Abstract
Background: In most staging systems, 45 years of age is used to differentiate low risk thyroid cancer from high risk thyroid cancer. However, recent studies have questioned both the precise 45 year age point and the concept of using a binary cut off as accurate predictors of disease specific mortality.
Methods: A cohort of 3664 thyroid cancer patients that received surgery and adjuvant treatment at Memorial Sloan Kettering Cancer Center (MSKCC) from the years 1985 to 2010 were analyzed to determine the significance of age at diagnosis as a categorical variable at a variety of age cutoffs (5 year intervals between 30 and 70 years of age). The unadjusted and adjusted hazard ratio for the association between disease-specific survival and age was determined using a Cox proportional hazards model adjusted for other predictive variables sex, histology, and pathological T, N, and M status. Furthermore, predictive nomograms of disease-specific mortality were created and validated on an external dataset of 4551 patients to evaluate the impact of age at diagnosis as both a categorical and continuous variable.
Results: In the MSKCC cohort, with a median follow-up time of 54 months (range 1–332), there were 59 deaths from thyroid cancer with a 10 year disease-specific survival of 96%. Adjusted hazard ratios for all age cutoffs from age 30 to age 70 years were significant. There was no specific cutoff age which risk stratifies patients with differentiated thyroid cancer (DTC). Categorizing age into five strata (<40, 40–49, 50–59, 60–69 and >70 years) showed a 37-fold increase in hazard ratio from age <40 years to age >70 years. A predictive nomogram using age as a continuous variable with other predictive variables had a high concordance index of 96%. Validation on the external cohort had a concordance index of 73%.
Conclusions: Mortality from DTC increases progressively with advancing age. There is no specific cutoff age which risk stratifies patients with DTC. A predictive nomogram using age as a continuous variable may be a more appropriate tool for stratifying patients with DTC and for predicting outcome.
Introduction
The incidence of thyroid cancer has shown a rapid increase over the last decade (1–5). This is largely due to increased detection of subclinical disease by the incidental finding of thyroid nodules on computed tomography, magnetic resonance imaging, positron emission tomography, and ultrasound imaging (3,6). This has resulted in a large increase in the number of thyroidectomy operations as well as an increased number of patients being treated with adjuvant radioactive iodine therapy. There are many factors that determine outcome, including age of the patient, size of the primary tumor, presence of gross extrathyroid extension, presence of regional lymph nodes, and the presence of distant metastatic disease (7). These factors are all included in the American Joint Committee on Cancer (AJCC) TNM classification system for differentiated thyroid cancer (8). The 5 year survival figures for stages 1, 2, 3, and 4 are 100%, 100%, 93% and 51% respectively (8).
Unlike any other adult cancer, thyroid cancer is the only cancer that has age as a prognostic factor in the TNM staging system. The current staging system uses the age of 45 as the cutoff with patients 45 years of age or over having poorer outcome. The reason why 45 years of age was selected as the prognostic cutoff is unclear. One reason could be based upon the observation that 45 years of age is the median age of patients in multiple series. However, a report by Byar et al. (9) in 1979 was the first to show survival was poorer at the age of 45 years. It is widely thought that this age cutoff is too young and that a better cutoff age may be age over 55 years. There is also some argument that a binary variable for age may not be appropriate and that a staging system utilizing age as a continuous variable may be more appropriate. For example, a 44-year-old man with a T1N1b thyroid cancer is currently staged as stage 1 with a survival of 100% at 5 years. However, a 46-year-old man with an identical tumor is staged as stage 4 with an estimated 5-year survival of 51%. Clearly the difference of 1 year should not move patients from stage 1 to stage 4 disease, reducing the estimate of survival by 49%. The aim of our study was to challenge the traditional concept that there is a distinct age cutoff around which outcomes are significantly different.
Methods
After internal review board approval, a database of 3664 patients with differentiated thyroid cancer who received their primary therapy at Memorial Sloan Kettering Cancer Center (MSKCC) was created. These patients all received surgery and adjuvant treatment at MSKCC during the years 1985 to 2010. Patient, tumor, and treatment characteristics were extracted from patient electronic records by clinicians. Patient, tumor, and treatment characteristics for all patients are summarized in Table 1. Characteristics of an external dataset of 4551 patients, comprising patients from Brazil (n = 646), Toronto (n = 925), Sydney (n = 1129), and University of California, San Francisco (n = 1851) used for validation are also shown.
Table 1.
