Abstract
Purpose
A fluorophotometer designed to measure aqueous flow in murine eyes was tested with artificial fluorescein chambers and in live mice with different anesthesia regimens, aqueous flow suppressants, and an anterior chamber cannulation method.
Methods
Two hours following topical fluorescein application, one group of CD-1 mice was anesthetized with ketamine/xylazine, 2,2,2-tribromoethanol, or ketamine alone. Cornea and anterior chamber fluorescein concentrations were measured periodically for 60 to 90 minutes by fluorophotometric scans to calculate aqueous flow. Later, a subgroup of mice underwent aqueous flow measurement by anterior chamber cannulation. A third group was treated with timolol, dorzolamide, and vehicle in a crossover manner 1 hour prior to fluorophotometric scans.
Results
Aqueous flow with ketamine/xylazine anesthesia (0.09 ± 0.05 μL/min, mean ± SD, n = 24) was slower than with tribromoethanol or ketamine alone (P < 0.001). Timolol reduced aqueous flow from 0.20 ± 0.07 μL/min to 0.07 ± 0.03 μL/min (P = 0.001) under tribromoethanol anesthesia and from 0.14 ± 0.03 μL/min to 0.10 ± 0.02 μL/min (P = 0.004) under ketamine anesthesia but not under ketamine/xylazine anesthesia. Dorzolamide reduced aqueous flow from 0.09 ± 0.03 to 0.06 ± 0.03 μL/min (P = 0.04) under ketamine/xylazine anesthesia. Aqueous flow by anterior chamber cannulation (0.20 ± 0.13 μL/min) was greater (P = 0.05) than by fluorophotometry (0.09 ± 0.07 μL/min).
Conclusions
A new noninvasive fluorophotometric method detected effects of general anesthesia and known aqueous suppressants on aqueous flow in mice. Aqueous flow measured by fluorophotometry was slower than by cannulation, and was technically easier with less variability. The mouse fluorophotometer is useful for repeated measurements of aqueous flow in the murine eye making crossover and longitudinal studies possible.
Keywords: fluorophotometry, aqueous flow, mouse
Intraocular pressure (IOP) is determined by the rate of aqueous humor production (flow), facility of trabecular outflow, rate of uveoscleral outflow, and pressure in the episcleral veins—collectively termed aqueous humor dynamics. Aqueous humor dynamics can vary under physiological and pathological conditions and with pharmacological and surgical interventions. Identification and interpretation of these varied patterns can help in designing better ways to return IOP to healthy levels and slow the progression of glaucoma. Investigating murine aqueous humor dynamics is becoming increasingly valuable because of the similarities of mice to human ocular physiology1 and because of the potential for genetic and experimental manipulation of mice.2–4 Numerous mutant and transgenic strains of mice mimicking specific aspects of glaucoma5,6 are the subject of ongoing study, with the potential for many more strains to be developed in the future.
The small size of the mouse eye poses challenges for accurate assessment of aqueous flow. This parameter is measured by fluorophotometry in larger eyes (rabbits,7 cats,8 dogs,9 nonhuman primates,10 and humans11) to monitor the clearance rate of topically applied fluorescein from the cornea and anterior chamber. Custom12 and commercial fluorophotometers (Fluorotron Master fluorophotometer; OcuMetrics, Inc., Mountain View, CA, USA)13,14 have been described by others; but until now, fluorophotometry in mice was not possible because these instruments had insufficient optical and mechanical resolution for the especially small dimensions of the murine eye. Here, we describe the first modified Fluorotron fluorophotometer to measure aqueous flow in mice. This work evaluates the performance of the instrument under different experimental conditions.
Materials and Methods
Fluorotron Design Modifications and Thin Film Tests
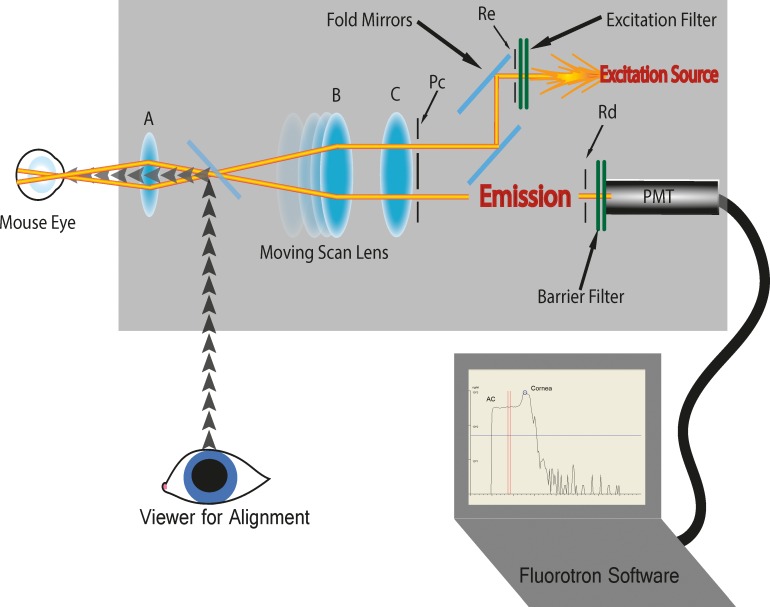
A commercially available scanning ocular fluorophotometer (Fluorotron Master; OcuMetrics, Inc.) was modified to accommodate the small size of the murine eye. This required increasing the resolution of both the optical and mechanical elements within the instrument. The resolution of the scanning lens was increased by a factor of 4. A special eye lens consisting of a 20× microscope objective and an achromatic field lens of 20 mm effective focal length was designed and fabricated to replace the usual front objective (Fig. 1, lens A), and the slit apertures reduced in size (508 μm × 102 μm; Fig. 1) to image a 143 μm × 29 μm slit in the eye. The mouse was positioned on a platform with a bite bar, nose plate, and body strap to keep the anesthetized animal motionless during the experiment. The platform was heated to prevent loss of body temperature during the study. The platform was clamped in front of the eye lens of the Prototype fluorophotometer to hold the animal in place. The platform had independent X, Y, and Z axis position adjustments, but could only accommodate measurements of the left eye of the animals. Experience with this Prototype instrument led to the development of a dedicated mouse fluorophotometer (Mouse Fluorotron; OcuMetrics, Inc.). This instrument is an integrated system that includes all of the functions required to position the animal and measure the fluorescence of the fluorescein-treated eye. It also includes a modified heated animal holder that can accommodate scanning either eye (Fig. 2).
Figure 1.
Diagram of mouse fluorophotometer. The excitation source irradiates through a bandpass filter and slit aperture Re, which is imaged by the optical system as a 143 μm × 29 μm slit in the eye. Light reemitted (reflection and fluorescence) by fluorescein dye is sampled from a 143 μm × 29 μm slit, aligned to the excitation, and defined by an aperture Rd (which is confocal to Re). Lens B is used to scan Rd and Re along the optical axis. The excitation and detection pupils are defined by the apertures Pc, located very close to lens C. These pupils are imaged anterior to the animal's cornea by the optics. This configuration minimizes contributions from the fluorescence outside of the measurement point by separating the excitation and detection paths. Another bandpass filter rejects reflected excitation light, and the fluorescence collected by aperture Rd is detected using an end-on photomultiplier tube selected for low (<100 count/s) dark noise (Hamamatsu Photonics K.K., Model 647P-SELECT). Measurements are made by photon counting which yields the greatest sensitivity, signal to noise ratio, and excellent linearity.
