Abstract
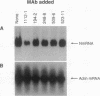
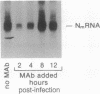
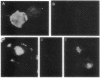
The in vitro biological activities of several rabies virus-neutralizing monoclonal antibodies (mAbs) were compared with their ability to prevent a lethal rabies virus encephalomyelitis. The protective activity of a particular mAb in vivo did not correlate with its virus-neutralizing activity in vitro; rather it was related to the mAb's ability to inhibit virus spread from cell to cell and to restrict rabies virus RNA transcription. Since treatment of rabies virus-infected cells with virus-neutralizing mAbs results in an endocytosis of the antibody, we hypothesize that an antibody may exert its inhibitory activity even after uptake by the cell. Post-exposure treatment of rats with a mAb that inhibited both virus spread and virus RNA transcription in vitro resulted in viral clearance from the central nervous system and protected the animals against a lethal rabies virus infection.
Full text
PDF
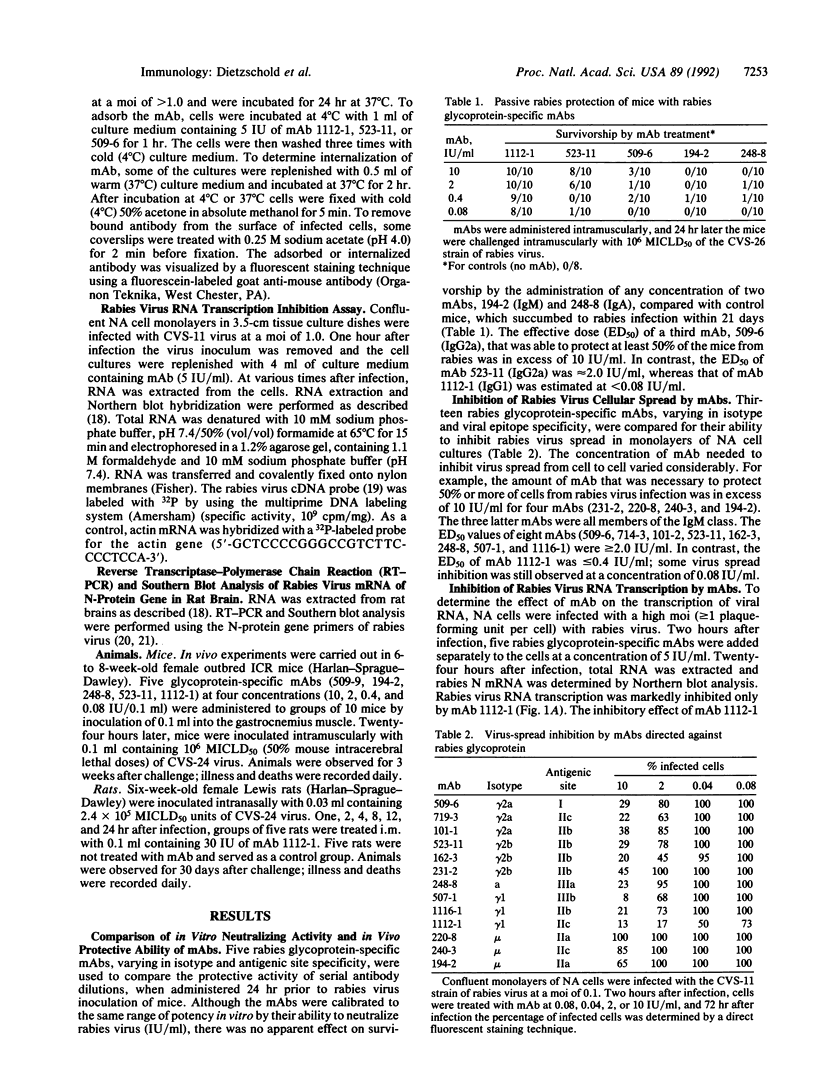

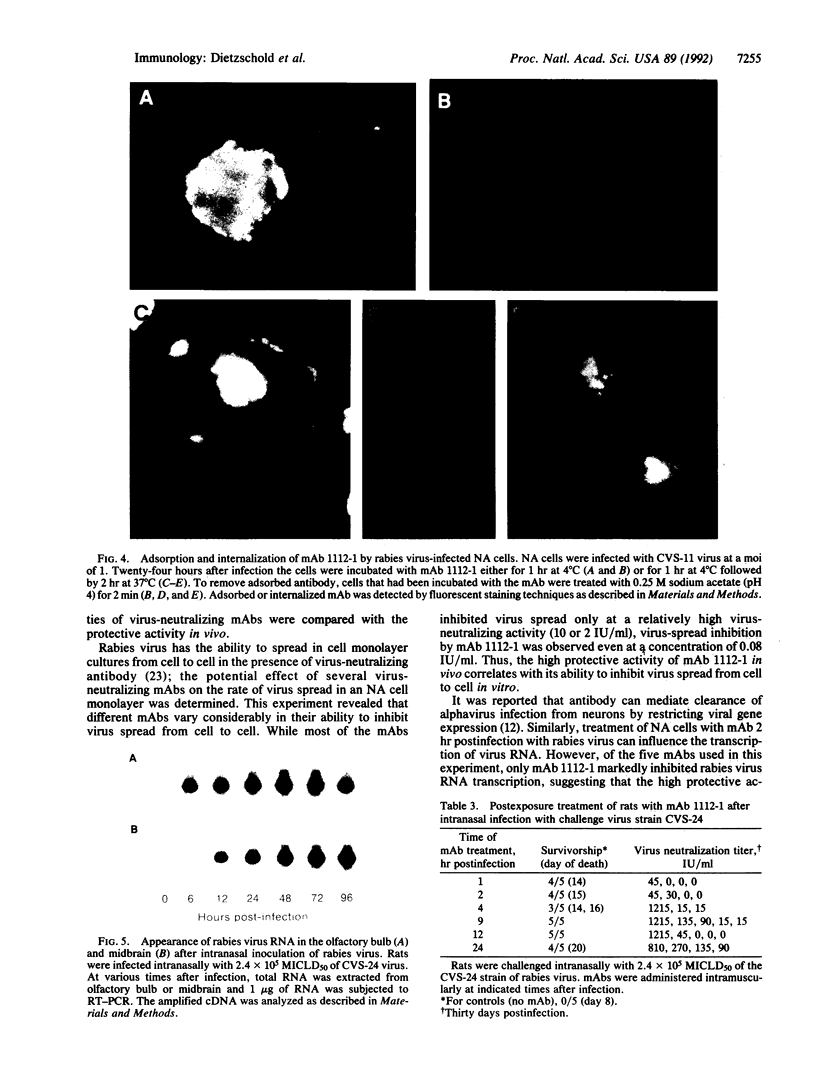

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Baldridge J. R., Buchmeier M. J. Mechanisms of antibody-mediated protection against lymphocytic choriomeningitis virus infection: mother-to-baby transfer of humoral protection. J Virol. 1992 Jul;66(7):4252–4257. doi: 10.1128/jvi.66.7.4252-4257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne J. A., Ahmed R., Oldstone M. B. Biology of cloned cytotoxic T lymphocytes specific for lymphocytic choriomeningitis virus. I. Generation and recognition of virus strains and H-2b mutants. J Immunol. 1984 Jul;133(1):433–439. [PubMed] [Google Scholar]
- Dietzschold B., Gore M., Casali P., Ueki Y., Rupprecht C. E., Notkins A. L., Koprowski H. Biological characterization of human monoclonal antibodies to rabies virus. J Virol. 1990 Jun;64(6):3087–3090. doi: 10.1128/jvi.64.6.3087-3090.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzschold B., Gore M., Marchadier D., Niu H. S., Bunschoten H. M., Otvos L., Jr, Wunner W. H., Ertl H. C., Osterhaus A. D., Koprowski H. Structural and immunological characterization of a linear virus-neutralizing epitope of the rabies virus glycoprotein and its possible use in a synthetic vaccine. J Virol. 1990 Aug;64(8):3804–3809. doi: 10.1128/jvi.64.8.3804-3809.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzschold B., Tollis M., Lafon M., Wunner W. H., Koprowski H. Mechanisms of rabies virus neutralization by glycoprotein-specific monoclonal antibodies. Virology. 1987 Nov;161(1):29–36. doi: 10.1016/0042-6822(87)90167-x. [DOI] [PubMed] [Google Scholar]