Patient, Tumor, and Treatment Characteristics for Internal and External Cohorts
| Variable | MSKCC (n = 3664) | % | External (n = 4551) | % | p-Value |
|---|---|---|---|---|---|
| Age at diagnosis | |||||
| <45 years | 1668 | 46% | 2203 | 48% | |
| ≥45 years | 1996 | 54% | 2348 | 52% | 0.009 |
| Sex | |||||
| Male | 995 | 27% | 1030 | 23% | |
| Female | 2669 | 73% | 3521 | 77% | <0.001 |
| Pathology | |||||
| Papillary carcinoma | 3436 | 94% | 4444 | 98% | |
| Follicular carcinoma | 129 | 4% | 70 | 2% | |
| Hürthle cell carcinoma | 99 | 3% | 37 | 1% | <0.001 |
| pT stage | |||||
| T1 | 1874 | 51% | 2361 | 52% | |
| T2 | 540 | 15% | 859 | 19% | |
| T3 | 1092 | 30% | 1047 | 23% | |
| T4 | 152 | 4% | 284 | 6% | |
| Unknown | 10 | 0% | – | – | <0.001 |
| pN stage | |||||
| N0Nx | 2556 | 70% | 2952 | 65% | |
| N1a | 548 | 15% | 793 | 17% | |
| N1b | 560 | 15% | 806 | 18% | <0.001 |
| M stage | |||||
| M0Mx | 3593 | 98% | 4403 | 97% | |
| M1 | 71 | 2% | 148 | 3% | <0.001 |
MSKCC, Memorial Sloan Kettering Cancer Center.
Causal hypothesis
Our causal hypothesis was that cancer-specific survival from thyroid cancer is associated with age after adjusting for other prognostic variables. Our outcome variable was disease (cancer) specific survival (DSS). There were 312 overall deaths, of which 59 were due to thyroid cancer. DSS was calculated using the date of last follow up with a MSKCC physician from the thyroid cancer multidisciplinary team. Details of death were determined from the social security death index and hospital records. All patients were cross-linked to the social security death index. The cause of death in patients who were still under follow up was determined from patient medical records. However, in patients who were lost to follow-up, death from thyroid cancer was defined if the patient had active structural disease at the last follow up date and died within three years of this follow-up date.
Assessment of age as a prognostic factor
Variables predictive of DSS were determined by univariable analysis using the Kaplan Meier method. The significance of age was determined as a categorical variable at a variety of age cutoffs from 30 to 70 years at 5-year intervals. The unadjusted and adjusted hazard ratio (HR) for the association between DSS and age was determined using a Cox proportional hazards model. In this model, age was adjusted for other predictive variables including sex, histology, pathological T (tumor) status, pathological N (node) status, and M (metastases) status. Pathological N status was categorized into N0, Nx, N1a, N1b. N0 was defined as negative pathological neck nodes. Nx was defined as no clinically enlarged or suspicious lymph nodes, N1a was defined as positive pathological nodes in the central neck, and N1b defined as positive pathological lymph nodes in the lateral neck. We further categorized the N class into three categorical variables N0/Nx, N1a, and N1b. M status was categorized into a binary variable M0/Mx and M1. M0/Mx was defined as no distant metastases at presentation, and M1 defined as distant metastases at presentation. To test the proportional hazards assumption, time-dependent variables of the interaction between the predictors, and the logarithm of time to event (DSS time) were introduced into the model. The proportional hazards assumption was reasonable in all models.
Creation of nomograms using Cox proportional regression analysis
Cox proportional hazards models were created for age as a categorical variable (stratified into the following strata age <40, 40–49, 50–59, 60–69 and >70 years) and also as a continuous variable. In each model age was adjusted for other variables prognostic for disease-specific mortality. Nomograms predictive of five-year disease-specific mortality were created from the models. Predictive accuracy was assessed by discrimination (the ability of a model to separate patients with different outcomes). Discrimination was measured with the concordance index, similar to the area under the receiver operating characteristic curve: values range from 0.5 (no discrimination) to 1.0 (perfect discrimination). The final nomogram was validated using the external dataset and concordance index determined.
Statistical analyses
Statistical analysis was carried out using SPSS (version 21, IBM Corporation). Patient, tumor, and treatment characteristics were compared using Pearson's chi-squared test. Survival outcomes were analyzed using the Kaplan-Meier method. Unadjusted and adjusted HRs were calculated using the Cox proportional hazard model. A p-value less than 0.05 was considered significant. R version 3.0.2 (The R Foundation for Statistical Computing) was used to create the nomograms and for validation.
Results
Table 1 describes the patient and tumor characteristics of the MSKCC and external cohorts in detail. The MSKCC cohort had more male patients, more patients over 45 years of age, and had a greater percentage of advanced T stage tumors. However, the MSKCC cohort tended to have fewer patients with positive neck disease and slightly less distant metastases. The distribution of age within all cohorts was similar (Supplementary Figs. S1, S2; Supplementary Data are available online at www.liebertpub.com/thy). In the MSKCC cohort, with a median follow up time of 54 months (range 1–332). This means 50% of patients had less than 54 months of follow-up due to censoring either due to the event (death from thyroid cancer) or due to loss to follow-up. There were 59 deaths from thyroid cancer with a 10 year DSS of 96%. Factors predictive of DSS on univariable analysis are shown in Supplementary Table S1. Age, sex, pathology of the primary tumor, pathological T status and N status as well as M status were all predictive of outcome. Patients with follicular or Hürthle cell pathology had poorer DSS compared with papillary pathology (87%, 87% versus 98%, p < 0.0001). Patients with T4 tumors had poorer DSS than those with T1 tumors (77% vs 98%, p < 0.0001), patients with N1b neck disease poorer DSS than those with N0Nx disease (92% vs. 97%, p < 0.0001) and patients with M1 disease poorer DSS than those with M0 disease (42% vs. 98%, p < 0.0001).