Figure 2.
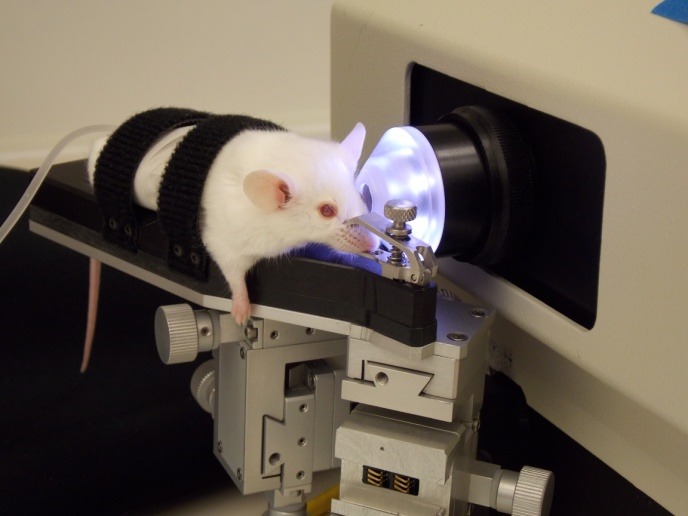
Mouse fluorophotometer. An anesthetized mouse placed on a warmed platform, secured with straps and a nose clamp, and positioned in front of the eyepiece lens of the Mouse Fluorotron.
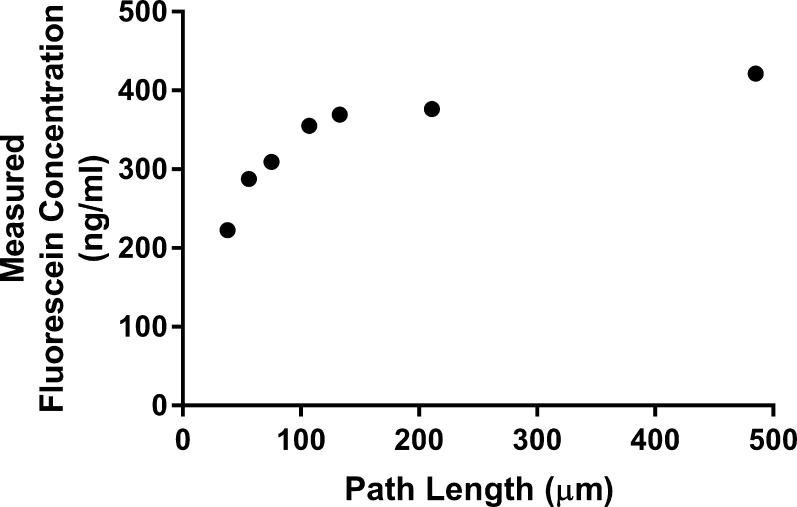
The resolution of the Mouse Fluorotron is not as fine as the thickness of the mouse cornea. This is by design as higher resolution optical designs were found to be too sensitive to movement of the cornea due to cardiac pulsation, and resulted in less repeatable measurements. However, by setting the axial length of the measurement volume to 100 μm as we did, the final instrument design resulted in fluorophotometer measurements that were lower than actual fluorescein concentrations in the cornea, which required correction based on cornea thickness. This was done by measuring the fluorescence signal of spectrophotometer cells with fluid spaces of 38, 56, 75, 107, 133, 211, and 485 μm filled with 400 ng/mL fluorescein (thin film test 1). These fluid spaces ranged from less than to greater than the thickness of a mouse cornea. Data obtained from this thin film test were empirically fitted to a correction formula.
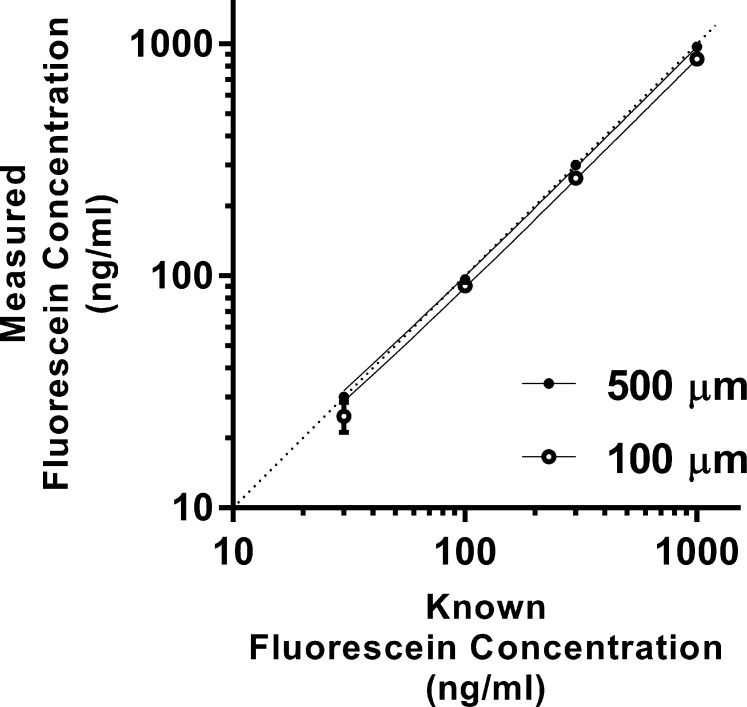
The performance of the Mouse Fluorotron was tested (thin film test 2) with glass cuvettes having fluid spaces of 100 and 500 μm filled with different concentrations of sodium fluorescein (fluorescein) solution. These cuvettes approximate the thickness of a mouse cornea (100 μm) and depth of its anterior chamber (500 μm).15 The test concentrations of fluorescein solution were 30, 100, 300, and 1000 ng/mL. Each fluorescein-filled cuvette was placed in the optical path of the Mouse Fluorotron and a fluorescence measurement was taken. The natural log of the measured fluorescein concentration was plotted against the natural log of the actual fluorescein concentrations in each cuvette. The natural log scale was chosen based upon standard fluorophotometric equations utilized by the Mouse Fluorotron to determine aqueous humor turnover rate.16,17 The slope of measured versus actual concentration was used to determine the accuracy of the Mouse Fluorotron in detecting the known fluorescein concentration. Five scans were acquired from each cuvette and the results averaged. From these data, the percent error of the Mouse Fluorotron was calculated.
Animals
Two separate groups of mice were studied; male CD-1 mice (Charles River Laboratories, Wilmington, MA, USA) between 12 and 16 weeks of age weighing between 35 and 45 g were used for the Prototype experiments, and female CD-1 mice greater than 6 months of age and weighing between 35 and 45 g were used for the Mouse Fluorotron experiments. These were extraneous mice from an independent project. Mice were housed under light-, humidity-, and temperature-controlled conditions. The housing room was lit for 12 hours each day (8 AM to 8 PM). Measurements were taken between 2 PM and 5 PM in consideration of daily aqueous flow fluctuations. All procedures were carried out in accordance with the regulations reported in the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research, and were approved by the University of Nebraska Medical Center Animal Care and Use Committee.
