Abstract
Transforming growth factor beta (TGF-β) has become one of the most widely utilized mediators of engineered cartilage growth. It is typically exogenously supplemented in the culture medium in its active form, with the expectation that it will readily transport into tissue constructs through passive diffusion and influence cellular biosynthesis uniformly. The results of this investigation advance three novel concepts regarding the role of TGF-β in cartilage tissue engineering that have important implications for tissue development. First, through the experimental and computational analysis of TGF-β concentration distributions, we demonstrate that, contrary to conventional expectations, media-supplemented exogenous active TGF-β exhibits a pronounced concentration gradient in tissue constructs, resulting from a combination of high-affinity binding interactions and a high cellular internalization rate. These gradients are sustained throughout the entire culture duration, leading to highly heterogeneous tissue growth; biochemical and histological measurements support that while biochemical content is enhanced up to 4-fold at the construct periphery, enhancements are entirely absent beyond 1 mm from the construct surface. Second, construct-encapsulated chondrocytes continuously secrete large amounts of endogenous TGF-β in its latent form, a portion of which undergoes cell-mediated activation and enhances biosynthesis uniformly throughout the tissue. Finally, motivated by these prior insights, we demonstrate that the alternative supplementation of additional exogenous latent TGF-β enhances biosynthesis uniformly throughout tissue constructs, leading to enhanced but homogeneous tissue growth. This novel demonstration suggests that latent TGF-β supplementation may be utilized as an important tool for the translational engineering of large cartilage constructs that will be required to repair the large osteoarthritic defects observed clinically.
Keywords: Transforming growth factor beta, cartilage tissue engineering, biochemical gradients, heterogeneous growth, growth factor delivery, finite element model
Introduction
Cartilage tissue engineering is a promising treatment strategy for osteoarthritis (OA). One approach involves cultivation of functional replacement tissue constructs that can be used to repair cartilage defects. Conventionally, this strategy consists of the in vitro encapsulation of chondrogenic cells (typically mature chondrocytes or mesenchymal progenitor cells) in a polymeric scaffold or other cell assembly, providing them with an environment that supports the synthesis and elaboration of a new extracellular matrix (ECM). As such, the technique aims to recapitulate a functional cartilaginous ECM, capable of supporting physiologic loads and successful functionality upon implantation.
To date, this strategy has exhibited growing success in the generation of small tissue constructs (~∅4 × 2 mm), which develop biochemical content approaching levels seen in native cartilage [1]. However, a major challenge remains in the fabrication of larger-sized, clinically-relevant-sized tissue constructs, which are required to repair OA defects [2]; symptomatic defects are typically 15-25 mm in diameter and can be as great as 5 mm thick. These larger engineered tissues suffer from highly inhomogeneous matrix deposition and, as such, possess inferior mechanical properties that are unable to support physiologic loads [3-5]. It is surmised that this heterogeneous growth generally results from limitations in nutrient supply; nutrients are rapidly consumed at the construct periphery and are unable to effectively reach cells in the interior [6-8]. However, a detailed mechanism identifying all the specific nutrients and/or other metabolic mediators responsible for this phenomenon has yet to be described in the literature.
Transforming growth factor beta (TGF-β) has become one of the most widely utilized mediators for cartilage tissue engineering [1, 9-11] in light of its ability to promote chondrogenesis and strongly enhance the synthesis of an assortment of cartilaginous structural matrix proteins, including proteoglycans, type-II collagen, and cartilage oligomeric matrix protein [12-16]. Typically, TGF-β is exogenously supplemented in culture media in its active form with the expectation that it will readily diffuse deep into constructs and uniformly enhance biosynthesis throughout the tissue. Interestingly, in native tissues, TGF-β is largely confined to its local environment as a result of a combination of binding interactions with ECM constituents, internalization from cell receptors, and degradation from proteases. For example, we have recently demonstrated that, due to the presence of nonspecific binding sites in the ECM of articular cartilage, active TGF-β transporting from synovial fluid into the tissue accumulates exclusively in the topmost 200 μm superficial zone and is unable to penetrate deeper [17]. However, in the early stages of engineered tissue growth, the influence of these interactions on TGF-β transport is assumed to be negligible, as constructs are initially devoid of ECM constituents to which TGF-β can bind and the culture medium is conventionally supplied with supra-physiologic levels of active TGF-β. Potentially, at later stages of tissue development, TGF-β binding may become considerable as large amounts of ECM are deposited in the construct, giving rise to concentration gradients in the tissue. However, the characterization of concentration gradients of exogenous TGF-β in tissue constructs has not been previously characterized in the literature. Thus, the influence of exogenous TGF-β on the heterogeneous growth of engineered cartilage remains unclear. In the first study of this investigation, we demonstrate that, contrary to conventional expectations, media-supplemented exogenous active TGF-β exhibits pronounced and sustained gradients in early stage (freshly-cast) tissue constructs, which result from a combination of binding to the construct scaffold and an unanticipated high rate of internalization by seeded cells. These TGF-β gradients give rise to a highly heterogeneous cartilaginous matrix elaboration and tissue growth, thus highlighting the importance of the development of alternative TGF-β delivery strategies.
An additional important contribution to TGF-β activity in tissue constructs can potentially arise from the action of endogenous TGF-β secreted locally by seeded chondrocytes. TGF-β is synthesized by a large variety of native tissues [18] and can act as an autocrine biosynthetic mediator [19]. Importantly, TGF-β produced by native tissues is secreted exclusively in an inactive complex, termed latent TGF-β, in contrast to the active form that is conventionally supplemented in culture medium. In this latent complex, the 25 kDa mature TGF-β peptide is linked non-covalently to a 70 kDa latency associated peptide (LAP), together forming the small latent complex (SLC). This complex may be disulfide-bonded to a latent TGF-β binding protein (LTBP, ~180 kDa), constituting a configuration termed the large latent complex (LLC). This latent complex is unable to bind to membrane receptors; thus, in order to induce a biological response, the TGF-β peptide must undergo release from this latent complex in a process termed TGF-β activation [20]. This process may be mediated by the action of mechanical forces [21-24] or cell-secreted enzymes acting on the latent molecule [25]. In native cartilage, large stores of latent TGF-β are present throughout the tissue depth (~300 ng/mL [26]), and are believed to act as an important autocrine regulator of chondrocytes that are beyond the reach of TGF-β from synovial fluid [17]. In tissue constructs, the activity of endogenous TGF-β may be similarly critical for biosynthesis. However, while isolated chondrocytes have been reported to secrete TGF-β [19, 27, 28], the level synthesized by tissue constructs or their ability to influence tissue biosynthesis has yet to be described in the literature. In the second study of this investigation, we demonstrate that seeded chondrocytes in tissue constructs secrete large amounts of latent TGF-β, which subsequently undergo cellular mediated activation and act as a strong autocrine mediator of tissue biosynthesis.
In consideration of the important role of latent TGF-β in native tissue biosynthesis, it is intriguing that exogenous TGF-β in tissue engineering is exclusively supplemented in its active form. Interestingly, TGF-β configured in its small latent complex form exhibits properties that can potentially improve its transport into tissue constructs, such as a low binding affinity for cartilaginous matrix constituents [17] and a delayed cellular internalization (internalization can only occur after the latent complex has been activated [20]). These properties, along with the understanding that chondrocytes have the capacity to activate their endogenous latent TGF-β (demonstrated in study 2), gives rise to the possibility that chondrocytes would additionally activate exogenously supplied latent TGF-β and that this activation may occur subsequent to its transport deep into tissue constructs. As such, this occurrence could potentially improve the uniformity of TGF-β activity in tissue constructs. Therefore, we hypothesize that the alternative supplementation of exogenous latent TGF-β in media will enhance biosynthesis uniformly throughout tissue constructs, leading to enhanced but homogeneous tissue growth.
Overall, this investigation attempts to perform several novel characterizations regarding the influence of TGF-β on the heterogeneities of engineered cartilage growth: 1) Measure the distribution of media-supplemented exogenous active TGF-β in tissue constructs and its influence on the heterogeneities of tissue growth, 2) Characterize the secretion rate of endogenous latent TGF-β in constructs and examine its autocrine influence on tissue growth, and 3) Examine the influence of exogenously supplemented latent TGF-β on construct biosynthesis and assess its ability to reduce heterogeneities of tissue growth.
The characterizations of this investigation are performed on constructs seeded with bovine chondrocytes, which represent a well-established experimental model for cartilage tissue engineering [1, 29-31] and serve as an important foundation for the use of autologous primary chondrocytes for the development of replacement tissues. Further, in order to better understand the relevance of these characterizations for other cell-based systems, the distribution of exogenous active TGF-β is additionally measured in constructs seeded with mesenchymal stem cells (MSCs). MSCs serve as a highly promising option for cartilage tissue engineering due to their abundant availability. Their use in tissue engineering applications has expanded considerably in recent years as a result of significant advances in differentiation induction strategies [11, 32]. Recent studies have shown MSC-seeded constructs to be highly responsive to exogenous TGF-β.
To examine the influence of exogenous active TGF-β supplementation on the heterogeneous growth of engineered cartilage, we first measured its distribution in tissue constructs using an experimental 1D culture system (Fig 1; Study 1A). This analysis was performed on chondrocyte-seeded constructs in varying developmental stages (freshly-cast or mature) and freshly-cast MSC-seeded constructs. Next, a finite element model was implemented in order to interpret these experimental results and theoretically assess the contribution of extracellular binding, cellular internalization, and protease-mediated degradation on TGF-β gradients in the tissue (Study 1B). Lastly, given TGF-β’s strong anabolic influence on cellular biosynthetic activity, exogenous gradients are likely to induce heterogeneous ECM deposition in constructs. In order to directly quantify this influence, our 1D culture system was further implemented to measure the distribution of ECM constituents in tissue constructs after long-term TGF-β exposure (Study 1C). In this study, we further assessed the ability of raised levels of active TGF-β to overcome extracellular interactions and enhance biosynthesis in deeper tissue regions.