Age as a prognostic variable
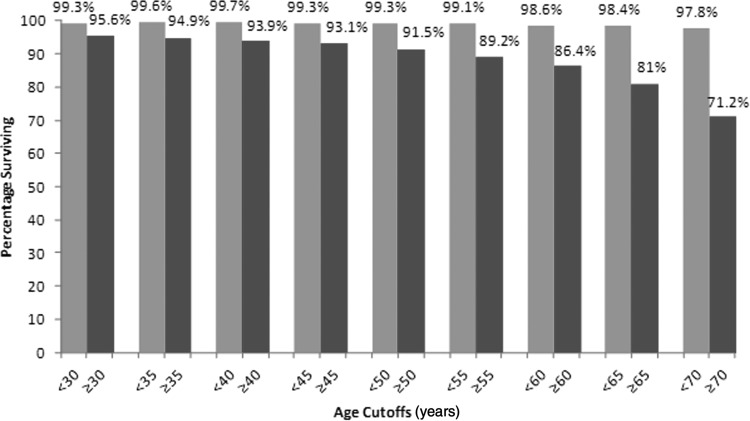
Table 2 shows the impact that age had on outcome as a binary variable. All cutoff ages from 30 to 70 years showed that the older cohort had poorer DSS (Fig. 1). Multivariable analysis for age at different cutoffs, adjusting for the other predictor variables using Cox proportional hazards regression, also showed that age was significant at all cutoff values from 30 to 70 years. The HRs for adjusted age cutoffs were ranging from 7.09 to 19.03.
Table 2.
Unadjusted and Adjusted Hazard Ratio of Different Age Cutoffs on Disease-Specific Survival
| Variable | Univariable analysis | Multivariable analysis* | |||||
|---|---|---|---|---|---|---|---|
| Age at diagnosis (years) | 10 year DSS (%) | Unadjusted HR | 95% CI | p Value | AdjustedHR | 95% CI | p Value |
| <30 | 99.3 | ||||||
| ≥30 | 95.6 | 5.16 | 1.26–21.18 | 0.023 | 10.77 | 2.57–45.05 | 0.001 |
| <35 | 99.6 | ||||||
| ≥35 | 94.9 | 6.74 | 2.10–21.57 | 0.001 | 9.35 | 2.88–30.37 | <0.001 |
| <40 | 99.7 | ||||||
| ≥40 | 93.9 | 12.26 | 3.83–39.24 | <0.001 | 13.42 | 43.450 | <0.001 |
| <45 | 99.3 | ||||||
| ≥45 | 93.1 | 11.06 | 4.42–27.69 | <0.001 | 9.75 | 3.85–24.72 | <0.001 |
| <50 | 99.3 | ||||||
| ≥50 | 91.5 | 13.97 | 6.00–32.52 | <0.001 | 19.03 | 7.71–46.94 | <0.001 |
| <55 | 99.1 | ||||||
| ≥55 | 89.2 | 12.64 | 6.39–24.00 | <0.001 | 13.35 | 6.40–27.84 | <0.001 |
| <60 | 98.6 | ||||||
| ≥60 | 86.4 | 9.4 | 5.43–16.28 | <0.001 | 7.96 | 4.38–14.46 | <0.001 |
| <65 | 98.4 | ||||||
| ≥65 | 81 | 12.12 | 7.15–20.55 | <0.001 | 7.72 | 4.32–13.79 | <0.001 |
| <70 | 97.8 | ||||||
| ≥70 | 71.2 | 17.76 | 10.57–29.86 | <0.001 | 7.09 | 3.96–12.67 | <0.001 |
Adjusted for sex, pathology, and T (tumor), N (node), and M (metastases).
FIG. 1.
Ten year disease-specific survival (DSS) at different age cutoffs from age 30 to age 70 years.
Age as a categorical variable
We then categorized age into the following categories: <40, 40–49, 50–59, 60–69, and >70 years and calculated the HR for these age strata after adjusting for the other prognostic variables. The unadjusted and adjusted HRs are shown in Table 3. The data demonstrate that the HR progressively increases with increasing age. Patients between 50–59 years had a 14-fold increased risk of death compared to patients less than 40 years after adjusting for sex, histology, and T, N, and M status. Patients over the age of 70 years had a 37-fold increased risk of death compared to patients less than 40 years after adjusting for sex, histology, and T, N, and M status. The unadjusted and adjusted HRs for the other variables in this model are also shown in Table 3. In our adjusted model, the other important variables predictive of DSS were a nonpapillary pathology (follicular HR = 4.36, Hürthle HR = 3), pathological T4 status (HR = 3.66), and M1 status (HR = 14.85).
