Abstract
Empirical tests of adaptive maternal sex allocation hypotheses have presented inconsistent results in mammals. The possibility that mothers are constrained in their ability to adjust sex ratios could explain some of the remaining variation. Maternal effects, the influence of the maternal phenotype or genotype on her developing offspring, may constrain sex allocation through physiological changes in response to the gestational environment. We tested if maternal effects constrain future parental sex allocation through a lowered gestational stress environment in laboratory mice. Females that experienced lowered stress as embryos in utero gave birth to female-biased litters as adults, with no change to litter size. Changes in offspring sex ratio was linked to peri-conceptual glucose, as those females that had increasing blood glucose peri-conceptionally gave birth to litters with a higher male to female sex ratio. There was, however, no effect of the lowered prenatal stress for developing male embryos and their sperm sex ratio when adult. We discuss the implications of maternal effects and maternal stress environment on the lifelong physiology of the offspring, particularly as a constraint on later maternal sex allocation.
Keywords: sex allocation, maternal, paternal, fluorescent in situ hybridization, sex ratio
1. Introduction
Adaptive sex allocation hypotheses predict variation in the sex ratio of offspring where sex-specific fitness returns vary with local conditions and/or parental ability to invest [1–4]. Such hypotheses are logically appealing and have resulted in numerous empirical tests, including in mammals (reviewed in [5–7]). Initial reviews in mammals suggested little consistency in support for adaptive hypotheses, but methodological inconsistencies between studies explain some of the variation [5,7]. Nonetheless, unexplained variability both between and within species in empirical studies occurs, especially in mammals [8]. The unpredictability of effect sizes suggests that parents may be physiologically constrained in their ability to skew the sex of their offspring [9,10].
An increasing understanding of the underlying physiological mechanisms for maternal sex allocation suggests factors that might constrain maternal ability to skew sex ratios [10]. Lifelong and inter-generational modifiers of maternal physiology may constrain an individual's ability to respond to the current local conditions [10–12], particularly through maternal effects, the causal influence of the maternal phenotype or genotype on developing offspring [13–15]. Several factors have been linked to sex ratio skews through their physiological actions, including circulating glucose [5], testosterone [16–18] and stress hormones [19]. Each of these factors is influenced by the local conditions a mother experiences and can directly affect the developing fetus. Thus, the environment experienced in utero can alter physiological pathways, thereby changing the individual's response to the environment as adults [20]. Such maternal effects may result in parents that are physiologically constrained in their ability to alter sex ratios in response to current conditions.
Stress responses provide a link between the proposed mechanisms of sex ratio adjustment [19,21] and can have profound physiological impacts on developing offspring as a maternal effect [22]. Stressors experienced by the mother are mediated through internal hormone fluctuations; stressors stimulate the release of corticotropin-releasing hormone from the hypothalamus, which in turn stimulates the release of adrenocorticotropic hormone from the pituitary gland, resulting in the release of glucocorticoids (GCs; [23]). GCs then bind to receptors, which allow the body to return to homeostasis through acute stress events [23–25]. Fetuses are extremely sensitive to GCs [26,27], and so protective enzymes (e.g. 11 beta-hydroxysteroid dehydrogenase type 2) in the placenta metabolize roughly 80% of naturally occurring GCs, thereby buffering the fetus from high levels of GCs [28,29]. However, the remaining proportion can cross the placenta, and thereby influence offspring development [30]. These changes can be either deleterious or advantageous to the offspring (e.g. [31,32]) and can last a lifetime [31], potentially even persisting across generations [33,34]. Offspring fitness may be increased, for example by matching poor-quality mothers with reduced offspring demand [35] and offspring traits that increase survival [32]. However, changes that create a mismatch with the local environment are likely to result in offspring relatively less suited for the current environment, thus decreasing their fitness [36,37].
The physiological effects of maternal gestational stress on developing offspring include changes in the hypothalamic-pituitary-adrenal (HPA) axis function, immunity, glucose and insulin tolerance and regulation, body condition and adult reproductive behaviour and function in the offspring [38–40]. Stress probably influences maternal sex allocation, through increased susceptibility of male offspring to adverse conditions during late gestation [41], and more subtly through physiological changes persisting into adulthood. Changes to the HPA axis (and thereby sensitivity to stress) as a result of maternal effects during late gestation could influence offspring sex ratios and survival once that offspring itself reaches breeding age. Furthermore, such changes may influence maternal sex allocation through interactions with free glucose [5], because hepatic gluconeogenesis results from increased cortisol [42], and gestational stress can alter glucose levels and insulin tolerance lifelong [43,44]. Increases in peri-conceptual glucose increase the proportion of male offspring [5,45], due to interactions between free glucose and X-linked proteins and metabolic pathways [46], where female conceptus development is compromised under high glucose conditions [45,47] but enhanced under low glucose conditions. GCs also inhibit the secretion of reproductive hormones, including testosterone, also linked to sex ratio skews in mammals [48]. High levels of maternal testosterone have been linked to an increasing proportion of male offspring [49,50], hypothetically altering the receptivity of the egg to either X- or Y-chromosome-bearing spermatozoa in relation to follicular testosterone [17]. Hormonal differences between adult males have also been linked to variation in the X to Y ratio in sperm (reviewed in [9]) potentially also influencing paternal sex allocation. Therefore, maternal stress levels can influence offspring development during gestation in ways that could alter sex allocation when they reproduce, irrespective of current local conditions.
