Abstract
Regulating adherens junction complex assembly/disassembly is critical to maintaining epithelial homeostasis in healthy epithelial tissues. Consequently, adherens junction structure and function is often perturbed in clinically advanced tumors of epithelial origin. Some of the most studied factors driving adherens junction complex perturbation in epithelial cancers are transcriptional and epigenetic down-regulation of E-cadherin expression. However, numerous reports demonstrate that post-translational regulatory mechanisms such as endocytosis also regulate early phases of epithelial-mesenchymal transition and metastatic progression. In already assembled healthy epithelia, E-cadherin endocytosis recycles cadherin-catenin complexes to regulate the number of mature adherens junctions found at cell-cell contact sites. However, following de novo epithelial cell-cell contact, endocytosis negatively regulates adherens junction assembly by removing E-cadherin from the cell surface. By contrast, following de novo epithelial cell-cell contact, spatially localized β-actin translation drives cytoskeletal remodeling and consequently E-cadherin clustering at cell-cell contact sites and therefore positively regulates adherens junction assembly. In this report we demonstrate that dynamin-mediated endocytosis and β-actin translation dependent cadherin-catenin complex anchoring oppose each other following epithelial cell-cell contact. Consequently, the final extent of adherens junction assembly depends on which of these processes is dominant following epithelial cell-cell contact. We expressed β-actin transcripts impaired in their ability to properly localize monomer synthesis (Δ3′UTR) in MDCK cells to perturb actin filament remodeling and anchoring and demonstrate the resulting defect in adherens junction structure and function is rescued by inhibiting dynamin mediated endocytosis. Therefore, we demonstrate balancing spatially regulated β-actin translation and dynamin mediated endocytosis regulates epithelial monolayer structure and barrier function.
Keywords: Endocytosis, β-actin mRNA zipcode, adherens junctions, Pearson’s correlation coefficient, actin cytoskeleton
Introduction
Adherens junctions are a major class of cell adhesion complexes observed in epithelial tissues (Nagafuchi 2001; Takeichi 2014). These complexes contain E-cadherin, a classical cell-cell adhesion molecule, and the adaptor proteins β-catenin and α-catenin. Dynamic interactions between cadherin-catenin complexes and linear actin filaments regulate epithelial homeostasis (Cavey et al. 2008; van Roy and Berx 2008). For instance, in several epithelial cancers, reduced E-cadherin expression correlates with increased metastatic potential (Blanco et al. 2002; Rosivatz et al. 2002). In addition to methylation of the E-cadherin promoter (Lombaerts et al. 2006) and down-regulation by transcriptional repression (Blanco et al. 2002; Cano et al. 2000; Korpal et al. 2008), post-translational control of E-cadherin regulates epithelial-mesenchymal transition and cancer invasion (Thiery 2003). Phosphorylation decreases the residence time of cadherin-catenin complexes at the cell surface reducing cell-cell adhesion strength and barrier integrity (Bertocchi et al. 2012). Additionally, activation of the Src oncogene or growth factors, such as TGF-β or HGF, cause targeted E-cadherin degradation in lysosomes which drives tumor and metastasis progression (Fujita et al. 2002; Janda et al. 2006; Kimura et al. 2006; Shen et al. 2008), as reviewed in (de Beco et al. 2012; Mosesson et al. 2008). Conversely, blocking E-cadherin recycling at the cell surface by inhibiting endocytosis increases E-cadherin trans-dimer formation (Troyanovsky et al. 2006) and F-actin anchoring thereby stabilizing adherens junction complexes (Harris and Tepass 2010). The contribution of E-cadherin endocytosis to the maintenance of mature adherens junctions in epithelia has been well studied (de Beco et al. 2009). However, its role in balancing actin anchoring of cadherin-catenin complexes during de novo adherens junction formation is yet to be explored. We demonstrate that dynamin mediated endocytosis opposes F-actin anchoring of cadherin-catenin complexes, thus negatively regulating adherens junction assembly.
Epithelial cell-cell contact stimulates E-cadherin clustering causing a local increase in α-catenin levels and driving actin filament remodeling from branched networks to linear bundles (Drees et al. 2005; Yamada et al. 2005). Linear actin bundles anchor cadherin-catenin complexes, and hence positively regulate adherens junction assembly (Gloushankova et al. 1997; Krendel and Bonder 1999; Vasioukhin et al. 2000; Wu et al. 2015). E-cadherin clustering on the cell surface and actin filament remodeling, following de novo epithelial cell-cell contact, are interdependent processes driving adherens junction assembly/disassembly dynamics. Actin cytoskeleton remodeling required for adherens junction assembly, is driven by a local increase in β-actin monomer concentration. Localizing β-actin mRNA translation governs monomer concentration at epithelial cell-cell contacts and regulates actin filament polymerization (Condeelis and Singer 2005; Gutierrez et al. 2014; Rodriguez et al. 2006). Knocking down β-actin (Baranwal et al. 2012) or mistargeting its synthesis (Gutierrez et al. 2014; Kislauskis et al. 1994) specifically impairs adherens junction assembly. Additionally, expressing β-actin mRNA lacking its 3′UTR (Δ3′UTR), and thus the mRNA zipcode, impairs actin filament remodeling at the cell periphery (Lyubimova et al. 1999), which results in reduced cadherin clustering at cell-cell contacts (Rodriguez et al. 2006).
In this report, using Dynamin inhibitors: dynasore (Macia et al. 2006) and hydroxy-dynasore (McCluskey et al. 2013), we block endocytosis in MDCK cells with partial mislocalization of β-actin translation in order to increase cadherin clustering at the cell surface (Troyanovsky et al. 2006). We demonstrate that this tips the balance towards actin anchoring of cadherin-catenin complexes and rescues adherens junction assembly defects in cells with partial mislocalization of β-actin translation. We also show that monolayer barrier integrity correlates with adherens junction assembly, as quantified with a new fluorescence microscopy-based covariance analysis. In addition, we demonstrate that a more complete mislocalization of β-actin translation is sufficient to prevent the rescue of adherens junction assembly by inhibiting endocytosis in cells. Finally, we demonstrate that the rescue of adherens junction assembly in monolayers composed of cells, which have partial mislocalization of β-actin translation, requires E-cadherin function using a function blocking antibody (Vestweber and Kemler 1985).
Results and Discussion
Partially mislocalizing β-actin translation in MDCK cells impairs their ability to assemble functional epithelial monolayers
The β-actin mRNA zipcode is a 28 nucleotide sequence present in the 3′ UTR. Expressing 3′ UTR deleted β-actin in cells results in partial mislocalization of β-actin translation (Gutierrez et al. 2014; Rodriguez et al. 2006). We observed significant morphological differences in MDCK monolayers assembled from cells able to target β-actin translation to cell-cell contacts versus cells impaired in their ability to target β-actin translation, even though the total actin expression in both populations is similar (Ballestrem et al. 1998; Gutierrez et al. 2014). MDCK monolayers assembled from cells that properly localize β-actin translation to cell-cell contacts exhibit strong E-cadherin and F-actin colocalization at cell-cell contact sites. These cells also contain stress fibers in the basal cytoplasm (Figure 1A). By contrast, we observed few stress fibers in the cytoplasm of MDCK monolayers composed of cells with partially mislocalized β-actin translation. However, these cells still showed significant, albeit weaker, E-cadherin and F-actin colocalization at cell-cell contacts. Importantly, in these cells we also observed lamellar protrusions that were largely absent in confluent monolayers assembled from MDCK cells that properly localize β-actin translation to cell-cell contacts (Figure 1B). E-cadherin clustering at cell-cell contact sites increases α-catenin recruitment, which is required for inhibiting lamellar protrusions (Drees et al., 2005). However, cells impaired in their ability to locally synthesize monomers exhibit reduced cadherin localization at the cell-cell contacts (Rodriguez et al. 2006). Thus epithelial monolayers composed of cells with partially mislocalized β-actin translation are unable to recruit and anchor α-catenin to cell-cell contacts and turn off lamellar protrusions following de novo contact.