Aqueous Flow Measurements
Aqueous flow was determined using a fluorophotometry method described in detail previously.17 One 2-μL drop of 1% fluorescein was administered to the left eye of each mouse. The drop remained on the cornea for 10 minutes followed by a thorough rinse with eye irrigation solution (Eye Stream; Alcon Laboratories, Inc., Fort Worth, TX, USA). Starting 2 hours after dosing, fluorescence signals in the cornea and anterior chamber decayed exponentially over several hours, and the two decay slopes were similar in magnitude on a logarithmic scale and remained constant for the duration of the 90-minute aqueous flow assessment. The fluorescence of the cornea and anterior chamber were measured in anesthetized animals secured on a platform that was warmed to 35°C for the duration of the experiment. The animal was placed in front of the modified fluorophotometer and the eye aligned with the instrument lens. Five-second scans of the left eye were collected at 15-minute intervals for 60 to 90 minutes. OcuMetrics software calculated flow via the Yablonski method17 using published biometric parameters for the mouse.20 Corrections for corneal thickness were made from results of the thin film test 1.
The program calculates the aqueous turnover directly from the equation:
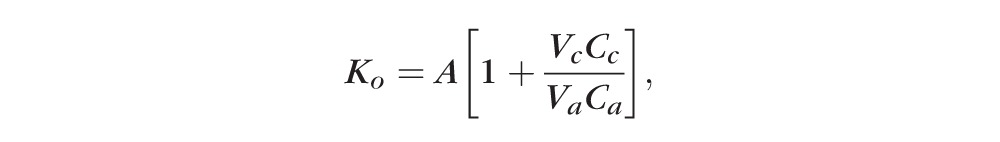 |
where
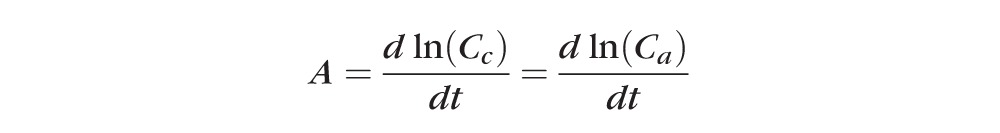 |
The program uses the average of these two concentration decay rates as computed using least squares linear regression.
Vc = volume of the cornea (μL)
Va = volume of the anterior chamber (μL)
Cc = cornea fluorescein concentration (ng/mL)
Ca = anterior chamber fluorescein concentration (ng/mL)
The ratio of corneal fluorescein concentration versus anterior chamber fluorescein concentration at the midexperiment time point is used as predicted by the best fit equations of the data determined by least squares linear regression. Aqueous flow is computed as:
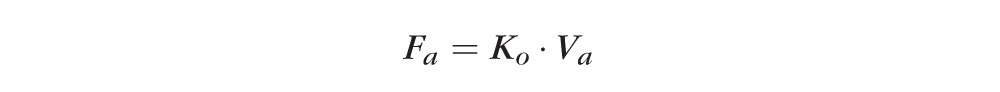 |
In another set of experiments, aqueous flow by fluorophotometry was compared with aqueous flow by a microneedle anterior chamber cannulation method, which has been described in detail previously.1 Ten ketamine/xylazine anesthetized mice underwent the aqueous flow assessment using the Fluorotron Prototype, and the microneedle cannulation experiment was done in the same mice 1 to 2 days later. Borosilicate glass capillary tubes (ID = 1.0 mm, OD = 1.5 mm; Garner Glass & Co., Claremont, CA, USA) were pulled and beveled to create microneedles with tips between 50 and 100 μm in diameter. One microneedle was connected to an aspiration system and a second microneedle was connected to an infusion system. Both microneedles were inserted into the anterior chamber through the cornea, with care taken to avoid the lens and iris. Fluorescein isothiocyanate (FITC) dextran (70 kD, 2%) was infused into the anterior chamber at 3 μL/min through one needle and aqueous humor was removed at the same rate through the second needle. The perfusion pressure was set at 9.5 mm Hg, which was considered episcleral venous pressure in the mouse. With this pressure, one could assume that the outflow through the trabecular meshwork was zero. After 20 minutes of perfusion, the aspiration tubing was divided into segments equivalent to 6 μL of fluid collected in each 2-minute period. The fluorescence of fluid collected between 10 and 20 minutes was measured with a spectrofluorometer (SpectraMax GeminiEM; Microplate Fluorescence Reader, Sunnyvale, CA, USA). During the perfusion, the fluid infused into the eye was diluted by the secreted aqueous humor and the magnitude of dilution serves as a measure of aqueous flow. The formulas used in the calculation of aqueous flow have been described in detail elsewhere.17
Anesthesia
Anesthesia was necessary to immobilize the animal and properly align the eye during the fluorophotometry scans. Three different regimens were tested for effects on aqueous flow: (1) ketamine alone (150–200 mg/kg; Hospira, Inc., Lake Forest, IL, USA; n = 13); (2) a combination of ketamine HCI (100 mg/kg) and xylazine (9 mg/kg, X-Ject SA; Burns Veterinary Supply, Inc., Westbury, NY, USA; n = 24); and (3) 2,2,2-tribromoethanol (40 mg/kg; Sigma-Aldrich Corp., St. Louis, MO, USA; n = 14). Anesthetic was administered by intraperitoneal injection. Supplementary doses were administered as needed, at 30- to 40-minute intervals, to maintain adequate sedation. Isoflurane gas also was tested but it caused nystagmus that disturbed the aqueous flow measurement by fluorophotometry. This anesthetic was not studied further.
Aqueous Flow Suppressants
The effect of topical timolol on aqueous flow was evaluated in mice anesthetized with 2,2,2-tribromoethanol (n = 7), with ketamine alone (n = 10), and with a mixture of ketamine + xylazine (n = 10). One 10-μL drop of 0.5% timolol maleate ophthalmic solution (Bausch & Lomb, Inc., Tampa, FL, USA) was placed on the cornea of the left eye of conscious mice 1 hour prior to fluorophotometry scans and 1 to 1.5 hours after fluorescein administration. Animals were restrained or distracted for 5 minutes following timolol administration to keep the drop in contact with the cornea allowing corneal penetration of the drug. Aqueous flow rate was measured 1 hour after drug administration and compared to baseline aqueous flow rate measurements obtained 2 days previously.
Ketamine + xylazine proved to be the best anesthetic regimen in terms of keeping the mouse and its eye still for the required period of time. In addition, animals easily recovered from this anesthetic making them available for future study. Therefore, this ketamine + xylazine mixture was used in further studies of aqueous flow changes with aqueous flow suppressants. In the Mouse Fluorotron study, one 10-μL drop of saline, 0.5% timolol, or 2% dorzolamide (Alcon Laboratories, Inc.) was applied to the cornea of the left eye at 1 hour before fluorophotometric scans began and 30 minutes after fluorescein administration (n = 10). The animals were anesthetized with ketamine + xylazine and aqueous flow was determined by fluorophotometry.
Intraocular pressure was measured in conscious mice by rebound tonometry (TonoLab Tonometer TV02; Tiolat Oy, Finland)18,19 immediately before and at 1 hour after topical administration of timolol.
Statistical Analysis
Paired t-tests were used to compare aqueous flow determined by the fluorophotometric method to aqueous flow determined by the anterior chamber cannulation, and to compare the effects of timolol or dorzolamide on IOP and aqueous flow from the same animals. Values are reported as mean ± SD unless otherwise indicated. A comparison of anesthesia effects was performed with ANOVA and Tukey posthoc tests to determine significance between groups of unequal sample sizes. P-values < 0.05 were considered to be statistically significant.
