Abstract
Replication-competent (oncolytic) viruses (OV) as cancer immunotherapeutics have gained an increasing level of attention over the last few years while the clinical evidence of virus-mediated antitumor immune responses is still anecdotal. Multiple clinical studies are currently ongoing and more immunomonitoring results are expected within the next five years. All viruses can be recognized by the immune system and are therefore potential candidates for immune therapeutics. However, each virus activates innate immune system by using different combination of recognition receptors/pathways which leads to qualitatively different adaptive immune responses. This review summarizes immunological findings in cancer patients following treatment with replication-competent viruses.
Introduction
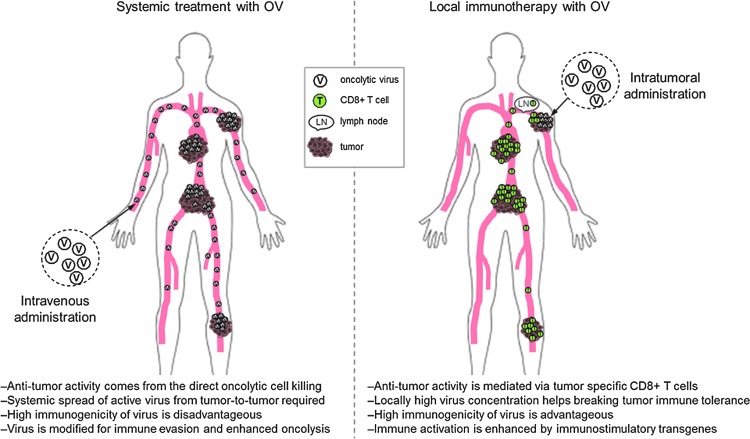
The use of currently approved immunotherapeutic vaccines relies on the knowledge of the expression pattern of the target antigens. As a result, their clinical use is restricted to only a few oncology indications. Replication-competent (oncolytic) viruses (OV) are not similarly limited to certain indications, as they can, via oncolysis, release the unique tumor epitopes from each patient's own tumor and can, therefore, be considered as in situ vaccines. Development of OVs has earlier been focusing on enhancing replication, lytic cell death, and systemic distribution of the virus to distant lesions in the body. Induction of antitumor immunity has often been mentioned but little effort to demonstrate treatment-induced tumor-targeted immune responses has been given until very recently. The challenge has been to decide whether to focus on maximally effective oncolysis and systemic distribution of the virus, where immune evasion is critical, or to enhance the visibility of the virus to the immune system for enhanced antitumor immunity to occur. It is not possible to both have the cake and eat it, and one can provocatively say that even today companies that develop virus-based cancer treatments have not clearly decided on which mechanism to focus: robust oncolysis and systemic spread of active virus, where effective immune evasion is a prerequisite (Fig. 1A), or local immunotherapy, where the goal is to use an immunogenic virus to make tumors visible to the immune system and induce systemic antitumor immune response, thereby eliminating the need to deliver the virus systemically to each tumor site (Fig. 1B). Oncolytic viruses under current clinical development are fundamentally different in many ways and most of them are naturally more suited to the former than the latter.
Figure 1.
Two concepts of using oncolytic virus for cancer treatment. (A) Systemically administered oncolytic viruses possess either natural tumor tropism or can be genetically modified for enhanced tumor cell transduction. High virus dose and/or repeated administration is needed for tumor penetration as majority of the virus is rapidly cleared by liver, spleen, and other organs. High oncolytic potency of the virus is beneficial and a systemic spread of infective viral progeny from one tumor to another is required for clinical efficacy. To this end, antivirus immune response needs to be hindered either by endogenous viral genes, via genetic engineering of the virus, or with concomitant immune modulatory medication. (B) Locally administered replication-competent virus creates a strong “danger signal” at tumor site and helps immune system to see tumor as a threat. Cancer cell death mediated by (some) oncolytic viruses is immunologically active phenomenon and attracts immune cells to tumors. Immune activation can be further enhanced and tailored by immune-stimulating transgenes coded by the virus. Antigen-presenting cells pick up tumor antigens released from dying cancer cells and present these antigens to T-cells in the draining lymph node. Tumor-specific CD8+ T-cells recognize and kill cancer cells in both injected and noninjected distant tumors.
Preclinical testing of immunological properties of virus candidates is not an easy task. Mimicking complex immunosuppressive networks present in advanced human tumors is not possible in preclinical models where tumors develop rapidly. Furthermore, the range of species that viruses can infect varies from strict species specificity to wide host range, which may result in qualitatively different immune responses in animals and humans. Because of these fundamental differences in the (tumor) immunology between preclinical models and the actual human disease, relevant animal models are often not available. This review focuses on describing immunological observations in cancer patients following treatment with replication-competent viruses.
Vesicular Stomatitis Virus
Vesicular stomatitis virus (VSV) is an enveloped RNA virus that is highly lytic and causes fatal infections in immunocompromised animals.1 VSV can infect a wide variety of cell types. VSV has been shown to cause viral encephalitis in animal models because of its neurotropism, and therefore natural tropism of VSV needs to be altered for safe clinical use. Development of VSV as a cancer treatment has been primarily focused on improving tumor specificity, oncolytic potency, and systemic virus spread. To this end, preclinical studies have demonstrated that genetically modified VSV can escape from humoral immunity allowing repeated systemic administration without the loss of therapeutic efficacy.2 Some studies, however, have also explored the potential immunotherapeutic properties of VSV and demonstrated that the virus can act as an efficient tumor vaccine agent in a prime-boost strategy with adenovirus vector in a murine model of melanoma,3 and with adoptive T-cell transfer.4
Clinical experience
A phase I clinical study is currently underway in patients with liver cancer (NCT01628640). The primary objective of this study is to evaluate the safety of intratumoral administration of VSV expressing human interferon beta (IFNβ). The recruitment of patients was initiated in 2012 and the first results are expected in late 2015. ClinicalTrials.gov describes assessment of general and virus-specific CD8+T-cell and NK cell responses as additional objectives.
Maraba Virus
Maraba virus is an enveloped rhabdovirus related to VSV and has shown a strong oncolytic activity in preclinical studies.5 Rhabdoviruses have been shown to infect all organisms except bacteria6 but they are rarely associated with disease in humans. In preclinical studies, engineered maraba viruses have shown improved cancer cell selectivity and a potential as oncolytic virus and vaccine vector.5,7
Clinical experience
A clinical trial utilizing the virus as a MAGE A3-encoding oncolytic vector in a heterologous prime-boost strategy with adenovirus-encoded MAGE A3 is currently recruiting (NCT02285816). The trial has a series of secondary objectives, including the assessment of virus- and tumor-specific immune responses. No results are so far available.