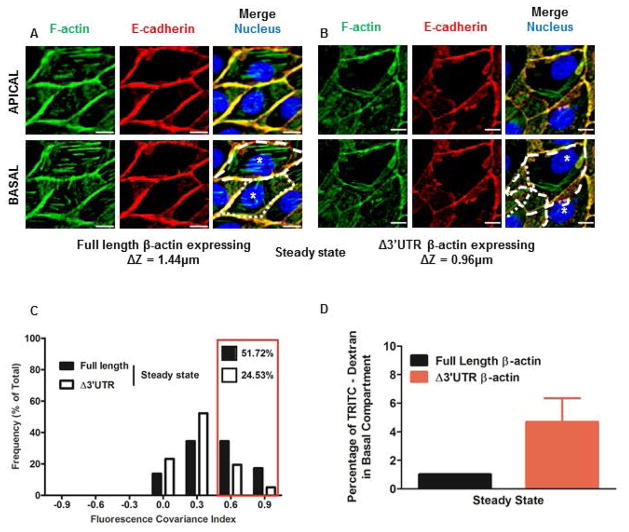
Figure 1. MDCK cells with partially mislocalized β-actin translation show adherens junction defects measured using Fluorescence Covariance Index.
(A) MDCK cells with proper localization of β-actin translation (expressing full length β-actin mRNA) fixed at steady state and immunostained for F-actin (Green), E-cadherin (Red), and Nucleus (Blue). Stress fibers are visible in the basal compartment, while E-cadherin and F-actin co-stain cell-cell interfaces in the apical and basal compartments. (B) MDCK cells with partially mislocalized β-actin translation (expressing Δ3′UTR β-actin mRNA) fixed at steady state and stained for F-actin (Green), E-cadherin (Red) and Nucleus (Blue). Lamellar overlap from neighboring cells is visible in the basal compartment although E-cadherin and F-actin still stain the cell-cell interfaces. Scale bars = 10μm. Dotted lines indicate cell boundaries and * indicates nucleus of individual cells. ΔZ represents the distance between the apical and basal planes in μm. Cells that properly localize β-actin translation (expressing full length β-actin mRNA) are taller than those with partially mislocalized β-actin translation (expressing Δ3′UTR β-actin mRNA), indicating defects in apical lifting in these cells. (C) Frequency distributions of FCI values for E-cadherin and F-actin for cells fixed in steady state (expressing full length β-actin expressing cells N = 29 cells and Δ3′UTR β-actin expressing cells N= 314 cells). Bin size = 0.3. Red box indicates the proportion of cells with high FCI values. (D) Percentage of TRITC-Dextran in the bottom compartment in an in vitro permeability assay for MDCK cells in steady state with and without partial defects in localizing β-actin translation. Bars represent Mean ± S.E.M.
Actin anchored cadherin-catenin complexes induce a myosin II dependent feed forward loop that drives additional cadherin-catenin/actin filament anchoring. The resulting expansion and maturation of adherens junctions is required for epithelial structure and function (Smutny et al. 2010; Yonemura et al. 2010). Conversely, E-cadherin that is not anchored at the cell surface is internalized by dynamin mediated endocytosis, and gets either recycled or degraded (Cavey et al. 2008; Harris and Tepass 2010). To quantify the extent of E-cadherin anchoring to F-actin, we used compartment specific Pearsons Correlation Coefficient (PCC) analysis (Barlow et al. 2010; Costes et al. 2004). Other global pixel intensity-based statistics, such as Manders Overlap Coefficients or Manders Colocalization Coefficients (Dunn et al. 2011; Manders et al. 1993), cannot distinguish between bona fide protein-protein interactions and non-specific overlap of the two fluorescent signals (Adler and Parmryd 2010). Briefly, we obtained PCC values in the cell-cell contact zone (periphery) and the cytoplasm. Since a PCC value of 0.1 represents a very weak correlation, we set a lower bound on all PCC values to 0.1, (Zinchuk et al. 2013). We then derived a covariance measure, Fluorescence Covariance Index (FCI), by obtaining the logarithm transformed ratio of the PCC value in the periphery to the PCC value in the cytoplasm (Supporting Information Figure S1). MDCK monolayers impaired in their ability to localize β-actin translation had fewer cells with high FCI values (0.5 – 1.0) compared to monolayers composed of cells that properly localize β-actin translation (Figure 1C). In addition, compared to steady state monolayers, fewer cells had high FCI values following EGTA-induced junction disassembly, indicating this frequency range is a useful measure of adherens junction assembly/disassembly (Supporting Information Figure S2). Cells with partially mislocalized β-actin translation had high cytoplasmic correlations between E-cadherin and F-actin, generating low FCI values. These cytoplasmic correlations arise from overlap of lamellar protrusions, which are inhibited in cells that properly localize β-actin translation. Additionally, monolayers assembled from cells partially impaired in their ability localize β-actin translation were only ≈66% the height of monolayers assembled from cells that have no defects in localizing β-actin translation (Figure 1A and 1B). These results indicate that partial impairment in localizing β-actin translation causes defects in the junction maturation stage of adherens junction assembly. Additionally, the in vitro permeability to fluorescent dextran was ≈4 times higher in confluent monolayers assembled from cells with partially mislocalized β-actin translation (Figure 1D). Importantly, low FCI values correlate with reduced barrier integrity demonstrating this metric has value in quantifying gain or loss of monolayer function.
Inhibiting Dynamin mediated endocytosis rescues the epithelial adherens junction assembly defect caused by partially mislocalizing β-actin translation
To study adherens junction assembly dynamics in epithelial MDCK monolayers composed of cells with partially mislocalized β-actin translation, we performed calcium switch experiments (Volberg et al. 1986) and carried out FCI analysis for E-cadherin and F-actin. Typically, epithelial adherens junction assembly in vitro occurs in three phases – contact initiation, contact expansion and junction maturation (Adams et al. 1998; Baum and Georgiou 2011; Kovacs et al. 2002). Following calcium repletion, cells with partially mislocalized β-actin translation form initial contact within 1 hour and expand these contacts within 2 hours (Supporting Information Figure S3A). However, significant lamellar protrusions were observed 3 hours after calcium repletion. In addition, these cells fail to reach the height of monolayers assembled from cells which have no defects in localizing β-actin translation (Figure 2A). Between 2 and 3 hours after calcium repletion, fluorescence correlations between E-cadherin and F-actin increased in the cytoplasm, while correlations at the periphery remained unchanged (Figure 2B). The changes in FCI values correlated with morphological changes, increasing between 1 hour and 2 hours after calcium repletion and decreasing significantly 3 hours after calcium repletion (Figure 2C). These results indicate that following contact expansion, these cells form extensive lamellar protrusions, which contain nascent cadherin-catenin complexes anchored to F-actin (en face adhesions). Thus, cells with partial mislocalization of β-actin translation undergo contact expansion but fail to progress to junction maturation due to lamellar overlap.
