Abstract
Many G protein-coupled receptors (GPCRs) internalize after agonist-induced activation. While endocytosis has long been associated with homeostatic attenuation of cellular responsiveness, accumulating evidence from study of a wide range of eukaryotes reveals that the endocytic pathway also contributes to generating receptor-initiated signals themselves. Here we review recent progress in this area, discussing primarily but not exclusively GPCR signaling in mammalian cells.
Introduction
Agonist-induced endocytosis of GPCRs was initially recognized as a phenomenon coinciding with rapid desensitization of G protein-mediated cellular responses [1,2]. The discovery that arrestins (also called β-arrestins) can promote GPCR endocytosis as well as desensitization provided a mechanistic link between these phenomena (reviewed in [3]). The identification of later molecular sorting operations that govern whether endocytosis facilitates reversal of the desensitized state (resensitization) or persistent attenuation (down-regulation) of cellular responsiveness further solidified a close relationship between endocytosis and reduced GPCR signaling (reviewed in [4]) (Figure 1). The present article discusses accumulating evidence suggesting that the endocytic pathway also functions, conversely, to promote receptor-mediated signaling responses. We will discuss G protein-independent signaling mechanisms only briefly, due to limited space and because authoritative reviews discussing aspects of this topic have appeared recently (e.g. [5,6]). We focus here on roles of the endocytic pathway in promoting cellular signaling mediated by heterotrimeric G proteins.
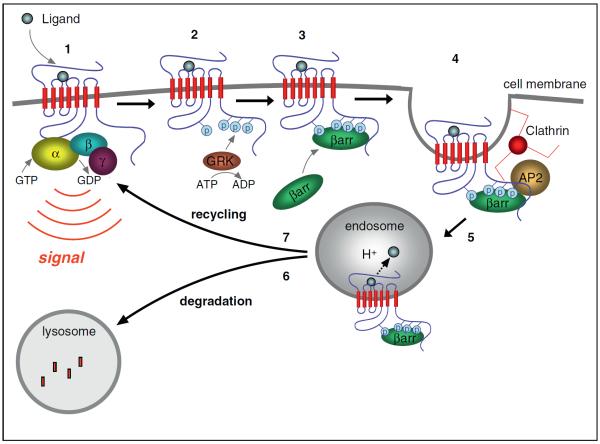
Figure 1.
Major cellular events of GPCR signaling and trafficking. (1) Binding of agonist ligand to the GPCR initiates signaling by increasing guanine nucleotide exchange activity for cognate heterotrimeric G protein, activating the alpha subunit (Gα) and releasing the beta-gamma subunit (Gβγ), each of which can transduce signals downstream. (2) Many GPCRs are phosphorylated after agonist-induced activation, often by members of the GPCR kinase (GRK) family that selectively recognize agonist-occupied receptors. (3) Phosphorylated receptors are preferred substrates for association with arrestins. (4) Several arrestin isoforms also bind components of endocytic lattices (clathrin heavy chain, AP2 alpha subunit and PIP2), promoting agonist-dependent clustering of GPCRs in clathrin-coated pits. (5) GPCRs that engage the clathrin-dependent endocytic machinery internalize and are delivered to early endosomes, where it is thought that ligand dissociation and receptor dephosphorylation events occur, and where receptors engage distinct molecular sorting machineries that determine later trafficking fate. (6) Receptor sorting to lysosomes results in their proteolytic destruction and contributes to a long-term attenuation of cellular responsiveness (`down-regulation') that is often observed after prolonged agonist exposure. (7) Receptor sorting into a recycling pathway mediates non-destructive return of receptors to the plasma membrane. This is thought to support sustained cellular responsiveness, or promote functional recovery of cellular responsiveness from a desensitized state (`resensitization') after repeated agonist exposure.