Polio Virus
Polio virus is a highly lytic nonenveloped RNA virus. The poliovirus receptor Necl-5 is broadly expressed in malignant cells but also in normal central nervous system (CNS). For ablation of viral cytotoxicity for normal CNS, poliovirus internal ribosomal entry site (IRES) has been exchanged with its counterpart from human rhinovirus type 2 to generate polio/rhinovirus chimera, RIPO.8 Efficient tumor cell killing capacity and tumor specificity have been demonstrated in preclinical models.9–11
Clinical experience
Live attenuated serotype 1 poliovirus (PVS-RIPO) is currently in phase I clinical testing in patients with recurrent glioblastoma multiforme (NCT01491893). The purpose of the study is to determine the maximally tolerated dose (MTD) and dose-limiting toxicity (DLT) of PVSRIPO when delivered intracerebrally. Furthermore, this study aims to evaluate immunologic, virologic, and histopathologic parameters related to the viral infection. Among total of 13 patients treated thus far, one dose limiting toxicity was reported (grade 4 intracranial hemorrhage at catheter removal).12 No data describing the effects on human immune system following PVS-RIPO administration are yet available.
Reovirus
Reovirus is a nonenveloped double-stranded RNA virus. Productive replication of reovirus occurs in cells harboring an activated Ras mutation making reovirus naturally targeted to tumor cells.13,14 Oncolytic potency of local and systemic reovirus treatment has been demonstrated in both immunodeficient and immunocompetent animal models (reviewed in ref.15).
Clinical experience
Safety of unmodified wild-type reovirus (Reolysin) has been tested in cancer patients with a variety of malignancies in phase I studies utilizing both intratumoral (reviewed in ref.16) and systemic17 administration routes. Treatments were well-tolerated, and 37% and 52% of patients experienced grade 1 or 2 fever following local or systemic administration, respectively.18,19 Fever and concomitant increase in systemic cytokines indicate activation of the innate immune system following virus administration. Paired pre- and posttreatment tumor biopsies were collected from three intravenously dosed patients and all showed replication competent virus in posttreatment biopsies17 suggesting that biologically active virus is systemically available for the transduction of tumor cells in metastatic disease even in the presence of high antiviral neutralizing antibody titer in serum. Increase in antiviral neutralizing antibodies in serum has been reported systematically in all clinical trials with reovirus regardless of the route of administration.17,18,20,21 In addition to antiviral antibody responses, changes in lymphocyte subsets in peripheral blood following intravenous reovirus administration have been reported.21 In a study of 21 patients, one-third of them showed a posttreatment increase in CD4+ and CD8+ T-cells as well as in natural killer cells (CD3−/CD56+) in peripheral blood. In addition, five patients showed an increase in activated CD8+ T-cells (CD8+/granzyme B+/perforin+) in peripheral blood. The number of circulating regulatory T-cell (CD3+/CD4+/CD25+; Treg) was mostly unaffected even though two patients showed a posttreatment increase in systemic Tregs. However, antigen specificity of T-cells was not assessed, and therefore it remains unclear whether circulating T-cells recognized viral or tumor epitopes.
Measles Virus
Measles virus is a negative-strand enveloped RNA virus causing a highly contagious exanthemous measles disease. Attenuated measles virus vaccine strains have been shown to selectively infect, replicate in, and lyse cancer cells while causing minimal cytopathic effect on normal tissues (reviewed in ref.22).
Clinical experience
Measles virus encoding for tumor antigen CEA (MV-CEA) is currently in phase 1 testing in glioblastoma multiforme (NCT00390299). Several safety and dose-finding clinical trials are currently ongoing to test either local or systemic administration of measles virus encoding the human thyroidal sodium iodide symporter (MV-NIS) in patients with various malignancies. In addition, phase 2 study is recruiting ovarian cancer patients to test how well intraperitoneally administered MV-NIS works in comparison to chemotherapy (NCT02364713). For this study, antiviral and antitumor immune responses are mentioned as outcome measures by ClinicalTrials.gov but no results are yet available.
Newcastle Disease Virus
Newcastle disease virus (NDV) is a negative-strand enveloped RNA virus that is responsible for highly contagious disease in avian species, but does not cause disease in humans. Individual strains of NDV are classified as lytic or nonlytic. Early studies with the virus demonstrated its predilection to replicate in and lyse human cancer cells.23–27 Additional preclinical studies showed that the virus has a capability to activate both innate and adaptive immune responses and synergized with antibody targeting CTLA-4.28–30
Clinical experience
To explore the immunotherapeutic potential of NDV in patients, several studies utilized NDV-infected autologous or allogeneic tumor cells for immunization of patients with advanced metastatic disease.31–36 The majority of the studies demonstrated prolongation of survival when compared with historical controls, suggesting the involvement of immune response. The PV701 strain of NDV was tested with intravenous administration in 79 patients with advanced solid tumors.37 Objective responses were reported in the study and tumor biopsies demonstrated evidence of immune infiltration into the tumors. However, no pretreatment biopsies were available for comparison and it was unclear whether the observed immune responses were primarily targeting tumor or viral antigens.37
Coxsackievirus A21
Coxsackievirus A21 is a common cold-causing nonenveloped lytic enterovirus with a single-strand RNA genome. It is naturally targeted to cells expressing intercellular adhesion molecule 1 (ICAM-1) and decay-accelerating factor (DAF). ICAM-1 expression is upregulated in several human cancers, allowing coxsackievirus A21 to preferentially infect and, subsequently, replicate in tumor cells. Preclinical antitumor activity of CAVATAK was characterized by highly efficient systemic spread of progeny viral particles and oncolytic tumor cell death in tumors distant to administration site.38
Clinical experience
The safety of an unmodified wild-type coxsackievirus, CAVATAK, has been tested as a local and systemic oncolytic therapy in phase I studies in patients with ICAM-1-expressing solid tumors, and no safety concerns have been reported.39,40 Some patients have shown a reduction in lesions and/or disease stabilization after local or systemic CAVATAK administration.39,40 Infectious CAVATAK particles were routinely detected in serum 30 min after systemic administration.40 A phase II study (NCT01227551) of intratumoral CAVATAK in patients with malignant melanoma is currently ongoing. Responses in both injected and noninjected lesions in the presence of high serum antiviral neutralizing antibody levels and in the absence of circulating infectious CAVATAK have been reported.41 Even though direct clinical evidence of CAVATAK-induced antitumor humoral or cellular immune responses is currently missing, the existing data strongly suggest involvement of the immune system. To further evaluate CAVATAK-mediated immune responses in melanoma patients, this phase II study is currently recruiting an additional cohort of 12 patients for immunoprofiling. In addition, intratumoral CAVATAK administration in combination with the immune checkpoint inhibitor ipilimumab is currently in phase I clinical testing in patients with advanced melanoma (NCT02307149).
Parvovirus
Parvovirus is a small nonenveloped virus with a single-stranded DNA genome.42 Parvovirus is a human-specific virus and its lytic replication cycle takes place only in actively proliferating cells (e.g., cancer cells), while the infection is normally asymptomatic in adults.43 Parvovirus can selectively shut down antiviral innate immune mechanisms in malignant cells,44 making it highly oncolytic. Indeed, preclinical studies have demonstrated complete eradication of established brain tumors following either local or systemic parvovirus administration without deleterious side effects or major signs of local inflammation.45,46 This preclinical finding led to the initiation of clinical studies of parvovirus as an oncolytic treatment for brain tumors.