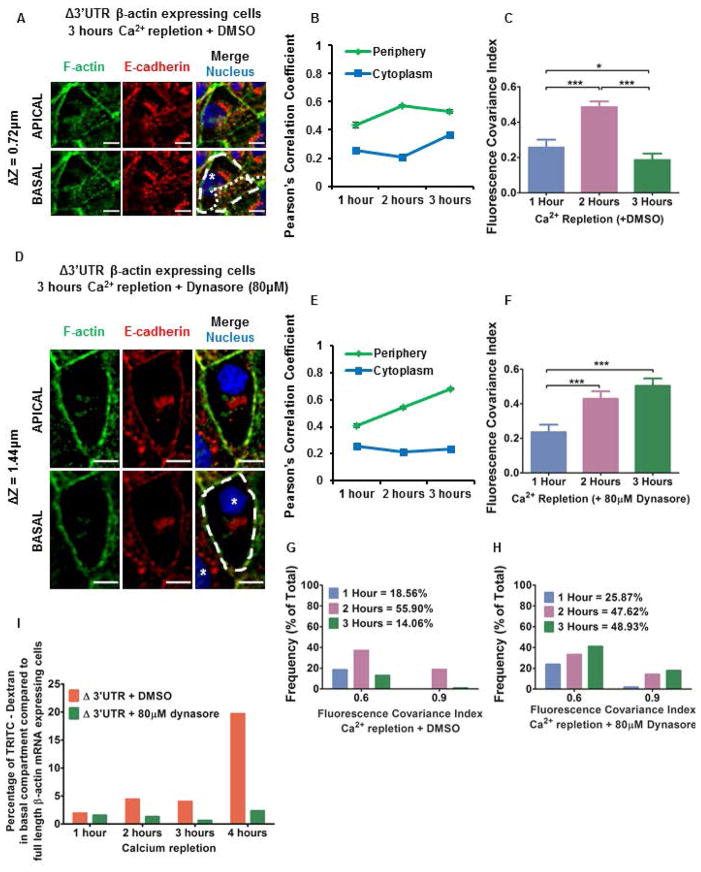
Figure 2. Inhibiting dynamin mediated endocytosis with 80μM dynasore rescues the adherens junction assembly defects caused by partially mislocalizing β-actin translation.
(A) MDCK cells with partially mislocalized β-actin translation (expressing Δ3′UTR β-actin mRNA) fixed 3 hours after calcium repletion with DMSO (0.5% v/v) and immunostained for F-actin (Green), E-cadherin (Red), and Nucleus (Blue). (B) Change in PCC values for F-actin and E-cadherin in the periphery and the cytoplasm measured for cells with partially mislocalized β-actin translation (expressing Δ3′UTR β-actin mRNA) fixed 1, 2 and 3 hours after calcium repletion with DMSO (0.5% v/v). Points represent Mean ± SEM. Note 3 hours after calcium repletion PCC values in the periphery do not increase while PCC values in the cytoplasm increase due to lamellar overlap from neighboring cells. (C) Mean FCI values. Bars represent Mean ± 95% C.I. One-way ANOVA result: p value < 0.0001. Tukey’s post-hoc multiple comparison test results with * p < 0.05, *** p < 0.0001 are indicated on the graph. (D) MDCK cells with partially mislocalized β-actin translation (expressing Δ3′UTR β-actin mRNA) fixed 3 hours after calcium repletion with 80μM dynasore and immunostained for F-actin (Green), E-cadherin (Red), and Nucleus (Blue). (A, D) Scale bars = 10μm. Dotted lines indicate cell boundaries and * indicates nucleus of individual cells. ΔZ represents the distance between the apical and basal planes in μm. Note the lamellar overlap of cells treated with 0.5% v/v DMSO. Cells treated with 80μM dynasore show no such overlap. (E) Change in PCC values for F-actin and E-cadherin in the periphery and the cytoplasm measured for cells with partially mislocalized β-actin translation (expressing Δ3′UTR β-actin mRNA) fixed 1, 2 and 3 hours after calcium repletion with 80μM dynasore. Points represent Mean ± SEM. Note 3 hours after calcium repletion PCC values in the periphery increase while PCC values in the cytoplasm remains low. (F) Mean FCI values. Bars represent Mean ± 95% C.I. One-way ANOVA result: p value < 0.0001. Tukey’s post-hoc multiple comparison test results with *** p < 0.0001 are indicated on the graph. (G) Frequency plots for high FCI values of F-actin and E-cadherin in cells with partially mislocalized β-actin translation (expressing Δ3′UTR β-actin mRNA) following calcium repletion with DMSO (0.5% v/v). 1 hour (N = 97); 2 hours (N = 229); 3 hours (N = 192). Bin size = 0.3. Note 3 hours after calcium repletion with DMSO the proportion of cells with high FCI values decreases. (H) Frequency plots for high FCI values of E-cadherin and F-actin in cells with partially mislocalized β-actin translation (expressing Δ3′UTR β-actin mRNA) following calcium repletion with 80μM dynasore. 1 hour (N = 201); 2 hours (N = 147); 3 hours (N = 112). Bin size = 0.3. Note 3 hours after calcium repletion with 80μM dynasore the proportion of cells with high FCI values is maintained indicating adherens junction recovery. (I) Percentage of TRITC-Dextran in the bottom compartment in an in vitro permeability assay for MDCK cells with partially mislocalized β-actin translation in steady state (expressing Δ3′UTR β-actin mRNA), treated with DMSO or 80μM dynasore. The bars represent values for cells with partially mislocalized β-actin translation (expressing Δ3′ UTR β-actin mRNA) relative to cells without any defects in localizing β-actin translation (expressing full length β-actin mRNA). Note treating cells with partially mislocalized β-actin translation with 80μM dynasore rescues the barrier integrity defect.
To inhibit Dynamin mediated endocytosis, we used dynasore, a small molecule inhibitor of Dynamin’s GTPase function (Macia et al. 2006). At 80μM dynasore, we observed colocalization between E-cadherin and F-actin at cell-cell contacts and few lamellar protrusions 3 hours after calcium repletion (Figure 2D). Additionally, these cells were as tall as cells in monolayers assembled from cells with no defects in localizing β-actin translation (Figure 2D). The initial phases of adherens junction assembly: cell-cell contact formation, contact initiation and contact expansion were unaffected by endocytosis inhibition (Supporting Information Figure S3C). Fluorescence correlations for E-cadherin and F-actin remained low in the cytoplasm, while correlations at cell-cell contacts progressively increased from 1 to 3 hours after calcium repletion (Figure 2E). Consequently, FCI values remained high 3 hours after calcium repletion, indicating these cells successfully completed adherens junction maturation (Figure 2F). Treatment with vehicle only showed significantly fewer cells with high FCI values (0.5 – 1.0) 3 hours after calcium repletion. In contrast, cells treated with 80μM dynasore showed a progressive increase in high FCI values following calcium repletion (Figure 2G and 2H). These results indicate that inhibiting endocytosis rescues adherens junction assembly in cells with partially mislocalized β-actin translation.