G protein-independent signaling from endosomes
The idea that endosomes might be sites of receptor-mediated signaling emerged from studies of growth factor receptors, in which subcellular fractionation (and later live cell imaging) experiments detected tyrosine-phosphorylated receptors, together with signaling adaptors and associated kinases, in endosomes (reviewed in [7,8]). The discovery that arrestins, like traditional adaptor proteins involved in growth factor signaling, bind various kinases in addition to receptors motivated the hypothesis that GPCRs initiate G protein-independent signals through arrestin-mediated scaffolding of downstream kinase cascades [9]. Many concurrent and subsequent studies, with particularly extensive contributions from the Lefkowitz laboratory, strongly support the `arrestin scaffolding' hypothesis (reviewed in [5,6]). However, the subcellular location of these events has been less clear. An early study, based on the effects of endocytic inhibitors, suggested that the β2-adrenergic GPCR (βb2AR) initiates G protein-dependent activation of adenylyl cyclase specifically from the plasma membrane and G protein-independent activation of MAP kinase signaling specifically from endosomes [10]. Shortly later it was found that β2AR-elicited activation of MAP kinase is mediated by arrestin scaffolding of the non-receptor tyrosine kinase c-Src but that this occurs in the plasma membrane, during or around the time of receptor clustering in clathrin-coated pits [9]. Terrillon and Bouvier then showed, using a clever chemical strategy, that plasma membrane recruitment of arrestin is sufficient to activate MAP kinase signaling [11]. These latter observations are in line with the general observation that β2ARs (like many other GPCRs) associate with arrestins primarily in the plasma membrane but not strongly in endosomes. However there is a subset of GPCRs that do robustly recruit arrestin to endosomes as well as the plasma membrane, apparently because they remain persistently phosphorylated after endocytosis [12]; for several of these GPCRs, endosome recruitment of MAP kinase components has also been demonstrated and is thought to contribute to localized cellular responses (e.g. [13–15]).
A distinct mechanism of signal control is by effective depletion of arrestin activity from the cytoplasm through its recruitment to endosomes, thereby reducing arrestin engagement with GPCRs in the plasma membrane and increasing the G protein-mediated response. This `arrestin sequestration' mechanism, first recognized in transfected cells [16], was later verified in native neurons expressing GPCRs at endogenous levels and linked to several relevant signaling consequences [17,18]. An altogether different mechanism, but related in principle and revealing remarkable natural diversity in the use of endosomes to deplete a key pathway regulator, occurs by accumulation in endosomes of an RGS protein rather than arrestin; this mechanism triggers activation of downstream signaling by reducing (RGS-dependent) shutoff of a constitutively active G protein present in the plasma membrane. The `RGS sequestration' mechanism was discovered through detailed study of nutrient signaling in Arabidopsis that identified an unusual seven-trans-membrane RGS protein capable of ligand-induced endocytosis [19,20•], and this mechanism appears to be conserved across vascular plants [21•]. Accordingly, there are multiple mechanisms that mediate endosome-based control of signaling without requiring direct engagement of G proteins in this compartment. However, at the cellular level, these mechanisms may significantly (e.g. arrestin sequestration) or primarily (e.g. RGS sequestration) affect G protein-mediated pathways (Figure 2).
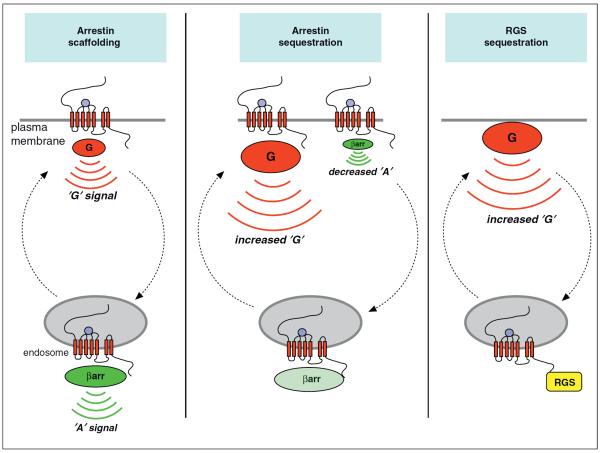
Figure 2.
`G protein-independent' signal control from endosomes. Arrestin scaffolding refers to the ability of GPCR-arrestin complexes present in endosomes to associate with components of MAP kinase modules and thereby initiate downstream kinase signaling separate from the traditional G protein-mediated response. In early studies, as described in the text, the G protein-mediated signal (`G' signal) was believed to be restricted to the plasma membrane and the distinct arrestin-mediated response (`A' signal) was believed to be restricted to the endosome membrane. Arrestin sequestration refers to signal control by effectively depleting the cytoplasmic arrestin pool through association with the endosome membrane, resulting in an increased `G' signal and reduced `A' response from the plasma membrane. RGS sequestration refers to an analogous depletion of a membrane-embedded RGS protein rather than arrestin, reducing shutoff of a constitutively activated G protein present in the plasma membrane and thereby increasing the `G' signal.