Clinical experience
Phase I/IIa clinical testing of H-1 parvovirus (H-1PV) is currently ongoing in patients with glioblastoma multiforme (NCT01301430). H-1PV (ParvOryx01) is administered at three dose levels either intratumorally or intravenously followed, 10 days later, by intracerebral administration of the walls where the tumor had previously been resected. The study was initiated in 2011 and the primary objectives of the trial are safety, MTD, viremia, and virus shedding. No immunological assessments have been listed as objectives by clinicalTrials.gov. We speculate that the natural ability of parvovirus to inhibit recognition by innate immune system and type 1 interferon response may hamper its use as a cancer immunotherapy agent and render primarily as a cytotoxic (oncolytic) agent. To this end, only 3 out of 12 patients showed a moderate and transient fever briefly after administration of H-1PV in a pilot study, suggesting very limited activation of innate immune system.47 Importantly, however, the treatment was well tolerated and MTD was not reached.
Vaccinia Virus
Vaccinia virus (VV) is a large and complex enveloped double-stranded DNA virus that replicates within the cytoplasm of host cells. VV is highly lytic and can replicate in a wide variety of host species.48 VV produces two different types of infective viral particles of which the extracellular enveloped viruses (EEV) are important in the systemic dissemination of VV as they are coated by a host cell-derived membrane and are therefore invisible to the immune system.49,50 VV has evolved numerous strategies to shut down the host defense mechanisms and to escape from the detection by the immune system,51 one example being encoding intracellular and secreted proteins that block intracellular innate immune responses in infected cells or mimic the extracellular binding domain of host cytokine receptors. Despite the multiple immune evasion mechanisms, VV has been researched as a vaccine vector, and highly promising results have been reported with the nonreplicating PROSTVAC vaccine in patients with prostate cancer.52,53 Humoral (antibody) response to virus was shown to correlate with overall survival in two independent patient cohorts52 and a survival benefit of 8.5 months was demonstrated in a randomized phase 2 study.53 Pivotal phase 3 trial is currently ongoing.
VV has no natural tumor-targeting mechanism at the level of cell entry, but systemically delivered VV targets tumors via leaky vasculature. Tumor-selective replication is achieved by deleting viral genes that are necessary for replication in normal cells (e.g., thymidine kinase and vaccinia growth factor), leaving genetically engineered vectors able to replicate only in cancer cells that complement the functions of deleted genes.54–56 Genetically engineered GM-CSF-coding vaccinia viruses JX-594 and JX-963 have shown a significant oncolytic potential after systemic administration in immunocompetent animal models.56,57
Clinical experience
Several clinical studies have been conducted with Western Reserve TK gene-inactivated oncolytic vaccinia viruses expressing human GM-CSF as an immunostimulatory transgene (JX-594 and JX-929) to assess the safety and dosing in various indications following intratumoral injections.58–60 Patients treated with JX-594 and JX-929 typically experienced fever and flu-like symptoms shortly after treatment and developed antiviral antibodies within 1 month from treatment initiation, indicating that innate and adaptive immune responses toward VV were elicited. Further evidence for innate immunity evoked by JX-594 was seen as a rapid cytokine response posttreatment depicted by elevation of Interleukin-6, interleukin-10, and TNF-alpha levels in serum.58 Infective VV in noninjected tumors and concomitant clinical responses in some of these noninjected tumors was reported for both viruses,58–60 suggesting that responses are because of systemic spread of infectious virus and virus replication at distant sites. Importantly, treatments with these GM-CSF-expressing VVs resulted in increased serum level of GM-CSF with a concomitant increase in absolute neutrophil and eosinophil counts, indicating expression and systemic availability of the transgene on a level that was sufficient for biological effect.
Interestingly, an induction of humoral antitumor immune response has been seen in the form of antibody-mediated complement-dependent cytotoxicity (CDC) in patients' serum against allogenic liver cancer cell lines.59 To this end, evidence for antitumor humoral immune response with VV has been shown both in animal models and in humans.61 While the study did not assess for adaptive tumor-specific cellular immune responses, CD4 and CD8 T-cell proliferation was seen in peripheral blood after treatment with JX-929. Furthermore, posttreatment tumor biopsies collected from a patient with metastatic melanoma showed higher expression levels of immune cell markers, cytokines, and chemokines in injected tumor than in noninjected tumor. Finally, cytotoxic T-cell response against vaccinia and viral transgene (β-gal) was induced by treatment with JX-594.59 Any reported clinical studies with vaccinia, however, did not look for tumor-specific T-cell responses, and it is unclear whether the observed antitumor effects were mediated by direct oncolysis or whether the therapy was capable of inducing tumor-specific cytotoxic T-cell responses.
Herpes Simplex Virus Type 1
Herpes simplex virus type 1 (HSV-1) is a highly lytic large double-stranded enveloped DNA virus. Wild-type HSV-1 has several mechanisms to interfere with host immune responses and it can establish latency. Wild-type HSV-1 has evolved multiple mechanisms to avoid antigen presentation on major histocompatibility (MHC) molecules and to circumvent antibody and complement responses. It expresses multiple proteins (e.g., ICP0, ICP34.5, and vhs) interfering with IFN-response.62–64
Clinical experience
T-VEC (Talimogene Laherparepvec) is a human GM-CSF-coding herpes simplex virus type 1 in which neurovirulence factor ICP34.5 and ICP47 genes have been deleted for increased virus growth, tumor selective replication, and increased immunogenicity.65 In a phase 1 study, one-third of the patients with refractory cutaneous or subcutaneous metastases of various cancer types experienced grade 1–2 fever after treatment with single intratumoral administration of T-VEC.66 Even though a repeated dosing increased the proportion of patients experiencing fever up to 65%, the lack of fever in over one-third of the patients may suggest that the innate immune reaction toward T-VEC is suboptimal for breaking the tumor immunotolerance. All patients who were seronegative for T-VEC neutralizing antibodies at the baseline seroconverted within 3–4 weeks from treatment initiation. Furthermore, baseline seropositive patients showed a general trend for increased antibody titers during the treatment. These results indicate an active humoral immune response against the virus. Serum samples remained negative for GM-CSF in all patients and viral DNA was not routinely detected in the blood or urine with the authors concluding that the virus was retained at the injection site. Several patients showed disappearance or shrinkage of individual injected or noninjected lesions. A concomitant presence of necrotic tissue and viral particles in injected tumors suggested T-VEC-mediated tumor cell death.
T-VEC has also been tested as a monotherapy in phase II study in patients with unresectable metastatic melanoma.67,68 A total of 50 patients were treated intratumorally with repeated T-VEC injections. Objective clinical response was detected in 13 out of 50 (26%) patients, which included responses in both virus-injected and distant lesions. Biological materials—tumor biopsies and PBMCs—were compared with samples from a cohort of nontrial cancer patients with resectable melanoma (tumor samples) or from healthy donors (PBMCs). Injected and noninjected posttreatment tumor biopsies were compared as pairs. Extensive lymphocyte infiltration was reported generally in T-VEC-treated tumors, and the tumor-derived T-cells showed activated CD45RO+phenotype with upregulation of CD45RO, CD25, and HLA-DR. Comparison of injected and noninjected lesions showed significantly lower number of CD4+FoxP3+CD25+T regulatory cells in injected lesions, suggesting that local T-VEC treatment was able to modulate tumor microenvironment to become less immunosuppressive. Finally, T-cells derived from tumors undergoing regression recognized melanoma antigen MART-1 more often than T-cells isolated from tumors of nonstudy control melanoma patients. Unfortunately, these analyses failed to capture pretreatment biopsies and PBMCs, making it difficult to assess the magnitude of immune response induced by T-VEC.
