ABSTRACT
Isorhapontigenin (ISO) is a new derivative of stilbene isolated from the Chinese herb Gnetum cleistostachyum. Our recent studies have revealed that ISO treatment at doses ranging from 20 to 80 μM triggers apoptosis in multiple human cancer cell lines. In the present study, we evaluated the potential effect of ISO on autophagy induction. We found that ISO treatment at sublethal doses induced autophagy effectively in human bladder cancer cells, which contributed to the inhibition of anchorage-independent growth of cancer cells. In addition, our studies revealed that ISO-mediated autophagy induction occurred in a SESN2 (sestrin 2)-dependent and BECN1 (Beclin 1, autophagy related)-independent manner. Furthermore, we identified that ISO treatment induced SESN2 expression via a MAPK8/JNK1 (mitogen-activated protein kinase 8)/JUN-dependent mechanism, in which ISO triggered MAPK8-dependent JUN activation and facilitated the binding of JUN to a consensus AP-1 binding site in the SESN2 promoter region, thereby led to a significant transcriptional induction of SESN2. Importantly, we found that SESN2 expression was dramatically downregulated or even lost in human bladder cancer tissues as compared to their paired adjacent normal tissues. Collectively, our results demonstrate that ISO treatment induces autophagy and inhibits bladder cancer growth through MAPK8-JUN-dependent transcriptional induction of SESN2, which provides a novel mechanistic insight into understanding the inhibitory effect of ISO on bladder cancers and suggests that ISO might act as a promising preventive and/or therapeutic drug against human bladder cancer.
KEYWORDS: autophagy, bladder cancer, isorhapontigenin, MAPK8, sestrin 2
Introduction
Bladder cancer is a common urological cancer that ranks as the sixth most common cancer in the United States following breast cancer, lung cancer, prostate cancer, colon cancer and lymphoma.1 It is the third most common cancer in men, and the eleventh most common cancer in women.1 There were an estimated 74,000 new cases diagnosed with bladder cancer in 2015; and 16,000 of these patients died from this disease in the United States according to a report from the National Cancer Institute. The majority of human bladder cancer is transitional cell carcinomas derived from the uroepithelium, which accounts for more than 90% of bladder carcinomas.2 The depth of invasion of the bladder wall is closely associated with the clinical treatment of bladder cancers.3 Since high-grade invasive bladder cancer can progress to a life-threatening metastases and a 5-year overall survival in patients with lymph node-only disease was 20.9%,4 the discovery and evaluation of a new alternative medication will be of tremendous importance for reducing the mortality of this disease.
The Chinese herb Gnetum cleistostachyum has been used for treatment of human bladder cancers for centuries, but its bio-active components and anticancer mechanisms have been barely explored.5 Isorhapontigenin (ISO) is a new derivative of a stilbene compound isolated from this herbal medicine with multiple anticancer activities demonstrated in our previous studies.5-7 We found that ISO at low dosages (5–10 μM) effectively induces G0/G1 growth arrest through an inhibition of the key cell-cycle regulatory protein CCND1 (cyclin D1) expression,5,6 whereas it causes an apoptotic response of cancer cells by inhibiting transcription of the anti-apoptotic XIAP (X-linked inhibitor of apoptosis) gene at relatively high dosages (20–60 μM).7 Our most recent studies demonstrate that in vivo ISO (150 mg/kg/d) can markedly inhibit xenograft tumor growth in nude mice injected with high invasive human bladder cancer T24T cells (p < 0.01, n = 6).8 All these studies suggest that ISO may be a promising agent for the prevention and/or treatment of human bladder cancer.
Macroautophagy/autophagy is a highly evolutionarily conserved catabolic process characterized by the formation of phagophores, a double-membrane compartment that sequesters long-lived cytoplasmic proteins and organelles; the phagophore matures into an autophagosome allowing cargo degradation by further fusing with a lysosome.9 There is emerging evidence for the role of autophagy during development and differentiation,10 and in diseases such as cancer.11 In combination with various conventional chemotherapeutic drugs, inhibition of autophagy has emerged as an attractive and promising approach to sensitize malignancies to chemotherapy in cancer therapy, which is based on the fundamental premise that autophagy plays a protective role in the anticancer action of these chemotherapeutic agents.12 However, a recent report has also shown that there are at least 4 functional forms of autophagy that may occur in response to chemotherapeutic drugs in a context (cancer cell line and agent)-specific manner: cytoprotective, cytostatic, cytotoxic and nonprotective.12 Therefore, it is necessary and essential to recognize the functional type of autophagy induced by chemotherapeutic drugs and to further determine the underlying mechanism prior to their application in clinical trials.
SESN2 (sestrin 2) encodes a member of the sestrin family of PA26-related proteins, which plays major roles in suppression of oxidative stress and regulation of adenosine 5′-monophosphate-activated protein kinase (AMPK)-MTOR (mechanistic target of rapamycin [serine/threonine kinase]) signaling.13,14 The SESN2 protein may be involved in complex regulation of cell viability in response to different stress conditions.15 Ectopic SESN2 expression could inhibit cell growth and proliferation, whereas it also results in protection from apoptotic cell death induced by oxygen- and/or glucose-deprivation or H2O2 treatment in a breast cancer cell line.16 In the present study, we found that SESN2 was dramatically downregulated in human bladder cancer tissues. We also found that ISO treatment effectively induced autophagy in an SESN2-dependent manner, and such ISO-induced autophagy was required for the ISO inhibition of anchorage-independent cancer cell growth, demonstrating that autophagy induced by ISO is cytotoxic to human bladder cancer cells. Moreover, we determined that JUN was a direct transcriptional factor that was responsible for the induction of SESN2 expression by ISO treatment.
Results
ISO effectively induced autophagy in human cancer cells
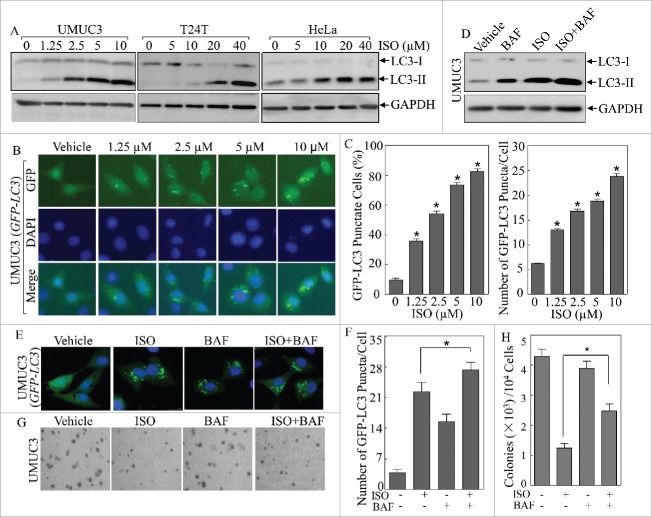
The in vitro anticancer activity of ISO has been well documented by our previous studies showing that treatment of ISO effectively inhibits anchorage-independent growth of human bladder and colon cancer cells via modulating the expression of CCND1 and XIAP. However, potential effect of ISO treatment on autophagy and the role of autophagy in the anticancer activity of ISO have never been explored. To evaluate the potential effect of ISO on autophagy, we therefore treated several human cancer cell lines including human bladder cancer UMUC3 and T24T cells, as well as human cervical cancer HeLa cells with various concentrations of ISO for 24 h. As shown in Fig. 1A, ISO treatment resulted in a significant increase in the conversion of LC3 from LC3-I to LC3-II, indicating that ISO effectively induces autophagy in these cancer cells in a dose-dependent manner.
