Abstract
Structural studies on living cells by conventional methods are limited to low resolution because radiation damage kills cells long before the necessary dose for high resolution can be delivered. X-ray free-electron lasers circumvent this problem by outrunning key damage processes with an ultra-short and extremely bright coherent X-ray pulse. Diffraction-before-destruction experiments provide high-resolution data from cells that are alive when the femtosecond X-ray pulse traverses the sample. This paper presents two data sets from micron-sized cyanobacteria obtained at the Linac Coherent Light Source, containing a total of 199,000 diffraction patterns. Utilizing this type of diffraction data will require the development of new analysis methods and algorithms for studying structure and structural variability in large populations of cells and to create abstract models. Such studies will allow us to understand living cells and populations of cells in new ways. New X-ray lasers, like the European XFEL, will produce billions of pulses per day, and could open new areas in structural sciences.
Subject terms: Molecular biophysics, X-rays, Imaging
Background & Summary
Imaging living cells at resolutions higher than the resolution of optical microscopy is challenging. A dose in excess of hundred million Grays (Gy: Jkg−1) is required to reach sub-nanometer resolution on a micron-sized cell, using X-rays or electrons, and no cell can survive this level of irradiation; a dose of only hundred Grays kills most cells1,2. What is known about cells today at high resolution comes from dead cells.
Ultra-short and extremely intense coherent X-ray pulses from X-ray lasers offer the possibility to outrun key damage processes3 and deliver a molecular-level snapshot of a cell that is alive at the time of image formation4 but explodes a few picosecond later5. ‘Diffraction-before-destruction’3,4,6 has been successfully demonstrated on a wide range of biological samples, including protein nanocrystals7, living cells8, cell organelles9 and virus particles10. Recent results also show 3D reconstruction of reproducible virus particles11. The data sets described in this paper are from similar ultra-fast imaging experiments.
The ability to measure millions of diffraction patterns in a day at X-ray free-electron lasers (XFELs) offers new avenues for experiments on cells. The femtosecond illumination ‘freezes’ all motion in the sample on the time scale of atomic vibrations. The massive amount of data emerging from XFELs will represent more than just individual projection images of cells. There is a need to develop algorithms to create abstract models of cells from the data, where no individual image gives us complete information but rather nudges the abstraction to describe common features and common internal interactions. Conversely, the data can be used to describe structural variability in populations. With so many images per day, even statistically rare events could be pinpointed and studied. The data sets also offer innovative avenues for data-driven discovery, and helping this effort was one of our motivations in releasing the data sets.
Cyanobacteria were used in this experiment because of their small size and for being remarkably robust. Solitary C. gracile cells are between 0.25–0.4 μm in diameter and 0.4–2.4 μm long12. The S. elongatus cells are similar in diameter but are longer on average by up to a micron. Both species divide symmetrically by binary fission. The two daughter cells separate from each other after reaching the size and shape of the mother cell13. We used non-synchronized cell cultures in our studies, undergoing active growth and providing cells in various stages of their cell cycle.
The live cells were delivered into the pulse train of the Linac Coherent Light Source (LCLS) in an aerosol at a reduced pressure using methods developed for studies on giant viruses10. This type of sample injection delivers truly isolated samples into the X-ray beam and gives diffraction patterns with practically no background noise. In addition, the contrast between the sample and its surrounding (wet helium gas expanding into a vacuum chamber) is also exceptionally high. Injected cells arrive in random order and are imaged in random orientations. The data sets include images with signal extending beyond 4 nm resolution.
At these wavelengths and the scattering angles of the strongest patterns, a single diffraction pattern contains limited depth information, and this information may be retrieved by a numerical propagation of the complex-valued wave front4,6,14,15. There is a need to explore possibilities to extract depth information from the patterns, and a community effort would speed up progress here.
