Abstract
Since its discovery, Euparkeria capensis has been a key taxon for understanding the early evolution of archosaurs. The braincase of Euparkeria was described based on a single specimen, but much uncertainty remained. For the first time, all available braincase material of Euparkeria is re-examined using micro-computed tomography scanning. Contrary to previous work, the parabasisphenoid does not form the posterior border of the fenestra ovalis in lateral view, but it does bear a dorsal projection that forms the anteroventral half of the fenestra. No bone pneumatization was found, but the lateral depression of the parabasisphenoid may have been pneumatic. We propose that the lateral depression likely corresponds to the anterior tympanic recess present in crown archosaurs. The presence of a laterosphenoid is confirmed for Euparkeria. It largely conforms to the crocodilian condition, but shows some features which make it more similar to the avemetatarsalian laterosphenoid. The cochlea of Euparkeria is elongated, forming a deep cochlear recess. In comparison with other basal archosauromorphs, the metotic foramen is much enlarged and regionalized into vagus and recessus scalae tympani areas, indicating an increase in its pressure-relief mechanism. The anterior semicircular canal is extended and corresponds to an enlarged floccular fossa. These aspects of the braincase morphology may be related to the development of a more upright posture and active lifestyle. They also indicate further adaptations of the hearing system of Euparkeria to terrestriality.
Keywords: Euparkeria, diapsid, archosaur, computer tomography scan, inner ear, braincase
1. Introduction
Archosauria, a crown group of diapsid reptiles represented today by birds and crocodilians and including the extinct dinosaurs, is highly speciose (with over 9000 species of modern birds and crocodilians [1]) and has been so since its origin in the Late Triassic. Archosaurs filled most terrestrial ecological niches for large-bodied vertebrates for over 150 Myr [2–4], from the Late Triassic to the end of the Cretaceous. The rise of the archosaurs to this position of ecological dominance took place following diversity decline among therapsids, which had previously filled most macroscale terrestrial niches (e.g. [2,4–12]). This faunal transition began at the end of the Permian and continued through the Triassic [4,12,13]. The rise of archosaurs is a landmark terrestrial faunal transition and an outstanding example of an ecological radiation over geological timescales [3].
Euparkeria capensis is a small (known individuals reaching approx. 1 m in length [14]) stem archosaur represented by the remains of over 10 individuals collected from a single locality in Subzone B of the Cynognathus Assemblage Zone [15,16] (the uppermost biozone of the Burgersdorp Formation and the Beaufort Group), close to Aliwal North, Eastern Cape, South Africa [14,17]. Subzone B is probably Anisian (Middle Triassic) in age [16]. Since its discovery, Euparkeria has been considered to be an important taxon for our understanding of the rise and early evolution of archosaurs. Euparkeria is nearly universally found to be either the sister taxon to, or a very close relative of, Archosauria in phylogenetic analyses [3,18–28]. For this reason Euparkeria is often used as an outgroup in phylogenetic and evolutionary analyses of crown taxa (e.g. [29–41]), allowing the sequence and direction of morphological changes during the radiation of Archosauria to be understood.
Given its phylogenetic position and lack of unique autapomorphies, the morphology of Euparkeria has been considered to potentially approach that of the ancestor of Archosauria, and thus may shed light on the early evolution of archosaurs [42]. The gracile, cursorial body plan of Euparkeria represents a morphological stage intermediate between more ‘sprawling’ non-archosaurian archosauromorph taxa and fully erect, and often bipedal [12,43,44] crown taxa. Beyond this, Euparkeria itself represents a part of the radiation of archosauromorphs, within which the crown radiation is nested. Although often used as a phylogenetic outgroup to Archosauria, Euparkeria can also be seen as displaying a relatively derived braincase morphology in comparison to many stem taxa (e.g. relatively high, dorsoventrally elongated parabasisphenoid, elongated semicircular canals, discussed below), representing a continuation of morphological developments which begin further down the archosaur stem.
The braincase of Euparkeria was originally described by Ewer [14], based on the holotype (SAM-PK-5867), SAM-PK-7696 and UMZC T.692 (‘Watson's specimen A’; formerly R 527), in a monographic treatment of the taxon. Subsequently, an isolated braincase from specimen SAM-PK-7696 was further acid prepared and was described by Cruickshank [45]. Evans [46] figured this same isolated braincase and used it as a comparator in her treatment of the braincase of Prolacerta broomi. Welman [47] figured both SAM-PK-7696 and the braincase of the holotype, which had been further mechanically prepared in the interim. Welman [47] compared the morphology of the braincase of Euparkeria to that of birds, dinosaurs and crocodilians, and came to the controversial conclusion that Euparkeria was more closely related to birds than to dinosaurs or crocodilians, resurrecting the idea that birds and dinosaurs had separate origins among the ‘thecodonts', a paraphyletic assemblage of stem archosaurs and early pseudosuchians [48]. Gower & Weber [42] thoroughly redescribed the braincase of Euparkeria, based primarily on UMZC T.692. In addition to providing a comprehensive reference work, these authors presented evidence refuting the presence or importance of most of the anatomical features used by Welman [47] to link Euparkeria to birds to the exclusion of other archosaurs.
Here, we provide a thorough redescription of the osteology of the braincase of Euparkeria, building on the work of Gower & Weber [42] and bringing new clarification to points of doubt, documenting new information and confirming areas where our understanding is limited by the material. Although the work of Gower & Weber [42] was thorough, given the material and methods available to the authors, recent advances in computed tomography (CT) allow new insights into the braincase and inner ear anatomy. All material pertaining to the braincase of Euparkeria was available for us to examine, and we were able to CT scan the specimen available to Gower & Weber [42] (UMZC T.692), the holotype (SAM-PK-5867), specimen SAM-PK-6047A and the isolated braincase SAM-PK-7696.
Table 1.
Nomenclature.
| ? | uncertainty regarding identification | ip | interparietal |
| aa | anterior ampulla | is | interorbital septum |
| aip | anterior inferior process of prootic | ld | lateral depression |
| ap | ascending process of parabasisphenoid | lg.cr | lagenar crest |
| arts | articular surface | lj | lower jaw |
| asc | anterior semicircular canal | ls | laterosphenoid |
| bb | bridge of bone | ls.btr | laterosphenoid buttress |
| bo | basioccipital | lsc | lateral semicircular canal |
| bp | basipterygoid process | m | maxilla |
| bt | basal tuber | md.rd | median ridge |
| cc | common crus | mf | metotic foramen |
| cl | cochlea | mpr | median pharyngeal recess |
| CN I | foramen for cranial nerve I | mx | matrix |
| CN II | foramen for cranial nerve II | oc | occipital condyle |
| CN III | foramen for cranial nerve III | op | opisthotic |
| CN IV | foramen for cranial nerve IV | ov.dp | oval depression |
| CN V | foramen for cranial nerve V | pa | parietal |
| CN VI | foramen for cranial nerve VI | pbs | parabasisphenoid |
| CN VII | foramen for cranial nerve VII | pf | perilymphatic foramen |
| CN VIIhym | groove for hyomandibular branch of cranial nerve VII | pp | paroccipital process |
| CN VIIpal | groove for palatine branch of cranial nerve VII | pr | prootic |
| CN XII | foramen for cranial nerve XII | psc | posterior semicircular canal |
| CN XIIa | foramen for anterior branch of cranial nerve XII | psa | posterior ampulla |
| CN XIIp | foramen for posterior branch of cranial nerve XII | pt | pterygoid |
| cap | capitate process | ptf | posttemporal fenestra |
| cp | cultriform process | q | quadrate |
| cr1 | crest 1 | rd | ridge |
| cr2 | crest 2 | s | suture |
| ds | dorsum sellae | sd | semilunar depression |
| eo | exoccipital | st.gr | stapedial groove |
| f | frontal | so | supraoccipital |
| fc.pa | facet for parietal | sp | slender process |
| ff | floccular fossa | st | stapes |
| fm | foramen magnum | su | sulcus |
| fo | fenestra ovalis | tc | tensor crest |
| gr.ga | groove for Gasserian ganglion | tu | tuber |
| gr | groove | ug | unossified gap |
| gr.ut | groove marking ventral connection between common crus and utriculus | vcd | vena capitis dorsalis channel |
| gr.ov.dp.VII | groove connecting oval depression with foramen for cranial nerve VII | ve | vestibule |
| hf | hypophyseal fossa | vr.op | ventral ramus of the opsithotic |
| ica | path of internal carotid artery | vt | vertebra |
CT scanning allows us to provide additional information on sutures and contacts between elements, as well as details of the internal structures of the braincase and the morphology of the inner ear. Furthermore, we provide thorough documentation of the element generally regarded as a laterosphenoid in Euparkeria, describing for the first time its morphology in SAM-PK-5867 and conducting an extensive discussion on its morphology and potential homology.
Our work makes the braincase of Euparkeria one of the best-documented early archosauriform braincases and provides a reference point for archosauriform morphologists that will contribute to a growing understanding of the rise and evolutionary radiation of the archosaurs.
2. Material and methods
SAM-PK-7696 and UMCZ T.692 (electronic supplementary material, figures S1 and S2) were scanned at the Museum für Naturkunde, Berlin, using a Phoenix|x-ray Nanotom (GE Sensing and Inspection Technologies GmbH, Wunstorf, Germany). The scans comprised a total of 1440 slices, using a tungsten target and a Cu filter of 0.1 mm thickness in modus 0 with averaging 3 and skip 2. The scans of SAM-PK-7696 were reconstructed with the software datos|x-reconstruction v. 1.5.0.22, whereas scans of UMCZ T.692 were reconstructed using datos|x 2 reconstruction v. 2.2.1.739 (both from GE Sensing and Inspection Technologies GmbH, Phoenix|x-ray). Scan settings were as follows - SAM-PK-7696: 80 kV, 250 µA, 1000 ms, 16.34 µm voxel size; UMCZ T.692: 120 kV, 250 µA, 1000 ms, 24.49 µm voxel size.
Specimens SAM-PK-5867 (electronic supplementary material, figure S3) and SAM-PK-6047A were scanned at the Evolutionary Studies Institute (formerly Bernard Price Institute for Palaeontological Research), University of the Witwatersrand. Scanning was conducted with an X Tek HMX ST 225 (Nikon Metrology Inc.), comprising 3000 projections, using a tungsten target with gain 4 and binning 0. Files were reconstructed using CT Pro 3D software (Nikon Metrology, Inc.). Scan settings were as follows - SAM-PK-5867: 70 kV, 140 µA, 1000 ms, 57.50 µm voxel size, 1.8 mm Al filter; SAM-PK-6047A: 120 kV, 95 µA, 2000 ms, 60.10 µm voxel size, 1.2 mm Cu filter.
In addition, four braincases of extant species were scanned at the Museum für Naturkunde Berlin for comparative purposes. Machine settings were the same as described earlier, except 1000 slices were made with the function Fast Scan and no filter (except if stated otherwise). Scan setting were as follows - Meleagris gallopavo (ZMB 1793 792): 75 kV, 240 µA, 750 ms, 17.05 µm voxel size; Sphenodon punctatus (ROM R9298): 75 kV, 280 µA, 750 ms, 19.44 µm voxel size; Struthio camelus (ZMB 2000 2769): 90 kV, 400 µA, 750 ms, 30 µm voxel size; Osteolaemus tetraspis (ZMB 23467): 90 kV, 350 µA, 1000 ms, 32.37 µm voxel size and Cu filter.
All scans were post-processed and segmented using VG Studio Max 2.1 and 2.2 (Volume Graphics, Heidelberg, Germany).
3. Institutional abbreviations
- BP
Evolutionary Studies Institute (formerly Bernard Price Institute for Palaeontological Research), University of the Witwatersrand, Johannesburg, South Africa
- NM
National Museum, Bloemfontein, South Africa
- PIN
Paleontological Institute of the Russian Academy of Sciences, Moscow, Russia
- PVSJ
División de Paleontología, Museo de Ciencias Naturales de la Universidad Nacional de San Juan, Argentina
- ROM
Royal Ontario Museum, Toronto, Canada
- SAM
Iziko South African Museum, Cape Town, South Africa
- UCMP
University of California Museum of Paleontology, Berkeley, USA
- UMZC
University Museum of Zoology, University of Cambridge, Cambridge, UK
- ZMB
Museum für Naturkunde Berlin, Berlin, Germany
- ZPAL
Institute of Paleobiology of the Polish Academy of Sciences, Warsaw, Poland
4. Description
4.1. Basioccipital
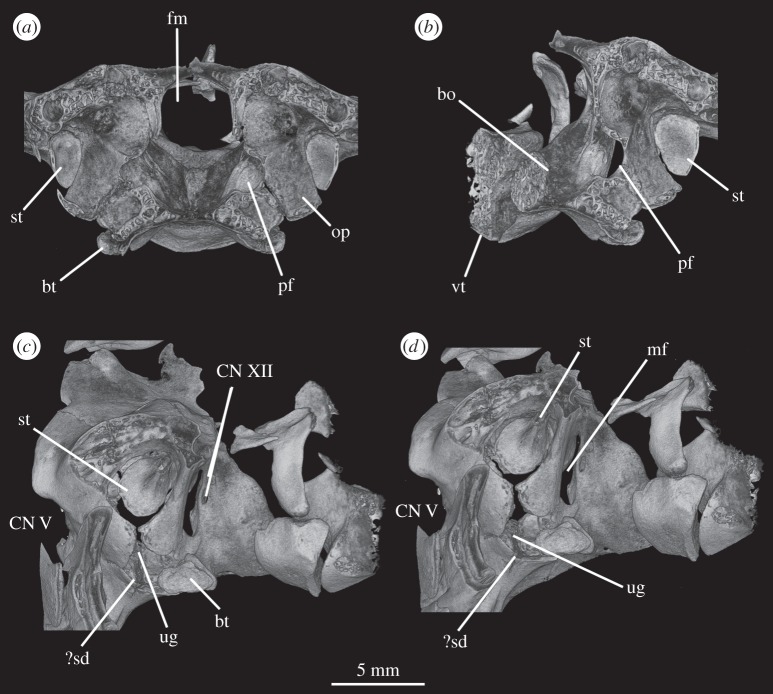
The basioccipital forms the majority of the occipital condyle, with only the dorsolateral corners of the condyle formed by the exoccipitals. The entire occipital condyle (including the exoccipital contribution) is hemispherical, with the dorsal margin being very gently concave in posterior view (figures 1b, 7b and 11b). There is no pronounced ridge delimiting the condyle from the condyle neck (figure 11b), unlike in Dorosuchus neoetus [49], nor is there a notochordal pit like in Youngina capensis [50]. The contribution of the basioccipital to the border of the foramen magnum is very limited, not accounting for more than the middle third of the ventral border of the foramen (figures 1b, 6a and 11b). Thus, the interpretation of Cruickshank [45, fig. 2] (also Gower & Weber [42, fig. 1b]) to some extent exaggerated the basioccipital contribution to the foramen magnum. The basioccipital articulates with the exoccipital in a dorsomedial–ventrolateral orientated plane, below the foramen for cranial nerve (CN) XII (figure 6a,c).
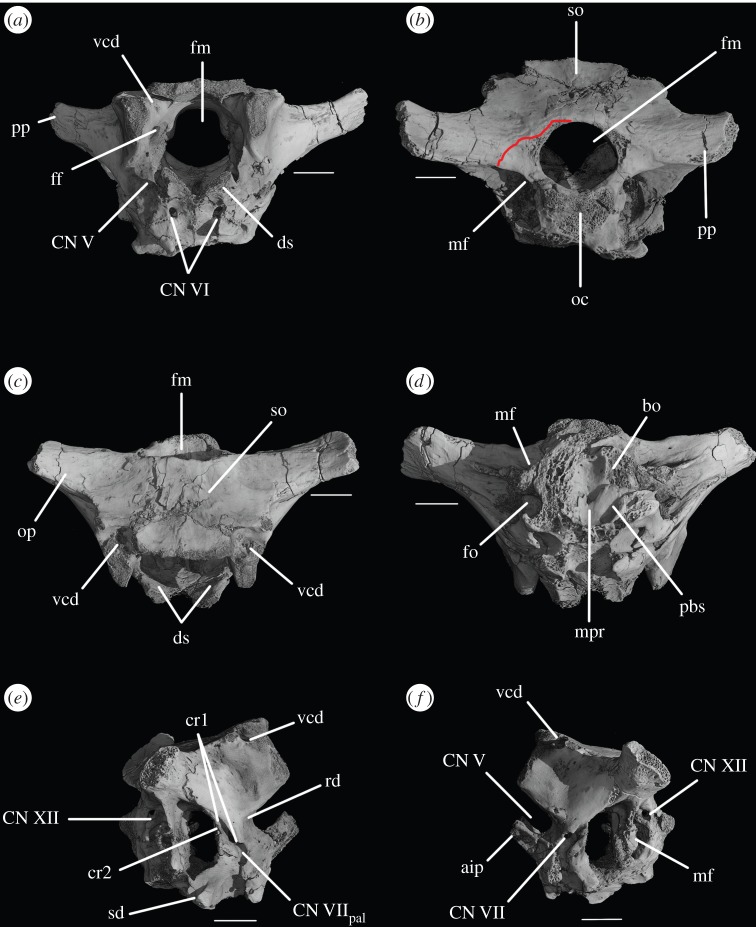
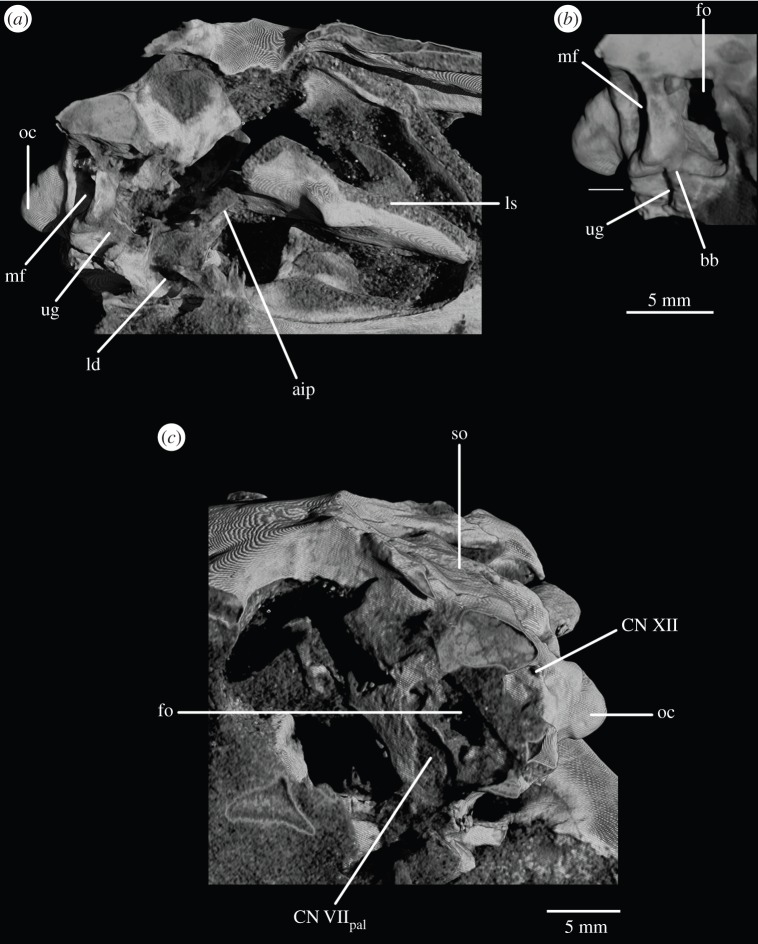
Figure 1.
CT reconstruction of the braincase of SAM-PK-7696 in (a) anterior, (b) posterior, (c) dorsal, (d) ventral, (e) right lateral and (f) left lateral views. Red line in (b) indicates the suture line between exoccipital and opisthotic/supraoccipital based on CT scans (see figure 6). For abbreviations, see table 1.
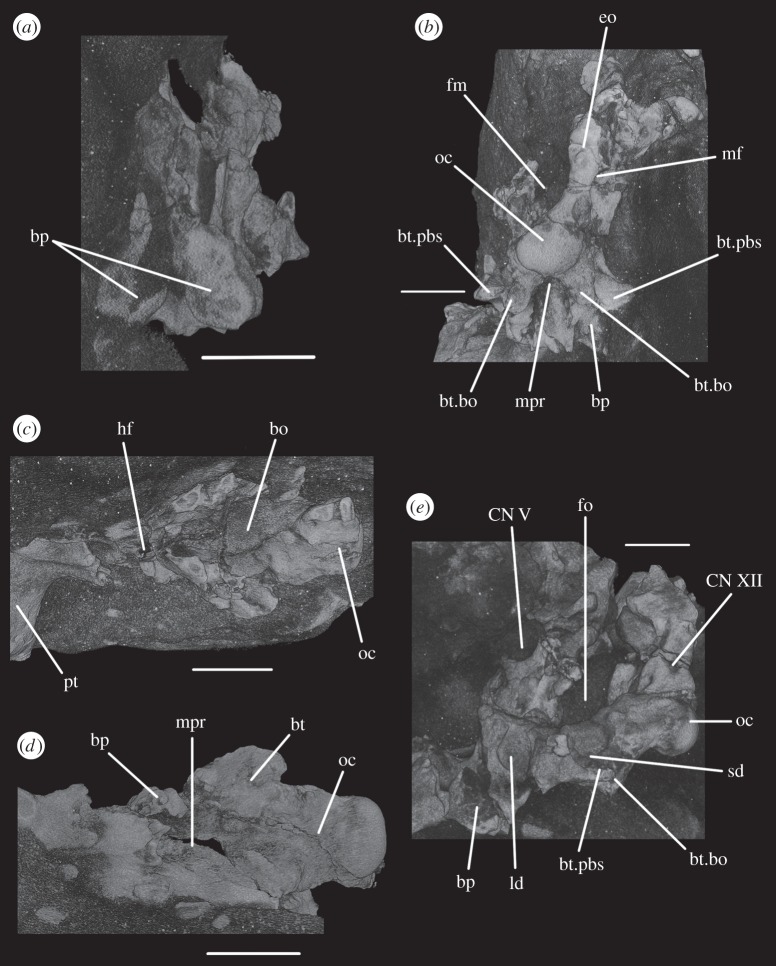
Figure 7.

