ng/dl, Normo vs Sham; p < 0.01). The number of FJB positive cells in early hypothermia group was significantly smaller than in normothermia group (Normo vs Early: 774,588 ± 162,173 vs 180,903 ± 42,212, p<0.05). Also, the injury area and volume were smaller in the early hypothermia group in which hypothermia was induced before craniotomy and cerebral reperfusion (115.2±15.4 mm3 in early hypothermia group, 344.7±29.1 mm3 in late hypothermia group, 311.2±79.2 mm3 in Normothermia group p<0.05).
Conclusion
In conclusion, the data suggests early, preoperatively induced hypothermia could mediate the reduction of neuronal and glial damage in reperfusional phase of ischemic/reperfusional brain injury.
Keywords: ischemic/reperfusional injury, hypothermia, traumatic brain injury, microdialysis, biomarker, neuronal degeneration
INTRODUCTION
A wealth of basic and clinical research exists regarding the pathomechanisms and treatment of severe traumatic brain injury (TBI). However, despite much effort, the prognosis of TBI patients with acute subdural and intracranial hematomas remains poor 41. About 50,000 people die and at least 5.3 million live with disabilities related to TBI in the USA, and subdural hematoma is a large component of this mortality and morbidity22,73. Further, it is recognized as a leading cause of mortality and morbidity in young adults globally.13,16,46 One therapeutic method, posttraumatic hypothermia, has been shown in previous studies to improve histopathological and behavioral consequences of TBI using many experimental models,10,19,20,27,32,34,55 and different delivery paradigms. Unfortunately, however therapeutic hypothermia has not shown overall efficacy in multi-center trials probably due, at least in part, to the heterogeneous nature of the brain damage mechanisms in TBI patients12,20,28,47,49,65,70. In a recent large severe TBI clinical trial,13 post-hoc subgroup analysis demonstrated the possibility that early induced hypothermia might have a specific beneficial effect in the patients with acute subdural hematomas (ASDH). This patient subgroup with ASDH demonstrate the worst outcome of any category of severe TBI, and 60% will die or remain severity disabled48,76,77.
Based upon this data from the Clifton study, we hypothesized that hypothermia may be beneficial to acute intracranial hematomas via blunting the effects of ischemic /reperfusional (I/R) injury. To date, no well-controlled preclinical studies have been undertaken to test the efficacy of early cooling in an acute hematoma model of severe TBI. We therefore used the well characterized rat ASDH model, to evaluate the efficacy of temperature management in reducing brain damage after ASDH.
We measured the effect of early and delayed moderate hypothermia (33°C) by using cell counts (Fluorojade staining) of neuron dying due to activation of both necrotic and apoptotic cell death pathways, and by an independent volumetric estimation of the amount of brain damage, using the mitochondria triphenyltetrazolium chloride (TTC) staining method55. Since there is no effective method to predict outcome in ASDH patients, we also tested the ability of a new neural biomarker, Ubiquitin Carboxyl-Terminal Hydrolase-L1 (UCH-L1) and a glial marker, Glial Fibrillary Acidic Protein (GFAP) to determine the amount of cell death, and the effect of the hypothermic therapy, in this model.
The prognostic utility of brain biomarkers, such as S100β or NSE, has been studied extensively in severe TBI patients 6,37,43,63,67. However, due to lack of organ specificity and a short half-life 1,64, serum sampling of these biomarkers maybe unable to differentiate brain injury from multiple trauma, including skeletal injuries60,62. Additional influences such as blood brain barrier dysfunction8,61 or renal dysfunction 30,31 make using serum concentrations alone problematic, for S100β.
For a more direct estimation of brain damage, the microdialysis (MD) technique can be utilized. MD has demonstrated promise in neuromonitoring of severely brain injured patients. Extracellular biomarkers, such as glucose, lactate, pyruvate, glycerol, and glutamate have been measured with 20 kD cut-off MD probes in the clinical setting. Lactate/Pyruvate ratio (LPR) has been said to be effective in the detection of ischemic events4,71,80. The LPR, however, is influenced by the primary injury and delayed events, and it is often difficult to correlate LPR values with cellular/ neuronal survival and therefore patient outcome 42,54. For more direct understanding of neuronal and glial viability, we measured two recently discovered biomarkers using 100kD cut-off MD probes in the rat ASDH model. Glial Fibrillary Acidic Protein (GFAP) and Ubiquitin Carboxyl-Terminal Hydrolase-L1 (UCH-L1) were studied as markers of glial and neuronal cell damage respectively. GFAP is said to be one of the more clinically reliable serum biomarkers for head injury 24,45,75. Furthermore, the molecular weight of GFAP is 50 kD, and this allows a high recovery rate with the 100kD MD technique. UCH-L1 has also been said to be a sensitive and specific neuronal biomarker, able to predict injury severity and mortality after severe TBI in humans44,50,51,56. UCH-L1 is a compact cytosolic protein with a low molecular weight (~24kD), allowing measurement of UCH-L1 concentrations in extracellular fluid, relatively easily with 100kD MD techniques. In the past there have been no studies, laboratory or clinical that correlate MD levels of UCH-L1 and GFAP with their predictive ability for outcome. Our secondary aim was therefore to analyze these biomarkers in serum and extracellular fluid (measured with MD), comparing their levels before and after craniotomy and establishing their value as markers for monitoring injury and therapeutic response in this subdural hematoma brain injury model.
MATERIALS AND METHODS
Animal Groups
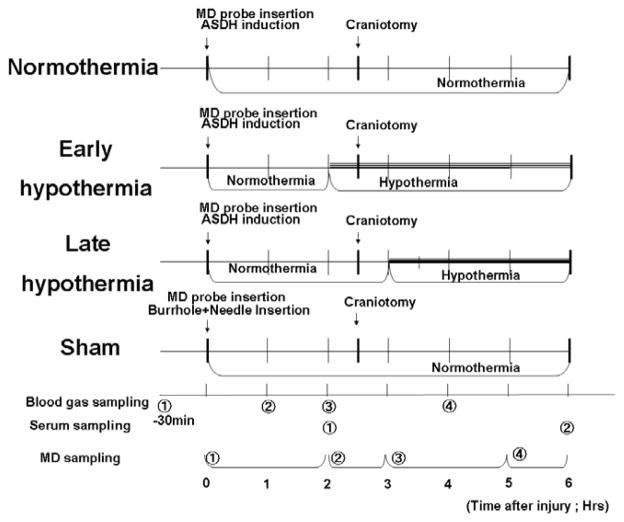
Adult male Sprague-Dawley rats (300 to 350 g; Harlan Laboratories, Indianapolis, IN) were randomly divided into four treatment groups. They underwent subdural hematoma induction, brain microdialysis catheter placement, tail artery cannulation, and temperature manipulation as described below (Fig. 1); (1) In the normothermia group, head temperature was maintained at normothermic levels (37°C) during the course of the experiment. (2) The early hypothermia group underwent hypothermia induction (33°C) thirty minutes prior to decompressive craniotomy and removal of the hematoma to mimic a clinical situation in which hypothermia induction could be started as soon as ASDH is diagnosed by CT scan and while the operating room is prepared. Hypothermic treatment was continued 3 hours after decompression. (3) The late hypothermia group received hypothermia induction (33°C) thirty minutes after decompression surgery and it was maintained for 3 hours. (4) The sham group did not receive induced subdural hematoma but underwent craniotomy. Their head temperature was maintained at normothermic levels (37°C) during the course of the experiment.
Fig. 1.
Definition of each treatment group and their time course.
Experimental rats were randomly allocated into four groups. In normothermia group and sham groups, the head temperature was controlled at 37°C during the course of experiment. In the early hypothermia group, mild hypothermia (33°C) was induced thirty minutes before craniotomy. In the late hypothermia group, the same level of hypothermia (33°C) was induced thirty minutes after craniotomy. Microdialysate were collected in the ischemic phase (0–2h after hematoma induction), the craniotomy phase (30min before and after craniotomy), early reperfusion phase (0.5–2.5h after craniotomy) and late reperfusion phase (2.5–3.5h). Abbreviations in this figure: ASDH; acute subdural hematoma, MD; Microdialysis.