Cells partially defective in their ability to localize β-actin translation also exhibited ≈6 fold higher permeability to fluorescent dextran 4 hours after calcium repletion compared to cells with no defects in localizing β-actin translation (Figure 2I and Supporting Information Figure S4). Inhibiting endocytosis during calcium repletion using dynasore (80μM) in cells with partially mislocalized β-actin translation rescued the barrier integrity defects within 3 hours after calcium repletion (Figure 2I). These results further demonstrate that FCI values are predictive of monolayer barrier function.
Adding 40 μM dynasore, following calcium repletion, to cells with partial defects in localizing β-actin translation showed normal contact initiation followed by contact expansion (Supporting Information S5A). 3 hours after calcium repletion, the cells reached a height of 1.44μM similar to cells that target β-actin translation properly (Supporting Information S5A). However, these cells remained impaired in their ability to shut off lamellar protrusive activity on the basal surface (Supporting Information S5A). Consistent with the presence of lamellar protrusions (en face adhesions), E-cadherin and F-actin fluorescence correlations increased in the cytoplasm, which resulted in low FCI values, although correlations at cell-cell contacts increased (Supporting Information S5B and S5C). The proportion of cells with high FCI values (0.5 – 1.0) between 2 hours and 3 hours after calcium repletion with 40μM dynasore decreased, demonstrating a defect in adherens junction maturation at this concentration of dynasore (Supporting Information S5D).
Further, we increased the concentration of dynasore to 160μM during calcium repletion in cells with partially mislocalized β-actin translation. Morphologically, during contact initiation and contact expansion, these cells showed no significant changes compared to cells treated with lower doses of dynasore (Supporting Information Figure S6A). There were no observable lamellar overlaps during junction maturation, and the height of the cells was comparable to cells that have no defects in localizing β-actin translation (Supporting Information Figure S6A). However, fluorescence correlations between E-cadherin and F-actin in the periphery 3 hours after calcium repletion were lower with 160μM dynasore as compared to 80μM dynasore. This indicates very high doses of dynasore increase F-actin and E-cadherin localization to the cell-cell interface without a corresponding increase in the association of these two proteins (Supporting Information Figure S6B). Moreover, mean FCI values and the proportion of cells with high FCI (0.5 – 1.0) values increased between 2 and 3 hours after calcium repletion, consistent with adherens junction maturation in cells treated with 160μM dynasore (Supporting Information S6C and S6D).
To further validate our observations, we used a new generation Dynamin inhibitor – Hydroxy-Dynasore aka Dyngo 4a (McCluskey et al. 2013), and the results obtained were similar to those observed following dynasore treatment. Contact initiation and contact expansion were observed 1 and 2 hours following calcium repletion with 80μM hydroxy-dynasore in cells with partially mislocalized β-actin translation (Supporting Information Figure S7A). 3 hours after calcium repletion, these cells had few lamellar protrusions, and reached a height similar to cells that had no defects in localization of β-actin translation (Supporting Information Figure S7B). Fluorescence correlations between E-cadherin and F-actin remained low in the cytoplasm, while correlations at the cell-cell contact increased. Consequently, FCI values increased between 1, 2 and 3 hours after calcium repletion (Supporting Information Figure S7B and S7C). Lastly, the proportion of cells with high FCI values (0.5 – 1.0) increased between 2 and 3 hours after calcium repletion (Supporting Information Figure S7D). These results confirm that inhibiting Dynamin mediated endocytosis rescues adherens junction assembly defects caused by partially mislocalizing β-actin translation.
β-actin mRNA zipcode function and Dynamin mediated endocytosis balance each other to drive adherens junction maturation
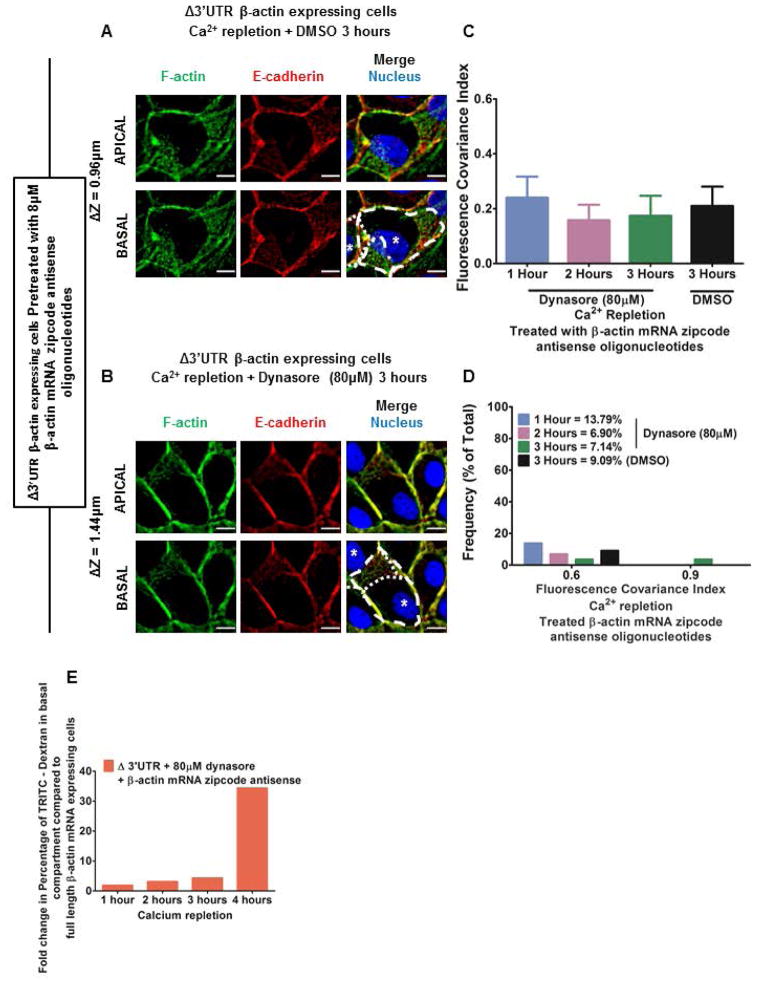
Previously we showed expression of the 3′UTR truncated β-actin contributes to approximately 20% of the total β-actin content in the cells (Gutierrez et al. 2014). Consequently, 80% of β-actin mRNA in these cells is endogenous and contains the zipcode. This endogenous β-actin mRNA localizes to cell-cell contacts to produce actin filaments, which when combined with decreased endocytosis, tips the balance towards anchoring cadherin-catenin complexes. In order to achieve mislocalization of endogenous β-actin mRNA we used antisense oligonucleotides that mask the β-actin mRNA zipcode (Gutierrez et al. 2014; Shestakova et al. 2001). Pretreating MDCK cells partially defective in localizing β-actin translation with β-actin mRNA zipcode antisense oligonucleotides resulted in significant lamellar protrusive activity 3 hours after calcium repletion (Figure 3A). Adding 80μM dynasore to cells treated with antisense oligonucleotides resulted in taller cells, but lamellar protrusive activity was still present 3 hours after calcium repletion (Figure 3B). FCI values remained low after calcium repletion even in the presence of 80μM dynasore (Figure 3C, 3D and Supporting Information Figure S8). A more complete mislocalization of β-actin translation using β-actin mRNA zipcode antisense oligonucleotides abolished the rescue of barrier integrity observed by inhibiting endocytosis (Figure 3E c.f. Figure 2I). These results show that partial mislocalization of β-actin translation can be compensated by inhibiting endocytosis, whereas a complete mislocalization of β-actin translation blocks the rescue of adherens junction assembly and epithelial function.