CT reconstructions of braincase of SAM-PK-5867 in (a) anterior view in cross section through skull and (b) posteroventral view. For abbreviations, see table 1.
Figure 11.
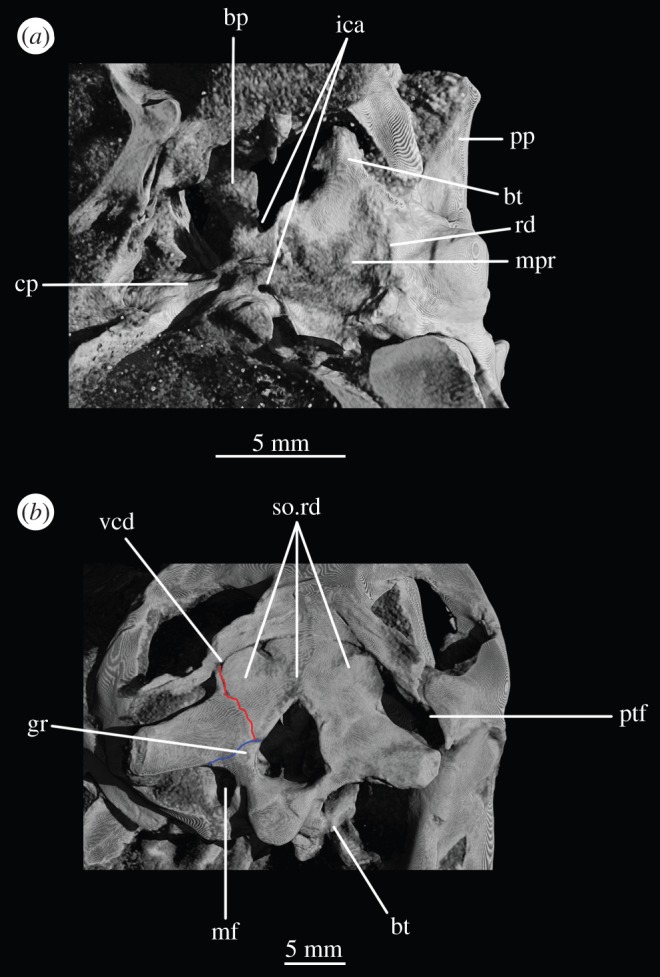
CT reconstructions of braincase of SAM-PK-5867 in (a) ventral and (b) posterior views. In (b), red line indicates the suture between supraoccipital and opisthotic and blue line indicates the suture between opisthotic and exoccipital. For abbreviations, see table 1.
Figure 6.
CT reconstructions of the braincase of SAM-PK-7696 showing sutures in (a) anterior, (b) ventral and (c) right lateral views. Bones of the braincase have been rendered transparent for better visualization of the suture lines. For abbreviations, see table 1.
Anterior to the occipital condyle the basioccipital expands laterally to form the basioccipital contribution to the basal tubera (figures 7b and 8b). A low, rounded ridge extends obliquely from the occipital condyle to about half the distance to the ventrolateral extreme of the contribution on each side, separating a more horizontally orientated ventral surface of the basioccipital from a more vertically orientated dorsal surface (figure 7b, rd). In UMCZ T.692, the dorsal parts of the expanded part of the basioccipital contribution on each side appear to be missing. This ridge seems to be the posterior counterpart of the concave articular surface (for the parabasisphenoid) that is located on the anterior face of the contribution of the basioccipital to the basal tuber, as seen in Prolacerta [46].
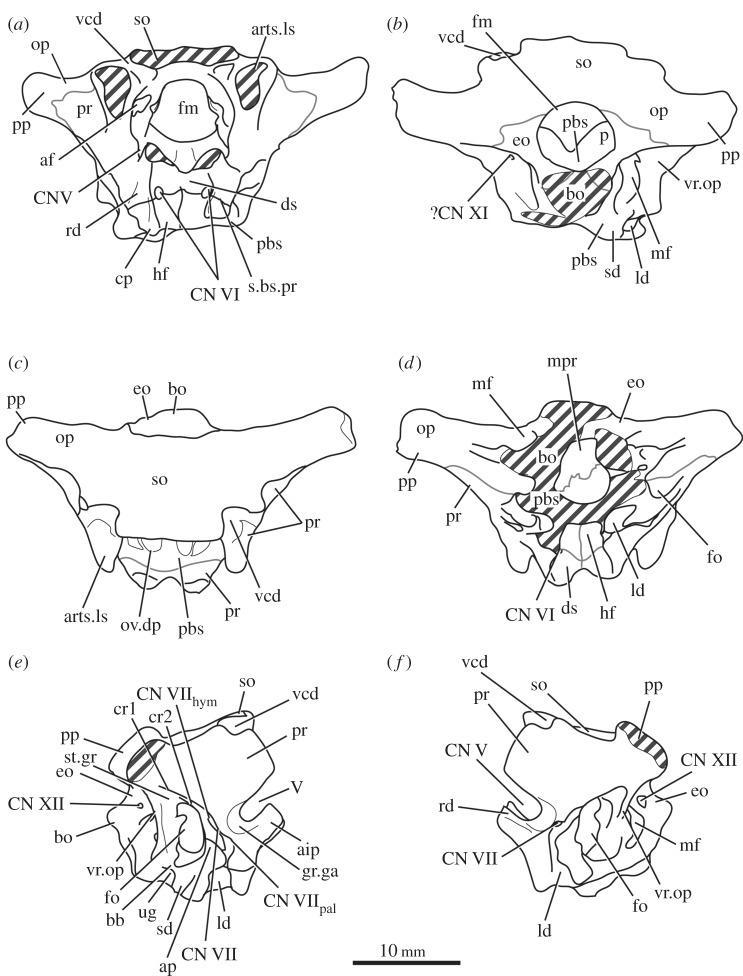
Figure 8.
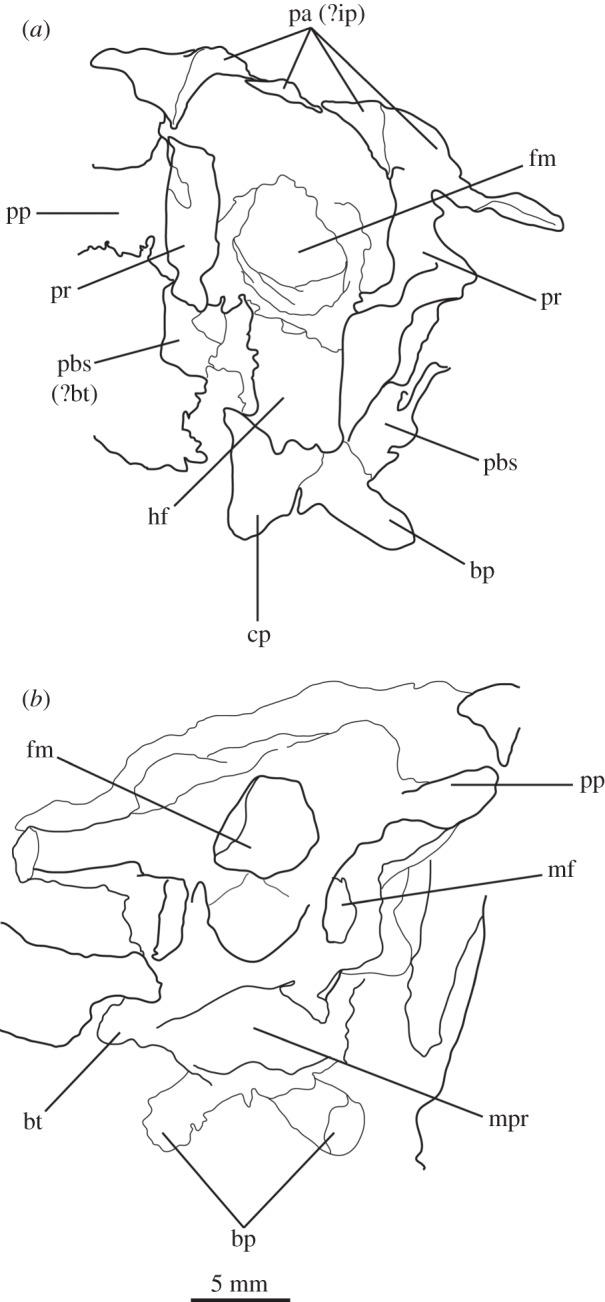
Line drawings of figure 7. Braincase of SAM-PK-5867 in (a) anterior view and (b) posteroventral view. For abbreviations, see table 1.
The basal tubera are separated in posterior view, but are connected to each other by a low ridge (figures 1d and 11a, rd) which formed the posterior margin of the basioccipital–basisphenoid fossa [51]. This fossa forms the posterior part of the ventral median pharyngeal recess (sensu Witmer [52]; figure 7b, mpr); the posterior surface of the parabasisphenoid lacks the ‘intertuberal plate’ that separates the basioccipital–basisphenoid fossa from the rest of the median pharyngeal recess in some other Triassic archosauriforms (e.g. [51]).
The basioccipital also forms the floor of the metotic foramen. The suture between basioccipital and parabasisphenoid extends in a gently meandering line transversely across the braincase, ending laterally close to the posteroventral corner of the fenestra ovalis (figure 3d). Thus, the basioccipital contributes to the posterior portion of the floor of the fenestra ovalis; in lateral view, the suture line extends straight ventrally (figure 6c).
Figure 3.
CT reconstructions of the braincase of SAM-PK-7696 (a) in right medial view, (b) in left medial view, (c) in anterior view (only posterior part showing, anterior cut off), (d) showing braincase floor in dorsal view, (e) in cross section to right of midline through opisthotic, to show basisphenoid contribution to ATR and (f) in cross section showing braincase floor in dorsal view, more ventral than (d), showing detail of basisphenoid posterior contact with ventral ramus of the opisthotic. For abbreviations, see table 1.
The lateral margin of the basioccipital dorsal to the basal tuber forms the posterior margin of the ‘unossified gap’ of Gower & Weber [42] (figures 1e and 2e, ug) also bounded by the ventral ramus of the opisthotic and the parabasisphenoid; the gap is well preserved as an open channel on the right-hand side of SAM-PK-7696 and in SAM-PK-5867 (discussed later; figure 9a,b, ug).
Figure 2.
Line drawings of figure 1. Braincase of SAM-PK-7696 in (a) anterior, (b) posterior, (c) dorsal, (d) ventral, (e) right lateral, and (f) left lateral views. For abbreviations, see table 1.
Figure 9.
CT reconstructions of braincase of SAM-PK-5867 in (a) right lateral view (cross section through skull), (b) right lateral view showing the ‘bridge of bone’ (basisphenoid–opisthotic contact) and (c) left lateral view (cross section through skull). For abbreviations, see table 1.
4.2. Parabasisphenoid
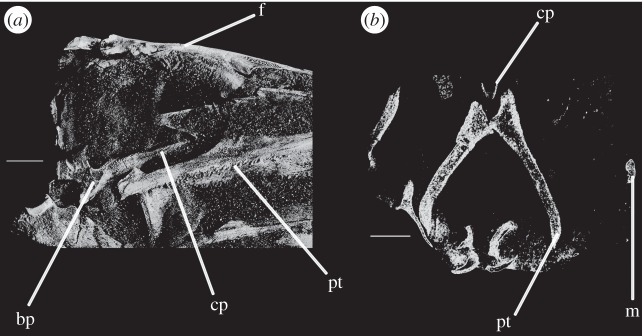
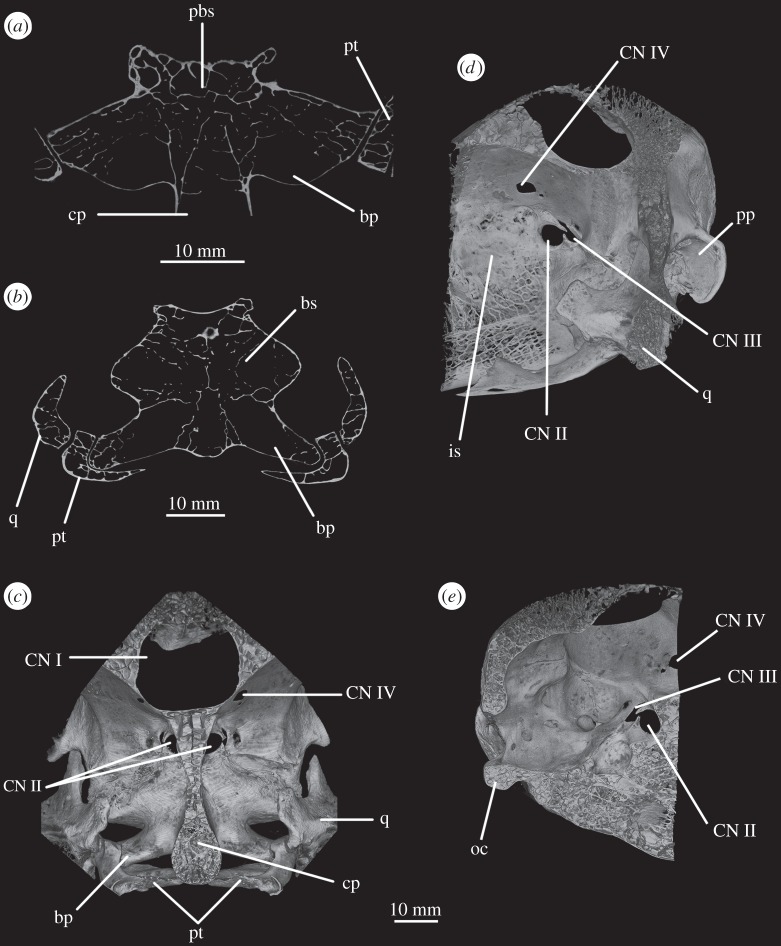
The parabasisphenoid forms the ventral part of the braincase anterior to the basioccipital, ventral to the prootics. The basal tubera are displaced dorsally in comparison to the basipterygoid processes (figure 14b), and the part of the parabasisphenoid between them can thus be described as vertically rather than horizontally aligned (following Gower & Sennikov [51]). The basipterygoid processes are well preserved in SAM-PK-K6047A (figure 12b,c,e,f), in SAM-PK-5867 (figures 7b and 11a) and in UMCZ T.692 (figure 14a,b,e). They are slightly anteroposteriorly elongated ovals in ventral view, and anterodorsally–posteroventrally elongated ovals in lateral view. The distal tips of the basipterygoid processes are ventrolaterally and slightly posteriorly directed.
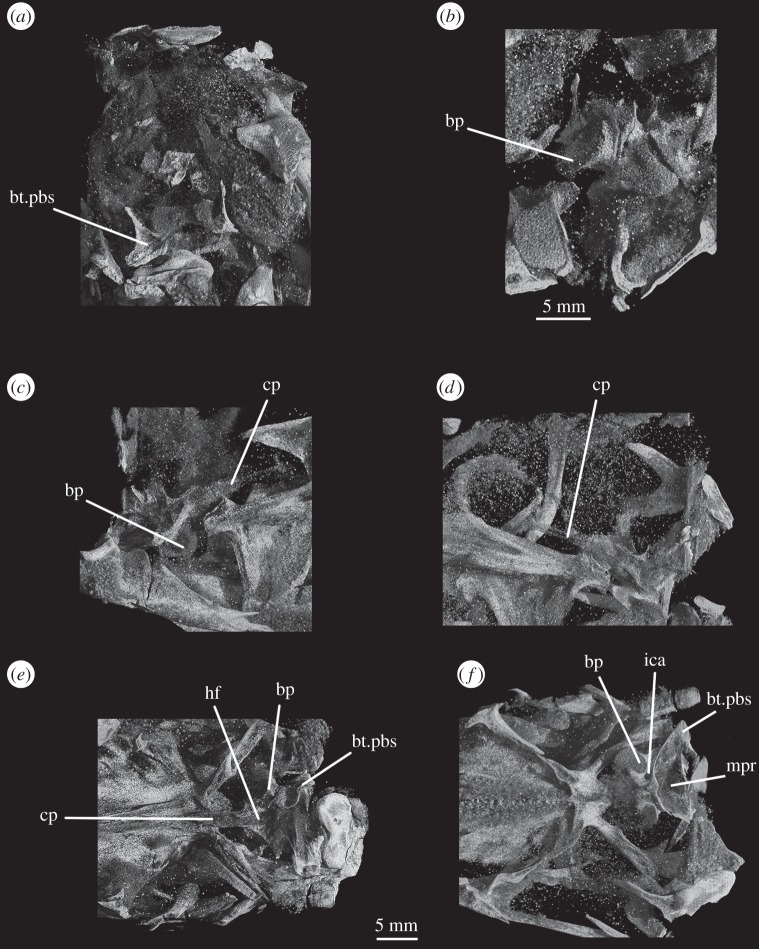
Figure 14.
CT reconstructions of braincase of UMZC T.692 in (a) anterior, (b) posterior, (c) dorsal (in cross section to expose braincase floor), (d) ventral and (e) left lateral views. For abbreviations, see table 1.
Figure 12.
CT reconstructions of braincase of SAM-PK-6047A in (a) posterior, (b) anterior, (c) right lateral, (d) left lateral, (e) dorsal and (f) ventral views. For abbreviations, see table 1.
The ventral surface of the parabasisphenoid forms the anterior two-thirds of the median pharyngeal recess (figures 1d and 7b, mpr) between the basal tubera and the basipterygoid processes. The recess bears no foramina. The suture with the basioccipital extends across the recess in a gently meandering line which is slightly anteriorly convexly curved in overall trajectory in ventral view (figures 1d, 3d and 7b). The anterior bases of the basal tubera are connected to each other by rounded lips of bone that meet in the midline, forming the anterior border of the median pharyngeal recess. They join with a median ridge extending from the ventral surface of the cultriform process, and together form a tubercle which projects posteriorly under the anterior part of the median pharyngeal recess (figure 7b).
CT data show that the suture between parabasisphenoid and prootic extends obliquely from posterolaterally to anteromedially in dorsal view (figure 6b, s.pbs.pr). However, the parabasisphenoid bears an ascending process posteriorly that conceals part of the lateral surface of the prootic and which forms the anteroventral border of the fenestra ovalis (figure 5a). Thus, in lateral view, the contact between prootic and parabasisphenoid can be described in two parts: the first, more posterior part, is anteroventrally inclined and extends from the fenestra ovalis to the groove for CN VII; the second, more anterior part, is anterodorsally inclined and starts anterior to the ‘lateral depression’ of the parabasisphenoid and the groove for CN VII (figure 5a). This interpretation of the relationships between these two bones differs from that of previous authors and is discussed in a later section.
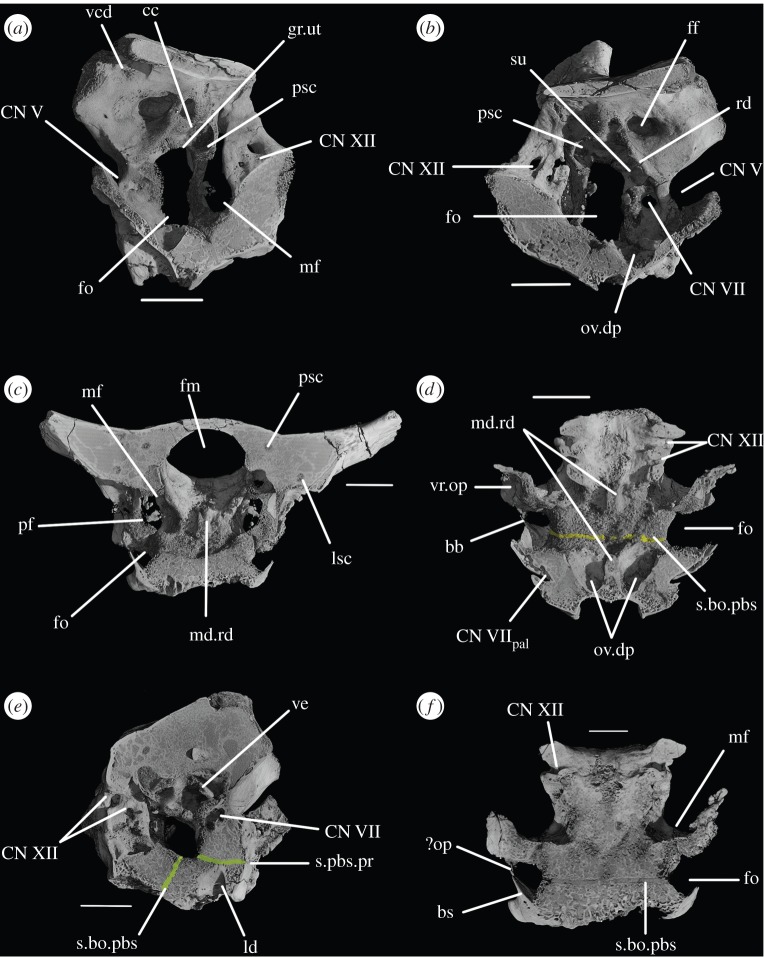
Figure 5.
CT reconstructions of the braincase of SAM-PK-7696 (a) in right posterolateral and slightly ventral view and (b) in left posterolateral and slightly dorsal view. For abbreviations, see table 1.
The basal tubera are mostly lost in SAM-PK-5867 and completely lost in SAM-PK-7696. However, based on what remains in those two specimens and on UMZC T.692 and SAM-PK-6047A, the parabasisphenoid contribution to the basal tubera extends posteroventrally and laterally from near the anteroventral margin of the fenestra ovalis (figures 12f and 14b,d,e). On the right-hand side of SAM-PK-7696, the lateral surface of the parabasisphenoid contribution to the basal tuber bears a deep, posteroventrally open sulcus—the semilunar depression of Gower & Weber [42] and Evans [53] (figures 1e and 2e, sd). This cannot have been an articulation for the ventral ramus of the opisthotic (as suggested by Evans [53]), as the braincase is articulated and the ventral ramus of the opisthotic instead ends more posteriorly, close to the basioccipital contribution to the basal tuber, and connected to the parabasisphenoid laterally by a thin strip of bone. Posterior to the semilunar depression, and anterior to the distal end of the ventral ramus of the opisthotic, is the ‘unossified gap’ of Gower & Weber [42] (figures 1e and 2e, ug).