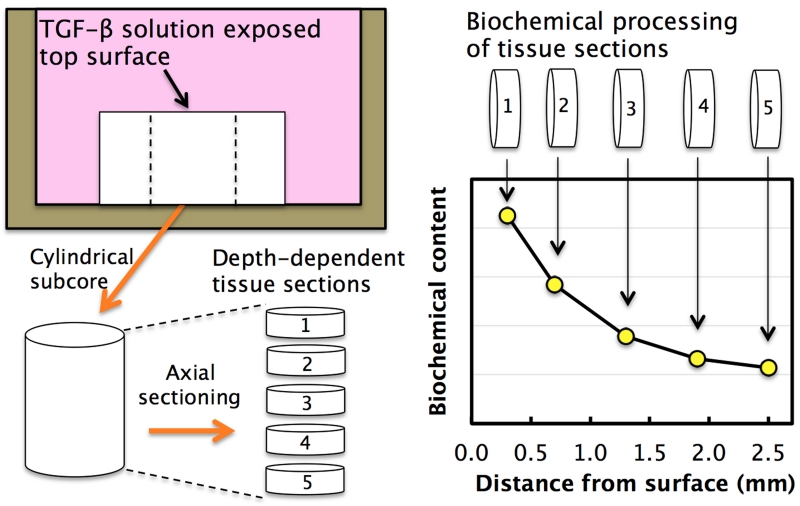
Figure 1.
Schematic of experimental procedure to analyze the 1D spatial distribution of exogenous active TGF-β and long-term biochemical ECM constituents in ∅6 × 3.2 mm tissue constructs. A ∅3 mm axial cylindrical subcore was extracted from the center of each construct and then transversely cut into thin depth-dependent tissue sections. Each section was subsequently processed for its content of active TGF-β (Study 1A) or ECM constituents (GAG, collagen, DNA; Studies 1C & 3), yielding the concentration at discrete positions as a function of distance from the media exposed construct top surface. This 1D analysis assumes a negligible contribution of TGF-β transporting into the 3mm axial core from the exposed lateral surface of the original ∅6 mm construct during the experiment, as confirmed later by experimental results (Figs 3 & 5).
In order to examine the autocrine role of endogenous TGF-β in tissue constructs, we first measured the rate at which latent TGF-β1 (the dominant isoform for chondrocytes [26]) is synthesized and incorporated into the matrix of tissue constructs (Study 2A). Based on TGF-β’s well-documented autoinduction response [17, 28, 33], TGF-β1 synthesis was measured in the presence of exogenous active TGF-β3 (supplemented transiently or continuously). Next, we attempted to directly characterize the biosynthetic effect of the secreted endogenous TGF-β in tissue constructs by examining ECM synthesis in the presence of a TGF-β specific inhibitor (Study 2B).
In order to test the hypothesis that exogenous latent TGF-β supplementation can promote uniform biosynthetic enhancements in constructs, our 1D culture system was similarly implemented to measure the distribution of ECM constituents in constructs in response to long-term latent TGF-β exposure (Study 3).
Methods
Tissue Constructs
Immature primary articular chondrocytes were isolated from bovine calf carpometocarpal joints and seeded in 2% type VII agarose at nominal densities of (30 or 60) × 106 cells/mL, as described previously [30]. Human primary MSCs (Lonza) were passaged twice while cultured in DMEM supplemented with 10% FBS, 1ng/mL TGF-β1, 10 ng/mL PDGF-ββ, and 5 ng/mL bFGF-2, as described previously [34] and seeded in agarose at 60 × 106 cells/mL. All experiments utilized a chondrogenic media formulation [1], consisting of high glucose DMEM supplemented with 100 nM dexamethasone, 100 μg/mL sodium pyruvate, 50 μg/mL L-proline, 1% ITS+ premix (6.25 μg/mL human recombinant insulin, 6.25 μg/mL human holotransferrin, 6.25 ng/mL selenous acid), 1% PS/AM antibiotic-antimycotic, and 173 μM ascorbic acid 2-phosphate. Unless otherwise noted, constructs were maintained at a 20:1 media to tissue volume ratio and media was changed thrice weekly. For all experiments, exogenously supplemented active TGF-β1, active TGF-β3, and latent TGF-β1 (100 kDa small latent complex form) were human recombinant (R&D Systems). While TGF-β3 was used for long-term tissue culture (Studies 1C, 2A, & 3), TGF-β1 was used for exogenous distribution analyses (Study 1A & 1B). Consistent with prior documentations [35], we have concluded that both isoforms exhibit similar biosynthetic effects on tissue constructs (Supplementary Data). For long-term culture studies, TGF-β was applied either transiently (for only the first two weeks) or continuously (for the entire culture duration). The term ‘freshly-cast tissue constructs’ refers to samples cast at 60×106 cells/mL that were used in experiments after three days of TGF-β-free culture, while the term ‘mature tissue constructs’ refers to samples that were used after 28 days of culture with continuous 10 ng/mL TGF-β3 supplementation. All cultures were performed at 37°C on an orbital shaker to avoid the formation of unstirred TGF-β boundary layers, which could potentially confound our transport analyses.
TGF-β measurements
Concentrations of exogenous and endogenous TGF-β1 in media and tissue constructs were determined through the use of a TGF-β1 ELISA kit (Duoset, R&D Systems). This assay specifically detects the TGF-β1 isoform (human and bovine) but only recognizes it in the active form. Therefore, to assess the endogenous TGF-β secretion rate (study 2A), conditioned media samples were assayed before and after an acid activation treatment, allowing for independent measures of their active and total (active + latent) TGF-β1 content. Active and latent TGF-β1 contents of tissue constructs (Studies 1A, 1B, & 2A) were extracted through the use of a previously validated protocol [36]. Briefly, active TGF-β (bound and soluble) was extracted with exposure to a Tris/CHAPS buffer solution (50mM Tris-HCl, 0.5% CHAPS, 150mM NaCl at pH 7.2) at 4°C overnight. Total TGF-β (active + latent) was extracted with 4M guanidine-HCl solution, which acts by activating and extracting all latent TGF-β in the tissue. All cultures were performed in non-treated polystyrene well plates and samples were collected in silanized polypropylene tubes, both to which active TGF-β exhibits negligible binding [36].
Study 1: Influence of exogenous active TGF-β on heterogeneous construct growth
Study 1A: Exogenous active TGF-β distribution in tissue constructs: Experimental analysis
Tissue constructs (∅6 × 3.2 mm seeded at 60 × 106 cells/mL) were exposed to 10 ng/mL of exogenous active TGF-β1 for 72 hours while maintained under free swelling conditions in media (4 mL per sample). Constructs were maintained on an orbital shaker with media replenished once (at 48 hours) over this period. At the end of this exposure period, samples were analyzed as described in Fig. 1, and ∅3 mm sub-cores were transversely cut into ~300 μm sections, yielding the concentration of exogenous active TGF-β1 at discrete positions as a function of distance from the media-exposed construct top surface. This experiment was performed with four different tissue types: 1) freshly-cast MSC-seeded constructs, 2) freshly-cast chondrocyte-seeded constructs, 3) mature chondrocyte-seeded constructs, and 4) acellular agarose scaffolds as a control (n=6 per group).
It is important to note that the TGF-β1 ELISA detects both the exogenous human and endogenous bovine active TGF-β1 in constructs. Therefore, a set of control samples (n=4 per group) was exposed to active exogenous TGF-β3, which is not detected by the assay (TGF-β3 exhibits less than 1% cross reactivity with TGF-β1 Duoset ELISA) to independently assess the distribution of endogenous active TGF-β1 in constructs.
Study 1B: Exogenous active TGF-β distribution in tissue constructs: Theoretical analysis
A finite-element (computational) simulation was performed using the open-source finite element program FEBio (www.febio.org) to theoretically predict the distribution of exogenous active TGF-β in freshly-cast and mature constructs under the conditions implemented in Study 1A. This simulation accounted for the mass transport of soluble TGF-β governed by:
| (1) |
where CF is the solute TGF-β concentration, D is the soluble diffusivity, and is the molar supply rate of TGF-β due to chemical reactions. This molar supply consists both of the reversible binding kinetics of TGF-β with the tissue matrix [17, 37] and TGF-β internalization [6] according to the relations of Eqns. 2 and 3:
| (2) |
| (3) |
where CB is the concentration of bound active TGF-β, Nt is the total concentration of binding sites in the tissue, kf and kr are the respective forward and reverse binding reaction rates and RTGFβ is the TGF-β internalization rate constant. The model required knowledge of the transport, binding, and cellular internalization properties of active TGF-β in the tissue, i.e. the diffusivity, D; partition coefficient, κ; the concentration of binding sites, Nt; the dissociation constant, KD; and the active TGF-β internalization rate constant, RTGFβ. For acellular agarose and freshly-cast constructs, which are predominantly devoid of ECM, κ was estimated at a value of unity based on a prior characterization for similar sized molecules in 2% agarose [38]. For mature tissue constructs, which possess GAG content similar to native cartilage, a κ value of unity was also used, based on a prior characterization for macromolecules that possess TGF-β’s molecular weight and similar positive charge in articular cartilage [39]. The diffusivity of TGF-β in mature tissue constructs was estimated at a value of 6 μm2/s, from the interpolation of similarly sized proteins and polysaccharides in the tissue [40]. The experimental and theoretical procedures implemented to measure the remaining parameters in acellular agarose, freshly-cast constructs, and mature constructs are described in the following experiments below.
Simulations using these independently measured parameters were compared to the experimental results of Study 1A. Further, to assess the relative impacts of binding and internalization on the uptake response, additional simulations were performed in the absence of internalization (RTGFβ = 0) and in the absence of both internalization and binding (RTGFβ = 0 & Nt = 0).
