Abstract
Objective
It is unclear how specific periods of gestational weight gain (GWG) during pregnancy relate to childhood adiposity. We aimed to assess the differential impact of GWG timing on childhood body composition.
Methods
In 979 mother-child pairs from the pre-birth Project Viva cohort, we calculated trimester-specific GWG using clinically recorded weights. Outcomes included body mass index (BMI) z-score, dual X-ray absorptiometry fat mass index (FMI, kg/m2) and fat free mass index (FFMI, kg/m2) in mid-childhood. We used linear regression models to assess associations of each trimester’s GWG (per 0.2 kg/week) with childhood outcomes, adjusted for maternal pre-pregnancy BMI, socio-demographic variables, lifestyle, and GWG in prior trimester(s).
Results
Mean (SD) 1st trimester GWG was 0.22(0.22)kg/week, 2nd trimester 0.49(0.18)kg/week, and 3rd trimester 0.47(0.20)kg/week. Faster 1st trimester GWG was associated with higher BMI z-score (0.06 units [95% CI:0.01, 0.12] per 0.2kg/week) and with higher adiposity according to all indices; associations were strongest in women with pre-pregnancy BMI>30kg/m2. Faster 2nd trimester GWG was associated with higher BMI z-score (0.11[0.04, 0.18]), fat mass (FMI=0.16[0.02, 0.31] kg/m2) and also lean mass (FFMI=0.11[0.01, 0.22] kg/m2). 3rd trimester GWG was not associated with childhood adiposity.
Conclusion
These results reinforce the importance of addressing appropriate GWG in early pregnancy.
Keywords: gestational weight gain, trimester-specific, pregnancy, adiposity, dual X-ray absorptiometry
Introduction
Increasing evidence supports that in utero and early life events contribute to risk of childhood obesity.1 Better characterisation of these events will help to define opportunities for interventions to prevent lifetime risk of obesity. Excessive maternal gestational weight gain (GWG) is one prenatal exposure that is associated with higher risk of obesity in later life.2 Recent studies and meta-analyses have consistently shown that greater total GWG is associated with higher body mass index (BMI) in offspring, in childhood, adolescence, and adulthood.2–4 This literature is limited in that few of these studies have assessed childhood adiposity using measures other than BMI,5–7 which incorporates lean mass as well as fat mass.
Furthermore, excessive GWG might have different programming effects depending on timing of the weight gain during pregnancy. Greater GWG in early pregnancy represents mainly maternal fat gain and might influence placental nutrient transfer differently than later GWG, which reflects fetal and placental growth and maternal vascular expansion in addition to maternal fat gain. Late pregnancy GWG has been consistently reported to be associated with birth weight.8, 9 Recently, greater GWG specifically in 2nd and 3rd trimester were associated with larger birth weight in some studies,6, 10 while other studies showed significant associations for all 3 trimesters.11, 12 Given that higher birth weight has been associated with later childhood obesity, the timing of GWG might influence childhood anthropometric outcomes through its impact on birth weight, or via other pathways.
However, only a handful of studies have assessed specific pregnancy periods of GWG rate and risk of childhood excess weight and adiposity.5, 6, 11–13 Most of these studies suggested that greater 1st trimester GWG is associated with offspring risk of obesity, while data on 2nd trimester is inconsistent.5, 6, 11–13 One of the main limitations of these previous studies is the use of only BMI for child assessment, which captures both lean and fat mass together. One notable exception, Fraser A et al., reported that greater early pregnancy GWG was associated with higher childhood adiposity assessed by dual X-ray absorptiometry (DXA).5 However, they defined “mid-pregnancy” as 14 to 36 weeks of gestation,5 which limited the discrimination of a possible 2nd or 3rd trimester-specific effect on childhood body composition.
To address this gap in the literature, we investigated the risk of excess adiposity in mid-childhood (median 7.7 years, full range i.e. min-max 6.6 to 10.9 years) according to rate of GWG by trimester. In addition, we evaluated whether trimester-specific associations differed according to maternal pre-pregnancy BMI, which is also known to influence both GWG rate and risk of excess weight in childhood.14 We consider that careful attention to maternal BMI is essential not only because of the increasing rate of women entering pregnancy with overweight or obesity, but also to tease out the separate contributions of these two often correlated factors.
Methods
Description of participants
Project Viva is a pre-birth cohort of mothers and children. Between 1999 and 2002, we recruited pregnant women at 8 obstetric offices of Atrius Harvard Vanguard Medical Associates, a multispecialty group practice in Massachusetts.15 All participating pregnant women provided informed consent; institutional review boards reviewed and approved the project in line with ethical standards established by the Declaration of Helsinki.16
We followed women throughout their pregnancy and collected clinical data on obstetric and delivery outcomes on 2,128 live singleton births. For this analysis, we excluded 1,012 children without anthropometry data at mid-childhood. Among the remaining 1,116, we excluded 8 women with pre-gestational diabetes (type 1 and type 2), 51 women with gestational diabetes (because its management affects subsequent GWG), 17 women missing weight data, 37 women with a pre-pregnancy BMI <18.5kg/m2 and 14 deliveries at <34 weeks. Thus the final sample for our main analyses included 989 children with at least one anthropometric measurement at mid-childhood, among which 780 children attended DXA scans. We included the maximal sample size available for each trait of interest. We compared characteristics of the 989 included in our current analyses with the 1139 excluded participants. Overall, we found similar mean maternal age (32 years in both), pre-pregnancy BMI (24.7 vs. 25.0kg/m2) and total GWG (0.39kg/week in both). Women included were more likely to have completed a college degree (69 vs. 61%); had higher annual household income (65 vs. 58% reported ≥$70,000/year); and less likely to report smoking during pregnancy (10 vs. 15%). For analyses where we examined associations of trimester-specific GWG with birth outcomes, we included 1,885 newborns whose mothers did not have pre-gestational diabetes, gestational diabetes, or BMI <18.5kg/m2.