- Dietzschold B., Wiktor T. J., Trojanowski J. Q., Macfarlan R. I., Wunner W. H., Torres-Anjel M. J., Koprowski H. Differences in cell-to-cell spread of pathogenic and apathogenic rabies virus in vivo and in vitro. J Virol. 1985 Oct;56(1):12–18. doi: 10.1128/jvi.56.1.12-18.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P. C., Gerhard W. Breakdown of the blood--cerebrospinal fluid barrier to immunoglobulin in mice injected intracerebrally with a neurotropic influenza A virus. Post-exposure treatment with monoclonal antibody promotes recovery. J Neuroimmunol. 1981 Sep;1(3):227–237. doi: 10.1016/0165-5728(81)90027-8. [DOI] [PubMed] [Google Scholar]
- Ertl H. C., Dietzschold B., Gore M., Otvos L., Jr, Larson J. K., Wunner W. H., Koprowski H. Induction of rabies virus-specific T-helper cells by synthetic peptides that carry dominant T-helper cell epitopes of the viral ribonucleoprotein. J Virol. 1989 Jul;63(7):2885–2892. doi: 10.1128/jvi.63.7.2885-2892.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z. F., Dietzschold B., Schumacher C. L., Wunner W. H., Ertl H. C., Koprowski H. Rabies virus nucleoprotein expressed in and purified from insect cells is efficacious as a vaccine. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):2001–2005. doi: 10.1073/pnas.88.5.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski H. Laboratory techniques in rabies. Mouse inoculation test. Monogr Ser World Health Organ. 1966;23:69–80. [PubMed] [Google Scholar]
- Levine B., Hardwick J. M., Trapp B. D., Crawford T. O., Bollinger R. C., Griffin D. E. Antibody-mediated clearance of alphavirus infection from neurons. Science. 1991 Nov 8;254(5033):856–860. doi: 10.1126/science.1658936. [DOI] [PubMed] [Google Scholar]
- Momburg F., Koch N., Möller P., Moldenhauer G., Hämmerling G. J. In vivo induction of H-2K/D antigens by recombinant interferon-gamma. Eur J Immunol. 1986 May;16(5):551–557. doi: 10.1002/eji.1830160516. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Bauer S. P., Harrison A. K., Winn W. C., Jr Comparative pathogenesis of rabies and rabies-like viruses. Viral infection and transit from inoculation site to the central nervous system. Lab Invest. 1973 Mar;28(3):361–376. [PubMed] [Google Scholar]