Parameter characterization: Binding site density and dissociation constant
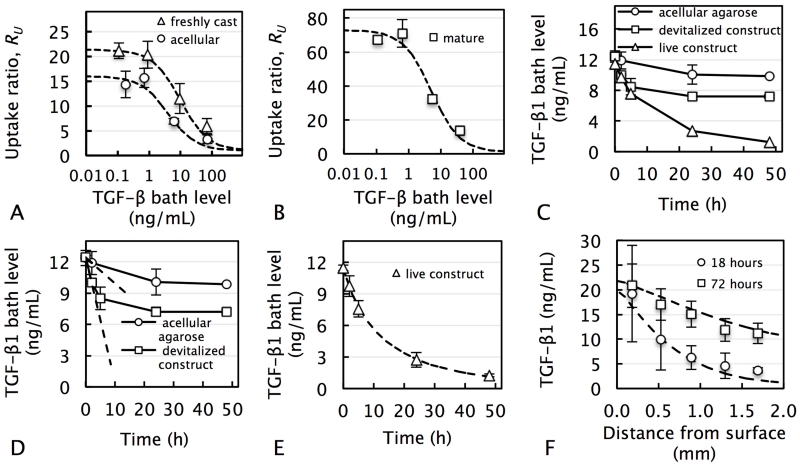
The following experiment was adopted from our previous work [17] in order to measure Nt and KD in acellular agarose, freshly-cast tissue constructs, and mature tissue constructs. Here, small samples (∅ 3 × 1 mm) of each type were devitalized through a freeze-thaw cycle and individually exposed to a bath of exogenous active TGF-β1 over a wide concentration (CBath) range (0.1, 1, 10, and 100 ng/mL) for 24 h (n=5 per concentration). At the end of testing, the exogenous active TGF-β1 concentration of each sample (CB + CF) and its corresponding bath solution (CBath) was measured, giving rise to the normalized uptake ratio RU (Figs 2.A & 2.B). The data were fit to Eq. 4, as described previously [17], yielding the quantities Nt and KD for each sample type.
Figure 2.
Measurement of binding, transport and internalization parameters of active TGF-β in tissue constructs. Normalized equilibrium uptake ratio, RU, of exogenous active TGF-β1 versus applied bath level in (A) acellular agarose and freshly-cast tissue constructs, and (B) mature tissue constructs. Dashed curve represents theoretical fit of Eq. 4 allowing for the measurement of Nt and KD. (C) Representative experimental transient decrease of exogenous active TGF-β1 concentration in bath solution due to either binding and internalization (for live construct) or binding only (for acellular agarose and devitalized tissue construct). (D) Dashed lines represent initial slopes, dCBath / dt, which were used to determine kf and kr according to Eq. 5. (E) Dashed line represents theoretical fit of finite element model to the data, allowing for the measure of the TGF-β internalization rate constant, RTGFβ. (F) Distribution of exogenous active TGF-β1 in acellular agarose after 18 and 72 h. Dashed curves represent theoretical curve-fits (R2=0.61) used to extract TGF-β diffusivity value.
| (4) |
Parameter characterization: Binding reaction rate constants
The following experiment was adopted [17] in order to measure kf and kr. Here, small samples (∅ 3 × 1 mm) from five groups (acellular agarose, live or devitalized freshly-cast tissue constructs, and live or devitalized mature tissue constructs) were individually exposed to an 80 μL bath of 10 ng/mL exogenous active TGF-β1. Initially and after 2, 6, 24, or 48 hours of exposure, the concentration of exogenous active free TGF-β1 in the bath was measured (Fig 2.C). For acellular agarose and devitalized samples, the initial rate of change of TGF-β1, dCBath / dt, was determined (Fig 2.D) and used to calculate the quantity Ntkf through Eq. 5, as described previously [17]:
| (5) |
Subsequently, this quantity was used to calculate kf and kr using the values for Nt and KD obtained from the prior experiment.
Parameter characterization: Internalization rate constant
To measure the internalization rate RTGFβ of active TGF-β, the concentration decrease with live constructs was curve-fitted to a finite element model (FEBio), which incorporated the experimental geometries, TGF-β binding (using the previously acquired parameters; Table 1) and TGF-β internalization (Fig 2.E). The TGF-β internalization rate, dCF / dt, was modeled according the relation of Eq. 3.
Table 1.
Reversible binding, transport, and internalization parameters of active TGF-β1 in acellular agarose, freshly-cast, and mature (28-day cultured) chondrocyte-seeded tissue constructs.
| Binding site density, Nt [ng/mL] |
Dissociation constant, KD [ng/mL] |
Forward rate constant, kf [× 102 s.ng/mL−1] |
Reverse rate constant, kr [× 103 s−1] |
Partition coefficient κ |
Diffusivity, D [μm2/s] |
Internalization rate, RTGFβ [× 104 s−1] |
|
|---|---|---|---|---|---|---|---|
| Acellular agarose | 60 | 15 | 1.8 | 2.7 | 1.0 | 23 | NA |
| Fresh construct | 200 | 10 | 2.7 | 2.5 | 1.0 | 23 | 4.5 |
| Mature construct | 370 | 5.1 | 1.6 | 8.3 | 1.0 | 6.8 | 3.0 |
Parameter characterization: Diffusivity
The following experiment was performed to measure the diffusivity, D, of TGF-β in acellular agarose, which was then used as an estimate of D in freshly-cast tissue constructs. Here, acellular agarose disks (∅6 × 3.2 mm) were subjected to the experimental conditions and analysis of Study 1A for two separate time points (18 and 72 hours), yielding the exogenous concentration at discrete positions as a function of distance from the TGF-β-exposed top surface. This experimental data was curve-fitted with a finite element model (FEBio), while accounting for TGF-β reversible binding kinetics (parameters in Table 1), in order to extract the TGF-β diffusivity (Fig 2.F).
Study 1C: Biochemical gradients in response to exogenous active TGF-β supplementation
Chondrocyte-seeded constructs (∅6 × 3.2 mm; 30 × 106 cells/mL) were individually cultured under free swelling conditions in a 24-well plate. Constructs were exposed to TGF-β3 (transient supplementation) at either standard levels (10 ng/mL), enhanced levels (50 ng/mL), or maintained TGF-β free for the entire culture period (n=6 per group). After 56 days of culture, the 1D analytical technique (Fig 1) was applied and ∅3 mm sub-cores were transversely cut into ~800 μm sections. Each section was subsequently processed for its glycosaminoglycan (GAG), collagen, and DNA contents, as described previously [1], yielding the concentration of each constituent as a function of distance from the media-exposed construct top surface. This 1D analysis assumed a negligible contribution to biosynthesis from TGF-β3 transporting into the 3 mm axial core from the exposed lateral surface of the full ∅6 mm construct, as subsequently confirmed by results. Further, samples from each group were subjected to the Live/Dead cytotoxicity assay (Invitrogen) and imaged via a confocal microscope (Leica) to assess cell viability and distribution.
Study 2: Autocrine role of endogenous TGF-β on engineered cartilage growth
Study 2A: Synthesis and extracellular deposition of endogenous TGF-β
Chondrocyte-seeded constructs (∅4 × 2.3 mm; 30 × 106 cells/mL) were cultured with either transient or continuous TGF-β3 supplementation (10 ng/mL) or maintained TGF-β free. At each media change, fresh and conditioned media was collected from all groups and analyzed for its endogenous active and total (active + latent) TGF-β1 content. After 7, 14, 28, and 45 days of culture, constructs (n=4 per group) were diametrically halved, with each half used to analyze either active or total (active + latent) levels of endogenous TGF-β1.
Study 2B: Autocrine role of endogenous TGF-β on matrix synthesis
Chondrocyte-seeded constructs (∅3 × 1mm; 30 × 106 cells/mL) were exposed to increasing concentrations of a highly specific TGF-β inhibitor, soluble TGF-β type III receptor (solRIII, R&D Systems). This soluble inhibitor acts as a scavenger by binding to activated TGF-β in the tissue, thereby preventing TGF-β-cell receptor interactions [41]. Constructs were cultured for two weeks in the absence of exogenous TGF-β and in the presence of solRIII at either 0, 1.5, or 10 μg/mL (n=10 constructs per group). Upon completion of culture, constructs were analyzed for their GAG and collagen contents.
Study 3: Biochemical gradients in response to exogenous latent TGF-β
A new batch of chondrocyte-seeded constructs (∅6 × 3.2mm; 60 × 106 cells/mL) was cultured under identical conditions as in Study 1C while exposed to either the transient supplementation of latent TGF-β1 (180 ng/mL on the basis of the total latent TGF-β protein mass; 43 ng/mL on the basis of the active peptide mass), the transient supplementation of active TGF-β3 (10 ng/mL), or maintained TGF-β free (n=8 constructs per group). Latent TGF-β was supplemented at levels that exceed those conventionally used for active TGF-β in order to compensate for its expected low activation rate by cells [42]. After 56 days of culture, the 1D analytical technique (Fig 1) was applied, yielding the concentration of GAG and collagen as a function of distance from the media-exposed construct top surface. Further, samples from each group were fixed, paraffin-embedded, and sectioned as described previously [3]. Sections were stained for GAG (0.01% Safranin-O, 0.1% Fast Green and hematoxylin), collagen (0.1% Picrosirius Red), or with hematoxylin and eosin (H&E).
Lastly, a recently developed finite element model [43] was implemented to determine the depth dependent distribution and whole-construct Young’s moduli of the ∅ 3mm central subcores (Fig. 1) extracted from active TGF-β, latent TGF-β, and TGF-β-free constructs. The model accounted for the proteoglycan-induced Donnan osmotic swelling pressure and utilized a material collagen fiber model based on the experimentally determined depth dependent biochemical content of samples, as described in Supplementary Data.
Statistical analyses
Two-way ANOVAs (α=0.05) were performed to determine the effects of exogenous TGF-β exposure conditions and distance from the media-exposed construct surface on either construct TGF-β levels (Study 1A), biochemical content (Studies 1C & 3), or endogenous latent TGF-β levels (Study 2A). A one-way ANOVA was performed to determine the effect of endogenous TGF-β inhibition on GAG and collagen synthesis (Study 2B).