Measures
Exposures – definitions of total and trimester-specific GWG
We collected self-reported pre-pregnancy weight at the initial prenatal visit. Among 343 women who had weight recorded in the medical record in the 3 months before their last menstrual period, the association between self-reported and clinically measured weight was linear (r=0.997).17 We extracted serial prenatal weights from medical records – we obtained a median of 13 (range 5 to 27) clinical weights recorded per woman over the course of the index pregnancy. We calculated total GWG rate as the difference between the last prenatal weight recorded (within 4 weeks of delivery) minus the pre-pregnancy weight, divided by number of gestational weeks at delivery. We defined 1st trimester as the date of last menstrual period to day 91, 2nd trimester as days 91–182, and 3rd trimester as day 182 to the time of the last prenatal weight recorded (within 4 weeks of delivery). As previously reported,18 we performed linear interpolation between the 2 closest weight measures to estimate weights at day 91 and day 182 and calculated GWG rates (kg/wk) for each trimester.
Outcomes – Child anthropometry and adiposity indices
At a research visit in mid-childhood (median 7.7 years), trained research assistants measured children’s weights (TBF-300A; Tanita, Arlington Heights, IL) and heights (calibrated stadiometer; Shorr Productions, Olney, MD). We calculated age- and sex-specific BMI percentiles and z-scores using U.S. national reference data.19 Normal weight was defined as <85th percentile, and obesity as ≥95th percentile. Using standardized protocols, our research assistants measured subscapular (SS) and triceps (TR) skinfold thicknesses using Holtain calipers (Holtain, Crosswell, U.K.); we calculated the sum (SS+TR) of skinfolds to represent overall adiposity. As a measure of central adiposity, we measured waist circumference just above the right iliac crest at the midaxillary line to the nearest 0.1 cm (Hoechstmass Balzer, Sulzbach, Germany) using standardized procedures.20, 21
At visits performed at our research center, research assistants administered whole-body DXA scans with Hologic model Discovery A (Hologic, Bedford, MA). We used Hologic software version 12.6 for scan analysis. A single trained research assistant checked all scans for positioning, movement, and artifacts and defined body regions for analysis. Intra-rater reliability was high (r=0.99). We calculated the DXA fat mass (FMI) and fat-free mass (FFMI) indexes using the formula: [total DXA measured mass (fat or fat free mass) in kg]/(height in meters)2..22 We also calculated trunk fat mass (trunk FMI).23, 24 We calculated the percent fat by dividing total fat mass by total weight.
Birth Outcomes
We obtained birth weight and delivery date from hospital medical records and research assistants measured birth length. We determined sex-specific birth length z-scores and birth weight for-gestational-age (BW/GA) z-scores using US reference data.19, 25 We included all newborns (N= 1885) with birth data available that met our maternal inclusion criteria to maximize our power for analyses of birth outcomes.
Co-variables
Using questionnaires, we collected information on maternal race/ethnicity, age, education, parity, household income, and smoking status during pregnancy. In early pregnancy, we collected maternal dietary patterns, using validated food frequency questionnaires,26 and physical activity, using a questionnaire modified from the Physical Activity Scale for the Elderly.27 We collected additional prenatal clinical information from the mother’s medical record, including glucose tolerance and mode of delivery. At the mid-childhood visit, we repeated lifestyle questionnaires for the mothers, as well as indicators of children’s dietary behaviors including consumption of convenience based fast food (frequency/week), and average weekly physical activity across light, moderate and vigorous intensities, as reported by the mother.15
Statistical analyses
We conducted linear regression analyses modeling weekly rate of GWG – per 0.2kg/week – for the total period of pregnancy and for each trimester to examine their associations with mid-childhood anthropometric and DXA indices. We selected 0.2kg/week as the effect size since this was the approximate SD of GWG rate at each trimester in our cohort. We conducted logistic models to predict risk of childhood obesity (BMI≥95th percentile) versus normal weight (<85th percentile). We adjusted models for child sex and age at outcome, and maternal pre-pregnancy BMI, parity, education, pregnancy smoking status and race/ethnicity. We additionally adjusted 2nd trimester models for GWG during the 1st trimester, and 3rd trimester models for 1st and 2nd trimester GWGs. We also conducted analyses stratified by pre-pregnancy BMI: normal weight (BMI 18.5 to <25.0kg/m2), overweight (BMI 25.0 to <30.0kg/m2), and obesity (BMI ≥30kg/m2). We conducted subsidiary analyses to further adjust for maternal lifestyle during pregnancy and for child lifestyle at the time of outcome measurements. We also further adjusted childhood outcomes models for BW/GA z-score to examine the extent to which size at birth would be on the pathway leading to later adiposity.
Among participants with data at birth, we examined associations of trimester-specific GWG with birth outcomes and we adjusted models for child sex, maternal pre-pregnancy BMI, parity, education, pregnancy smoking status and race/ethnicity (plus gestational age for birth length). We performed all analyses using software SAS version 9.3 (SAS Institute Inc, Cary, NC).
Results
We recruited women at a mean (SD) age of 32.1(5.4) years, 48% were nulliparous and 36% had pre-pregnancy BMI≥25kg/m2 (Table 1). Approximately a third (31%) were non-white, 91% were married or cohabitating, 69% had attained college degree or higher education, and 65% reported a household income of >US$70,000 per year at enrollment 1999–2002. Total mean (SD) GWG was 15.6(5.3)kg, while 1st, 2nd, and 3rd trimester GWG were 2.8(2.8)kg, 6.3(2.4)kg, and 6.5(2.7)kg respectively. Normal weight women gained 16.1(4.6)kg, overweight women gained 16.1(5.2)kg, while women with BMI≥30kg/m2 gained 12.6(7.3)kg over the full period of pregnancy; rates of excess GWG were 50.3%, 79.5%, and 71.6% respectively, as classified according to IOM recommendations. Children were born at a mean (SD) of 39.7(1.4) weeks of gestation, the mean (SD) birth weight was 3514(519)g and 50% were female. At mid-childhood, 12% had a BMI≥95th percentile.