Figure 3. Completely mislocalizing β-actin translation blocks adherens junction assembly even with endocytosis inhibition.
MDCK cells with partially mislocalized β-actin translation (expressing Δ3′UTR β-actin mRNA) were pretreated with β-actin mRNA zipcode antisense oligonucleotides and fixed 3 hours after calcium repletion with: (A) DMSO (0.5% v/v) or (B) 80μM dynasore; and immunostained for F-actin (Green), E-cadherin (Red), and Nucleus (Blue). Scale bars = 10μm. Dotted lines indicate cell boundaries and * indicates nucleus of individual cells. ΔZ represents the distance between the apical and basal planes in μm. Note the lamellar overlap in the cells seen in the basal compartment. (C) Mean FCI values. Bars represent Mean ± 95% C.I. One-way ANOVA results for Dynasore with β-actin mRNA zipcode antisense oligonucleotides treatment indicates no significant difference with time in FCI values. Unpaired Student t test indicates no significant variation between Dynasore with antisense oligonucleotides and DMSO with antisense oligonucleotides treatment 3 hours following calcium repletion. (D) Frequency distribution of FCI values for E-cadherin and F-actin following calcium repletion with either Dynasore (1 hour N = 29; 2 hours N = 29; 3 hours N = 28) or DMSO treatment (3 hours N = 22). Bin size = 0.3. Note the proportion of cells with high FCI values remains very low during calcium repletion. (E) Percentage of TRITC-Dextran in the bottom compartment in an in vitro permeability assay following calcium repletion for cells with partially mislocalized β-actin translation (expressing Δ3′UTR β-actin mRNA) and pretreated with β-actin mRNA zipcode antisense oligonucleotides as well as 80μM Dynasore following calcium repletion. The bars are relative to values for cells without any defects in localization of β-actin translation (expressing full length β-actin mRNA). Note barrier integrity fails to recover following calcium repletion upon a more complete mislocalization of β-actin translation with zipcode antisense oligonucleotides.
The dynasore dependent rescue of adherens junction assembly in Δ3′UTR β-actin mRNA expressing cells requires functional E-cadherin
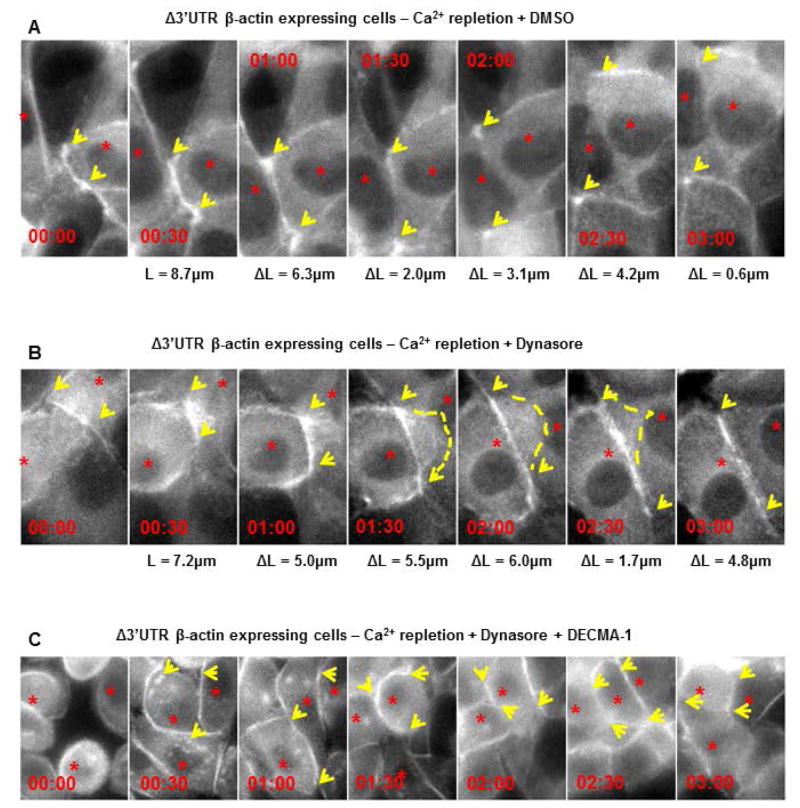
Dynamin inhibitors block the recycling of several transmembrane proteins including E-cadherin. To test whether global inhibition of dynamin mediated endocytosis or more specifically, E-cadherin function at the cell surface, was required for rescuing adherens junction assembly in cells with partial defects in localizing β-actin translation, we used DECMA-1, an E-cadherin function blocking antibody (Vestweber and Kemler 1985). We performed live cell imaging experiments following calcium repletion with cells expressing Δ3′UTR β-actin mRNA that encodes β-actin with an eGFP –fusion tag. In cells treated with vehicle alone, contact initiation followed by contact expansion was observed as an increase in distance between cell-cell vertices (Figure 4A Arrows). However, soon after this phase, the cells continued to overlap contacting neighboring cells and failed to reach the junction maturation phase (Figure 4A). Cells treated with 80μM dynasore during calcium repletion, also exhibited normal contact initiation followed by normal contact expansion. This was followed by junction maturation seen as the accumulation of linear actin cables running along the cell-cell interface. Additionally, the lamellar protrusive activity was shut off as the cells reached the junction maturation stage 3 hours after calcium repletion (Figure 4B). By contrast, treating cells with DECMA-1 and 80μM of dynasore during calcium repletion not only abolished the rescue of junction maturation, but also inhibited contact expansion (Figure 4C). These data show that inhibiting Dynamin mediated endocytosis requires E-cadherin function to rescue adherens junction assembly in cells with partially mislocalized β-actin translation.
Figure 4. Blocking endocytosis and E-cadherin function are both required for rescuing adherens junction assembly in cells with partially mislocalized β-actin translation.
Montage of MDCK cells with partially mislocalized β-actin translation (expressing Δ3′UTR β-actin mRNA that encodes an eGFP-fusion tag), following calcium repletion. Cells were treated with: (A) 0.5% (v/v) DMSO or (B) 80μM Dynasore or (C) 80μM DMSO + 100μg/mL DECMA-1, following calcium repletion. Time is indicated as duration post initial contact (hh:mm). L is an estimate of contact length and ΔL represents the increase in length with respect to the previous frame. Arrows point to expanding contacts. Note in cells treated with DMSO, there is overlap of neighboring cells while in cells treated with 80μM dynasore, contact expansion is followed by retraction of the protrusion (dotted line). Treating cells with DECMA-1 completely abolishes contact expansion as seen by the lack of continued expansion of the filamentous actin along the cell-cell interface. The data represents qualitative assessment of retraction followed by contact expansion upon inhibition of endocytosis in cells expressing Δ3′UTR β-actin mRNA following calcium repletion. Three independent experiments were performed for each condition in A, B and two independent experiments for C.