In a randomized phase 3 clinical trial (OPTiM) in unresectable melanoma comparing intralesional T-VEC to subcutaneous GM-CSF, T-VEC achieved its primary endpoint of statistically significant improvement in durable response rate.69 While there was a trend toward improvement in overall survival, this failed to meet statistical significance (p=0.051).69 As with CAVATAK, responses in distant lesions implicate the role of the immune system, providing rationale for further exploration of T-VEC in combination with other immune-modulating agents. Indeed, multiple clinical trials for testing the combinatorial use of T-VEC with other cancer therapeutics, including immune checkpoint inhibitors ipilimumab (NCT01740297) and anti-PD-1 pembrolizumab (NCT02263508), are currently ongoing in melanoma patients with promising preliminary data.70
HF-10 is a naturally mutated HSV-1 virus derived from syncytia-forming parent virus. UL43, 49.5, 55, and 56 and latency-associated transcripts are confirmed to be inactivated in HF-10. Toxicity of HF-10 is significantly lower in comparison to wild-type virus but the virus still remains effective in killing tumor cell lines.71 Intratumoral administration of HF-10 was shown to be safe in solid tumor patients72–75 and it is currently in phase 2 clinical testing in combination with checkpoint inhibitor anti-CTLA-4 ipilimumab (NCT02272855).
Adenovirus
Adenovirus is a common cold-causing nonenveloped lytic double-stranded DNA virus. Adenoviruses are divided into 51 human serotypes and into 6 different groups from A to F. Adenovirus shows the biggest clinical experience among all viral vectors used for treatment of human diseases.76 It has been used as a nonreplicating gene transfer vector in various human diseases,76 as a prophylactic vaccination vehicle,77 as well as an oncolytic cancer treatment.78 Adenovirus can be genetically engineered for tumor-specific transduction and replication. Numerous replication-competent mutated viruses have been developed and the discussion will be limited to the vectors that are currently in clinical testing.
Clinical experience
ColoAd1 is a chimeric unarmed Ad11p/Ad3 group B adenovirus. It has been generated by bioselection from a library of chimeric adenoviruses for rapid replication in colorectal cancer cells.79 Safety of intravenously delivered ColoAd1 has been tested in a phase 1 study in patients with metastatic solid tumors of epithelial origin (NCT02028442). A total of 34 patients were treated in this dose-escalation study, and DLT was detected in 2 patients at a dose level of 10e13 viral particles over 5 min infusion.80 Systemic delivery of ColoAd1 into tumor tissue and replication in tumor cells following repeated intravenous administration has been demonstrated in colorectal patients in two separate clinical trials, NCT02028442 and NCT02053220.81,82 In addition, tumor infiltrating CD8+ T-cells were reported in the region of virus infection in posttreatment colorectal biopsies,82 but in the absence of pretreatment sample the significance of this finding remains unclear. A phase I/II study to evaluate the safety and tolerability of intraperitoneal administration of ColoAd1 is currently ongoing in platinum-resistant epithelial ovarian cancer (NCT02028117). While feasibility of systemic administration of ColoAd1 and productive virus replication in metastatic disease has been established, no evidence for ColoAd1-induced tumor-targeted immunological responses has been reported as yet.
CG0070 is a replication-competent serotype 5 adenovirus that has been engineered to preferentially replicate in and destroy retinoblastoma (Rb) pathway-defective cancer cells using the E2F-1 promoter to control virus replication and expression of GM-CSF. G0070 is administered via intravesical infusion after preconditioning the bladder with a detergent to enhance viral infection of bladder epithelium.83 In a phase I study with single or repeated intravesical administrations, treatments were well-tolerated and the MTD was not reached. Only 11% of the patients experienced flu-like symptoms and no fever was reported, suggesting that systemic exposure to virus was low. This is further supported by the fact that only three patients had CG0070 present in plasma at any time point. On the other hand, high levels of GM-CSF and CG0070 genomes were detected in urine 2–5 days following CG0070 administration, suggesting transgene expression and virus replication in bladder cancer tissue. Detectable GM-CSF levels in plasma were measured in 15 out of 35 patients. No virus- or transgene-mediated anticancer immune responses were evaluated. CG0070 is currently in phase III testing for the treatment of nonmuscle invasive bladder cancer.
DNX2401 is an unarmed replication-competent serotype 5 adenovirus that has been engineered to replicate only in cells exhibiting mutated Rb signaling pathway. Enhanced infection of tumor cells is achieved via RGD capsid modification targeting the virus to cell surface integrins. A phase I study to assess safety of intratumoral DNX2401 treatment in patients with recurrent malignant gliomas (NCT00805376) has recently been completed.84 Treatment was well tolerated and evidence of virus replication and tumor cell killing was detected in histological analysis of posttreatment surgical specimens. Magnetic resonance imaging (MRI) revealed increased tumor volume before tumor regression, consistent with an inflammatory response. Three patients (12%) showed a complete response. Analysis of serum cytokine levels demonstrated that responders had 10- to 10 000-fold increases in serum IL-12p70 in comparison to nonresponders. IL-12p70 mediates Th1 polarization and cellular immunity. Posttreatment increase in this cytokine suggests that the antitumor immune response was contributing to these clinical responses.84 Two additional clinical trials are currently ongoing in patients with glioblastoma or gliosarcoma to assess safety and efficacy of intratumoral DNX2402 treatment in combination with temozolomide (NCT01956734) or interferon-gamma (NCT02197169).
ICOVIR-5 is an unarmed replication-competent serotype 5 adenovirus. It is transductionally targeted to tumor cells via RGD modification in the virus capsid. Tumor-selective replication is achieved via 24 bp deletion in viral E1A gene. Insertion of two E2F-1-responsive element promoter preceded by an insulator upstream of the E1A decreases E1A-medited toxicity. ICOVIR-5 is currently in phase I testing to evaluate the safety of weekly infusion of ICOVIR-5-infected bone marrow-derived autologous mesenchymal cells in children and adults with metastatic solid tumors (NCT01844661). Another phase I study is ongoing to test the safety of intravenous ICOVIR-5 administration in patients with locally advanced or metastatic melanoma (NCT01864759). No results from these studies are yet available and there does not seem to be any immunological assessments as endpoints according to ClinicalTrials.gov.
