Abstract
Lithium carbonate, a drug for the treatment of bipolar disorder, provides mood stability to mitigate recurrent episodes of mania and/or depression. Despite its long-term and widespread use, the mechanism by which lithium acts to elicit these psychological changes has remained unknown. Using nuclear magnetic resonance (NMR) methods, in this study we characterized the association of lithium with adenosine triphosphate (ATP) and identified a bimetallic (Mg·Li) ATP complex. Lithium’s affinity to form this complex was found to be relatively high (Kd ∼1.6 mM) compared with other monovalent cations and relevant, considering lithium dosing and physiological concentrations of Mg2+ and ATP. The ATP·Mg·Li complex reveals, for the first time, to the best of our knowledge, that lithium can associate with magnesium-bound phosphate sites and then act to modulate purine receptor activity in neuronal cells, suggesting a molecular mode for in vivo lithium action.
Introduction
Lithium (Li+) has been used for treating various ailments since the 1850s and was first repurposed to treat “psychotic excitement” by John Cade in 1949 (1, 2). Shortly after its approval by the Food and Drug Administration (FDA) in 1970, it was proposed that Li+ may act through a “challenge” to other biological cations [e.g., sodium (Na+), potassium (K+), calcium (Ca2+), magnesium (Mg2+)] and thereby cause a biological effect (3). Support for the challenge hypothesis came from early research that showed that under experimental conditions that simulated complex biological media where many molecules compete for binding to these cations, Li+ could compete with Mg2+ for its binding sites. Under such simulated conditions created with cation-sequestering agents, like ATP, it was shown that Li+ could challenge Mg2+ for binding to uramildiacetate (4), a complexing agent derived from urea and an iminopolycarboxylic acid, where Mg2+ is normally preferred (3).
Subsequently, the interpretation of these experiments was challenged on the basis of a failure to consider that Li+ might also bind to the ATP present in the medium (5). Further nucleotide binding studies (6) suggested that Li+ could associate with nucleotides in the presence of Mg2+, which led to the hypothesis that Li+ may act therapeutically by interrupting ATP/ADP equilibria in key enzymes, such as ATPases. Although these preliminary studies suggested the possibility of a ternary complex comprising Li+, Mg2+, and nucleotide, confirmation of the exact nature of this complex required more direct physical evidence (6). Moreover, despite subsequent biochemical and biophysical studies (7, 8, 9), a consensus on the mode of Li+ interaction was never reached, nor have the enzymes found to be inhibited by Li+, such as glycogen synthase kinase 3β (GSK3β) and inositol monophosphatase (IMPase) (10), been validated as the target of Li+ in treatment of bipolar disorder.
In this study we demonstrate, using solution-state NMR techniques, that Li+ can indeed associate with magnesium-chelated nucleotides (NDP/NTP) under physiological cation concentrations. This has led to the hypothesis that a purine receptor response might be modulated by an ATP·Mg·Li complex and more generally provides a common molecular framework for how lithium may adopt its bioactive form by cobinding to magnesium-bound phosphorylated ligands or cofactors.
Materials and Methods
Certain commercial equipment, instruments, and materials are identified in this article to specify the experimental procedure. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology (NIST), nor does it imply that the material or equipment identified is necessarily the best available for the purpose.
NMR sample preparation
NMR samples were prepared in 5 mm NMR tubes (Wilmad Labglass, Vineland, NJ) with a H2O/D2O (9:1) mixture to a final volume of 500 μL (D2O from Cambridge Isotope Laboratories, Tewksbury, MA). Dry stocks of the sodium salts of adenosine triphosphate, adenosine diphosphate, guanosine triphosphate, guanosine diphosphate, triphosphate (Sigma-Aldrich, St. Louis, MO) were dissolved in water at 100 mM, adjusted to pH 8.0 with NaOH and stored at −20°C until ready for use. A standard pH meter and probe were used (series Φ pH meter from Beckman, Brea, CA; Orion pH electrode from Thermo Scientific, Waltham, MA). Samples were prepared in NMR buffer (25 mM sodium chloride and 1 mM sodium cacodylate, pH 6.5) from a 10× stock. Sodium chloride, sodium cacodylate, lithium chloride solution (8 M), magnesium chloride hexahydrate, and manganese (II) chloride tetrahydrate were purchased from Sigma-Aldrich. Unless otherwise stated, NMR samples contained 10 mM ATP (50 μL of 100 mM stock), 11 mM MgCl2 (5.5 μL of 1M MgCl2 stock), 10 mM LiCl (5 μL of 1M LiCl stock), with or without 50 μM MnCl2 (2.5 μL from 10 mM MnCl2 stock), 50 μL D2O, and brought up to 500 μL with NMR buffer.
NMR data collection
Data were collected on a Bruker (Billerica, MA) 600 MHz AVANCE spectrometer with a broadband observe (BBFO) probe, a Bruker 500 MHz DRX spectrometer with a broadband probe, or a Bruker 600 MHz AVANCE spectrometer equipped with a broadband inverse probe. Datasets were collected at 283 K (9.85°C), 298 K (24.85°C), 300 K (26.85°C), 303 K (29.85°C), or 310 K (36.85°C) and data are presented in the text with specific temperatures of acquisition.