Actin-anchoring and endocytosis: The Yin and Yang of adherens junction assembly
E-cadherin endocytosis contributes significantly to the dynamics of adherens junctions (Troyanovsky et al. 2006). While endocytosis is required for redistributing E-cadherin at mature junctions (de Beco et al. 2009), constitutively active Rac1 depletes E-cadherin from cell-cell contacts by increasing its internalization (Akhtar and Hotchin 2001), suggesting two roles for E-cadherin endocytosis. The first is a rapid turnover during the initial phase of adherens junction assembly following cell-cell contact where Rac1 activity is high, and the second is to recycle E-cadherin at mature junctions following disassembly of trans dimers (Figure 5). In fact, non-trans interacting E-cadherin is preferentially internalized (Izumi et al. 2004). We report a model of adherens junction assembly where in, β-actin translation dependent actin filament remodeling drives E-cadherin clustering at the cell surface to oppose the negative contributor, endocytosis of E-cadherin, and drives adherens junction assembly in epithelial cells (Figure 5). The balance tips if filament assembly and remodeling is blocked either pharmacologically using Latrunculin A (Wu et al. 2015) and Cytochalasin B (Krendel and Bonder 1999) or by inhibiting cell-cell contact localized β-actin monomer synthesis (Gutierrez et al. 2014). The result is that cells are unable to transition from contact initiation to contact maturation owing to endocytosis of cadherin-catenin complexes, which now dominates actin anchoring of these complexes. Utilizing a new fluorescence microscopy based covariance analysis to determine the extent of adherens junction assembly, we show that FCI values correlate with barrier integrity and therefore are a useful measure of epithelial functionality.
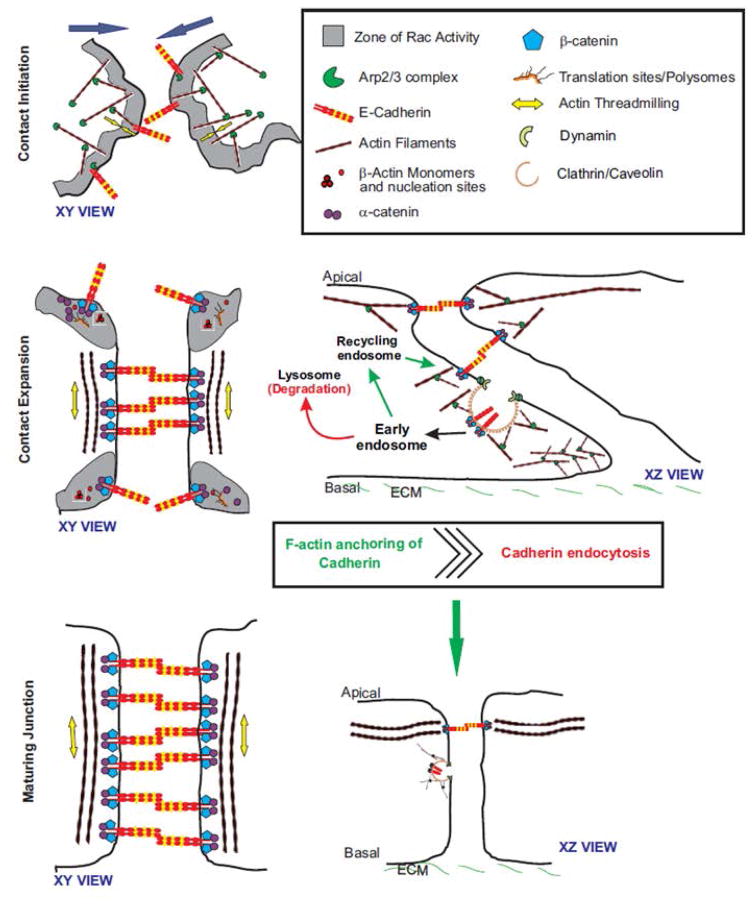
Figure 5. Actin mediated E-cadherin clustering and E-cadherin endocytosis balance each other during adherens junction assembly.
Schematic showing the three stages of de novo adherens junction assembly – contact initiation, contact expansion and junction maturation. Contact initiation is characterized by branched actin network at the leading edge of the cells. E-cadherin clustering mediates actin filament remodeling from branched array to linear filaments which anchor adherens junctions complexes, leading to the contact expansion phase. This is followed by an inhibition of protrusive activity and junction maturation. Endocytosis acts to remove E-cadherin-catenin complexes that are not actin anchored during contact expansion thus contributing negatively to contact expansion. The actin driven clustering supersedes endocytosis mediated removal of E-cadherin at the cell surface to drive adherens junction assembly.
Materials and Methods
Cell culture and stable cell lines
MDCK – NBL2 cells (ATCC® CCL-34™) were cultured in DMEM supplemented with 10% (v/v) FBS and 100 I.U./mL Penicillin and 100μg/mL Streptomycin. Transfection of these cells with eTC GFP β-actin full length and eTC GFP β-actin ΔZip – (Δ3′UTR) (Addgene Plasmid #27123, #27124 and (Rodriguez et al. 2006)) was carried out using Lipofectamine® 2000 (Life Technologies) as per manufacturer’s specifications. Transfected cells were selected using 500μg/mL G418 (Sigma-Aldrich). Flow cytometry was used to sort cells and obtain a homogeneous population of cells expressing GFP-β-actin.
Calcium switch experiments
Cells were plated at a density of 250,000 cells per well in a 6 well plate onto a 22X22mm No.1 coverslip (Fisher Scientific) and grown 48 hours. Cells washed with 1XPBS and medium without serum and supplemented with 4mM EGTA was added for 1 hour. Regular growth medium was added to initiate de novo adherens junction assembly. Additionally, either DMSO (0.5% v/v) (Sigma-Aldrich) or Dynasore (Sigma-Aldrich) at a concentration of 40μM/80μM/160μM or 4-Hydroxy Dynasore at a concentration of 80μM (Sigma-Aldrich) was added.
Pretreatment of cells with β-actin mRNA zipcode antisense oligonucleotide treatment was carried out in regular growth medium with 8μM of oligonucleotides for 12 hours prior to start of the experiment with fresh oligonucleotides being added every 4 hours. This was followed by a calcium switch as described above, maintaining oligonucleotides in the medium throughout the course of the experiment.
Live cell imaging
Cells were seeded at a density of 250,000 in 35mm MatTek dishes with glass cover bottom (MatTek Corp.) for 48 hours and treated subject to low calcium treatment as described above. FluorBrite DMEM (Life Technologies) supplemented with 10% FBS and OxyFluor (Oxyrase) at 1% (v/v) was used as calcium repletion medium to minimize photoxicity and photobleaching. Images were acquired on an inverted wide field Zeiss microscope (Zeiss AxioObserver.Z1) equipped with an incubator chamber (37 ºC and 5% CO2) was used. A 63X water immersion objective, NA 1.33 was used. QuantEM 512SC camera (Photometrics) was used with no binning. Time-lapse images were taken in GFP channel (Filter Set 38HE, item number 489038-9901-000, Carl Zeiss, Inc) at 5 minute intervals for 12 hours. DECMA-1 antibody (Sigma-Aldrich) was used at 100μg/mL following calcium repletion.