Results
Thin Film Tests
The measured concentration of fluorescein in spectrophotometer cells was slightly lower than the actual concentration at all spectrophotometer cell depths (38, 56, 75, 107, 133, 211, 485 μm). Values deviated the most from the actual concentration when measuring the cells with the smallest depth (Fig. 3). These data were fitted to a formula that was incorporated into the Fluorotron program to automatically correct corneal fluorescein concentration measurements according to the corneal thickness that can be specified by the investigator. These corrected values are used in the calculations of aqueous flow:
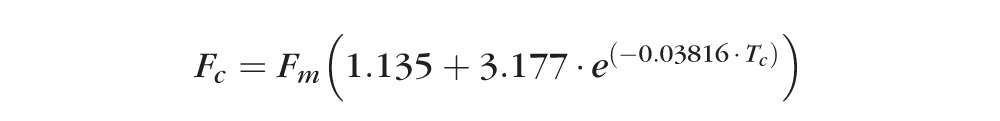 |
where
Figure 3.
Mouse Fluorotron measurements of fluorescein in small chambers. The performance of the Mouse Fluorotron was assessed by measuring fluorescence in spectrophotometer cells of varying depth (path length) when loaded with fluorescein of a known concentration (420 ng/mL). These sizes simulate the cornea thickness and anterior chamber depth.
Fc = Actual fluorescein concentration in cornea (ng/mL)
Fm = Measured fluorescein concentration in cornea (ng/mL)
Tc = Thickness of cornea (microns)
The anterior chamber is sufficiently deep for measured concentrations to be accurate and included in calculations without correction. The concentration of fluorescein measured by the Prototype was lower than the actual concentration for the 100-μm cell (11% error) and for the 500-μm cell (12% error). The Mouse Fluorotron (with improved cornea concentration corrections) also measured lower than actual concentrations, but by only 2% with both spectrophotometer cell depths. The ability of the Mouse Fluorotron to measure change in concentration was determined from the slope of the concentrations measured by the Mouse Fluorotron as compared to the actual concentrations on a natural log scale, with slope = 1 and R2 = 1.0 being perfectly accurate (Fig. 4). The Mouse Fluorotron generated a slope with <1% error with both cell depths.
Figure 4.
Mouse Fluorotron measurements of known amounts of fluorescein. The accuracy of the Mouse Fluorotron to detect the fluorescein concentration and the change in concentration in a mouse eye was determined by simulating the thickness of a mouse cornea (100 μm) and the depth of the anterior chamber (500 μm) using two cuvettes. The Mouse Fluorotron measured fluorescein concentration in both cuvettes at 2% above actual concentration. A linear fit yielded correlation values of > 0.999 for both cuvettes. Dashed line represents slope = 1.
Measurement of Aqueous Flow in Mice
The fluorescence of the cornea and anterior chamber in the mouse measured by fluorophotometry, plus central corneal thickness, corneal volume, and anterior chamber volume, are used by the fluorophotometer software to determine aqueous flow (Fig. 5). Individual values can be inputted into the program. However, for the current study, previously published values of 93 μm for cornea thickness, 0.5 μL for cornea volume, and 5.9 μL for anterior chamber volumes were used.20 With ketamine/xylazine anesthetized mice, (n = 21) aqueous flow ranged between 0.01 and 0.24 μL/min with the Prototype Fluorotron and between 0.03 and 0.17 μL/min with the Mouse Fluorotron.
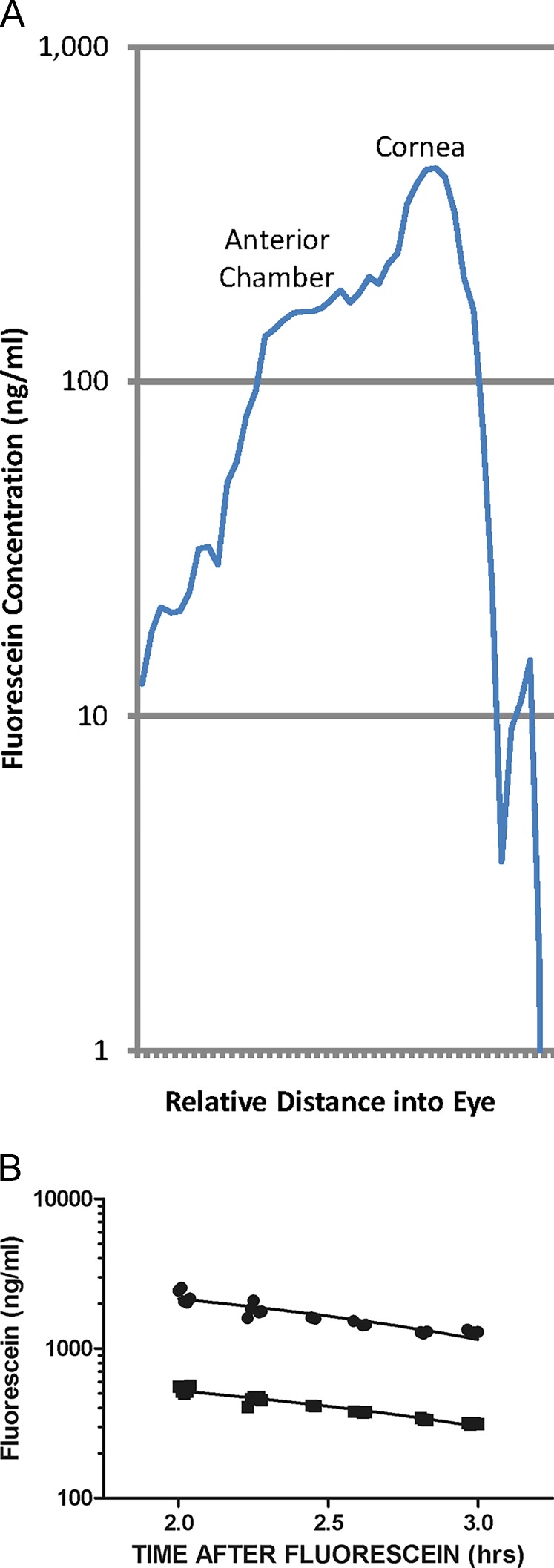
Figure 5.
Examples of fluorophotometer scans and cornea and anterior chamber decay curves of a mouse eye. (A) Scan of the mouse eye shows fluorescein distribution with characteristics very similar to those of humans and other animals. The largest peak is the cornea fluorescence taken 1 hour after fluorescein was administered topically to the cornea. The plateau to the left of the cornea peak is the anterior chamber fluorescence. The x-axis is the distance into the eye, and the y-axis is the fluorescein concentration in ng/mL. (B) Decay curves of cornea (upper curve) and anterior chamber (AC; lower parallel curve). The time of initial application of the fluorescein to the eye was 2 hours before scans commenced. The circles are cornea measurements, and the squares are the anterior chamber measurements. Good data are considered to be two parallel lines with all data falling on or very near the lines.