Anterior to the anterodorsal extremity of the basal tuber, the lateral surface of the parabasisphenoid is deeply concave (the ‘lateral depression’ of Gower & Weber [42]; figure 2e, ld). This concavity is confluent with the groove for the palatine branch of CN VII (figure 2e, CN VIIpal), which extends down the lateral surface of the prootic and would have continued down the anterolateral surface of the basipterygoid process as an osseous groove, as in other reptilians (e.g. Captorhinus [54]; Ctenosaura pectinata [55]; Dysalotosaurus lettowvorbecki, [56]), but is not observable due to preservation.
In lateral view, the posterior third of the braincase floor is subhorizontal, though convex (figure 3b). More anteriorly, the floor slopes ventrally (figure 3b), and a low median ridge (figure 3d, md.rd) divides this sloping section into left and right halves, both of which are gently concave. The anterior third of the floor shows two large, oval depressions (figure 3b,d, ov.dp) with their longer axes extending posterolaterally–anteromedially. These depressions are a little deeper anteriorly than posteriorly, and they are separated by a thick, dorsally flat strip of the braincase floor, which may have connected to the ridge seen more posteriorly on the braincase floor (this cannot be ascertained because of damage to the braincase floor in SAM-PK-7696).
The ventral surface of the parabasiphenoid between the basipterygoid processes is very gently concave, with a pronounced median ridge extending from the anterior margin of the median pharyngeal recess to the base of the cultriform process (=rostrum) of the parabasisphenoid (figure 11a). This ventral surface bears, on each side, a foramen for the internal carotid artery (figure 11a, ica), placed at the posteromedial base of the basipterygoid process, immediately anterior to the lips of bone connecting the basal tubera (as mentioned earlier). The cultriform process (figures 12c–e, cp and 14) is elongated and tapers to a distal point, and its dorsal margin dips slightly ventrally close to its base then rises dorsally again yet further proximally. In cross section, the cultriform process is deeply excavated dorsally, forming a U-shape in anterior view.
In anterior view, the suture between the parabasisphenoid and the prootic extends from ventrolaterally to dorsomedially, through the foramen of CN VI on each side (the margin of which is thus formed half by the parabasisphenoid and half by the prootic), meeting in an apex at the midline close to the dorsal border of the dorsum sellae (figure 2a). The posterior wall of the hypophyseal fossa is gently concave transversely and has a central, low ridge extending dorsoventrally. The clinoid processes (figures 1a and 2a, cp) protrude a small distance medially over the posterior wall of the hypophyseal fossa, concealing the lateral borders of the foramina for CN VI in anterior view.
4.3. Exoccipital
The contact between exoccipital and basioccipital is very short (figure 6), confirming the observation of Gower & Weber [42] that the exoccipital is restricted to the pillar between the foramen magnum and the metotic foramen. The lateral surface of this pillar is smooth and shows no lateral ridge (sensu Gower [57]; figure 1e). The exoccipitals form the dorsolateral corners of the occipital condyle, and in SAM-PK-7696 do not meet at the midline to exclude the basioccipital from the foramen magnum (figures 1b and 2b). On first inspection, SAM-PK-5867 presents the impression that the exoccipitals did exclude the basioccipital from the foramen magnum (figure 7b), but this appears to be due to mediolateral compression of the braincase compounded by a preparation artefact. In UMZC T.692, the left side of the braincase is disarticulated and was not scanned with the main block of the material. The right exoccipital, however, is preserved in contact with the basioccipital (figure 14b) and a line of fracture likely represents their contact. In this specimen, the exoccipital extends further medially than in SAM-PK-5867, almost reaching the midline, but does not seem to contact its counterpart, as noted by Gower & Weber [42]. Although the braincase is not laterally compressed, the exoccipital seems to be somewhat displaced medially from its original position, decreasing the distance between left and right elements. In addition, the exoccipital facets on the basioccipital are directed slightly outwards, again indicating lack of contact. We thus conclude that there is a basioccipital contribution to the foramen magnum, but this contribution seems to be smaller in SAM-PK-5867 and UMZC T.692 than in SAM-PK-7696. The basioccipital is missing in SAM-PK-6047A.
The anterior two-thirds of the suture between the exoccipital and opisthotic are identifiable in CT scans of SAM-PK-7696, and in posterior view the suture is slanted dorsomedially–ventrolaterally (figure 6b, s.eo.op). Exactly where this suture emerges posteriorly is not entirely clear in any specimen, but based on the part of the suture visible internally in the scans, its path would roughly describe an arch that begins at the base of the paroccipital process and reaches up to the dorsal border of the foramen magnum. On the left side of SAM-PK-7696, there is a meandering line that broadly follows this same arch (figures 1b, red line and 2b). This line separates a more ventral, smoother and depressed area from a more dorsal, rougher and more convex area and almost certainly represents the exoccipital–opisthotic suture as it follows its expected trajectory, though its continuation cannot be traced internally; a slight groove is visible in SAM-PK-5867 in a similar position to the line in SAM-PK-7696 (figure 11b, blue line). In SAM-PK-7696, the posterior surface of the exoccipital is damaged at the point where this probable suture line would contact the border of the foramen magnum, preventing clarity regarding the exact contribution of the exoccipital to the foramen. However, it is probable, based on the suture line within the bone, that the exoccipital formed the lateral rim of the foramen magnum as well as the lateral parts of its dorsal rim. In SAM-PK-7696, the supraoccipital–opisthotic suture cannot be located, but in SAM-PK-5867, the supraoccipital–opisthotic suture line hits the probable exoccipital–opisthotic suture line immediately lateral to the border of the foramen magnum (figure 11b, red line), meaning that the opisthotic is excluded from the foramen, and the suture between the supraoccipital and exoccipital consists of a brief point contact at the border of the foramen magnum.
A depressed area (as mentioned earlier) below the exoccipital–opisthotic suture line is clearly present on the posterior surface of the exoccipital in SAM-PK-7696, being especially pronounced on the right-hand side (figure 1b). This feature is less apparent in SAM-PK-5867 (figure 11b). Whether this area represents a particular functional feature is unclear, but it certainly does not represent any exit foramina, as the CT scans show no traces of internal paths. In SAM-PK-7696, on the right lateral surface of the exoccipital, there are two well-marked foramina for the anterior and posterior branches of CN XII (figure 5a, CN XIIa, CN XIIp)—the posterior foramen (CNXIIp) is somewhat larger and more dorsally located. A short distance anterior and ventral to these, there seems to be a third foramen, which, in the CT scans, does not penetrate far into the bone (figure 5a, ?CN XI). If a real feature, however, this foramen could represent an independent exit for the accessory nerve (CN XI). On the left side, the foramen for the posterior branch of CN XII is clearly visible, but because the area anterior to it is somewhat damaged, the foramen for the anterior branch of CN XII is located more medially. There is no corresponding foramen to the third foramen seen on the right-hand side. In SAM-PK-5867, only one foramen is visible in this area of the exoccipital, corresponding to the exit of the posterior branch of CN XII (CN XIIp).
4.4. Opisthotic
The opisthotic forms most of the paroccipital processes and the lateral wall of the braincase between the fenestra ovalis and the metotic foramen. Contrary to Cruickshank [45], the opisthotic does not participate in the borders of the foramen magnum. The contact between opisthotic and prootic is broad (sensu Nesbitt [28], state 0 of character 105), being dorsoventrally extended and reaching up to half the length of the paroccipital process (figure 6). However, the prootic contribution to the paroccipital process is restricted to a sheet of bone covering the anterior surface of the process, the bulk of the process being formed by the opisthotic (figure 1a,e).
The paroccipital processes protrude posterolaterally and dorsally (figures 1, 7, 11). They are oval in cross section and the shaft is gently twisted along its length, so that the dorsal surface twists to face more posterodorsally at the distal end. The distal tips are gently rounded and separated from the main shaft of the processes by a slight constriction. The distalmost tip of the right process is missing in SAM-PK-7696. The paroccipital processes are excavated postero- and anteroventrally by the dorsal borders of the metotic foramen and the fenestra ovalis (recessus stapedialis), respectively. These excavations are separated by a ridge (corresponding to the crista interfenestralis of Oelrich [55]) ascending from the ventral ramus of the opisthotic and ending approximately half way along the paroccipital process shaft (figure 5a, ci).
The ventral ramus of the opisthotic (figures 1b,f and 2b,f, vr.op) descends ventrally from the base of the paroccipital process and bends gently posteriorly. It separates the fenestra ovalis anteriorly from the metotic foramen posteriorly. In posterior view, the ventral ramus of the opisthotic is clearly visible, with its lateral margin offset laterally from that of the exoccipital by a distance roughly equal to the width of the exoccipital (figures 1b and 2b, vr.op). In posterior view, the lateral margin of the ventral ramus of the opisthotic is laterally concave and extends from ventromedially to dorsolaterally. The distal end of the ventral ramus of the opisthotic is roughly level with the dorsoventral midpoint of the occipital condyle. In transverse cross section, the long axis of the ramus is anteromedially to posterolaterally directed; the perilymphatic duct would thus have extended anterolaterally to posteromedially (discussed later). On the anterior surface of the left ventral ramus of SAM-PK-7696, the well-marked lagenar crest protrudes anteriorly (figure 5a,b, lg.cr), separating the vestibular region dorsally from the cochlear region ventrally. This structure is missing on the right side. The distal end of the ventral ramus of the opisthotic is expanded laterally, anteriorly and posteriorly compared with the rest of the shaft, but this expansion does not compare with that seen in some other stem archosaur taxa (e.g. Garjainia prima [51]).
On the ventral border of the right fenestra ovalis in SAM-PK-7696, there is a thin sagittal bony contact between the ventral ramus of the opisthotic and the posterior region of the parabasisphenoid (figure 2e, bb). This bony contact forms the lateral limit of a small foramen (figure 2e, ug), the medial edge of which is delimited by the lateral surfaces of the basioccipital and the parabasisphenoid forming the braincase floor. The ventrolaterally open area ventral to this foramen was identified by Cruickshank [45] as the lagenar recess, whereas Gower & Weber [42] identified it as an ‘unossified gap’. This area was probably covered by cartilage, with the tip of the lagena projecting through the foramen (discussed later).
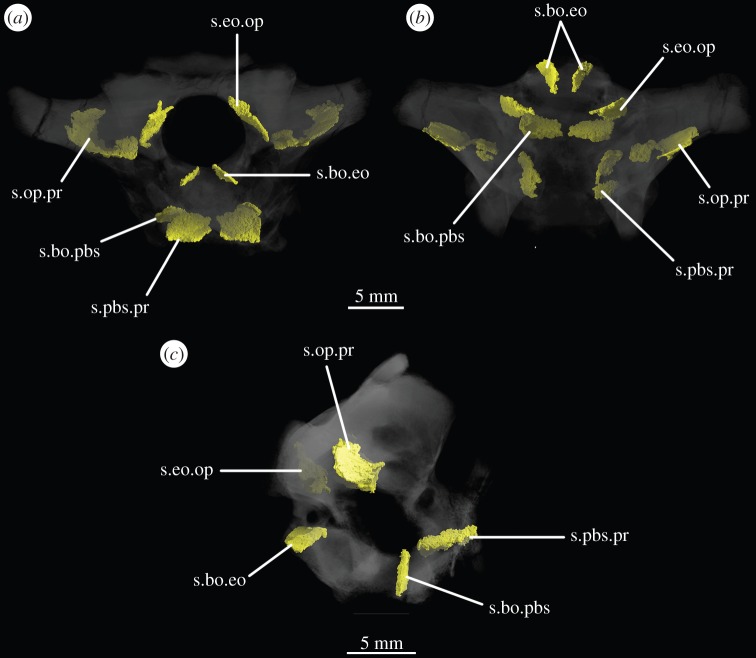
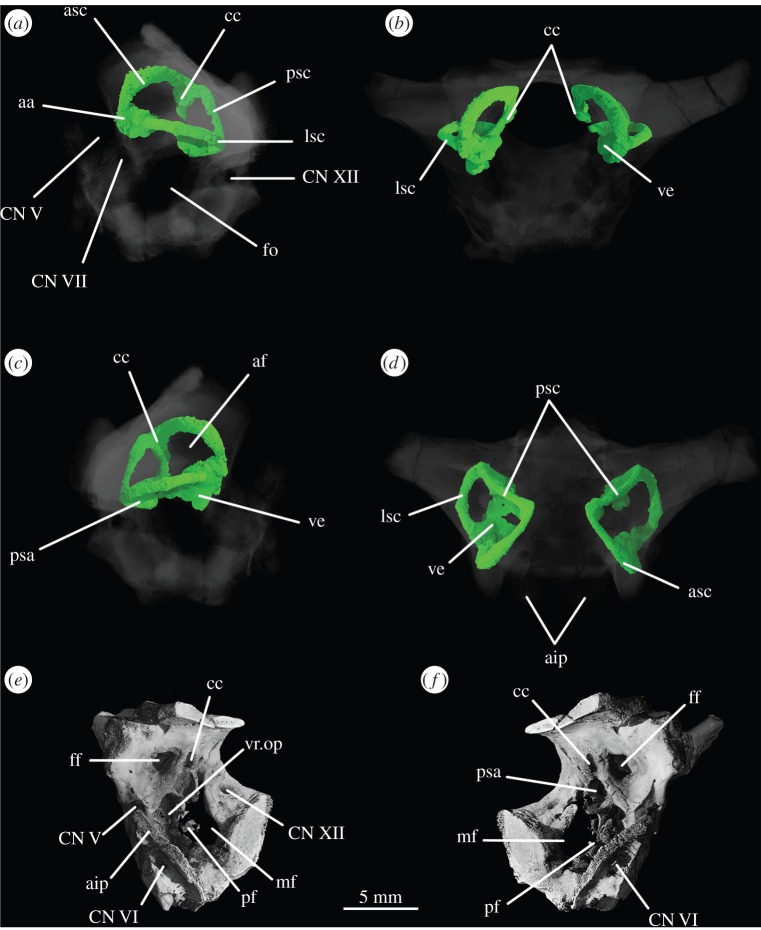
On the dorsal part of the medial wall of the opisthotic, there are two confluent, medially open depressions (figure 3a,b, cc, psc). One is smaller and positioned more anterodorsally than the other. The first corresponds to the common, dorsal openings of the anterior and posterior semicircular canals, termed common crus. The second, posteroventral one corresponds to the posterior ampulla, from which the posterior semicircular canal leaves the vestibule. On the right-hand side of SAM-PK-7696, the ventral ramus of the opisthotic has a large, rounded notch occupying all the region ventral to the confluence of these two depressions (figures 3c and 16e,f, pf). This notch marks the border between the otic capsule and the occipital region, through which passed the perilymphatic duct. The notch is, however, too large to be considered only the lateral border of the perilymphatic foramen, and it may have housed other structures such as part of the perilymphatic sac (see inner ear and discussion sections below).
Figure 16.
CT reconstructions of braincase of SAM-PK-7696 showing structures of inner ear in (a) left lateral, (b) anterior, (c) right lateral and (d) dorsal views, and showing (e) right medial wall in anteromedial view and (f) left medial wall in anteromedial view. In (a–d), bones of the braincase have been rendered transparent for better visualization of the suture lines. For abbreviations, see table 1.
4.5. Prootic
The prootic forms the lateral wall of the braincase posterior to the laterosphenoid and anterior to the fenestra ovalis (figures 1e,f and 2e,f, pr). Posterolaterally, the prootic extends onto the anterior surface of the paroccipital process in a laterally tapering sheet that reaches to just under half way along the paroccipital process (figure 1a,e,f). Anteriorly, the prootic contacts the laterosphenoid, and forms the dorsal, posterior and ventral margins of the large foramen for the trigeminal nerve (CN V), with the laterosphenoid forming the anterior margin. The CN V foramen (figure 1f, CN V) is oval, with its long axis extending posteroventrally to anterodorsally. The anterodorsal extremity of the prootic forms the ventral floor of a recess (figures 1a,c,e,f, 3a and 5b, vcd) which was roofed by the supraoccipital dorsomedially and, when in articulation, by the parietal dorsolaterally. The laterosphenoid may have formed the anterior wall of this channel, though it is disarticulated in all specimens. The smooth and rounded nature of this recess, and the relationships of the cited elements as seen in other taxa suggest that it was not simply an articulation for the parietal and/or laterosphenoid, but a channel for the vena capitis dorsalis [56,58–60], which would have connected the braincase cavity with the temporal region. In the CT scans of SAM-PK-5867, whether such a channel is present is difficult to assess, but there does appear to be a rounded opening which may represent its lateral extreme (figure 11b). A corresponding structure identified as a venous sinus is found on the supraoccipital of Osmolskina czatkowicensis [61]. Immediately posterior to the lateral part of this recess is a small posterolaterally directed depression with marked borders which appears to have been a facet for the parietal (figure 5a, fc.pa).
Posteroventral to the foramen for CN V, the prootic is slightly depressed, indicating the position of a Gasserian ganglion external to the brain cavity (figures 1e,f and 2e,f, gr.ga). A sharp crest (here referred to as crest 1, to avoid terminological confusion) extends ventrally down from the paroccipital process (figure 2e, cr1), forming the anterior margin of the stapedial groove and then, ventral to the dorsal margin of the fenestra ovalis, the anterior margin of the groove for the hyomandibular branch of CN VII (CN VIIhym, figure 1e). A lower crest (crest 2; figure 2e, cr2) originates from this crest at the dorsal margin of the fenestra ovalis, and forms the posterior margin of the dorsalmost part of the groove for CN VIIhym and then the anterior margin of the fenestra ovalis. Crest 1 becomes much lower and arcs anteriorly then posteriorly again just below the exit for CN VII, before descending directly ventrally and approaching the posterior wall of the groove for the palatal branch of CN VII (CN VIIpal). Further ventrally still, the wall of the groove for CN VIIpal and crest 1 diverge once again below to form the posterior and anterior margins of the lateral depression, respectively. A bulging ridge marking the path of the lateral semicircular canal follows the line of crest 1 dorsal to the foramen for CN VII, but is inset anterodorsally from the crest. Once it reaches a point level with the foramen for CN VII, this ridge curves sharply anterodorsally, bordering the dorsal margin of the depression for the Gasserian ganglion (figure 2e). In lateral view, the posterior surface of the prootic forming the anterior border of the fenestra ovalis of SAM-PK-7696 bears a lagenar crest (figure 5a,b, lg.cr), a smoothly rounded protuberance that marks the division of the vestibular and cochlear regions of the inner ear.
The medial wall of the prootic possesses a large, round and very deeply marked fossa immediately dorsal and a short distance posterior to the foramen for CN V—the floccular fossa (= fossa subarcuata, auricular fossa; figures 1a and 3a,b, af). The posterior wall of the fossa has a marked, deeper subregion that seems to enter the bone but does not lead off within it. Ventral to the floccular fossa, the left foramen for CN V is separated from the inner ear by an elevated and rounded ridge (figures 3b, rd and 4). Posterior to this ridge, close to its base, there seems to be a dorsoventral sulcus, perhaps leading dorsally to the foramen for CN VII or ventrally to the brain cavity (figure 3b, su). The foramen for CN VII is connected to the oval depressions (as mentioned earlier) on the anterior third of the braincase floor by a groove (figure 4, gr.ov.dp.VII).
Figure 13.
Details of the cultriform process of SAM-PK-6047A in (a) right lateral and (b) transverse views. For abbreviations, see table 1.
Figure 4.
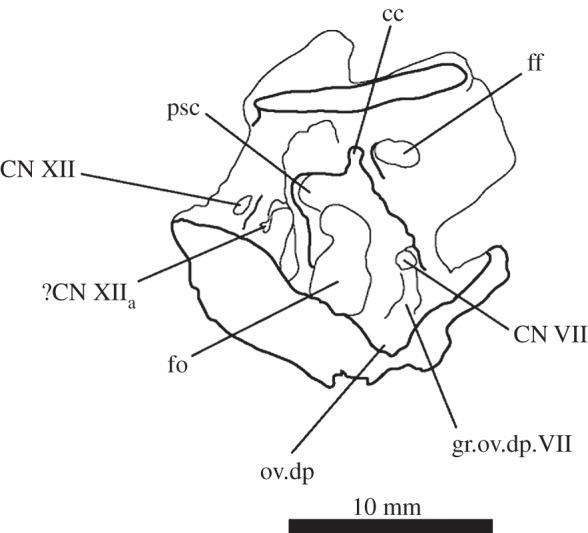
Line drawing of figure 3b. Braincase of SAM-PK-7696 in left medial view. For abbreviations, see table 1.
Ventral to the foramen for the trigeminal nerve, the prootic projects as a thin process—the anterior inferior process (figure 2e, aip). The left and right processes are connected by a sheet of bone, the dorsum sellae (figure 2a, ds). The dorsum sellae is bordered laterally by ridges, which connect ventrally to the protruded margins of the clinoid processes (figure 2a, rd; see parabasisphenoid). The middle third of the dorsal margin of the dorsum sellae dips ventrally to the midline, thus forming a V-shape in anterior view (figure 1a). The dorsum sellae forms the dorsal rim of the foramen of the abducens nerve (figure 2a, CN VI), and is directed anteroventrally rather than anteriorly, unlike, for example, in Erythorosuchus africanus [62]. On the dorsal part of the lateral surface of the anterior inferior process, immediately ventral to the trigeminal opening, is a low, very slight anteroposteriorly extending narrow ridge (figure 2f, rd); it is less pronounced than in Dorosuchus (PIN 1579/62) and is perhaps better described as a thickening of the bone along the margin of the trigeminal foramen rather than a true ridge.
4.6. Supraoccipital
The supraoccipital is a broad, flat element that forms the posterodorsal part of the roof of the braincase and forms the medial third of the dorsal border of the foramen magnum (figures 1b,c, 2, 7b, 9c, so and 11b). The supraoccipital partially housed the common crus, the posterior portion of the anterior semicircular canal and the anterior part of the posterior semicircular canal.
Laterally and posterolaterally, the supraoccipital contacts the opisthotics. The entire line of this suture is unclear in SAM-PK-7696. In SAM-PK-5867, however, in dorsal view, this suture extends in a laterally concave arc from the lateral margin of the contact between the exoccipital and the supraoccipital at the lateral margin of the foramen magnum to the anteromedial margin of the paroccipital process (figure 11b). Anterior to the end of this suture, the supraoccipital contacts the prootic along the dorsolateral extreme of the braincase (figures 2e,f and 11b). The suture line could not be identified in SAM-PK-7696, but in SAM-PK-5867 it describes a gentle, medially concave arc from the lateralmost point of the suture between supraoccipital and opisthotic to the anterior border of the supraoccipital, at the dorsomedial corner of the recess of the vena capitis dorsalis (figures 3a, 11b, vcd). The lateral part of the dorsal margin of the supraoccipital thus forms the medial and posterior margins of the recess (see ‘Prootic’ section), which is anteriorly and dorsally open; immediately medial to this, the supraoccipital also roofs the medial section of this hollow.