7Li T1 relaxation measurements
One-dimensional (1D) 7Li NMR inversion recovery experiments were used to measure T1 relaxation with the following experimental acquisition parameters unless otherwise noted in the text: predelay (D1) of roughly five times the 7Li T1, which was measured at each temperature and typically exceeded 100 s. The total digitization (TD) was 4096 points, sweep width 699.63, dummy scans (DS) = 4, number of scans (NS) = 8, with a spectrometer frequency of 233.233 MHz for lithium at 14.4 Tesla (600 MHz proton frequency spectrometer). The variable delay list was kept constant for comparable sets of experiments but may otherwise be different depending on the expected relaxation rate (i.e., experiments at different temperatures). Spectra collected at different delay times were processed with the same zero filling to 2× the number of data points, apodized with exponential multiplication using 2.0 Hz line broadening, and phase corrected based on the zero time delay of the variable delay. The lithium signal was peak picked, the peak height and integrated volume of the signal was then measured and plotted for each experiment at the different variable delays to allow fitting to a T1 relaxation rate using Topspin 1.3 with the following commands: “xf2,” “t1guide,” “extract slice” from FID number 32, “define ranges” to obtain integral, export region to relaxation module, “relaxation window” to plot data, and fit the data.
Lithium titration binding measurements by NMR
Li+ binding affinity to ATP was determined by a series of 7Li inversion recovery experiments measuring the 7Li T1 relaxation (2 mM LiCl in buffer) with increasing concentrations of ATP·Mg (Mn). The Bruker automation software, IconNMR, was used with the Bruker automation hardware, NMR Case, for acquisition of the multiple samples required to generate each 7Li T1 relaxation data point and the T1 relaxation was determined using the Topspin “T1Guide” software. Further details on the measurement of 7Li T1 relaxation is as described in the previous experimental section. The 7Li T1 relaxation data were plotted and fit using a nonlinear equation in GraFit 5 (Erithacus Software, East Grinstead, UK). Each observed 7Li T1 signal (Tobs) was interpreted using a two-state model where the two states refer to free and ATP·Mg-bound Li+, and treated as the population-weighted average of the signals from the following two states:
| (1) |
where Tf represents the free Li+ NMR 7Li T1 constant, ff represents the fraction of free Li+, Tb represents the ATP·Mg-bound Li+ NMR 7Li T1 constant, and fb represents the fraction of ATP·Mg-bound Li+.
| (2) |
where
| (3) |
and [Li0] is the total Li+ concentration and [ATP0] is the total concentration of ATP·Mg, except in the Li+ control experiment (Fig. S3 in the Supporting Material), where [ATP0] is the total concentration of ATP.
31P NMR measurements
The 31P 1D experiments used a zero go pulse program with the broadband probe tuned to 242.93 MHz for phosphorus at 14.4 Tesla (600 MHz proton frequency spectrometer), number of scans (NS) = 1024, dummy scans (DS) = 4, and total digitization (TD) = 8192. Spectra were processed with zero filling to 2× the number of data points and apodized with a cosine function over 512 points.
[Ca2+]i measurements on rat neurons
Male Sprague-Dawley rats, weighing 150–250 g, were purchased from Harland Laboratories (Frederick, MD) and killed by CO2 asphyxiation, as approved by the Institutional Animal Care and Use Committee of the University of Maryland Biotechnology Institute. Dissociation of nodose ganglion neurons (NGNs) was performed as described previously (11) with the exception that sterile technique was used and the final neuronal pellet was resuspended in Leibovitz L-15 medium (Gibco-BRL, Grand Island, NY) containing 10% fetal bovine serum (FBS; JRH Bioscience, Lenexa, KS). The resulting cell suspension was plated as 0.2 mL aliquots onto 25 mm glass cover slips (Fisher Scientific, Newark, DE) coated with poly-D-lysine (0.1 mg/mL; Sigma). NGNs were incubated at 37°C for 24 h, then maintained at room temperature to prevent neurite outgrowth, and used for experiments for 72 h. NGNs were loaded with fluo-3 by incubation with the acetoxymethyl (AM) ester of fluo-3, as previously described (12). During imaging experiments, the neurons were superfused with Locke solution containing the following (in mM): 120 NaCl, 3.0 KCl, 1.5 MgCl2, 1.0 NaH2PO4, 25 NaHCO3, 2.5 CaCl2, and 10.0 dextrose; the solution was equilibrated with 95% O2/5% CO2 to reach a final pH of 7.4. Where nominally Ca2+-free solution was required, CaCl2 was replaced with an equivalent amount of MgCl2. P2 receptor activation was induced by exposure to 100 μM ATP, 100 μM MgCl2, and 1 mM LiCl in the superfusate as specified in the data. A laser scanning confocal microscope (LSM 510 NLO; Carl Zeiss Microscopy, Jena, Germany) was used to image changes in fluo-3 fluorescence elicited by agonist challenges in the NGNs. Fluorescence excitation was at 490 nm and other experimental parameters with fluo-3 detection were performed as described previously (12). After background correction, fluorescence intensity change was reported relative to the baseline fluorescence (ΔF/F0). Student’s t-test was applied to the data to determine significance between two means.