Surgical Procedure
The animals were maintained on a 12-h/12-h light/dark cycle and given food ad libitum. All animal procedures followed guidelines established by the National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals and were approved by the University of Miami’s Institutional Animal Care and Use Committee.
All animals were anesthetized initially with 3% isoflurane, 70% N2O and a balance of 30% O2 delivered in a Perspex chamber, with subsequent endotracheal intubation and mechanical respiration as previously described33,38. The tail artery was cannulated with a polyethylene catheter for blood pressure monitoring, blood sampling and obtaining the autologous blood needed for ASDH induction. Blood gas analysis was performed four times throughout the procedure (Fig. 1, Table 1), to control ventilation of the animal. A PaO2 of around 100–150 mmHg and a PaCO2 of 30–40mmHg were aimed for to mimic clinical conditions.
TABLE 1.
Physiologic variables in experimental time course (Mean±SEM).
| 0.5 hr before ASDH induction |
1 hr after ASDH induction |
2 hr after ASDH induction (0.5 hr after craniotomy) |
4 hr after ASDH induction (1.5 hr after craniotomy) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normo | Early | Late | Sham | Normo | Early | Late | Sham | Normo | Early | Late | Sham | Normo | Early | Late | Sham | |
| Mean Arterial Pressure (mmHg) | 116.8 ± 20.5 | 112.5 ± 18.3 | 119.5 ± 17.4 | 116.8 ± 20.5 | 110.7 ± 14.8 | 116.0 ± 18.8 | 116.4 ± 14.9 | 109.8 ± 9.7 | 116.5 ± 12.6 | 112.8 ± 13.5 | 116.4 ± 13.3 | 109.6 ± 8.2 | 105.2 ± 13.7 | 116.5 ± 12.1 | 110.9 ± 16.6 | 109.3 ± 9.2 |
| pH | 7.463 ± 0.062 | 7.459 ± 0.034 | 7.467 ± 0.033 | 7.500 ± 0.046 | 7.484 ± 0.035 | 7.454 ± 0.032 | 7.456 ± 0.050 | 7.484 ± 0.035 | 7.459 ± 0.029 | 7.454 ± 0.028 | 7.433 ± 0.067 | 7.459 ± 0.029 | 7.432 ± 0.041 | 7.401 ± 0.050 | 7.388 ± 0.043 | 7.442 ± O.039 |
| PaO2 (mmHg) | 103.1 ± 15.8 | 100.8 ± 12.5 | 122.9 ± 34.7 | 97.4 ± 12.6 | 125.6 ± 16.9 | 117.9 ± 14.8 | 129.1 ± 22.8 | 125.6 ± 16.9 | 128.1 ± 26.8 | 122.9 ± 13.7 | 123.2 ± 29.2 | 128.1 ± 26.8 | 137.4 ± 26.9 | 130.2 ± 18.5 | 146.8 ± 32.4 | 137.4 ± 26.9 |
| PaCO2 (mmHg) | 35.5 ± 2.5 | 36.7 ± 2.9 | 36.3 ± 4.2 | 34.0 ± 4.0 | 33.5 ± 3.5 | 34.4 ± 4.1 | 33.7 ± 4.7 | 32.7 ± 4.3 | 33.7 ± 1.5 | 33.0 ± 2.2 | 35.9 ± 4.9 | 32.4 ± 3.3 | 34.1 ± 3.1 | 34.3 ± 2.3 | 34.5 ± 2.9 | 34.1 ± 3.1 |
Abbreviations: Normo; normothermia group, Early; early hypothermia group, Late; late hypothermia group, Sham; sham-operated group, ASDH; acute subdural hematoma.
Temperature Manipulations
The head and rectal temperatures were maintained at 33°C in the early- and late- hypothermia groups by a combination of a cooling / heating system (Gaymar Medi-Therm III, Gaymar Industries, Inc. NY, USA) and local cooling fan/heating lamp. In the normothermia and sham rat groups, head and rectal temperatures were maintained at 37°C during the course of the experiment. Head temperature was measured by a thermistor probe placed in the right temporalis, and estimated as brain temperature29.
Subdural hematoma induction
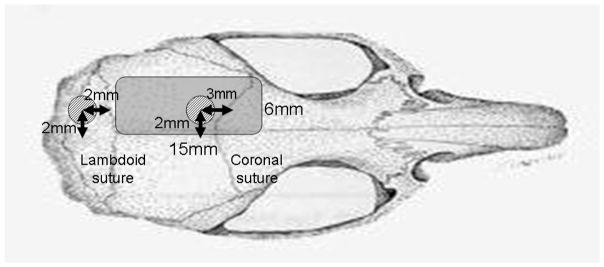
Details of the method used to produce subdural hematoma are described in previous reports by our group18,38,39. A midline scalp incision was made and a 3 mm diameter burrhole was drilled 2 mm to the left of the sagittal suture and 3 mm behind the coronal suture (Fig. 2). With the aid of an operating microscope, the dura was incised and a blunt-tipped, J-shaped, No. 23 gauge needle inserted into the subdural space. Quick-setting cyanoacrylate glue was used to set the needle and seal the burrhole. The hematoma was then induced by injecting 350 μl non-heparinized autologous blood into the subdural space over a period of 7 minutes, allowing it to clot in situ. After injection, the induction needle was cut off and sealed. In the sham-treated group, the needle was set in place but no blood was injected.
Fig. 2.
Schematic representation of craniotomy and burrhole placement.
The area of craniotomy extended from the lambdoid suture to 15 mm ahead of the lambdoid. The medial and lateral borders were the sagittal suture and superior temporal line, respectively. This made the width approximately 8 mm. The first burrhole, for hematoma induction, was 3mm in diameter and placed 2 mm to the left of the sagittal suture and 3 mm behind the coronal suture. The second burrhole, which allowed for insertion of the microdialysis probe, was 3 mm in diameter was placed 2 mm to the left of the sagittal suture and 2 mm behind the lambdoid suture. The probe was inserted toward the front of the head, at a 10 degree angle from the horizontal and at a 6mm depth from the brain surface.
Two and a half hours after induction of the subdural hematoma, a craniotomy measuring 15 × 6 mm was made using a saline cooled-dental drill (Fig. 2). The hematoma was then removed using saline irrigation and forceps after widely opening the dura. Hemostasis of superficial blood vessels was achieved using bipolar diathermy if needed. The scalp was closed over the craniotomy without replacing the bone to mimic clinical practice of decompressive craniotomy.
Extracellular biomarker measurement with microdialysis (n=7, each group)
We used a CMA 12 MD probe (CMA Microdialysis, Solna, Sweden), which had an active membrane length of 4 mm and a molecular weight cut-off at 100 kD. One hour before ASDH induction, a second burrhole was drilled 2 mm to the left of the sagittal suture and 2 mm behind the lambdoid suture for microdialysis probe insertion (Fig 2). The probe was inserted into this burrhole at 10 degrees from the horizontal plane and at a depth of 6 mm, as previously described 40. This insertion technique made for optimal placement of the dialyzing membrane within the cerebral subcortical “penumbra” area40. Probes were precalibrated in vitro to ensure that interprobe variation was minimal. The dialysis probes were continuously perfused with physiological saline with 4% BSA added at 0.3 μl/min. Microdialysis (MD) sampling was delayed by 1 hour after insertion to allow the brain to adapt to the presence of the probe. Dialysate samples were then started to produce from 2.5 hours before craniotomy and sampled four times, i.e., ischemic phase (0–2h after ASDH induction), craniotomy phase (0.5h before and after craniotomy), early reperfusion phase (0.5–2.5 after craniotomy), and late reperfusion phase (2.5–3.5h after craniotomy) (Fig. 1). Frozen microdialysate vials were later analyzed for biomarkers. Also, at similar two time points in the ischemic and reperfusional phase, 0.6 ml of blood was collected (Fig. 1), and centrifuged at 2,500 rpm for 10 min. From these centrifuged blood samples, serum were saved and stored at − 80°C for biomarker analyses, in parallel with the MD samples.