Results
Study 1: Influence of exogenous active TGF-β on heterogeneous construct growth
Study 1A: Exogenous active TGF-β distribution in tissue constructs – Experimental analysis
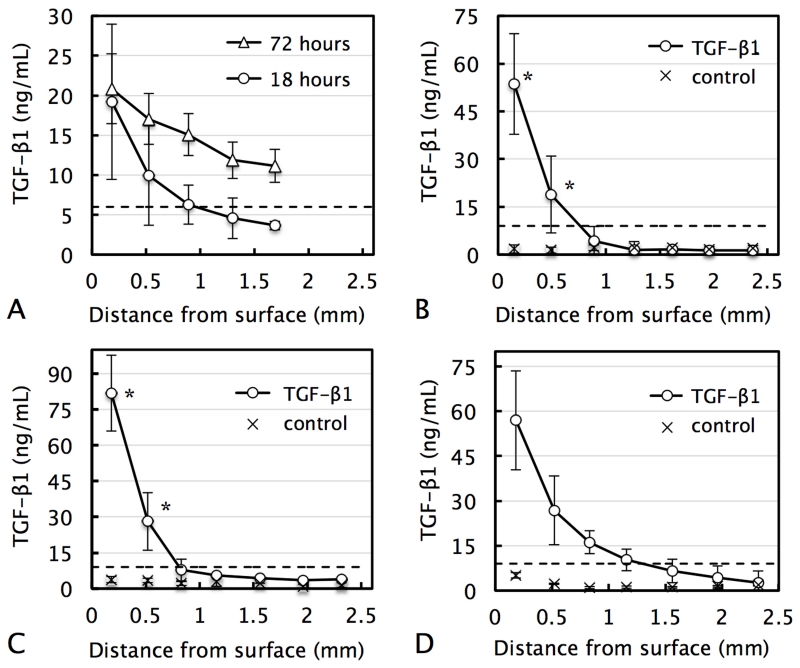
In acellular agarose gels, media-supplemented exogenous active TGF-β1 exhibited binding to the sample (manifested by 3-fold concentration increase above bath level at surface), but was still able to penetrate deep into these scaffolds after 72 h of exposure (Fig 3.A). Control chondrocyte-seeded freshly-cast tissue constructs exhibited low levels of endogenous active TGF-β1 (mean value= 1.9±1.0 ng/mL) at all positions throughout their depth (Fig 3.B control). In contrast to acellular agarose, after 72 h, the exogenous active TGF-β1 concentration in tissue constructs exhibited a sharp gradient: levels near the solution-exposed surface (150 μm deep) increased significantly, reaching a value of 53.6±15.8 ng/mL (~6-fold rise above the bath concentration), while no increase above endogenous control levels were observed below 490 mm deep into the construct (Fig 3.B). Mature constructs possessed a substantially higher content of GAG than freshly-cast constructs (3.2 ± 0.5% versus 0.11 ± 0.01 % GAG per wet weight). Exogenous active TGF-β1 uptake by mature constructs exhibited a similar but more pronounced gradient with a concentration of 81.7 ± 29.2 ng/mL at the surface and no increase above endogenous control levels below 520 μm into the construct (Fig 3.C). Uptake in MSC-seeded constructs exhibited a more modest gradient; active TGF-β1 was present at deeper regions of the tissue but at only a fraction of the peripheral levels (Fig 3.D).
Figure 3.
Distribution of media-supplemented exogenous active TGF-β1 as a function of distance from the media-exposed construct surface in (A) acellular agarose after 18 and 72 h, (B) freshly-cast chondrocyte-seeded constructs after 72 h, (C) mature (28-day cultured) chondrocyte-seeded constructs after 72 h, and (D) freshly-cast MSC-seeded constructs after 72 h. Control levels represent endogenous active TGF-β1 levels in constructs. Dashed line represents active TGF-β1 media bath concentration. *p<0.05 represents significant increase above corresponding control value.
Study 1B: Exogenous active TGF-β distribution in tissue constructs – Theoretical analysis
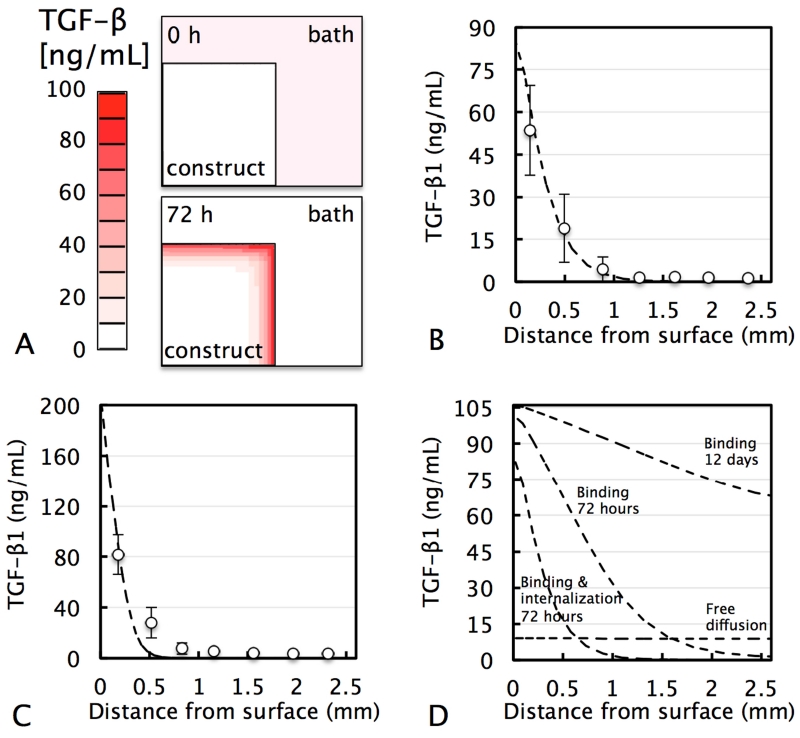
Experimental measurements indicated that active TGF-β undergoes binding and internalization in freshly-cast tissue constructs (Table 1). The theoretical prediction of the distribution of exogenous active TGF-β in freshly-cast tissue constructs, while accounting for these experimentally-determined TGF-β binding and internalization rates, exhibited a strong agreement with experimental data (R2=0.87; Fig 4.B). In mature tissue constructs, active TGF-β exhibited an increased binding site density and decreased dissociation constant and internalization rate (Table 1). Based on these measured parameters, this theoretical prediction exhibited a similar strong agreement with experimental data (Fig 4.C; R2=0.74), further validating the model.
Figure 4.
(A) FE model of cross-sectional distribution of media-supplemented exogenous active TGF-β1 in ∅6 × 3.2 mm tissue construct (half construct depicted) after 72 h. Experimental active TGF-β1 distribution as a function of distance from the media-exposed construct surface in (B) freshly-cast and (C) mature chondrocyte-seeded constructs and theoretical simulation (dashed curve) based on experimentally measured TGF-β1 binding, internalization, and transport properties summarized in Table 1. (D) Hypothetical theoretical simulations for penetration depth in freshly-cast constructs for free diffusion (absence of binding and internalization) after 72 h, or binding alone (absence of internalization) after 72 h and 12 days. In the presence of binding and internalization, theoretical curves after 72 h and 12 days are nearly identical (R2=0.99; 12 day curve not shown).
To develop a further understanding of the contribution of each of these mechanisms, an additional set of hypothetical simulations was performed (Fig 4.D). In the hypothetical absence of both binding and internalization interactions, active TGF-β1 distribution reached steady state after only 72 h, at a value equal to the bath concentration (10 ng/mL). As a result of binding interactions, active TGF-β accumulated at high levels at the construct periphery and exhibited delayed penetration deeper into the tissue. For the hypothetical case where binding interactions are present and cellular internalization absent, active TGF-β eventually transported further into the tissue, nearing steady state at a concentration ~10-fold above the bath level after a prolonged 12 day exposure period. Internalization inhibited TGF-β penetration even further. In fact, in the presence of both binding and internalization (at experimentally measured rates), TGF-β exhibited no further penetration into the tissue between 72 h and 12 day exposure time points.
Study 1C: Biochemical gradients in response to exogenous active TGF-β supplementation
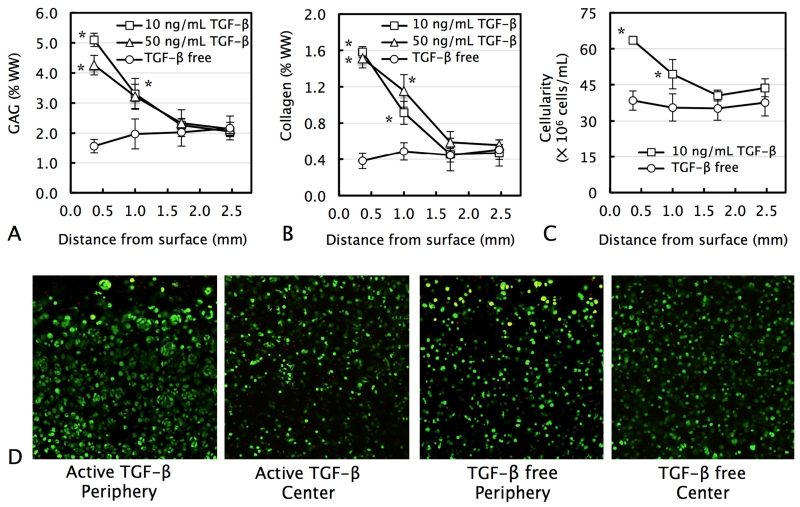
Tissue constructs grown for 56 days in TGF-β-free medium exhibited near-uniform biochemical content (GAG and collagen) and cellularity throughout their depth (Figs 5.A, 5.B, & 5.C). The media supplementation of active TGF-β3 at either standard (10 ng/mL) or enhanced (50 ng/mL) levels led to an increase in construct biochemical content, in a highly heterogeneous fashion: while the concentration of biochemical constituents (GAG, collagen, and DNA) increased up to 4-fold above TGF-β-free levels near the construct periphery (0.35 mm deep), at regions deeper in the tissue (1.7 and 2.5 mm deep) levels of all constituents remained statistically not different from TGF-β-free levels (p>0.05).
Figure 5.
Distribution of (A) GAG, (B) collagen, and (C) cell content as a function of distance from the media-exposed construct surface in chondrocyte-seeded ∅6 × 3.2 mm tissue constructs cultured for 56 days with the transient supplementation of 10 ng/mL or 50 ng/mL exogenous active TGF-β3 or maintained TGF-β free. *p<0.05 represents significant increase above corresponding TGF-β-free levels. (D) Live/Dead viability imaging of periphery or central region of constructs.
Cell viability imaging revealed that TGF-β3 supplementation induced the formation of dense cell clusters at the periphery of constructs (< 0.5 mm from the media-exposed surface; Fig 5.D). In contrast, in the central region of TGF-ß-supplemented constructs and in all regions of TGF-ß-free constructs, cells appeared predominantly isolated.