Table 1.
Project Viva mothers and children characteristics
| Characteristics | N=989 Mean (SD) or N (%) |
|---|---|
| Mothers | |
| Age, years | 32.1 (5.4) |
| Pre-pregnancy BMI, kg/m2 | 24.7 (5.0) |
| Pre-pregnancy BMI category, % | |
| 18.5 – <25.0 kg/m2 | 640 (65) |
| 25.0 – <30.0 kg/m2 | 215 (22) |
| ≥30.0 kg/m2 | 134 (14) |
| Race/ethnicity, % | |
| Black | 161 (16) |
| Hispanic | 62 (6) |
| White | 676 (69) |
| Other | 86 (9) |
| College graduate or higher, % | 676 (69) |
| Married or cohabitating, % | 895 (91) |
| Household income >$70K/y, % | 583 (65) |
| Smoking status, % | |
| Never | 702 (71) |
| Former | 191 (19) |
| During pregnancy | 94 (10) |
| Nulliparous, % | 471 (48) |
| 1st trimester GWG, kg/wk | 0.22 (0.22) |
| 2nd trimester GWG, kg/wk | 0.49 (0.18) |
| 3rd trimester GWG, kg/wk | 0.47 (0.20) |
| Total GWG, kg/wk | 0.39 (0.13) |
| Total GWG, kg | 15.6 (5.3) |
| Children | |
| Female, % | 492 (50) |
| At birth | |
| Birth weight, gm | 3514 (519) |
| Gestation length, wk | 39.7 (1.4) |
| BW/GA z-score | 0.20 (0.97) |
| C-section, % | 219 (22) |
| At Mid-childhood | |
| Age, years | 7.9 (0.8) |
| BMI z-score | 0.41 (0.99) |
| BMI percentile, % | |
| . <85th | 733 (75) |
| . 85th–<95th | 129 (13) |
| . ≥95th | 121 (12) |
| DXA FMI, kg/m2 | 4.4 (1.9) |
| DXA trunk FMI, kg/m2 | 1.5 (0.9) |
| DXA FFMI, kg/m2 | 13.1 (1.4) |
| DXA percent fat | 24.6 (6.4) |
| Waist circumference, cm | 60.0 (8.2) |
| Sum of skinfolds (SS+TR), mm | 19.9 (9.8) |
BMI: body mass index; GWG: gestational weight gain; BW/GA: birth weight for gestational age; DXA: dual X-ray absorptiometry; FMI: fat mass index; FFMI: fat free mass index; SS: sub-scapular; TR: triceps
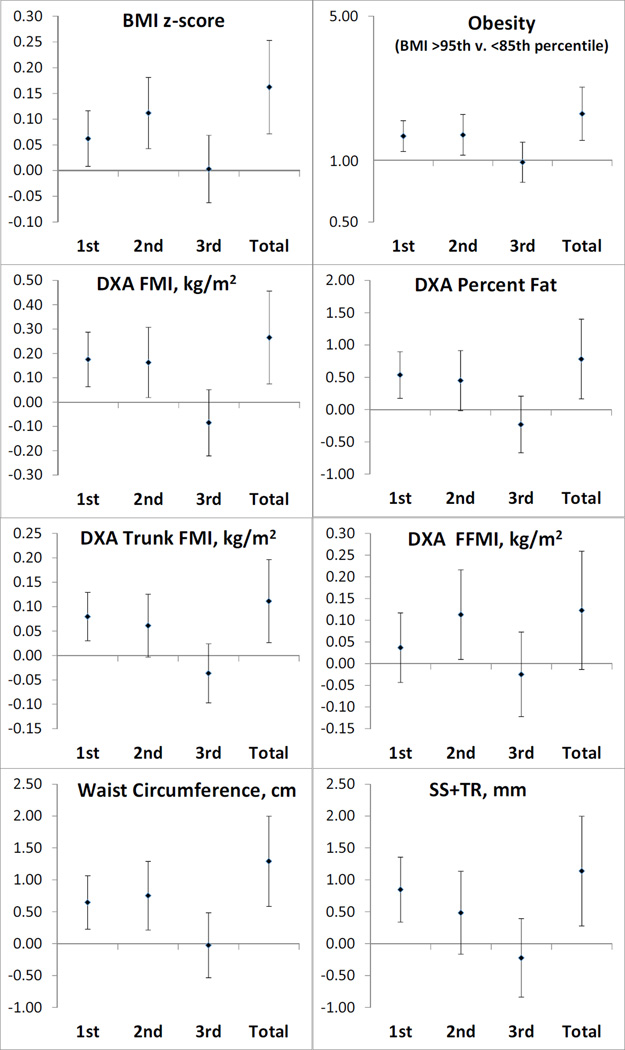
GWG and childhood anthropometric measures (Figure 1)
Figure 1. Anthropometric measurements and risk of obesity in mid-childhood according to rate of GWG by 0.2kg/week) for each trimester and total duration of pregnancy.
We adjusted all models for child sex and age at outcome and maternal pre-pregnancy BMI, parity, education, pregnancy smoking status and race/ethnicity; 2nd trimester models were further adjusted for 1st trimester GWG; 3rd trimester models were adjusted for 1st and 2nd trimester GWG. BMI: body mass index; GWG: gestational weight gain; DXA: dual X-ray absorptiometry; FMI: fat mass index; FFMI: fat free mass index; SS+TR; sum of skinfolds.