Structures and graphs
ATP structures were built using ChemBioDraw (Perkin Elmer, Waltham, MA) and annotated with CorelDraw X7 (Corel, Ottawa, Canada).
Results and Discussion
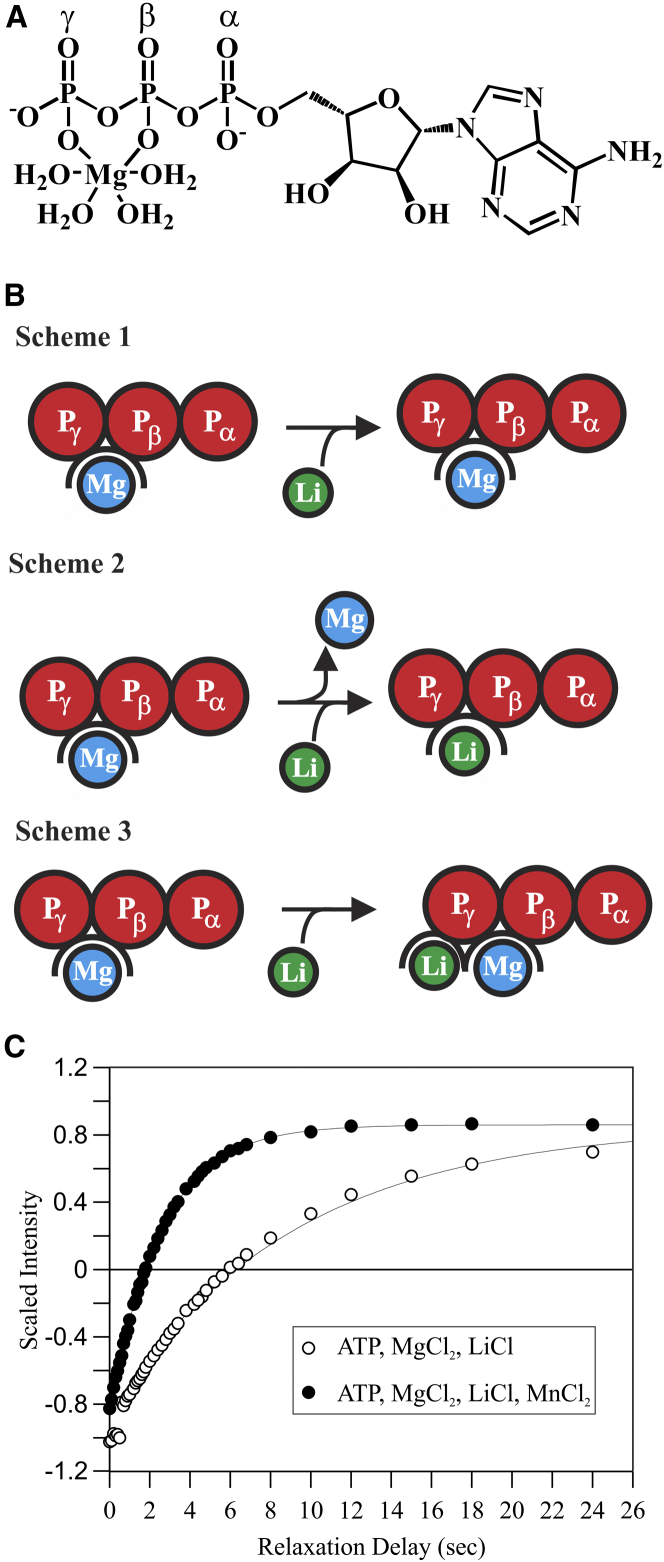
In this study, the question of Li+ binding to ATP (13) in the presence of Mg2+ has been readdressed using 7Li NMR (Fig. 1 A). To ensure a known and invariant ATP·Mg concentration, Mg2+ was used at saturating concentration (10 mM ATP, 11 mM MgCl2); saturation was confirmed by 31P NMR, which can monitor Mg2+ binding through changes in the chemical shifts of the nucleotide phosphorus atoms (Fig. S1). Under these conditions, addition of a stoichiometric amount of Li+ can have three possible outcomes (Fig. 1 B): 1) no Li+ binding to ATP·Mg; 2) Li+ binding to ATP by displacing the Mg2+; or 3) Li+ binding to ATP·Mg without displacing the Mg2+. To distinguish these possibilities, the 7Li T1 relaxation time was measured in the presence of ATP·Mg and compared with reference values obtained in buffer alone. If Li+ binds to ATP·Mg and the Li+ population shifts from being free to nucleotide-bound, then T1 would be expected to be shorter than that measured in buffer alone. Fig. 1 C shows representative 7Li T1 inversion-recovery data (open circles) where Li+ peak intensity is plotted as a function of relaxation delay, which reveals the extent that the signal has returned to the equilibrium value after application of a 180-degree inversion pulse. Single-exponential fits to the data yielded T1 values. Thus, whereas the reference 7Li T1 for 10 mM LiCl and 11 mM MgCl2 in buffer alone was 12.96 s at 10°C (Table S1), the 7Li T1 for 10 mM LiCl in the presence of 10 mM ATP and 11 mM MgCl2 was 7.16 s. The nearly twofold change in T1 indicates measurable Li+ binding to ATP.