Figure 1.
ISO induced autophagy in human cancer cells. (A) Human bladder cancer UMUC3 and T24T cells and human cervical cancer HeLa cells, were treated with ISO at the indicated doses for 24 h. The cells were extracted and cell lysates were subjected to western blotting assay by using the indicated antibodies. (B) The GFP-LC3 construct was stably transfected into UMUC3 cells, and the transfectants were treated with various doses of ISO for 24 h. LC3 puncta formation was observed and images were captured using fluorescence microscopy. (C) Percentage of cells with GFP-LC3 puncta (left) and number of puncta per positive cell (right) were calculated and presented as described in Materials and Methods. The symbol (*) indicates a significant increase from the vehicle control (p < 0.05). (D) UMUC3 cells were treated with ISO (10 μM), together with or without 5 nM BAF for 24 h and the protein levels of LC3 were assessed by western blotting. (E) UMUC3 cells were transfected with the GFP-LC3 construct and the transfectants were treated with ISO (10 μM), with or without 5 nM BAF for 24 h. The representative images of GFP-LC3 puncta were captured using a confocal fluorescence microscope. (F) Number of puncta per GFP-LC3-positive cell was calculated and presented as described in Materials and Methods. The symbol (*) indicates a significant increase from the ISO treatment group (p < 0.05). (G) Representative images of colonies of UMUC3 treated with 10 μM ISO, 1 nM BAF or both in soft-agar assay were captured as described in Materials and Methods. (H) Colonies shown in (G) were counted under a microscope with more than 32 cells of each colony. The results are presented as colonies per 104 cells, and the bars show mean ± SD from 3 independent experiments. The symbol (*) indicates a significant increase from the ISO treatment group (p < 0.05).
To further demonstrate autophagy induction by ISO treatment, we established stable UMUC3 cells transfected with a GFP-LC3 plasmid, encoding a fusion protein that would allow us to observe the formation of autophagosomes.17 Following the treatment of UMUC3 (GFP-LC3) cells with various concentrations of ISO for 24 h, the percentage of GFP-LC3 puncta-positive cells and numbers of GFP-LC3 puncta per cell increased along with the doses of ISO treatment (Fig. 1B and C), again revealing that ISO treatment led to the formation of autophagosomes and the induction of autophagy in a dose-dependent manner. In addition, autophagy flux was determined by treatment with bafilomycin A1 (BAF; a vacuolar-type H+-ATPase inhibitor that blocks autophagosome and lysosome fusion). ISO treatment led to further increase of LC3-II (Fig. 1D) and GFP-LC3 puncta levels (Fig. 1E and F) in the presence of BAF, suggesting that ISO increases the autophagy flux level.
To determine the physiological relevance of ISO-induced autophagy in ISO-mediated inhibition of anchorage-independent cancer cell growth, a soft-agar assay was performed using UMUC3 cells treated with ISO alone or in combination with BAF. As seen in Fig. 1G and H, the presence of BAF obviously abolished the inhibitory effects of ISO on anchorage-independent growth of UMUC3 cells. These results demonstrate that ISO is able to induce autophagy effectively, which might be associated with its inhibition of anchorage-independent growth of human bladder cancer cells.
SESN2, but not BECN1, was crucial for autophagy induction and inhibition of anchorage-independent growth of human bladder cancer cells by ISO
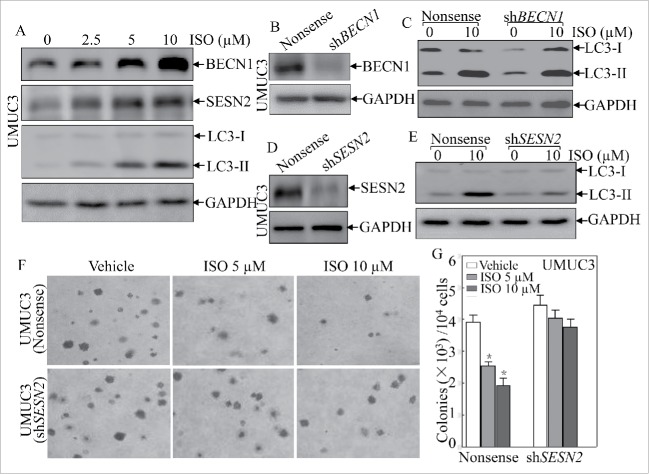
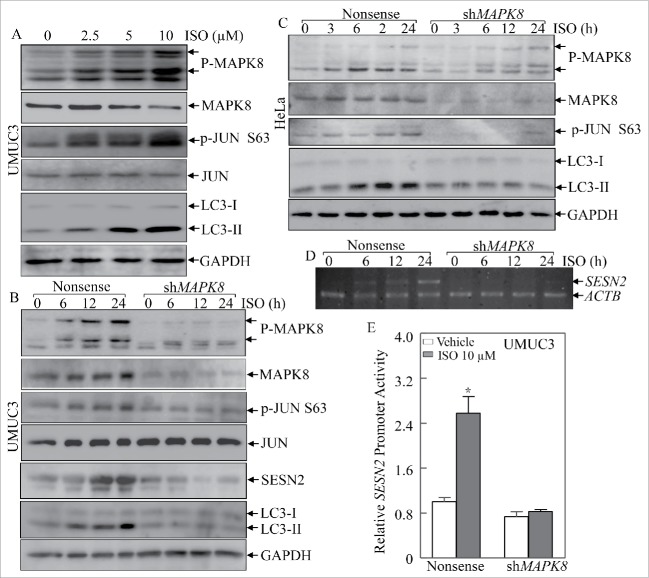
BECN1 is a critical upstream regulator of autophagy, and heterozygous disruption of BECN1 causes a high incidence of spontaneous tumors,18,19 and SESN2 has also been reported to participate in serum deprivation-induced autophagy and cell death in cancer cells.20 Thus, we determined whether BECN1 and/or SESN2 were involved in ISO-associated autophagy. Interestingly, ISO treatment increased expression of both BECN1 and SESN2 at the autophagy induction doses (2.5∼10 μM; Fig. 2A). To evaluate the roles for BECN1 and SESN2 in ISO-induced autophagy, we employed short-hairpin RNAs (shRNAs) targeting human BECN1 or SESN2 to knock down expression of endogenous BECN1 or SESN2 in UMUC3 cells. The corresponding stable transfectants were established and identified as shown in Fig. 2B and D. Unexpectedly, knockdown of BECN1 expression had no observable effects on ISO-induced conversion of LC3 from LC3-I to LC3-II (Fig. 2C). However, SESN2 depletion significantly attenuated ISO-mediated induction of LC-3II (Fig. 2E). These data suggested that ISO-induced autophagy occurred in a SESN2-dependent and BECN1-independent manner. Furthermore, we assessed the effects of SESN2 depletion on ISO-mediated inhibition of anchorage-independent growth, and found that SESN2 knockdown pronouncedly abolished the inhibitory effects of ISO on anchorage-independent growth of human bladder cancer cells (Fig. 2F and G). Collectively, these results suggest that induction of SESN2, but not BECN1, plays an essential role in ISO-mediated autophagy induction and anchorage-independent growth inhibition.