In order to facilitate developments, we present two data records containing a total of 199,000 diffraction patterns from living cells (Data Citation 1), making it the largest freely available X-ray diffraction data set on cells collected at an X-ray FEL. A subset of 11 diffraction patterns from these data sets was used in a recent publication on imaging live cells8. We hope the release of these very large data sets will stimulate interest and help software development.
Methods
Experimental setup
The experiment was executed using the CFEL-ASG Multi-Purpose (CAMP) instrument16, at the AMO end station17 of the Linac Coherent Light Source (LCLS)18, using an experimental configuration identical to that used in ref. 10. The bandwidth of the LCLS is approximately 0.5%. The length of the electron bunch was ~70 fs (full-duration at half-maximum) and the length of the photon bunch is believed to be shorter. The size of the focal spot was 3 μm×7 μm (full width at half maximum).
The two data records presented in this paper come from two experiments, using different experimental parameters (Table 1). In Experiment 1 we collected diffraction patterns from C. gracile cells. The patterns are presented in data record 1. The photon energy of experiment 1 was 517 eV (2.40 nm wavelength). In Experiment 2 we collected diffraction patterns from S. elongatus, presented in data record 2. The photon energy of experiment 2 was 1,100 eV (1.13 nm).
Table 1. Experimental setup.
| Sample | Experiment 1 | Experiment 2 |
|---|---|---|
| This table describes the experimental parameters used in experiment 1 and experiment 2. The sample, the source parameters, and the detector properties. | ||
| Cell type | Cyanobium gracile | Synechococcus elongatus |
| Source Parameters |
||
| End station | AMO | AMO |
| Repetition rate | 120 Hz | 120 Hz |
| Pulse duration | ~70 fs | ~70 fs |
| Photons per pulse | ~1.5×1013 (1.26 mJ) | 1.2×1013 (2.18 mJ) |
| Optical efficiency | 15% | 15% |
| Bandwidth | 0.5% | 0.5% |
| Photon energy | 517 eV (2.4 nm) | 1,100 eV (1.13 nm) |
| Focal size | 3 μm×7 μm | 3 μm×7 μm |
| Flux in the focus | 1.1×1011 photons/μm2 | 8.6×1010 photons/μm2 |
| Detector Properties |
||
| Distance from interaction point (front detector) | 220 mm | 220 mm |
| Gap size (front detector) | 55.6 mm | 22.8 mm |
| Full-well capacity (front detector) | 3,500 photons | 1,600 photons |
| Distance from interaction point (back detector) | 741 mm | 741 mm |
| Gap size (back detector) | 0 mm | 0 mm |
| Full-well capacity (back detector) | 3,500 photons | 1,600 photons |
The interaction chamber was equipped with two pairs of pnCCD16 X-ray area detectors (front and back detectors), each consisting of two movable detector panels (Fig. 1). The front detector assembly was placed 220 mm from the interaction point, and the back detector assembly at 741 mm in both experiments. The gap between the two front detector panels was 55.6 mm for experiment 1 and 22.8 mm for experiment 2. The gap between the back detector panels was closed in both experiments. The direct beam exited through openings between the two detector halves and was absorbed in a beam dump behind the back detectors. Each detector panel contained 512×1,024 pixels with 75 μm edge lengths and a full-well capacity of 500,000 electrons/pixel, corresponding to 3,500 photons in experiment 1 and 1,600 photons in experiment 2. The read-out rate matched the 120 Hz repetition rate of the LCLS.
Figure 1. The experimental arrangement.
C. gracile and S. elongatus cells were injected into the pulse train of the LCLS18 at 10−6 mbar pressure, using an aerosol sample injector built in Uppsala. The cells are in different stages of division, and arrive in random order and are imaged in random orientations. The diffracted signal is recorded on two detector pairs (front detector and back detector). The direct beam passes through an opening between the two detector halves of each detector pair16. The opening or gap between the front detector halves is 55.6 mm for experiment 1 (C. gracile cells), and 22.8 mm for experiment 2 (S. elongatus cells). The gap between the back detector halves is closed for both experiments.