In posterior view (figure 11b), the dorsal margin of the supraoccipital is raised into a convexity at the midline and into another, smaller convexity laterally on either side. Gentle ridges or raised strips extend back from each of these convexities (figure 11b, so.rd), with those extending from the lateral convexities moving towards the midline posteriorly (figure 11b); all three of these ridges disappear around half way to the border of the foramen magnum.
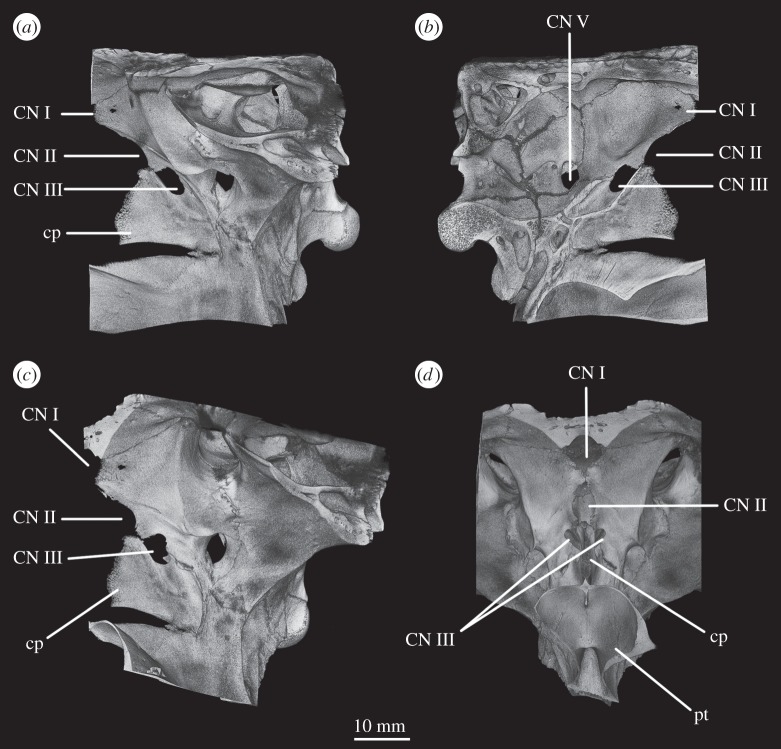
4.7. Laterosphenoid
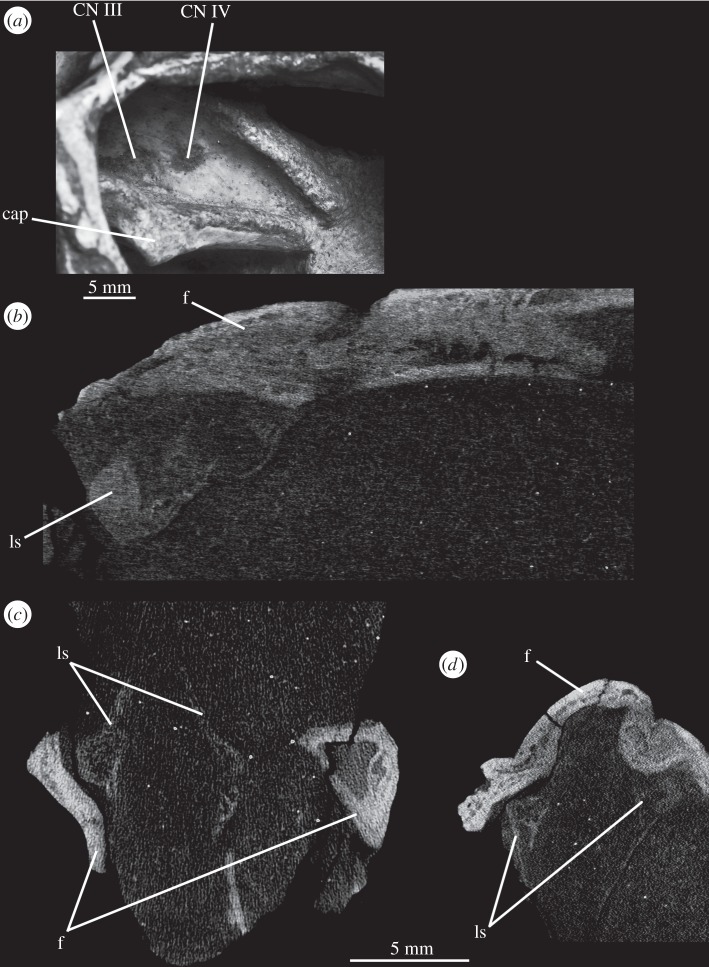
In SAM-PK-5867, the braincase wall anterior to the prootic is fully ossified as the laterosphenoid, although disarticulated from the remainder of the braincase (figure 15), indicating that it may not have been firmly sutured in life. In SAM-PK-5867, the laterosphenoid is displaced anteriorly and (probably associated with the lateral compression of the specimen seen on its left side) greatly tilted to the right in relation to the occipital area of the skull, so it is visible in dorsal view through the right orbit (figures 15 and 17a). A single disarticulated bone identified as a right laterosphenoid has been described from SAM-PK-7696 [63]. Given its fragmentary nature and lack of convincingly diagnostic features, we find that whether this is indeed a laterosphenoid (and/or the same ossification of the anterior braincase wall as preserved in SAM-PK-5867) is difficult to assess. Fragments of bone were attributed to the laterosphenoid in UMZC T.692 [42,63] and this attribution is confirmed by CT scans, but their poor preservation prevents morphological information being obtained from them. The laterosphenoid of SAM-PK-6047A is missing entirely. The presence of a laterosphenoid was noted by Clark et al. [63] for SAM-PK-5867, but that specimen was not fully prepared at that time to allow a more complete description. Although we agree with these authors on the identification of this structure as a laterosphenoid, we note some differences to the laterosphenoid of extant crocodilians, potentially indicating the presence of more elements (fused together) in Euparkeria than the ‘true’ laterosphenoid (discussed later). Irrespective of homology, the laterosphenoid of Euparkeria will be described here as a single element. The description is based on the right side of the laterosphenoid of SAM-PK-5867, unless stated otherwise, as this is more clearly visible both visually and in the CT scans.
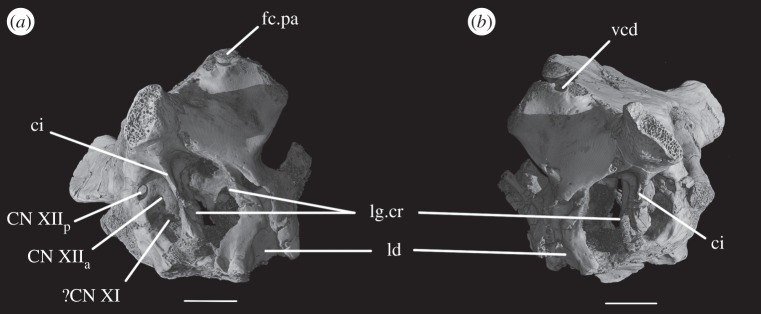
Figure 15.
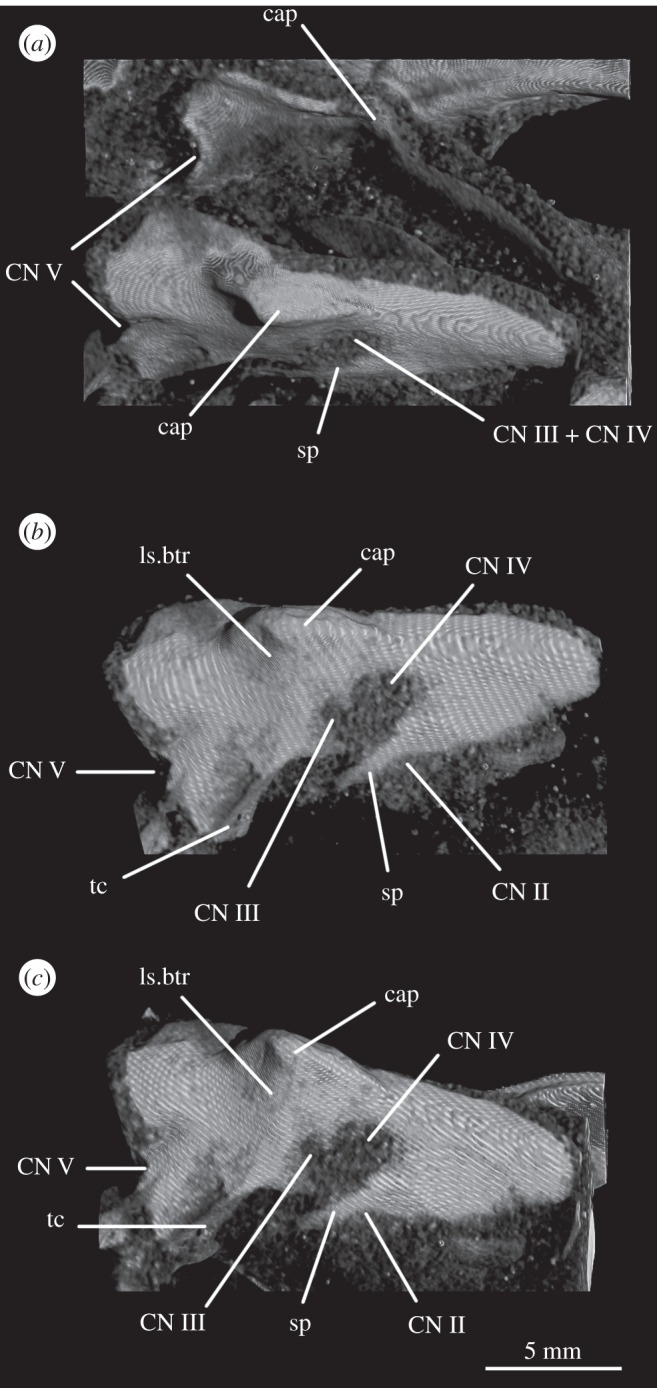
CT reconstructions of laterosphenoid(s) of SAM-PK-5867 in (a) right dorsolateral, (b) right lateral and (c) right ventrolateral views. For abbreviations, see table 1.
Figure 17.
Laterosphenoid of SAM-PK-5867 (a) in left dorsolateral view, and CT slices on laterosphenoids of UMZC T.692 in (b) right lateral, (c) dorsal and (d) posterior view. For abbreviations, see table 1.
The laterosphenoid of Euparkeria is fundamentally similar to that of Proterosuchus alexanderi (NMQR 1484), but less anteroposteriorly elongated. The laterosphenoid of Euparkeria does not reach further anteriorly than half the length of the orbit, while that of Proterosuchus alexanderi does. The anterior part of the laterosphenoid is also more ventrally extended (figure 15b,c). In dorsal view, the posterior part of the dorsal rim extends straight anteriorly, then bends sharply anterolaterally to form the posterior part of the capitate process, and then curves smoothly anteromedially again (figure 15a). In lateral view (figure 15b), the dorsal half of the posterior margin of the laterosphenoid is convexly rounded. Although it shows a subtle projection, it has no posteriorly extending process as developed as that seen in Proterosuchus alexanderi [63]. The dorsal half of the posterior margin of the laterosphenoid is separated from the ventral half by a notch, which forms the anterior rim of the foramen for CN V (figure 15b, CN V). The border of this notch is depressed laterally, as seen in the prootic, marking the position of the Gasserian ganglion. The ventral half of the posterior margin of the laterosphenoid is still in articulation with the anterior inferior process of the prootic (figures 9a and 15b,c); the laterosphenoid does not extend below the articulation with the prootic, and would not have contacted the parabasisphenoid. The lateral surface of the posterior part of laterosphenoid is smooth and delimited anteriorly by two rounded crests: a more dorsal crest, the laterosphenoid buttress (=cotylar crest of Clark et al. [63]; figure 15b,c, ls.btr), and a more ventral crest, the tensor crest (sensu Holliday & Witmer [64]; figure 15b,c, tc).
The laterosphenoid buttress curves first anteroventrally and then posteroventrally from the capitate process (figure 15, cp) to form an anteriorly convex outline with the tensor crest. Both structures, however, do not contact each other, leaving a space between them flush with the lateral surface of the laterosphenoid, at about its midheight. The capitate process (= postorbital process of Holliday & Witmer [64]:718; figure 15, cp) is very robust in comparison to the rest of the laterosphenoid, and protrudes from the main body of the laterosphenoid laterally. The tensor crest (figure 15b,c, tc) of Euparkeria is very well marked compared with that of Proterosuchus alexanderi, where it appears to be absent [63]. Anterior to the tensor crest, the laterosphenoid extends as a medially directed process, the dorsoventral extension of which is about half that of the tensor crest.
Anterior to the buttress and to the tensor crest, there is a large opening. The posterior margin of this opening bears one sharp, distinct anterior projection, just ventral to the end of the laterosphenoid buttress. On the right-hand side, this projection does not appear to reach the anterior margin of the opening, but on the left-hand side, it reaches the anterior margin to form a small foramen dorsal to it. Although visible in the specimen, the complete bar forming the foramen of the left-hand side could not be segmented out in the three-dimensional model because, despite having a different coloration from the matrix, the densities are not easily distinguishable. This bar would represent the separation between the foramina of CN III ventrally from CN IV dorsally.
The anteroventral borders of the CN III and IV foramina are formed by the slender process. Compared with Proterosuchus alexanderi (NMQR 1484; Clark et al. [63]), the slender process of Euparkeria (figure 15, sp) is longer and more posteriorly directed. The anterodorsal border of the slender process seems to be slightly notched, probably corresponding to the point of exit of CN II (figure 15b,c, CN II). No ventral crest that would correspond to that identified in Proterosuchus alexanderi [63] is visible. The lateral surface of the anteriormost region of the laterosphenoid is smooth and gently concave. The laterosphenoid tapers dorsoventrally at its anteriormost end (figure 15b,c), but, in relation to the width of the slender process, it does not extend as far anteriorly as that in Proterosuchus alexanderi [63].
4.8. Inner ear
The inner ear (figure 16) is very well preserved and when the overall size of the braincase is taken into account, it is much enlarged (82.44 mm3; table 2) when compared with that of Youngina (62.69 mm3 [50]; the only non-saurian diapsid for which the inner ear is segmented). The fenestra ovalis (figure 16a, fo) is well defined and dorsoventrally elongate when compared with that of Prolacerta [46, fig. 1] and laterally it is formed mostly by the prootic anteriorly and the opisthotic posteriorly, with participation of the parabasisphenoid ventrally and anteroventrally (figure 6c). The basioccipital also contributes to the posteroventral part of the medial margin (figure 4c). The lagenar crests (figure 5a,b, lg.cr) are situated on the anterior and posterior borders of the fenestra ovalis, at about its midheight. The lagenar crests mark the dorsalmost limit of the lagenar recess and separate the vestibular and cochlear regions of the inner ear (figure 5a,b, lg.cr). The anterior lagenar crest is low and rounded, while the posterior one is more prominent and thinner. The ventralmost tip of the lagenar recess appears to lie in the ‘unossified gap’ (sensu Gower & Weber [42]), with the cochlea having passed medial to the bony bar connecting the ventral ramus of the opisthotic and the posterodorsal region of the parabasisphenoid, and lateral to the braincase floor (figure 3d,f). The region connecting the otic capsule and the occipital region in Euparkeria is marked by a rounded notch on the medial side of the ventral ramus of the opisthotic (figures 3 and 16e,f, pf). The perilymphatic duct passed through this notch, likely through its narrowest part. This part is located more laterally in comparison to that of Sphenodon (ROM R9298) and the duct ran in a more anteroposterior direction in Euparkeria as opposed to anterolateral to posteromedial in Sphenodon (as discussed in later sections). The notch as a whole is too large to have housed the perilymphatic duct alone. Thus, the perilymphatic foramen was only partially laterally surrounded by bone (see ‘Opisthotic’ section).
Table 2.
Measurements of the inner ear of Euparkeria. The measurements of the vestibule used to compare the volume with Youngina in the text were taken from the right side because that is the only information available for Youngina. The measurements for the semicircular canals are taken from one ampulla to the other, or to the common crus, thus corresponding to the circumference of the canals at their longest point.
| right (mm) | left (mm) | |
|---|---|---|
| vestibule, height | 4.34 | 4.31 |
| vestibule, length | 6.29 | 5.93 |
| vestibule, width | 3.02 | 2.85 |
| asc | 6.64 | 6.88 |
| psc | 5.83 | 5.89 |
| lsc | 5.32 | 6.01 |
The semicircular canals (figure 15a–d) are of roughly the same length as one another. The posteroventral part of the vestibule is not enclosed by bone, in part because of the elongated fenestra ovalis, and medially the internal auditory meatus is largely unossified. The anterior semicircular canal exits the anterior ampulla anteriorly and slightly laterally (figure 16, asc), extending immediately dorsally and posteriorly, and then medially, around the floccular fossa, entering the common crus anterodorsally. Only the dorsalmost part of the common crus is surrounded by bone (figure 16e,f, cc), but its ventral connection to the utriculus is marked by a groove on the right medial wall of the braincase (figure 3a, gr.ut), allowing its course to be reconstructed. The anterior and posterior semicircular canals meet at approximately the midlength of the vestibule (figure 16a, asc, psc), but the common crus enters the utriculus just anterior to the dorsal rim of the fenestra ovalis, extending ventrally and posteriorly. The posterior semicircular canal leaves the posterior portion of the vestibule dorsolaterally and extends anteromedially into the common crus. In dorsal view (figure 16d), the paths of the anterior and posterior semicircular canals describe arches whose concavities face opposite directions. The lateral semicircular canal is a little shorter than the other semicircular canals. It leaves the posterolateral portion of the anterior ampulla, describing a gentle arc in dorsal view, and enters the vestibule laterally (figure 16d).
Like the fenestra ovalis, the metotic foramen (figures 1f, 7b and 16c--f, mf) is also dorsoventrally elongated compared with Youngina [50, online animated figure] and Prolacerta [46, fig. 1]. The lateral border of the metotic foramen is fairly uniform, but the posterior rim of the ventral part of the medial border protrudes, so that the foramen is wider at its medial margin ventrally than dorsally. The ventral portion comprises more than half of the foramen. The metotic foramen is not subdivided into a fenestra pseudorotunda and a vagus foramen and there is also no indication of an independent exit for the glossopharyngeal nerve, thus it is certain that CN X and CN IX exited the braincase through the metotic foramen. The accessory nerve (CN XI) and the vena cephalica posterior could have exited the braincase through the metotic foramen, or, alternatively, through the faint, anteriormost foramen found on the right exocciptal of SAM-PK-7696 (as discussed earlier; figure 5a, ?CN XI) and through the foramen magnum, respectively. The irregular medial shape of the metotic foramen potentially indicates the positions of the structures housed by, and exiting through, the foramen: the ventral portion is wide and rounded in posterolateral view, and likely represents the area where the perilymphatic sac sat and bulged into—the area corresponding to the recessus scalae tympani in taxa where the fenestra pseudorotunda is present. The perilymphatic foramen, which connects this area to the lagenar recess, is only ossified laterally, as described earlier. The nerves and the vein would have left dorsal to the perilymphatic sac, where the metotic foramen is narrower medially.
4.9. Cranial nerves and some vascular elements
The optic nerve (CN II) would have entered the braincase anteromedially either through a single foramen or through separate foramina for the right and left nerves. The laterosphenoid of Euparkeria is fairly well preserved, thus the absence of any medial structure, or indication thereof, seems to indicate that the CN II left the braincase through a single medial foramen (figure 16b,c, CN II), similar to the situation in Proterosuchus alexanderi [63]. The oculomotor (CN III) and trochlear (CN IV) nerves are closely related motor nerves that may have confluent foramina in some archosaur clades [65], with the CN III lying ventral to the CN IV. The nerves in Euparkeria, like in Proterosuchus alexanderi [63], had separate foramina (figure 16b,c, CNIII, CNIV), but the ventrolateral border of the foramen for CN III in Euparkeria seems to have been more complete than in Proterosuchus alexanderi. This is indicated by the presence of a small projection of the laterosphenoid (figure 15c) absent in Proterosuchus alexanderi.
The trigeminal nerve (CN V) innervates a diverse group of tissues and muscles that include the regions of the nose, mouth, facial skin, cornea, teeth, palate and pharynx, among others, and is, therefore, the largest of the cranial nerves [64]. In Euparkeria, the margin of its foramen is formed by the prootic (figure 1f, CN V), except for the anterodorsal border, which was formed by the laterosphenoid (figure 15, CN V). The lateral surface of the prootic bordering the posterior region of the foramen is gently depressed, indicating the external position of the Gasserian ganglion in relation to the brain cavity (figure 2e, gr.ga). The ventral and anteroventral borders of the trigeminal foramen are formed by the ossification of part of the base of the embryonic pila antotica—the anterior inferior process of the prootic (figure 2e, aip).
As the suture between prootic and parabsisphenoid extends through the foramina for the abducens nerve (CN VI), the prootic and parabasisphenoid thus form the laterodorsal and ventromedial borders of these foramina, respectively (figure 2a, CN VI). The foramina for CN VI are located on the dorsum sellae, near its lateral margin at about its midheight. The lateral borders of the foramina are partially concealed in anterior view by the clinoid processes of the parabasisphenoid.
On the right-hand side of SAM-PK-7696, the foramen for the facial nerve (CN VII) is largely concealed by crest 1 of the prootic (figure 1e, cr1). On the left-hand side, crest 1 does not obscure the foramen (there appears to have been post-mortem distortion on the right-hand side), but the foramen sits in a groove formed by crest 1 anteriorly (figure 1f, CN VII; also seen on SAM-PK-5867, figure 9c). This groove extends dorsally and ventrally, marking the routes of the hyomandibular (figure 2e, CN VIIhym) and palatine (figures 2e and 11c, CN VIIpal) branches of CN VII, respectively. As described earlier, the hyomandibular branch of the facial nerve would have left the braincase from the posterodorsal part of the foramen, and continued posterodorsally towards the base of the paroccipital process, whereas the palatine branch would have exited the ventral part of the foramen, and extended ventrally and then medially along the posterior margin of the basipterygoid process [46,54–56].