Study 2: Influence of endogenous TGF-β on construct growth
Study 2A: Synthesis and extracellular deposition of endogenous TGF-β
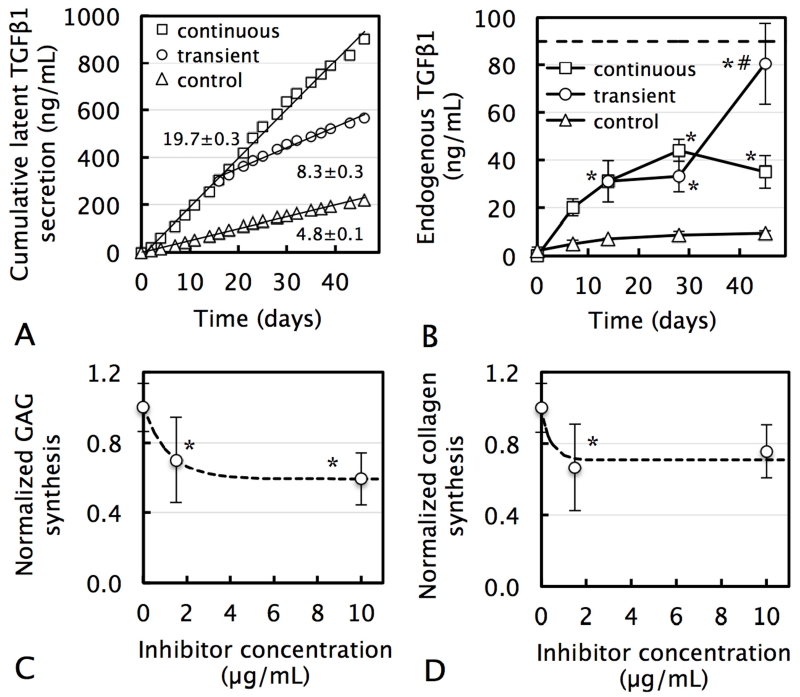
For all groups, tissue constructs secreted large amounts of TGF-β1 into their surrounding media at constant rates over a 45-day culture period (Fig 6.A). Exogenous active TGF-β3 supplementation increased this rate 4-fold; upon cessation of TGF-β supplementation after 14 days, the secretion rate immediately decreased by 60%, consistent with TGF-β’s well-documented autoinduction response. Active TGF-β1 was not detected in conditioned media, indicating that all secreted TGF-β1 was in its latent form, as observed in native tissue [17, 26]. Levels of TGF-β1 incorporated in the construct ECM increased with time for all TGF-β supplementation groups (Fig 6.B; p<0.001) and were significantly higher with continuous TGF-β3 supplementation over no-supplementation controls at all time points (p<0.01). After 45 days of culture, the concentrations in transient supplemented constructs increased significantly above those subjected to continuous supplementation (p<0.001), and reached levels seen in the native tissue. For all groups, only low levels of active TGF-β1 were detected (0.3 ± 0.2 ng/mL), indicating that measured TGF-β1 in the ECM was also predominantly in the latent form. These latent levels present in constructs were only a fraction of the cumulative mass released into media (control: 4%, continuous: 4%, transient: 14% at day 45), indicating that only a fraction of cell-secreted TGF-β1 gets incorporated into the tissue ECM.
Figure 6.
(A) Cumulative secretion of endogenous latent TGF-β1 by chondrocyte-seeded constructs into their surrounding culture medium in response to continuous TGF-β3 supplementation (10 ng/mL for the entire culture duration), transient supplementation (10 ng/mL for only the first two weeks), or in TGF-β-free (control) culture. Slope represents latent TGF-β1 secretion rate (ng/mL per day; mean ± standard estimate). (B) Incorporation of endogenous latent TGF-β1 into the ECM of tissue constructs. Dashed line represents native levels of latent TGF-β1 [36]. All concentrations are normalized to day 0 construct volume. For all groups, active TGF-β1 was not detected in conditioned media or tissue constructs. *p<0.05 represents significant increase above corresponding control value. #p<0.05 represents significant increase above corresponding continuous TGF-β value. Decrease of (C) GAG and (D) collagen synthesis by chondrocyte-seeded tissue constructs in response to endogenous TGF-β inhibition through the action of the highly specific solRIII inhibitor. Dashed curves represent exponential curve-fits to experimental data. *p<0.05 represents significant decrease below 0 μg/mL value.
Study 2B: Autocrine role of endogenous TGF-β on matrix synthesis
In the absence of exogenous TGF-β supplementation, the administration of the TGF-β-specific inhibitor (solRIII) induced a dose-dependent exponential decrease in biosynthesis. At 10 μg/mL, this response plateaued, reaching respective 40% and 24% depressions of GAG and collagen deposition levels (Figs 6.A & 6.B).
Study 3: Biochemical gradients in response to exogenous latent TGF-β
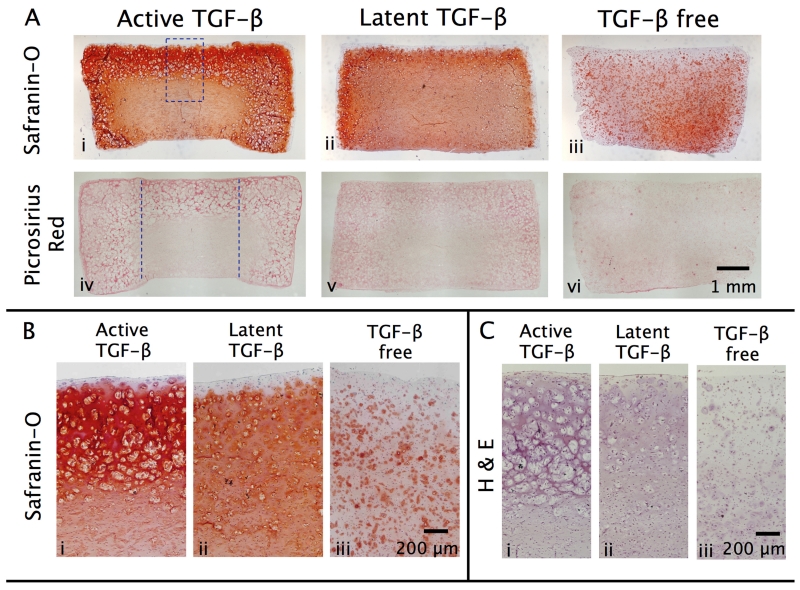
In response to the supplementation of exogenous active TGF-β3, tissue constructs exhibited a highly heterogeneous distribution of GAG and collagen, consistent with results from Study 1C. At the construct periphery, levels of GAG and collagen increased 5-fold and 4-fold above TGF-β-free levels, respectively, but no statistical increase was observed in deeper regions. Histology showed intense staining for GAG (Fig 8.A.i) and collagen (Fig 8.A.iv) at the tissue periphery, but far less staining in the tissue interior. Furthermore, consistent with Study 1C, active TGF-β induced a highly heterogeneous distribution of cells. In the construct periphery, H&E staining showed dense clusters of cells on the order of ∅100-200 μm in size (Fig 8.C.i). Each of these clusters possessed multiple nuclei, indicating that several cells are present. These regions were devoid of extracellular matrix (GAG [Figs 8.A.i & 8.B.i] and collagen [Fig 8.A.iv]). In the tissue central region, cells appeared predominantly isolated and disperse, consistent with cells in TGF-β-free constructs.
Figure 8.
Representative histological staining of chondrocyte-seeded ∅6 × 3.2 mm tissue constructs cultured for 56 days with the transient supplementation of exogenous active TGF-β3 (10 ng/mL), exogenous latent TGF-β1 (43 ng/mL), or maintained TGF-β free. (A) Safranin-O and Picrosirius Red staining for GAG and collagen distribution in transverse cross-sections of full constructs. High magnification images of (B) Safranin-O and (C) hematoxylin and eosin staining of constructs. Dashed rectangle represents approximate region of interest used for high magnification imaging. Dashed parallel lines illustrate subcore boundary used for biochemical analysis implemented in Fig 7.
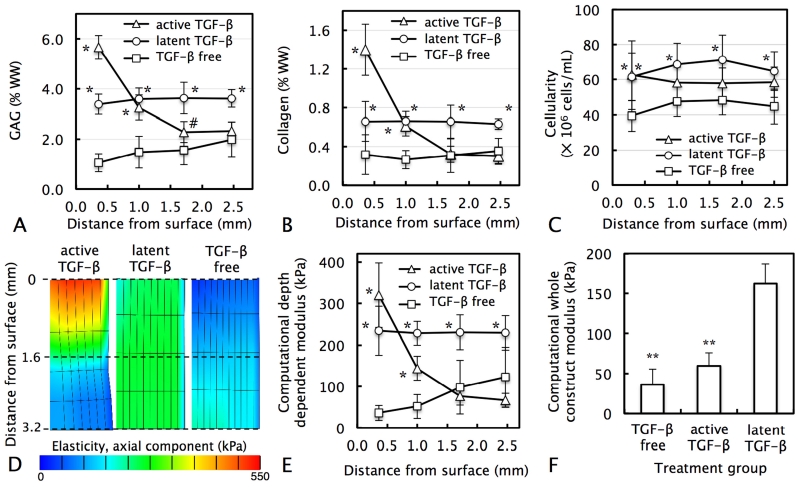
In contrast, in response to latent TGF-β supplementation, tissue constructs exhibited uniform biochemical enhancements in all regions throughout the tissue depth (mean 2.7-fold increase for GAG content and 2.1-fold increase for collagen over control; Figs 7.A & 7.B), yielding tissue constructs with homogeneous biochemical content. These results were consistent with histological images; staining for GAG (Fig 8.A.ii) and collagen (Fig 8.A.v) was far more homogeneous. Further, latent TGF-β supplementation led to a far lower degree of cell clustering; cells appeared predominantly isolated throughout all regions of the tissue. Computational mechanical modeling results demonstrated that TGF-β supplementation increased the whole-construct Young’s modulus of constructs. While active TGF-β increased the modulus by 1.7-fold, latent TGF-ß led to a 4.5-fold increase (Fig 7.F).
Figure 7.