Table 3.
Categorical Age Variable: Factors Predictive of Disease-Specific Survival on Cox Proportional Hazards Model
| Variable | Unadjusted HR | 95% CI | p value | Adjusted HR | 95% CI | p value |
|---|---|---|---|---|---|---|
| Age at diagnosis (years)* | ||||||
| <40 | reference | reference | ||||
| 40–49 | 1.88 | 0.38–9.34 | 0.439 | 2.093 | 0.42–10.4 | 0.367 |
| 50–59 | 8.59 | 2.45–30.18 | 0.001 | 14.34 | 3.89–52.88 | <0.001 |
| 60–69 | 11.43 | 3.18–41.08 | <0.001 | 21.52 | 5.61–82.52 | <0.001 |
| >70 | 76.43 | 23.1–252–92 | <0.001 | 37.34 | 10.92–127.76 | <0.001 |
| Sex | ||||||
| Female | reference | reference | ||||
| Male | 2.93 | 1.75–4.89 | <0.001 | 1.19 | 0.66–2.15 | 0.567 |
| Pathology | ||||||
| Papillary Ca | reference | reference | ||||
| Follicular Ca | 2.86 | 1.22–6.71 | 0.015 | 4.32 | 1.56–11.95 | 0.005 |
| Hürthle cell Ca | 2.94 | 1.17–7.4 | 0.022 | 2.88 | 1.02–8.17 | 0.047 |
| pT stage | ||||||
| T1 | reference | reference | ||||
| T2 | 1.04 | 0.33–3.32 | 0.95 | 0.93 | 0.28–3.06 | 0.9 |
| T3 | 2.94 | 1.38–6.28 | 0.005 | 1.41 | 0.62–3.21 | 0.412 |
| T4 | 19.3 | 9.26–40.3 | <0.001 | 4.86 | 2.04–11.55 | <0.001 |
| pN stage | ||||||
| N0NX | reference | reference | ||||
| N1a | 1.48 | 0.68–3.2 | 0.325 | 1.99 | 0.80–4.93 | 0.14 |
| N1b | 3.6 | 2.0–6.3 | <0.001 | 2.22 | 1.02–4.86 | 0.046 |
| M stage | ||||||
| M0 | reference | reference | ||||
| M1 | 45.38 | 27.11–75.96 | <0.001 | 17.79 | 9.63–32.85 | <0.001 |
Age (categorical) adjusted for sex, pathology, and T, N, and M stage.
Age as a continuous variable
We then calculated the unadjusted and adjusted HRs for age as a continuous variable, again adjusting for the same variables of sex, pathology, and T, N, and M stage. This is shown in Table 4. The adjusted HR was 1.076 indicating that for every additional year the risk of death progressively increased.
Table 4.
Continuous Age Variable: Factors Predictive of Disease-Specific Survival on Cox Proportional Hazards Model
| Variable | Unadjusted HR | 95% CI | p Value | Adjusted HR | 95% CI | p Value |
|---|---|---|---|---|---|---|
| Age at diagnosis (years)* | 1.114 | 1.09–1.14 | <0.001 | 1.076 | 1.06–1.1 | <0.001 |
| Sex | ||||||
| Female | reference | reference | ||||
| Male | 2.93 | 1.75–4.89 | <0.001 | 1.27 | 0.70–2.28 | 0.43 |
| Pathology | ||||||
| Papillary Ca | reference | reference | ||||
| Follicular Ca | 2.86 | 1.22–6.71 | 0.015 | 4.36 | 1.56–12.07 | 0.004 |
| Hürthle Cell Ca | 2.94 | 1.17–7.4 | 0.022 | 3.01 | 1.09–8.32 | 0.034 |
| pT stage | ||||||
| T1 | reference | reference | ||||
| T2 | 1.04 | 0.33–3.32 | 0.95 | 0.78 | 0.24–2.54 | 0.67 |
| T3 | 2.94 | 1.38–6.28 | 0.005 | 1.26 | 0.56–2.84 | 0.58 |
| T4 | 19.3 | 9.26–40.3 | <0.001 | 3.65 | 1.54–8.63 | 0.003 |
| pN stage | ||||||
| N0NX | reference | reference | ||||
| N1a | 1.48 | 0.68–3.2 | 0.325 | 1.85 | 0.75–4.55 | 0.18 |
| N1b | 3.6 | 2.0–6.3 | <0.001 | 2.01 | 0.91–4.45 | 0.08 |
| M stage | ||||||
| M0 | reference | reference | ||||
| M1 | 45.38 | 27.11–75.96 | <0.001 | 14.88 | 8.27–26.78 | <0.001 |
Age (continuous) adjusted for sex, pathology, and T, N, and M stage.