Figure 1.
Mode of Li+ binding to ATP·Mg. (A) Schematic representation of ATP with Mg2+ bound to the β and γ phosphates is shown. (B) Three possible modes of Li+ binding to ATP·Mg (phosphates, red; Mg2+, blue; Li+, green) are shown. Scheme 1 represents Lif + ATP·Mg → ATP·Mg + Lif. Scheme 2 represents Lif + ATP·Mg → ATP·Li + Mgf. Scheme 3 represents Lif + ATP·Mg → ATP·Mg·Li. (C) Representative 7Li T1 relaxation data in the absence (open circles) and presence (closed circles) of 50 μM MnCl2 in a sample of 10 mM ATP, 11 mM MgCl2, and 10 mM LiCl; smooth curves are single-exponential fits.
Although the change in 7Li T1 indicates that Li+ binds ATP in the presence of Mg2+, this measurement does not reveal whether Li+ binding displaces Mg2+ (competition). To address the latter question, manganese (Mn2+), which can bind nucleotide triphosphates in a manner similar to Mg2+, was spiked into the buffer as a paramagnetic probe (14). Because of the fast exchange kinetics (> 10,000 s−1) of Mg2+/Mn2+ binding to ATP, rapid sampling of all the ATP molecules by the two divalent cations was observed even at a Mg2+:Mn2+ molar ratio of 220:1 (Fig. S2). Since the electron magnetic dipole moment of Mn2+ is known to cause a paramagnetic relaxation enhancement (PRE) to all NMR-active nuclei within ∼30 Å, observation of a 7Li T1 PRE would indicate that the sites being sampled by Mn2+ are proximal to the Li+ binding sites, and is thus evidence of two-metal binding (Fig. 1 B, Scheme 3). Conversely, 7Li T1 PRE would not be observed if Mg2+/Mn2+ is displaced into solution upon Li+ binding (Fig. 1 B, Scheme 2). Addition of 50 μM MnCl2 to a sample of 10 mM ATP, 11 mM MgCl2, and 10 mM LiCl caused marked PRE—shortening the 7Li T1 from 7.16 to 2.30 s (Fig. 1 C; Table S1). This observation provides direct evidence that under conditions of saturating, stoichiometric binding of Mg2+, Li+ does not displace Mg2+ from ATP, but rather cobinds in proximity to the divalent cation (Mg2+/Mn2+). In contrast, the addition of Mn2+ to a sample of Li+ and Mg2+ in buffer without ATP yielded a 7Li T1 of 12.24 s—only slightly shorter (∼5%) than the T1 measured in the absence of Mn2+ (Table S1); such a small change is within the uncertainty of the measurement. The observation of the Li+ binding to ATP·Mg led to the question of whether a nucleoside diphosphate would similarly bind Mg2+ and Li+, and whether the adenine and ribose moieties are necessary for metal ion binding. To address these questions, 7Li T1 relaxation inversion-recovery experiments were performed with adenosine 5′-diphosphate (ADP) and triphosphate (TP) (Table S1) in the presence of Mg2+. Like ATP, these molecules are known to chelate Mg2+ (15, 16) and to interact with Li+ in its presence. Just as was observed for ATP, trace Mn2+ in these samples caused 7Li T1 PRE. These experiments showed that Li+ binds to ATP·Mg, ADP·Mg, and TP·Mg, as well as other nucleotides (Table S1) under saturating, stoichiometric Mg2+ binding conditions. Together, the data support an interaction of Li+ through the polyphosphates that, as for Mg2+, is electrostatically driven and guided by the cation’s coordination geometry. Taken together, these results are important to how Li+ may interact to form a “bioactive” species, as biological ATP is normally found in the Mg2+ bound state.