Evidence for G protein-dependent signaling from intracellular membranes
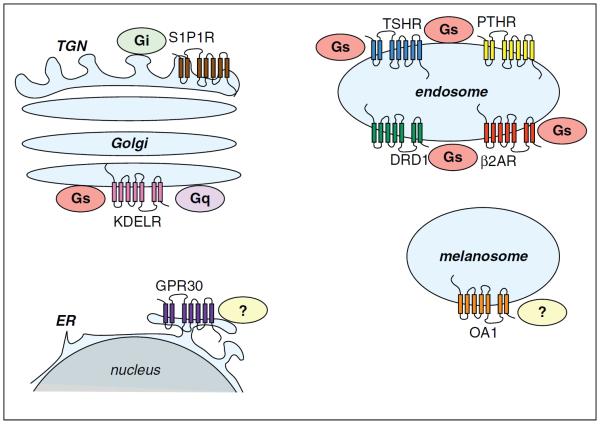
Heterotrimeric G proteins have long been recognized at intracellular membrane locations as well as at the plasma membrane (reviewed in [22,23]), but it is largely unknown if internal heterotrimeric G proteins are substrates for activation by GPCRs. Because G protein subunits present on internal membranes can themselves redistribute and functionally interact with membrane trafficking machineries, the presence of activated G proteins on internal membranes would not necessarily require their direct activation at these sites. However, a few GPCRs that localize prominently to intracellular membranes have emerged as plausible candidates for such signaling. GPR30 localizes to the endoplasmic reticulum and is thought to mobilize calcium and generate phosphoinositides from this compartment, although the coupling mechanism mediating the response remains undefined [24]. The KDEL receptor is a seven-trans-membrane protein that functions in biosynthetic cargo retrieval by shuttling between the endoplasmic reticulum and Golgi. KDELRs have long been thought to regulate transport vesicle formation by locally controlling activity of the small GTP-binding protein ARF1 through recruitment of an ARF-GAP [25]. It was proposed more recently that the KDELR functions as a conventional GPCR activating Gq and Gs at the Golgi [26]. OA1, a seven transmembrane protein expressed in specialized pigment-producing cells, can be activated by l-DOPA (L-3,4-dihydroxyphenylalanine) and promote calcium mobilization when over-expressed in transfected cells, apparently by coupling to Gq in the plasma membrane [27,28]. l-DOPA is an intermediate in the pathway of melanin pigment biosynthesis and OA1 prominently localizes to melanosomes of pigment cells. Thus OA1 has been proposed to control melanosome biogenesis by locally sensing biosynthetic intermediate concentration [29]. However, to our knowledge, it is not established whether OA1 couples to a G protein in the melanosome membrane (Figure 3).
Figure 3.
`G protein-dependent' signal control from intracellular membranes. Individual examples are discussed in the text.
A function of endosomes as specific sites of bona fide G protein-mediated signaling was identified in studies of cellular responses initiated by a pheromone-activated GPCR (encoded by the Ste2 gene) in budding yeast. Ste2p activation in the plasma membrane catalyzes guanine nucleotide exchange on the G protein alpha subunit (Gα) encoded by Gpa1, releasing the beta-gamma subunit (Gβγ, encoded by Ste4 and Ste18) from the heterotrimer; it is Gβγ that transduces a major portion of the mating response (reviewed in [30]). Using a clever genetic strategy, Slessareva et al. identified a second component of the GPCR-initiated signal that is carried by Gα and occurs from endosomes through its association with a distinct transduction complex including the endosome-associated PI3 kinase encoded by Vps34 [31]. It was not determined if this second component of the signal requires GPCR-G protein activation at the endosome per se or is stimulated in another manner, such as by redistribution of activated Gα from the plasma membrane or GPCR-independent activation at the endosome. Supporting the latter possibility, the same group subsequently identified a non-GPCR guanine nucleotide exchange factor (encoded by Arr4), whose deletion reduces the pheromone-induced mating response to a similar degree as disrupting Vps34 [32]. The ability of the Arr4 protein to function as a guanine nucleotide exchange factor for Gpa1 was directly established with purified proteins in vitro, but Arr4 (also called Get3) also functions in a discrete mechanism of tail-anchored membrane protein insertion to the endoplasmic reticulum [33,34]; thus, to our knowledge, it remains unclear to what extent Arr4's contribution to endosome-based signaling in vivo is direct or indirect.
Sustained GPCR-G protein signaling from endosomes
Evidence for GPCR-G protein signaling from endosomes in mammalian cells emerged from study of the delayed therapeutic effects of an immunomodulatory drug used in the treatment of multiple sclerosis, FTY720 (fingolimod). FTY720 is an agonist of sphingosine 1-phosphate (S1P) receptors (S1PRs) and elicits Gi-mediated signaling from one receptor subtype (the S1P1R) that is much more sustained than that elicited by the natural agonist S1P. This sustained drug response was associated with persistent and extensive internalization of S1P1Rs, which localized primarily to the trans-Golgi network, suggesting that FTY720-bound S1P1Rs remain persistently activated in this compartment after endocytosis. Because the sustained Gi-mediated signal increased endothelial cell motility in culture, and because FTY720 is thought to promote endothelial barrier closure to influence lymphocyte migration in vivo, it was proposed that the clinical efficacy of this drug might be mediated specifically by an endosome-derived signal [35].