ONCOS-102 is a serotype 5 adenovirus that features a chimeric capsid for enhanced gene delivery to cancer cells, a 24 bp deletion in Rb binding site of E1A for cancer cell-restricted replication, and armed with GM-CSF. Safety and immune activation profile following repeated intratumoral administration of ONCOS-102 have been assessed in phase I study in 12 patients with refractory solid tumors (NCT01598129). Treatment was well tolerated and no MTD was detected.85 Biological materials—tumor biopsies and PBMCs—were systematically collected both before and during the study. Intratumoral ONCOS-102 triggered an innate immune response in every patient as measured by a transient increase in systemic proinflammatory cytokines and induction of fever in all patients within 6–10 hr after administration.85 Infiltration of innate immune cells into tumors posttreatment was detected in 11 out of 12 patients. Concomitant infiltration of T-cells was detected in 11 out of 12 patients with the most prominent increase seen in CD8+ T-cells.86 Biopsies from a noninjected distant tumor were obtained from one patient with a 2.5-fold increase in CD8+ T-cells following ONCOS-102 treatment was detected.87 Two patients showing the most robust increase in tumor-infiltrating CD8+ T-cells posttreatment also showed a prominent induction of tumor-specific CD8+ T-cells in peripheral blood.88,89 Multiple CD8+ T-cell subpopulations recognizing several tumor antigens (MAGE-A3, MAGE-A1, NY-ESO-1, mesothelin) were detected posttreatment, while none of these CD8+ T-cell populations were present at baseline. Gene expression profiling of tumor biopsies before and after ONCOS-102 treatment showed an increased expression of Th1 markers and Th1 cytokines posttreatment, suggesting that local ONCOS-102 treatment was able to polarize tumor microenvironment toward Th1 type immunity.90 The correlation between OS and increase in tumor-infiltrating lymphocytes (TILs) was analyzed where CD8+ T-cells and CD68+ macrophages showed a statistically significant correlation with OS.87 Gene expression profiling of posttreatment biopsies suggested that macrophages had cytotoxic M1 phenotype.90 Concomitant trafficking of innate and adaptive immune cells to the tumors and the induction of tumor-specific CD8+ T-cells suggest that ONCOS-102 is able to induce de novo antitumor immune responses in refractory metastatic cancer patients and act as an immune primer. Correlation between TILs following treatment and OS, as well as CD8+ T-cell infiltration into a noninjected distant lesion, suggest an involvement of a systemic immune activation. To our knowledge, this phase 1 study with ONCOS-102 is the only clinical trial among all viral vectors where the virus-induced immune activation profile has been systematically evaluated by collecting pre- and posttreatment tumor and blood samples. Even more importantly, ONCOS-102 is so far the only viral vector that has been formally clinically demonstrated to prime de novo antitumor cytotoxic CD8+ T-cell response in the situation where no baseline antitumor activity was detected.
Outlook/Conclusions
The great majority of the immunological assessments done in the past clinical trials are focusing on antiviral immune responses—almost exclusively on antiviral neutralizing antibodies—and significantly less or no attention has been paid to tumor-targeted immune responses. All viruses tested, regardless of administration route, have been shown to systematically induce significant antiviral antibody responses in cancer patients. This should be seen as a positive signal as it clearly demonstrates that viruses are able to activate the immune system per se. Preclinical evidence shows that enhancing the antiadenoviral immune response also enhances the protective antitumor immunity.91
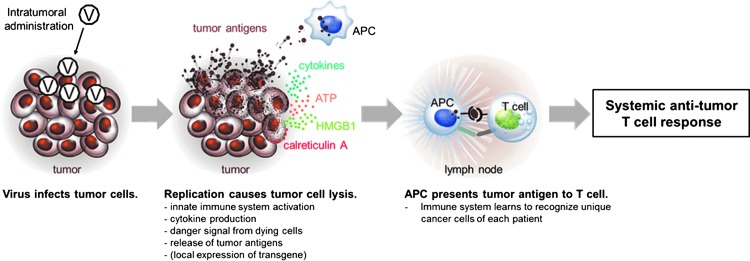
Optimally, oncolytic virus can provide an adjuvant effect with local cytokine production and danger signal resulting from immunogenic cancer cell death, and simultaneously function as a “personalized” cancer vaccine by releasing patient-specific unique tumor antigens from dying cancer cells (Fig. 2). The former can be further enhanced by arming viruses with immunostimulatory transgenes, like GM-CSF, CD40L or other co-stimulatory modulators. Local administration may be preferable to systemic for several reasons. First, danger signal is needed at tumor site to break the immunotolerance, and, second, the release of tumor antigens has to occur at the same place and time with the activation of innate immune cells in order to maximize the likelihood of successful priming of tumor-targeted adaptive immune response. Preclinical evidence confirms that the activation signal for the innate immune system needs to be provided locally, that is, at the same place where antigen is presented, while the separate presentation of antigen and adjuvant fails to provide equally effective adjuvant effect.92
Figure 2.
Schematic presentation of virus initiated antitumor T-cell response. Locally administered replication-competent virus activates the innate immune system via pathogen-associated molecular pattern (PAMP) and danger-associated molecular pattern (DAMP) receptors. Virus replication causes an immunogenic cancer cell death leading to exposure of calreticulin A on the outer surface of tumor cells, and release of ATP, HMGB1, and tumor antigens from dying cancer cells. All these signals increase the activity of antigen-presenting cells (APCs), which take up and process tumor antigens. Maturation of APCs and local immunostimulation can be further enhanced by virus-coded transgene. APCs present antigens to T-cells in the draining lymph node leading to systemic T-cell attack against tumors.
Third, systemic dosing requires higher viral doses in comparison to local administration since virus clearance mechanisms, regardless of choice of virus, significantly limit the bioavailability of virus at the tumor environment following intravenous administration. This may prove to be a significant limitation to virus manufacturing toward commercialization. Importantly, the vector needs to be carefully chosen. Via TLRs and TLR-independent detectors, different viruses activate innate immune system differently, leading to qualitatively and quantitatively distinct responses of humoral (antibody) and cellular (cytotoxic T-cells) arms of adaptive immune system.77,93–96 These fundamental and complex interactions between virus and host immune system likely cannot be dramatically changed even with genetic engineering and modern molecular technologies. Critical differences in immune activation profiles of each virus can only be studied in cancer patients and may ultimately define the feasibility and viability of each virus platform as a cancer immunotherapeutic. Systematic evaluation of tumor biopsies and blood samples collected before and after treatment should be prioritized while planning phase I and II clinical trials with viral vectors in the future.
Safe handling of replicating viruses requires a proper education of hospital pharmacies and nursing staff. Consequently, increasing awareness of the clinical use of oncolytic viruses lowers the threshold for sites to participate in clinical trials and this experimental therapy becomes available for more cancer patients. Importantly, safety profile of most oncolytic viruses is relatively benign in comparison to current standard treatments (e.g., chemotherapy) and nonoverlapping adverse event profiles allow combination treatments. Unlike adoptive cell transfer, oncolytic virus therapy is essentially off-the-shelf immunotherapy that can provide a tailored immune response against each patients' own tumors. Furthermore, it is significantly less labor-intensive and less expensive than adoptive cell transfer. Provided that we learn how viruses can be optimally harnessed to reveal tumors to the immune system, viral immunotherapy may provide a completely new treatment arsenal and hope for patients suffering from cancer indications that are not treatable or responding to currently available immunotherapies.
Acknowledgments
We thank Tuuli Ranki and Laura Suoranta for technical writing support and assistance in data collection.
Author Disclosure
No competing financial interests exist.