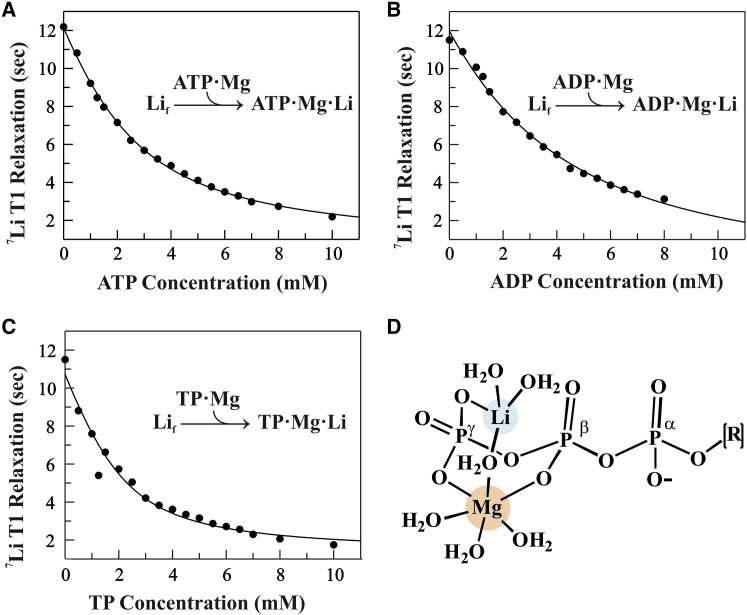
To measure the affinity of Li+ for its binding site, the T1 of 2 mM LiCl was determined as a function of the concentration of the substrate (i.e., ATP·Mg, ADP·Mg, or TP·Mg). The data in Fig. 2, A–C, show that increasing the substrate concentration shortened the 7Li T1, reflecting a shift from free to substrate-bound Li+. Nonlinear least-squares fitting of the data yielded the apparent affinity of Li+ for each substrate (Fig. 2, A–C). For example, the equilibrium dissociation constant (Kd) of Li+ from ATP·Mg·Li was found to be 1.6 ± 0.21 mM at 10°C. Interestingly, the measured Li+ affinity for ATP·Mg is higher than the affinity of Li+ for ATP alone (Kd ∼7.1 mM) (Fig. S3) or the Li+ affinity for ATP previously reported using another competitive binding method (13). More importantly, the affinity determined here is relevant in view of the therapeutic Li+ dosage (target serum level 0.8–1.1 mM (17)) as well as physiological concentrations of ATP and Mg2+. Similarly, the affinity of Li+ for ADP and TP in the presence of Mg2+ was measured and Kd’s are in the low-millimolar range (Fig. 2, A–C; Table S2). As expected, the affinity of Li+ for ADP·Mg at 10°C (Kd = 3.24 ± 0.56 mM) was weaker than for ATP·Mg, likely attributable to ADP having one fewer phosphate group and thus a reduced negative charge. Interestingly, Li+ affinity for TP·Mg was higher (Kd = 0.71 ± 0.23 mM) relative to ATP·Mg, and further increased with temperature (Kd = 0.31 ± 0.16 mM at 37°C). The temperature dependence of the binding affinity supports a model where binding is entropically driven, primarily through reorganization of the Li+ hydration sphere. Further, the observation of an apparent higher affinity of Li+ for TP·Mg relative to nucleotide·Mg could be explained by the fact that the TP is conformationally unconstrained by the nucleoside, has one extra negative charge on the second terminal phosphate and has effectively two terminal (PP) moieties for binding Mg2+ and Li+ thus giving TP complex formation a statistical advantage. Together, this and other binding data (Table S2) support a model where Mg2+ binding to a phosphate moiety creates a secondary, “high-affinity” Li+ binding site (Fig. 2 D), thus generating a two-metal-phosphate complex. The identified phosphate·Mg·Li interaction points to the possibility, proposed in earlier work (5), that the pharmacologic mechanism of action of Li+ could involve modulating normal ATP functions. The concept of a core phosphate·Mg·Li interaction provides a common link between a number of previous studies that have identified seemingly unrelated kinases, phosphatases and other enzymes that have been proposed as potential targets for the action of Li+ (10, 18). Alternatively, the identified ATP·Mg·Li complex raises the possibility that Li+ could act by modulating ATP’s normal function as a receptor ligand. For example, purine ligands ATP or ADP bind and activate cell-surface purine receptors, of which there are two subtypes (Fig. 3 A): P2X, which are ion channels that mediate influx of extracellular Ca2+ into the cytoplasm, and P2Y, which are G-protein-coupled receptors (GPCRs) that activate the inositol trisphosphate second messenger pathway to release Ca2+ from intracellular stores (19, 20, 21, 22, 23). Modulation of purine receptor activity has been shown with different nucleotide analogs and metal cofactors (19, 20, 24).
Figure 2.
Measured affinity of Li+ binding to ATP·Mg, ADP·Mg, and TP·Mg. 7Li T1 relaxation times (black circles) measured for 2 mM LiCl at 10°C as a function of the concentration of (A) ATP·Mg, (B) ADP·Mg, or (C) TP·Mg are shown. Fits using a quadratic binding equation (curves) yielded Li+ equilibrium dissociation constants, Kd: 1.60 ± 0.21 mM for ATP·Mg; 3.24 ± 0.56 mM for ADP·Mg; and 0.71 ± 0.23 mM for TP·Mg (reported uncertainties are standard errors calculated from least-squares fitting). Sample integrity was monitored using 31P NMR (Fig. S4). (D) A proposed molecular model for the ternary ATP·Mg·Li complex is shown. Mg2+ is 6-coordinate, with the β and γ phosphate oxygens replacing two water molecules. Li+ is 4-coordinate, with a γ phosphate oxygen replacing one water and a water bridge shared with Mg2+. To see this figure in color, go online.