Nomograms predictive of disease specific mortality using age as a categorical and continuous variable
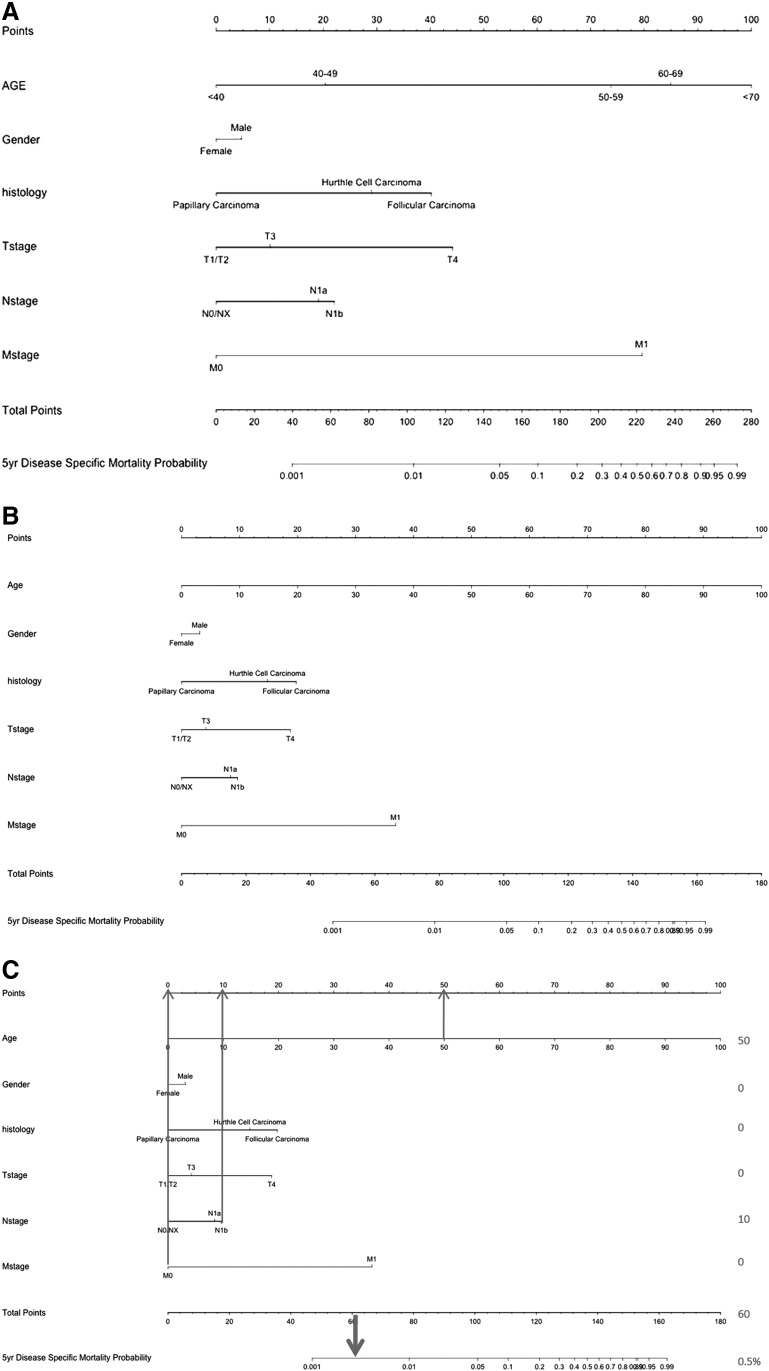
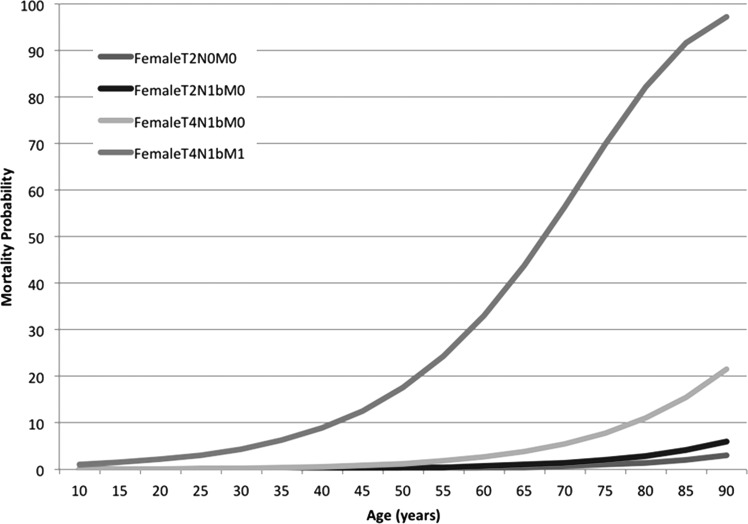
A nomogram was created using the age categories described above (<40, 40–49, 50–59, 60–69, and >70 years) (Fig. 2A). The concordance index for this nomogram was 96%. We then constructed a nomogram using age as a continuous variable along with the other prognostic variables. The nomogram for this model is shown in Figure 2B. The concordance index for the nomogram was 96%. Validation of this nomogram with the external dataset had a concordance index of 73%. Using this nomogram, the predicted 5-year mortality for 4 hypothetical patients at different ages and stages is illustrated in Figure 3. This shows how patients with an identical stage of disease have a progressively poorer outcome as age increases. To illustrate how such a nomogram works, Figure 2C illustrates a hypothetical patient, a 50-year-old female with a T2N1bM0 papillary thyroid cancer (PTC) tumor. The total score for this patient is 60, which equates to a 50 year predicted mortality of 0.5%.