References
- 1.Huneycutt BS, Bi Z, Aoki CJ, et al. Central neuropathogenesis of vesicular stomatitis virus infection of immunodeficient mice. J Virol 1993;67:6698–6706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muik A, Stubbert LJ, Jahedi RZ, et al. Re-engineering vesicular stomatitis virus to abrogate neurotoxicity, circumvent humoral immunity, and enhance oncolytic potency. Cancer Res 2014;74:3567–3578 [DOI] [PubMed] [Google Scholar]
- 3.Bridle BW, Stephenson KB, Boudreau JE, et al. Potentiating cancer immunotherapy using an oncolytic virus. Mol Ther 2010;18:1430–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wongthida P, Diaz RM, Pulido C, et al. Activating systemic T-cell immunity against self tumor antigens to support oncolytic virotherapy with vesicular stomatitis virus. Hum Gene Ther 2011;22:1343–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brun J, McManus D, Lefebvre C, et al. Identification of genetically modified Maraba virus as an oncolytic rhabdovirus. Mol Ther 2010;18:1440–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fauquet CM, Mayo AA, Maniloff J, et al. Virus Taxonomy: Eighth Report of the International Committee on Taxonomy of Viruses. (International Committee on Taxonomy of Viruses, London: ), 2005 [Google Scholar]
- 7.Pol JG, Zhang L, Bridle BW, et al. Maraba virus as a potent oncolytic vaccine vector. Mol Ther 2014;22:420–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gromeier M, Alexander L, Wimmer E. Internal ribosomal entry site substitution eliminates neurovirulence in intergeneric poliovirus recombinants. Proc Natl Acad Sci USA 1996;93:2370–2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gromeier M, Lachmann S, Rosenfeld MR, et al. Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proc Natl Acad Sci USA 2000;97:6803–6808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dobrikova EY, Broadt T, Poiley-Nelson J, et al. Recombinant oncolytic poliovirus eliminates glioma in vivo without genetic adaptation to a pathogenic phenotype. Mol Ther 2008;16:1865–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goetz C, Everson RG, Zhang LC, et al. MAPK signal-integrating kinase controls cap-independent translation and cell type-specific cytotoxicity of an oncolytic poliovirus. Mol Ther 2010;18:1937–1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desjardins A, Sampson JH, Peters KB, et al. Final results of a phase 1 trial of an oncolytic polio/rhinovirus recombinant (PVSRIPO) against recurrent glioblastoma (GBM). 19th Annual Scientific Meeting of the Society for Neuro-Oncology, Miami, Florida, 2014 [Google Scholar]
- 13.Coffey MC, Strong JE, Forsyth PA, et al. Reovirus therapy of tumors with activated Ras pathway. Science 1998;282:1332–1334 [DOI] [PubMed] [Google Scholar]
- 14.Meurs E, Chong K, Galabru J, et al. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell 1990;62:379–390 [DOI] [PubMed] [Google Scholar]
- 15.Comins C, Heinemann L, Harrington K, et al. Reovirus: Viral therapy for cancer “as nature intended.” Clin Oncol (R Coll Radiol) 2008;20:548–554 [DOI] [PubMed] [Google Scholar]
- 16.Harrington KJ, Vile RG, Melcher A, et al. Clinical trials with oncolytic reovirus: Moving beyond phase I into combinations with standard therapeutics. Cytokine Growth Factor Rev 2010;21:91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vidal L, Pandha HS, Yap TA, et al. A phase I study of intravenous oncolytic reovirus type 3 Dearing in patients with advanced cancer. Clin Cancer Res 2008;14:7127–7137 [DOI] [PubMed] [Google Scholar]
- 18.Morris DG, Feng X, DiFrancesco LM, et al. REO-001: A phase I trial of percutaneous intralesional administration of reovirus type 3 dearing (Reolysin(R)) in patients with advanced solid tumors. Invest New Drugs 2013;31:696–706 [DOI] [PubMed] [Google Scholar]
- 19.Galanis E, Markovic SN, Suman VJ, et al. Phase II trial of intravenous administration of Reolysin((R)) (Reovirus Serotype-3-dearing Strain) in patients with metastatic melanoma. Mol Ther 2012;20:1998–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gollamudi R, Ghalib MH, Desai KK, et al. Intravenous administration of Reolysin, a live replication competent RNA virus is safe in patients with advanced solid tumors. Invest New Drugs 2010;28:641–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White CL, Twigger KR, Vidal L, et al. Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing type 3) during a phase I clinical trial. Gene Ther 2008;15:911–920 [DOI] [PubMed] [Google Scholar]
- 22.Msaouel P, Iankov ID, Dispenzieri A, et al. Attenuated oncolytic measles virus strains as cancer therapeutics. Curr Pharm Biotechnol 2012;13:1732–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zamarin D, Palese P. Oncolytic Newcastle disease virus for cancer therapy: Old challenges and new directions. Future Microbiol 2012;7:347–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omar AR, Ideris A, Ali AM, et al. An overview on the development of newcastle disease virus as an anti-cancer therapy. Malays J Med Sci 2003;10:4–12 [PMC free article] [PubMed] [Google Scholar]
- 25.Zulkifli MM, Ibrahim R, Ali AM, et al. Newcastle diseases virus strain V4UPM displayed oncolytic ability against experimental human malignant glioma. Neurol Res 2009;31:3–10 [DOI] [PubMed] [Google Scholar]
- 26.Ali R, Alabsi AM, Ali AM, et al. Cytolytic effects and apoptosis induction of Newcastle disease virus strain AF2240 on anaplastic astrocytoma brain tumor cell line. Neurochem Res 2011;36:2051–2062 [DOI] [PubMed] [Google Scholar]
- 27.Abdullah JM, Mustafa Z, Ideris A. Newcastle disease virus interaction in targeted therapy against proliferation and invasion pathways of glioblastoma multiforme. Biomed Res Int 2014;2014:386470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zorn U, Dallmann I, Grosse J, et al. Induction of cytokines and cytotoxicity against tumor cells by Newcastle disease virus. Cancer Biother 1994;9:225–235 [DOI] [PubMed] [Google Scholar]
- 29.Kato H, Sato S, Yoneyama M, et al. Cell type-specific involvement of RIG-I in antiviral response. Immunity 2005;23:19–28 [DOI] [PubMed] [Google Scholar]
- 30.Zamarin D, Holmgaard RB, Subudhi SK, et al. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med 2014;6:226ra232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cassel WA, Garrett RE. Newcastle disease virus as an antineoplastic agent. Cancer 1965;18:863–868 [DOI] [PubMed] [Google Scholar]
- 32.Cassel WA, Murray DR. A ten-year follow-up on stage II malignant melanoma patients treated postsurgically with Newcastle disease virus oncolysate. Med Oncol Tumor Pharmacother 1992;9:169–171 [DOI] [PubMed] [Google Scholar]