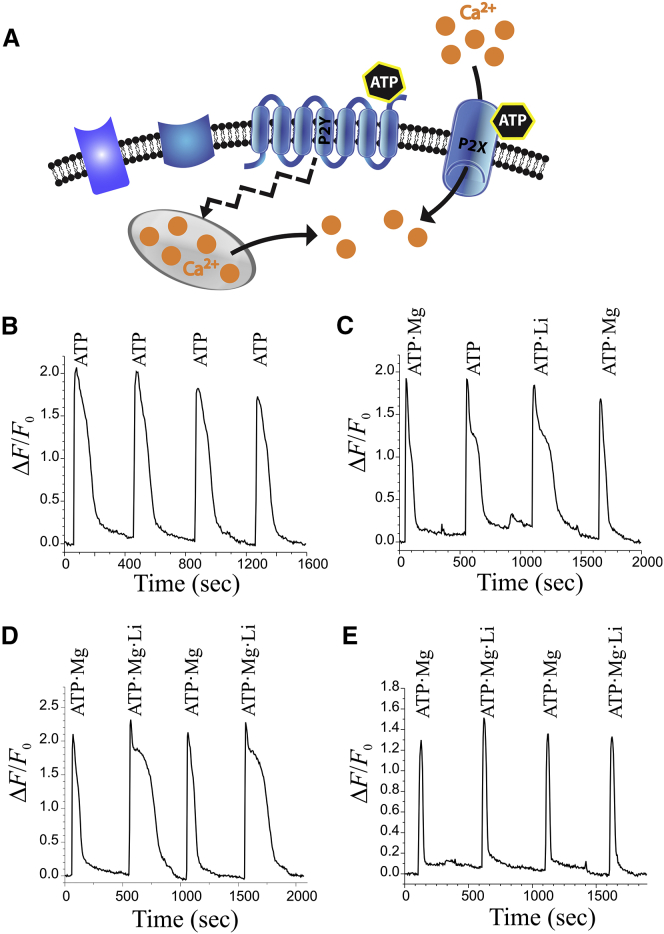
Figure 3.
Intracellular Ca2+ signals evoked by stimulating neuronal purinergic receptors with ATP, ATP·Mg, ATP·Li, and ATP·Mg·Li. (A) Schematic representation of purinergic receptors on the cell surface: P2X receptors are ion channels that permit influx of Ca2+ ions when activated by purinergic ligands like ATP; P2Y receptors are G-protein-coupled and trigger Ca2+ release from intracellular stores when activated by ATP. (B) Ca2+ signals are reliably triggered by consecutive 20 s pulses of 100 μM ATP. Repeated responses in the same cell are not significantly different (compare 1st and 2nd responses: t100-20 = 114, 112 s, respectively, p = 0.83, n = 9). (C) Ca2+ signals elicited by 20 s pulses of 100 μM ATP·Mg, 100 μM ATP, or 100 μM ATP·Li. ATP·Li without Mg2+ lengthens the Ca2+ response by 39% (compare 2nd and 3rd responses (ATP vs. ATP·Li, [Mg2+] = 0 mM): mean t100-20 = 139, 193 s, respectively, p = 0.015, n = 12). (D) Ca2+ responses evoked by 20 s pulses of 100 μM ATP·Mg or 100 μM ATP·Mg·Li. Response evoked by ATP·Mg·Li was longer than that evoked by ATP·Mg by 2.2-fold (compare 1st and 2nd responses (ATP·Mg vs. ATP·Mg·Li): t100-20 = 86, 191 s, respectively, p = 2.4 × 10−5, n = 20; compare 1st and 3rd response (1st ATP·Mg vs. 2nd ATP·Mg; internal control): t100-20 = 86, 88 s, respectively, p = 0.78, n = 20). (E) Ca2+ responses triggered by 100 s pulses of 100 μM ATP·Mg or 100 μM ATP·Mg·Li in Ca2+-free buffer. The P2Y component of ATP response does not depend significantly on Li+ (compare 1st and 2nd responses (ATP·Mg vs. ATP·Mg·Li): t100-20 = 41, 50 s, respectively, p = 0.17338, n = 28; compare 1st and 3rd responses (1st ATP·Mg vs. 2nd ATP·Mg; internal control): t100-20 = 41, 38 s, respectively, p = 0.73, n = 28). In panels B–D, extracellular Ca2+ was 2.5 mM. Application of Li+ alone elicited no response (not shown).
To test the effect of Li+ on P2 receptor activation, receptor responses in rat nodose ganglion neurons (12) were measured upon stimulation with 100 μM ATP in presence of 1.5 mM MgCl2, 1 mM LiCl, or both salts at these concentrations. Under these different conditions and using the binding affinities for each metal-ATP complex, the stimulating solution would be expected to contain 100 μM ATP·Mg, and 63 μM ATP·Mg·Li, respectively. The change in cytosolic Ca2+ concentration ([Ca2+]c) upon P2 receptor activation was monitored with a fluorescent Ca2+ indicator, fluo-3 (25). After establishing that repeated ATP stimuli could reliably elicit P2 responses (Fig. 3 B), the responses to ATP·Mg and ATP·Li were compared with the response of ATP alone (Fig. 3 C). Although the Ca2+ response amplitudes were comparable for the three agents, the response elicited by ATP·Li is ∼40% longer in duration than that elicited by ATP alone. Comparing the responses with ATP·Mg and ATP·Mg·Li (Fig. 3 D) again showed comparable amplitudes, but notably, the response to the ternary complex, ATP·Mg·Li, lasted about twice as long as the response to ATP·Mg. The longer response could be due to prolonged Ca2+ influx through P2X receptors, or prolonged P2Y receptor signaling triggering greater intracellular Ca2+ release. These alternatives can be distinguished by applying the stimuli in the absence of extracellular Ca2+, which abolishes Ca2+ influx (Fig. 3 E). In Ca2+-free medium, not only were the responses to ATP·Mg and ATP·Mg·Li comparable in amplitude, their temporal durations were also not significantly different. As absence of extracellular Ca2+ permits only P2Y-mediated Ca2+ responses, this experiment indicates that ATP·Mg and ATP·Mg·Li activate P2Y receptors comparably. Therefore, that ATP·Mg·Li evoked a longer Ca2+ response than ATP·Mg in Ca2+-containing medium implies that P2X receptor activation is prolonged when ATP·Mg·Li is the stimulus.