Cells
Cyanobium gracile PCC 6307 and Synechococcus elongatus PCC 7942 cells were grown in the standard Bg11 medium in batch cultures under constant light. The cell cultures were non-synchronized providing cells in various stages of division. Before the imaging experiments, cells were centrifuged at 6,500 g for 10 min, creating a soft pellet. The pellet was resuspended in 25 mM ammonium acetate, and this buffer exchange was repeated twice to remove salt and contaminants.
Sample injection
The suspension of live cells was aerosolized with helium in a gas dynamic nebulizer19. The aerosols were delivered into the pulse train of the X-ray laser through an aerodynamic lens20. This method delivers cells in free flight without substrate or other supporting medium, thereby minimizing background scattering, and can produce millions of exposures per day. Most of the nebulizing gas, and vapours of the volatile buffer were pumped away through a differential pumping stage.
Data recording
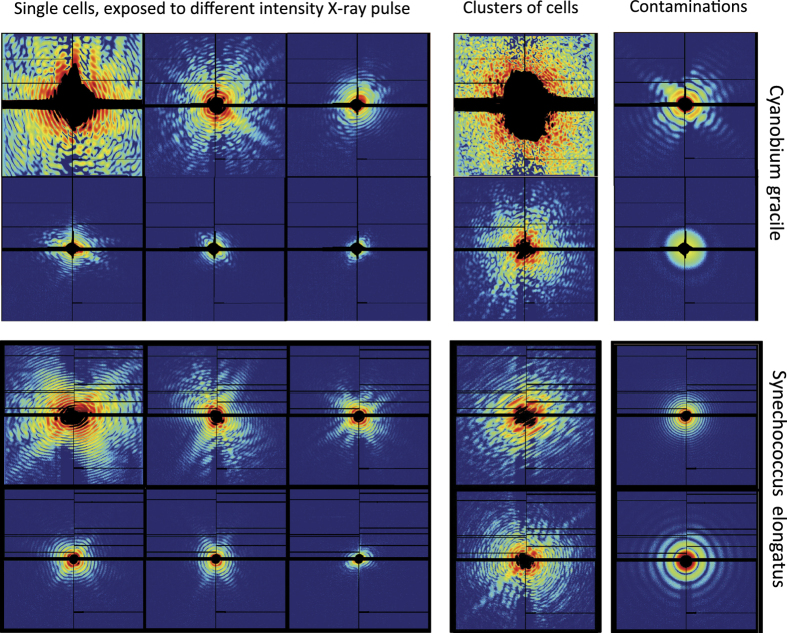
We recorded a variety of diffraction patterns, originating from single cells, clusters of cells, droplets of buffer or contaminants. The patterns have a large variation in recorded intensity, depending on X-ray pulse-intensity, where in the pulse the particle was hit, and the size of the particle. Figure 2 shows a representative set of diffraction patterns from both experiments.
Figure 2. Compilation of representative sampling of diffraction patterns from both experiments.
Ten representative diffraction patterns from each data record were selected. Both data sets contain diffraction patterns from single living cells, multiple cells, large clusters of cells, as well as from contaminants such as spherical droplets, or virus-like particles (possibly being an earlier injected sample). All patterns are normalized individually; dark blue is no scattered signal, dark red is most intense signal in the pattern.
Data preprocessing
We also supply a minimally preprocessed dataset, which includes only diffraction patterns with significant scattered signal (199,000 out of 540,000 patterns). The preprocessing included generation and subtraction of calibration levels, the masking of faulty pixels, the application of the experimental geometry, and a background subtraction. Frames were considered hits if more than 300 pixels record a value above 45 arbitrary detector units (ADU) for experiment 1, and 4,000 pixels recording a value above 45 ADU for experiment 2. The increased threshold in experiment 2 compensates for a stronger background scattering present in experiment 2. All preprocessing steps were done automatically using the Cheetah software package21. The Cheetah configuration files, calibration data, the bad pixel masks, and the respective geometry files are also included into the Data records (Tables 2 and 3).