Studies of Gs-mediated signaling initiated by the thyroid-stimulating hormone GPCR (TSHR) strongly support the concept of endosome-based GPCR-G protein signaling and provide insight to its impact on the actions of an endogenous agonist. In an elegant series of experiments using a transgenic mouse expressing a biosensor of cyclic AMP (cAMP), the critical second messenger chemical generated by GPCR-Gs signaling via adenylyl cyclase, Calebiro et al. observed that cAMP accumulation initiated by binding of thyroid stimulating hormone (TSH) to the TSH receptor (TSHR) in native thyroid follicle cells is prolonged and poorly reversible after TSH washout. This sustained signaling component coincided with persistent internalization of TSH and the TSHR, which partially colocalized with both Gs and adenylyl cyclase in endosomes or an endosome-associated pre-Golgi compartment. Remarkably, experimental manipulations that inhibit TSH/TSHR endocytosis rendered the TSH-induced cAMP signal reversible after agonist washout. The investigators concluded that activated TSHRs elicit a transient cAMP signal from the plasma membrane, similar to other Gs-coupled GPCRs, and a discrete component of sustained Gs-coupled cAMP signaling after endocytosis that is receptor-specific. Because endocytic inhibition reduced the ability of TSH to regulate actin organization, Calebiro et al. further suggested that this specialized form of endosome-based G protein activation directs signaling to qualitatively different downstream effectors [36]. A potential caveat to this interpretation is that a sustained response might occur as a consequence of slow dissociation of TSH from the TSHR irrespective of endocytosis [37]. A follow-up study supported the endosome signaling hypothesis by showing that the duration of the sustained response exceeds the time required for TSH dissociation from TSHRs. It also emphasized the specialized nature of the sustained signal by showing that it does not occur in human embryonal kidney cells [38•].
Additional support for a role of endosomes in generating sustained G protein-mediated signals came from study of a particular form of parathyroid hormone polypeptide (PTH1-34) that binds to the parathyroid hormone (PTH)/PTH-related peptide receptor (PTHR) almost irreversibly. Application of PTH1-34 to human embryonal kidney cells expressing recombinant PTHRs produced persistent internalization of the PTHR that corresponded in time to a sustained cAMP response. Internalized PTHRs colocalized with PTH1-34 as well as Gs, and inhibiting PTHR internalization rendered the cAMP response transient even in the continuous presence of PTH1-34. This led the authors to conclude that PTHRs mediate a transient cAMP response upon coupling to Gs in the plasma membrane and a distinct, sustained component upon coupling to Gs in endosomes [39]. The ability of PTHRs to produce a sustained cAMP signal appeared to be specific to this GPCR because activation in the same cells of β2ARs, which also couple to Gs, produced a transient response [40].
An unresolved question is how sustained G protein activation from endosomes is controlled. An interesting and possibly relevant observation is that over-expression of arrestin, which one might expect to accelerate signal termination, further prolonged the PTHR-mediated cAMP response [40]. It was initially suggested that sustained cAMP accumulation occurs as a consequence of reduced cAMP destruction rather than enhanced cAMP production, through G protein-independent signaling mediated by arrestin scaffolding of a MAP kinase module on the endosome membrane that inhibits cellular phosphodiesterase activity [40]. Notably, this proposed mechanism of sustained cAMP signaling does not require heterotrimeric G protein activation in endosomes. It was later proposed, by the same group, that sustained signaling is indeed a consequence of increased cAMP production but occurs through formation of an `alternate' signaling complex (presumably on endosomes), composed of arrestin-bound receptors together with the entire G protein heterotrimer, which could stabilize the activated Gα after its formation or scaffold Gβγ to facilitate repeated rounds of Gα activation [41•]. A similar hypothesis was subsequently proposed to explain a persistent cAMP signal elicited by the V2 vasopressin receptor (V2R) [42].