Quantitative detection of UCH-L1 in serum and microdialysate was performed using proprietary SW enzyme-linked immunosorbent assay (ELISA) (Banyan Biomarkers, Inc. FL, USA) and recombinant UCH-L1 as standard 68. For quantification of GFAP in serum and microdialysate, a novel rat ELISA assay (Banyan Biomarkers, Inc. FL, USA) was used.
Degenerative neuron counting with Fluoro-Jade B staining (n=7, each group)
Twenty four hours after the surgery, animals were anesthetized (3% isoflurane, 70% nitrous oxide, and 30% oxygen for 5 min) and perfused transcardially with isotonic saline for 2 min (80mL), and then with 4% paraformaldehyde in 0.1M sodium phosphate buffer (PB), pH 7.4 (360mL). The brains were embedded in paraffin and serial sectioned (20 μm thick). One in five of the serial sections was stained with Fluoro-Jade B (FJB). Sections were air dried at 50°C, deparaffinized by incubation in 100% ethanol for 3 min and 70% ethanol for 1 min and then washed with distilled water. Sections were then incubated for 10 min in 0.06% potassium permanganate for 10 min followed by 30 min in FJB solution (Millipore., USA). Sections were dried on a slide warmer, cleared with xylene, and coverslipped using cytoseal (Richard Allan Scientific USA).
To determine neuronal degeneration in bilateral cortex and hippocampus, serial sections (16μm thick after shrinkage, 360 μm apart) from −2.2 to −4.0 mm to bregma were quantified in an unbiased, systematic manner by a blind observer using the physical fractionator method following the workflow in StereoInvestigator 7.50.1 software (MicroBrightField, Inc.) with an Axiophot microscope (Carl Zeiss MicroImaging, Inc.). In each rat, cortex area to be counted is defined as the subcortical parietal area between midline to rhinal fissure. The hippocampus and subcortex were contoured at 5×, and then a counting grid of 450×450 μm was placed. Using a 60×60-μm counting frame, FJB positive cells were counted in randomly placed sampling sites with a 63×objective.
Measurement of Injury Volume with 2,3,5-triphenyltetrazolium chloride (TTC) staining (n=7, each group)
We quantified the ischemic area and volume of injury by 2,3,5-triphenyltetrazolium chloride (TTC) staining was performed at 24 h after the craniotomy in each of the four treatment groups. The brain was cut coronally every 2 mm using a lucite brain matrix (Tedpella), and each section was stained with 2% of TTC for 30 min 55. The injured area was defined as an unstained, white area in each section. For every section, a high-resolution picture was taken by digital camera (DSC-T70, Sony, Japan) and the volume of injury was calculated by using an image-analyzing software (lenaraf220, Vector Japan Co, Tokyo). The total volume of injury was calculated by multiplying the area in each section by the distance between the sections (2mm).
Statistical analysis
All data were expressed as mean ± standard error of the mean (SEM). FJB positive cell counts and injury volume were analyzed with a one-way analysis of variance (ANOVA), followed by a post-hoc Bonferroni’s test, Physiological data and ischemic area were compared using a two-way ANOVA followed by a post-hoc Bonferroni’s test. Differences were considered significant at p<0.05. Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA USA)
RESULTS
Physiological Parameters
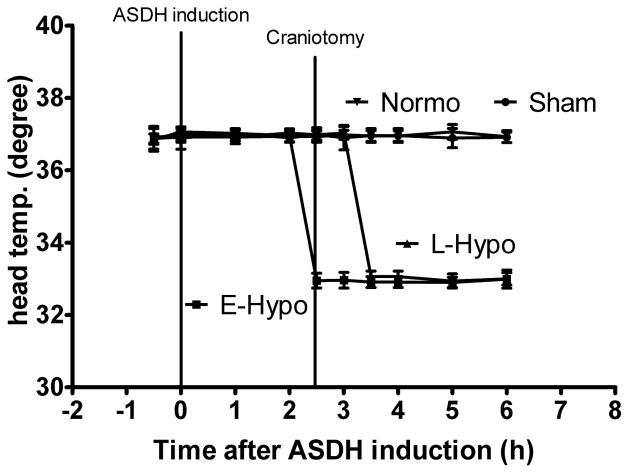
Physiological parameters of mean arterial blood pressure (MABP), arterial blood pH, PaO2 and PaCO2 are given in Table.1. Over the course of the experiments, all physiological values were within the normal range, and there were no significant differences between the various experimental groups in terms of MABP, pH, PaO2, and PaCO2. (Table.1). As expected, head (brain) temperatures were significantly lower in the early hypothermia group than in the other at the time of decompressive craniotomy and 30 min after craniotomy (p<0.001) and in the early hypothermia and the late hypothermia groups compared to the sham and normothermia groups at 1h, 1.5h, 2.5h and 3.5h after craniotomy (Fig. 3).
Fig. 3.
Head temperature manipulation. In the early hypothermia group, mild hypothermia (33°C) was induced 30 minutes before craniotomy. In the late hypothermia group, cooling began 30 minutes after craniotomy. At the time of decompressive craniotomy, head temperature was significantly different between early and late hypothermia groups. Values are expressed as mean ± SEM.
Abbreviations; ASDH; acute subdural hematoma, E-Hypo; early hypothermia therapy (33°C), L-Hypo; late hypothermia therapy, Normo; normothermia therapy, Sham; sham operated group.
Degenerative neuron counting
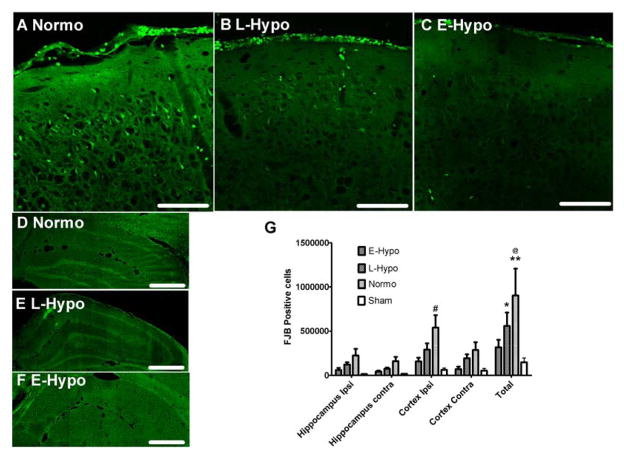
The number of FJB positive cells was predominantly greater in the ipsilateral and subcortical regions under the ASDH (Fig. 4G). Compared to the normothermia group, the number of FJB positive cells in the early hypothermia group was significantly less, especially in the ipsilateral subcortical region (Fig. 4G). (Normo vs Early: 774,588 ± 162,173 vs 180,903 ± 42,212, p<0.05).
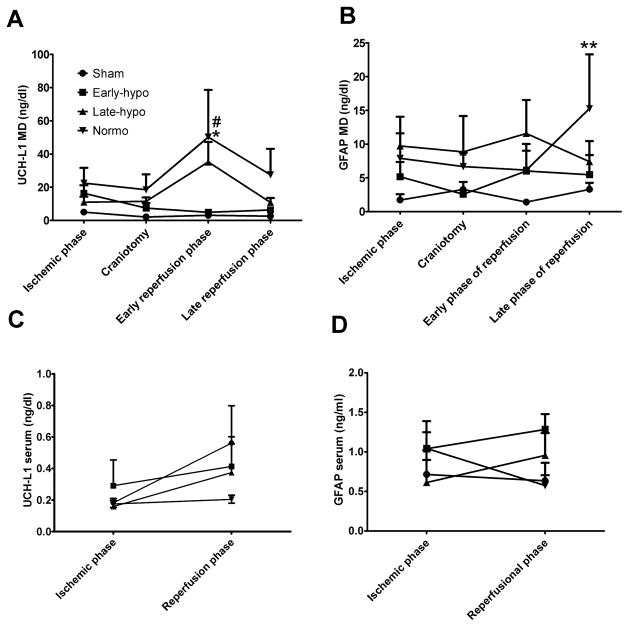
FIG. 4.