Table 2. Deposited data and configuration files.
| Data type | Example filename | File format |
|---|---|---|
| This table describes the files deposited on the CXIDB under accession number ID-37. ID-37 consists of two data records, from two experiments. Both records contain all these files. | ||
| Experimental Data |
||
| Diffraction data (all exposures) | e54-r0207-s00-c00.xtc | XTC |
| Preprocessed data (only hits) | preprocessed_hits_exp1-r0207.cxi | CXI |
| Calibration data (dark run) | calibration_data_back_detector_exp1.h5 | Hdf5 |
| Preprocessing |
||
| Cheetah initialization file (generate calibration data) | cheetah_calibration_exp1.ini | Text |
| Cheetah configuration file (generate calibration data) | psana_calibration_exp1.cfg | Text |
| Calibration data (back detector) | calibration_data_back_detector_exp1.h5 | Hdf5 |
| Calibration data (front detector) | calibration_data_front_detector_exp1.h5 | Hdf5 |
| Cheetah initialization file (preprocessing) | cheetah.ini | Text |
| Cheetah configuration file (preprocessing) | psana.cfg | Text |
| Bad pixel map (back detector) | badpixelmap_back_detector_exp1.h5 | Hdf5 |
| Bad pixel map (front detector) | badpixelmap_front_detector_exp1.h5 | Hdf5 |
| Experimental geometry (back detector) | geometry_back_detector_exp1.h5 | Hdf5 |
| Experimental geometry (front detector) | geometry_front_detector_exp1.h5 | Hdf5 |
Table 3. Description of the experimental data sets.
| Records | Cell species | Data Type | Run number | Size (# exposures) | Hit ratio | Duration run |
|---|---|---|---|---|---|---|
| This table describes the experimental data of the two data records; the species of cyanobacteria imaged, the data type, the run number of the data sets, the size of the data set, the hit ratio, and the duration of the run. | ||||||
| Data Record 1 | No sample | Calibration data | r0206 | 885 | N.A. | 1 min |
| C. gracile | Diffraction data | r0207 | 195,491 | 42% | 27 min | |
| C. gracile | Diffraction data | r0210 | 67,685 | 39% | 9 min | |
| C. gracile | Diffraction data | r0212 | 4,222 | 46% | 1 min | |
| C. gracile | Diffraction data | r0214 | 206,049 | 40% | 29 min | |
| Data Record 2 | No Sample | Calibration data | r0140 | 2,597 | N.A. | 2 min |
| S. elongatus | Diffraction data | r0142 | 66,442 | 10% | 9 min |
Data Records
Data record 1
Data record 1 contains the raw and preprocessed data of 473,447 snapshots from C. gracile measured during 77 min of beam-time. The X-ray photon energy was 512 eV and both front and back detector panels were included. The snapshots include blank shots, hits of contaminants, and hits of single and multiple C. gracile cells in random orientation and in random stages of the cell cycle, exposed to different pulse intensities. We estimate the hit ratio of C. gracile cells to be 41% (192,370 diffraction patterns).
The raw data is provided in extended tagged container (XTC) format, and the preprocessed data is provided in CXI format. Both are available for download from the Coherent X-ray Imaging Data Bank (CXIDB)22.
Data record 2
Data record 2 contains the raw and preprocessed data of 66,442 snapshots from S. elongatus measured during 9 min of beam-time. The X-ray wavelength was 1,100 eV and both front and back detector panels were included. The snapshots include blank shots, hits of contaminants, and hits of single and multiple S. elongatus cells in random orientation and in random stages of the cell cycle, exposed to different pulse intensities. We estimate the hit ratio of S. elongatus cells to be 10% (6394 diffraction patterns).
The raw data is provided in XTC format, and the preprocessed data is provided in CXI format, both available at the CXIDB (Data Citation 1).