Immunofluorescence and imaging
Cells were fixed at room temperature in 4% (w/v) paraformaldehyde in 1XPBS (pH 7.2) for 20 minutes. 0.5% Triton-X was used to permeablize the cells for 1 minute at room temperature. Followed by blocking in 1% (w/v) BSA, cells were incubated in mouse monoclonal anti-E-cadherin primary antibody (1:200 dilution in blocking solution, BD Biosciences, #610181) overnight at 4°C. Cells were then incubated with goat anti-mouse Cy5 (Life Technologies) conjugated secondary antibody (1:1000 dilution in blocking solution, Life Technologies) for 1½ hours at room temperature. Rhodamine-Phalloidin (1:40 dilution in 1X PBS, Life technologies) was used to stain F-actin for 40 minutes at room temperature. DAPI or Hoechst 33342 was used to stain the nucleus following which coverslips were mounted using Prolong® Gold (Life Technologies). Images were acquired using a Zeiss AxioObserver.Z1 microscope and a 63X oil immersion lens (NA 1.4). Z-stacks were acquired using QuantEM 512SC camera (Photometrics) or Cool Snap HQ2 (Photometrics) at a Z-depth of 240μm per slice.
Image processing and quantitative analysis
Images were processed using the Zeiss AxioVision 4.8.2 Deconvolution algorithm with the following parameters – Autolinear normalization, Noise regularization: Manual Strength – 6 and Constrained Iterative deconvolution. Processed images were exported into Volocity® 6.1 (PerkinElmer). Two distinct cellular compartments – cell periphery and the cytoplasm (interior of the cell excluding the periphery and the nucleus) were defined using the ROI tool and the Pearson’s Correlation Coefficient (for E-cadherin and F-actin) was computed using the object co-localization tool. The data for all the cells was analyzed using MATLAB and graphed using GraphPad Prism 5 software.
In vitro permeability assay
MDCK cells expressing Δ3′UTR β-actin were plated at 700,000 cells per Transwell® support (0.4μm pore size, Corning) in a 6 well plate and incubated for 48 hours. A calcium switch was performed as described above and 5mg/mL of 3000MW TRICT-dextran was added in 1, 2, 3 or 4 hours after calcium repletion in the top compartment for 2 hours. 200 μl samples were taken from the top and bottom compartments and fluorescence intensity was measured using a GloMax plate reader (Promega). The ratio of the fluorescence intensities in the bottom and top compartments was taken. The contribution of TRITC-dextran in the bottom chamber was used as a proxy to assess the barrier functionality of a monolayer.
Supplementary Material
Acknowledgments
This work was supported by NIH R25 60825-06 to N.G. and L.C and by NIH R25 GM096161-04 to B.A. The authors are grateful to Dr. Edward Bonder for critically reading the manuscript.
References
- Adams CL, Chen YT, Smith SJ, Nelson WJ. Mechanisms of epithelial cell-cell adhesion and cell compaction revealed by high-resolution tracking of E-cadherin-green fluorescent protein. J Cell Biol. 1998;142(4):1105–19. doi: 10.1083/jcb.142.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler J, Parmryd I. Quantifying colocalization by correlation: the Pearson correlation coefficient is superior to the Mander’s overlap coefficient. Cytometry A. 2010;77(8):733–42. doi: 10.1002/cyto.a.20896. [DOI] [PubMed] [Google Scholar]
- Akhtar N, Hotchin NA. RAC1 regulates adherens junctions through endocytosis of E-cadherin. Mol Biol Cell. 2001;12(4):847–62. doi: 10.1091/mbc.12.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballestrem C, Wehrle-Haller B, Imhof BA. Actin dynamics in living mammalian cells. J Cell Sci. 1998;111(Pt 12):1649–58. doi: 10.1242/jcs.111.12.1649. [DOI] [PubMed] [Google Scholar]
- Baranwal S, Naydenov NG, Harris G, Dugina V, Morgan KG, Chaponnier C, Ivanov AI. Nonredundant roles of cytoplasmic beta- and gamma-actin isoforms in regulation of epithelial apical junctions. Mol Biol Cell. 2012;23(18):3542–53. doi: 10.1091/mbc.E12-02-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow AL, Macleod A, Noppen S, Sanderson J, Guerin CJ. Colocalization analysis in fluorescence micrographs: verification of a more accurate calculation of pearson’s correlation coefficient. Microsc Microanal. 2010;16(6):710–24. doi: 10.1017/S143192761009389X. [DOI] [PubMed] [Google Scholar]
- Baum B, Georgiou M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J Cell Biol. 2011;192(6):907–17. doi: 10.1083/jcb.201009141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertocchi C, Vaman Rao M, Zaidel-Bar R. Regulation of adherens junction dynamics by phosphorylation switches. J Signal Transduct. 2012;2012:125295. doi: 10.1155/2012/125295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco MJ, Moreno-Bueno G, Sarrio D, Locascio A, Cano A, Palacios J, Nieto MA. Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene. 2002;21(20):3241–6. doi: 10.1038/sj.onc.1205416. [DOI] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2(2):76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Cavey M, Rauzi M, Lenne PF, Lecuit T. A two-tiered mechanism for stabilization and immobilization of E-cadherin. Nature. 2008;453(7196):751–6. doi: 10.1038/nature06953. [DOI] [PubMed] [Google Scholar]
- Condeelis J, Singer RH. How and why does beta-actin mRNA target? Biol Cell. 2005;97(1):97–110. doi: 10.1042/BC20040063. [DOI] [PubMed] [Google Scholar]
- Costes SV, Daelemans D, Cho EH, Dobbin Z, Pavlakis G, Lockett S. Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys J. 2004;86(6):3993–4003. doi: 10.1529/biophysj.103.038422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beco S, Amblard F, Coscoy S. New insights into the regulation of E-cadherin distribution by endocytosis. Int Rev Cell Mol Biol. 2012;295:63–108. doi: 10.1016/B978-0-12-394306-4.00008-3. [DOI] [PubMed] [Google Scholar]
- de Beco S, Gueudry C, Amblard F, Coscoy S. Endocytosis is required for E-cadherin redistribution at mature adherens junctions. Proc Natl Acad Sci U S A. 2009;106(17):7010–5. doi: 10.1073/pnas.0811253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123(5):903–15. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KW, Kamocka MM, McDonald JH. A practical guide to evaluating colocalization in biological microscopy. Am J Physiol Cell Physiol. 2011;300(4):C723–42. doi: 10.1152/ajpcell.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HE, Behrens J, Sommer T, Birchmeier W. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol. 2002;4(3):222–31. doi: 10.1038/ncb758. [DOI] [PubMed] [Google Scholar]
- Gloushankova NA, Alieva NA, Krendel MF, Bonder EM, Feder HH, Vasiliev JM, Gelfand IM. Cell-cell contact changes the dynamics of lamellar activity in nontransformed epitheliocytes but not in their ras-transformed descendants. Proc Natl Acad Sci U S A. 1997;94(3):879–83. doi: 10.1073/pnas.94.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez N, Eromobor I, Petrie RJ, Vedula P, Cruz L, Rodriguez AJ. The beta-actin mRNA zipcode regulates epithelial adherens junction assembly but not maintenance. RNA. 2014;20(5):689–701. doi: 10.1261/rna.043208.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11(7):502–14. doi: 10.1038/nrm2927. [DOI] [PubMed] [Google Scholar]