- 33.Cassel WA, Murray DR, Torbin AH, et al. Viral oncolysate in the management of malignant melanoma. I. Preparation of the oncolysate and measurement of immunologic responses. Cancer 1977;40:672–679 [DOI] [PubMed] [Google Scholar]
- 34.Cassel WA, Weidenheim KM, Campbell WG Jr., et al. Malignant melanoma. Inflammatory mononuclear cell infiltrates in cerebral metastases during concurrent therapy with viral oncolysate. Cancer 1986;57:1302–1312 [DOI] [PubMed] [Google Scholar]
- 35.Murray DR, Cassel WA, Torbin AH, et al. Viral oncolysate in the management of malignant melanoma. II. Clinical studies. Cancer 1977;40:680–686 [DOI] [PubMed] [Google Scholar]
- 36.Liebrich W, Schlag P, Manasterski M, et al. In vitro and clinical characterisation of a Newcastle disease virus-modified autologous tumour cell vaccine for treatment of colorectal cancer patients. Eur J Cancer 1991;27:703–710 [DOI] [PubMed] [Google Scholar]
- 37.Pecora AL, Rizvi N, Cohen GI, et al. Phase I trial of intravenous administration of PV701, an oncolytic virus, in patients with advanced solid cancers. J Clin Oncol 2002;20:2251–2266 [DOI] [PubMed] [Google Scholar]
- 38.Shafren DR, Au GG, Nguyen T, et al. Systemic therapy of malignant human melanoma tumors by a common cold-producing enterovirus, coxsackievirus a21. Clin Cancer Res 2004;10:53–60 [DOI] [PubMed] [Google Scholar]
- 39.Shafren DR, Smithers BM, Formby M. A phase I, open label, cohort study of two doses of coxsackievirus A21 given intratumorally in stage IV melanoma. ASCO, Chicago, IL, 2011 [Google Scholar]
- 40.Liauw WS, Chern B, Shafren DR. Phase I, open-label cohort study of CAVATAKTM (Coxsackievirus 21) given intravenously to stage IV patients bearing ICAM-1 expressing solid tumors. EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics, Dublin, Ireland, 2012 [Google Scholar]
- 41.Andtbacka RHI, Curti B, Kaufman HL, et al. CALM study: Secondary endpoints of a phase II study of a novel oncolytic immunotherapeutic agent, Coxsackievirus A21, delivered intratumorally in patients with advanced malignant melanoma. European Society for Medical Oncology, Madrid, Spain, 2014 [Google Scholar]
- 42.Tattersall P. The evolution of parvovirus taxonomy. In: Parvoviruses. Kerr JR, Cotmore SF, Bloom ME, et al., eds. (Hodder Arnold, London: ). 2006; pp. 5–14 [Google Scholar]
- 43.Cotmore SF, Tattersall P. Parvoviral host range and cell entry mechanisms. Adv Virus Res 2007;70:183–232 [DOI] [PubMed] [Google Scholar]
- 44.Randall RE, Goodbourn S. Interferons and viruses: An interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol 2008;89:1–47 [DOI] [PubMed] [Google Scholar]
- 45.Rommelaere J, Geletneky K, Angelova AL, et al. Oncolytic parvoviruses as cancer therapeutics. Cytokine Growth Factor Rev 2010;21:185–195 [DOI] [PubMed] [Google Scholar]
- 46.Geletneky K, Herrero y Calle M, Herold-Mende C, et al. Complete remission of advanced autologous intracranial gliomas by oncolytic parvovirus H1. Eur J Cancer 2005;3:53 [Google Scholar]
- 47.Le Cesne A, Dupressoir T, Jamin N, et al. Intra-lesional administration of a live virus, parvovirus H-1, in cancer patients: A feasibility study. Proc Am Soc Clin Oncol 1993;12:297 [Google Scholar]
- 48.Alcami A, Smith GL. Soluble interferon-gamma receptors encoded by poxviruses. Comp Immunol Microbiol Infect Dis 1996;19:305–317 [DOI] [PubMed] [Google Scholar]
- 49.Smith GL, Vanderplasschen A, Law M. The formation and function of extracellular enveloped vaccinia virus. J Gen Virol 2002;83:2915–2931 [DOI] [PubMed] [Google Scholar]
- 50.Roberts KL, Smith GL. Vaccinia virus morphogenesis and dissemination. Trends Microbiol 2008;16:472–479 [DOI] [PubMed] [Google Scholar]
- 51.Mossman K, Upton C, Buller RM, et al. Species specificity of ectromelia virus and vaccinia virus interferon-gamma binding proteins. Virology 1995;208:762–769 [DOI] [PubMed] [Google Scholar]
- 52.Campbell CT, Gulley JL, Oyelaran O, et al. Humoral response to a viral glycan correlates with survival on PROSTVAC-VF. Proc Natl Acad Sci USA 2014;111:E1749–E1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol 2010;28:1099–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buller RM, Smith GL, Cremer K, et al. Decreased virulence of recombinant vaccinia virus expression vectors is associated with a thymidine kinase-negative phenotype. Nature 1985;317:813–815 [DOI] [PubMed] [Google Scholar]
- 55.Gnant MF, Noll LA, Irvine KR, et al. Tumor-specific gene delivery using recombinant vaccinia virus in a rabbit model of liver metastases. J Natl Cancer Inst 1999;91:1744–1750 [DOI] [PubMed] [Google Scholar]
- 56.Thorne SH, Hwang TH, O'Gorman WE, et al. Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963. J Clin Invest 2007;117:3350–3358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim JH, Oh JY, Park BH, et al. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol Ther 2006;14:361–370 [DOI] [PubMed] [Google Scholar]
- 58.Park BH, Hwang T, Liu TC, et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: A phase I trial. Lancet Oncol 2008;9:533–542 [DOI] [PubMed] [Google Scholar]
- 59.Heo J, Reid T, Ruo L, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med 2013;19:329–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zeh HJ, Downs-Canner S, McCart JA, et al. First-in-man study of western reserve strain oncolytic vaccinia virus: Safety, systemic spread, and antitumor activity. Mol Ther 2015;23:202–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim MK, Breitbach CJ, Moon A, et al. Oncolytic and immunotherapeutic vaccinia induces antibody-mediated complement-dependent cancer cell lysis in humans. Sci Transl Med 2013;5:185ra163. [DOI] [PubMed] [Google Scholar]
- 62.Broberg EK, Hukkanen V. Immune response to herpes simplex virus and gamma134.5 deleted HSV vectors. Curr Gene Ther 2005;5:523–530 [DOI] [PubMed] [Google Scholar]
- 63.Lubinski JM, Jiang M, Hook L, et al. Herpes simplex virus type 1 evades the effects of antibody and complement in vivo. J Virol 2002;76:9232–9241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chee AV, Roizman B. Herpes simplex virus 1 gene products occlude the interferon signaling pathway at multiple sites. J Virol 2004;78:4185–4196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu BL, Robinson M, Han ZQ, et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther 2003;10:292–303 [DOI] [PubMed] [Google Scholar]
- 66.Hu JC, Coffin RS, Davis CJ, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res 2006;12:6737–6747 [DOI] [PubMed] [Google Scholar]
- 67.Senzer NN, Kaufman HL, Amatruda T, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol 2009;27:5763–5771 [DOI] [PubMed] [Google Scholar]
- 68.Kaufman HL, Kim DW, DeRaffele G, et al. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol 2010;17:718–730 [DOI] [PubMed] [Google Scholar]