Figure 2.
SESN2 was required for autophagic induction and anchorage-independent growth inhibition by ISO in human bladder cancer UMUC3 cells. (A) UMUC3 cells were treated with various concentrations of ISO for 24 h. The cells were extracted and cell lysates were subjected to western blotting to detect the expression of BECN1, SESN2 and LC3. (B and D) shRNA BECN1 (B) and shRNA SESN2 (D) were stably transfected into UMUC3 cells, and the stable transfectants were identified. (C and E) The indicated stable transfectants were subjected to ISO treatment for 24 h for determination of LC3 in ISO-induced autophagy in UMUC3 cells. (F) Representative images of colonies of UMUC3 (shSESN2) and UMUC3 (Nonsense) cells in soft-agar assay in the presence or absence of various concentrations of ISO were captured using a microscope. (G) Colonies shown in (F) were counted under a microscope with more than 32 cells of each colony. The results are presented as colonies per 104 cells, and the bars shows mean ± SD from 3 independent experiments. The symbol (*) indicates a significant decrease from vehicle control (p < 0.05).
ISO increased SESN2 transcription via a JUN-dependent mechanism
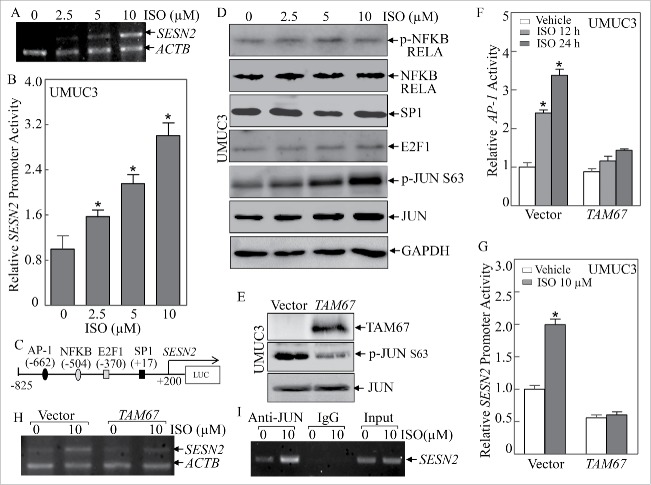
In order to characterize the molecular mechanism underlying the modulation of SESN2 expression by ISO treatment, we first checked the alteration of SESN2 mRNA expression in UMUC3 upon ISO treatment by using RT-PCR (Fig. 3A). Consistent with the effects of ISO on SESN2 protein expression, ISO treatment increased SESN2 mRNA expression in a dose-dependent manner, suggesting that the regulatory effect of ISO on SESN2 expression may occur at the transcriptional level. As expected, ISO treatment indeed led to a ∼2 to 3-fold increase in the activity of a SESN2 promoter-driven luciferase reporter (Fig. 3B). We then performed a bioinformatics search for putative transcription factors that are potentially responsible for the induction of SESN2 transcription by ISO, and found that there were several potential binding sites for transcription factors in the SESN2 proximal promoter region including AP-1, NFKB, E2F1 and SP1 (Fig. 3C). Therefore, we examined the expression alterations of those potential transcription factors.
Figure 3.
JUN activation and its binding to the AP-1 binding consensus in SESN2 promoter-mediated SESN2 transcriptional expression in human bladder cancer cells. (A) UMUC3 cells were treated with various concentrations of ISO for 12 h. RT-PCR was carried out to evaluate the expression of SESN2 mRNA. (B) The SESN2 promoter-driven luciferase reporter was transfected into UMUC3 cells. The stable transfectants were subjected to ISO treatment for 12 h to evaluate SESN2 promoter transcriptional activity. The induction fold was normalized using pRL-TK as an internal control. The results are presented as SESN2 promoter activity relative to vehicle control (relative SESN2 promoter activity). The bars show mean ± SD from 3 independent experiments. The symbol (*) indicates a significant increase from the vehicle control (p < 0.05). (C) The putative transcription factor consensus binding site in the SESN2 proximal promoter region was predicted using bioinformatics analysis. (D) The expression levels of potential transcription factors were determined following ISO treatment for 24 h using western blotting. (E) TAM67 was stably transfected into UMUC3 cells and the stable transfectants were identified by western blotting. (F and G) AP-1 luciferase reporter (F) or SESN2 promoter luciferase reporter (G) was transiently transfected into UMUC3 (TAM67) and UMUC3 (Vector) cells in combination with pRL-TK. The transfectants were treated with 10 μM ISO for 12 or 24 h and luciferase activity was determined and presented as relative AP-1 activity (F) or relative SESN2 promoter activity (G). The symbol (*) indicates a significant increase from the vehicle control (p < 0.05). (H) RT-PCR was carried out to determine the mRNA changes of SESN2 following ISO treatment for 12 h in the indicated cells. (I) ChIP was carried out using anti-JUN antibody to detect the interaction of JUN with the SESN2 promoter following ISO treatment for 12 h.
As shown in Fig. 3D, ISO treatment had no observable effects on the expression levels of phospho-NFKB RELA, NFKB RELA, SP1 or E2F1, but profoundly increased JUN phosphorylation at Ser63, suggesting that JUN might be involved in the transcriptional induction of SESN2 by ISO treatment. To test this notion, we blocked JUN activation in UMUC3 cells by using a well-characterized JUN dominant negative mutant, TAM67 (Fig. 3E),21 and treated these stable transfectants with 10 μM ISO for 12 or 24 h. As expected, ectopic expression of TAM67 successfully inhibited the induction of endogenous AP-1-dependent transcriptional activity by ISO (Fig. 3F), and further abolished the ISO-mediated transcriptional induction of SESN2 promoter activity (Fig. 3G). Moreover, blockade of endogenous JUN activation by TAM67 also significantly attenuated the increased effects of ISO on SESN2 mRNA levels (Fig. 3H). These results strongly demonstrate that JUN-AP-1 plays an essential role in ISO-mediated induction of SESN2 expression.
To provide direct evidence for the involvement of JUN in ISO-induced SESN2 expression, we performed a chromatin immunoprecipitation (ChIP) assay using anti-JUN antibody, and found that JUN directly bound to the putative AP-1 binding site in the SESN2 promoter even in the absence of ISO treatment (Fig. 3I). More importantly, ISO treatment further resulted in a remarkable increase in the binding of JUN to the AP-1 site in the SESN2 promoter (Fig. 3I). Taken together, our results reveal that ISO treatment increases SESN2 expression via a JUN-AP-1-dependent mechanism, in which JUN is activated upon ISO exposure and further upregulates SESN2 transcription by directly binding to the putative AP-1 site in the SESN2 promoter.