FIG. 2.
(A) Nomogram for disease-specific mortality with age as a categorical variable. Concordance index 0.958. (B) Nomogram for disease specific mortality with age as a continuous variable. Concordance index 0.958. (C) A 50 year old female with a T2N1bM0 papillary thyroid cancer has a predicted disease specific mortality of 0.5%.
FIG. 3.
Five year disease-specific mortality for four hypothetical patients with advancing age.
Discussion
The aim of our study was to investigate the association between age and survival in thyroid cancer to determine if there is a specific age cutoff predictive of outcome. We show that the HR for mortality increased with increasing age. Based on this observation we were able to create statistical models predictive of mortality, one using age as a categorical variable and the other using age as a continuous variable. These nomograms may be a more appropriate method of predicting outcome in patients with PTC and may supersede the current AJCC staging classification system.
The first study that showed age to be important in thyroid cancer was reported by Byar et al. in 1979 (9). Since then, several different staging systems for differentiated thyroid cancer have been described all of which use an age cutoff as a prognostic variable. The Mayo Clinic's Metastasis, Age, Completeness of resection, Invasion, and Size classification has an age of 40 years as the cutoff (10). The Lahey Clinic's Age, Metastases, Extent, and Size system has different cutoffs for men (age 40) and women (age 50) (11). The Grade, Age, Metastases, Extent, and Size staging developed at Memorial Sloan Kettering assigned patients above age 45 a higher score (12). The most important staging system is the AJCC staging protocol for differentiated thyroid cancer (DTC) (Supplementary Table S1). In this system, patients under 45 years are either stage 1 (absence of metastatic disease) or 2 (presence of metastatic disease). In contrast, patients age 45 and above are divided into stages 1 through 4c (8). This 45 year age cutoff has been a component since the second edition of the AJCC Cancer Staging Manual (13). However, there is evidence that age adds to the risk of mortality starting at age 35 (14,15). Furthermore, recent studies by Orosco et al. (16) and by Londero et al. (17) have suggested that although age is an important prognostic factor, there is no specific age cut off which predicts survival. Recent studies by Yang et al. (18) and Banerjee et al. (19) using SEER data have also reported evidence that death increases progressively with increasing age and that age as a continuous variable may be more appropriate.
The initial age division for our multivariable analysis was chosen according to the current AJCC staging protocol (45 years). Our adjusted HR showed this age cutoff to be significant, but all other age cutoffs were also significant. This supports recent studies that have indicated that there may not be a sudden increase in mortality risk at one age point as the AJCC staging implies, but that the relationship between increasing age and risk might be more complex (20,21). It also supports reports that survival disadvantages for DTC patients may come into effect as early as age 35 (9,22). Based on our analysis, it is evident that older age increases the likelihood of thyroid cancer–specific mortality. However, age as a binary variable does not show a specific age cutoff suggesting that while age is an important factor to assess an individual patient's prognosis, a specific binary age cutoff cannot be used to stratify patients into unique risk categories. Indeed, our study shows that mortality increased with older age, with patients over 70 years having a 37-fold increased risk of death compared to patients less than 40 years. The current TNM staging system therefore seems to be insufficient in predicting the prognosis for patients with thyroid cancer. Our data suggests that the utilization of age either as a categorical variable stratified into different age ranges or age as a continuous variable would be a more rational approach for predicting outcome in these patients. Such a system would require the use of age in a predictive model containing the other important prognostic factors such as T status, N status, and M status.