- Izumi G, Sakisaka T, Baba T, Tanaka S, Morimoto K, Takai Y. Endocytosis of E-cadherin regulated by Rac and Cdc42 small G proteins through IQGAP1 and actin filaments. J Cell Biol. 2004;166(2):237–48. doi: 10.1083/jcb.200401078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda E, Nevolo M, Lehmann K, Downward J, Beug H, Grieco M. Raf plus TGFbeta-dependent EMT is initiated by endocytosis and lysosomal degradation of E-cadherin. Oncogene. 2006;25(54):7117–30. doi: 10.1038/sj.onc.1209701. [DOI] [PubMed] [Google Scholar]
- Kimura T, Sakisaka T, Baba T, Yamada T, Takai Y. Involvement of the Ras-Ras-activated Rab5 guanine nucleotide exchange factor RIN2-Rab5 pathway in the hepatocyte growth factor-induced endocytosis of E-cadherin. J Biol Chem. 2006;281(15):10598–609. doi: 10.1074/jbc.M510531200. [DOI] [PubMed] [Google Scholar]
- Kislauskis EH, Zhu X, Singer RH. Sequences responsible for intracellular localization of beta-actin messenger RNA also affect cell phenotype. J Cell Biol. 1994;127(2):441–51. doi: 10.1083/jcb.127.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpal M, Lee ES, Hu G, Kang Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem. 2008;283(22):14910–4. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs EM, Ali RG, McCormack AJ, Yap AS. E-cadherin homophilic ligation directly signals through Rac and phosphatidylinositol 3-kinase to regulate adhesive contacts. J Biol Chem. 2002;277(8):6708–18. doi: 10.1074/jbc.M109640200. [DOI] [PubMed] [Google Scholar]
- Krendel MF, Bonder EM. Analysis of actin filament bundle dynamics during contact formation in live epithelial cells. Cell Motil Cytoskeleton. 1999;43(4):296–309. doi: 10.1002/(SICI)1097-0169(1999)43:4<296::AID-CM3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Lombaerts M, van Wezel T, Philippo K, Dierssen JW, Zimmerman RM, Oosting J, van Eijk R, Eilers PH, van de Water B, Cornelisse CJ, et al. E-cadherin transcriptional downregulation by promoter methylation but not mutation is related to epithelial-to-mesenchymal transition in breast cancer cell lines. Br J Cancer. 2006;94(5):661–71. doi: 10.1038/sj.bjc.6602996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubimova A, Bershadsky AD, Ben-Ze’ev A. Autoregulation of actin synthesis requires the 3′-UTR of actin mRNA and protects cells from actin overproduction. J Cell Biochem. 1999;76(1):1–12. doi: 10.1002/(sici)1097-4644(20000101)76:1<1::aid-jcb1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10(6):839–50. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Manders EMM, Verbeek FJ, Aten JA. Measurement of Colocalization of Objects in Dual-Color Confocal Images. Journal of Microscopy-Oxford. 1993;169:375–382. doi: 10.1111/j.1365-2818.1993.tb03313.x. [DOI] [PubMed] [Google Scholar]
- McCluskey A, Daniel JA, Hadzic G, Chau N, Clayton EL, Mariana A, Whiting A, Gorgani NN, Lloyd J, Quan A, et al. Building a better dynasore: the dyngo compounds potently inhibit dynamin and endocytosis. Traffic. 2013;14(12):1272–89. doi: 10.1111/tra.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosesson Y, Mills GB, Yarden Y. Derailed endocytosis: an emerging feature of cancer. Nat Rev Cancer. 2008;8(11):835–50. doi: 10.1038/nrc2521. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A. Molecular architecture of adherens junctions. Curr Opin Cell Biol. 2001;13(5):600–3. doi: 10.1016/s0955-0674(00)00257-x. [DOI] [PubMed] [Google Scholar]
- Rodriguez AJ, Shenoy SM, Singer RH, Condeelis J. Visualization of mRNA translation in living cells. J Cell Biol. 2006;175(1):67–76. doi: 10.1083/jcb.200512137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosivatz E, Becker I, Specht K, Fricke E, Luber B, Busch R, Hofler H, Becker KF. Differential expression of the epithelial-mesenchymal transition regulators snail, SIP1, and twist in gastric cancer. Am J Pathol. 2002;161(5):1881–91. doi: 10.1016/S0002-9440(10)64464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Hirsch DS, Sasiela CA, Wu WJ. Cdc42 regulates E-cadherin ubiquitination and degradation through an epidermal growth factor receptor to Src-mediated pathway. J Biol Chem. 2008;283(8):5127–37. doi: 10.1074/jbc.M703300200. [DOI] [PubMed] [Google Scholar]
- Shestakova EA, Singer RH, Condeelis J. The physiological significance of beta -actin mRNA localization in determining cell polarity and directional motility. Proc Natl Acad Sci U S A. 2001;98(13):7045–50. doi: 10.1073/pnas.121146098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smutny M, Cox HL, Leerberg JM, Kovacs EM, Conti MA, Ferguson C, Hamilton NA, Parton RG, Adelstein RS, Yap AS. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat Cell Biol. 2010;12(7):696–702. doi: 10.1038/ncb2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Dynamic contacts: rearranging adherens junctions to drive epithelial remodelling. Nat Rev Mol Cell Biol. 2014;15(6):397–410. doi: 10.1038/nrm3802. [DOI] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15(6):740–6. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Troyanovsky RB, Sokolov EP, Troyanovsky SM. Endocytosis of cadherin from intracellular junctions is the driving force for cadherin adhesive dimer disassembly. Mol Biol Cell. 2006;17(8):3484–93. doi: 10.1091/mbc.E06-03-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Roy F, Berx G. The cell-cell adhesion molecule E-cadherin. Cell Mol Life Sci. 2008;65(23):3756–88. doi: 10.1007/s00018-008-8281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100(2):209–19. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- Vestweber D, Kemler R. Identification of a putative cell adhesion domain of uvomorulin. EMBO J. 1985;4(13A):3393–8. doi: 10.1002/j.1460-2075.1985.tb04095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volberg T, Geiger B, Kartenbeck J, Franke WW. Changes in membrane-microfilament interaction in intercellular adherens junctions upon removal of extracellular Ca2+ ions. J Cell Biol. 1986;102(5):1832–42. doi: 10.1083/jcb.102.5.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Kanchanawong P, Zaidel-Bar R. Actin-delimited adhesion-independent clustering of E-cadherin forms the nanoscale building blocks of adherens junctions. Dev Cell. 2015;32(2):139–54. doi: 10.1016/j.devcel.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123(5):889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol. 2010;12(6):533–42. doi: 10.1038/ncb2055. [DOI] [PubMed] [Google Scholar]
- Zinchuk V, Wu Y, Grossenbacher-Zinchuk O. Bridging the gap between qualitative and quantitative colocalization results in fluorescence microscopy studies. Sci Rep. 2013;3:1365. doi: 10.1038/srep01365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.