As the medial wall of the otic capsule is mostly unossified, there are no foramina for the branches of the auditory nerve (CN VIII). The glossopharyngeal (CN IX), vagus (CN X), and likely also the accessory (CN XI) nerves exited the braincase through the metotic foramen (figures 1e,f, 3a,f, 6, 7b, 11a,b and 12b, mf). The presence of a shallow recess that closely resembles a foramen on the right-hand side of SAM-PK-7696 may indicate a separate and independent route for the CN XI (figure 5a, ?CN XI). The dorsal portion of the metotic foramen is somewhat narrower than its ventral portion, and the cranial nerves would have left the brain cavity through the former, while the perilymphatic sac would have been sited on the latter.
In squamates, the anterior branch of the hypoglossal nerve (CN XII) has smaller fibres and innervates the tongue, whereas the posterior branch is morphologically very similar to the first and second spinal nerves [55,66]. The hypoglossal nerve may leave the braincase as a single ramus, in which case the exoccipital is pierced by only one foramen. However, the presence of two independent foramina (figure 6a, CN XIIa, CN XIIp) indicates that the divergence between the branches took place before the CN XII exited the braincase in Euparkeria.
Foramina for the ethmoidal and ophthalmic arteries were found in Proterosuchus alexanderi [63], but no similar structures could be identified in Euparkeria, possibly due to the poorer preservation of the laterosphenoid. The vidian canals are preserved in all specimens of Euparkeria except SAM-PK-7696. The canals are not well preserved in any of the specimens although it is possible to verify that they make a simple connection with the hypophyseal fossa, perforating the basisphenoid ventrally (figures 11a and 12f, ica), posteromedial to the bases of the basipterygoid processes and extending anterodorsally into the fossa. The vena capitis dorsalis exited the brain cavity through a foramen located between the prootic, supraoccipital, parietal and laterosphenoid (figures 1a,c, 3a, 5b, vcd and 11b).
5. Discussion
In the light of the new data provided by CT scanning, and re-examination of all available material of Euparkeria, it is now possible to clarify a number of points of uncertainty remaining from previous studies regarding the braincase anatomy of Euparkeria. We thus address these points, roughly in the morphological order of description used above, in the discussion. Additionally, we examine and discuss Euparkeria in the broader context of diapsid braincase evolution, with our understanding again facilitated by the new data available to us. Furthermore, we suggest new directions for future studies of archosauriform and diapsid evolution.
5.1. Previous literature
5.1.1. Basioccipital
The basioccipital of Euparkeria was described by Gower & Weber [42] as contributing to the basal tubera with ‘relatively long and slender’ [42, p. 373] projecting tongues of bone in comparison to ‘other earliest archosaurs’ [42, p. 373]. Examination of SAM-PK-5867 (figures 7b and 11a), however, shows that while the occiput of Euparkeria is overall more gracile than those of other crownward archosauriforms, the basal tubera nonetheless have similar proportions to those of, for instance, Xilousuchus sapingensis [51]. The basal tubera of Xilousuchus were described as similar to those of Garjainia prima and Fugusuchus hejiapensis, which, in turn, were considered ‘small, simple and ventrally projecting’ [51, p. 884] and ‘broad, flat and simple’ [51, p. 889] ventral projections respectively. Nesbitt [28] introduced a new character describing the nature of the anteroposterior thickness of the basioccipital portions of the basal tubera (character 106), in which non-pseudosuchian archosauriforms including Euparkeria show basal tubera which are ‘rounded and anteroposteriorly elongated’ [28, p. 89] (state 0, contrasting with the ‘bladelike and anteroposteriorly shortened’ basal tubera of some crocodile-line taxa). We agree with the scoring of Nesbitt [28] and find that the basal tubera of Euparkeria cannot be described as exceptionally long and slender among stem and early archosarus either in lateral or posterior view.
5.1.2. Parabasisphenoid
Welman [47] labelled the cerebral branch of the internal carotid artery as extending into the parabasisphenoid through foramina located in the semilunar depression. As indicated in the previous section, the entrance foramina of the artery are located on the ventral surface of the bone, posteromedial to the base of the basipterygoid processes (figures 11a and 12f, ica). This is in agreement with the scoring of character 95 of Euparkeria in Nesbitt [28]. Welman [47] also stated that the basipterygoid processes of Euparkeria are more similar in shape to those of palaeognath birds than to those of dinosauromorphs and pseudosuchians. We found it difficult to assess this statement as little explanation of exactly what is regarded as similar is provided. However, the basipterygoid processes of Euparkeria have little particular resemblance with those of palaeognath birds. The processes of Struthio camelus (ZMB 2000 2769), with which Welman [47] made extensive comparisons, are short and laterally orientated (figure 19a, bt). They are highly pneumatic and anteroposteriorly elongated in cross section, with the long axis directed slightly ventrally. This general morphology is in fact quite similar to that of Sphenosuchus acutus [67], while the processes of Coelophysis bauri [28] are also small and anteroposteriorly elongate, but ventrally orientated and with their long axes dorsally directed.
Figure 19.
Braincase of Struthio camelus (ZMB 2000 2769): (a) transverse and (b) coronal CT cut of the anterior part of the braincase showing morphology of basipterygoid processes; CT reconstructions of braincase in (c) anterior (with posterior part of braincase removed for clarity), (d) left anterolateral and (e) left medial views. For abbreviations, see table 1.
The route of the suture line between the prootic and parabasisphenoid described earlier contrasts with that described by Cruickshank [45], who indicated that the parabasisphenoid formed part of the posterior margin of the fenestra ovalis (a ‘small posteriormost process in front of the ventral ramus of the opisthotic’ [45, p. 684]) and a large part of its anterior margin. The suture between the prootic and the parabasisphenoid was identified in the CT scans as extending directly posterodorsally to anteroventrally from the anteroventral margin of the fenestra ovalis (figures 3e and 6c). However, the basisphenoid does have a small dorsal process laterally that forms the anteroventral border of the fenestra ovalis (figures 1e, 5a). The suture identified by Cruickshank [45] is in fact the groove for the palatal branch of CN VII, and the ‘posteriormost process' indicated by Cruickshank is absent. Ewer [14] indicated the parabasisphenoid–prootic suture line broadly correctly, with the parabasisphenoid ascending dorsally as a short posterior process anterior to the fenestra ovalis, then descending again along the groove for CN VII. Gower & Weber [42] excluded the dorsal process of the parabasisphenoid that contributes to the fenestra ovalis from that element entirely, indicating the prootic–parabasisphenoid suture line as extending anteroposteriorly; the posterior portion of the line indicated by Gower & Weber [42] is likely a crack, with the fragment of bone immediately dorsal to this crack also being part of the parabasisphenoid; it is also possible to identify the ascending process of the parabasisphenoid on the left side of SAM-PK-5867. A tall posterior ascending process of the parabasisphenoid was depicted by Dzik [68] in the braincase of Silesaurus. Upon examination of the relevant specimens, however, it is difficult to verify exactly the contribution of the parabasisphenoid to the fenestra ovalis. On the right-hand side of ZPAL Ab III/364 the process seems robust and tall, but on the left side it is was not found. There are no signs of processes on a second Silesaurus specimen, ZPAL Ab III/361. Stagonolepis olenkae (ZPAL AbIII/466/17, right-hand side) also shows the parabasisphenoid participating in the anteroventral border of the fenestra ovalis, but the prootic–parabasisphenoid suture is difficult to follow anterior to this and an ascending process may have been absent.
We confirm the statement of Gower & Weber [42] that the lateral depression is formed entirely by the parabasisphenoid (figure 3e) and there is no participation of another element, such as the alaparasphenoid [47] or the prootic [46]. We also confirm that the lateral depression is not connected to any cavity within the parabasisphenoid itself or within the basioccipital. This is confirmed not only upon examination of SAM-PK-7696, but also of SAM-PK-5867 and UMZC T.692, and is thus not a result, as suggested by Gower & Weber [42], of individual variation. However, the braincase as a whole is lightly constructed; while this is exaggerated to some extent in SAM-PK-7696 due to acid preparation, the entire braincase is formed of trabeculate bone. In UMZC T.692 and SAM-PK-6047A, the preserved parts of the basipterygoid processes appear hollow in the CT scans; this may be either because the delicate trabeculae were damaged or because the scan made was not sensitive enough to detect them. We were not able to locate any foramen or channel connecting these or other internal spaces to the outside (discussed later). With the reevaluation of the dorsal extent of the parabasisphenoid and its subsequent contribution to the anterior part of the lateral braincase wall, our perception of the roles of the parabsisphenoid and prootic is also altered and is discussed in a later section. Other aspects of the lateral depression and of potentially pneumatic features, such as its homologous nature with the anterior tympanic recess (ATR), will also be discussed in a later section.
Scans of SAM-PK-5867 and UMZC T.692 confirm the conclusions of Gower & Weber [42] that Euparkeria possesses no intertuberal plate, in contrast with Nesbitt [28], who scores the intertuberal plate as present and arched anteriorly. The anterior border of the medial pharyngeal recess of Euparkeria is, however, arched, and we thus suppose Nesbitt [28] must have mistaken this border for an intertuberal plate; the intertuberal plate of some taxa, e.g. Arizonasaurus babbitti (see [69]), is indeed both present and arched anteriorly, but the distribution of this morphology is much more restricted than indicated by Nesbitt [28].
5.1.3. Exoccipital
Gower & Weber [42] tentatively described the exoccipitals as not contacting medially. This was reconsidered in Gower [57], and based on the scoring of this character for Euparkeria, Nesbitt [28, p. 91] stated that ‘[p]lesiomorphically among archosauriforms, the exoccipitals meet along the midline preventing the basioccipital from participating in the endocranial cavity’, also scoring Euparkeria as having contacting exoccipitals. By tracing the suture lines in CT data, we can confirm that the exoccipitals did not meet at the midline in SAM-PK-7696, with the ventral border of the foramen magnum thus formed by the basioccipital (figure 6a). In SAM-PK-5867, the exoccipitals also do not meet, but they approach each other more than in SAM-PK-7696 (figure 7b), and we consider this to be the result of the lateral compression suffered by the specimen. In UMZC T.692, however, the right exoccipital extends further medially than in SAM-PK-5867 and almost reaches the midline (figures 11b, 14b), but does not contact its antimere. We can also confirm that there are two foramina for CN XII in both SAM-PK-7696 and SAM-PK-5867, with a third foramen in the region of SAM-PK-7696 potentially attributable to CN XI (figure 5a). We agree with Gower & Weber [42] that CN X would have left the braincase via the metotic foramen as in all modern amniotes without a proper vagus foramen, contra Ewer [14], who identified the upper portion of the CN XII foramina as being for CN X.
5.1.4. Opisthotic
The suture between opisthotic and exoccipital was identified with the assistance of CT scans (figure 6a), and it can be confirmed that the exoccipital is restricted to the pillar between the metotic foramen and the foramen magnum, not participating in the paroccipital process. We can confirm that Cruickshank [45] and Welman [47] were correct in their identification of a delicate bony bridge connecting the distal end of the ventral ramus of the opisthotic and the parabasisphenoid on the right-hand side of SAM-PK-7696 (figures 2e and 3d,f, bb). We also identify this feature on the right-hand side in SAM-PK-5867 (figure 9b, bb). Damage prevents its identification on the left-hand side in both specimens and in UMZC T.692 (as discussed later).
5.1.5. Prootic
Gower & Sennikov [51] reported a small ridge on the anterior inferior process of the prootic of Euparkeria, but further examination of UMZC T.692 led Gower & Weber [42] to reidentify it as a preservational artefact and to consider the ridge absent in Euparkeria. The absence of such a ridge was also identified by Nesbitt [28] as a synapormorphy of Archosauria (character 94). We confirm, however, that the ridge is indeed present in Euparkeria in SAM-PK-7696 (figure 2f, rd) and on the left-hand side of SAM-PK-5867 (although poorly preserved; damage prevents accurate assessment in UMZC T.692 and in SAM-PK-6047A the prootic is not preserved) and marks the ventral edge of the depression for the Gasserian ganglion. Such a ridge also forms part of the raised border of the depression for the Gasserian ganglion in other archosauriforms (e.g. Trilophosaurus buettneri [70]) and in most achosaur taxa where the entire border of the trigeminal foramen is well known, e.g. Dysalotosaurus [56], Stagonolepis olenkae (ZPAL AbIII/466/17) and Adeopapposaurus mognai (PVSJ 568). We also disagree that this feature is absent in Erythrosuchus (BP/1/3893; contra [28,62]), although the ridge is much less prominent than in crown taxa. The phylogenetic informativeness of this feature is thus, in our opinion, doubtful.
Two separate foramina for the palatine and hyomandibular branches of the facial nerve were identified first by Ewer [14], and this was followed by Cruickshank [45]. We agree with Gower & Weber [42] that only one foramen is present in UMZC T.692, and could also identify only one opening for CN VII in SAM-PK-7696 (figures 1e,f and 3b,e) and on the left-hand side of SAM-PK-5867 (the right-hand side is too damaged for assessment). As correctly noted by Gower & Weber [42], the area identified as housing the palatine foramen by Ewer [14] and Cruickshank [45] is simply a blind depressed area on the lateral surface of the parabasisphenoid. Separate foramina for the hyomandibular and palatine branches of the facial nerve are not found with certainty in any taxa outside Theropoda, and it appears safe to state that such a separation did not occur until much later in archosaurian evolutionary history, and that there is no individual variation in Euparkeria (contra the suggestion of Gower & Weber [42]).
5.1.6. Supraoccipital
Gower & Weber [42, p. 379] stated that ‘[i]f the medial suture between the prootic and supraoccipital has been correctly identified, then the posterodorsal end of the floccular recess just extends onto the supraoccipital on the left of UMZC T.692, and the broken surface exposed above the recess on the right side represents the prootic surface for articulation with the supraoccipital’. We disagree that the floccular fossa extends onto the supraoccipital, but we agree that the suture between prootic and supraoccipital should extend just dorsal to the recess. We also find it difficult to understand how, if the floccular fossa extends dorsally onto the supraoccipital, the articular surface of the prootic would be exposed dorsal to the recess. Gower & Weber [42] also state that ‘[a] shallow groove on the left side immediately anterior to the floccular recess is interpreted as indicating the probable path of the middle cerebral vein’. What we identify as the hollow for the transverse sinus, more than the middle cerebral vein itself (as mentioned earlier), is not preserved in UMZC T.692. The area indicated by Gower & Weber [42] instead corresponds to the anterior part of the subarcuate fossa which has been anteroposteriorly compressed, creating the appearance of a shallow groove.
Welman [47] identified an epiotic bone anterior to the dorsal part of the base of the paroccipital processes in both in SAM-PK-5867 and SAM-PK-7696, though only indicated the suture between it and the opisthotic and prootic in the latter. We can find no evidence for a separate ossification in this region both using CT data and on re-examination of the specimens. The anterolateral margins of the supraoccipital in SAM-PK-5867 appear to be more rounded and extended than in SAM-PK-7696, and this could be potentially indicative of an ossification separate from the supraoccipital in this position that is absent in SAM-PK-7696. However, we can find no sutural distinction between these areas of the skull roof and the rest of the supraoccipital in SAM-PK-5867, and these differences in shape may be more readily explained by mediolateral compression and the articulation with the interparietal and parietal in SAM-PK-5867. We thus find no good evidence for the existence of a separate epiotic in Euparkeria.
5.1.7. Fenestra ovalis and metotic foramen
Welman [47] used the size of the fenestra ovalis as a synapormorphy uniting Euparkeria and birds with the exclusion of dinosaurs. We agree with Gower & Weber [42] that, without quantification of ‘large’ or ‘small’, it is difficult to compare the state in Euparkeria with that in other taxa. Taxa on both the archosaur stem, e.g. Mesosuchus browni (SAM-PK-6536), and on the crocodile line, e.g. Stagonolepis olenkae (ZPAL AbIII/466/17), show a fenestra ovalis just as extensive as that of Euparkeria, and we find Welman's [47] conclusions thus doubtful. We also agree with Gower & Weber [42] that there is no fenestra pseudorotunda, rather only an unsubdivided metotic foramen. There is thus no metotic strut—the structure which would subdivide the foramen [42,52], formed from the metotic cartilage and separating CN IX from CN X [71]. Although neither feature is present in Euparkeria, we also point out that the metotic strut is a distinct feature from the lateral ridge of the exoccipital (which separates CN XIIa from CN XIIp), contra Nesbitt [28].
Character 114 of Nesbitt [28] synonymizes the lateral ridge of the exoccipital of Gower [57] with the metotic strut of theropods. However, these two structures differ in their topological position, and cannot be homologous. The term ‘metotic strut’ appears to have been introduced into the fossil avialan literature by Witmer [72] to refer to the ossification of the metotic cartilage, a structure related to the formation of the recessus scalae tympani and located between the nerves glossopharyngeal (CN IX) and vagus (CN X) [71]. The lateral ridge of the exoccipital as used by Gower [57], is, by contrast, located between the anterior and posterior branches of the hypoglossal (CN XII) nerve. Gower [57] was clearly aware of the developmental context in which the term ‘metotic strut’ was coined and used, for it had been part of the base of the argument of Gower & Weber [42, section 3a-V] for the absence of a fenestra pseudorotunda in Euparkeria. We thus disagree with the synonymization proposed by Nesbitt.
5.1.8. Inner ear
We are also able to shed light on several aspects of the anatomy of the inner ear. Gower & Weber [42, p. 389] state that ‘there is no clearly ossified differentiation between the canalicular and cochlear parts of the inner ear’ in UMZC T.692, but we do find a lagenar crest to be present in SAM-PK-7696 (figure 5a,b). However, we agree with Gower & Weber [42] that evidence regarding shape of the cochlea is inconclusive. The otic capsule of diapsids is not extensively ossified as in mammals [73], and thus the exact shape and length of the cochlea cannot be assessed based on osteology alone, and Euparkeria is no exception. We also agree with Gower & Weber [42, p. 389] that ‘part of the anteroventral limit of the vestibule can be detected in Euparkeria as a subhorizontal ridge in UMZC T.692, on the medial surface of the braincase immediately above the facial foramen’. The lagenar recess is formed equally by the basioccipital and the basisphenoid medially, and thus differs from the description of Welman [47]. Gower & Weber [42] were unsure if the unossified gap would indicate the ventralmost part of the lagenar recess, but, as we can confirm that it does (discussed later), the orientation of the cochlea can be more precisely reconstructed based on the lagenar crests and the unossified gap as being straight ventral. Nesbitt [28] (character 118) identified Euparkeria as having no well-defined lagenar recess. This is, however, based on Gower [57], which is in turn based on the reluctance of Gower & Weber [42] to identify the unossified gap as part of the lagenar recess.
A marked notch on the right medial wall of the opisthotic of SAM-PK-7696 is likely an artefact. The surfaces of the ventral ramus of the opisthotic around this notch are damaged on both sides, and CT scans show that disarticulated fragments of bone are attached to them with what appears to be glue (figure 16e,f). However, the ventralmost portion of the medial surface of the ramus does appear to bear a gently rounded broad notch, and a similar structure is seen on the left-hand side of SAM-PK-5867. This leads to the conclusion that the lateral border of the perilymphatic foramen is identifiable, but it is more ventrally located than the original area indicated by Gower & Weber [42]. The perilymphatic foramen can be confirmed to lack a bony medial border, as is also the case in Sphenodon (ROM R9298, figure 20). However, the structures of Euparkeria and Sphenodon differ in several respects. Firstly, the perilymphatic foramen in Euparkeria would have been more laterally located than in Sphenodon. Secondly, the dorsal and ventral borders of the foramen in Euparkeria are less extensively ossified, and altogether the medial extension of the ossified part of the ramus in Sphenodon is greater. Furthermore, the axis of the ventral ramus is slightly twisted in Sphenodon, so that the perilymphatic duct would have extended in an anterolateral to posteromedial direction, whereas in Euparkeria it is straighter, and the duct would thus have extended roughly anteroposteriorly. Finally, in Sphenodon, the ventral half of the perilymphatic foramen is formed by the basioccipital, while in Euparkeria it is formed by the opisthotic.
Figure 20.
CT reconstructions of braincase of Sphenodon punctatus (ROM R9298): (a) anterior view in transverse cross section, (b) anteromedial view in cross section, (c) left lateral view and (d) ventroposterolateral view. For abbreviations, see table 1.
5.1.9. Unossified gap
The open area between the ventralmost tip of the ventral ramus of the opisthotic, the anterodorsal part of the basioccipital, and the posterodorsal region of the parabasisphenoid was identified by Cruickshank [45] as the lagenar recess and by Welman [47] as the fenestra pseudorotunda. Gower & Weber [42] termed this structure as an ‘unossified gap’ and homologized it with similarly positioned unossified gaps of Sphenodon and other diapsids. Gower & Weber [42] corrected Welman's [47] interpretation of the presence of a fenestra pseudorotunda, as this structure relates to the bony subdivision of the metotic foramen and the formation of a true recessus scalae tympani. The metotic foramen of Euparkeria is not subdivided, and thus no fenestra pseudorotunda is present.
Although Gower & Weber [42] indicated that this unossified gap could represent part of the lagenar recess, there remained some uncertainty. The specimen UMCZ T.692 provides only a medial and a damaged lateral view of the braincase, and Gower & Weber [42] had only a cast of SAM-PK-7696 and the work of Cruickshank [45] at their disposal. With CT scans of SAM-PK-7696 and of SAM-PK-5867 at hand, in the light of the growing literature on braincase and neuroanatomy of fossil archosaurs [56,60,74] and based on comparisons to extant lepidosaurs [55], we confirm that this space represents the ventralmost part of the lagenar recess.
We are also able to confirm that the bony bridge separating the aperture of the gap from the margin of the fenestra ovalis is mostly formed by a thin, but marked posterolateral process of the basisphenoid (figure 3d,f). What unfortunately remains unclear is whether there is a minor anterior contribution of the opisthotic. On the right side of SAM-PK-7696, the cortex of the anterior surface of the distal part of the ventral ramus of the opisthotic seems to have been worn away during preparation (figure 1f), together with the medial border of the unossified gap formed by basioccipital and basisphenoid, whereas on the left side this structure is not preserved. The left side of SAM-PK-5867 is severely damaged and on the right side only the posterolateral process of the basisphenoid remains. If the opisthotic contributed to this bridge, then it was probably just a small eminence for the articulation with the basisphenoid.