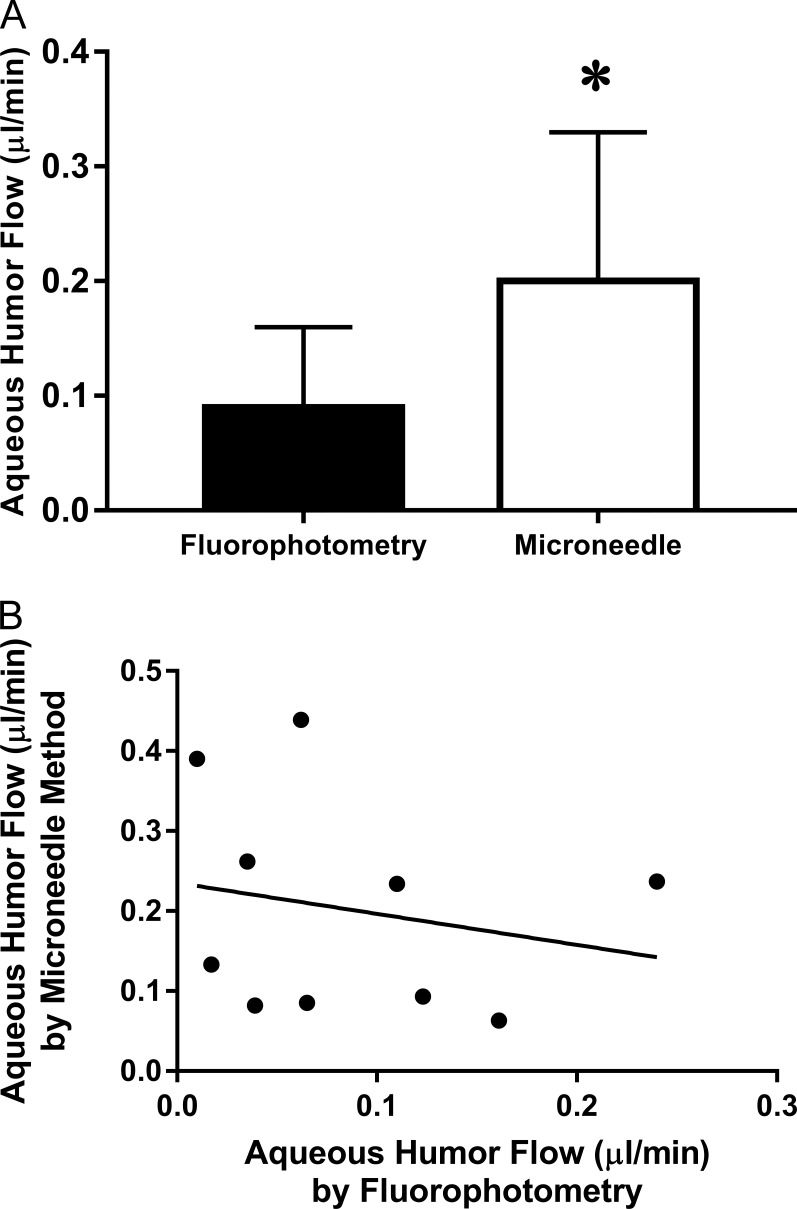
The mean aqueous flow measured by the Prototype was 0.09 ± 0.07 μL/min (mean ± SD, n = 10) and by anterior chamber cannulation was 0.20 ± 0.13 μL/min (P = 0.05; Fig. 6A). A linear regression analysis demonstrated no correlation between these two methods (Fig. 6B).
Figure 6.
Comparison of aqueous flow by two methods. Aqueous flow was measured by fluorophotometry and anterior chamber cannulation in 10 mice. (A) Aqueous flow was significantly (P = 0.05) lower when measured by fluorophotometry (0.09 ± 0.07 μL/min, error bars are SD) than by anterior chamber cannulation (0.20 ± 0.13 μL/min, *P = 0.05). (B) No correlation existed between the two aqueous flow measurement methods (R2 = 0.04, P > 0.05).
Effects of Anesthesia on Aqueous Flow
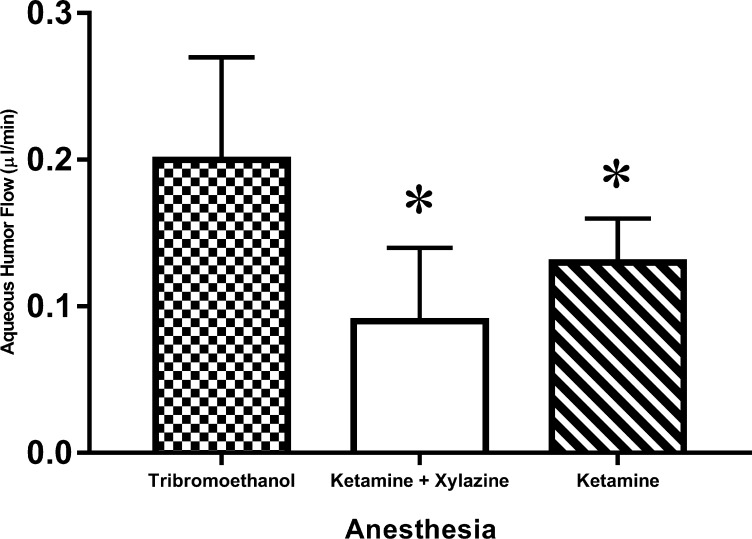
Aqueous flow was 0.09 ± 0.05 μL/min (n = 21) under ketamine/xylazine anesthesia. This was significantly (P < 0.001) lower than with 2,2,2-tribromoethanol (0.20 ± 0.07 μL/min, n = 10) or with ketamine (0.13 ± 0.03 μL/min, n = 7) anesthesia (Fig. 7).
Figure 7.
Effect of anesthesia on aqueous flow measured by fluorophotometry. Aqueous flow as measured by fluorophotometry under ketamine/xylazine anesthesia was 0.09 ± 0.05 μL/min (mean ± SD, n = 21); under ketamine anesthesia was 0.13 ± 0.03 μL/min (n = 10); and under 2,2,2-tribromoethanol anesthesia was 0.20 ± 0.07 μL/min (n = 7). Differences in flow were detected across anesthesia groups (P = 0.001). Ketamine only (P < 0.05) and ketamine/xylazine anesthesia were associated with lower aqueous flow rates than tribromoethanol (*P = 0.001).
Effect of a Topical Beta Blocker and a Carbonic Anhydrase Inhibitor on Aqueous Flow
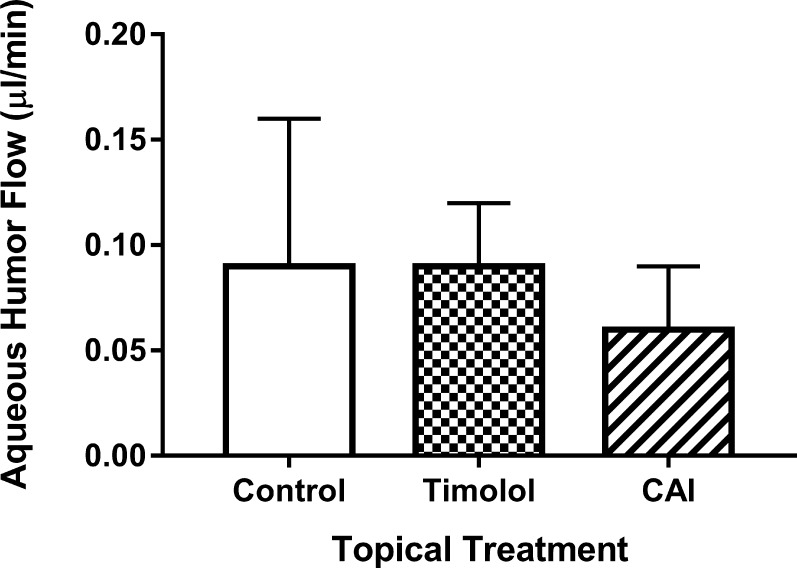
One hour after topical administration of timolol, IOP was significantly (P < 0.001) lower in conscious mice (14.5 ± 2.4 vs. 8.9 ± 1.5 mm Hg; Fig. 8). Under ketamine + xylazine anesthesia, timolol did not change aqueous flow (0.09 ± 0.07 vs. 0.09 ± 0.03 μL/min; Fig. 9), but dorzolamide reduced aqueous flow from 0.09 ± 0.07 to 0.06 ± 0.03 μL/min (P = 0.04; Fig. 9).