JUN activation was crucial for ISO-mediated autophagy induction and anchorage-independent growth inhibition
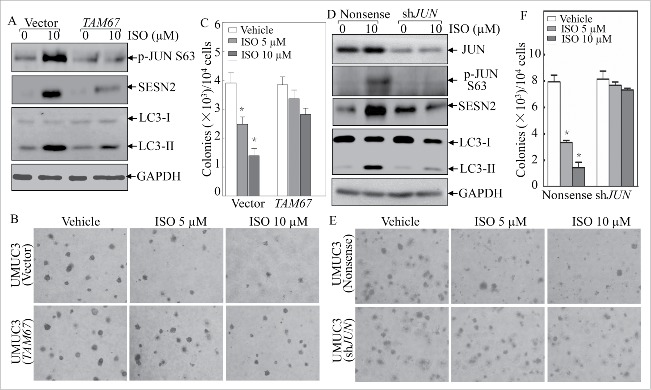
To evaluate the role for JUN in ISO inhibition of anchorage-independent growth of human bladder cancer cells, we established stable transfectants by using the dominant mutant of JUN (TAM67) or shRNA specifically targeting human JUN. As shown in Fig. 4A, ectopic expression of TAM67 significantly abolished the induction of SESN2 protein expression upon ISO treatment. As expected, blockade of endogenous JUN activation by TAM67 also dramatically inhibited the ISO-mediated conversion of LC3-I to LC3-II (Fig. 4A), suggesting that TAM67 blocked the autophagy induction by ISO treatment. Additionally, ISO treatment at 5 and 10 μM led to an obvious reduction in colony formation of vector control cells in soft agar, while ISO treatment at the same doses failed to inhibit anchorage-independent growth in UMUC3 cells stably transfected with TAM67 (Fig. 4B and C). This notion was greatly supported by the results obtained from utilization of stable transfectants of shRNA specifically targeting human JUN (Fig. 4D–F). Collectively, JUN expression and activation are essential for ISO-mediated SESN2 protein expression, autophagy induction and cancer cell anchorage-independent growth inhibition.
Figure 4.
JUN activation was crucial for autophagy induction and anchorage-independent growth inhibition by ISO treatment. (A and D) The stable transfectants, UMUC3 (TAM67) vs. UMUC3 (Vector) or UMUC3 (shJUN) vs. UMUC3 (Nonsense) cells, were treated with 10 μM ISO for 24 h. The cells were then extracted and the cell lyses were subjected to western blotting for determination of JUN phosphorylation, SESN2 induction and LC3-II generation as indicated. (B and E) Representative images of anchorage-independent growth of UMUC3 (TAM67) vs. UMUC3 (Vector) or UMUC3 (shJUN) vs. UMUC3 (Nonsense) in the absence or presence of various concentrations of ISO were visualized and captured using a microscope. (C and F) The colony formation was counted under a microscope with more than 32 cells of each colony, and the results presented as colonies/104 cells. The bars indicate mean ± SD from 3 independent experiments. The symbol (*) shows a significant decrease from the vehicle control (p < 0.05).
MAPK8 is the upstream kinase responsible for JUN-dependent SESN2 induction and SESN2 was downregulated in human bladder cancer tissues
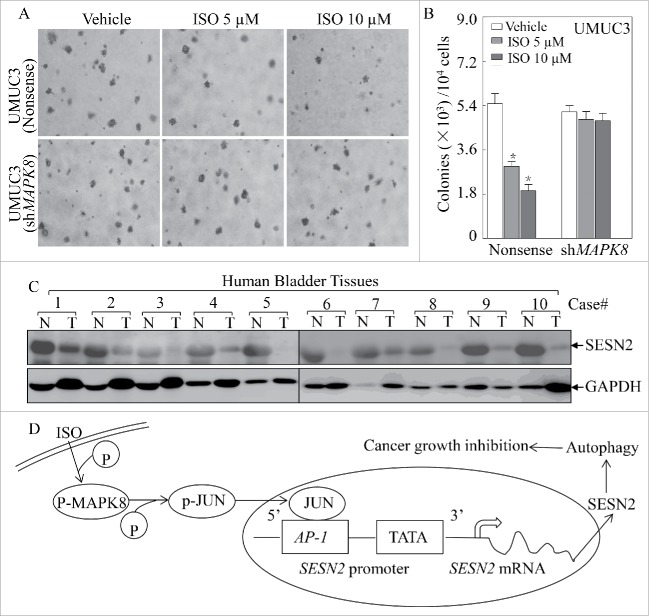
MAPKs22 are rapidly activated in response to various extracellular stimuli including growth factors, cytokines and cellular stresses.23 Therefore we determined whether a MAPK was the upstream kinase responsible for JUN-dependent SESN2 induction by ISO treatment. As shown in Fig. 5A, activation of MAPK8 was observed in accordance with JUN activation as well as LC3 conversion. When shRNA to MAPK8 was introduced to knock down endogenous MAPK8 expression in UMUC3 cells, it not only blocked JUN activation, but also attenuated SESN2 induction and LC3-II formation following ISO treatment (Fig. 5B). Similar results were also obtained from human cervical cancer HeLa cells (Fig. 5C). These results suggest that MAPK8 is crucial for ISO-induced JUN activation and SESN2-dependent autophagy. In addition, we also found that knockdown of endogenous MAPK8 abolished the transcriptional induction of SESN2 mRNA following ISO treatment (Fig. 5D and E), again supporting the crucial role for MAPK8 in ISO-mediated transcriptional induction of SESN2. More importantly, 5 and 10 μM of ISO treatment led to a significant inhibition of anchorage-independent cell growth in nonsense control UMUC3 cells, but lost this inhibition in MAPK8 knockdown transfectants (Fig. 6A and B), emphasizing the indispensable role for the MAPK8-JUN axis in ISO inhibition of anchorage-independent cell growth of human bladder cancer cells.
Figure 5.
MAPK8 is the upstream kinase mediating for JUN activation and SESN2 expression following ISO treatment. (A) UMUC3 cells were treated with various concentrations of ISO for 24 h. The cells were extracted and cell lyses were subjected to western blotting to determine the activation of JUN and MAPK8 as well as generation of LC3-II. (B and C) shRNA MAPK8 was stably transfected into UMUC3 cells (B) and HeLa cells (C). Following treatment with ISO (10 μM) for the indicated time points, proteins were extracted and cell extracts were then subjected to western blotting for evaluation of the changes of JUN, MAPK8, SESN2 and LC3. (D) UMUC3 (shMAPK8) and UMUC3 (Nonsense) transfectants were subjected to ISO treatment and SESN2 mRNA level was detected by RT-PCR. (E) SESN2 promoter-driven luciferase reporter together with pRL-TK was transiently transfected into UMUC3 (shMAPK8) and UMUC3 (Nonsense) cells. The transfectants were treated with ISO (10 µM) for 12 h and then analyzed for luciferase activity assay. The bars show mean ± SD from 3 independent experiments. The symbol (*) indicates a significant difference between the vehicle control and ISO treatment (p < 0.05).
Figure 6.
MAPK8 is required for ISO inhibition of anchorage-independent growth in UMUC3 cells and SESN2 was downregulated in human bladder cancer tissues. (A) Representative images of colonies of UMUC3 (shMAPK8) and UMUC3 (Nonsense) cells in a soft-agar assay in the absence or presence of various concentrations of ISO were captured using a microscope. (B) The colony formation was counted under a microscope with more than 32 cells of each colony, and the results presented as colonies/104 cells. The bars indicate mean ± SD from 3 independent experiments. The symbol (*) shows a significant decrease from the vehicle control (p < 0.05). (C) Clinically freshly collected human bladder cancer samples in comparison with the paired adjacent normal bladder tissue were subjected to western blotting to analyze SESN2 expression. N, normal; T, tumor. (D) The proposed model for ISO induction of SESN2 and autophagy and inhibitory effect on human bladder cancer cells.