Distribution of (A) GAG, (B) collagen, and (C) cell content as a function of distance from the media-exposed construct surface in chondrocyte-seeded ∅6 × 3.2 mm tissue constructs cultured for 56 days with the transient supplementation of either exogenous active TGF-β3 (10 ng/mL), exogenous latent TGF-β1 (43 ng/mL), or maintained TGF-β free. (D) Distribution of axial elasticity (half construct depicted), (E) depth dependent Young’s moduli, and (F) whole-construct Young’s moduli in ∅3 mm central core (Fig.1) for active TGF-β, latent TGF-β, and TGF-β-free constructs as determined by computational mechanical simulations based on experimentally determined biochemical content. *p<0.001 #p<0.05 represent significant increase above corresponding TGF-β-free value. **p<0.05 represents significant decrease below latent TGF-β value.
Discussion
This investigation advances three novel concepts regarding the role of TGF-β in cartilage tissue engineering that have important implications for cartilage development: 1) media-supplemented exogenous active TGF-β exhibits pronounced and sustained gradients in tissue constructs, leading to highly heterogeneous tissue growth; 2) tissue constructs continuously secrete large amounts of endogenous latent TGF-β that undergo cellular-mediated activation and uniformly enhance tissue biosynthesis; and 3) the alternative supplementation of exogenous latent TGF-β enhances biosynthesis uniformly throughout tissue constructs, leading to enhanced but homogeneous tissue growth.
The pronounced concentration gradients of media-supplemented active TGF-β observed in freshly-cast tissue constructs (Fig 3.B) is a highly unanticipated outcome, given that they are initially devoid of an ECM and are supplied with supraphysiologic levels of active TGF-β. The ability of our theoretical model to faithfully describe the TGF-β uptake response in both freshly-cast and mature tissue constructs (Figs 4.B & 4.C) strengthens our interpretation that these concentration gradients result from a combination of matrix binding interactions and cell-mediated internalization. The mechanistic details responsible for the establishment of these gradients can be understood through the analysis of binding and internalization kinetic measurements (Table 1) and our set of hypothetical theoretical simulations (Fig 4.D). According to binding kinetics measurements, TGF-β binding to tissue constructs results from interactions with the agarose scaffold as well as cellular and matrix constituents, as indicated by the rise in binding site density for agarose after seeding with cells (Nt = 60 ng/mL for acellular agarose versus Nt = 200 ng/mL for cellularized constructs; Table 1). This rise in binding site density is likely attributed to interactions with cellular membrane proteins with a high affinity for TGF-β, such as betaglycan [44]. As a result of these binding interactions, active TGF-β accumulates at high levels at the construct periphery and exhibits delayed penetration deeper into the tissue. These binding interactions are, on their own, insufficient to indefinitely engender TGF-β gradients; our hypothetical simulation demonstrates that in the absence of internalization active TGF-β will reach nearly uniform distribution in the construct after 12 days of exposure. However, in the added presence of cellular internalization, TGF-β concentration gradients become more pronounced and are sustained indefinitely, thus strongly suggesting that the experimentally measured active TGF-β gradients (Fig 4.B) are indeed sustained throughout the entire culture duration. Although it is well documented that TGF-β undergoes degradation following receptor-mediated endocytosis (for a detailed review see [45]), the high internalization rate by construct-seeded chondrocytes is quite striking, as evidenced by the marked >90% reduction in the TGF-β media concentration after only two days of exposure (Fig 2.C). The alternative explanation that this TGF-β loss can be explained by extracellular degradation induced by chondrocyte-secreted proteases has been discounted through the observed stability of TGF-β when exposed to construct conditioned media (Supplementary Data).
Mature constructs exhibit a more pronounced TGF-β concentration gradient (Fig 3.C) that results from an increased active TGF-β binding site density (Table 1) due to their higher ECM content. Interestingly, however, the TGF-β binding site density of these mature constructs remains orders of magnitude below levels measured in native cartilage [17], despite having a GAG content that approaches native values. This observation strongly suggests that it is type-II collagen, rather than proteoglycans, that serves as the dominant binding constituent for active TGF-β in the native cartilage.
In order to assess the impact of active TGF-β gradients on the development of biochemical heterogeneities in tissue constructs, we employed a novel 1D culture system, allowing for the fully quantitative assessment of the distribution of biochemical constituents as a function of distance from the media exposed construct surface (Fig 1). Consistent with experimentally measured gradients of media-supplemented TGF-β, this system explicitly demonstrates that TGF-β enhances the content of GAG, collagen, and DNA solely at the construct periphery; enhancements drop substantially beyond 0.5 mm from the media-exposed construct surface and no enhancements are observed beyond 1.0 mm from the surface (Figs 5 & 7). The presence of these biochemical heterogeneities is further supported by our histological analysis (Fig 8). Further, this highly heterogeneous matrix deposition is similarly observed in response to enhanced levels of exogenous active TGF-β (50 ng/mL; Fig 5), indicating that increased concentrations are unable to saturate the TGF-β internalization process and improve penetration into the tissue.
The supplementation of active TGF-β in media further induced large heterogeneities in cell distribution. This is most strikingly evident through the observed presence of dense clusters of cells in the periphery of active TGF-β supplemented constructs (Figs 5.D & 8.C). These clusters appear to result from rapid proliferation induced by active TGF-β; clusters are not present in the central regions of the tissue, which are devoid of high active TGF-β levels, or in TGF-β-free control samples. Morphologically, these clusters resemble the hypertrophic chondrocytes in the growth plate [46], suggesting that active TGF-β may be inducing a transition of chondrocytes towards a hypertrophic phenotype. In support of this concept, prior work has shown that the intraarticular administration of high doses of active TGF-β in vivo induces the hypertrophy of articular chondrocytes and leads to bone formation in the joint space [47]. Given the supraphysiologic levels of active TGF-β utilized for cartilage tissue engineering, the cell clustering observed in this study appears consistent with prior documentation. The presence of these cell clusters is consistent with prior tissue engineering reports on primary chondrocytes and MSCs [11, 48]. While those studies have speculated that cells are undergoing hypertrophic events, traditional hypertrophic markers (e.g. type I & X collagen) were not observed in tissues, suggesting that these cells may not have fully differentiated. Regardless of the precise differentiation state of these cells, these dense clusters do not resemble the morphology of articular chondrocytes and can be considered a highly undesirable outcome for cartilage tissue engineering. While the administration of lower, physiologic doses of active TGF–β may be considered as a potential alternative to mitigate cell cluster formation, these doses will exhibit even poorer penetration into the tissue, thus further exacerbating biochemical heterogeneities.
The transport analysis presented here strongly suggests that large biochemical and cellular heterogeneities even exist in the small tissue constructs (∅4-5mm) that are routinely utilized in cartilage tissue engineering investigations. These TGF-β gradients likely exist and have important implications for tissue development in a broad range of tissue engineering systems. Based on active TGF-β’s well-characterized ability to undergo high-affinity binding interactions with a wide range of molecules (e.g., proteins, polysaccharides, and synthetic polymers) and internalization by a wide range of cell types [49], sustained gradients are likely to exist in engineering systems for many other scaffold materials (e.g. hydrogels, synthetic polymers, and protein-based scaffolds) and musculoskeletal tissues (e.g. meniscus, intervertebral disk, bone, tendon, and ligament). In this study, gradients are observed in constructs consisting of two distinct and highly relevant cell sources for cartilage tissue engineering: primary articular chondrocytes (Fig 3.B) and MSCs (Fig 3.D). Although the biosynthetic effect of these TGF-β gradients was not directly examined in MSC-seeded constructs, prior work has shown that heterogeneities exist in these tissues as well [50]. Future work will need to determine the contribution of TGF-β to these heterogeneities, as other nutrients could potentially play a greater role for this cell type.
The results of this study have important implications for the development of large tissue constructs, which are needed to repair large cartilage defects (∅15-25 mm) that are characteristic of symptomatic OA. Sustained TGF-β gradients will result in the formation of tissues with pronounced biochemical heterogeneities, which will be less likely to support physiologic loads upon implantation [3-5]. Furthermore, cartilage tissue engineering strategies are evolving towards the use of higher cell densities for construct growth in an attempt to recapitulate the native developmental process, as characteristic of growing fetal and immature cartilage [51]. For example, the fabrication of chondrocyte self-assembled tissue constructs at a density of 350 × 106 cells/mL has been shown to give rise to tissues with native collagen levels [10]. These increased cell densities will likely intensify TGF-β gradients and biochemical heterogeneities in the tissue. These considerations strongly advocate for the development of alternative TGF-β delivery strategies in order to promote high biosynthetic rates while avoiding pronounced heterogeneous growth. It should further be noted that while TGF-β gradients appear to be the dominant contributing factor towards construct heterogeneity, as tissue construct size increases further (e.g. approaching that of an entire articular surface), other nutrients, such as oxygen and glucose, may contribute as well [52]. Interestingly, in the absence of exogenous TGF-ß (Fig. 7A), cells appear to yield increased GAG biosynthesis in regions further from the media exposed construct surface, a trend that may be explained by oxygen gradients as supported by prior work [53].
The second novel concept advanced in this investigation is that endogenous TGF-β plays an important role in construct growth and development. Although it has been previously shown that articular chondrocytes synthesize latent TGF-β, this represents the first study to characterize the levels secreted by tissue constructs and demonstrate its autocrine influence on tissue biosynthesis. Here we show that seeded chondrocytes continuously synthesize and secrete large amounts of latent TGF-β; this latent TGF-β is predominantly released into the culture medium and a smaller fraction (4-14%) gets incorporated into the ECM. This secreted latent TGF-β likely consists of a combination of the small latent complex and large latent complex configurations [54]. As a whole, these endogenous TGF-β secretions promote a strong autocrine biosynthetic response, as evidenced by large enhancements in both GAG (Fig 6.C) and collagen (Fig 6.D) relative to fully inhibited TGF-β conditions. Furthermore, the demonstrated homogeneity of constructs in the absence of exogenous TGF-β (Figs 7.A & 7.B) strongly suggests that these endogenous stores of latent TGF-β enhance GAG and collagen biosynthesis uniformly throughout the tissue. Based on the understanding that latent TGF-β is unable to interact with cell membrane receptors, these enhancements indicate that seeded chondrocytes are promoting the activation of endogenous latent TGF-β. Based on investigations on other tissue systems, it is likely that activation is caused by cell-secreted enzymes, such as serine proteases [25] or matrix metalloproteinases [55, 56]. However, the identification of the precise mechanism implicated here was beyond the scope of the current investigation. It is important to note that our inability to directly observe active TGF-β in constructs or the culture medium is consistent with the high internalization rate of active TGF-β by chondrocytes, as endogenous TGF-β is likely rapidly internalized following activation.