Acute GPCR-G protein signaling from endosomes
There is emerging evidence supporting the hypothesis that endosomes also function in acute G protein-mediated signaling, distinguished from sustained signaling by its onset within minutes after agonist application and its rapid reversal after agonist washout. A first hint came from study of cytoplasmic cAMP accumulation elicited by agonist-induced activation of the D1 dopamine receptor (DRD1). This Gs-mediated biochemical response was found to occur with a comparable time course as regulated endocytosis of the DRD1, and internalized receptors partially colocalized with both Gs and adenylyl cyclase in early endosomes. Remarkably, experimental manipulations that inhibit DRD1 endocytosis blunted the acute cAMP response in human embryonal kidney cells as well as in neurons cultured from rodent brain. Further, endocytic inhibition prevented DRD1-dependent regulation of neuronal excitability assessed by electrophysiological analysis of a brain slice preparation, representing an integrated G protein-dependent signaling response that is linked to learning and motivated behavior in vivo [43•]. Endocytic inhibition was also found to attenuate acute Gs-mediated cAMP accumulation elicited by the β2AR [44•]. Significantly, this acute signaling component was observed in human embryonal kidney cells and was elicited by activation of the β2AR, which is generally considered representative of the largest group (family A) of GPCRs. These observations suggest that a function of endosomes in promoting GPCR-G protein signaling might be widespread rather than specialized.
A major limitation to definitive elucidation of endosome-based signaling from GPCRs has been the inability to directly localize fundamental processes of receptor and G protein activation as they occur in intact cells. Recent inroads have been made using single-domain antibody reagents (nanobodies) that were developed initially for in vitro study of GPCR [45,46••] and G protein [47•] structure and conformational change. By adapting these molecules to function as genetically encoded biosensors, a form of the β2AR representing an activated conformation, and a form of Gs corresponding to an activation intermediate, were recently detected in living cells. Both signals appeared at the plasma membrane as well as at early endosomes, and did so sequentially and in discrete phases after acute agonist application [44•]. These observations provide arguably direct evidence that GPCR-G protein activation occurs at early endosomes as well as the plasma membrane.
Outlook
We suggest that there is now reasonably strong evidence supporting the hypothesis that the endocytic pathway, in addition to its well-established roles in homeostatic control of GPCR number and activity, contributes to the receptor-mediated signaling response itself. Endosomes appear to support multiple mechanisms of signaling, occurring over different time scales and differing in reversibility. While much of our current understanding remains limited to indirect evidence, there is now direct support for the presence of activated β2ARs and dynamic activation of Gs at early endosomes. Endosome-generated signals may produce qualitatively different effects compared to those generated from the plasma membrane, as suggested by TSHR-mediated regulation of the cytoskeleton, or shape the acute response in space and time, as suggested by sequential and discrete phases of Gs and arrestin engagement by the β2AR (Figure 4). We anticipate that endosomal signaling is particularly important to specialized cell types such as neurons and cardiac muscle cells that exhibit a high degree of structural specialization and support compartmentalized, time-limited responses. Much remains to be learned about spatiotemporally distributed GPCR signaling and its functional significance; indeed, the signaling-trafficking nexus seems to be entering an exciting period of discovery. Further investigations will likely shed new light on the fundamental cell biology of receptor-mediated signaling and provide insight to the actions of presently known drugs, as suggested already from studies of FTY720. We also hope that continued progress in this area will spark development of improved GPCR-targeted therapies.
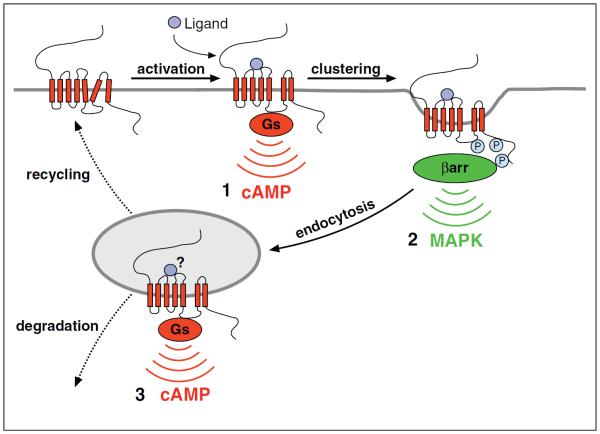
Figure 4.
Proposed model for `multi-phase' GPCR signaling organized by the endocytic pathway, using the β2AR as an example. (1) β2AR-Gs coupling in the plasma membrane promotes cAMP production from the plasma membrane. (2) Local accumulation of β2AR-arrestin complexes, potentially activating MAP kinase(s) by arrestin scaffolding, occurs during or around the time of receptor clustering in clathrin-coated pits. (3) β2AR-Gs coupling occurs again in the early endosome limiting membrane, promoting a discrete phase of cAMP production from endosomes. The `?' indicates that it is presently unknown if the endosome signal requires continued agonist engagement of receptors, and how endosome-initiated signal strength or duration is controlled.