We had defined the crucial role for the MAPK8-JUN axis in ISO-mediated transcriptional induction of SESN2 in vitro as mentioned above. However, the expression pattern of SESN2 in human bladder cancer tissues remained unknown. Therefore, we compared the protein levels of SESN2 in 10 pairs of human bladder cancer tissues and matched adjacent normal bladder tissues. As shown in Fig. 6C, SESN2 expression was greatly downregulated or even lost in human bladder cancer tissues as compared to the corresponding adjacent normal bladder tissues. Our results reveal that SESN2 downregulation is associated with bladder cancer formation, and further suggest that elevation of SESN2 expression by ISO might be related to its anticancer effects.
Discussion
ISO is a new derivative of stilbene isolated from the Chinese herb Gnetum cleistostachyum.6 Our recent studies have revealed that ISO triggers apoptosis in multiple human cancer cell lines.5,7 In the current studies, we found that at sublethal doses ISO treatment induced autophagy via upregulation of SESN2 in a MAPK8-JUN-dependent manner, which further contributed to ISO inhibition of anchorage-independent growth of cancer cells. An interesting observation made from this study is that SESN2 was downregulated in human bladder cancer tissues as compared to the paired adjacent normal human bladder tissues, while ISO treatment induced upregulation of SESN2 expression in bladder epithelial cells. Collectively, our results not only identify the participation of autophagy in the anticancer activity of ISO, but also further provide a novel molecular insight into understanding autophagy induction upon ISO treatment as diagrammed in Fig. 6D.
Autophagy is a critical process that senses intracellular homeostasis changes and helps cells to survive during nutrient depletion, energy insufficiency or other cellular stress conditions.24 Under stress circumstances, autophagy can also target the degradation of larger protein complexes and organelles, such as ribosomes and mitochondria, leading to the inhibition of cell growth.25,26 Conventional cytotoxic chemotherapeutic drugs and irradiation induce autophagy, and depending on the extent of induction, duration, and cellular context, autophagy further causes cell growth arrest, senescence and apoptosis.27,28 In line with this, our recent studies showed that treatment with relatively higher doses of ISO (20–60 μM) trigger obvious cell death in T24T cells,7 whereas lower doses of ISO treatment (5–20 μM) induce cell cycle arrest in UMUC3 cells.5
In the current study, we further found that ISO treatment activated the autophagy process. Using loss-of-function analyses, we found that SESN2 played an essential role in ISO-induced autophagy. In addition, ISO treatment increased MAPK8-JUN phosphorylation under the same experimental conditions, and introduction of shRNA to MAPK8, shRNA to JUN or TAM67, attenuated SESN2 expression as well as autophagy induction upon ISO treatment. The results obtained from using specific shRNA targeting MAPK8 and JUN or TAM67 indicate that these render UMUC3 cells resistant to ISO inhibition of anchorage-independent growth. All results demonstrate that autophagy induction plays an important role in mediating inhibitory effect of ISO on anchorage-independent growth of human bladder cancer cells.
SESN2 is reported to be associated with oxidative stress-induced autophagy.29 TP53/p53 is defined as a major transcriptional regulator of SESN2 under DNA damage and oxidative stress.30 In our current studies, we found that ISO treatment increased SESN2 expression at the transcriptional level demonstrated by using RT-PCR and a SESN2 promoter-driven luciferase reporter. Since UMUC3 cells carry a TP53 F113C missense mutation, which inactivates TP53-dependent transcriptional regulation,31 we focused on the TP53-independent transcriptional regulation of SESN2 induction. In our case, AP-1 was identified as the key transcription factor that was activated by ISO. Knockdown of JUN or overexpression of TAM67 impaired SESN2 induction, and the physical binding of JUN to the promoter region of SESN2 was also proven by ChIP assay. Our results demonstrate that ISO treatment results in activation of JUN-AP-1, which in turn upregulates SESN2 transcription and expression.
MAPK8 is known as a stress-activated protein kinase (SAPK) of the MAPK family, and is initially activated in response to a variety of stress signals and has been implicated in many cellular events including apoptosis and autophagy.32,33 It has been reported that the activation of the MAPK8-JUN pathway involves the upregulation of SESN2 induced by oxidized LDL.15 Our previous findings revealed that ISO treatment activates the MAPK8-JUN pathway, which plays an important role in suppression of transformation of normal cells to cancerous cells promoted by growth factors.6 Our findings here indicate that the MAPK8-JUN pathway contributes to autophagy induction by ISO in UMUC3 cells, suggesting that the MAPK8-JUN pathway is a new key pathway for SESN2 induction that can lead to autophagy induction and repression of transformation under stress conditions.6
In summary, our current studies reveal that ISO treatment at sublethal dose triggers autophagy of human bladder cancer cells in a MAPK8-JUN-SESN2-dependent manner, which contributes to abrogation of cancer cell anchorage-independent growth. The results generated from the current studies, taken together with our most recent data demonstrating ISO inhibition of human bladder tumor growth in xenograft nude mice,8 not only improve our understanding of ISO induction of autophagy and the underlying mechanisms of this important biological effect, but also provide us with valuable information for our future investigation of the potential application of ISO in prevention and/or therapy of human bladder cancer.
Materials and methods
Cell culture and reagents
UMUC3 and HeLa cells as well as their stable transfectants were maintained at 37°C in a 5% CO2 incubator with Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich Corporation, D6429) supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals, S12450), 2 mmol L-glutamine (GIBCO, 25030–081), and 1% penicillin/streptomycin (Life Technologies, 15140163). T24T cells were cultured in DMEM:F12 (1:1; Corning Inc., 10-090-CV) with 5% FBS. The cultures were dissociated with trypsin (GIBCO, 15400–054) and transferred to new 75-cm2 culture flasks twice a wk. ISO (Higher Biotech, 10200109) with over 98% purity was dissolved in dimethyl sulfoxide (Sigma-Aldrich Corporation, 67-68-5) to make a stock concentration at 20 mmol and the same concentration (0.1%, v/v) of dimethyl sulfoxide was used as a negative control in all experiments. BAF (sc-201550) was from Santa Cruz Biotechnology.
Constructs and transfection
GFP-LC3 and its vector control were a kind gift from Dr. Gang Chen (University of Kentucky, Lexington, KY). ShRNA constructs against human BECN1 (V3LHS-349509), SESN2 (V2LHS-117405), JUN/C-JUN (V2LHS-262965) and MAPK8/JNK1 (V2LHS-170499) were purchased from Open Biosystems. An expression construct for the JUN dominant negative mutant (pcDNA3.1/TAM67) was kindly provided by Dr. Tim G. Bowden (College of Pharmacy, University of Arizona, Tucson, AZ) and Dr. Matthew Young (The Center for Cancer Research, National Cancer Institute, Frederick, MD). The AP-1-Luc plasmid (219074) was purchased from Stratagene. The cells were stably transfected by using PolyJet™ DNA In Vitro Transfection Reagent (SignaGen Laboratories, SL100468), according to the manufacturer's instruction, as described in our previous studies.34,35 The transfections were subjected to selection with either puromycin (Santa Cruz Biotechnology, Sc-108071B) or hygromycin B (Gold Biotechnology, H-270-1) and all surviving transfectants from the selection were pooled as stable mass cultures. These stable transfectants were cultured in selective antibiotic-free medium for at least 2 passages before utilization for experiments.