This observed capacity of chondrocytes to activate their endogenous latent TGF-β has served as a key motivation for the hypothesis that additional supplies of latent TGF-β through exogenous supplementation can yield uniform biosynthetic enhancements throughout constructs. Our results have confirmed this hypothesis, demonstrating that, in contrast to exogenous active TGF-β, the supplementation of latent TGF-β gives rise to elevated uniform biochemical enhancements throughout the entire construct (2.5 fold for GAG; 2.1 fold for COL relative to TGF-β-free levels), yielding more homogeneous engineered cartilage; these enhancements reach at least as far as 2.5 mm away from the media exposed construct surface. Because latent TGF-β is unable to undergo cellular internalization until activation, it is presumably undergoing activation (mediated by the activity of seeded chondrocytes) at an optimal rate: sufficiently high to induce biochemical enhancements, but sufficiently low to allow for penetration into constructs. Our recently developed mechanical computational framework confirms that the uniform biochemical enhancements induced by latent TGF-β give rise to the formation of a tissue with highly improved mechanical properties, featuring a 2.2-fold increase in whole-construct Young’s modulus above that achieved via active TGF-β supplementation. This mechanical stability may be critical for the survival of the tissue upon native implantation. Furthermore, the concentrations of latent TGF-β activated by cells are inducing sufficient biosynthetic enhancements while avoiding the production of dense cellular clusters, as seen when TGF-β is supplemented in the active form. As such, latent TGF-β supplementation is potentially capable of generating large homogeneous tissue constructs with native biochemical contents and mechanical properties, while maintaining a healthy morphology of articular chondrocytes. It is important to note that while this technique yields native GAG levels (~4% per tissue wet weight) throughout the tissue, native collagen levels are not achieved, consistent with results from other tissue engineering strategies.
The supplementation of exogenous latent TGF-β may potentially serve as an important alternative to, or may complement existing TGF-β delivery strategies that are currently under investigation. For example, in other studies, we have been investigating a delivery strategy consisting of the introduction of nutrient channels to cartilage tissue constructs, which have the potential to increase exogenous TGF-β penetration [3]. The use of latent TGF-β in concert with this strategy may reduce the required channel density and greatly facilitate this research direction. Another example is the use of TGF-β-releasing scaffolds [57], which allow for a continuous supply of exogenous TGF-β originating uniformly throughout the tissue. While this strategy exhibits large potential, it is suitable for only a subset of scaffold materials. It also introduces the potential of highly adverse side effects resulting from high levels of active TGF-β that continue to undergo release from the tissue following implantation [47]. Alternatively, the media supplementation of exogenous TGF-β is compatible with any scaffold material, and can be easily discontinued at the completion of tissue development, allowing for the avoidance of excessive growth factor administration upon implantation. As such, latent TGF-β supplementation can serve as an invaluable, easily implemented, strategy allowing for the generation of large-sized, clinically relevant functional articular cartilage constructs.
Conclusions
This investigation demonstrates that contrary to conventional expectations, media-supplemented exogenous active TGF-β exhibits pronounced concentration gradients in cartilage tissue constructs. These gradients promote highly heterogeneous tissue growth, characterized by biochemical enhancements occurring solely in the tissue periphery and the presence of undesirable dense cell clustering in this peripheral region. Alternatively, the supplementation of exogenous TGF-β in its native latent form promotes uniform biosynthetic enhancements and mechanical property development throughout constructs while maintaining chondrocytes with an isolated morphology, as characteristic of native cartilage. Together, these findings illustrate an important limitation of using active TGF-β to stimulate engineered cartilage growth and suggest that latent TGF-β can be used as an important tool for the generation of large-sized, clinically relevant functional articular cartilage constructs.
Supplementary Material
Acknowledgements
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute of General Medical Sciences of the National Institutes of Health under Award Numbers AR060361, AR043628, and GM083925. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- [1].Byers BA, Mauck RL, Chiang IE, Tuan RS. Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissue-engineered cartilage. Tissue engineering Part A. 2008;14(11):1821–34. doi: 10.1089/ten.tea.2007.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Moisio K, Eckstein F, Chmiel JS, Guermazi A, Prasad P, Almagor O, et al. Denuded subchondral bone and knee pain in persons with knee osteoarthritis. Arthritis and rheumatism. 2009;60(12):3703–10. doi: 10.1002/art.25014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bian L, Angione SL, Ng KW, Lima EG, Williams DY, Mao DQ, et al. Influence of decreasing nutrient path length on the development of engineered cartilage. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2009;17(5):677–85. doi: 10.1016/j.joca.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hung CT, Lima EG, Mauck RL, Takai E, LeRoux MA, Lu HH, et al. Anatomically shaped osteochondral constructs for articular cartilage repair. Journal of biomechanics. 2003;36(12):1853–64. doi: 10.1016/s0021-9290(03)00213-6. [DOI] [PubMed] [Google Scholar]
- [5].Hung CT, Mauck RL, Wang CC, Lima EG, Ateshian GA. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann Biomed Eng. 2004;32(1):35–49. doi: 10.1023/b:abme.0000007789.99565.42. [DOI] [PubMed] [Google Scholar]
- [6].Nims RJ, Cigan AD, Albro MB, Hung CT, Ateshian GA. Synthesis rates and binding kinetics of matrix products in engineered cartilage constructs using chondrocyte-seeded agarose gels. Journal of biomechanics. 2014;47(9):2165–72. doi: 10.1016/j.jbiomech.2013.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cigan AD, Nims RJ, Albro MB, Esau JD, Dreyer MP, Vunjak-Novakovic G, et al. Insulin, ascorbate, and glucose have a much greater influence than transferrin and selenous acid on the in vitro growth of engineered cartilage in chondrogenic media. Tissue engineering Part A. 2013;19(17-18):1941–8. doi: 10.1089/ten.tea.2012.0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cigan AD, Nims RJ, Albro MB, Vunjak-Novakovic G, Hung CT, Ateshian GA. Nutrient channels and stirring enhanced the composition and stiffness of large cartilage constructs. Journal of biomechanics. 2014;47(16):3847–54. doi: 10.1016/j.jbiomech.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Awad HA, Wickham MQ, Leddy HA, Gimble JM, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25(16):3211–22. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- [10].Revell CM, Reynolds CE, Athanasiou KA. Effects of initial cell seeding in self assembly of articular cartilage. Ann Biomed Eng. 2008;36(9):1441–8. doi: 10.1007/s10439-008-9524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Huang AH, Stein A, Tuan RS, Mauck RL. Transient exposure to transforming growth factor beta 3 improves the mechanical properties of mesenchymal stem cell-laden cartilage constructs in a density-dependent manner. Tissue engineering Part A. 2009;15(11):3461–72. doi: 10.1089/ten.tea.2009.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Galera P, Vivien D, Pronost S, Bonaventure J, Redini F, Loyau G, et al. Transforming growth factor-beta 1 (TGF-beta 1) up-regulation of collagen type II in primary cultures of rabbit articular chondrocytes (RAC) involves increased mRNA levels without affecting mRNA stability and procollagen processing. Journal of cellular physiology. 1992;153(3):596–606. doi: 10.1002/jcp.1041530322. [DOI] [PubMed] [Google Scholar]
- [13].Hiraki Y, Inoue H, Hirai R, Kato Y, Suzuki F. Effect of transforming growth factor beta on cell proliferation and glycosaminoglycan synthesis by rabbit growth-plate chondrocytes in culture. Biochim Biophys Acta. 1988;969(1):91–9. doi: 10.1016/0167-4889(88)90092-4. [DOI] [PubMed] [Google Scholar]
- [14].Morales TI, Roberts AB. Transforming growth factor beta regulates the metabolism of proteoglycans in bovine cartilage organ cultures. The Journal of biological chemistry. 1988;263(26):12828–31. [PubMed] [Google Scholar]
- [15].Recklies AD, Baillargeon L, White C. Regulation of cartilage oligomeric matrix protein synthesis in human synovial cells and articular chondrocytes. Arthritis and rheumatism. 1998;41(6):997–1006. doi: 10.1002/1529-0131(199806)41:6<997::AID-ART6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- [16].Redini F, Galera P, Mauviel A, Loyau G, Pujol JP. Transforming growth factor beta stimulates collagen and glycosaminoglycan biosynthesis in cultured rabbit articular chondrocytes. FEBS Lett. 1988;234(1):172–6. doi: 10.1016/0014-5793(88)81327-9. [DOI] [PubMed] [Google Scholar]
- [17].Albro MB, Nims RJ, Cigan AD, Yeroushalmi KJ, Alliston T, Hung CT, et al. Accumulation of exogenous activated TGF-beta in the superficial zone of articular cartilage. Biophysical journal. 2013;104(8):1794–804. doi: 10.1016/j.bpj.2013.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Roberts AB, Frolik CA, Anzano MA, Sporn MB. Transforming growth factors from neoplastic and nonneoplastic tissues. Fed Proc. 1983;42(9):2621–6. [PubMed] [Google Scholar]
- [19].Rosier RN, O’Keefe RJ, Crabb ID, Puzas JE. Transforming growth factor beta: an autocrine regulator of chondrocytes. Connect Tissue Res. 1989;20(1-4):295–301. doi: 10.3109/03008208909023900. [DOI] [PubMed] [Google Scholar]