Higher rate of 1st trimester GWG (β [95%CI] per 0.2kg/week) was associated with higher BMI z-score (0.06 [0.01, 0.12]), as well as with more direct indices of overall adiposity – FMI (0.18 [0.06, 0.29]kg/m2), percent fat (0.54 [0.17, 0.90]%), and sum of skinfolds (0.85 [0.34, 1.36]mm) – as well as with indices of higher central adiposity– trunk FMI (0.08 [0.03, 0.13]kg/m2) and waist circumference (0.64 [0.23, 1.06]cm), but was not associated with fat free mass index (FFMI; 0.04 [−0.04, 0.12]kg/m2). Higher rate of GWG in 1st trimester was also associated with greater odds risk of obesity in mid-childhood (OR 1.31; 95%CI 1.10, 1.55).
Similarly, higher rate of 2nd trimester GWG was associated with higher BMI z-score (0.11 [0.04, 0.10]) and higher FMI (0.16 [0.02, 0.31]kg/m2) and with central adiposity indices (waist circumference: 0.75 [0.21, 1.29]cm, and trunk FMI: 0.06 [0.00, 0.13]kg/m2). Higher rate of GWG in 2nd trimester was associated with greater odds risk of obesity in mid-childhood (OR 1.33; 95%CI 1.06, 1.67). Second trimester GWG was also associated with greater lean mass: FFMI (0.11 [0.01, 0.22]kg/m2 per 0.2kg/week of gain).
We did not find associations between 3rd trimester GWG rate and any anthropometric measurements at mid-childhood or with risk of obesity (Figure 1). Given the effects of GWG in the first 2 trimesters, total GWG was associated with higher adiposity indices and with risk of obesity in mid-childhood (Figure 1). In additional models adjusted for maternal and childhood lifestyle behaviors, results remained essentially the same (Table S1).
GWG and childhood anthropometric measures by maternal pre-pregnancy weight status
Associations of 1st trimester GWG rate with childhood adiposity appeared stronger among women with a pre-pregnancy BMI≥30 kg/m2 than among normal weight and overweight women. For example, we noted associations between higher rate of 1st trimester GWG and greater sum of skinfolds: 1.04 [0.44, 3.24]mm in children from women with pre-pregnancy BMI≥30 kg/m2 compared to 0.64 [−0.51, 1.79]mm in overweight and 0.54 [−0.10, 1.19]mm in normal weight mothers. Associations of higher rate of 1st trimester GWG with higher FMI, trunk FMI, percent fat, and waist circumference also demonstrated greater effect sizes in women with BMI≥30kg/m2 compared to effect sizes among normal weight and overweight women (Table 2). The association of faster 2nd trimester GWG with greater lean mass was mainly observed in normal weight women: FFMI=0.18 [0.04, 0.32]kg/m2 in children from normal weight women versus 0.13 [−0.09, 0.35] in overweight women and 0.05 [−0.22, 0.31] in women with BMI≥30kg/m2.
Table 2.
Associations of rate of GWG (per 0.2 kg/wk) with anthropometry and adiposity indices at midchildhood, stratified by pre-pregnancy BMI category
| Pre-pregnancy BMI category, kg/m2 | ||||
|---|---|---|---|---|
| Outcome | Exposure GWG (per 0.2 kg/wk) |
18.5 – <25.0 N = 640 |
25.0 – <30.0 N = 215 β (95% CI) |
≥30.0 N = 134 |
| BMI z-score | 1st trimester | 0.05 (−0.03, 0.13) | 0.05 (−0.06, 0.16) | 0.09 (−0.02, 0.20) |
| 2nd trimester | 0.11 (0.02, 0.21) | 0.13 (−0.01, 0.28) | 0.12 (−0.03, 0.26) | |
| 3rd trimester | 0.04 (−0.04, 0.13) | −0.04 (−0.18, 0.09) | −0.02 (−0.18, 0.14) | |
| Total GWG | 0.18 (0.05, 0.31) | 0.15 (−0.04, 0.35) | 0.19 (−0.01, 0.38) | |
| DXA FMI, kg/m2 | 1st trimester | 0.15 (0.00, 0.29) | 0.07 (−0.14, 0.27) | 0.33 (0.00, 0.66) |
| 2nd trimester | 0.14 (−0.05, 0.32) | 0.19 (−0.09, 0.47) | 0.27 (−0.17, 0.72) | |
| 3rd trimester | 0.06 (−0.10, 0.23) | −0.17 (−0.42, 0.09) | −0.28 (−0.77, 0.20) | |
| Total GWG | 0.32 (0.08, 0.57) | 0.14 (−0.23, 0.50) | 0.44 (−0.15, 1.03) | |
| DXA trunk FMI, kg/m2 | 1st trimester | 0.05 (−0.01, 0.12) | 0.03 (−0.06, 0.13) | 0.17 (0.02, 0.32) |
| 2nd trimester | 0.05 (−0.03, 0.13) | 0.06 (−0.07, 0.18) | 0.13 (−0.07, 0.33) | |
| 3rd trimester | 0.04 (−0.03, 0.11) | −0.07 (−0.18, 0.05) | −0.17 (−0.38, 0.05) | |
| Total GWG | 0.13 (0.03, 0.24) | 0.04 (−0.12, 0.20) | 0.21 (−0.06, 0.48) | |
| DXA FFMI, kg/m2 | 1st trimester | 0.04 (−0.07, 0.15) | 0.03 (−0.13, 0.19) | 0.04 (−0.16, 0.24) |
| 2nd trimester | 0.18 (0.04, 0.32) | 0.13 (−0.09, 0.35) | 0.05 (−0.22, 0.31) | |
| 3rd trimester | 0.05 (−0.08, 0.17) | −0.09 (−0.29, 0.11) | −0.05 (−0.34, 0.24) | |
| Total GWG | 0.24 (0.05, 0.42) | 0.10 (−0.19, 0.38) | 0.05 (−0.30, 0.39) | |
| DXA percent fat | 1st trimester | 0.50 (−0.01, 1.00) | 0.17 (−0.54, 0.89) | 0.96 (0.04, 1.89) |
| 2nd trimester | 0.29 (−0.34, 0.92) | 0.59 (−0.38, 1.57) | 0.79 (−0.44, 2.02) | |
| 3rd trimester | 0.09 (−0.47, 0.65) | −0.53 (−1.42, 0.37) | −0.63 (−1.98, 0.72) | |
| Total GWG | 0.84 (0.00, 1.68) | 0.37 (−0.89, 1.64) | 1.39 (−0.25, 3.03) | |
| Waist circumference, cm | 1st trimester | 0.35 (−0.21, 0.90) | 0.64 (−0.28, 1.56) | 1.17 (0.06, 2.29) |
| 2nd trimester | 0.72 (0.05, 1.39) | 1.32 (0.11, 2.53) | 1.01 (−0.52, 2.54) | |
| 3rd trimester | 0.58 (−0.02, 1.17) | −0.52 (−1.71, 0.67) | −0.83 (−2.43, 0.76) | |
| Total GWG | 1.40 (0.52, 2.28) | 1.61 (−0.06, 3.27) | 1.65 (−0.36, 3.67) | |
| Sum of skinfolds, mm | 1st trimester | 0.54 (−0.10, 1.19) | 0.64 (−0.51, 1.79) | 1.84 (0.44, 3.24) |
| 2nd trimester | 0.35 (−0.44, 1.14) | 1.16 (−0.34, 2.66) | 0.79 (−1.08, 2.66) | |