- Prabhakar B. S., Fischman H. R., Nathanson N. Recovery from experimental rabies by adoptive transfer of immune cells. J Gen Virol. 1981 Sep;56(Pt 1):25–31. doi: 10.1099/0022-1317-56-1-25. [DOI] [PubMed] [Google Scholar]
- Reddehase M. J., Cox J. H., Koszinowski U. H. Frequency analysis of cytolytic T lymphocyte precursors (CTL-P) generated in vivo during lethal rabies infection of mice. II. Rabies virus genus specificity of CTL-P. Eur J Immunol. 1984 Nov;14(11):1039–1043. doi: 10.1002/eji.1830141114. [DOI] [PubMed] [Google Scholar]
- Rosenthal K. L., Oldstone M. B., Hengartner H., Zinkernagel R. M. Specificity of in vitro cytotoxic T cell clones directed against vesicular stomatitis virus. J Immunol. 1983 Jul;131(1):475–478. [PubMed] [Google Scholar]
- Schumacher C. L., Dietzschold B., Ertl H. C., Niu H. S., Rupprecht C. E., Koprowski H. Use of mouse anti-rabies monoclonal antibodies in postexposure treatment of rabies. J Clin Invest. 1989 Sep;84(3):971–975. doi: 10.1172/JCI114260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar V., Dietzschold B., Koprowski H. Direct entry of rabies virus into the central nervous system without prior local replication. J Virol. 1991 May;65(5):2736–2738. doi: 10.1128/jvi.65.5.2736-2738.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tsiang H., Lagrange P. H. In vivo detection of specific cell-mediated immunity in street rabies virus infection in mice. J Gen Virol. 1980 Mar;47(1):183–191. doi: 10.1099/0022-1317-47-1-183. [DOI] [PubMed] [Google Scholar]
- Wiktor T. J., Koprowski H. Monoclonal antibodies against rabies virus produced by somatic cell hybridization: detection of antigenic variants. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3938–3942. doi: 10.1073/pnas.75.8.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., Macfarlan R. I., Foggin C. M., Koprowski H. Antigenic analysis of rabies and Mokola virus from Zimbabwe using monoclonal antibodies. Dev Biol Stand. 1984;57:199–211. [PubMed] [Google Scholar]
- Wiktor T. J., Macfarlan R. I., Foggin C. M., Koprowski H. Antigenic analysis of rabies and Mokola virus from Zimbabwe using monoclonal antibodies. Dev Biol Stand. 1984;57:199–211. [PubMed] [Google Scholar]
- Wright K. E., Buchmeier M. J. Antiviral antibodies attenuate T-cell-mediated immunopathology following acute lymphocytic choriomeningitis virus infection. J Virol. 1991 Jun;65(6):3001–3006. doi: 10.1128/jvi.65.6.3001-3006.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]