Acknowledgments
The authors thank present and former members of the von Zastrow lab, as well as many other colleagues and collaborators at UCSF and elsewhere, for enormous contributions of data, reagents, ideas and critical discussion. We regret not being able to acknowledge these individuals by name and highlighting only a subset of relevant research articles due to space constraints. Work in the authors' laboratory is supported by the U.S. National Institutes of Health. R.I. is supported by a postdoctoral fellowship from the American Heart Association.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Waldo GL, Northup JK, Perkins JP, Harden TK. Characterization of an altered membrane form of the beta-adrenergic receptor produced during agonist-induced desensitization. J Biol Chem. 1983;258:13900–13908. [PubMed] [Google Scholar]
- 2.Staehelin M, Simons P, Jaeggi K, Wigger N. CGP-12177. A hydrophilic beta-adrenergic receptor radioligand reveals high affinity binding of agonists to intact cells. J Biol Chem. 1983;258:3496–3502. [PubMed] [Google Scholar]
- 3.Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 4.Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol. 2008;48:537–568. doi: 10.1146/annurev.pharmtox.48.113006.094830. [DOI] [PubMed] [Google Scholar]
- 5.Shenoy SK, Lefkowitz RJ. Seven-transmembrane receptor signaling through beta-arrestin. Sci STKE. 2005;2005:cm10. doi: 10.1126/stke.2005/308/cm10. [DOI] [PubMed] [Google Scholar]
- 6.Reiter E, Ahn S, Shukla AK, Lefkowitz RJ. Molecular mechanism of beta-arrestin-biased agonism at seven-transmembrane receptors. Annu Rev Pharmacol Toxicol. 2012;52:179–197. doi: 10.1146/annurev.pharmtox.010909.105800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baass PC, Di Guglielmo GM, Authier F, Posner BI, Bergeron JJ. Compartmentalized signal transduction by receptor tyrosine kinases. Trends Cell Biol. 1995;5:465–470. doi: 10.1016/s0962-8924(00)89116-3. [DOI] [PubMed] [Google Scholar]
- 8.Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10:609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 10.Daaka Y, Luttrell LM, Ahn S, Della Rocca GJ, Ferguson SS, Caron MG, Lefkowitz RJ. Essential role for G protein-coupled receptor endocytosis in the activation of mitogen-activated protein kinase. J Biol Chem. 1998;273:685–688. doi: 10.1074/jbc.273.2.685. [DOI] [PubMed] [Google Scholar]
- 11.Terrillon S, Bouvier M. Receptor activity-independent recruitment of betaarrestin2 reveals specific signalling modes. EMBO J. 2004;23:3950–3961. doi: 10.1038/sj.emboj.7600387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Association of beta-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J Biol Chem. 1999;274:32248–32257. doi: 10.1074/jbc.274.45.32248. [DOI] [PubMed] [Google Scholar]
- 13.DeFea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, Bunnett NW. beta-Arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeFea KA, Vaughn ZD, O'Bryan EM, Nishijima D, Dery O, Bunnett NW. The proliferative and antiapoptotic effects of substance P are facilitated by formation of a beta-arrestin-dependent scaffolding complex. Proc Natl Acad Sci U S A. 2000;97:11086–11091. doi: 10.1073/pnas.190276697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tohgo A, Pierce KL, Choy EW, Lefkowitz RJ, Luttrell LM. beta-Arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. J Biol Chem. 2002;277:9429–9436. doi: 10.1074/jbc.M106457200. [DOI] [PubMed] [Google Scholar]
- 16.Klein U, Muller C, Chu P, Birnbaumer M, von Zastrow M. Heterologous inhibition of G protein-coupled receptor endocytosis mediated by receptor-specific trafficking of beta-arrestins. J Biol Chem. 2001;276:17442–17447. doi: 10.1074/jbc.M009214200. [DOI] [PubMed] [Google Scholar]
- 17.Yu YJ, Arttamangkul S, Evans CJ, Williams JT, von Zastrow M. Neurokinin 1 receptors regulate morphine-induced endocytosis and desensitization of mu-opioid receptors in CNS neurons. J Neurosci. 2009;29:222–233. doi: 10.1523/JNEUROSCI.4315-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidlin F, Dery O, Bunnett NW, Grady EF. Heterologous regulation of trafficking and signaling of G protein-coupled receptors: beta-arrestin-dependent interactions between neurokinin receptors. Proc Natl Acad Sci U S A. 2002;99:3324–3329. doi: 10.1073/pnas.052161299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen JG, Willard FS, Huang J, Liang J, Chasse SA, Jones AM, Siderovski DP. A seven-transmembrane RGS protein that modulates plant cell proliferation. Science. 2003;301:1728–1731. doi: 10.1126/science.1087790. [DOI] [PubMed] [Google Scholar]
- 20.Urano D, Phan N, Jones JC, Yang J, Huang J, Grigston J, Taylor JP, Jones AM. Endocytosis of the seven-transmembrane RGS1 protein activates G-protein-coupled signalling in Arabidopsis. Nat Cell Biol. 2012;14:1079–1088. doi: 10.1038/ncb2568. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper describes a remarkable variation of the principle of signal control by endosome-based sequestration of a negative pathway regulator, mediated by ligand-induced endocytosis of a membrane-embedded RGS protein.