Biomarker concentrations. Ubiquitin carboxyl-terminal hydrolase -L1 (UCH-L1) and GFAP concentrations in microdialysate (A,B) and in serum (C,D). In early phase of reperfusion UCH-L1 MD concentration in normothermia group was highest and significantly higher than sham rat group ( *; p<0.01). Also, UCH-L1 MD in early hypothermia group was significantly lower than normothermia group ( #; p<0.01). GFAP concentration in normothermia was highest and peaked in the late phase of reperfusion (**; p<0.05 vs sham). The differences between means of serum values were not significantly different.
Abbreviations; E-Hypo; early hypothermia group, L-Hypo; late hypothermia group, Normo; normothermia group.
Injury area and volume measurement with TTC staining
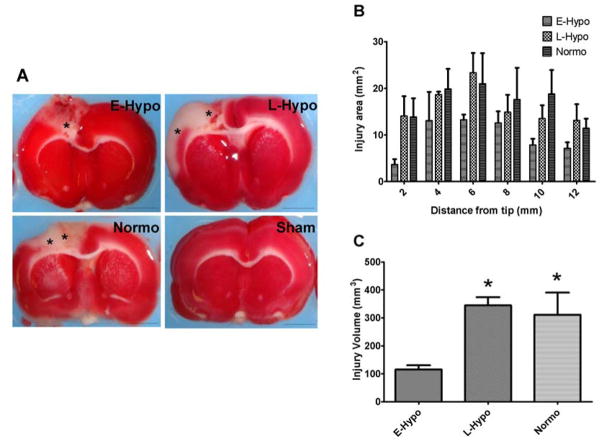
In the early hypothermia group, the injury area was smaller than in the late hypothermia and normothermia groups in each section (Fig. 5A, B). Moreover, the total injury volume in the early hypothermia group was significantly smaller than in the other treatment groups (115.2±15.4 mm3 in early hypothermia group, 344.7±29.1 mm3 in late hypothermia group, 311.2±79.2 mm3 in Normothermia group p<0.05, Fig. 5C).
Fig. 5.
Quantification of degenerating cells with Fluoro Jade B (FJB) staining.
Images of FJB staining cells in ipsilateral (Injured) cortex (−3.2mm from bregma) in normothermia (A), late (B), and early induced hypothermia (C) (Scale bars =50μm). (D,E,F) Ipsilateral hippocampus images in −3.2mm from bregma (CA1–3, and dentate gyrus, Scale bars = 500μm) in each treatment group. (G) FJB positive cell counting in each region (Mean±SEM). In normothermia group, many degenerating cells were observed in all groups, especially ipsilateral cortex. Early induced hypothermia appears to mitigate the I/R injury. (#p<0.05 vs E-Hypo, @p<0.01 vs E-Hypo, *p< 0.01 vs Sham, **p< 0.001 vs Sham)
Abbreviations; E-Hypo; early hypothermia group, L-Hypo; late hypothermia group, Normo; normothermia group.
Concentrations of biomarkers in serum and cerebral microdialysate
In the microdialysate UCH-L1 concentration in the early induced hypothermia group was lower than in the normothermia and late hypothermia groups on the early phase of reperfusion (30min- 2.5 hrs after decompression; Early; 4.9±1.0 ng/dl, Late; 35.2±12.1 ng/dl, Normo; 50.20±28.3 ng/dl, Sham; 3.1±1.3 ng/dl, Early vs Normo; p < 0.01, Sham vs Normo; p < 0.01 : Fig. 6A). Also, on the late phase of reperfusion (> 2.5hrs after decompression), extracellular GFAP in the early hypothermia group was lower than in the normothermia and late hypothermia groups (Early; 5.5±2.9 ng/dl, Late; 7.4±3.4 ng/dl, Normo; 15.3±8.4 ng/dl, Sham; 3.3±1.0 ng/dl, Normo vs Sham; p < 0.01 ; Fig. 6B).
Fig. 6.
TTC (2,3,5-triphenyltetrazolium chloride) volumetry in each treatment group
(A) An examples of TTC injury area in each treatment group, 6mm from the tip (±1.0 mm from bregma). In TTC staining, ischemic injuries were identified with unstained, white areas (asterisk). In sham rat, we could not find any ischemia in these pictures. On the other hand, in late induced hypothermia and normothermia group, we could identify large ischemic lesions compared to early hypothermia group. (scale bars = 5mm ) Abbreviation, E-Hypo; early induced hypothermia group, L-Hypo; late induced hypothermia group, Normo; normothermia group,
(B) Ischemic area in each sections (Mean±SEM). In early induced hypothermia group, injury volume was smaller across all sections.
(C) Ischemic volume. In early- hypothermia group, ischemic area was significantly smaller than late hypothermia and normothermia group (*p<0.05).
Abbreviation, E-Hypo; early induced hypothermia group, L-Hypo; late induced hypothermia group, Normo; normothermia group.
In serum UCH-L1 and GFAP concentrations, there were no significant differences among treatment groups on the ischemic and reperfusional phase (Fig. 6C, D)
DISCUSSION
Early-induced hypothermia reduces neuronal degeneration and injury volume after acute subdural hematoma in a rat model
We have shown that early-induced, pre-reperfusional hypothermia was associated with reduced neuronal degeneration and injury volume when compared to normothermia or late hypothermia therapy in this ASDH decompression rat model, based upon both FJB positive cell counts (Fig. 4), and injury volumetry (Fig. 5)
The pathology of I/R injury can be separated into two mechanisms that play out over time. The ischemia-induced cellular dysfunction is followed by reperfusion-induced free radical production57,72. Reperfusion following ischemia results in a short period of excessive free radical production. Experimental measurements of post I/R free radical production demonstrate that oxygen- and carbon- centered free radical production peaks within 5 min of reperfusion 9 and that hydroxyl generation peaks within 15 minutes 35. Thus, mitochondrial free radical production is an important target and provides the first theoretical “window of opportunity” for hypothermia treatment. We speculate that intra-ischemic cooling was beneficial in our model because it achieve lower blood temperature at the time of reperfusion (see Fig. 3).
A second window of opportunity for hypothermia targets the inflammatory cascade and cell death pathways of apoptosis and necrosis, which is initiated by reperfusion. These probably center on mitochondrial dysfunction and previous data has suggested that a transition in mitochondrial permeability may be the “point of no return” in both cell death pathways 52. Apoptosis is ATP dependent, whereas necrosis is not. Activation of caspases and proteases, and the release of mitochondrial cytochrome c, are features of apoptosis 52. These cell death processes represent the second “ therapeutic opportunity” for hypothermia. Despite much basic and clinical research concerning I/R brain injury, the mechanisms of neuronal protection in hypothermia therapy remain unknown. Therapeutic hypothermia is believed to confer protection against I/R injury through multiple mechanisms, such as by reducing cellular metabolism and oxygen demand while maintaining acceptable ATP levels21.
Additionally, hypothermia attenuates abnormal free radical production 66, improves cellular ion handling, and improves cellular pH balance 57. Hypothermia also reduces cell death and inflammatory signaling 79. Although different tissues have different sensitivities to ischemia, I/R injury has been observed in many tissue types3.
In our results, we were able to show the beneficial effects of early induced hypothermia, upon brain damage associated with an acute subdural hematoma. Early mild hypothermia may reduce reperfusional neuronal damage after decompression of focal mass in ASDH rat model, by reducing neurotoxic mechanisms including oxidative stress, free radical generation and vascular perturbations. Based on these data it can be proposed that hypothermia should be initiated before reperfusion injury whenever possible.