| 3rd trimester | 0.32 (−0.38, 1.02) | −0.46 (−1.93, 1.02) | −1.38 (−3.35, 0.59) | |
| Total GWG | 1.11 (0.07, 2.15) | 1.50 (−0.55, 3.56) | 1.88 (−0.64, 4.39) | |
BMI: body mass index; GWG: gestational weight gain; DXA: dual X-ray absorptiometry; FMI: fat mass index; FFMI: fat free mass index. We adjusted all models for child sex and age at outcome and maternal pre-pregnancy BMI, parity, education, pregnancy smoking status and race/ethnicity; 2nd trimester models were further adjusted for 1st trimester GWG; 3rd trimester models were adjusted for 1st and 2nd trimester GWG.
GWG and birth outcomes
Higher rate of GWG during each trimester were associated with higher BW/GA z-score (Table 3), with 2nd trimester GWG presenting a larger effect size and distinct confidence intervals (0.21units per 0.2kg/wk [0.16, 0.26]) compared with 1st (0.07 [0.03, 0.10]) or 3rd trimester gain (0.06 [0.02, 0.11]). Associations between higher rates of GWG during the 1st and 2nd trimesters and higher mid-childhood adiposity indices were not explained by their effects on BW/GA, since adjusting mid-childhood outcome models for BW/GA did not attenuate the results (Table S1). Higher rate of 2nd trimester GWG was associated greater birth length (0.11 [0.05, 0.16] z-score) but GWG during the 1st or 3rd trimester were not (Table 3).
Table 3.
Associations of rate of GWG (per 0.2 kg/wk) with birth outcomes (N=1855)
| All participants | Stratified by pre-pregnancy BMI category, kg/m2 | ||||
|---|---|---|---|---|---|
| Outcome | Exposure GWG (per 0.2 kg/wk) |
N=1855 β (95% CI) |
18.5 – <25.0 N = 1169 |
25.0 – <30.0 N = 408 β (95% CI) |
≥30.0 N = 278 |
| BW/GA z-score | 1st trimester | 0.07 (0.03, 0.10) | 0.09 (0.04, 0.15) | 0.08 (0.01, 0.16) | 0.01 (−0.07, 0.08) |
| 2nd trimester | 0.21 (0.16, 0.26) | 0.30 (0.23, 0.37) | 0.15 (0.05, 0.24) | 0.12 (0.02, 0.21) | |
| 3rd trimester | 0.06 (0.02, 0.11) | 0.07 (0.01, 0.12) | 0.09 (0.00, 0.18) | 0.03 (−0.08, 0.14) | |
| Total GWG | 0.28 (0.22, 0.35) | 0.39 (0.30, 0.48) | 0.26 (0.13, 0.38) | 0.12 (−0.01, 0.25) | |
| Birth length z-score* | 1st trimester | 0.03 (−0.01, 0.07) | 0.03 (−0.03, 0.09) | 0.05 (−0.03, 0.13) | −0.01 (−0.08, 0.07) |
| 2nd trimester | 0.11 (0.05, 0.16) | 0.14 (0.06, 0.22) | 0.04 (−0.07, 0.14) | 0.09 (−0.01, 0.19) | |
| 3rd trimester | 0.04 (−0.01, 0.09) | 0.03 (−0.04, 0.09) | −0.01 (−0.12, 0.10) | 0.12 (0.00, 0.23) | |
| Total GWG | 0.14 (0.07, 0.21) | 0.16 (0.06, 0.27) | 0.08 (−0.06, 0.22) | 0.13 (0.00, 0.27) | |
| Gestational age, wk | 1st trimester | 0.05 (−0.03, 0.13) | 0.10 (−0.02, 0.21) | 0.03 (−0.12, 0.17) | −0.04 (−0.22, 0.14) |
| 2nd trimester | 0.06 (−0.03, 0.15) | 0.17 (0.04, 0.31) | −0.11 (−0.28, 0.06) | 0.01 (−0.20, 0.21) | |
| 3rd trimester | 0.03 (−0.06, 0.11) | 0.04 (−0.07, 0.15) | 0.10 (−0.07, 0.26) | −0.14 (−0.37, 0.09) | |
| Total GWG | 0.19 (0.07, 0.32) | 0.33 (0.15, 0.51) | 0.08 (−0.17, 0.33) | 0.03 (−0.27, 0.33) | |
GWG: gestational weight gain; BW/GA: birth weight z-score for sex and gestational age;
N=922 with birth length z-score
We adjusted all models for child sex and maternal pre-pregnancy BMI, parity, education, pregnancy smoking status and race/ethnicity; birth length z-score models were additionally adjusted for gestational age
Discussion
In this observational study of a modern cohort of US mothers and children, we found that a faster rate of GWG during the 1st trimester was associated with greater overall and central adiposity among children at 7–10 years of age. This association is in contrast with an existing literature suggesting that mid/late pregnancy GWG is more strongly predictive of size at birth, but in line with the limited, more recent literature examining weight in childhood. We are adding to the literature by using refined phenotyping to assess child body composition including gold-standard DXA, as well as showing that the association between 1st trimester GWG and childhood adiposity is stronger in children born to women with obesity prior to entering pregnancy. We also demonstrated for the first time that faster 2nd trimester GWG is associated with greater lean mass, particularly in normal weight women, which might explain some prior reports of an association between mid-pregnancy GWG and childhood BMI,11, 28 a measure that reflects both lean and fat mass. Women with obesity entering pregnancy require special attention: in addition to their higher own obstetric risk,29 their offspring are more likely to be large at birth30 and have excess weight later in life.14 We demonstrate here that greater 1st trimester GWG is associated with offspring risk of excess adiposity during childhood. Limiting GWG in early pregnancy in women with obesity is also essential for their own health since excessive GWG in early pregnancy is also associated with greater risk of pre-eclampsia,31 gestational diabetes,32, 33 and post-partum weight retention.18 Since many women are often entering obstetric care in early 2nd trimester, there is a critical need to develop effective pre-conception interventions for women with obesity to promote weight reduction before pregnancy and to limit early pregnancy GWG to improve short and long-term outcomes in both mothers and offspring.