5.1.10. Semilunar depression
On the right-hand side of SAM-PK-7696, immediately anterior to the unossified gap there is a clear depression on the lateral surface of the parabasisphenoid contribution to the basal tuber (figure 1e). The posterodorsal border of this depression is open. The anteroventral border is delimited by a crest of bone of the parabasisphenoid on the proximal end of the basal tuber. This structure was identified as the semilunar depression by Gower & Weber [42]. The term was introduced by Evans [46, p. 186] for a similar feature in Prolacerta and Mesosuchus and subsequently identified in other archosauriforms [51,61,62]. Evans [46, fig. 7] illustrated the above-mentioned feature in SAM-PK-7696, but did not label it. We are not able to locate this structure with certainty in SAM-PK-5867 because the basal tubera of the parabasisphenoid are damaged. The form of the semilunar depression in SAM-PK-7696 differs notably from other taxa, being much more pronounced and more dorsally, as opposed to laterally, open than in Prolacerta (BP/1/2675), Proterosuchus alexanderi (NMQR 880), Osmolskina (ZPAL RV/413 and ZPAL RV/424) and Dorosuchus (PIN 1579/62), but this may be exaggerated by loss of the posteroventralmost part in this specimen.
Of the putative functions suggested for this structure, the function as an articular facet for the ventral ramus of the opisthotic can probably be excluded as in articulated specimens of both Euparkeria (SAM-PK-7696) and Proterosuchus goweri (NMQR 880) the semilunar depression is exposed. Furthermore, in Prolacerta (BP/1/2675) the ventral ramus is somewhat bent at its mid-portion, so that the distalmost part is clearly ventrally directed and could not have articulated with the semilunar depresion. Likewise, Evans [46, fig. 7] illustrated the ventral ramus of Euparkeria as anteroventrally directed towards the semilunar depression, but this is inaccurate. The ventral ramus of the opisthotic is only slightly anteriorly directed and bends very gently, extending ventrally until its distal tip (figure 5a); no articulation with the semilunar depression is present. The alternative functional suggestion of Evans [46] for the semilunar depression is to serve as a ‘line of attachment for connective tissue filling in the lower part of the overlying fenestra ovalis'. Considering this and the definition of Gower & Weber [42] that a ‘lateral opening between opisthotic, parabasisphenoid and basioccipital […] represents an unossified area […] that would probably have been covered by cartilage in life’, then it would make the semilunar depression of Evans [46] the anteroventral part of the unossified gap of Gower & Weber [42]—or the parabasisphenoid contribution to the unossified gap. However, a similar depression appears to be absent in crown archosaurs, non-archosauriform archosauromorphs and Sphenodon (ROM R9298, figure 20c,d), although an unossified gap is often present. With the absence of a homologue in extant taxa we cannot be certain as to the function of the semilunar depression.
5.1.11. Pneumatization
No true pneumatic cavity was found in the sense of an internal space within a bone connected through a foramen to other external spaces such as the middle ear cavity or the pharyngeal sinus. However, CT scanning revealed trabeculate, rather than compact, bone histology in the braincases of Euparkeria. The pneumatic system often includes shallow recesses that do not necessarily perforate adjacent bones (hereafter termed pneumatic sinuses), and in the case of Euparkeria, these may include the ventral median pharyngeal recess (figures 1d, 7b, 11a, 12f and 14b,d, mpr) and the lateral depression of the parabasisphenoid (figures 2e,f, 3e, 5a,b, 9a and 14e, ld). The median pharyngeal recess (basisphenoid recess of Witmer & Ridgely [75]) is present in a number of non-archosauriform reptiles such as Captorhinus laticeps [54], Youngina [50] and Prolacerta [46], whereas the lateral depression is probably homologous with the ATR of theropods and birds (as discussed later; sensu Witmer & Ridgley [75]), but unlike in coelurosaurs [75], it shows no pneumatization of the surrounding bones. Nesbitt [28] scored a median pharyngeal recess as absent in Euparkeria (character 107), but his definition of this structure seems to correspond to a pronounced depression at the anterior extreme of the ventral fossa at the midline. Such a depression is indeed absent in Euparkeria, although appears to also be absent in some of the taxa where this is scored as present by Nesbitt [28] (e.g. Turfanosuchus dabanensis [76]). We would advocate using a different nomenclature (e.g. pronounced midline fossa at anterior of median pharyngeal recess) to describe this more pronounced recess to avoid confusion with the more broadly applicable ‘median pharyngeal recess'.
Gower & Weber [42] described the deep lateral depression of the parabasisphenoid as not being homologous with the ATR of birds (contra [47]), and Nesbitt [28] restricted the presence of a true ATR to dinosauromorphs (character 101). However, while no pneumatic sinuses leading from the lateral depression can be identified, we find that the lateral depression in Euparkeria corresponds topologically to the ATR of dinosaurs and birds. In dinosaurs and birds, the ATR arises in the region of the internal carotid foramen, between the alaparasphenoid and the basisphenoid. The facial nerve exits the braincase within or just posterior to the ATR and its palatine branch traverses the recess [72,75]. Similarly, the ATR of Euparkeria is located on the lateral surface of the parabasisphenoid, posterodorsal to the basipterygoid process and just ventral to the exit of the CN VII, and the palatine branch of the nerve also crosses the area. In Euparkeria, the internal carotid artery does not enter the parabasisphenoid in the ATR area, but in taxa where the internal carotid artery pierces the bone laterally instead of ventrally it does so in this same region (e.g. [77], as discussed later). The ATR identified in Silesaurus lacks pneumatic sinuses [28,68], but is still classed as an ATR [68] and is extremely similar both in terms of morphology and topology to the lateral depression of Euparkeria. It is also located on the lateral surface of the parabasisphenoid, posterodorsal to the basipterygoid process and ventral to the foramen of the CN VII and on the course of its palatine branch. It is, however, worth noting that the ATR of Silesaurus opolensis is deeper and larger than that of Euparkeria, with a lateral expansion of the parabasisphenoid marking its anterior limit [77].
We feel that the only meaningful distinction to be made between the ATR and the lateral depression is whether pneumatic sinuses are present leading off into the braincase wall from the depression. Following this distinction, both Silesaurus and Euparkeria would have a lateral depression while some dinosaurs would show an ATR. A more straightforward way of describing this difference, reflecting better the homology of these structures, may be an ATR lacking pneumatic sinuses versus one showing pneumatic sinuses. While we understand that caution is warranted in homologizing pneumatic structures [72], we do not see justification for homologizing the recess of Silesaurus (and other taxa lacking pneumatic pneumatic sinuses, e.g. Lewisuchus [78]) with that of dinosaurs if that of Euparkeria is not homologized similarly. We would advocate two separate homology statements: one homologizing the recess of Euparkeria, dinosaurs and Silesaurus based on its topological correspondence, and a second homologizing the presence of pneumatic pneumatic sinuses (i.e. the condition seen in theropods). We also note that a very similar structure, which we would homologize with the lateral depression of Euparkeria, is present in some non-crocodylomorph pseudosuchians (e.g. Stagonolepis olenkae [77], Prestosuchus chiniquensis [79], Shuvosaurus inexpectatus [80,81], Postosuchus [57]), and thus the presence of a lateral depression (=ATR) does not necessarily support avemetatarsalian affinities for Euparkeria.
5.1.12. Laterosphenoid
The presence or absence of an ossified element forming the anterior braincase wall of stem archosauriforms was a topic of some uncertainty until the presence of such ossifications was demonstrated in Euparkeria and Proterosuchus alexanderi by Clark et al. [63]. However, at the time of that publication, further preparation to reveal the laterosphenoids of the holotype of Euparkeria was not complete, and an apparent disarticulated laterosphenoid in SAM-PK-7696 was relied upon to describe the element. On inspection of SAM-PK-7696, we find it difficult both to confirm the identification of the element as a laterosphenoid and to identify morphological features thereof with certainty. Examination of the laterosphenoids of SAM-PK-5867 and UMZC T.692 provides confirmation of the morphology of this element in specimens where there is no doubt regarding the identification as a laterosphenoid.
The first usage of the term ‘laterosphenoid’ was to refer to the anteriormost ossification of the braincase of crocodilians [63], but subsequent usage of the term included a non-homologous ossification in snakes, a partially homologous ossification in non-ophidian lepidosaurs, and a probably homologous structure in birds. In fact, the developmental definition of a laterosphenoid was based on the embryology of non-ophidian lepidosaurs, a group in which this element seldomly ossifies [82]. According to this, the laterosphenoid represents the ossification of part of the embryonic pila antotica (figure 18), a cartilaginous structure located between the exits of CN III and CN IV anteriorly and of the CN V posteriorly [82–84]. In addition to the laterosphenoid, another element may ossify (or just calcify) in the anterior braincase wall of non-ophidian lepidosaurs: the orbitosphenoid. The orbitosphenoid is formed by the ossification of the pila metoptica (which forms the anteriormost part of the embryonic braincase, between CN II anteriorly and CN III and IV posteriorly) with some contribution from the taenia medialis—which connects the pila metoptica to the structure supporting the olfactory bulb, the planum supraseptale [82] (figure 18). By contrast, the laterosphenoid of crocodilians does not quite conform to this definition. It is composed of the pila antotica, the pila metoptica and part of the taenia medialis [82,85]. If preference is given to the first usage, the laterosphenoid and orbitosphenoid of non-ophidian squamates can thus be looked upon as ossifications of subsets of the ‘true’ laterosphenoid.
Figure 18.
Schematic drawings of the lizard chondrocranium: (a) dorsal view, late stage, illustrating connections between sensory capsules and basal plate; (b) left lateral view, showing otic capsule and connections to basal plate. Both redrawn from [84, fig. 1.2]. For abbreviations, see table 1.
The ossifications of Euparkeria and Proterosuchus alexanderi appear to fundamentally conform to the crocodilian condition, and thus presumably to include all three embryonic elements. However, the laterosphenoids of Euparkeria and Proterosuchus alexanderi differ from those of crocodilians in that a slender process is present, markedly separating the foramen of CN III and CN IV from that of CN II (figure 15, sp). By contrast, in crocodilians, the separation of these nerves by the laterosphenoid is made by a very modest process, the ventral portion of which is completed by a dorsal extension of the cultriform process of the parabasisphenoid (figure 21a–c). These differences can be interpreted as indicating a greater degree of ossification of the pila metoptica in Euparkeria and Proterosuchus alexanderi than in crocodilians. Furthermore, the foramina for the olfactory and optic nerves are only separated by a brief contact of the right and left laterosphenoids in crocodilians (figure 21d).
Figure 21.
CT reconstructions of braincase of Osteolaemus tetraspis (ZMB 23467) in (a) left lateral, (b) left medial, (c) left anterolateral, and (d) anterior views. For abbreviations, see table 1.
By contrast, in Euparkeria, and yet further so in Proterosuchus alexanderi, the contact is more extensive and the laterosphenoids of the fossil taxa extend further anteriorly between the orbits than the ossification in crocodilians, reaching as far as the anterior third of the orbit (in crocodilians the laterosphenoid does not reach the middle of the orbit, e.g. Alligator sp. specimen 238 of the Biological Sciences Collection of the University of Birmingham, UK). Such an anterior extension suggests a greater degree of ossification of the taenia medialis than in crocodilians, and also potentially that another embryonic element may, at least in part, be involved in the formation of the laterosphenoid of Euparkeria and Proterosuchus alexanderi, namely the planum supraseptale (figure 18). The planum supraseptale results from the fusion of the embryonic orbital cartilages and, as mentioned earlier, supports the forebrain. The planum supraseptale may form a ventral keel, the interorbital septum, to connect to the basal plate and together interorbital septum and planum supraseptale are considered to ossify as a third element, the sphenethmoid [82,84].
Sphenethmoids are common ossifications identified in reptiliomorphs close to amniotes such as diadectomorphs [86,87], and also in basal reptilians such as captorhinids and parareptiles [54,88,89] that are thought to be subsequently lost in saurians [90]. Although in many instances the sphenoid ossification does not ossify farther posteriorly at the anterior region of the braincase (e.g. [91]), the term ‘sphenethmoid’ does include ossifications that would in theory comprise parts of the laterosphenoid—the pila antotica, the pila metoptica and the taenia medialis [90,92]. Thus, the sphenethmoid would be homologous to the laterosphenoid of stem archosaurs and crocodilians. As revealed by the CT scans of SAM-PK-5867 and UMCZ T.692, ossifications of the anterior braincase wall are very thin and delicate structures that can be easily prepared away and it may well be that such structures are indeed present in other basal diapsids. In fact, a sphenethmoid has been identified in the diapsid of uncertain affinities Elachistosuchus huenei [93] and also tentatively in Youngina [94] (although it was not mentioned by Gardner et al. [50]).
The anterior braincase wall ossification of some basal pseudosuchians (e.g. Stagonolepis olenkae [77], Shuvosaurus [80,81]) appears to have been more similar to that of Euparkeria and Proterosuchus alexanderi than to that of modern crocodilians, in lacking a contact between the cultriform process and the slender process. However, other basal pseudosuchians do show such a contact (Stagonolepis robertsoni [72], Desmatosuchus spurensis [95]), though whether the parabasisphenoid formed part of the margin of the CN II foramen is not clear. The anterior braincase wall of other fossil taxa closer to (e.g. Prestosuchus [79]) or within (e.g. Sphenosuchus [67]) Crocodylomorpha are more similar to that of crocodilians in that the ventral border of the foramen of CN II is formed by the basisphenoid.
The condition in extant birds is more similar to the one found in Euparkeria and Proterosuchus alexanderi than to that in crocodilians, in that all the embryonic structures (namely the pila antotica, pila metoptica, taenia medialis, planum supraseptale and interorbital septum) ossify [82,84] (figures 19 and 22). However, in avian terminology, instead of sphenethmoid it is more common to find the terms ‘interorbital septum’ and/or ‘mesethmoid’ for the ossifications anterior to the foramen of CN II [96,97]. The presence of these ossifications in dinosaurs is well documented in derived ornithischians [65], sauropods [98–100] and theropods [60]. An orbitosphenoid distinguished from the laterosphenoid is often described, although in most cases sutures are difficult to identify.
Figure 22.
CT reconstructions of the braincase of Meleagris gallopavo (ZMB 1793 792) in (a) anterior, (b) right anterolateral, and (c) left medial views. For abbreviations, see table 1.
A laterosphenoid is documented for a number of less derived crown taxa [28], but well preserved and complete elements suitable for a more detailed analysis are still somewhat rare. The laterosphenoid of Heterodontosaurus tucki appears similar to that of some pseudosuchians [101], in that a short spur makes the contact with the basisphenoid. However, the contribution of the basisphenoid to the foramen of CN II is unclear. The degree of ossification, as well as the anterior extension of this structure, seems to include not only the pila antotica, but also the pila metoptica and taenia medialis—but not the planum supraseptale. A distinction is made between laterosphenoid and orbitosphenoid in figs 2B and 15B of Norman et al. [101], but no description is provided.
On the other hand, the laterosphenoids of Coelophysis bauri [28], Lesothosaurus diagnosticus [20], Tawa hallae [26], as well as those of basal ornithopods Dysalotosaurus [56], Thescelosaurus neglectus [102] and Hypsilophodon foxii [103] are strikingly different. In these taxa, the ossification seems to be restricted to the posterior part of the pila antotica only. As a consequence, the exits of the cranial nerves I–IV are not represented by foramina and there is also no contact to the cultriform process of the parabasisphenoid. A foramen for CN III is identified for Lesothosaurus [20] but we regard that as unlikely due to its posterior position. In these taxa, the laterosphenoid seems to correspond to the ossification of the pila antotica only, resembling the laterosphenoid sensu Bellairs & Kamal [82]. Whether the anterior part of the ossification was not present or has been prepared away (or if these represent juvenile individuals) is unknown, but it is likely that a reduction in the degree of ossification of the anterior braincase wall occurred in some taxa. Laterosphenoids of more derived theropods such as Alioramus altai [104] or Troodon formosus [105] are again represented by complex and extensive structures, likely encompassing multiple embryonic elements.
Irrespective of the terminology employed, our main intention here is to demonstrate that the laterosphenoids of Euparkeria, Proterosuchus alexanderi, and other basal archosaurs do not appear to be exactly the same structure as the laterosphenoid of extant crocodilians and may be more similar to that of extant birds than previously acknowledged. Also, given the delicate nature of the ossifications of the anterior braincase wall and the presence of such structures in a diapsid incertae sedis (Elachistosuchus huenei [93]), it may be that these elements were not lost in the evolutionary history of reptilians and subsequently reappeared in archosauriforms, but that it has been present throughout Diapsida, being lost only at a certain stage within Lepidosauromorpha.
5.2. Euparkeria in the context of wider diapsid braincase evolution
Since the seminal work of Gower & Weber [42], our knowledge of fossil archosauromorph braincases has increased substantially thanks to a large number of new descriptions [50,56,57,68,69,77,98,106–109], as has our understanding of the phylogenetic relationships of early and stem archosaurs [3,28,110–112]. It is thus appropriate to attempt to place the braincase of Euparkeria in the context of the wider archosauromorph and eureptilian radiation, and in the morphological trends seen both stemward and crownward of the taxon.
Increasing braincase height relative to anteroposterior length is identifiable as a trend in archosauromorph and diapsid braincase evolution, with Gower & Sennikov [51] first attempting to capture this change via the character of a verticalized parabasisphenoid, with the basal tubera dorsal to the basipterygoid processes. However, another degree of verticalization is found in non-archosauromorph diapsids in the contact between basioccipital and parabasisphenoid, so that the occipital condyle lies dorsal to the basal tubera. The braincase of basal eureptilians is anteroposteriorly flat in spite of a very subtle angle formed by the contact between parasphenoid and basioccipital (e.g. Captorhinus [54]), and the dorsoventral distance between occipital condyle and the basal tubera increases in basal diapsids (e.g. Youngina [50,85]). The angulation of the parabasisphenoid first appears in archosauromorphs.
In Euparkeria, the basal tubera are placed noticeably dorsal to the basipterygoid processes in lateral view, and the occipital condyle is in turn dorsal to the basal tubera (figures 9c, 10b and 14e). This contrasts with the state in the archosauromorph Prolacerta [46,51], and the archosauriforms Proterosuchus fergusi [51], and Fugusuchus [51], which show much more horizontal braincases. A relatively tall, verticalized parabasisphenoid is typical of crown archosaurs (e.g. Coelophysis rhodesiensis [113]; Stagonolepis robertsoni [67]), but it is also found in many archosauriforms (e.g. Erythrosuchus [62]; Sarmatosuchus otschevi [51]) and some archosauromorphs (Trilophosaurus—UCMP V6374; Mesosuchus—SAM-PK-6536). Some archosaurs, however, do show a lesser degree of angulation between basioccipital and parabasisphenoid (e.g. Desmatosuchus spurensis [95], Lewisuchus admixtus [78], Silesaurus [68], Stagonolepis olenkae [77] and proterochampsids [109]).
Figure 10.
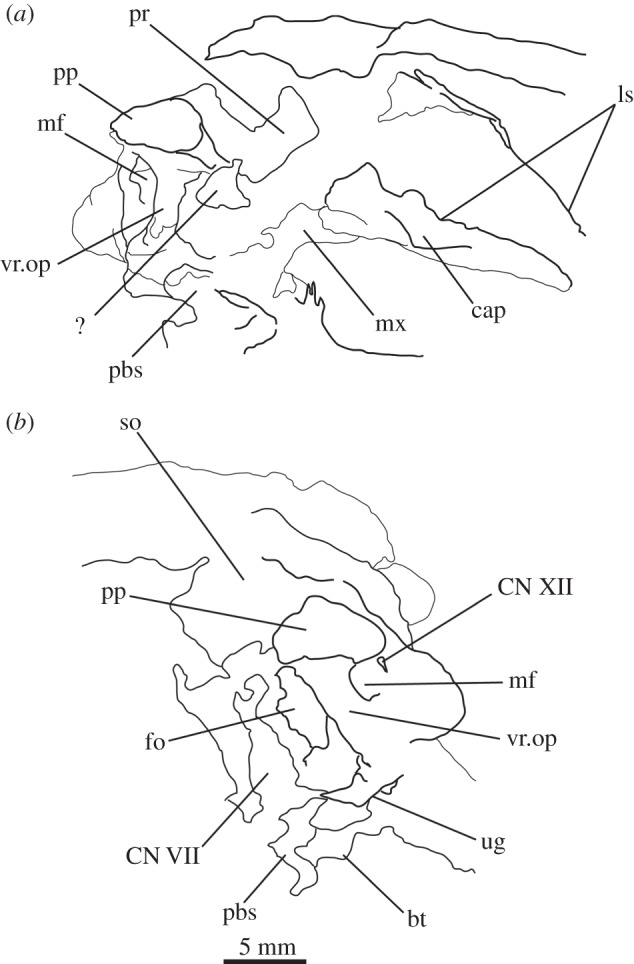
Line drawings of figure 9a,c. Braincase of SAM-PK-5867 in (a) right lateral view and (b) left lateral view. For abbreviations, see table 1.
Another trend in diapsid and archosauromorph evolution is the increase in the participation of the basioccipital in the braincase floor. As the myelencephalon appears to correlate with the basioccipital and the parabasisphenoid with the metencephalon, this may reflect an increase in the posterior part of the hindbrain relative to the anterior part. Basal eureptilians have a parasphenoid as long as two-thirds of the anteroposterior length of the braincase (e.g. Captorhinus [54]). The contribution of both elements is more equal in basal diapsids (e.g. Araeoscelis gracilis [114]; Youngina [50,115]). The situation is similar in the archosauromorph Prolacerta [46], but the basioccipital contribution is somewhat greater in the archosauromorphs Trilophosaurus [70] and Mesosuchus (SAM-PK-6536), demonstrating that this ‘trend’ is not uniform, given that these taxa are generally placed lower on the stem than Prolacerta (e.g. [116]). In Euparkeria, and many crown taxa (e.g. Silesaurus [68]; Stagonolepis robertsoni [117]) the contribution of the parabasisphenoid to the braincase floor is limited to at most the anterior third (figure 6c).