Fluorescence microscopy
UMUC3 cell transfectants were cultured on cover slides in 10% FBS DMEM medium for 24 h. The cells were exposed to ISO at the indicated dose and time and fixed with 4% paraformaldehyde (Sigma-Aldrich Corporation, 158127) in PBS (135 mM NaCl, 4.7 mM KCl, 10 mM Na2HPO4, 2.0 mM NaH2PO4, pH 7.4) at room temperature for 10 min, then washed 3 times with PBS (Corning Inc., 55-031-PC). Next, the membrane was permeabilized with PBS containing 0.1% Triton X-100 (Thermo Fisher Scientific Inc., 28314) for 10 min, and then stained with 0.1 mg/ml DAPI (Sigma-Aldrich Corporation, 9542) for 30 min. The slides were washed 3 times with PBS and mounted with antifade reagent (Molecular Probes, P36930). All cell images were captured using an inverted Leica fluorescence microscope (Wetzlar, Germany). Cells with 5 or more intense GFP-LC3 puncta were considered autophagic cells, whereas those with diffuse cytoplasmic GFP-LC3 staining were considered nonautophagic cells. The percentage of GFP-LC3-positive cells were calculated based on at least 200 counted cells. The number of GFP-LC3 puncta per cell was counted for at least 50 cells.
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from the cells using Trizol reagent (Invitrogen Corporation, 15596-018). Total cDNAs were synthesized by ThermoScript™ RT-PCR system (Invitrogen Corporation, 11146–057). The mRNA amount present in the cells was measured by semiquantitative RT-PCR. The primers for human SESN2 were 5′-AGA GGG CAC AGG AAA GAA-3′ and 5′-TCA AGC ATA AAG GAC CAA A-3′; human ACTB/β- ACTIN were 5′-CTC GCC TTT GCC GAT CCG CCG-3′ and 5′-AGT GGT ACG GCC AGA GGC GTA C-3′. The PCR products were separated on 2% agarose gels, stained with ethidium bromide (Fisher Scientific Corporation, 1239-45-8), and scanned for images under UV light. The results were displayed with an Αlpha Innotech SP image system (Αlpha Innotech Corporation, San Leandro, CA, USA).
Construction of SESN2 promoter-driven luciferase reporter
A fragment spanning from −825 to +200 relative to the transcription start site of the human SESN2 genomic sequence was produced by PCR with the forward primer 5′-TCA GGT ACC GAA TTT AGC TTG GAG GTG C-3′ and the reverse primer 5′-CCG CTC GAG GCT TTG GTG CTG GAC TCT T-3′. The PCR products were subcloned into the KpnI and XhoI sites of pGL3-Basic vector (Promega Corporation, E1751) to generate the SESN2 promoter-driven luciferase reporter (SESN2-Luc). The construct was confirmed by DNA sequencing.
Luciferase reporter assay
AP-1 luciferase reporter (AP-1-Luc) was purchased for determining AP-1-dependent transcriptional activity. In this plasmid, the expression of the firefly luciferase gene is controlled by a synthetic promoter that contains direct repeats of the transcription recognition sequences for AP-1. The SESN2-Luc construct was generated as described above for determining relative SESN2 promoter activity. UMUC3 cells were transfected with an AP-1-Luc plasmid or SESN2-Luc constructs in combination with the pRL-TK vector (Promega Corporation, E2241) as an internal control. The dual luciferase assay kit (E1960) was purchased from Promega Corporation. The luciferase activities were determined using a luminometer (Wallac 1420 Victor 2 multilabel counter system) as described in previous studies.36,37
Anchorage-independent growth assay
The soft-agar assay was performed as described previously.6,38 Briefly, 2.5 ml of 0.5% agar in basal modified Eagle's medium supplemented with 10% FBS with or without ISO was layered onto each well of 6-well tissue culture plates. 1 × 104 UMUC3 cells or their transfectants were mixed with 1 ml of 0.5% agar (Becton, Dickinson and Company, 214010) in basal modified Eagle's medium supplemented with 10% FBS with or without ISO, and then layered on top of the 0.5% agar layer. The plates were incubated at 37°C in 5% CO2 for 2 weeks. The colonies with more than 32 cells were scored and the results were presented as colonies/104 cells.
ChIP assay
ChIP was performed using the ChIP kit (Millipore Technologies, 17–295) as described in our previous publication.7 Briefly, UMUC3 cells were treated with or without 10 μM ISO for 24 h. Then genomic DNA and the proteins were cross-linked with 1% formaldehyde (Protocol Corporation, 245–684). The cross-linked cells were pelleted, resuspended in lysis buffer, and sonicated to generate 200–500 base pair chromatin DNA fragments. After centrifugation, the supernatant fractions were diluted 10-fold and then incubated with anti-JUN/C-JUN antibody (Santa Cruz Biotechnology, sc-45X) or the control rabbit IgG (Santa Cruz Biotechnology, sc-2027) overnight at 4°C. The immune complex was captured by protein G-agarose (Santa Cruz Biotechnology, C1014) saturated with salmon sperm DNA (Upstate Biotechnology Inc., 0606031838), then eluted with the elution buffer. DNA-protein crosslinking was reversed by heating overnight at 65°C. DNA was purified and subjected to PCR analysis. To specifically amplify the region containing the putative responsive elements on the human SESN2 promoter, PCR was performed with the following pair of primers: 5′-AAA AGG GTC AGA TAA AAC AT-3′ and 5′-TTA GTA AAT AGA GAC AGG GT-3′. The PCR products were separated on 2% agarose gels and stained with ethidium bromide; the images were then scanned with a UV light.39
Western blotting assay
The cells were washed twice with ice-cold PBS and collected with cell lysis buffer (10 mM Tris-HCl, pH 7.4, 1% SDS [Thermo Fisher Scientific Inc., BP16650], 1 mM Na3VO4). The cell extracts were sonicated, denatured by heating at 100°C for 5 min, and protein concentrations were determined by Nano Drop 2000 (Thermo Fisher Scientific Inc., Waltham, MA). Equal aliquots of cell extracts were separated on SDS-polyacrylamide gels. The proteins were then transferred to PVDF membranes, blocked, and probed with one of the antibodies against phospho-JUN S63 (2361S), total-JUN (9165S), phospho-MAPK8 T183/Y185 (4668S), total-MAPK8 (9252S), E2F1 (3742S), BECN1 (3495S), total NFKB RELA (4764S), phospho-NFKB RELA S536 (3033S), LC3 (2775S) or GAPDH (5174S) (Cell Signaling Technology); SESN2 (sc-292558) or SP-1 (sc-59) (Santa Cruz Biotechnology); or ACTB (Sigma-Aldrich Corporation, A5441). Immunoreactive bands were detected using the alkaline phosphatase-linked secondary antibody and ECF western blotting system (Amersham Biosciences, Piscataway, NJ). The images were acquired using a Typhoon FLA 7000 imager (GE Healthcare, Pittsburgh, PA). The results shown are representative of 3 independent experiments.