Technical Validation
Viability of cells
In ref. 8 we show that the injection method was not disruptive to the cells and that the cells were alive at the moment of exposure to the X-ray pulse.
Contamination
We have observed contaminants to be present in each data set, i.e., spherical droplets, and virus-like particles. The former is a common artifact from the injection method, and the latter is most likely samples injected earlier that remained in the pipeline. Both contaminants are easily distinguished by their diffraction patterns (see Fig. 2).
Reconstruction validation
It is shown in ref. 8 that diffraction patterns from this data set can be phased and that the resulting electron densities are matching expectations.
Usage Notes
The data is available in CXI format22 (Table 4). CXI uses the HDF5 format23 which is readable in many computational environments such as matlab, python and C.
Table 4. Examples of data stored in the cxi format.
| Description | Location descriptor |
|---|---|
| The examples in this table summarize the structure of the cxi files deposited under ID-37. There are three main groups in the cxi format: LCLS, Cheetah, and entry_1. LCLS contains various experimental parameters, such as the photon energy for each exposure (photon_energy_eV). Cheetah contains cheetah-calculated variables, for example the number of lit pixels (peakNpix). Entry_1 contains preprocessed front and back detector readouts. It might be useful to use the number of lit pixels to sort your data for instance, or perhaps some of the experimental parameters could be useful for as sort as well. For a detailed description of the data format see ref.22. | |
| Preprocessed back detector data of hits | /entry_1/image_1/data |
| Preprocessed front detector data of hits | /entry_1/image_2/data |
| Photon energy of hits | /LCLS/photon_energy_eV |
| Number of lit pixels in hits | /cheetah/event_data/peakNpix |
Additional Information
How to cite this article: van der Schot, G. et al. Open data set of live cyanobacterial cells imaged using an X-ray laser. Sci. Data 3:160058 doi: 10.1038/sdata.2016.58 (2016).
Supplementary Material
Acknowledgments
This work was supported by the Swedish Research Council (621-2011-5710, 2015-05007, 628-2008-1109, 828-2012-108, 822-2012-5260, 822-2010-6157, 621-2012-3404, ICA 12-0064, 621-2012-3570), the Knut and Alice Wallenberg Foundation (KAW-2011.081), the European Research Council (ERC-291602), the Röntgen-Ångström Cluster (349-2011-6488, 2015-06107, 349-2011-6497, 349-2013-574, 05K12CH1), Stiftelsen Olle Engkvist Byggmästare, ELI—Extreme Light Infrastructure—phase 2 (CZ.02.1.01/0.0/0.0/15_008/0000162) from European Regional Development Fund, ‘The Hamburg Center for Ultrafast Imaging’ of the Deutsche Forschungsgemeinschaft (CUI, DFG-EXC1074), and the Helmholtz Association through program funds to DESY. Portions of this research were carried out at the Linac Coherent Light Source, a national user facility operated by Stanford University on behalf of the U.S. Department of Energy, Office of Basic Energy Sciences. We are grateful to the scientific and technical staff of the LCLS for support. We thank the CAMP collaboration for giving us access to their experimental setup and for supporting the experiment at the LCLS. We also acknowledge the Max Planck Society for funding the development and operation of the CAMP instrument.
Footnotes
The authors declare no competing financial interests.
Data Citations
- van der Schot G. 2016. Coherent X-ray Imaging Data Bank. http://dx.doi.org/10.11577/1245696
References
- Fairand B. P. Radiation Sterilization for Health Care Products: X-Ray, Gamma, and Electron Beam. 6, 6–7 (2001).