Figure 8.
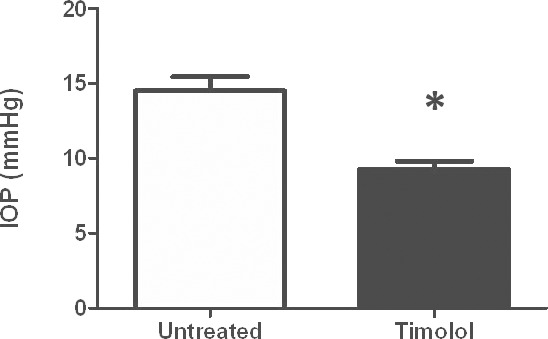
Effect of timolol on IOP. Intraocular pressure in conscious, restrained mice (n = 7) was significantly (*P < 0.001) reduced from 14.5 ± 2.4 to 8.9 ± 1.5 mm Hg (mean ± SD) following topical administration of timolol 0.5%.
Figure 9.
Effect of aqueous flow suppressants measured by fluorophotometry. Mice were anesthetized with ketamine/xylazine. Timolol, or dorzolamide (CAI), was applied topically and fluorophotometry was performed 1 hour later. On a separate day, fluorophotometry was done in the same mice similarly anesthetized but without topical eye drop (Control). Treatment affected aqueous flow (Fa); by 1-way ANOVA encompassing all three treatments (P = 0.04). CAI treatment caused a decrease in Fa compared to control (*P = 0.03, by posthoc analysis).
Figure 10 compares the timolol effect on aqueous flow under three different anesthetic regimens. With ketamine alone, aqueous flow in eyes treated with timolol (0.10 ± 0.02 μL/min) was significantly (P = 0.004) lower than in the same eyes without timolol treatment (0.14 ± 0.03 μL/min). In 2,2,2-tribromoethanol-anesthetized animals, timolol treatment significantly (P = 0.001) reduced aqueous flow from 0.20 ± 0.06 μL/min to 0.07 ± 0.02 μL/min. In animals anesthetized with ketamine/xylazine, timolol had no effect on aqueous flow.
Figure 10.
Effect of timolol plus anesthesia on aqueous flow measured by fluorophotometry. Timolol was applied topically to the corneas of mice compared to those with no topical treatment (Baseline). One hour later, mice were anesthetized using 2,2,2-tribromoethanol (blue bars), ketamine alone (red bars), or ketamine/xylazine combination (green bars). There was a difference in aqueous flow (Fa) amongst anesthesia and treatment groups in general (P < 0.0001). †P < 0.05 vs. 2,2,2-tribromoethanol alone. *P < 0.05 versus ketamine alone.
Effects of Age on Aqueous Flow
Young male CD1 mice measured using the Prototype had a faster rate of aqueous flow than older female CD1 mice measured using the Mouse Fluorotron (0.13 ± 0.03 vs. 0.09 ± 0.03 μL/min, respectively; P = 0.03).
Discussion
We report a mouse fluorophotometer with photometric sensitivity and spatial resolution capable of detecting changes in fluorescein concentration over time in the cornea and anterior chamber of mice, thereby allowing the calculation of aqueous flow in these small animals. Trials using the Prototype detected very small errors in the slope of measured versus known concentrations of sodium fluorescein in simulated corneas and anterior chambers. The errors with the Mouse Fluorotron were smaller than with the Prototype, not because of design changes but rather because of the added correction factors obtained from the Prototype thin film tests. Overall, however, these errors are negligible in the estimate of aqueous humor flow rate. Since most studies are designed to detect changes in aqueous flow with an experimental manipulation such as treatment with aqueous flow suppressants, this measurement error would apply similarly to control and experimental conditions within the limits of the instrumentation. Any flow changes caused by the experimental manipulation should be detectable provided there are meaningful changes in aqueous flow. In our study, a sample size of 10 animals had a power of .8 to detect a change in aqueous flow of 0.03 μL/min (SD of 0.03).
It should be noted that diffusional loss of fluorescein into the iris and other ocular tissues may occur in mice as it does in other animals. In large eyes, this loss may be negligible, but in the mouse eye, unaccounted diffusional loss may be manifest as a more significant overestimate of flow. This loss should be taken into consideration, particularly if an experimental manipulation is thought to affect fluorescein diffusion. Corrections for diffusional loss were not made in this study because it was assumed that it was unchanged during the experiments and unaffected by the drugs studied. Diffusional loss of fluorescein in the mouse eye does need to be carefully investigated in the future.
The aqueous flow from the fluorophotometry method did not correlate with that from an invasive anterior chamber cannulation method when comparing measurements taken from the same animals. On average, aqueous flow in the mouse measured by fluorophotometry was lower than by the microneedle cannulation method and there was no correlation between these two methods. The microneedle cannulation method is technically challenging requiring the insertion of two microneedles through the cornea into the anterior chamber without touching the iris or lens or disturbing flow through either microneedle. Tracer that flows into the connecting tubing must be captured with minimal loss as even a fraction of a microliter loss is a large percentage of the total volume of the murine anterior chamber. Additionally, any contamination of the fluorescent sample by fluorescein other than that in the anterior chamber could overestimate aqueous flow. A previous study21 in rats described difficulties with the microneedle cannulation method for determining aqueous flow. By changing the flow rate, the difference between the fluorescence of the starting infusion and the fluid in the drainage tube changes. If the experiment is done quickly, there is little collected fluid to analyze and the tracer may not be well mixed, but if performed over an extended period of time, aqueous humor dynamics may have changed in response to cannulation or other experimental manipulation. The microneedle method is by no means the gold standard, but it was felt to be the most direct method available. The comparison presented here does not prove that one method measures true aqueous flow better than the other. However, it did show that one method is easier and less invasive than the other and remained sensitive enough to detect statistically significant reductions in aqueous flow from administration of aqueous flow suppressants. Further experiments are required in order to clarify the relationship between these two methods. Accuracy of the flow estimates from these methods cannot be assessed because there is no independent measurement of flow that is known to be correct.
In our study, aqueous flow in the mouse under ketamine-xylazine anesthesia measured by fluorophotometry was 0.09 ± 0.05 μL/min. This is the baseline flow in the timolol and dorzolamide experiments. The published literature reports aqueous humor production rates measured noninvasively as 0.06 ± 0.0035 (SEM) μL/min using iontophoresis and confocal microscopy methods in CD1 mice anesthetized with ketamine alone (250 mg/kg)22 and measured invasively as 0.18 ± 0.05 μL/min and 0.14 ± 0.04 μL/min using an anterior chamber cannulation method in NIH Swiss white mice anesthetized with a mixture of ketamine and xylazine.23 The data reported in the current study are in good agreement with data from other methods for measuring aqueous flow in the mouse.
Our experiments show that aqueous flow in mice is greatly affected by different general anesthetic agents. Previous studies strongly suggest that anesthetics alone can lower IOP in humans, monkeys, dogs, cats, rats, and rabbits22,24–33 and can decrease aqueous humor flow rate.22,33–36 Our data demonstrate that the combination of ketamine and xylazine decreases aqueous flow in mice more significantly than ketamine alone or 2,2,2-tribromoethanol. A reduction in aqueous flow caused by the addition of xylazine may be due to its suppressive effect on sympathetic nerve function,27 which would explain why timolol did not reduce aqueous flow in animals given ketamine + xylazine. The combination of ketamine and xylazine provides a more stable level of anesthesia when compared to ketamine alone or 2,2,2-tribromoethanol, but one must consider the study design before choosing the anesthetic regimen.