The contribution of the parabasisphenoid to the lateral braincase wall also tends to decrease towards the archosaur crown, as it is replaced by the anterior inferior process of the prootic. This development reflects assimilation of the embryonic pila antotica by the bone (discussed later). The parabasisphenoid forms the anteroventral border of the trigeminal notch in basal eureptilians (e.g. Captorhinus [54]), but in diapsids its contribution decreases due to the development of a small anterior inferior process (e.g. Youngina [50,115]). Participation of the parabasisphenoid disappears completely in archosauromorphs (e.g. Trilophosaurus [70]; Mesosuchus—SAM-PK-6536; Prolacerta [46]), including Euparkeria.
Further development of the prootic is exemplified by increased ossification dorsal to the foramen for CN V, by the relative positions of the trigeminal foramen and that for CN VII and by the prootic contribution to the dorsum sellae. These are all connected to assimilations of further embryonic structures by the prootic. The region of the prootic dorsal to the trigeminal notch and anterior to the otic capsule is the alar process [84], and appears to be absent, or very weakly developed, in early eureptilians (e.g. Captorhinus [54]). The process becomes more ossified towards the crown and in Euparkeria, Fugusuchus and Xilousuchus [51], and Erythrosuchus [62] (BPI 3893), it shows an extensive degree of ossification. The prootic may continue to expand anterodorsally in crown archosaurs, and eventually enclose the foramen of CN V entirely (e.g. Dysalotosaurus [56]).
The ossification of the body of the prootic is related to the otic capsule and to the prefacial and basicapsular commissures—connections of cartilage formed between the otic capsule and the basal plate [84]. Its lateral wall is characterized by the presence of the crista prootica, ventral to which the trigeminal and facial nerves exit the braincase. In basal eureptilians, the foramina for the nerves lie in the same horizontal plane, also indicated by a horizontal crest (e.g. Captorhinus [54]). In diapsids (e.g. Youngina [115]), a shift occurs in the relative positions of these foramina, with the facial foramen lying a short distance ventral to the trigeminal foramen. The crest thus curves gently in an anteroventral direction. In archosauromorphs the morphology is more varied, and some taxa show a roughly horizontal crest (e.g. Prolacerta [46]), while the crest of others is strongly inclined (e.g. Mesosuchus, SAM-PK-6536). The trigeminal and facial foramina in archosauriforms, including Euparkeria, are also asymmetrically positioned, albeit not to the same degree as in Mesosuchus (e.g. Fugusuchus, Garjainia prima [51], Erythrosuchus [62]). A stronger anteroventral inclination of the crista is also found in crown archosaurs (e.g. Silesaurus [68]; Stagonolepis olenkae [77]; although less so in Xilousuchus [51]).
The exact extent of the contribution of the prootic and parabasisphenoid to the dorsum sellae is difficult to confirm in some taxa. It is formed by the basisphenoid only in basal eureptilians (e.g. Captorhinus [54]), in basal diapsids (e.g. Youngina [50]), and at least in some archosauromorphs (e.g. Prolacerta [46]). The participation of the prootic in Euparkeria indicates assimilation of the embryonic basal plate by the prootic [84].
The hearing system also shows considerable changes along the diapsid and archosauromorph lineages. A pattern that has been extensively discussed in the evolution of the hearing system is the identification of the elements forming the border of the fenestra ovalis. The relative contributions of these bones are of particular interest in the construction of homology statements [118]. In basal eureptilians [54] and the basal diapsid Araeoscelis gracilis [114], the parasphenoid, basisphenoid and basioccipital contribute significantly to the ventral, anterior and posterior rims of the fenestra ovalis, respectively, with limited contribution by the prootic anterodorsally. In Euparkeria, the prootic and opisthotic form most of the anterior and posterior borders of the fenestra ovalis, respectively, with a small posteroventral contribution by the basioccipital on the medial side of the fenestra, and a contribution to the anterior rim by the posterodorsal process of the parabasisphenoid (figure 6c). In extant archosaurs, the prootic and opisthotic alone form the border of the fenestra ovalis [42], but in several extinct crown archosaurs (e.g. [56,68]) the basioccipital and parabasisphenoid are not completely excluded from the fenestra ovalis, with very restricted contributions at its ventralmost extent, while in other extinct crown taxa [95] and in erythrosuchids [62], a small basioccipital contribution at least is reported. However, this trend is not clear cut, as in the basal diapsid Youngina, and the archosauromorphs Prolacerta and Trilophosaurus the basioccipital appears to have been totally excluded from the fenestra ovalis by the opisthotic [46,50,70,115].
Elongation of the semicircular canals is seen in crown archosaurs in comparison to the only fossil basal diapsid for which the structure of the semicircular canals is well known—Youngina [50]. Strongly elongated semicircular canals are seen in flying and gliding taxa [119]. It indicates that elongation facilitates improved locomotor agility, with elongation of a particular semicircular canal corresponding to increased sensitivity in its plane of action [60,74]. The inner ear of Euparkeria shows relatively elongated semicircular canals with larger radii of curvature when compared with Youngina, especially the posterior semicircular canal. They are, however, not as strongly elongated as those of coelurosaurian theropods (especially birds [74,119,120]) and pterosaurs [119], where the anterior semicircular canal is particularly elongated. Similarly, the size of the floccular lobe, and thus the floccular fossa in which it sits, has been hypothesized to facilitate agility [121], as it emits coordination-related responses important in movements of the head and eyes [122]—although the floccular size alone may not be a good proxy for predicting the flying ability in birds [123]. An enlarged foccular lobe may be a result not only of the increase amount of floccular tissue itself but also of other parts of the vestibulocerebellum involved in postural and locomotor reflexes [123]. Expansion of these parts may be responsible for the expression of the floccular lobe through the anterior semicircular canal, and its secondary enlargement. In Euparkeria, the floccular fossa is much smaller than in modern birds [120], but larger than in Youngina, as shown not only by the increased radius of curvature of the anterior semicircular canal but also by the depth of the fossa on the medial side of the prootic. Taken together, both the form of the semicircular canals and the size of the floccular lobe may be indicative of navigation in more complex, three-dimensional environments, thus supporting a more upright, agile locomotory pattern seen in Euparkeria [17] than in ‘sprawling’ diapsids such as Youngina.
In non-saurian diapsid taxa such as Captorhinus and Youngina, there is no recess on the dorsal surface of the basioccipital/parabasisphenoid indicating that the cochlea would extend further ventrally than the limit of the braincase floor. As we interpret the distal tip of the cochlea of Euparkeria passing lateral to the braincase floor and medial to the bony bar connecting the ventral ramus of the opisthotic and the posterodorsal region of the parabasisphenoid, an increase in cochlear length is also evident in Euparkeria, indicating an improved hearing ability. Different groups of hair cells have stereocilia with varying degrees of length and stiffness, and the mass of the basilar membrane itself changes topologically. Thus, different parts of the cochlea resonate differently to the same sound frequency and the auditory epithelium is said to be tonotopically arranged along its axis [124,125]. In order to expand the hearing range, an increased number of cells are necessary, resulting in a longer cochlea. This is called mechanical tuning and is assumed to correspond to the plesiomorphic type of tonotopic discrimination in amniotes [126,127]. It has been demonstrated that cochlear length is predictive of both auditory capabilities and behaviour in extant archosaurs [128]. However, physiological tuning mechanisms such as electrical resonance became predominant during the evolutionary history of birds and turtles. In these, hair cells set up a voltage gradient via active K+/Ca2+ channels, oscillating in response to a depolarizing stimulus [126]. Although the elongation of the cochlea is also present in birds, and to a lesser extent also in crocodylians, it seems to be less important for sound discrimination than the physiological properties of the inner ear.
Because mechanical tuning is an important property of the ear of extant mammals and squamates [123,125], but not so much for birds and turtles [126], it is likely that basal archosauromorphs also relied on such mechanisms. A further, indirect indication of the importance of mechanical tuning in hearing is extensive ossification of the otic capsule. Increased ossification raises the stiffness of the system and influences frequency response by reducing energy loss due to flexion [129]. Increased ossification also promotes acoustic isolation, hindering sound conduction along routes other than those where sound detecting tissues are located. However, acoustic isolation without a compensatory mechanism for pressure relief can limit hearing capacity [73,130]. Crown group archosaurs developed a specialized pressure-relief window, a fenestra pseudorotunda, in which the metotic foramen becomes subdivided into anterior and posterior regions [42]. The compensatory mechanism of the fenestra pseudorotunda can, to a lesser extent, be carried out by the undivided metotic foramen [84]. The medial wall of the otic capsule of most eureptilians is extensively unossified, but the metotic foramen becomes increasingly enlarged. The metotic foramen of Euparkeria is even more enlarged than that of Captorhinus [54], Youngina [115] or Prolacerta [46], and there is further differentiation of a pressure-relief region ventrally.
Some of the changes seen in the lineage leading to Euparkeria, and subsequently within the archosaur crown, relate to the changing ecomorphology of the taxa. A general increase in ossification of the braincase may be indicative of development of the forebrain and improvement of terrestrial hearing. Furthermore, increased length of the semicircular canals and increased size of the floccular lobe are probably indicative of the change from a sprawling to upright gait. The changes witnessed along the lineage leading to Euparkeria and beyond can thus be framed as part of the broader terrestrial amniote pattern of increasing adaptation to terrestriality, including locomotion and hearing, and of development of increased cognitive abilities.
6. Conclusion
For the first time, a complete description of the braincase of Euparkeria is undertaken based on all available material. We were able confirm and correct several details of descriptions published previously. For instance, we confirm the presence of a laterosphenoid in the anterior braincase region of Euparkeria, and find that the element may not be fully homologous with that present in extant crocodylians, with the crocodilian condition being less ossified. We also homologize the ATR of Eupakeria with that of other basal archosaurs. The elongation of the semicircular canals (the anterior in particular) and the enlargement of the floccular fossa may correspond to development of a more upright quadrupedal posture and a more active lifestyle in Euparkeria than in basal diapsids and taxa further down the archosaur stem. The enlargement of the fenestra ovalis and of the metotic foramen, together with the regionalization of the latter and the elongation of the cochlea, are considered to be related to extension of the hearing range and improvements in the impedance matching functions of the inner ear, pointing to further development of a sense of hearing more adapted to terrestrial environments.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Jennifer Clack (Univeristy of Cambridge, UK) and Mathew Lowe (University Museum of Zoology, Cambridge, UK), Philipe Havlik (Universität Tübingen, Germany), and Sheena Kaal and Zaituna Erasmus (Iziko South African Museum, Cape Town, South Africa) for access to and/or loan of specimens in their care. Authors are also grateful to Felipe Montefeltro, Gabe S. Bever, Jason D. Pardo, Mario Bronzatti and Rodrigo G. Figueiredo for discussion, and H. Zaher for technical support.
Data accessibility
Given the large size of the individual three-dimensional models, all data generated by computed tomography scanning of the materials are deposited in the virtual database of the Museum für Naturkunde Berlin, and are available upon request.
Authors' contributions
G.S. designed the study, performed CT scans, segmented three-dimensional models, analysed and interpreted the data, and drafted the manuscript; R.B.S. designed the study, performed CT scans, analysed and interpreted the data, and drafted the manuscript; B.-A.S.B. performed CT scans, analysed and interpreted the data, and revised the manuscript; R.J.B. conceived the study, analysed and interpreted the data, and revised the manuscript; R.S. performed CT scans and revised the manuscript; J.M. conceived and designed the study, analysed and interpreted the data, and revised the manuscript. All authors gave final approval for publication.
Competing interests
The authors have no competing interests.
Funding
G.S. was supported by the DAAD and CAPES programme with funding provided by CAPES (BEX 3474/09-7). R.B.S. and R.J.B. were supported by the DFG Emmy Noether Programme (BU 2587/3-1 to R.J.B.) and a Marie Curie Career Integration Grant (PCIG14-GA-2013-630123 ARCHOSAUR RISE to R.J.B.) during completion of the work. B.-A.S.B. acknowledges the American Ornithological Union, the American Museum of Natural History Chapman Fund, the Harvard Museum of Natural History, and Yale University for funding.
References
- 1.Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO. 2012. The global diversity of birds in space and time. Nature 491, 444–448. (doi:10.1038/nature11631) [DOI] [PubMed] [Google Scholar]
- 2.Irmis RB, Nesbitt SJ, Padian K, Smith ND, Turner AH, Woody D, Downs A. 2007. A Late Triassic dinosauromorph assemblage from New Mexico and the rise of the dinosaurs. Science 317, 358–361. (doi:10.1126/science.1143325) [DOI] [PubMed] [Google Scholar]
- 3.Brusatte SL, Benton MJ, Desojo JB, Langer MC. 2010. The higher-level phylogeny of Archosauria (Tetrapoda: Diapsida). J. Syst. Palaeontol. 8, 3–47. (doi:10.1080/14772010903537732) [Google Scholar]
- 4.Brusatte SL, Benton MJ, Lloyd GT, Ruta M, Wang SC. 2011. Macroevolutionary patterns in the evolutionary radiation of archosaurs (Tetrapoda: Diapsida). Earth Environ. Sci. Trans. R. Soc. Edinb. 101, 367–382. (doi:10.1017/S1755691011020056) [Google Scholar]
- 5.Charig AJ. 1980. Differentiation of lineages among Mesozoic tetrapods. Mém. Soc. Géol. Fr. 139, 207–210. [Google Scholar]
- 6.Benton MJ. 1984. Dinosaur success in the Triassic: a noncompetitive ecological model. Q. Rev. Biol. 58, 29–55. (doi:10.1086/413056) [Google Scholar]
- 7.Tucker ME, Benton MJ. 1982. Triassic environments, climates and reptile evolution. Palaeogeogr. Palaeoclimatol. Palaeoecol. 40, 361–379. (doi:10.1016/0031-0182(82)90034-7) [Google Scholar]
- 8.Benton MJ. 1984. Dinosaurs’ lucky break. Nat. Hist. 93, 54–59. [Google Scholar]
- 9.Benton MJ. 1984. Rauisuchians and the success of dinosaurs. Nature 310, 101 (doi:10.1038/310101a0) [Google Scholar]
- 10.Sues H-D, Fraser NC. 2013. Triassic life on land: the great transition. New York, NY: Columbia University Press. [Google Scholar]
- 11.Sahney S, Benton MJ. 2008. Recovery from the most profound mass extinction of all time. Proc. R. Soc. B 275, 759–765. (doi:10.1098/rspb.2007.1370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brusatte SL, Benton MJ, Ruta M, Lloyd GT. 2008. Superiority, competition, and opportunism in the evolutionary radiation of the dinosaurs. Science 321, 1485–1488. (doi:10.1126/science.1161833) [DOI] [PubMed] [Google Scholar]
- 13.Sookias RB, Benson RBJ, Butler RJ. 2012. Biology, not environment, drives major patterns in maximum tetrapod body size through time. Biol. Lett. 8, 674–677. (doi:10.1098/rsbl.2012.0060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ewer RF. 1965. The anatomy of the thecodont reptile Euparkeria capensis Broom. Phil. Trans. R. Soc. Lond. B 248, 379–435. (doi:10.1098/rstb.1965.0003) [Google Scholar]
- 15.Hancox PJ, Shishkin MA, Rubidge BS, Kitching JW. 1995. A threefold subdivision of the Cynognathus Assemblage Zone (Beaufort Group, South Africa) and its palaeogeographical implications. S. Afr. J. Sci. 91, 143–144. [Google Scholar]
- 16.Hancox PJ. 2000. The continental Triassic of South Africa. Zbl. Geol. Paläontol. I 1998, 1285–1324. [Google Scholar]
- 17.Sookias RB, Butler RJ. 2013. Euparkeriidae. In Anatomy, phylogeny and palaeobiology of early archosaurs and their kin (eds Nesbitt SJ, Desojo JB, Irmis RB), pp. 35–48. Special Publication 379. London, UK: Geological Society of London. [Google Scholar]
- 18.Benton MJ, Clark JM. 1988. Archosaur phylogeny and the relationships of the Crocodylia. In The phylogeny and classification of tetrapods, volume 1: amphibians, reptiles, birds (ed. Benton MJ.), pp. 289–332. Oxford, UK: Clarendon Press. [Google Scholar]
- 19.Sereno PC, Arcucci AB. 1990. The monophyly of crurotarsal archosaurs and the origin of bird and crocodile ankle joints. N. Jb. Geol. Paläontol. Abh. 180, 21–52. [Google Scholar]
- 20.Sereno PC. 1991. Basal archosaurs: phylogenetic relationships and functional implications. Mem. Soc. Vert. Paleontol. 2, 1–53. (doi:10.2307/3889336) [Google Scholar]
- 21.Parrish JM. 1993. Phylogeny of the Crocodylotarsi, with reference to archosaurian and crurotarsan monophyly. J. Vert. Paleontol. 13, 287–308. (doi:10.1080/02724634.1993.10011511) [Google Scholar]
- 22.Juul L. 1994. The phylogeny of basal archosaurs. Palaeontol. Afr. 31, 1–38. [Google Scholar]
- 23.Bennett SC. 1996. The phylogenetic position of the Pterosauria within the Archosauromorpha. Zool. J. Linn. Soc. 118, 261–308. (doi:10.1080/08912963.2012.725727) [Google Scholar]
- 24.Benton MJ. 1999. Sceromochlus taylori and the origin of dinosaurs and pterosaurs. Phil. Trans. R. Soc. Lond. B 354, 1423–1446. (doi:10.1098/rstb.1999.0489) [Google Scholar]
- 25.Parker WG, Barton BJ. 2008. New information on the Upper Triassic archosauriform Vancleavea campi based on new material from the Chinle Formation of Arizona. Palaeontol. Electron. 11, 14A. [Google Scholar]
- 26.Nesbitt SJ, Stocker MR, Small BJ, Downs A. 2009. The osteology and relationships of Vancleavea campi (Reptilia: Archosauriformes). Zool. J. Linn. Soc. 157, 814–864. (doi:10.1111/j.1096-3642.2009.00530.x) [Google Scholar]
- 27.Ezcurra MD, Lecuona A, Martinelli A. 2010. A new basal archosauriform diapsid from the Lower Triassic of Argentina. J. Vert. Paleontol. 30, 1433–1450. (doi:10.1080/02724634.2010.501446) [Google Scholar]
- 28.Nesbitt SJ. 2011. The early evolution of archosaurs: relationships and the origin of major clades. Bull. Am. Mus. Nat. Hist. 352, 1–292. (doi:10.1206/352.1) [Google Scholar]
- 29.Perry SF. 1992. Gas exchange strategies in reptiles and the origin of the avian lung. In Physiological adaptations in vertebrates: respiration, circulation, and metabolism (eds Wood SC, Weber RE, Hargens AR, Millard RW), pp. 149–167. New York, NY: Marcel Dekker. [Google Scholar]
- 30.Carrier DR, Farmer CG. 2000. The evolution of pelvic aspiration in archosaurs. Paleobiology 26, 271–293. (doi:10.1666/0094-8373(2000)026<0271:TEOPAI>2.0.CO;2) [Google Scholar]
- 31.Hutchinson JR. 2001. The evolution of femoral osteology and soft tissues on the line to extant birds (Neornithes). Zool. J. Linn. Soc. 131, 169–197. (doi: 10.1111/j.1096-3642.2001.tb01314.x) [Google Scholar]
- 32.Hutchinson JR. 2001. The evolution of pelvic osteology and soft tissues on the line to extant birds (Neornithes). Zool. J. Linn. Soc. 131, 123–168. (doi:10.1111/j.1096-3642.2001.tb01313.x) [Google Scholar]
- 33.Marugán-Lobón J, Buscalioni AD. 2003. Disparity and geometry of the skull in Archosauria (Reptilia: Diapsida). Zool. J. Linn. Soc. 80, 67–88. (doi:10.1046/j.1095-8312.2003.00219.x) [Google Scholar]