- 21.Urano D, Jones JC, Wang H, Matthews M, Bradford W, Bennetzen JL, Jones AM. G protein activation without a GEF in the plant kingdom. PLoS Genet. 2012;8:e1002756. doi: 10.1371/journal.pgen.1002756. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper explores evolutionary diversity among mechanisms that control signaling via G proteins, and provides evidence suggesting that signal initiation by RGS sequestration is conserved throughout vascular plants.
- 22.Hewavitharana T, Wedegaertner PB. Non-canonical signaling and localizations of heterotrimeric G proteins. Cell Signal. 2012;24:25–34. doi: 10.1016/j.cellsig.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wedegaertner PB. G protein trafficking. Subcell Biochem. 2012;63:193–223. doi: 10.1007/978-94-007-4765-4_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 25.Aoe T, Cukierman E, Lee A, Cassel D, Peters PJ, Hsu VW. The KDEL receptor, ERD2, regulates intracellular traffic by recruiting a GTPase-activating protein for ARF1. EMBO J. 1997;16:7305–7316. doi: 10.1093/emboj/16.24.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giannotta M, Ruggiero C, Grossi M, Cancino J, Capitani M, Pulvirenti T, Consoli GM, Geraci C, Fanelli F, Luini A, et al. The KDEL receptor couples to Galphaq/11 to activate Src kinases and regulate transport through the Golgi. EMBO J. 2012;31:2869–2881. doi: 10.1038/emboj.2012.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Innamorati G, Piccirillo R, Bagnato P, Palmisano I, Schiaffino MV. The melanosomal/lysosomal protein OA1 has properties of a G protein-coupled receptor. Pigment Cell Res. 2006;19:125–135. doi: 10.1111/j.1600-0749.2006.00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez VM, Decatur CL, Stamer WD, Lynch RM, McKay BS. l-DOPA is an endogenous ligand for OA1. PLoS Biol. 2008;6:e236. doi: 10.1371/journal.pbio.0060236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giordano F, Bonetti C, Surace EM, Marigo V, Raposo G. The ocular albinism type 1 (OA1) G-protein-coupled receptor functions with MART-1 at early stages of melanogenesis to control melanosome identity and composition. Hum Mol Genet. 2009;18:4530–4545. doi: 10.1093/hmg/ddp415. [DOI] [PubMed] [Google Scholar]
- 30.Slessareva JE, Dohlman HG. G protein signaling in yeast: new components, new connections, new compartments. Science. 2006;314:1412–1413. doi: 10.1126/science.1134041. [DOI] [PubMed] [Google Scholar]
- 31.Slessareva JE, Routt SM, Temple B, Bankaitis VA, Dohlman HG. Activation of the phosphatidylinositol 3-kinase Vps34 by a G protein alpha subunit at the endosome. Cell. 2006;126:191–203. doi: 10.1016/j.cell.2006.04.045. [DOI] [PubMed] [Google Scholar]
- 32.Lee MJ, Dohlman HG. Coactivation of G protein signaling by cell-surface receptors and an intracellular exchange factor. Curr Biol. 2008;18:211–215. doi: 10.1016/j.cub.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schuldiner M, Metz J, Schmid V, Denic V, Rakwalska M, Schmitt HD, Schwappach B, Weissman JS. The GET complex mediates insertion of tail-anchored proteins into the ER membrane. Cell. 2008;134:634–645. doi: 10.1016/j.cell.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powis K, Schrul B, Tienson H, Gostimskaya I, Breker M, High S, Schuldiner M, Jakob U, Schwappach B. Get3 is a holdase chaperone and moves to deposition sites for aggregated proteins when membrane targeting is blocked. J Cell Sci. 2013;126:473–483. doi: 10.1242/jcs.112151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mullershausen F, Zecri F, Cetin C, Billich A, Guerini D, Seuwen K. Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat Chem Biol. 2009;5:428–434. doi: 10.1038/nchembio.173. [DOI] [PubMed] [Google Scholar]
- 36.Calebiro D, Nikolaev VO, Gagliani MC, de Filippis T, Dees C, Tacchetti C, Persani L, Lohse MJ. Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol. 2009;7:e1000172. doi: 10.1371/journal.pbio.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boutin A, Allen MD, Geras-Raaka E, Huang W, Neumann S, Gershengorn MC. Thyrotropin receptor stimulates internalization-independent persistent phosphoinositide signaling. Mol Pharmacol. 2011;80:240–246. doi: 10.1124/mol.111.072157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Werthmann RC, Volpe S, Lohse MJ, Calebiro D. Persistent cAMP signaling by internalized TSH receptors occurs in thyroid but not in HEK293 cells. FASEB J. 2012;26:2043–2048. doi: 10.1096/fj.11-195248. [DOI] [PubMed] [Google Scholar]; • This study is a recent contribution supporting the hypothesis that sustained G protein-dependent signaling can be initiated from endosomes, and emphasizes that this is true for a subset of GPCRs and in particular cell types.