We demonstrate that the mouse fluorophotometry method can detect a significant reduction in aqueous flow rates caused by aqueous flow suppressants. Timolol, a nonselective β-adrenergic antagonist, is one of the most commonly used antiglaucoma medications. It reduces IOP by suppressing aqueous flow in humans.17,23,37–40 The current results demonstrate a similar IOP lowering by a similar mechanism of action in mice provided xylazine is not used as an anesthetic; although in a separate study,41 betaxolol, a β1 selective adrenergic antagonist, decreased aqueous flow in ketamine/xylazine/acepromazine sedated BALB/c mice in which aqueous flow was measured indirectly by a microneedle method. Another class of aqueous flow suppressant includes carbonic anhydrase inhibitors such as dorzolamide.42 In our study, dorzolamide reduced aqueous flow in mice by 30% when using ketamine + xylazine for sedation. The xylazine does not interfere with the inhibitory effect of dorzolamide on carbonic anhydrase in the ciliary processes. In agreement with our results, mice rendered ocular hypertensive by injection of microbeads into the anterior chamber had an IOP decrease when treated with the carbonic anhydrase inhibitor, brinzolamide.43
Interestingly, the difference in aqueous flow between mice measured with the Prototype and mice measured with the Mouse Fluorotron under the same experimental conditions was found to be significant. This cannot be explained by intrainstrument variability as both Prototype and Mouse Fluorotron performed identically in detecting changes in known concentrations of fluorescein in the spectrophotometry cells. We speculate that the difference is an age effect as this is an identifiable difference between the two groups. Old mice have numerous physiological differences in IOP and aqueous humor dynamics when compared to young mice, including slowing of aqueous flow.44 Similarly, aqueous flow is slower in older adult humans compared to younger adults11,45 and older rabbits compared to juveniles.7 These findings emphasize that age should be carefully considered when using mice to study IOP and aqueous humor dynamics.
Several challenges with the use of fluorophotometry in the mouse should be mentioned. The resolution of the modified fluorophotometer is greatly enhanced to accommodate the small size of the mouse eye but when the mouse eye moves during the 90-minute experiment, a different region of cornea and anterior chamber are measured and further measurements do not fall on the original fluorescein decay curve. Hence turnover cannot be calculated accurately. This is contrary to our experience performing fluorophotometry in larger animals where repositioning of the eye is possible between readings and all scans before and after alignment of the eye fall on a linear decay curve. Fluorophotometry in mice works as long as the repeat scans are made at exactly the same region of the cornea and anterior chamber. The animal cannot move, which means only one eye of one mouse can be measured at a time. The sensitivity of the instrument to eye movement precludes the use of isoflurane as an anesthetic, because this easy to use gas causes tiny eye movements sufficient to prevent useable readings (data not shown).
The modified Fluorotron provides a new, noninvasive means to measure aqueous flow in the mouse. The method enables longitudinal or crossover studies of individual mice. The Mouse Fluorotron is sensitive enough to detect small changes in aqueous flow in mice under different general anesthetic regimens and by topical aqueous flow suppressants. The Mouse Fluorotron holds great potential for advancing the study of aqueous flow in the murine eye.
Acknowledgments
Supported by National Institutes of Health (Bethesda, MD, USA) Grant EY016902 (BMI) and Research to Prevent Blindness (University of Nebraska Medical Center).
Disclosure: C.B. Toris, None; S. Fan, None; T.V. Johnson, None; L.J. Camras, None; C.L. Hays, None; H. Liu, None; B.M. Ishimoto, OcuMetrics, Inc. (E, I), P
References
- 1. Aihara M,, Lindsey JD,, Weinreb RN. Aqueous humor dynamics in mice. Invest Ophthalmol Vis Sci. 2003; 44: 5168–5173. [DOI] [PubMed] [Google Scholar]
- 2. Paigen K. A miracle enough: the power of mice. Nat Med. 1995; 1: 215–220. [DOI] [PubMed] [Google Scholar]
- 3. John SW,, Anderson MG,, Smith RS. Mouse genetics: a tool to help unlock the mechanisms of glaucoma. J Glaucoma. 1999; 8: 400–412. [PubMed] [Google Scholar]
- 4. Silver LM. Mouse Genetics: Concepts and Applications. New York NY: Oxford University Press; 1995. [Google Scholar]
- 5. Anderson MG,, Libby RT,, Mao M,, et al. Genetic context determines susceptibility to intraocular pressure elevation in a mouse pigmentary glaucoma. BMC Biol. 2006; 4: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weinreb RN,, Lindsey JD. The importance of models in glaucoma research. J Glaucoma. 2005; 14: 302–304. [DOI] [PubMed] [Google Scholar]
- 7. Zhao M,, Hejkal JJ,, Camras CB,, Toris CB. Aqueous humor dynamics during the day and night in juvenile and adult rabbits. Invest Ophthalmol Vis Sci. 2010; 51: 3145–3151. [DOI] [PubMed] [Google Scholar]
- 8. Wang YL,, Toris CB,, Zhan G,, Yablonski ME. Effects of topical epinephrine on aqueous humor dynamics in the cat. Exp Eye Res. 1999; 68: 439–445. [DOI] [PubMed] [Google Scholar]
- 9. Toris CB,, Lane JT,, Akagi Y,, Blessing KA,, Kador PF. Aqueous flow in galactose-fed dogs. Exp Eye Res. 2006; 83: 865–870. [DOI] [PubMed] [Google Scholar]
- 10. Toris CB,, Zhan GL,, Wang YL,, et al. Aqueous humor dynamics in monkeys with laser-induced glaucoma. J Ocul Pharmacol Ther. 2000; 16: 19–27. [DOI] [PubMed] [Google Scholar]
- 11. Toris CB,, Yablonski ME,, Wang YL,, Camras CB. Aqueous humor dynamics in the aging human eye. Am J Ophthalmol. 1999; 127: 407–412. [DOI] [PubMed] [Google Scholar]
- 12. Topper JE,, McLaren J,, Brubaker RF. Measurement of aqueous humor flow with scanning ocular fluorophotometers. Curr Eye Res. 1984; 3: 1391–1395. [DOI] [PubMed] [Google Scholar]
- 13. Zeimer RC,, Blair NP,, Rusin MM,, Cunha-Vaz JG. The performance of a new commercial ocular fluorophotometer in the clinical environment. Graefes Arch Clin Exp Ophthalmol. 1985. ; 222: 223–224. [DOI] [PubMed] [Google Scholar]