- [20].Hyytiainen M, Penttinen C, Keski-Oja J. Latent TGF-beta binding proteins: extracellular matrix association and roles in TGF-beta activation. Crit Rev Clin Lab Sci. 2004;41(3):233–64. doi: 10.1080/10408360490460933. [DOI] [PubMed] [Google Scholar]
- [21].Albro MB, Cigan AD, Nims RJ, Yeroushalmi KJ, Oungoulian SR, Hung CT, et al. Shearing of synovial fluid activates latent TGF-beta. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2012;20(11):1374–82. doi: 10.1016/j.joca.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Annes JP, Chen Y, Munger JS, Rifkin DB. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. The Journal of cell biology. 2004;165(5):723–34. doi: 10.1083/jcb.200312172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tenney RM, Discher DE. Stem cells, microenvironment mechanics, and growth factor activation. Curr Opin Cell Biol. 2009;21(5):630–5. doi: 10.1016/j.ceb.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. The Journal of cell biology. 2007;179(6):1311–23. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jenkins G. The role of proteases in transforming growth factor-beta activation. Int J Biochem Cell Biol. 2008;40(6-7):1068–78. doi: 10.1016/j.biocel.2007.11.026. [DOI] [PubMed] [Google Scholar]
- [26].Morales TI, Joyce ME, Sobel ME, Danielpour D, Roberts AB. Transforming growth factor-beta in calf articular cartilage organ cultures: synthesis and distribution. Arch Biochem Biophys. 1991;288(2):397–405. doi: 10.1016/0003-9861(91)90212-2. [DOI] [PubMed] [Google Scholar]
- [27].Gelb DE, Rosier RN, Puzas JE. The production of transforming growth factor-beta by chick growth plate chondrocytes in short term monolayer culture. Endocrinology. 1990;127(4):1941–7. doi: 10.1210/endo-127-4-1941. [DOI] [PubMed] [Google Scholar]
- [28].Villiger PM, Lotz M. Differential expression of TGF beta isoforms by human articular chondrocytes in response to growth factors. Journal of cellular physiology. 1992;151(2):318–25. doi: 10.1002/jcp.1041510213. [DOI] [PubMed] [Google Scholar]
- [29].Buschmann MD, Gluzband YA, Grodzinsky AJ, Kimura JH, Hunziker EB. Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J Orthop Res. 1992;10(6):745–58. doi: 10.1002/jor.1100100602. [DOI] [PubMed] [Google Scholar]
- [30].Mauck RL, Soltz MA, Wang CC, Wong DD, Chao PH, Valhmu WB, et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122(3):252–60. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- [31].Mouw JK, Case ND, Guldberg RE, Plaas AH, Levenston ME. Variations in matrix composition and GAG fine structure among scaffolds for cartilage tissue engineering. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2005;13(9):828–36. doi: 10.1016/j.joca.2005.04.020. [DOI] [PubMed] [Google Scholar]
- [32].Bhumiratana S, Eton RE, Oungoulian SR, Wan LQ, Ateshian GA, Vunjak-Novakovic G. Large, stratified, and mechanically functional human cartilage grown in vitro by mesenchymal condensation. Proc Natl Acad Sci U S A. 2014;111(19):6940–5. doi: 10.1073/pnas.1324050111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Van Obberghen-Schilling E, Roche NS, Flanders KC, Sporn MB, Roberts AB. Transforming growth factor beta 1 positively regulates its own expression in normal and transformed cells. The Journal of biological chemistry. 1988;263(16):7741–6. [PubMed] [Google Scholar]
- [34].Sampat SR, O’Connell GD, Fong JV, Alegre-Aguaron E, Ateshian GA, Hung CT. Growth factor priming of synovium-derived stem cells for cartilage tissue engineering. Tissue engineering Part A. 2011;17(17-18):2259–65. doi: 10.1089/ten.tea.2011.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ng KW, O’Conor CJ, Kugler LE, Cook JL, Ateshian GA, Hung CT. Transient supplementation of anabolic growth factors rapidly stimulates matrix synthesis in engineered cartilage. Ann Biomed Eng. 2011;39(10):2491–500. doi: 10.1007/s10439-011-0356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Albro MB, Nims RJ, Cigan AD, Yeroushalmi KJ, Shim JJ, Hung CT, et al. Dynamic mechanical compression of devitalized articular cartilage does not activate latent TGF-beta. Journal of biomechanics. 2013;46(8):1433–9. doi: 10.1016/j.jbiomech.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Garcia AM, Szasz N, Trippel SB, Morales TI, Grodzinsky AJ, Frank EH. Transport and binding of insulin-like growth factor I through articular cartilage. Arch Biochem Biophys. 2003;415(1):69–79. doi: 10.1016/s0003-9861(03)00215-7. [DOI] [PubMed] [Google Scholar]
- [38].Albro MB, Rajan V, Li R, Hung CT, Ateshian GA. Characterization of the Concentration-Dependence of Solute Diffusivity and Partitioning in a Model Dextran-Agarose Transport System. Cell Mol Bioeng. 2009;2(3):295–305. doi: 10.1007/s12195-009-0076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Quinn TM, Morel V, Meister JJ. Static compression of articular cartilage can reduce solute diffusivity and partitioning: implications for the chondrocyte biological response. Journal of biomechanics. 2001;34(11):1463–9. doi: 10.1016/s0021-9290(01)00112-9. [DOI] [PubMed] [Google Scholar]
- [40].Maroudas A. Distribution and diffusion of solutes in articular cartilage. Biophysical journal. 1970;10(5):365–79. doi: 10.1016/S0006-3495(70)86307-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bandyopadhyay A, Lopez-Casillas F, Malik SN, Montiel JL, Mendoza V, Yang J, et al. Antitumor activity of a recombinant soluble betaglycan in human breast cancer xenograft. Cancer Res. 2002;62(16):4690–5. [PubMed] [Google Scholar]
- [42].Pedrozo HA, Schwartz Z, Gomez R, Ornoy A, Xin-Sheng W, Dallas SL, et al. Growth plate chondrocytes store latent transforming growth factor (TGF)-beta 1 in their matrix through latent TGF-beta 1 binding protein-1. Journal of cellular physiology. 1998;177(2):343–54. doi: 10.1002/(SICI)1097-4652(199811)177:2<343::AID-JCP16>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- [43].Nims RJ, Durney KM, Cigan AD, Dusseaux A, Hung CT, Ateshian GA. Continuum theory of fibrous tissue damage mechanics using bond kinetics: Applications to cartilage tissue engineering. Interface Focus. 2015 doi: 10.1098/rsfs.2015.0063. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Boyd FT, Cheifetz S, Andres J, Laiho M, Massague J. Transforming growth factor-beta receptors and binding proteoglycans. J Cell Sci Suppl. 1990;13:131–8. doi: 10.1242/jcs.1990.supplement_13.12. [DOI] [PubMed] [Google Scholar]
- [45].Chen YG. Endocytic regulation of TGF-beta signaling. Cell Res. 2009;19(1):58–70. doi: 10.1038/cr.2008.315. [DOI] [PubMed] [Google Scholar]
- [46].Poole AR, Pidoux I, Rosenberg L. Role of proteoglycans in endochondral ossification: immunofluorescent localization of link protein and proteoglycan monomer in bovine fetal epiphyseal growth plate. The Journal of cell biology. 1982;92(2):249–60. doi: 10.1083/jcb.92.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].van Beuningen HM, van der Kraan PM, Arntz OJ, van den Berg WB. Transforming growth factor-beta 1 stimulates articular chondrocyte proteoglycan synthesis and induces osteophyte formation in the murine knee joint. Lab Invest. 1994;71(2):279–90. [PubMed] [Google Scholar]
- [48].Ng KW, DeFrancis JG, Kugler LE, Kelly TA, Ho MM, O’Conor CJ, et al. Amino acids supply in culture media is not a limiting factor in the matrix synthesis of engineered cartilage tissue. Amino acids. 2008;35(2):433–8. doi: 10.1007/s00726-007-0583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Massague J, Cheifetz S, Boyd FT, Andres JL. TGF-beta receptors and TGF-beta binding proteoglycans: recent progress in identifying their functional properties. Ann N Y Acad Sci. 1990;593:59–72. doi: 10.1111/j.1749-6632.1990.tb16100.x. [DOI] [PubMed] [Google Scholar]
- [50].Kim M, Erickson IE, Choudhury M, Pleshko N, Mauck RL. Transient exposure to TGF-beta3 improves the functional chondrogenesis of MSC-laden hyaluronic acid hydrogels. J Mech Behav Biomed Mater. 2012;11:92–101. doi: 10.1016/j.jmbbm.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Klein TJ, Chaudhry M, Bae WC, Sah RL. Depth-dependent biomechanical and biochemical properties of fetal, newborn, and tissue-engineered articular cartilage. Journal of biomechanics. 2007;40(1):182–90. doi: 10.1016/j.jbiomech.2005.11.002. [DOI] [PubMed] [Google Scholar]
- [52].Nims RJ, Cigan AD, Albro MB, Vunjak-Novakovic G, Hung CT, Ateshian GA. Matrix Production in Large Engineered Cartilage Constructs Is Enhanced by Nutrient Channels and Excess Media Supply. Tissue engineering Part C, Methods. 2015;21(7):747–57. doi: 10.1089/ten.tec.2014.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Strobel S, Loparic M, Wendt D, Schenk AD, Candrian C, Lindberg RL, et al. Anabolic and catabolic responses of human articular chondrocytes to varying oxygen percentages. Arthritis research & therapy. 2010;12(2):R34. doi: 10.1186/ar2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Pedrozo HA, Schwartz Z, Mokeyev T, Ornoy A, Xin-Sheng W, Bonewald LF, et al. Vitamin D3 metabolites regulate LTBP1 and latent TGF-beta1 expression and latent TGF-beta1 incorporation in the extracellular matrix of chondrocytes. J Cell Biochem. 1999;72(1):151–65. [PubMed] [Google Scholar]
- [55].D’Angelo M, Billings PC, Pacifici M, Leboy PS, Kirsch T. Authentic matrix vesicles contain active metalloproteases (MMP). a role for matrix vesicle-associated MMP-13 in activation of transforming growth factor-beta. J Biol Chem. 2001;276(14):11347–53. doi: 10.1074/jbc.M009725200. [DOI] [PubMed] [Google Scholar]
- [56].Maeda S, Dean DD, Gay I, Schwartz Z, Boyan BD. Activation of latent transforming growth factor beta1 by stromelysin 1 in extracts of growth plate chondrocyte-derived matrix vesicles. J Bone Miner Res. 2001;16(7):1281–90. doi: 10.1359/jbmr.2001.16.7.1281. [DOI] [PubMed] [Google Scholar]
- [57].Madry H, Rey-Rico A, Venkatesan JK, Johnstone B, Cucchiarini M. Transforming growth factor Beta-releasing scaffolds for cartilage tissue engineering. Tissue engineering Part B, Reviews. 2014;20(2):106–25. doi: 10.1089/ten.TEB.2013.0271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.