Having established that age—either as a categorical variable or as a continuous variable—is more appropriate for predicting prognosis, we therefore designed two nomograms to create tools for individual risk prediction. Nomograms are statistical tools shown to accurately predict outcome in an individual patient by utilizing multiple variables in addition to the standard TNM variables. These nomograms are created using regression analysis (23). Well-designed nomograms have outperformed the projections of experienced clinicians (24,25) and have been incorporated into clinical trial inclusion criteria and National Comprehensive Cancer Network guidelines (26). Both nomograms developed here have extremely high concordance indexes of over 95% illustrating their predictive accuracy. Moreover, we were then able to validate the nomograms on a separate independent external dataset with a concordance index of 73%. A previous report by Yang et al. (18) has already described a nomogram for death in thyroid cancer using data from the SEER database. We would argue that our nomograms are more accurate because of the inherent limitations in the SEER database. In the MSKCC cohort, all case records were retrospectively reviewed by physicians with an interest in thyroid surgery and cancer. Therefore the T, N, and M status is extremely accurate. Importantly, all pathology has also been reviewed by a pathologist with a special interest in thyroid cancer. In the SEER data, clinical data is not reviewed by physicians and pathology review is not carried out. The data is collected from both academic and also nonacademic centers. Therefore, the SEER data may potentially consist of more poorly differentiated forms of thyroid cancer, anaplastic cancer, medullary cancer. The T, N, and M classifications in the SEER data may also be inaccurate. The data from single institutions specialized in thyroid cancer provides both a high volume of cases as well as expertise in treatment and pathology; this is expected to generate more accurate data but does limit the cohort size. Our study is also of importance in that it describes two nomograms that may have importance in the future risk estimation of individual patients with thyroid cancer. Since the AJCC is now planning on adopting individual risk estimation nomograms into the staging manual for all types of cancer, our study is both timely and relevant for these revisions.
It is important to discuss the limitations of our study. Firstly, this is a retrospective study and is therefore susceptible to the limitations of retrospective studies, such as incomplete data from chart review, and selection bias by physicians regarding surgical and adjuvant therapy; however, at MSKCC the surgeons and physicians treating these patients have many years of experience working as a multidisciplinary team managing several hundred patients annually. This unified treatment approach will therefore limit the selection bias present within the dataset. Secondly, the sample size may not be representative of the United States and international populations of patients with thyroid cancer. MSKCC is a tertiary care cancer center with an international reputation in the treatment of thyroid cancer. The quality of surgical treatment and adjuvant therapy with radioactive iodine or external beam radiation may be superior to that delivered by nonacademic centers and smaller institutions. This may be a threat to validity to our study, making any extrapolation to the general population invalid. To address this, we obtained external datasets from other international cancer centers specialized in thyroid cancer management (Brazil, Toronto, University of California–San Francisco, and Sydney). This external dataset consisted of 4551 patients from which there were 88 disease specific deaths. Comparison of the age distribution of patients with DTC in the internal and external cohorts showed a similar distribution with a median age of 45–46 years (Supplementary Figs. S1 and S2). This external dataset had different clinical and tumor characteristics compared to the MSKCC cohort. Despite this, our nomograms were validated with concordance indices of 73%. This indicates that our nomograms may be translatable to other institutions and populations. However, one could still argue that there is still a threat to external validity since these four external cohorts are also institutions with specialized high-volume thyroid cancer management. Applying our results to the general population may therefore still not be valid. A third limitation relates to the limited number of events. Even with the collaborative effort employed in this study, there were only 59 deaths in the MSKCC cohort and 88 deaths in the external cohort. However, despite limited events, our multivariable model converged and was stable, indicating that the conclusions from the model were justified.
In conclusion, our data show that mortality from DTC increases progressively with advancing age. There is no specific cutoff age which risk stratifies patients with DTC. Risk associated with age may be better expressed as a continuous function or as multiple categories. A predictive nomogram using age as a continuous variable may be a more appropriate tool for stratifying patients with DTC and for predicting outcome.
Supplementary Material
Acknowledgments
Ian Ganly, R. Michael Tuttle, Snehal G. Patel contributed to study design, data analysis and interpretation, and manuscript writing. Iain J. Nixon, Laura Y. Wang, Frank L. Palmer, Jocelyn C. Migliacci contributed data collection and analysis. Ahmad Aniss, Mark Sywak, Antoine E. Eskander, Jeremy L. Freeman, Michael J. Campbell, Wen T. Shen, Fernanda Vaisman, Denise Momesso, Rossana Corbo, Mario Vaisman, Ashok Shaha, and Jatin Shah participated in data collection and manuscript editing.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Chen AY, Jemal A, Ward EM. 2009. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer 115:3801–3807 [DOI] [PubMed] [Google Scholar]