- 69.Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 2015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 70.Puzanov I, Milhem MM, Andtbacka RHI, et al. Primary analysis of a phase 1b multicenter trial to evaluate safety and efficacy of talimogene laherparepvec (T-VEC) and ipilimumab (ipi) in previously untreated, unresected stage IIIB-IV melanoma. J Clin Oncol 2014;32:abstr. 9029 [Google Scholar]
- 71.Teshigahara O, Goshima F, Takao K, et al. Oncolytic viral therapy for breast cancer with herpes simplex virus type 1 mutant HF 10. J Surg Oncol 2004;85:42–47 [DOI] [PubMed] [Google Scholar]
- 72.Fujimoto Y, Mizuno T, Sugiura S, et al. Intratumoral injection of herpes simplex virus HF10 in recurrent head and neck squamous cell carcinoma. Acta Otolaryngol 2006;126:1115–1117 [DOI] [PubMed] [Google Scholar]
- 73.Nakao A, Kimata H, Imai T, et al. Intratumoral injection of herpes simplex virus HF10 in recurrent breast cancer. Ann Oncol 2004;15:988–989 [DOI] [PubMed] [Google Scholar]
- 74.Kimata H, Imai T, Kikumori T, et al. Pilot study of oncolytic viral therapy using mutant herpes simplex virus (HF10) against recurrent metastatic breast cancer. Ann Surg Oncol 2006;13:1078–1084 [DOI] [PubMed] [Google Scholar]
- 75.Nakao A, Kasuya H, Sahin TT, et al. A phase I dose-escalation clinical trial of intraoperative direct intratumoral injection of HF10 oncolytic virus in non-resectable patients with advanced pancreatic cancer. Cancer Gene Ther 2011;18:167–175 [DOI] [PubMed] [Google Scholar]
- 76.Giacca M. Gene Therapy. (Springer Science & Business Media, New York, NY: ), 2010; p. 303 [Google Scholar]
- 77.Draper SJ, Heeney JL. Viruses as vaccine vectors for infectious diseases and cancer. Nat Rev Microbiol 2010;8:62–73 [DOI] [PubMed] [Google Scholar]
- 78.Jiang H, Gomez-Manzano C, Rivera-Molina Y, et al. Oncolytic adenovirus research evolution: From cell-cycle checkpoints to immune checkpoints. Curr Opin Virol 2015;13:33–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuhn I, Harden P, Bauzon M, et al. Directed evolution generates a novel oncolytic virus for the treatment of colon cancer. PLoS One 2008;3:e2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Calvo E, Gil-Martin M, Machiels J-P, et al. A first-in-class, first-in-human phase I study of enadenotucirev, an oncolytic Ad11/Ad3 chimeric group B adenovirus, administered intravenously in patients with metastatic epithelial tumors. J Clin Oncol 2014a;32:abstr. 310325113756 [Google Scholar]
- 81.Calvo E, Martin MG, Cubillo A, et al. A phase I study of enadenotucirev, an oncolytic Ad11/Ad3 chimeric group B adenovirus, administered intravenously: Analysis of dose expansion and repeat cycle cohorts in patients with metastatic colorectal cancer (MCRC). Ann Oncol 2014;25:iv361–iv372 [Google Scholar]
- 82.Boni V, Portilla F, Cubillo A, et al. A phase I mechanism of action study of intra- tumoural (IT) or intravenous (IV) administration of enadenotucirev, an oncolytic Ad11/Ad3 chimeric group B adenovirus in colon cancer patients undergoing resection of primary tumour. Ann Oncol 2014;25:iv361–iv372 [Google Scholar]
- 83.Ramesh N, Memarzadeh B, Ge Y, et al. Identification of pretreatment agents to enhance adenovirus infection of bladder epithelium. Mol Ther 2004;10:697–705 [DOI] [PubMed] [Google Scholar]
- 84.Lang FF, Conrad C, Gomez-Manzaro C, et al. First-in-human phase I clinical trial of oncolytic delta-24-RGD (DNX-2401) with biological endpoints: Implications for viro- immunotherapy. Neuro Oncol 2014;16:iii39 [Google Scholar]
- 85.Pesonen S, Hemminki A, von Euler M, et al. Characterization of tumor-infiltrating lymphocytes following intratumoral administration of a chimeric adenovirus Ad5/3-D24-GMCSF (ONCOS-102) for refractory cancer patients with solid tumors. CIMT Annual Meeting, Mainz, Germany, 2014 [Google Scholar]
- 86.Pesonen S, Joensuu T, Jäger E, et al. Intratumoral ONCOS-102 shapes the tumor microenvironment in last line rafractory solid tumor patients. CRI Annual International Cancer Immunotherapy Symposium, New York, NY, 2014 [Google Scholar]
- 87.Pesonen S, Joensuu T, Jäger E, et al. Tumor-infiltrating lymphocytes (TILs) following intratumoral administration of ONCOS-102 are associated with prolonged overall survival in last line solid tumor patients. EORTC-NCI-AACR, Barcelona, Spain, 2014 [Google Scholar]
- 88.Ranki T, Joensuu T, Jager E, et al. Local treatment of a pleural mesothelioma tumor with ONCOS-102 induces a systemic antitumor CD8 T-cell response, prominent infiltration of CD8 lymphocytes and Th1 type polarization. Oncoimmunology 2014;3:e958937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vassilev L, Ranki T, Joensuu T, et al. Repeated intratumoral administration of ONCOS-102 leads to systemic antitumor CD8 T-cell response and robust cellular and transcriptional immune activation at tumor site in a patient with ovarian cancer. Oncoimmunology 2015;4:e1017702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Majumder M, Kumar A, Heckman C, et al. Gene expression analysis of tumors demonstrates an induction of Th1 type immune response following intratumoral administration of ONCOS-102 in refractory solid tumor patients. SITC 29th Annual Meeting, 2014 [Google Scholar]
- 91.Cerullo V, Diaconu I, Romano V, et al. An oncolytic adenovirus enhanced for toll-like receptor 9 stimulation increases antitumor immune responses and tumor clearance. Mol Ther 2012;20:2076–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weiner GJ, Liu HM, Wooldridge JE, et al. Immunostimulatory oligodeoxynucleotides containing the CpG motif are effective as immune adjuvants in tumor antigen immunization. Proc Natl Acad Sci USA 1997;94:10833–10837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bart PA, Huang Y, Karuna ST, et al. HIV-specific humoral responses benefit from stronger prime in phase Ib clinical trial. J Clin Invest 2014;124:4843–4856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Raftery MJ, Behrens CK, Muller A, et al. Herpes simplex virus type 1 infection of activated cytotoxic T cells: Induction of fratricide as a mechanism of viral immune evasion. J Exp Med 1999;190:1103–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Barcy S, Corey L. Herpes simplex inhibits the capacity of lymphoblastoid B cell lines to stimulate CD4+ T cells. J Immunol 2001;166:6242–6249 [DOI] [PubMed] [Google Scholar]
- 96.Villalba M, Hott M, Martin C, et al. Herpes simplex virus type 1 induces simultaneous activation of Toll-like receptors 2 and 4 and expression of the endogenous ligand serum amyloid A in astrocytes. Med Microbiol Immunol 2012;201:371–379 [DOI] [PubMed] [Google Scholar]