Early-induced hypothermia attenuates both neuronal and glial cell damage in reperfusional phase of I/R brain injury
We observed that concentrations of UCH-L1 and GFAP in microdialysate were low only in the early-induced hypothermia group. As far as we know, this is the first study that has demonstrated the utility of UCH-L1 and GFAP as measured by MD as a “BIOMARKER of ASDH induced brain damage”, and as a “measure” of moderate therapeutic hypothermia efficacy.
As shown in Fig. 6, the peak of UCH-L1 extracellular concentration was highest in the normothermia treatment group, as compared to the sham group. This indicates that the peak of neuronal injury occurs in the early reperfusion phase of ASDH (Fig 6A). Also, the peak of the extracellular GFAP concentration in the normothermia group was significantly higher than in the sham group and occurs in the late phase of reperfusion (Normothermia; 15.3±8.4 ng/dl, Sham; 3.3±1.0 ng/dl, Normothermia vs Sham; p < 0.01, Fig 6B).
The peak of the extracellular concentration of UCH-L1, and subsequent peak of GFAP seemed to be lowered by early hypothermia induction (Fig. 6A,B). However, late induced hypothermia could not to attenuate the neuronal damage in the early phase of reperfusion but reduced only the subsequent glial damage as determined by GFAP in the late phase of reperfusion. Thus, taken together, these data suggest that early induced hypothermia could reduce neuronal and subsequent glial injury in the delayed reperfusion phase of I/R pathophysiology. Based on the microdialysis data, the peak of glial cell damage might be occurring later than the neuronal damage, and this is consistent with different vulnerabilities between neurons and astrocytes in I/R injury, or an astrocytic response to neuronal death. In hypoxic, ischemic brain damage models, neurons have been shown to be much more sensitive and vulnerable than astrocytes 36,59,74,78. Astrocytes also might be more tolerant than neurons to I/R neurotoxicity, as seen in this ASDH rat model.
In the serum samples, we were unable to detect any significant difference between the ischemic and reperfusional phases, in the early vs, late hypothermia treatment groups (Fig. 6C,6D). Papa et al., reported that high UCH-L1 concentrations in CSF were highly related to injury severity and outcome in human TBI 56. UCH-L1 concentrations were in the range of 10–100 ng/dl in the human CSF samples, consistent with those results in our MD data. Further we also observed that MD extracellular concetrations of UCH-L1 and GFAP were 10–100 times higher than those in serum (Fig. 6). We think that microdialysis affords a direct measure of conditions within and around injured tissue and our MD data supports its use as a reliable biomarker, better than the cerebrospinal fluid (CSF), and unaffected by the status of the blood-brain barrier. Serum UCH-L1 concentration has been shown to correlate directly with the ratio of albumin in the serum to that in the CSF, implying that efflux of UCH-L1 into the blood would only be possible when the BBB was “open” 7. Thus, serum UCH-L1 levels might not directly indicate the severity of primary and secondary brain injury, because without BBB permeability, efflux of the biomarker maybe much reduced.
One more explanation for this discrepancy might be the difference of blood sampling time. In a lrecent report relating to serum UCH-L1 concentration, blood sampling was performed within 24hours after injury 5,17. In canine hypothermic cardiac arrest study, serum UCHL1 concentration peaked at 8 hours after cardiac arrest in heart surgery2. In our protocol, however, the blood sampling timing might be too early after injury, and thus serum UCH-L1 concentration might peak at a later time. An additional serum sample in the late phase after injury could have helped identify the serum UCH-L1 peak.
The appropriate timing and duration of hypothermia therapy
The appropriate and effective timing of hypothermia induction in brain injury remains controversial. Previous studies have shown that hypothermia should be achieved within 2 to 6 hours of severe hypoxic-ischemic injury in sheep, gerbils, and rats to afford protection57. For example, cooling sheep to 34°C for 72 hours gave good protection if started 90 minutes after the injury, was partly effective if started at 5.5 hours, and ineffective if started at 8.5 hours 23.
On the other hand, some experimental reports with delayed induction of therapeutic hypothermia in an ischemic rat model also exist. In the study of Colbourne et al. (1999), a 48-hour period of mild hypothermia was induced starting 6 hours after a 10 minute-long severe four-vessel occlusion ischemia in rats. Untreated normothermic ischemia resulted in total CA1 cell loss (99%), whereas delayed hypothermia treatment reduced neuronal loss to 14% at a 28-day survival. These results also indicate that the potential of late, but prolonged hypothermia in the reperfusional phase might be effective in the I/R rat model 14,15. In our study, late induced hypothermia did not reduce early ischemic/reperfusional injury (Fig. 5). However, in our study, the duration of hypothermia was short, only 3 hours. Based on these results, more prolonged hypothermia treatment in an I/R ASDH model may be useful to determine the minimum hypothermia duration that is beneficial.
The results of multicenter clinical trials of hypothermia and their interpretation
In a multicentre trial of hypothermia for neuroprotection 12, 392 patients with acute brain injury were randomized to normothermia or surface-induced hypothermia; hypothermia did not improve outcome12. However, there was weak evidence of improved outcomes in patients who were hypothermic on admission, and were treated with continued hypothermia 12. This same group then tried to confirm the efficacy of very early hypothermia in patients with severe brain injury, the National Acute Brain Injury Study:Hypothermia II ( NABISH: II ) 13.
In NABISH-II, early-induced hypothermia did not show efficacy as judged from mortality and morbidity data. On the other hand, in a post-hoc, sub-populational analysis separating diffuse brain injury patients and those with surgical hematoma evacuation, early-induced hypothermia appeared efficacious for the hematoma evacuation group. The authors thus concluded that one explanation was the different pathophysiology between diffuse brain injury and hematoma. In experimental models, ischemia occurs during acute subdural hematoma expansion and is followed by reperfusion after surgical removal of the hematoma 38. This is similar to the pathophysiology seen in patients with cardiac arrest—a group that has been successfully treated with hypothermia 26.
Experimentally, intra-ischemic hypothermia, prior to hematoma removal is associated with improved outcome 11. Diffuse brain injury is not characterized by ischemia in laboratory studies and may thus not be a good candidate for hypothermia treatment. In consideration of these clinical data, we decided to test early-induced hypothermia in the setting of an acute subdural hematoma rat model, which would simulate I/R injury to the brain. Supported by our present data, a further clinical trial, targeted upon focal mass/decompression injury is warranted, to investigate the effect of early hypothermia in I/R TBI, such as subdural hematoma. Several researchers have pointed out that the cooling rate, period of hypothermia, rewarming rate, and volumes of intravenous fluid for such a clinical trial are critically important variables 25,53,58,69.
Limitations and future implication
Some of the limitations of our current study must be considered. First, microdialysis is only a regional technique and cannot assess global brain damage with certainty. MD technique might be good at time-dependent analysis, however MD might be weak for spatial injury analysis. In this study, we applied FJB counting and TTC staining for this limitation. When we use a microdialysis technique as a monitor in clinical neurointensive care, additional data which represents global condition (ex, intracranial pressure monitoring, frequent CT examination, Xenon CT CBF mapping, PET, or MRI etc.) will be needed. The second limitation of this study was the natural rewarming from the hypothermia, due to technical constraints, rather than inducing a slow rewarming phase, as would be done in a clinical study13. Thirdly, our outcome assessments were limited to biomarker, histopathological, and volumetric end-points. In a clinical study, however, functional outcome (eg GOS-E) is a more informative measure.
We conclude that early-induced hypothermia can reduce neuronal degeneration and injury volume and to attenuate both neuronal and glial cell damage in this ASDH/decompression rat model. Our results support a clinical, multi-center trial to further examine the efficacy of very early, preoperative induced hypothermia, in this tightly related ASDH/TICH subgroup of patients with poor outcome.
Acknowledgments
Funding
This work was supported by funds from NINDS RO1 NS 042133 and the Miami Project to Cure Paralysis.
Footnotes
Author Disclosure Statement
Dr. Mondello and Dr. Mo are employees of Banyan Biomarkers, Inc.; Dr. Hayes owns stock, receives royalties from, and is employee of Banyan Biomarkers Inc., and as such may benefit financially as a result of the outcomes of this research or work reported in this publication.