Based on gold-standard DXA measurement, we were able to untangle the association of trimester-specific GWG with adiposity and lean mass components in children. Our findings showed that greater 2nd trimester GWG is associated with greater lean mass, in addition to some adiposity measures. This novel observation may be biologically explained by the fact that 2nd trimester is the most important period for organogenesis – including muscles, heart, liver, and bones – which are the main components of lean mass. Adipose tissue development also starts around the same period, as the first “fat lobules” are detectable around the 14–16th week of gestation.34 So, 2nd trimester GWG might promote development of both organs and adipose tissue, explaining our observation on lean mass and adiposity indices in childhood. We found a positive - but non-significant association - with percent body fat suggesting that 2nd trimester GWG does not overly affect relative adiposity – in contrast to 1st trimester GWG. We could speculate that the specific association of 1st trimester GWG with adiposity indices – and not lean mass - might be related to the fact this earlier time is the period of central nervous system (CNS) development, including appetite regulation centers such as the hypothalamus. Animal studies have suggested that the perinatal period might be important for appetite regulation centers,35, 36 but CNS development in humans is quite different from other mammals. Our finding of a 1st trimester-specific association with adiposity could also be related to placental development and therefore subsequent nutrient transfer. Placentas from women with obesity were found to have decreased system A neutral amino acid transporter activity and leptin resistance.37 Overall, it is still unknown whether these programming effects – either at 1st or 2nd trimester – occur because of molecular changes such as epigenetic modifications influencing cells/tissues differentiation, or through other mechanisms.
Previous studies have been inconsistent on the effect of 2nd trimester GWG and childhood BMI: one study found that greater 2nd trimester GWG was associated with higher childhood BMI,13 another study did not find significant association,12 while a third study found that greater 2nd trimester GWG was associated with higher risk of children being overweight, but not developing obesity.11 Fraser et al. is the only study that used DXA scans to evaluate children body composition: they did not find that “mid-pregnancy” GWG was associated with increased adiposity or lean mass, but they defined mid-pregnancy as 14 to 36 weeks, which includes both 2nd and 3rd trimesters, for which we did not find associations.5 This difference in exposure period might explain the difference in results with our study. Moreover, the population of pregnant women in Fraser et al.’s report included a small proportion of women with obesity prior to pregnancy (less than 7%), in whom our data suggest slightly larger effects of 2nd trimester GWG on childhood adiposity indices.
In our study, faster GWG rates over the total length of pregnancy and within each trimester were associated with higher BW/GA, similar to most – but not all - previous reports. Historically, lower GWG in the 2nd and 3rd trimester was associated with low birth weight – keeping in mind that these reports included no or very few women with excess weight.8, 9 Prior studies also reported slightly greater effect of 2nd trimester GWG on birth weight,9 concordant with our observations (Table 3). This is in line with the 2nd trimester being the most rapid period of fetal growth.38 Nevertheless, in our study, accounting for fetal growth did not explain associations between 1st and 2nd trimester GWG and child anthropometry as illustrated by the small changes in effect sizes in our models further adjusted for BW/GA (Table S1). We adjusted for maternal behaviours during pregnancy that were previously shown to be associated with risk of excessive GWG in our cohort and for child behaviours;39 however interestingly, this did not attenuate our findings, indicating that the observed findings here are not associated with these family lifestyle behaviors.
Strengths of our study include the prospective design, the collection of multiple maternal weights during pregnancy to assess trimester-specific GWG, and the use of DXA for child body composition estimation. Our main limitation was related to limited power in some of the analyses stratified by maternal pre-pregnancy BMI, as illustrated by the wider confidence intervals; sub-group results should therefore be interpreted with caution. Project Viva is composed in majority of women with relatively high socio-economic status and education levels, thus our findings might not be generalizable to lower income populations. Also, only 36% of women had a pre-pregnancy BMI>25kg/m2 – which is lower than current national estimates of overweight/obesity rate for women of reproductive age;40 having a greater proportion of women with excess weight would likely have increased effect sizes found in 1st trimester and power in sub-group analyses. Our loss to follow-up between pregnancy and mid-childhood was substantial; yet, we feel that our results are unlikely to be influenced by selection bias, as included and excluded participants were similar in GWG and pre-pregnancy BMI.