Patients and bladder cancer tissue specimens
Ten pairs of primary invasive bladder cancer specimens and their paired adjacent normal bladder tissues were obtained from patients who underwent radical cystectomy at the Department of Urology of the Union Hospital of Tongji Medical College (Wuhan, China) between 2012 and 2013. All specimens were immediately snap-frozen in liquid nitrogen after surgical removal. Histological and pathological diagnoses were confirmed and the specimens were classified by a certified clinical pathologist according to the 2004 World Health Organization Consensus Classification and Staging System for bladder neoplasms. All specimens were obtained with appropriate informed consent from the patients and a supportive grant obtained from the Medical Ethics Committee of China, and the experiments were carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Statistical analysis
The significance of the difference between the treated and untreated groups was determined with the Student t test. The results are expressed as mean ± SD.
Abbreviations
- AMPK
adenosine 5′-monophosphate-activated protein kinase
- BAF
bafilomycin A1
- BECN1
Beclin 1, autophagy related
- DAPI
4′,6-diamidino-2-phenylindole
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GFP
green fluorescent protein
- IgG
immunoglobulin G
- ISO
isorhapontigenin
- MAP1LC3/LC3
microtubule-associated protein 1 light chain 3
- MAPK
mitogen-activated protein kinase
- MTOR
mechanistic target of rapamycin (serine/threonine kinase)
- PBS
phosphate-buffered saline
- RT-PCR
reverse transcription-polymerase chain reaction
- SAPK
stress-activated protein kinase
- SESN2
sestrin 2
- shRNA
short hairpin RNA
- XIAP
X-linked inhibitor of apoptosis
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Gang Chen (University of Kentucky, Lexington, KY, USA) for providing the GFP-LC3 construct.
Funding
This work was partially supported by grants from NIH/NCI CA112557, CA177665 and CA165980, and NIH/NIEHS ES000260; the Natural Science Foundation of China (NSFC81229002), Key Project of Science and Technology Innovation Team of Zhejiang Province (2013TD10) and Wenzhou Science and Technology Bureau (Y20150008).
Reference
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: A Cancer J Clin 2015; 65:5-29; http://dx.doi.org/ 10.3322/caac.21254 [DOI] [PubMed] [Google Scholar]
- [2].Murphy WM, Soloway MS, Jukkola AF, Crabtree WN, Ford KS. Urinary cytology and bladder cancer. The cellular features of transitional cell neoplasms. Cancer 1984; 53:1555-65; PMID:6697294; http://dx.doi.org/ 10.1002/1097-0142(19840401)53:7%3c1555::AID-CNCR2820530723%3e3.0.CO;2-G [DOI] [PubMed] [Google Scholar]
- [3].Cowan NC, Crew JP. Imaging bladder cancer. Curr Opin Urol 2010; 20:409-13; PMID:20625298; http://dx.doi.org/ 10.1097/MOU.0b013e32833cbcb9 [DOI] [PubMed] [Google Scholar]
- [4].von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A, Arning M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol: Off J Am Soc Clin Oncol 2005; 23:4602-8; PMID:16034041; http://dx.doi.org/ 10.1200/JCO.2005.07.757 [DOI] [PubMed] [Google Scholar]
- [5].Fang Y, Cao Z, Hou Q, Ma C, Yao C, Li J, Wu XR, Huang C. Cyclin d1 downregulation contributes to anticancer effect of isorhapontigenin on human bladder cancer cells. Mol Cancer Ther 2013; 12:1492-503; PMID:23723126; http://dx.doi.org/ 10.1158/1535-7163.MCT-12-0922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gao G, Chen L, Li J, Zhang D, Fang Y, Huang H, Chen X, Huang C. Isorhapontigenin (ISO) inhibited cell transformation by inducing G0/G1 phase arrest via increasing MKP-1 mRNA Stability. Oncotarget 2014; 5:2664-77; PMID:24797581; http://dx.doi.org/ 10.18632/oncotarget.1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fang Y, Yu Y, Hou Q, Zheng X, Zhang M, Zhang D, Li J, Wu XR, Huang C. The Chinese herb isolate isorhapontigenin induces apoptosis in human cancer cells by down-regulating overexpression of antiapoptotic protein XIAP. J Biol Chem 2012; 287:35234-43; PMID:22896709; http://dx.doi.org/ 10.1074/jbc.M112.389494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zeng X, Xu Z, Gu J, Huang H, Gao G, Zhang X, Li J, Jin H, Jiang G, Sun H, et al.. Induction of miR-137 by isorhapontigenin (ISO) directly targets Sp1 protein translation and mediates its anticancer activity both in vitro and in vivo. Mol Cancer Ther 2016; http://dx.doi.org/ 10.1158/1535-7163.MCT-15-0606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science 2000; 290:1717-21; PMID:11099404; http://dx.doi.org/ 10.1126/science.290.5497.1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Melendez A, Talloczy Z, Seaman M, Eskelinen EL, Hall DH, Levine B. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science 2003; 301:1387-91; PMID:12958363; http://dx.doi.org/ 10.1126/science.1087782 [DOI] [PubMed] [Google Scholar]
- [11].Brech A, Ahlquist T, Lothe RA, Stenmark H. Autophagy in tumour suppression and promotion. Mol Oncol 2009; 3:366-75; PMID:19559660; http://dx.doi.org/ 10.1016/j.molonc.2009.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gewirtz DA. The four faces of autophagy: implications for cancer therapy. Cancer Res 2014; 74:647-51; PMID:24459182; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-2966 [DOI] [PubMed] [Google Scholar]
- [13].Lee JH, Budanov AV, Karin M. Sestrins orchestrate cellular metabolism to attenuate aging. Cell Metab 2013; 18:792-801; PMID:24055102; http://dx.doi.org/ 10.1016/j.cmet.2013.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Budanov AV, Lee JH, Karin M. Stressin' Sestrins take an aging fight. EMBO Mol Med 2010; 2:388-400; PMID:20878915; http://dx.doi.org/ 10.1002/emmm.201000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hu HJ, Shi ZY, Lin XL, Chen SM, Wang QY, Tang SY. Upregulation of Sestrin2 expression protects against macrophage apoptosis induced by oxidized low-density lipoprotein. DNA Cell Biol 2015; 34:296-302; PMID:25692450; http://dx.doi.org/ 10.1089/dna.2014.2627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Budanov AV, Shoshani T, Faerman A, Zelin E, Kamer I, Kalinski H, Gorodin S, Fishman A, Chajut A, Einat P, et al.. Identification of a novel stress-responsive gene Hi95 involved in regulation of cell viability. Oncogene 2002; 21:6017-31; PMID:12203114; http://dx.doi.org/ 10.1038/sj.onc.1205877 [DOI] [PubMed] [Google Scholar]