- 39.Ferrandon S, Feinstein TN, Castro M, Wang B, Bouley R, Potts JT, Gardella TJ, Vilardaga JP. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat Chem Biol. 2009;5:734–742. doi: 10.1038/nchembio.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feinstein TN, Wehbi VL, Ardura JA, Wheeler DS, Ferrandon S, Gardella TJ, Vilardaga JP. Retromer terminates the generation of cAMP by internalized PTH receptors. Nat Chem Biol. 2011;7:278–284. doi: 10.1038/nchembio.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wehbi VL, Stevenson HP, Feinstein TN, Calero G, Romero G, Vilardaga JP. Noncanonical GPCR signaling arising from a PTH receptor-arrestin-Gbetagamma complex. Proc Natl Acad Sci U S A. 2013;110:1530–1535. doi: 10.1073/pnas.1205756110. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper proposes an unorthodox function of arrestin in mediating sustained G protein signaling.
- 42.Feinstein TN, Yui N, Webber MJ, Wehbi VL, Stevenson HP, King JD, Hallows KR, Brown D, Bouley R, Vilardaga JP. Noncanonical control of vasopressin receptor type 2 signaling by retromer and arrestin. J Biol Chem. 2013 doi: 10.1074/jbc.M112.445098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kotowski SJ, Hopf FW, Seif T, Bonci A, von Zastrow M. Endocytosis promotes rapid dopaminergic signaling. Neuron. 2011;71:278–290. doi: 10.1016/j.neuron.2011.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper provides the first evidence that endocytosis can promote acute GPCR-G protein signaling, and links this component of the signal to behaviorally relevant control of the electrical excitability of native neurons by dopamine.
- 44.Irannejad R, Tomshine JC, Tomshine JR, Chevalier M, Mahoney JP, Steyaert J, Rasmussen SG, Sunahara RK, El-Samad H, Huang B, et al. Conformational biosensors reveal GPCR signalling from endosomes. Nature. 2013;495:534–538. doi: 10.1038/nature12000. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper provides arguably direct evidence for acute GPCR-G protein signaling from endosomes using nanobody-based biosensors to resolve, in living cells, an activated conformation of the β2AR and an activation intermediate of Gs.
- 45.Steyaert J, Kobilka BK. Nanobody stabilization of G protein-coupled receptor conformational states. Curr Opin Struct Biol. 2011;21:567–572. doi: 10.1016/j.sbi.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rasmussen SG, Choi HJ, Fung JJ, Pardon E, Casarosa P, Chae PS, Devree BT, Rosenbaum DM, Thian FS, Kobilka TS, et al. Structure of a nanobody-stabilized active state of the beta(2) adrenoceptor. Nature. 2011;469:175–180. doi: 10.1038/nature09648. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This paper provides important insight to the structural basis of GPCR activation, and describes a single domain antibody fragment (nanobody) that binds selectively to an agonist-activated conformation of the β2AR.
- 47.Westfield GH, Rasmussen SG, Su M, Dutta S, DeVree BT, Chung KY, Calinski D, Velez-Ruiz G, Oleskie AN, Pardon E, et al. Structural flexibility of the G alpha s alpha-helical domain in the beta2-adrenoceptor Gs complex. Proc Natl Acad Sci U S A. 2011;108:16086–16091. doi: 10.1073/pnas.1113645108. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper describes remarkable structural flexibity of the α-subunit of Gs that is associated with the guanine nucleotide-free state, and a nanobody that selectively recognizes nucleotide-free Gα because of this flexibility.