Conclusions
Understanding the impact of trimester-specific GWG on short and long-term outcomes is crucial in the current epidemic of obesity and related metabolic conditions. Recent trials aiming to limit GWG recruited pregnant women in late 1st or early 2nd trimester41 – missing the critical window of early pregnancy GWG. Our observations suggest that reducing GWG in late pregnancy, although it might influence birth weight, would have little impact on weight or adiposity in childhood, and that reducing GWG during the 2nd trimester could even have a negative impact on lean mass in childhood. If we want to lower the risk of excess adiposity in future children, interventions will need to address excess weight before conception and GWG in very early pregnancy, especially in women with obesity.
Supplementary Material
What is already known about this subject?
Greater total gestational weight gain (GWG) is associated with progression to a higher body mass index (BMI) and risk of obesity in childhood
Greater late pregnancy GWG predicts higher birth weight
Greater 1st trimester GWG is associated with higher childhood BMI, while data on 2nd trimester gain is inconsistent
What does this study add?
Greater 1st and 2nd trimester GWG is associated with greater childhood adiposity measured by gold-standard instruments
The association between 1st trimester GWG and childhood adiposity is stronger in women entering pregnancy with BMI>30 kg/m2
Greater 2nd trimester GWG is associated with greater lean mass in childhood
Acknowledgments
Grant Support: Project Viva is supported by NIH grant R37 HD34568. Dr. Oken received funding from K24 HD069408 and P30 DK092924.
Footnotes
DISCLOSURE: Authors have no conflict of interest to declare
References
- 1.Gillman MW, Ludwig DS. How early should obesity prevention start? N Engl J Med. 2013;369(23):2173–2175. doi: 10.1056/NEJMp1310577. [DOI] [PubMed] [Google Scholar]
- 2.Mamun AA, Mannan M, Doi SA. Gestational weight gain in relation to offspring obesity over the life course: a systematic review and bias-adjusted meta-analysis. Obes Rev. 2014;15(4):338–347. doi: 10.1111/obr.12132. [DOI] [PubMed] [Google Scholar]
- 3.Lau EY, Liu J, Archer E, McDonald SM. Maternal weight gain in pregnancy and risk of obesity among offspring: a systematic review. J Obes. 2014;2014:524939. doi: 10.1155/2014/524939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tie HT, Xia YY, Zeng YS, Zhang Y, Dai CL, Guo JJ, et al. Risk of childhood overweight or obesity associated with excessive weight gain during pregnancy: a meta-analysis. Arch Gynecol Obstet. 2014;289(2):247–257. doi: 10.1007/s00404-013-3053-z. [DOI] [PubMed] [Google Scholar]
- 5.Fraser A, Tilling K, Macdonald-Wallis C, Sattar N, Brion MJ, Benfield L, et al. Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation. 2010;121(23):2557–2564. doi: 10.1161/CIRCULATIONAHA.109.906081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karachaliou M, Georgiou V, Roumeliotaki T, Chalkiadaki G, Daraki V, Koinaki S, et al. Association of trimester-specific gestational weight gain with fetal growth, offspring obesity, and cardiometabolic traits in early childhood. Am J Obstet Gynecol. 2015;212(4):502 e1–502 e14. doi: 10.1016/j.ajog.2014.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol. 2007;196(4):322 e1–322 e8. doi: 10.1016/j.ajog.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hickey CA, Cliver SP, McNeal SF, Hoffman HJ, Goldenberg RL. Prenatal weight gain patterns and birth weight among nonobese black and white women. Obstet Gynecol. 1996;88(4 Pt 1):490–496. doi: 10.1016/0029-7844(96)00262-1. [DOI] [PubMed] [Google Scholar]
- 9.Strauss RS, Dietz WH. Low maternal weight gain in the second or third trimester increases the risk for intrauterine growth retardation. J Nutr. 1999;129(5):988–993. doi: 10.1093/jn/129.5.988. [DOI] [PubMed] [Google Scholar]
- 10.Ruchat S, Allard C, Doyon M, Lacroix M, Guillemette L, Patenaude J, et al. Timing of excessive weight gain during pregnancy modulates newborn anthropometry. J Obstet Gynaecol Can. doi: 10.1016/j.jogc.2015.12.014. in press. [DOI] [PubMed] [Google Scholar]
- 11.Gaillard R, Durmus B, Hofman A, Mackenbach JP, Steegers EA, Jaddoe VW. Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Obesity (Silver Spring) 2013;21(5):1046–1055. doi: 10.1002/oby.20088. [DOI] [PubMed] [Google Scholar]
- 12.Margerison-Zilko CE, Shrimali BP, Eskenazi B, Lahiff M, Lindquist AR, Abrams BF. Trimester of maternal gestational weight gain and offspring body weight at birth and age five. Matern Child Health J. 2012;16(6):1215–1223. doi: 10.1007/s10995-011-0846-1. [DOI] [PubMed] [Google Scholar]
- 13.Andersen CS, Gamborg M, Sorensen TI, Nohr EA. Weight gain in different periods of pregnancy and offspring's body mass index at 7 years of age. Int J Pediatr Obes. 2011;6(2-2):e179–e186. doi: 10.3109/17477166.2010.521560. [DOI] [PubMed] [Google Scholar]
- 14.Perng W, Gillman MW, Mantzoros CS, Oken E. A prospective study of maternal prenatal weight and offspring cardiometabolic health in midchildhood. Ann Epidemiol. 2014;24(11):793–800 e1. doi: 10.1016/j.annepidem.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oken E, Baccarelli AA, Gold DR, Kleinman KP, Litonjua AA, De Meo D, et al. Cohort profile: project viva. Int J Epidemiol. 2015;44(1):37–48. doi: 10.1093/ije/dyu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Medical Association declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277(11):925–926. [PubMed] [Google Scholar]