- [17].Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 2000; 19:5720-8; PMID:11060023; http://dx.doi.org/ 10.1093/emboj/19.21.5720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, et al.. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Investigat 2003; 112:1809-20; PMID:14638851; http://dx.doi.org/ 10.1172/JCI20039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A 2003; 100:15077-82; PMID:14657337; http://dx.doi.org/ 10.1073/pnas.2436255100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Budanov AV. Stress-responsive sestrins link p53 with redox regulation and mammalian target of rapamycin signaling. Antioxidants Redox Signaling 2011; 15:1679-90; PMID:20712410; http://dx.doi.org/ 10.1089/ars.2010.3530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cooper SJ, MacGowan J, Ranger-Moore J, Young MR, Colburn NH, Bowden GT. Expression of dominant negative c-jun inhibits ultraviolet B-induced squamous cell carcinoma number and size in an SKH-1 hairless mouse model. Mol Cancer Res: MCR 2003; 1:848-54; PMID:14517347 [PubMed] [Google Scholar]
- [22].Minden A, Karin M. Regulation and function of the JNK subgroup of MAP kinases. Biochimica et Biophysica Acta 1997; 1333:F85-104; PMID:9395283 [DOI] [PubMed] [Google Scholar]
- [23].Barr RK, Bogoyevitch MA. The c-Jun N-terminal protein kinase family of mitogen-activated protein kinases (JNK MAPKs). Int J Biochem Cell Biol 2001; 33:1047-63; PMID:11551821; http://dx.doi.org/ 10.1016/S1357-2725(01)00093-0 [DOI] [PubMed] [Google Scholar]
- [24].Altman BJ, Rathmell JC. Metabolic stress in autophagy and cell death pathways. Cold Spring Harbor Perspect Biol 2012; 4:a008763; PMID:22952396; http://dx.doi.org/ 10.1101/cshperspect.a008763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chen N, Eritja N, Lock R, Debnath J. Autophagy restricts proliferation driven by oncogenic phosphatidylinositol 3-kinase in three-dimensional culture. Oncogene 2013; 32:2543-54; PMID:22777351; http://dx.doi.org/ 10.1038/onc.2012.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Neufeld TP. Autophagy and cell growth–the yin and yang of nutrient responses. J Cell Sci 2012; 125:2359-68; PMID:22649254; http://dx.doi.org/ 10.1242/jcs.103333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yang ZJ, Chee CE, Huang S, Sinicrope FA. The role of autophagy in cancer: therapeutic implications. Mol Cancer Ther 2011; 10:1533-41; PMID:21878654; http://dx.doi.org/ 10.1158/1535-7163.MCT-11-0047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Santoni M, Amantini C, Morelli MB, Liberati S, Farfariello V, Nabissi M, Bonfili L, Eleuteri AM, Mozzicafreddo M, Burattini L, et al.. Pazopanib and sunitinib trigger autophagic and non-autophagic death of bladder tumour cells. Brit J Cancer 2013; 109:1040-50; PMID:23887605; http://dx.doi.org/ 10.1038/bjc.2013.420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ishihara M, Urushido M, Hamada K, Matsumoto T, Shimamura Y, Ogata K, Inoue K, Taniguchi Y, Horino T, Fujieda M, et al.. Sestrin-2 and BNIP3 regulate autophagy and mitophagy in renal tubular cells in acute kidney injury. Am J Physiol Renal Physiol 2013; 305:F495-509; PMID:23698117; http://dx.doi.org/ 10.1152/ajprenal.00642.2012 [DOI] [PubMed] [Google Scholar]
- [30].D'Amelio M, Cecconi F. A novel player in the p53-mediated autophagy: Sestrin2. Cell Cycle 2009; 8:1466-70; PMID:19411828; http://dx.doi.org/ 10.4161/cc.8.10.8767 [DOI] [PubMed] [Google Scholar]
- [31].Watanabe J, Nishiyama H, Matsui Y, Ito M, Kawanishi H, Kamoto T, Ogawa O. Dicoumarol potentiates cisplatin-induced apoptosis mediated by c-Jun N-terminal kinase in p53 wild-type urogenital cancer cell lines. Oncogene 2006; 25:2500-8; PMID:16518417; http://dx.doi.org/ 10.1038/sj.onc.1209162 [DOI] [PubMed] [Google Scholar]
- [32].Zhang D, Song L, Li J, Wu K, Huang C. Coordination of JNK1 and JNK2 is critical for GADD45alpha induction and its mediated cell apoptosis in arsenite responses. J Biol Chem 2006; 281:34113-23; PMID:16973625; http://dx.doi.org/ 10.1074/jbc.M602821200 [DOI] [PubMed] [Google Scholar]
- [33].Shimizu S, Konishi A, Nishida Y, Mizuta T, Nishina H, Yamamoto A, Tsujimoto Y. Involvement of JNK in the regulation of autophagic cell death. Oncogene 2010; 29:2070-82; PMID:20101227; http://dx.doi.org/ 10.1038/onc.2009.487 [DOI] [PubMed] [Google Scholar]
- [34].Huang H, Pan X, Jin H, Li Y, Zhang L, Yang C, Liu P, Liu Y, Chen L, Li J, et al.. PHLPP2 downregulation contributes to lung carcinogenesis following B[a]P/B[a]PDE exposure. Clin Cancer Res: Off J Am Assoc Cancer Res 2015; 21:3783-93; PMID:25977341; http://dx.doi.org/ 10.1158/1078-0432.CCR-14-2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jin H, Yu Y, Hu Y, Lu C, Li J, Gu J, Zhang L, Huang H, Zhang D, Wu XR, et al.. Divergent behaviors and underlying mechanisms of cell migration and invasion in non-metastatic T24 and its metastatic derivative T24T bladder cancer cell lines. Oncotarget 2015; 6:522-36; PMID:25402510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Che X, Liu J, Huang H, Mi X, Xia Q, Li J, Zhang D, Ke Q, Gao J, Huang C. p27 suppresses cyclooxygenase-2 expression by inhibiting p38beta and p38delta-mediated CREB phosphorylation upon arsenite exposure. Biochimica et Biophysica Acta 2013; 1833:2083-91; PMID:23639288; http://dx.doi.org/ 10.1016/j.bbamcr.2013.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Song L, Li J, Ye J, Yu G, Ding J, Zhang D, Ouyang W, Dong Z, Kim SO, Huang C. p85alpha acts as a novel signal transducer for mediation of cellular apoptotic response to UV radiation. Mol Cell Biol 2007; 27:2713-31; PMID:17242187; http://dx.doi.org/ 10.1128/MCB.00657-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhu J, Zhang J, Huang H, Li J, Yu Y, Jin H, Li Y, Deng X, Gao J, Zhao Q, et al.. Crucial role of c-Jun phosphorylation at Ser63/73 mediated by PHLPP protein degradation in the cheliensisin a inhibition of cell transformation. Cancer Prevent Res 2014; 7:1270-81; PMID:25281487; http://dx.doi.org/ 10.1158/1940-6207.CAPR-14-0233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zuo Z, Che X, Wang Y, Li B, Li J, Dai W, Lin CP, Huang C. High mobility group Box-1 inhibits cancer cell motility and metastasis by suppressing activation of transcription factor CREB and nWASP expression. Oncotarget 2014; 5:7458-70; PMID:25277185; http://dx.doi.org/ 10.18632/oncotarget.2150 [DOI] [PMC free article] [PubMed] [Google Scholar]