Portions of this work were presented in abstract at the National Neurotrauma Symposium, Fort Lauderdale, FL, July 10-13, 2011
References
- 1.Anderson RE, Hansson LO, Nilsson O, Dijlai-Merzoug R, Settergren G. High serum S100B levels for trauma patients without head injuries. Neurosurgery. 2001;48:1255–1258. doi: 10.1097/00006123-200106000-00012. discussion 1258–1260. [DOI] [PubMed] [Google Scholar]
- 2.Arnaoutakis GJ, George TJ, Wang KK, Wilson MA, Allen JG, Robinson CW, et al. Serum levels of neuron-specific ubiquitin carboxyl-terminal esterase-L1 predict brain injury in a canine model of hypothermic circulatory arrest. Journal of Thoracic and Cardiovascular Surgery. 2011;142:902–910. e901. doi: 10.1016/j.jtcvs.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovascular Research. 2004;61:461–470. doi: 10.1016/j.cardiores.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 4.Bellander BM, Cantais E, Enblad P, Hutchinson P, Nordstrom CH, Robertson C, et al. Consensus meeting on microdialysis in neurointensive care. Intensive Care Medicine. 2004;30:2166–2169. doi: 10.1007/s00134-004-2461-8. [DOI] [PubMed] [Google Scholar]
- 5.Berger RP, Hayes RL, Richichi R, Beers SR, Wang KK. Serum concentrations of ubiquitin C-terminal hydrolase-L1 and alphaII-spectrin breakdown product 145 kDa correlate with outcome after pediatric TBI. Journal of Neurotrauma. 2012;29:162–167. doi: 10.1089/neu.2011.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bloomfield SM, McKinney J, Smith L, Brisman J. Reliability of S100B in predicting severity of central nervous system injury. Neurocrit Care. 2007;6:121–138. doi: 10.1007/s12028-007-0008-x. [DOI] [PubMed] [Google Scholar]
- 7.Blyth BJ, Farahvar A, He H, Nayak A, Yang C, Shaw G, et al. Elevated serum ubiquitin carboxy-terminal hydrolase L1 is associated with abnormal blood-brain barrier function after traumatic brain injury. Journal of Neurotrauma. 2011;28:2453–2462. doi: 10.1089/neu.2010.1653. [DOI] [PubMed] [Google Scholar]
- 8.Blyth BJ, Farhavar A, Gee C, Hawthorn B, He H, Nayak A, et al. Validation of serum markers for blood-brain barrier disruption in traumatic brain injury. Journal of Neurotrauma. 2009;26:1497–1507. doi: 10.1089/neu.2008.0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolli R, Jeroudi MO, Patel BS, Aruoma OI, Halliwell B, Lai EK, et al. Marked reduction of free radical generation and contractile dysfunction by antioxidant therapy begun at the time of reperfusion. Evidence that myocardial “stunning” is a manifestation of reperfusion injury. Circulation Research. 1989;65:607–622. doi: 10.1161/01.res.65.3.607. [DOI] [PubMed] [Google Scholar]
- 10.Bramlett HM, Green EJ, Dietrich WD, Busto R, Globus MY, Ginsberg MD. Posttraumatic brain hypothermia provides protection from sensorimotor and cognitive behavioral deficits. Journal of Neurotrauma. 1995;12:289–298. doi: 10.1089/neu.1995.12.289. [DOI] [PubMed] [Google Scholar]
- 11.Burger R, Bendszus M, Vince GH, Solymosi L, Roosen K. Neurophysiological monitoring, magnetic resonance imaging, and histological assays confirm the beneficial effects of moderate hypothermia after epidural focal mass lesion development in rodents. Neurosurgery. 2004;54:701–711. doi: 10.1227/01.neu.0000108784.80585.ee. discussion 711–702. [DOI] [PubMed] [Google Scholar]
- 12.Clifton GL, Miller ER, Choi SC, Levin HS, McCauley S, Smith KR, Jr, et al. Lack of effect of induction of hypothermia after acute brain injury. New England Journal of Medicine. 2001;344:556–563. doi: 10.1056/NEJM200102223440803. [DOI] [PubMed] [Google Scholar]
- 13.Clifton GL, Valadka A, Zygun D, Coffey CS, Drever P, Fourwinds S, et al. Very early hypothermia induction in patients with severe brain injury (the National Acute Brain Injury Study. Hypothermia II). a randomised trial. Lancet Neurol. 2011;10:131–139. doi: 10.1016/S1474-4422(10)70300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colbourne F, Corbett D. Delayed postischemic hypothermia. a six month survival study using behavioral and histological assessments of neuroprotection. Journal of Neuroscience. 1995;15:7250–7260. doi: 10.1523/JNEUROSCI.15-11-07250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colbourne F, Li H, Buchan AM. Indefatigable CA1 sector neuroprotection with mild hypothermia induced 6 hours after severe forebrain ischemia in rats. Journal of Cerebral Blood Flow and Metabolism. 1999;19:742–749. doi: 10.1097/00004647-199907000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Cole TB. Global road safety crisis remedy sought. 1.2 million killed, 50 million injured annually. JAMA. 2004;291:2531–2532. doi: 10.1001/jama.291.21.2531. [DOI] [PubMed] [Google Scholar]
- 17.Czeiter E, Mondello S, Kovacs N, Sandor J, Gabrielli A, Schmid K, et al. Brain injury biomarkers may improve the predictive power of the IMPACT outcome calculator. Journal of Neurotrauma. 2012 doi: 10.1089/neu.2011.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di X, Bullock R. Effect of the novel high-affinity glycine-site N-methyl-D-aspartate antagonist ACEA-1021 on 125I-MK-801 binding after subdural hematoma in the rat. an in vivo autoradiographic study. Journal of Neurosurgery. 1996;85:655–661. doi: 10.3171/jns.1996.85.4.0655. [DOI] [PubMed] [Google Scholar]
- 19.Dietrich WD, Alonso O, Busto R, Globus MY, Ginsberg MD. Post-traumatic brain hypothermia reduces histopathological damage following concussive brain injury in the rat. Acta Neuropathol. 1994;87:250–258. doi: 10.1007/BF00296740. [DOI] [PubMed] [Google Scholar]
- 20.Dietrich WD, Bramlett HM. The evidence for hypothermia as a neuroprotectant in traumatic brain injury. Neurotherapeutics. 2010;7:43–50. doi: 10.1016/j.nurt.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erecinska M, Thoresen M, Silver IA. Effects of hypothermia on energy metabolism in Mammalian central nervous system. Journal of Cerebral Blood Flow and Metabolism. 2003;23:513–530. doi: 10.1097/01.WCB.0000066287.21705.21. [DOI] [PubMed] [Google Scholar]
- 22.Fu ES, Tummala RP. Neuroprotection in brain and spinal cord trauma. Curr Opin Anaesthesiol. 2005;18:181–187. doi: 10.1097/01.aco.0000162838.56344.88. [DOI] [PubMed] [Google Scholar]
- 23.Gunn AJ. Cerebral hypothermia for prevention of brain injury following perinatal asphyxia. Current Opinion in Pediatrics. 2000;12:111–115. doi: 10.1097/00008480-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Honda M, Tsuruta R, Kaneko T, Kasaoka S, Yagi T, Todani M, et al. Serum glial fibrillary acidic protein is a highly specific biomarker for traumatic brain injury in humans compared with S-100B and neuron-specific enolase. Journal of Trauma. 2010;69:104–109. doi: 10.1097/TA.0b013e3181bbd485. [DOI] [PubMed] [Google Scholar]
- 25.Honeybul S, Ho K, Lind C, Gillett G. Hypothermia in patients with brain injury. the way forward? Lancet Neurol. 2011;10:405–406. doi: 10.1016/S1474-4422(11)70086-2. author reply 406–407. [DOI] [PubMed] [Google Scholar]