Conclusions
The observation that Li+ can interact directly with magnesium-phosphates (e.g., ATP·Mg·Li) and modulate P2 receptor response, suggests a mode of in vivo Li+ action through ligand-activated cell surface receptors, which could modulate signaling in the central nervous system and the periphery. Although our data cannot rule out an off-target effect of Li+ in these experiments, the interpretation that Li+ exerts its effect through the ATP·Mg·Li complex is consistent with previous studies that have demonstrated a specific modulatory effect of magnesium binding to ATP on activation of different purine receptor subtypes (20, 21, 26).
For any putative bioactive form of Li+ to be clinically relevant, it should exist in appreciable proportions under the low dosing levels of Li+ (17, 27). Our studies indicate that a considerable fraction of the ATP·Mg·Li complex could form at normal ATP and Mg2+ concentrations found in the plasma or cytoplasm (Table S3). Moreover, elevated ATP levels corresponding to biological activity or stress response in the cytoplasm, organelles (e.g., mitochondria), and the extracellular matrix could serve as reservoirs for accumulating Li+.
In summary, the ATP·Mg·Li complex provides a way to broadly view lithium's bioactive form as acting through cobinding with Mg2+ to phosphate-containing ligands or cofactors of receptors and enzymes. This general molecular model provides a new perspective, to our knowledge, to direct the identification of Li+ targets, guide drug design, and contribute to advancing our understanding of lithium's pharmacology.
Author Contributions
K.T.B., G.G.G., and J.P.M. conceived the study. K.T.B. and G.G.G. performed the NMR experiments. G.L. and J.P.Y.K. performed the neuronal measurements. K.T.B., J.P.Y.K., and J.P.M. wrote the article.
Acknowledgments
We acknowledge NIST and W.M. Keck for support of NMR instrumentation. We thank Dr. Nese Sari (UMD) for support with NMR experiments.
This work was supported by grants from the NIH, GM056481 (J.P.Y.K.) and UMD Dean’s Matching Fund for NIH Training Grant in Cell and Molecular Biology, T32GM080201 (K.T.B.).
Editor: Jeff Peng
Footnotes
Four figures and three tables are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(16)30461-1.
Supporting Citations
References (28, 29, 30, 31) appear in the Supporting Material.
Supporting Material
References
- 1.Pearson I.B., Jenner F.A. Lithium in psychiatry. Nature. 1971;232:532–533. doi: 10.1038/232532a0. [DOI] [PubMed] [Google Scholar]
- 2.Cade J.F.J. Lithium salts in the treatment of psychotic excitement. Med. J. Aust. 1949;2:349–352. doi: 10.1080/j.1440-1614.1999.06241.x. [DOI] [PubMed] [Google Scholar]
- 3.Frausto da Silva J.J., Williams R.J. Possible mechanism for the biological action of lithium. Nature. 1976;263:237–239. doi: 10.1038/263237a0. [DOI] [PubMed] [Google Scholar]
- 4.Schwarzenbach G., Kampitsch E., Steiner R. Komplexone III1. Uramil-diessigsäure und ihr Komplexbildungsvermögen. Helv. Chim. Acta. 1946;29:364–370. [Google Scholar]
- 5.Birch N.J., Goulding I. Lithium-nucleotide interactions investigated by gel filtration. Anal. Biochem. 1975;66:293–297. doi: 10.1016/0003-2697(75)90750-2. [DOI] [PubMed] [Google Scholar]
- 6.Birch J. Possible mechanism for biological action of lithium. Nature. 1976;264:681. doi: 10.1038/264681a0. [DOI] [PubMed] [Google Scholar]
- 7.Abraha A., de Freitas D.E., Geraldes C.F.G.C. Competition between Li+ and Mg2+ for ATP and ADP in aqueous solution: a multinuclear NMR study. J. Inorg. Biochem. 1991;42:191–198. doi: 10.1016/0162-0134(91)84005-t. [DOI] [PubMed] [Google Scholar]
- 8.Mota de Freitas D., Castro M.M., Geraldes C.F. Is competition between Li+ and Mg2+ the underlying theme in the proposed mechanisms for the pharmacological action of lithium salts in bipolar disorder? Acc. Chem. Res. 2006;39:283–291. doi: 10.1021/ar030197a. [DOI] [PubMed] [Google Scholar]
- 9.Brown S.G., Hawk R.M., Komoroski R.A. Competition of Li(I) and Mg(II) for ATP binding: a 31P NMR study. J. Inorg. Biochem. 1993;49:1–8. doi: 10.1016/0162-0134(93)80044-a. [DOI] [PubMed] [Google Scholar]
- 10.Quiroz J.A., Gould T.D., Manji H.K. Molecular effects of lithium. Mol. Interv. 2004;4:259–272. doi: 10.1124/mi.4.5.6. [DOI] [PubMed] [Google Scholar]
