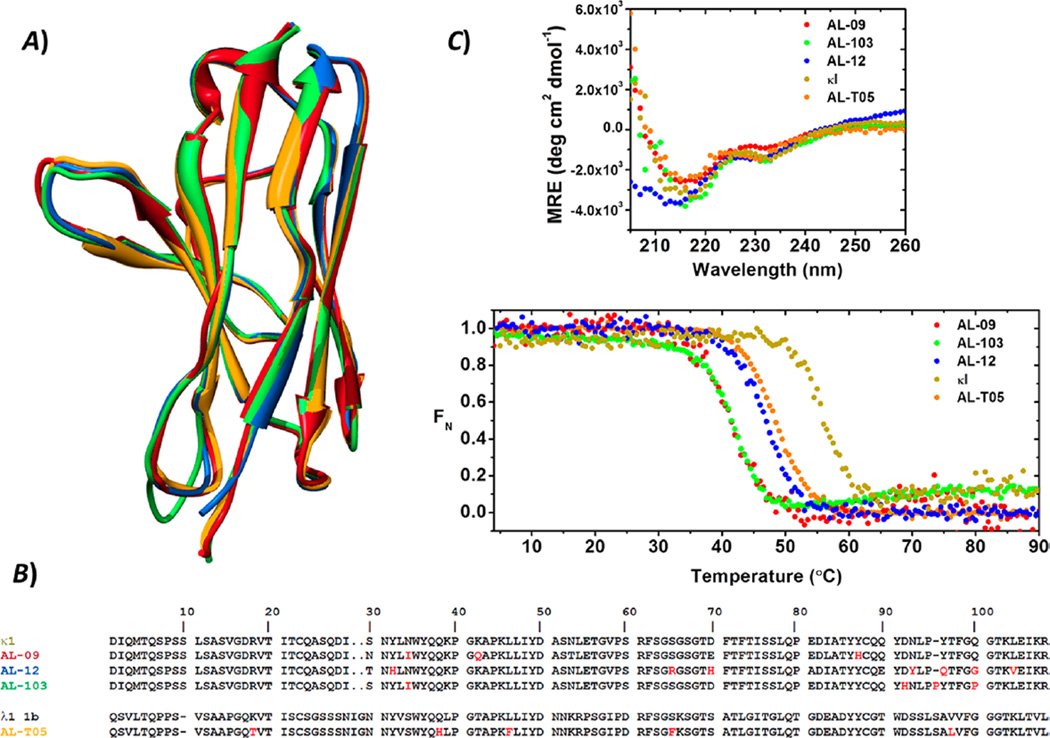
Figure 1.
Structural and stability properties of the VL proteins prior to amyloid formation reactions. (A) Structural and (B) sequence alignments of the VL immunoglobulin proteins. The sequence of the λ1 1b germline VL was included for the purpose of comparison. Nonconservative somatic mutations are colored red. (C) Far-UV CD spectra and thermal unfolding analysis of AL-09 (red), AL-103 (green), AL-12 (blue), AL-T05 (black), and κI (olive green). All proteins display β-sheet structure with the two characteristic minima (235 and ~217 nm) for these proteins. Experimental conditions were as follows: 20 µM protein in 10 mM Tris-HCl (pH 7.4). Far UV-CD spectra were recorded at 4 °C. Thermal denaturation experiments were performed from 4 to 90 °C at a rate of 0.5 °C min−1.