- 26.Janata A, Holzer M. Hypothermia after cardiac arrest. Progress in Cardiovascular Diseases. 2009;52:168–179. doi: 10.1016/j.pcad.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Jia F, Mao Q, Liang YM, Jiang JY. Effect of post-traumatic mild hypothermia on hippocampal cell death after traumatic brain injury in rats. Journal of Neurotrauma. 2009;26:243–252. doi: 10.1089/neu.2008.0670. [DOI] [PubMed] [Google Scholar]
- 28.Jiang JY. Clinical study of mild hypothermia treatment for severe traumatic brain injury. Journal of Neurotrauma. 2009;26:399–406. doi: 10.1089/neu.2008.0525. [DOI] [PubMed] [Google Scholar]
- 29.Jiang JY, Lyeth BG, Clifton GL, Jenkins LW, Hamm RJ, Hayes RL. Relationship between body and brain temperature in traumatically brain-injured rodents. Journal of Neurosurgery. 1991;74:492–496. doi: 10.3171/jns.1991.74.3.0492. [DOI] [PubMed] [Google Scholar]
- 30.Johnsson P, Backstrom M, Bergh C, Jonsson H, Luhrs C, Alling C. Increased S100B in blood after cardiac surgery is a powerful predictor of late mortality. Annals of Thoracic Surgery. 2003;75:162–168. doi: 10.1016/s0003-4975(02)04318-7. [DOI] [PubMed] [Google Scholar]
- 31.Jonsson H. S100B and cardiac surgery. possibilities and limitations. Restor Neurol Neurosci. 2003;21:151–157. [PubMed] [Google Scholar]
- 32.Karibe H, Zarow GJ, Graham SH, Weinstein PR. Mild intraischemic hypothermia reduces postischemic hyperperfusion, delayed postischemic hypoperfusion, blood-brain barrier disruption, brain edema, and neuronal damage volume after temporary focal cerebral ischemia in rats. Journal of Cerebral Blood Flow and Metabolism. 1994;14:620–627. doi: 10.1038/jcbfm.1994.77. [DOI] [PubMed] [Google Scholar]
- 33.Kawai N, Nakamura T, Okauchi M, Nagao S. Effects of hypothermia on intracranial hemodynamics and ischemic brain damage-studies in the rat acute subdural hematoma model. Acta Neurochir Suppl. 2000;76:529–533. doi: 10.1007/978-3-7091-6346-7_111. [DOI] [PubMed] [Google Scholar]
- 34.Kawai N, Nakamura T, Okauchi M, Nagao S. Effects of hypothermia on intracranial pressure and brain edema formation. studies in a rat acute subdural hematoma model. Journal of Neurotrauma. 2000;17:193–202. doi: 10.1089/neu.2000.17.193. [DOI] [PubMed] [Google Scholar]
- 35.Khalid MA, Ashraf M. Direct detection of endogenous hydroxyl radical production in cultured adult cardiomyocytes during anoxia and reoxygenation. Is the hydroxyl radical really the most damaging radical species? Circulation Research. 1993;72:725–736. doi: 10.1161/01.res.72.4.725. [DOI] [PubMed] [Google Scholar]
- 36.Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Research. 1982;239:57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- 37.Korfias S, Stranjalis G, Boviatsis E, Psachoulia C, Jullien G, Gregson B, et al. Serum S-100B protein monitoring in patients with severe traumatic brain injury. Intensive Care Medicine. 2007;33:255–260. doi: 10.1007/s00134-006-0463-4. [DOI] [PubMed] [Google Scholar]
- 38.Kuroda Y, Bullock R. Local cerebral blood flow mapping before and after removal of acute subdural hematoma in the rat. Neurosurgery. 1992;30:687–691. [PubMed] [Google Scholar]
- 39.Kwon TH, Chao DL, Malloy K, Sun D, Alessandri B, Bullock MR. Tempol, a novel stable nitroxide, reduces brain damage and free radical production, after acute subdural hematoma in the rat. Journal of Neurotrauma. 2003;20:337–345. doi: 10.1089/089771503765172291. [DOI] [PubMed] [Google Scholar]
- 40.Kwon TH, Sun D, Daugherty WP, Spiess BD, Bullock MR. Effect of perfluorocarbons on brain oxygenation and ischemic damage in an acute subdural hematoma model in rats. J Neurosurg. 2005;103:724–730. doi: 10.3171/jns.2005.103.4.0724. [DOI] [PubMed] [Google Scholar]
- 41.Langlois JA, Marr A, Mitchko J, Johnson RL. Tracking the silent epidemic and educating the public. CDC’s traumatic brain injury-associated activities under the TBI Act of 1996 and the Children’s Health Act of 2000. J Head Trauma Rehabil. 2005;20:196–204. doi: 10.1097/00001199-200505000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Larach DB, Kofke WA, Le Roux P. Potential non-hypoxic/ischemic causes of increased cerebral interstitial fluid lactate/pyruvate ratio. a review of available literature. Neurocrit Care. 2011;15:609–622. doi: 10.1007/s12028-011-9517-8. [DOI] [PubMed] [Google Scholar]
- 43.Li J, Li XY, Feng DF, Pan DC. Biomarkers associated with diffuse traumatic axonal injury. exploring pathogenesis, early diagnosis, and prognosis. Journal of Trauma. 2010;69:1610–1618. doi: 10.1097/TA.0b013e3181f5a9ed. [DOI] [PubMed] [Google Scholar]
- 44.Liu MC, Akinyi L, Scharf D, Mo J, Larner SF, Muller U, et al. Ubiquitin C-terminal hydrolase-L1 as a biomarker for ischemic and traumatic brain injury in rats. Eur J Neurosci. 2010;31:722–732. doi: 10.1111/j.1460-9568.2010.07097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lumpkins KM, Bochicchio GV, Keledjian K, Simard JM, McCunn M, Scalea T. Glial fibrillary acidic protein is highly correlated with brain injury. J Trauma. 2008;65:778–782. doi: 10.1097/TA.0b013e318185db2d. discussion 782–774. [DOI] [PubMed] [Google Scholar]
- 46.Mar J, Arrospide A, Begiristain JM, Larranaga I, Elosegui E, Oliva-Moreno J. The impact of acquired brain damage in terms of epidemiology, economics and loss in quality of life. BMC Neurol. 2011;11:46. doi: 10.1186/1471-2377-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marion D, Bullock MR. Current and future role of therapeutic hypothermia. Journal of Neurotrauma. 2009;26:455–467. doi: 10.1089/neu.2008.0582. [DOI] [PubMed] [Google Scholar]
- 48.Massaro F, Lanotte M, Faccani G, Triolo C. One hundred and twenty-seven cases of acute subdural haematoma operated on. Correlation between CT scan findings and outcome. Acta Neurochirurgica. 1996;138:185–191. doi: 10.1007/BF01411359. [DOI] [PubMed] [Google Scholar]
- 49.McIntyre LA, Fergusson DA, Hebert PC, Moher D, Hutchison JS. Prolonged therapeutic hypothermia after traumatic brain injury in adults. a systematic review. JAMA. 2003;289:2992–2999. doi: 10.1001/jama.289.22.2992. [DOI] [PubMed] [Google Scholar]
- 50.Mondello S, Jeromin A, Buki A, Bullock R, Czeiter E, Kovacs N, et al. Glial Neuronal Ratio (GNR). a Novel Index for Differentiating Injury Type in Patients with Severe Traumatic Brain Injury. J Neurotrauma. 2011 doi: 10.1089/neu.2011.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mondello S, Linnet A, Buki A, Robicsek S, Gabrielli A, Tepas J, et al. Clinical utility of serum levels of ubiquitin C-terminal hydrolase as a biomarker for severe traumatic brain injury. Neurosurgery. 2012;70:666–675. doi: 10.1227/NEU.0b013e318236a809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neumar RW. Molecular mechanisms of ischemic neuronal injury. Annals of Emergency Medicine. 2000;36:483–506. doi: 10.1067/mem.2000.110995. [DOI] [PubMed] [Google Scholar]