- 34.Nesbitt SJ. 2003. Arizonasaurus and its implications for archosaur divergence. Proc. R. Soc. Lond. B 270, S234–S237. (doi:10.1098/rsbl.2003.0066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rauhut OWM. 2003. The interrelationships and evolution of basal theropod dinosaurs. Spec. Pap. Palaeontol. 69, 1–213. [Google Scholar]
- 36.Seymour RS, Bennett-Stamper CL, Johnston SD, Carrier DR, Grigg GC. 2004. Evidence for endothermic ancestors of crocodiles at the stem of archosaur evolution. Physiol. Biochem. Zool. 77, 1051–1067. (doi:10.1086/422766) [DOI] [PubMed] [Google Scholar]
- 37.de Ricqles A, Padian K, Knoll F, Horner JR. 2008. On the origin of high growth rates in archosaurs and their ancient relatives: complementary histological studies on Triassic archosauriforms and the problem of a ‘phylogenetic signal’ in bone histology. Ann. Paleontol. 94, 57–76. (doi:10.1016/j.annpal.2008.03.002) [Google Scholar]
- 38.Sullivan C. 2010. The role of the calcaneal ‘heel’ as a propulsive lever in basal archosaurs and extant monitor lizards. J. Vert. Paleontol. 30, 1422–1432. (doi:10.1080/02724634.2010.501450) [Google Scholar]
- 39.Maidment SCR, Barrett PM. 2011. The locomotor musculature of basal ornithischian dinosaurs. J. Vert. Paleontol. 31, 1265–1291. (doi:10.1080/039.031.0609) [Google Scholar]
- 40.Butler RJ, Barrett PM, Gower DJ. 2012. Reassessment of the evidence for postcranial skeletal pneumaticity in Triassic archosaurs, and the early evolution of the avian respiratory system. PLoS ONE 7, e34094 (doi:10.1371/journal.pone.0034094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foth C, Rauhut OWM. 2013. Macroevolutionary and morphofunctional patterns in theropod skulls: a morphometric approach. Acta Palaeontol. Pol. 58, 1–16. (doi:10.4202/app.2011.0145) [Google Scholar]
- 42.Gower DJ, Weber E. 1998. The braincase of Euparkeria, and the evolutionary relationships of birds and crocodilians. Biol. Rev. 73, 367–411. (doi:10.1111/j.1469-185X.1998.tb00177.x) [Google Scholar]
- 43.Gauthier JA, Nesbitt SJ, Schachner ER, Bever GS, Joyce WG. 2011. The bipedal stem crocodilian Poposaurus gracilis: inferring function in fossils and innovation in archosaur locomotion. Bull. Peabody Mus. Nat. Hist. 52, 107–126. (doi:10.3374/014.052.0102) [Google Scholar]
- 44.Kubo T, Kubo MO. 2012. Associated evolution of bipedality and cursoriality among Triassic archosaurs: a phylogenetically controlled evaluation. Paleobiology 38, 474–485. (doi:10.1666/11015.1) [Google Scholar]
- 45.Cruickshank ARI. 1970. Early thecodont braincases. Proc. Intern. Gondwana Symp. 2, 683–685. [Google Scholar]
- 46.Evans SE. 1986. The braincase of Prolacerta broomi (Reptilia, Triassic). N. Jb. Geol. Paläontol. Abh. 173, 181–200. [Google Scholar]
- 47.Welman J. 1995. Euparkeria and the origin of birds. S. Afr. J. Sci. 91, 533–537. [Google Scholar]
- 48.Gauthier JA. 1986. Saurischian monophyly and the origin of birds. Mem. Cal. Acad. Sci. 8, 1–55. [Google Scholar]
- 49.Sookias RB, Sennikov AG, Gower DJ, Butler RJ. 2014. The monophyly of Euparkeriidae (Reptilia: Archosauriformes) and the origins of crown Archosauria: a revision of Dorosuchus neoetus from the Middle Triassic of Russia. Palaeontology 57, 1177–1202. (doi:10.5061/dryad.n525j) [Google Scholar]
- 50.Gardner NM, Holliday CM, O'Keefe FR. 2010. The braincase of Youngina capensis (Reptilia, Diapsida): new insights from high-resolution CT scanning of the holotype. Palaeontol. Electron. 13, 16. [Google Scholar]
- 51.Gower DJ, Sennikov AG. 1996. Morphology and phylogenetic informativeness of early archosaur braincases. Palaeontology 39, 883–906. [Google Scholar]
- 52.Witmer LM. 1997. The evolution of the antorbital cavity of archosaurs: a study in soft-tissue reconstruction in the fossil record with an analysis of the function of pneumaticity. Mem. Soc. Vert. Paleontol. 3, 1–73. (doi:10.1080/02724634.1997.10011027) [Google Scholar]
- 53.Evans SE. 1980. The skull of a new eosuchian reptile from the Lower Jurassic of South Wales. Zool. J. Linn. Soc. 70, 203–264. (doi:10.1111/j.1096-3642.1980.tb00852.x) [Google Scholar]
- 54.Heaton MJ. 1979. Cranial anatomy of primitive captorhinid reptiles from the late Pennsylvanian and Early Permian of Oklahoma and Texas. Oklah. Geol. Surv. Bull. 127, 1–81. [Google Scholar]
- 55.Oelrich TM. 1956. The anatomy of the head of Ctenosaura pectinata (Iguanidae). Misc. Publ. Mus. Zool. Univ. Mich. 94, 11–22. [Google Scholar]
- 56.Sobral G, Hipsley CA, Müller J. 2012. Braincase redescription of Dysalotosaurus lettowvorbecki (Dinosauria, Ornithopoda) based on computed tomography. J. Vert. Paleontol. 32, 1090–1102. (doi:10.1080/02724634.2012.693554) [Google Scholar]
- 57.Gower DJ. 2002. Braincase evolution in suchian archosaurs (Reptilia: Diapsida): evidence from the rauisuchian Batrachotomus kupferzellensis. Zool. J. Linn. Soc. 136, 49–76. (doi:10.1046/j.1096-3642.2002.00025.x) [Google Scholar]
- 58.Bruner HL. 1907. On the cephalic veins and sinuses of reptiles, with description of a mechanism for raising the venous blood-pressure in the head. Am. J. Anat. 7, 1–117. (doi:10.1002/aja.1000070102) [Google Scholar]
- 59.Dendy A. 1909. The intracranial vascular system of Sphenodon. Phil. Trans. R. Soc. B 200, 403–426. (doi:10.1098/rspb.1909.0025) [Google Scholar]
- 60.Sampson SD, Witmer LM. 2007. Craniofacial anatomy of Majungasaurus crenatissimus (Theropoda: Abelisauridae) from the Late Cretaceous of Madagascar. Mem. Soc. Vert. Paleontol. 8, 32–102. (doi:10.1671/0272-4634(2007)27[32:CAOMCT]2.0.CO;2) [Google Scholar]
- 61.Borsuk-Białynicka M, Evans SE. 2009. Cranial and mandibular osteology of the Early Triassic archosauriform Osmolskina czatkowicensis from Poland. Palaeontol. Pol. 65, 235–281. [Google Scholar]
- 62.Gower DJ. 1997. The braincase of the early archosaurian reptile Erythrosuchus africanus. Proc. Zool. Soc. Lond. 242, 557–576. (doi:10.1111/j.1469-7998.1997.tb03855.x) [Google Scholar]
- 63.Clark JM, Welman J, Gauthier JA, Parrish MJ. 1993. The laterosphenoid of bone of early archosauriforms. J. Vert. Paleontol. 13, 48–57. (doi:10.1080/02724634.1993.10011487) [Google Scholar]
- 64.Holliday CM, Witmer LM. 2009. The epipterygoid of crocodyliforms and its significance in the evolution of the orbitotemporal region of eusuchians. J. Vert. Paleontol. 29, 715–733. (doi:10.1671/039.029.0330) [Google Scholar]
- 65.Miyashita T, Arbour VM, Witmer LM, Currie PJ. 2011. The internal cranial morphology of an armoured dinosaur Euoplocephalus corroborated by X-ray computed tomographic reconstruction. J. Anat. 219, 661–675. (doi:10.1111/j.1469-7580.2011.01427.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Willard WA. 1915. The cranial nerves of Anolis carolinensis. Bull. Mus. Comp. Zool. Harv. Col. 59, 18–116. [Google Scholar]
- 67.Walker AD. 1990. A revision of Sphenosuchus acutus Haughton, crocodylomorph reptile from the Elliot Formation (Late Triassic or Early Jurassic) of South Africa. Phil. Trans. R. Soc. Lond. B 330, 1–120. (doi:10.1098/rstb.1990.0185) [Google Scholar]
- 68.Dzik J. 2003. A beaked herbivorous archosaur with dinosaur affinities from the early Late Triassic of Poland. J. Vert. Paleontol. 23, 556–574. (doi:10.1671/A1097) [Google Scholar]
- 69.Gower DJ, Nesbitt SJ. 2006. The braincase of Arizonasaurus babbitti—further evidence of the non-monophyly of Rauisuchia. J. Vert. Paleontol. 26, 79–87. (doi:10.1671/0272-4634(2006)26[79:TBOABE]2.0.CO;2) [Google Scholar]
- 70.Gregory JT. 1945. Osteology and relationships of Trilophosaurus. Univ. Tex. Publ. 4401, 273–359. [Google Scholar]
- 71.de Beer GR, Barrington EJW. 1934. The segmentation and chondrification of the skull of the duck. Phil. Trans. R. Soc. Lond. B 223, 411–467. (doi:10.1098/rstb.1934.0009) [Google Scholar]
- 72.Witmer LM. 1990. The craniofacial air sac system of Mesozoic birds (Aves). Zool. J. Linn. Soc. 100, 327–378. (doi:10.1111/j.1096-3642.1990.tb01865.x) [Google Scholar]
- 73.Wever EG. 1978. The reptile ear: its structure and function. Princeton, NJ: Princeton University Press. [Google Scholar]
- 74.Witmer LM, Ridgely RC, Dufeau DL, Semones MC. 2008. Using CT to peer into the past: 3D visualization of the brain and ear regions of birds, crocodiles, and nonavian dinosaurs. In Anatomical imaging: towards a new morphology (eds Endo H, Frey R), pp. 67–88. Tokyo, Japan: Springer. [Google Scholar]
- 75.Witmer LM, Ridgely RC. 2009. New insights into the brain, braincase, and ear region of tyrannosaurs, with implications for sensory organization and behavior. Anat. Rec. 292, 1266–1296. (doi:10.1002/ar.20983) [DOI] [PubMed] [Google Scholar]
- 76.Butler RJ, Sullivan C, Ezcurra MD, Liu J, Lecuona A, Sookias RB. 2014. New clade of enigmatic early archosaurs yields insights into early pseudosuchian phylogeny and the biogeography of the archosaur radiation. BMC Evol. Biol. 14, 128 (doi:10.1186/1471-2148-14-128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sulej T. 2010. The skull of an early Late Triassic aetosaur and the evolution of the stagonolepidid archosaurian reptiles. Zool. J. Linn. Soc. 158, 860–881. (doi:10.1111/j.1096-3642.2009.00566.x) [Google Scholar]
- 78.Bittencourt JS, Arcucci AB, Marsicano CA, Langer MC. 2014. Osteology of the Middle Triassic archosaur Lewisuchus admixtus Romer (Chañares Formation, Argentina), its inclusivity, and relationships among early dinosauromorphs. J. Syst. Paleontol. 13, 1–31. (doi:10.1080/14772019.2013.878758) [Google Scholar]
- 79.Mastrantonio BM, Schultz CL, Desojo JB, Garcia JB. 2013. The braincase of Prestosuchus chiniquensis (Archosauria: Suchia). In Anatomy, phylogeny and palaeobiology of early archosaurs and their kin (eds Nesbitt SJ, Desojo JB, Irmis RB), pp. 425–440. Special Publication 379. London, UK: Geological Society of London. [Google Scholar]
- 80.Chatterjee S. 1993. Shuvosaurus, a new theropod. Nat. Geo. Res. Expl. 9, 274–285. [Google Scholar]
- 81.Lehane J. 2005. Anatomy and relationships of Shuvosaurus, a basal theropod from the Triassic of Texas. MSc Thesis, Texas Tech University.
- 82.Bellairs A, Kamal AM. 1981. The chondrocranium and the development of the skull in recent reptiles. In Biology of the Reptilia, volume 11, morphology F (eds Gans C, Parsons TS), pp. 1–283. New York, NY: Academic Press. [Google Scholar]
- 83.de Beer GR. 1937. The development of the vertebrate skull. Oxford, UK: Oxford University Press. [Google Scholar]
- 84.Evans SE. 2008. The skull of lizards and tuatara. In Biology of the Reptilia: the skull of Lepidosauria, volume 20, morphology H (eds Gans C, Gaunt AS, Adler K), pp. 1–347. Salt Lake City, UT: Society for the Study of Amphibians and Reptiles. [Google Scholar]
- 85.Bhullar B-AS, Bever GS. 2009. An archosaur-like laterosphenoid in early turtles (Reptilia: Pantestudines). Breviora 518, 1–11. (doi:10.3099/0006-9698-518.1.1) [Google Scholar]
- 86.Case EC. 1911. A revision of the Cotylosauria of North America. Carn. Inst. Wash. Publ. 145, 122 (doi:10.5962/bhl.title.45604) [Google Scholar]
- 87.Fracasso MA. 1987. Braincase of Limnoscelis paludi Williston. Postilla 201, 1–22. [Google Scholar]
- 88.Modesto SP, Reisz RR. 2008. New material of Colobomycter pholeter, a small parareptile from the Lower Permian of Oklahoma. J. Vert. Paleontol. 28, 677–684. (doi:10.1671/0272-4634(2008)28[677:NMOCPA]2.0.CO;2) [Google Scholar]
- 89.Modesto SP, Scott DM, Berman DS, Müller J, Reisz RR. 2007. The skull and the palaeoecological significance of Labidosaurus hamatus, a captorhinid reptile from the Lower Permian of Texas. Zool. J. Linn. Soc. 149, 237–262. (doi:10.1111/j.1096-3642.2007.00242.x) [Google Scholar]
- 90.De Braga M, Rieppel O. 1997. Reptile phylogeny and the interrelationships of turtles. Zool. J. Linn. Soc. 120, 281–354. (doi:10.1111/j.1096-3642.1997.tb01280.x) [Google Scholar]
- 91.Pardo JD, Szostakiwskyj M, Anderson JS. 2015. Cranial morphology of the brachystelechid ‘microsaur’ Quasicaecilia texana Carroll provides new insights into the diversity and evolution of braincase morphology in recumbirostran ‘microsaurs’. PLoS ONE 10, e0130359 (doi:10.1371/journal.pone.0130359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Romer AS. 1956. Osteology of the reptiles. Chicago, IL: University of Chicago Press. [Google Scholar]
- 93.Sobral G, Sues H-D, Müller J. 2015. Anatomy of the enigmatic reptile Elachistosuchus huenei Janensch, 1949 (Reptilia: Diapsida) from the Upper Triassic of Germany and its relevance for the origin of Sauria. PLoS ONE 10, e0135114 (doi:10.1371/journal.pone.0135114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Olson EC. 1936. Notes on the skull of Youngina capensis Broom. J. Geol. 44, 523–533. (doi:10.1086/624447) [Google Scholar]
- 95.Small BS. 2002. Cranial anatomy of Desmatosuchus haplocerus (Reptilia: Archosauria: Stagonolepidae). Zool. J. Linn. Soc. 136, 97–111. (doi:10.1046/j.1096-3642.2002.00028.x) [Google Scholar]
- 96.Baumel JJ. 1993. Handbook of avian anatomy: nomina anatomica avium. Cambridge, UK: Nuttall Ornithological Club. [Google Scholar]
- 97.Livezey BC, Zusi RL. 2006. Higher-order phylogeny of modern birds (Theropoda, Aves: Neornithes) based on comparative anatomy. I. Methods and characters. Bull. Carn. Mus. Nat. Hist. 37, 1–544. (doi:10.1111/j.1096-3642.2006.00293.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Balanoff AM, Bever GS, Ikejiri T. 2010. The braincase of Apatosaurus (Dinosauria: Sauropoda) based on the computed tomography of a new specimen with comments on variation and evolution in sauropod neuroanatomy. Am. Mus. Nov. 3677, 1–29. (doi:10.1206/591.1) [Google Scholar]
- 99.Janensch W. 1935. Die Schädel der Sauropoden Brachiosaurus, Barosaurus und Dicraeosaurus aus der Tendaguru-Schichten Deutsch-Ostafrikas. Palaeontogr. Suppl. 7, 147–298. [Google Scholar]
- 100.Madsen JH, McIntosh JS, Berman DS. 1995. Skull and atlas-axis complex of the Upper Jurassic sauropod Camarasaurus Cope (Reptilia: Saurischia). Bull. Carn. Mus. Nat. Hist. 31, 1–43. [Google Scholar]
- 101.Norman DB, Crompton AW, Butler RJ, Porro LB, Charig AJ. 2011. The Lower Jurassic ornithischian dinosaur Heterodontosaurus tucki Crompton & Charig, 1962: cranial anatomy, functional morphology, taxonomy, and relationships. Zool. J. Linn. Soc. 163, 182–276. (doi:10.1111/j.1096-3642.2011.00697.x) [Google Scholar]
- 102.Boyd CA. 2014. The cranial anatomy of the neornithischian dinosaur Thescelosaurus neglectus. PeerJ 2, e669 (doi:10.7717/peerj.669) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Galton PM. 1974. The ornithischian dinosaur Hypsilophodon from the Wealden of the Isle of Wight. Bull. Br. Mus. Nat. Hist. Geol. 25, 1–152. [Google Scholar]
- 104.Bever GS, Brusatte SL, Carr TD, Xu X, Balanoff AM, Norell MA. 2013. The braincase anatomy of the Late Cretaceous dinosaur Alioramus (Theropoda: Tyrannosauroidea). Bull. Am. Mus. Nat. Hist. 376, 1–72. (doi:10.1206/810.1) [Google Scholar]
- 105.Currie PJ, Zhao X-J. 1993. A new troodontid (Dinosauria, Theropoda) braincase from the Dinosaur Park Formation (Campanian) of Alberta. Can. J. Earth Sci. 30, 2231–2247. (doi:10.1139/e93-194) [Google Scholar]
- 106.Carabajal AP, Coria RA, Chiappe LM. 2008. An incomplete Upper Cretaceous titanosaur (Sauropoda) braincase: new insights on the dinosaurian inner ear and endocranium. Cretaceous Res. 29, 643–648. (doi:10.1016/j.cretres.2008.01.011) [Google Scholar]
- 107.García RA, Carabajal AP, Salgado L. 2008. A new titanosaurian braincase from the Allen Formation (Campanian-Maastrichtian), Río Negro Province, Patagonia, Argentina. Geobios 41, 625–633. (doi:10.1016/j.geobios.2007.11.005) [Google Scholar]
- 108.Fernández MS, Carabajal AP, Gaspirini Z, Díaz GC. 2011. A metriorhynchid crocodyliform braincase from northern Chile. J. Vert. Paleontol. 32, 269–377. (doi:10.1080/02724634.2011.550361) [Google Scholar]
- 109.Trotteyn MJ, Haro JA. 2011. The braincase of a specimen of Proterochampsa Reig (Archosauriformes: Proterochampsidae) from the Late Triassic of Argentina. Paläontol. Z. 85, 1–17. (doi:10.1007/s12542-010-0068-7) [Google Scholar]
- 110.Benton MJ. 2004. Origin and relationships of Dinosauria. In Dinosauria, 2nd edn (eds Weishampel DB, Dobson P, Osmólska H), pp. 7–24. Berkeley, CA: University of California Press. [Google Scholar]
- 111.Nesbitt SJ, Norell MA. 2006. Extreme convergence in the body plans of an early suchian (Archosauria) and ornithomimid dinosaurs (Theropoda). Proc. R. Soc. B 273, 1045–1048. (doi:10.1098/rspb.2005.3426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nesbitt SJ. 2007. The anatomy of Effigia okeeffeae (Archosauria, Suchia), theropod convergence, and the distribution of related taxa. Bull. Am. Mus. Nat. Hist. 302, 1–84. (doi:10.1206/0003-0090(2007)302[1:TAOEOA]2.0.CO;2) [Google Scholar]
- 113.Raath MA. 1969. A new coelurosaurian dinosaur from the Forest Sandstone of Rhodesia. Arnoldia 28, 1–25. [Google Scholar]
- 114.Vaughn PP. 1955. The Permian reptile Araeoscelis re-studied. Bull. Mus. Comp. Zool. Harv. Col. 113, 305–467. [Google Scholar]
- 115.Evans SE. 1987. The braincase of Youngina capensis (Reptilia: Diapsida; Permian). N. Jb. Geol. Paläontol. Monatsh. 1987, 293. [Google Scholar]
- 116.Dilkes DW. 1998. The Early Triassic rhynchosaur Mesosuchus browni and the interrelationships of the basal archosauromorph reptiles. Phil. Trans. R. Soc. Lond. B 353, 501–541. (doi:10.1098/rstb.1998.0225) [Google Scholar]
- 117.Gower DJ, Walker AD. 2002. New data on the braincase of the aetosaurian archosaur (Reptilia: Diapsida) Stagonolepis robertsoni Agassiz. Zool. J. Linn. Soc. 136, 7–23. (doi:10.1046/j.1096-3642.2002.00023.x) [Google Scholar]
- 118.Clack JA, Allin E. 2004. The evolution of single- and multiple-ossicle ears in fishes and tetrapods. In Evolution of the vertebrate auditory system (eds Manley GA, Popper A, Fay RR), pp. 238–263. New York, NY: Springer. [Google Scholar]
- 119.Witmer LM, Chatterjee S, Franzosa J, Rowe T. 2003. Neuroanatomy of flying reptiles and implications for flight, posture and behaviour. Nature 425, 950–953. (doi:10.1038/nature02048) [DOI] [PubMed] [Google Scholar]
- 120.Balanoff AM, Bever GS, Norell MA. 2014. Reconsidering the avian nature of the oviraptorosaur brain (Dinosauria: Theropoda). PLoS ONE 9, e113559 (doi:10.1371/journal.pone.0113559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cox PG, Jeffery N. 2010. Semicircular canals and agility: the influence of size and shape measures. J. Anat. 216, 37–47. (doi:10.1111/j.1469-7580.2009.01172.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kardong K. 2001. Vertebrates: comparative anatomy, function, evolution. New York, NY: McGraw-Hill. [Google Scholar]
- 123.Walsh SA, Iwaniuk AN, Knoll MA, Bourdon E, Barrett PM, Milner AC, Nudds R, Abel RL, Dello Sterpaio P. 2013. Avian cerebellar floccular fossa size is not a proxy for flying ability in birds. PLoS ONE 8, e67176 (doi:10.1371/journal.pone.0067176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Manley GA, Clack JA. 2004. An outline of the evolution of vertebrate hearing organs. In Evolution of the vertebrate auditory system (eds Manley GA, Popper A, Fay RR), pp. 1–26. New York, NY: Springer. [Google Scholar]
- 125.Lewis ER, Leverenz EL, Koyama H. 1982. The tonotopic organization of the bullfrog amphibian papilla, an auditory organ lacking a basilar membrane. J. Comp. Physiol. 145, 437–445. (doi:10.1007/BF00612809) [Google Scholar]
- 126.Manley GA, Köppl C. 2008. What have lizard ears taught us about auditory physiology? Hear. Res. 238, 3–11. (doi:10.1016/j.heares.2007.09.011) [DOI] [PubMed] [Google Scholar]
- 127.Mann ZF, Kelley MW. 2011. Development of tonotopy in the auditory periphery. Hear. Res. 276, 2–15. (doi:10.1016/j.heares.2011.01.011) [DOI] [PubMed] [Google Scholar]
- 128.Walsh SA, Barrett PM, Milner AC, Manley G, Witmer LM. 2009. Inner ear anatomy is a proxy for deducing auditory capability and behaviour in reptiles and birds. Proc. R. Soc. B 276, 1355–1360. (doi:10.1098/rspb.2008.1390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lombard RE, Hetherington TE. 1993. Structural basis of hearing and sound transmission. In The skull, vol. 3 (eds Hanken J, Hall BK), pp. 241–302. Chicago, IL: University of Chicago Press. [Google Scholar]
- 130.Manley GA. 1990. Peripheral hearing mechanisms in reptiles and avialans. Berlin, Germany: Springer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Given the large size of the individual three-dimensional models, all data generated by computed tomography scanning of the materials are deposited in the virtual database of the Museum für Naturkunde Berlin, and are available upon request.