- 17.Provenzano AM, Rifas-Shiman SL, Herring SJ, Rich-Edwards JW, Oken E. Associations of maternal material hardships during childhood and adulthood with prepregnancy weight, gestational weight gain, and postpartum weight retention. J Womens Health (Larchmt) 2015;24(7):563–571. doi: 10.1089/jwh.2014.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walter JR, Perng W, Kleinman KP, Rifas-Shiman SL, Rich-Edwards JW, Oken E. Associations of trimester-specific gestational weight gain with maternal adiposity and systolic blood pressure at 3 and 7 years postpartum. Am J Obstet Gynecol. 2015;212(4):499 e1–499 e12. doi: 10.1016/j.ajog.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002;(246):1–190. [PubMed] [Google Scholar]
- 20.National Health and Nutrition Examination Survey (NHANES) Anthropometry Procedures Manual. 2000:3–30. and 33-31. [Google Scholar]
- 21.Pettitt DJ, Talton JW, Liese AD, Liu LL, Crimmins N, West NA, et al. Comparison of two waist circumference measurement protocols: the SEARCH for diabetes in youth study. Pediatr Obes. 2012;7(6):e81–e85. doi: 10.1111/j.2047-6310.2012.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.VanItallie TB, Yang MU, Heymsfield SB, Funk RC, Boileau RA. Height-normalized indices of the body's fat-free mass and fat mass: potentially useful indicators of nutritional status. Am J Clin Nutr. 1990;52(6):953–959. doi: 10.1093/ajcn/52.6.953. [DOI] [PubMed] [Google Scholar]
- 23.Ketel IJ, Volman MN, Seidell JC, Stehouwer CD, Twisk JW, Lambalk CB. Superiority of skinfold measurements and waist over waist-to-hip ratio for determination of body fat distribution in a population-based cohort of Caucasian Dutch adults. Eur J Endocrinol. 2007;156(6):655–661. doi: 10.1530/EJE-06-0730. [DOI] [PubMed] [Google Scholar]
- 24.Morimoto Y, Maskarinec G, Conroy SM, Lim U, Shepherd J, Novotny R. Asian ethnicity is associated with a higher trunk/peripheral fat ratio in women and adolescent girls. J Epidemiol. 2012;22(2):130–135. doi: 10.2188/jea.JE20110100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fawzi WW, Rifas-Shiman SL, Rich-Edwards JW, Willett WC, Gillman MW. Calibration of a semi-quantitative food frequency questionnaire in early pregnancy. Ann Epidemiol. 2004;14(10):754–762. doi: 10.1016/j.annepidem.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Pereira MA, Rifas-Shiman SL, Kleinman KP, Rich-Edwards JW, Peterson KE, Gillman MW. Predictors of change in physical activity during and after pregnancy: Project Viva. Am J Prev Med. 2007;32(4):312–319. doi: 10.1016/j.amepre.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bayer O, Ensenauer R, Nehring I, von Kries R. Effects of trimester-specific and total gestational weight gain on children's anthropometrics. BMC Pregnancy Childbirth. 2014;14:351. doi: 10.1186/1471-2393-14-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.ACOG Committee opinion no.549: obesity in pregnancy. Obstet Gynecol. 2013;121(1):213–217. doi: 10.1097/01.aog.0000425667.10377.60. [DOI] [PubMed] [Google Scholar]
- 30.Oken E, Kleinman KP, Belfort MB, Hammitt JK, Gillman MW. Associations of gestational weight gain with short- and longer-term maternal and child health outcomes. Am J Epidemiol. 2009;170(2):173–180. doi: 10.1093/aje/kwp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macdonald-Wallis C, Tilling K, Fraser A, Nelson SM, Lawlor DA. Gestational weight gain as a risk factor for hypertensive disorders of pregnancy. Am J Obstet Gynecol. 2013;209(4):327 e1–327 e17. doi: 10.1016/j.ajog.2013.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carreno CA, Clifton RG, Hauth JC, Myatt L, Roberts JM, Spong CY, et al. Excessive early gestational weight gain and risk of gestational diabetes mellitus in nulliparous women. Obstet Gynecol. 2012;119(6):1227–1233. doi: 10.1097/AOG.0b013e318256cf1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morisset AS, Tchernof A, Dube MC, Veillette J, Weisnagel SJ, Robitaille J. Weight gain measures in women with gestational diabetes mellitus. J Womens Health (Larchmt) 2011;20(3):375–380. doi: 10.1089/jwh.2010.2252. [DOI] [PubMed] [Google Scholar]
- 34.Poissonnet CM, Burdi AR, Bookstein FL. Growth and development of human adipose tissue during early gestation. Early Hum Dev. 1983;8(1):1–11. doi: 10.1016/0378-3782(83)90028-2. [DOI] [PubMed] [Google Scholar]
- 35.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304(5667):108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- 36.Bouyer K, Simerly RB. Neonatal leptin exposure specifies innervation of presympathetic hypothalamic neurons and improves the metabolic status of leptin-deficient mice. J Neurosci. 2013;33(2):840–851. doi: 10.1523/JNEUROSCI.3215-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farley DM, Choi J, Dudley DJ, Li C, Jenkins SL, Myatt L, et al. Placental amino acid transport and placental leptin resistance in pregnancies complicated by maternal obesity. Placenta. 2010;31(8):718–724. doi: 10.1016/j.placenta.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Moore KL. The fetal period. In: Moore KL, editor. The Developing Human: Clinically Oriented Embryology. Third. Philadelphia: WB Saunders; 1982. pp. 93–110. [Google Scholar]
- 39.Stuebe AM, Oken E, Gillman MW. Associations of diet and physical activity during pregnancy with risk for excessive gestational weight gain. Am J Obstet Gynecol. 2009;201(1):58 e1–58 e8. doi: 10.1016/j.ajog.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruifrok AE, van Poppel MN, van Wely M, Rogozinska E, Khan KS, de Groot CJ, et al. Association between weight gain during pregnancy and pregnancy outcomes after dietary and lifestyle interventions: a meta-analysis. Am J Perinatol. 2014;31(5):353–364. doi: 10.1055/s-0033-1352484. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.