- 11.Leal-Cardoso H., Koschorke G.M., Weinreich D. Electrophysiological properties and chemosensitivity of acutely isolated nodose ganglion neurons of the rabbit. J. Auton. Nerv. Syst. 1993;45:29–39. doi: 10.1016/0165-1838(93)90359-3. [DOI] [PubMed] [Google Scholar]
- 12.Hoesch R.E., Yienger K., Kao J.P.Y. Coexistence of functional IP3 and ryanodine receptors in vagal sensory neurons and their activation by ATP. J. Neurophysiol. 2002;88:1212–1219. doi: 10.1152/jn.2002.88.3.1212. [DOI] [PubMed] [Google Scholar]
- 13.Wilson J.E., Chin A. Chelation of divalent cations by ATP, studied by titration calorimetry. Anal. Biochem. 1991;193:16–19. doi: 10.1016/0003-2697(91)90036-s. [DOI] [PubMed] [Google Scholar]
- 14.Niccolai N., Tiezzi E., Valensin G. Manganese(II) as magnetic relaxation probe in the study of biomechanisms and of biomacromolecules. Chem. Rev. 1982;82:359–384. [Google Scholar]
- 15.Cowan J.A. Metallobiochemistry of magnesium. Coordination complexes with biological substrates: site specificity, kinetics and thermodynamics of binding, and implications for activity. Inorg. Chem. 1991;30:2740–2747. [Google Scholar]
- 16.Sari J.C., Belaich J.P. Microcalorimetric studies on the formation of magnesium complexes with 5 riboinucleotides of guanine, uracil, and hypoxanthine. J. Am. Chem. Soc. 1973;95:7491–7496. doi: 10.1021/ja00803a045. [DOI] [PubMed] [Google Scholar]
- 17.Yatham L.N., Kennedy S.H., Gorman C.P. Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines for the management of patients with bipolar disorder: consensus and controversies. Bipolar Disord. 2005;7(Suppl. 3):5–69. doi: 10.1111/j.1399-5618.2005.00219.x. [DOI] [PubMed] [Google Scholar]
- 18.Ryves W.J., Dajani R., Harwood A.J. Glycogen synthase kinase-3 inhibition by lithium and beryllium suggests the presence of two magnesium binding sites. Biochem. Biophys. Res. Commun. 2002;290:967–972. doi: 10.1006/bbrc.2001.6305. [DOI] [PubMed] [Google Scholar]
- 19.Burnstock G. An introduction to the roles of purinergic signalling in neurodegeneration, neuroprotection and neuroregeneration. Neuropharmacology. 2016;104:4–17. doi: 10.1016/j.neuropharm.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 20.Li M., Silberberg S.D., Swartz K.J. Subtype-specific control of P2X receptor channel signaling by ATP and Mg2+ Proc. Natl. Acad. Sci. USA. 2013;110:E3455–E3463. doi: 10.1073/pnas.1308088110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasuya G., Fujiwara Y., Nureki O. Structural insights into divalent cation modulations of ATP-gated P2X receptor channels. Cell Reports. 2016;14:932–944. doi: 10.1016/j.celrep.2015.12.087. [DOI] [PubMed] [Google Scholar]
- 22.Sperlágh B., Illes P. P2X7 receptor: an emerging target in central nervous system diseases. Trends Pharmacol. Sci. 2014;35:537–547. doi: 10.1016/j.tips.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Hattori M., Gouaux E. Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature. 2012;485:207–212. doi: 10.1038/nature11010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobson K.A., Jarvis M.F., Williams M. Purine and pyrimidine (P2) receptors as drug targets. J. Med. Chem. 2002;45:4057–4093. doi: 10.1021/jm020046y. [DOI] [PubMed] [Google Scholar]
- 25.Minta A., Kao J.P., Tsien R.Y. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J. Biol. Chem. 1989;264:8171–8178. [PubMed] [Google Scholar]
- 26.Salas E., Carrasquero L.M.G., Delicado E.G. Purinergic P2X7 receptors mediate cell death in mouse cerebellar astrocytes in culture. J. Pharmacol. Exp. Ther. 2013;347:802–815. doi: 10.1124/jpet.113.209452. [DOI] [PubMed] [Google Scholar]
- 27.Birch N.J. Academic Press; Boston: 1991. Lithium and the Cell: Pharmacology and Biochemistry. [Google Scholar]
- 28.Gorman M.W., Feigl E.O., Buffington C.W. Human plasma ATP concentration. Clin. Chem. 2007;53:318–325. doi: 10.1373/clinchem.2006.076364. [DOI] [PubMed] [Google Scholar]
- 29.Traut T.W. Physiological concentrations of purines and pyrimidines. Mol. Cell. Biochem. 1994;140:1–22. doi: 10.1007/BF00928361. [DOI] [PubMed] [Google Scholar]
- 30.Ando T., Imamura H., Suzuki T. Visualization and measurement of ATP levels in living cells replicating hepatitis C virus genome RNA. PLoS Pathog. 2012;8:e1002561. doi: 10.1371/journal.ppat.1002561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Idzko M., Ferrari D., Eltzschig H.K. Nucleotide signalling during inflammation. Nature. 2014;509:310–317. doi: 10.1038/nature13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.