- 53.Nichol AD, Trapani T, Murray L, Vallance S, Cooper DJ. Hypothermia in patients with brain injury. the way forward? Lancet Neurol. 2011;10:405. doi: 10.1016/S1474-4422(11)70085-0. author reply 406–407. [DOI] [PubMed] [Google Scholar]
- 54.Nielsen TH, Engell SI, Johnsen RA, Schulz MK, Gerke O, Hjelmborg J, et al. Comparison between cerebral tissue oxygen tension and energy metabolism in experimental subdural hematoma. Neurocrit Care. 2011;15:585–592. doi: 10.1007/s12028-011-9563-2. [DOI] [PubMed] [Google Scholar]
- 55.Okauchi M, Kawai N, Nakamura T, Kawanishi M, Nagao S. Effects of mild hypothermia and alkalizing agents on brain injuries in rats with acute subdural hematomas. J Neurotrauma. 2002;19:741–751. doi: 10.1089/08977150260139110. [DOI] [PubMed] [Google Scholar]
- 56.Papa L, Akinyi L, Liu MC, Pineda JA, Tepas JJ, 3rd, Oli MW, et al. Ubiquitin C-terminal hydrolase is a novel biomarker in humans for severe traumatic brain injury. Crit Care Med. 2010;38:138–144. doi: 10.1097/CCM.0b013e3181b788ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Critical Care Medicine. 2009;37:S186–202. doi: 10.1097/CCM.0b013e3181aa5241. [DOI] [PubMed] [Google Scholar]
- 58.Polderman KH, Andrews PJ. Hypothermia in patients with brain injury. the way forward? Lancet Neurol. 2011;10:404–405. doi: 10.1016/S1474-4422(11)70084-9. author reply 406–407. [DOI] [PubMed] [Google Scholar]
- 59.Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Annals of Neurology. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- 60.Raabe A, Grolms C, Keller M, Dohnert J, Sorge O, Seifert V. Correlation of computed tomography findings and serum brain damage markers following severe head injury. Acta Neurochirurgica. 1998;140:787–791. doi: 10.1007/s007010050180. discussion 791–782. [DOI] [PubMed] [Google Scholar]
- 61.Rosen H, Rosengren L, Herlitz J, Blomstrand C. Increased serum levels of the S-100 protein are associated with hypoxic brain damage after cardiac arrest. Stroke. 1998;29:473–477. doi: 10.1161/01.str.29.2.473. [DOI] [PubMed] [Google Scholar]
- 62.Ross SA, Cunningham RT, Johnston CF, Rowlands BJ. Neuron-specific enolase as an aid to outcome prediction in head injury. British Journal of Neurosurgery. 1996;10:471–476. doi: 10.1080/02688699647104. [DOI] [PubMed] [Google Scholar]
- 63.Rundgren M, Karlsson T, Nielsen N, Cronberg T, Johnsson P, Friberg H. Neuron specific enolase and S-100B as predictors of outcome after cardiac arrest and induced hypothermia. Resuscitation. 2009;80:784–789. doi: 10.1016/j.resuscitation.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 64.Savola O, Pyhtinen J, Leino TK, Siitonen S, Niemela O, Hillbom M. Effects of head and extracranial injuries on serum protein S100B levels in trauma patients. Journal of Trauma. 2004;56:1229–1234. doi: 10.1097/01.ta.0000096644.08735.72. discussion 1234. [DOI] [PubMed] [Google Scholar]
- 65.Shann F. Hypothermia for traumatic brain injury. how soon, how cold, and how long? Lancet. 2003;362:1950–1951. doi: 10.1016/S0140-6736(03)15083-0. [DOI] [PubMed] [Google Scholar]
- 66.Shao ZH, Sharp WW, Wojcik KR, Li CQ, Han M, Chang WT, et al. Therapeutic hypothermia cardioprotection via Akt- and nitric oxide-mediated attenuation of mitochondrial oxidants. Am J Physiol Heart Circ Physiol. 2010;298:H2164–2173. doi: 10.1152/ajpheart.00994.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stein DM, Kufera JA, Lindell A, Murdock KR, Menaker J, Bochicchio GV, et al. Association of CSF biomarkers and secondary insults following severe traumatic brain injury. Neurocrit Care. 2011;14:200–207. doi: 10.1007/s12028-010-9496-1. [DOI] [PubMed] [Google Scholar]
- 68.Svetlov SI, Prima V, Kirk DR, Gutierrez H, Curley KC, Hayes RL, et al. Morphologic and biochemical characterization of brain injury in a model of controlled blast overpressure exposure. Journal of Trauma. 2010;69:795–804. doi: 10.1097/TA.0b013e3181bbd885. [DOI] [PubMed] [Google Scholar]
- 69.Takeuchi S, Nawashiro H, Otani N. Hypothermia in patients with brain injury. the way forward? Lancet Neurol. 2011;10:404. doi: 10.1016/S1474-4422(11)70083-7. author reply 406–407. [DOI] [PubMed] [Google Scholar]
- 70.Timmons SD. Current trends in neurotrauma care. Critical Care Medicine. 2010;38:S431–444. doi: 10.1097/CCM.0b013e3181ec57ab. [DOI] [PubMed] [Google Scholar]
- 71.Timofeev I, Carpenter KL, Nortje J, Al-Rawi PG, O’Connell MT, Czosnyka M, et al. Cerebral extracellular chemistry and outcome following traumatic brain injury. a microdialysis study of 223 patients. Brain. 2011;134:484–494. doi: 10.1093/brain/awq353. [DOI] [PubMed] [Google Scholar]
- 72.Tuttolomondo A, Di Sciacca R, Di Raimondo D, Arnao V, Renda C, Pinto A, et al. Neuron protection as a therapeutic target in acute ischemic stroke. Curr Top Med Chem. 2009;9:1317–1334. doi: 10.2174/156802609789869646. [DOI] [PubMed] [Google Scholar]
- 73.Vink R, Nimmo AJ. Multifunctional drugs for head injury. Neurotherapeutics. 2009;6:28–42. doi: 10.1016/j.nurt.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Voloboueva LA, Suh SW, Swanson RA, Giffard RG. Inhibition of mitochondrial function in astrocytes. implications for neuroprotection. Journal of Neurochemistry. 2007;102:1383–1394. doi: 10.1111/j.1471-4159.2007.4634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vos PE, Jacobs B, Andriessen TM, Lamers KJ, Borm GF, Beems T, et al. GFAP and S100B are biomarkers of traumatic brain injury. an observational cohort study. Neurology. 2010;75:1786–1793. doi: 10.1212/WNL.0b013e3181fd62d2. [DOI] [PubMed] [Google Scholar]
- 76.Wilberger JE, Jr, Harris M, Diamond DL. Acute subdural hematoma. morbidity and mortality related to timing of operative intervention. Journal of Trauma. 1990;30:733–736. [PubMed] [Google Scholar]
- 77.Wilberger JE, Jr, Harris M, Diamond DL. Acute subdural hematoma. morbidity, mortality, and operative timing. Journal of Neurosurgery. 1991;74:212–218. doi: 10.3171/jns.1991.74.2.0212. [DOI] [PubMed] [Google Scholar]
- 78.Xu L, Sapolsky RM, Giffard RG. Differential sensitivity of murine astrocytes and neurons from different brain regions to injury. Experimental Neurology. 2001;169:416–424. doi: 10.1006/exnr.2001.7678. [DOI] [PubMed] [Google Scholar]
- 79.Yang D, Guo S, Zhang T, Li H. Hypothermia attenuates ischemia/reperfusion-induced endothelial cell apoptosis via alterations in apoptotic pathways and JNK signaling. FEBS Letters. 2009;583:2500–2506. doi: 10.1016/j.febslet.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 80.Yokobori S, Watanabe A, Matsumoto G, Onda H, Masuno T, Fuse A, et al. Time course of recovery from cerebral vulnerability after severe traumatic brain injury. a microdialysis study. J Trauma. 2011;71:1235–1240. doi: 10.1097/TA.0b013e3182140dd7. [DOI] [PubMed] [Google Scholar]