- Game J. C., Williamson M. S. & Baccari C. X-ray survival characteristics and genetic analysis for nine Saccharomyces deletion mutants that show altered radiation sensitivity. Genetics 169, 51–63 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neutze R., Wouts R., van der Spoel D., Weckert E. & Hajdu J. Potential for biomolecular imaging with femtosecond X-ray pulses. Nature 406, 752–757 (2000). [DOI] [PubMed] [Google Scholar]
- Bergh M. et al. Feasibility of imaging living cells at subnanometer resolutions by ultrafast X-ray diffraction. Q. Rev. Biophys. 41, 181–204 (2008). [DOI] [PubMed] [Google Scholar]
- Rath A. D. et al. Explosion dynamics of sucrose nanospheres monitored by time of flight spectrometry and coherent diffractive imaging at the split-and-delay beam line of the FLASH soft X-ray laser. Optics Express 22, 28914–28925 (2014). [DOI] [PubMed] [Google Scholar]
- Chapman H. N. et al. High-resolution ab initio three-dimensional X-ray diffraction microscopy. J. Opt. Soc. Am. A 23, 1179–1200 (2006). [DOI] [PubMed] [Google Scholar]
- Chapman H. N. et al. Femtosecond X-ray protein nanocrystallography. Nature 470, 73–77 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Schot G. et al. Imaging single cells in a beam of live cyanobacteria with an X-ray laser. Nature Communications 6, 5704 (2015). [DOI] [PubMed] [Google Scholar]
- Hantke M. F. et al. High-throughput imaging of heterogeneous cell organelles with an X-ray laser. Nature Photonics 8, 943–949 (2014). [Google Scholar]
- Seibert M. M. et al. Single mimivirus particles intercepted and imaged with an X-ray laser. Nature 470, 78–U86 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekeberg T. et al. Three-dimensional reconstruction of the giant mimivirus particle with an X-ray free-electron laser. Physical Review Letters 114, 098102 (2015). [DOI] [PubMed] [Google Scholar]
- Komarek J., Kopecky J. & Cepak V. Generic characters of the simplest cyanoprokaryotes Cyanobium, Cyanobacterium and Synechococcus. Cryptogam. Algol. 20, 209–222 (1999). [Google Scholar]
- Cohen-Bazire G. S. Fine-Structure of Cyanobacteria. Methods Enzymol. 167, 157–172 (1988). [Google Scholar]
- Spence J. C. H. et al. Phase recovery and lensless imaging by iterative methods in optical, X-ray and electron diffraction. Phil. Tran.s A Math. Phys. Eng. Sci 360, 875–895 (2002). [DOI] [PubMed] [Google Scholar]
- Raines K. S. et al. Three-dimensional structure determination from a single view. Nature 463, 214–217 (2010). [DOI] [PubMed] [Google Scholar]
- Strüder L. et al. Large-format, high-speed, X-ray pnCCDs combined with electron and ion imaging spectrometers in a multipurpose chamber for experiments at 4th generation light sources. Nucl. Instrum. Methods Phys. Res., Sect. A 614, 483–496 (2010). [Google Scholar]
- Bostedt C. et al. Ultra-fast and ultra-intense x-ray sciences: first results from the Linac Coherent Light Source free-electron laser. J. Phys. B 46, 164003 (2013). [Google Scholar]
- Emma P. et al. First lasing and operation of an angstrom-wavelength free-electron laser. Nat. Photonics 4, 641–647 (2010). [Google Scholar]
- DePonte D. P. et al. Gas dynamic virtual nozzle for generation of microscopic droplet streams. J. Phys. D: Appl. Phys. 41, 195505 (2008). [Google Scholar]
- Bogan M. J. et al. Single particle X-ray diffractive imaging. Nano Lett. 8, 310–316 (2008). [DOI] [PubMed] [Google Scholar]
- Barty A. et al. A new resource for processing serial X-ray diffraction data. J. Appl. Cryst. 47, 1118–1131 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia F. R. N. C. The Coherent X-ray Imaging Data Bank. Nat. Methods 9, 854–855 (2012). [DOI] [PubMed] [Google Scholar]
- The HDF Group. Hierarchical Data Format, version 5, 1997-2016. http://www.hdfgroup.org/HDF5/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- van der Schot G. 2016. Coherent X-ray Imaging Data Bank. http://dx.doi.org/10.11577/1245696