- 2.Davies L, Welch HG. 2006. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295: 2164–2167 [DOI] [PubMed] [Google Scholar]
- 3.Enewold L, Zhu K, Ron E, Marrogi AJ, Stojadinovic A, Peoples GE, Devesa SS. 2009. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev 18:784–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elaraj DM, Sturgeon C. 2009. Adequate surgery for papillary thyroid cancer. Surgeon 7:286–289 [DOI] [PubMed] [Google Scholar]
- 5.Mitchell I, Livingston EH, Chang AY, Holt S, Snyder WH, 3rd, Lingvay I, Nwariaku FE. 2007. Trends in thyroid cancer demographics and surgical therapy in the United States. Surgery 142:823–828; discussion 828.e1 [DOI] [PubMed] [Google Scholar]
- 6.Hughes DT, Haymart MR, Miller BS, Gauger PG, Doherty GM. 2011. The most commonly occurring papillary thyroid cancer in the United States is now a microcarcinoma in a patient older than 45 years. Thyroid 21:231–236 [DOI] [PubMed] [Google Scholar]
- 7.Shaha AR. 2004. Implications of prognostic factors and risk groups in the management of differentiated thyroid cancer. Laryngoscope 114:393–402 [DOI] [PubMed] [Google Scholar]
- 8.Edge SB, American Joint Committee on Cancer 2010. AJCC cancer staging manual. Seventh edition. Springer, New York: [DOI] [PubMed] [Google Scholar]
- 9.Byar DP, Green SB, Dor P, Williams ED, Colon J, van Gilse HA, Mayer M. 1979. A prognostic index for thyroid carcinoma. A study of the EORTC Thyroid Cancer Cooperative Group. Eur J Cancer 15:1033–1041 [DOI] [PubMed] [Google Scholar]
- 10.Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. 1993. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery 114:1050–1057; discussion 1057–1058 [PubMed] [Google Scholar]
- 11.Cady B, Rossi R. 1988. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery 104:947–953 [PubMed] [Google Scholar]
- 12.Shaha AR, Loree TR, Shah JP. 1994. Intermediate-risk group for differentiated carcinoma of thyroid. Surgery 116:1036–1040; discussion 1040–1041 [PubMed] [Google Scholar]
- 13.Beahrs O, Myers M. American Joint Committee on Cancer Manual for Staging of Cancer, 1983. Second edition. Lippincott, Philadelphia, PA [Google Scholar]
- 14.Oyer SL, Smith VA, Lentsch EJ. 2012. Reevaluating the prognostic significance of age in differentiated thyroid cancer. Otolaryngol Head Neck Surg 147:221–226 [DOI] [PubMed] [Google Scholar]
- 15.Lang BH, Lo CY, Chan WF, Lam KY, Wan KY. 2007. Staging systems for papillary thyroid carcinoma: a review and comparison. Ann Surg 245:366–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orosco RK, Hussain T, Brumund KT, Oh DK, Chang DC, Bouvet M. 2015. Analysis of age and disease status as predictors of thyroid cancer-specific mortality using the surveillance, epidemiology, and end results database. Thyroid 25:125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Londero SC, Krogdahl A, Bastholt L, Overgaard J, Pedersen HB, Hahn CH, Bentzen J, Schytte S, Christiansen P, Gerke O, Godballe C. 2015. Papillary thyroid carcinoma in Denmark, 1996–2008: outcome and evaluation of established prognostic scoring systems in a prospective national cohort. Thyroid 25:78–84 [DOI] [PubMed] [Google Scholar]
- 18.Yang L, Shen W, Sakamoto N. 2013. Population based study evaluating and predicting the probability of death resulting from thyroid cancer and other causes among patients with thyroid cancer. J Clin Oncol 31:468–474 [DOI] [PubMed] [Google Scholar]
- 19.Banerjee M, Muenz DG, Chang JT, Papaleontiou M, Haymart MR. 2014. Tree based model for thyroid cancer prognostication. J Clin Endocrinol Metab 99:3737–3745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston LE, Tran Cao HS, Chang DC, Bouvet M. 2012. Sociodemographic Predictors of Survival in Differentiated Thyroid Cancer: Results from the SEER Database. ISRN Endocrinol 2012:384707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilliland FD, Hunt WC, Morris DM, Key CR. 1997. Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973–1991. Cancer 79:564–573 [DOI] [PubMed] [Google Scholar]
- 22.Tran Cao HS, Johnston LE, Chang DC, Bouvet M. 2012. A critical analysis of the American Joint Committee on Cancer (AJCC) staging system for differentiated thyroid carcinoma in young patients on the basis of the Surveillance, Epidemiology, and End Results (SEER) registry. Surgery 152:145–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iasonos A, Schrag D, Raj GV, Panageas KS. 2008. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 26:1364–1370 [DOI] [PubMed] [Google Scholar]
- 24.Specht MC, Kattan MW, Gonen M, Fey J, Van Zee KJ. 2005. Predicting nonsentinel node status after positive sentinel lymph biopsy for breast cancer: clinicians versus nomogram. Ann Surg Oncol 12:654–659 [DOI] [PubMed] [Google Scholar]
- 25.Ross PL, Gerigk C, Gonen M, Yossepowitch O, Cagiannos I, Sogani PC, Scardino PT, Kattan MW. 2002. Comparisons of nomograms and urologists' predictions in prostate cancer. Semin Urol Oncol 20:82–88 [DOI] [PubMed] [Google Scholar]
- 26.Kawachi MH, Bahnson RR, Barry M, Busby JE, Carroll PR, Carter HB, Catalona WJ, Cookson MS, Epstein JI, Etzioni RB, Giri VN, Hemstreet GP, Howe RJ, Lange PH, Lilja H, Loughlin KR, Mohler J, Moul J, Nadler RB, Patterson SG, Presti JC, Stroup AM, Wake R, Wei JT. 2010. NCCN clinical practice guidelines in oncology: prostate cancer early detection. J Natl Compr Canc Netw 8:240–262 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.