- 14. Gray JR,, Mosier MA,, Ishimoto BM. Optimized protocol for Fluorotron Master. Graefes Arch Clin Exp Ophthalmol. 1985. ; 222: 225–229. [DOI] [PubMed] [Google Scholar]
- 15. Remtulla S,, Hallett PE. A schematic eye for the mouse, and comparisons with the rat. Vision Res. 1985. ; 25: 21–31. [DOI] [PubMed] [Google Scholar]
- 16. Jones RF,, Maurice DM. New methods of measuring the rate of aqueous flow in man with fluorescein. Exp Eye Res. 1966. ; 5: 208–220. [DOI] [PubMed] [Google Scholar]
- 17. Yablonski ME,, Zimmerman TJ,, Waltman SR,, Becker B. A fluorophotometric study of the effect of topical timolol on aqueous humor dynamics. Exp Eye Res. 1978. ; 27: 135–142. [DOI] [PubMed] [Google Scholar]
- 18. Wang WH,, Millar JC,, Pang IH,, Wax MB,, Clark AF. Noninvasive measurement of rodent intraocular pressure with a rebound tonometer. Invest Ophthalmol Vis Sci. 2005. ; 46: 4617–4621. [DOI] [PubMed] [Google Scholar]
- 19. Johnson TV,, Fan S,, Toris CB. Rebound tonometry in conscious conditioned mice avoids the acute and profound effects of anesthesia on intraocular pressure. J Ocul Pharmacol Ther. 2008. ; 24: 175–185. [DOI] [PubMed] [Google Scholar]
- 20. Smith RS,, John SWM,, Nishina PM,, Sundberg JP, Systematic evaluation of the mouse eye. : Sundberg J P,, Boggess D, eds. Systematic Approach to Evaluation of Mouse Mutations Boca Raton, FL: CRC Press; 2002: 3–24. [Google Scholar]
- 21. Mermoud A,, Baerveldt G,, Minckler DS,, Prata JA,, Jr, Rao NA. Aqueous humor dynamics in rats. Graefes Arch Clin Exp Ophthalmol. 1996. ; 234( suppl 1): S198 –S. [DOI] [PubMed] [Google Scholar]
- 22. Zhang D,, Vetrivel L,, Verkman AS. Aquaporin deletion in mice reduces intraocular pressure and aqueous fluid production. J Gen Physiol. 2002. ; 119: 561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Crowston JGWR. Glaucoma medication and aqueous humor dynamics. Curr Opin Ophthalmol. 2005. ; 16: 94–100. [DOI] [PubMed] [Google Scholar]
- 24. Danias J,, Kontiola AI,, Filippopoulos T,, Mittag T. Method for the noninvasive measurement of intraocular pressure in mice. Invest Ophthalmol Vis Sci. 2003. ; 44: 1138–1141. [DOI] [PubMed] [Google Scholar]
- 25. Jia L,, Cepurna WO,, Johnson EC,, Morrison JC. Effect of general anesthetics on IOP in rats with experimental aqueous outflow obstruction. Invest Ophthalmol Vis Sci. 2000. ; 41: 3415–3419. [PubMed] [Google Scholar]
- 26. Avila MY,, Carre DA,, Stone RA,, Civan MM. Reliable measurement of mouse intraocular pressure by a servo-null micropipette system. Invest Ophthalmol Vis Sci. 2001. ; 42: 1841–1846. [PubMed] [Google Scholar]
- 27. Burke JA,, Potter DE. The ocular effects of xylazine in rabbits, cats, and monkeys. J Ocul Pharmacol. 1986. ; 2: 9–21. [DOI] [PubMed] [Google Scholar]
- 28. Adams AK,, Barnett KC. Anaesthesia and intraocular pressure. Anaesthesia. 1966. ; 21: 202–210. [DOI] [PubMed] [Google Scholar]
- 29. Badrinath SK,, Vazeery A,, McCarthy RJ,, Ivankovich AD. The effect of different methods of inducing anesthesia on intraocular pressure. Anesthesiology. 1986. ; 65: 431–435. [DOI] [PubMed] [Google Scholar]
- 30. Ausinsch B,, Rayburn RL,, Munson ES,, Levy NS. Ketamine and intraocular pressure in children. Anesth Analg. 1976. ; 55: 773–775. [DOI] [PubMed] [Google Scholar]
- 31. Hahnenberger RW. Influence of cataleptoid anaesthetic agents on the intraocular pressure in monkeys (macaca fascicularis). Acta Ophthalmol (Copenh). 1976. ; 54: 491–499. [DOI] [PubMed] [Google Scholar]
- 32. Hahnenberger RW. Influence of various anesthetic drugs on the intraocular pressure of cats. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1976. ; 199: 179–186. [DOI] [PubMed] [Google Scholar]
- 33. Artru AA. Rate of anterior chamber aqueous formation trabecular outflow facility, and intraocular compliance during desflurane or halothane anesthesia in dogs. Anesth Analg. 1995. ; 81: 585–590. [DOI] [PubMed] [Google Scholar]
- 34. Erickson-Lamy KA,, Kaufman PL,, McDermott ML,, France NK. Comparative anesthetic effects on aqueous humor dynamics in the cynomolgus monkey. Arch Ophthalmol. 1984. ; 102: 1815–1820. [DOI] [PubMed] [Google Scholar]
- 35. Sperber GO,, Bill A. A method for near-continuous determination of aqueous humor flow; effects of anaesthetics, temperature and indomethacin. Exp Eye Res. 1984. ; 39: 435–453. [DOI] [PubMed] [Google Scholar]
- 36. Krupin T,, Feitl M,, Roshe R,, Lee S,, Becker B. Halothane anesthesia and aqueous humor dynamics in laboratory animals. Invest Ophthalmol Vis Sci. 1980. ; 19: 518–521. [PubMed] [Google Scholar]
- 37. Toris CB,, Zhan GL,, Yablonski ME,, Camras CB. Effects on aqueous flow of dorzolamide combined with either timolol or acetazolamide. J Glaucoma. 2004. ; 13: 210–215. [DOI] [PubMed] [Google Scholar]
- 38. Wayman L,, Larsson LI,, Maus T,, Alm A,, Brubaker R. Comparison of dorzolamide and timolol as suppressors of aqueous humor flow in humans. Arch Ophthalmol. 1997. ; 115: 1368–1371. [DOI] [PubMed] [Google Scholar]
- 39. Brubaker RF,, Nagataki S,, Bourne WM. Effect of chronically administered timolol on aqueous humor flow in patients with glaucoma. Ophthalmology. 1982. ; 89: 280–283. [DOI] [PubMed] [Google Scholar]
- 40. Coakes RL,, Brubaker RF. The mechanism of timolol in lowering intraocular pressure in the normal eye. Arch Ophthalmol. 1978. ; 96: 2045–2048. [DOI] [PubMed] [Google Scholar]
- 41. Millar JC,, Clark AF,, Pang IH. Assessment of aqueous humor dynamics in the mouse by a novel method of constant-flow infusion. Invest Ophthalmol Vis Sci. 2011. ; 52: 685–694. [DOI] [PubMed] [Google Scholar]
- 42. Sugrue MF. The preclinical pharmacology of dorzolamide hydrochloride a topical carbonic anhydrase inhibitor. J Ocul Pharmacol Ther. 1996. ; 12: 363–376. [DOI] [PubMed] [Google Scholar]
- 43. Yang Q,, Cho KS,, Chen H,, et al. Microbead-induced ocular hypertensive mouse model for screening and testing of aqueous production suppressants for glaucoma. Invest Ophthalmol Vis Sci. 2012. ; 53: 3733–3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Millar JC,, Phan TN,, Pang IH,, Clark AF. Strain and age effects on aqueous humor dynamics in the mouse. Invest Ophthalmol Vis Sci. 2015. ; 56: 5764 –57. [DOI] [PubMed] [Google Scholar]
- 45. Brubaker RF. Flow of aqueous humor in humans. Invest Ophthalmol Vis Sci. 1991. ; 32: 3145–3166. [PubMed] [Google Scholar]