Here, we test if downregulated stress during late gestation in laboratory mice impacts (i) the physical development and reproductive success of offspring and (ii) their sex allocation, in terms of sperm sex ratios in adult males and birth sex ratios in females. We predict that offspring born to treated mothers will have an increased number of glucocorticoid receptors [51], and therefore increased susceptibility to stress [26]. Female offspring may then experience increases in offspring sex ratios as a result of increased gluconeogenesis [5]; however, we do not predict that these changes should influence male sperm sex ratios.
2. Material and methods
We used BALB/c mice bred and housed at the University of Tasmania, Australia. They were kept under 12 L : 12 D photoperiod in a temperature and humidity controlled room and provided with mouse chow (Barastoc® irradiated food) and filtered water ad libitum.
2.1. Generating focal females and males
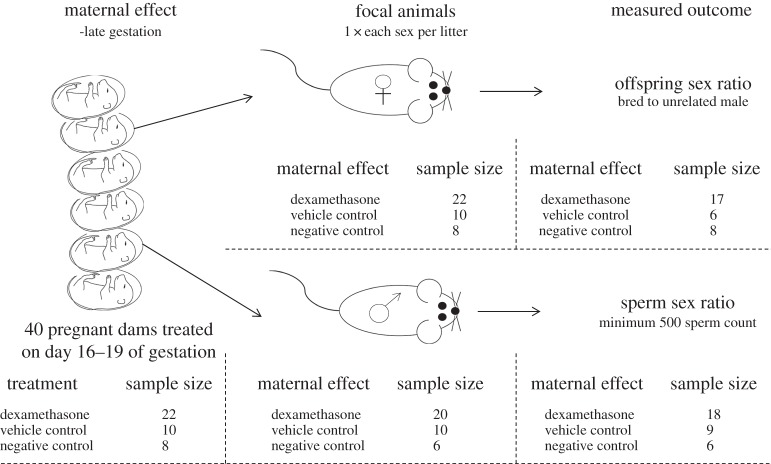
The experimental design is outlined in figure 1. Forty nulliparous dams were housed in groups of up to five until seven weeks of age when they were separated into pairs. One male was introduced to each cage, and each morning the dams were checked for the presence of a copulatory plug. Those dams that had a copulatory plug were removed from the cage and placed into group cages. The dams that did not have a copulatory plug were left with a male until a plug was observed.
Figure 1.
Diagram of the experimental design. The sample sizes at each stage of the experiment are listed based on treatment.
We used dexamethasone to reduce stress in these pregnant dams in late gestation. Dexamethasone is a synthetic GC that simulates an artificial low stress environment [52,53] and is used during late gestation in humans to reduce the risk of respiratory distress syndrome in premature babies [22]. Fetal effects from the simulated low stress environment are expected to be exaggerated because dexamethasone is not metabolized by the placenta [54]. Thus, there are fewer maternal GCs crossing the placenta as a result of dexamethasone interacting with the mother's body, as well as free dexamethasone entering the fetus and blocking its naturally occurring GCs. Combined, these effects result in perceived low stress levels for offspring.
At day 16 after the presence of a copulatory plug, 1.0 µg ml−1 of dexamethasone (as used by [52]) was added to the drinking water of 22 dams, and this was replaced with fresh water after 3 days. Although this method results in variable dosages, it eliminates any increase in GCs from the stress of handling and injections [53], which potentially could negate the treatment [52]. Water-soluble dexamethasone is provided in a complex with 2-hydroxypropyl-β-cyclodextrin. Therefore, we had 10 dams whose water was treated with 14.4 µg ml−1 2-hydroxypropyl-β-cyclodextrin as a vehicle control, to equally match the amount of vehicle that was required to deliver 1.0 µg ml−1 of dexamethasone. The water of eight dams was left untreated, as the negative control.
As close as possible to birth and at least within 10 h, the pups were counted to record litter size in case of infanticide. These pups are considered to be the focal animals; the sperm sex ratios and offspring sex ratios produced by them are a means of determining the influence that maternal stress had. At 21 days after birth, the focal pups were sexed via visual examination of the anogenital distance and separated into single sex group cages. To avoid pseudo-replication, only one focal female and one focal male from each litter were kept as the focal animals. At seven weeks of age, the focal pups were considered adult, and body measurements (table 1) were taken.
Table 1.
Variables measured from BALB/c mice, used in a mating trial to determine whether maternal effects (in utero treatment with dexamethasone) have the ability to constrain sex allocation in laboratory mice. Physical body measurements were taken at maturity (seven weeks of age).
| variable | description |
|---|---|
| body condition | calculated from the residuals of an ordinary least-squares linear regression of body mass and pes length [55]. Pes length is measured using digital callipers |
| anogenital distance | calculated as the distance between the anus and the genital opening. Measuring using digital callipers |
| digit ratio | digit ratio was calculated as the ratio of second to fourth digit on the hind right foot. Digit length is measured using digital callipers from the tip of the toe to the base of the footpad. Observers were blind to the treatment of the animal |
| blood glucose | blood glucose was measured using an Accu-Chek Performa Nano glucometer, from blood collected via tail tipping |
2.2. Breeding of focal females
Focal females were housed in pairs with an unrelated male until a copulatory plug was noted, after which females were weighed and blood glucose tested. Three days later the blood glucose test was repeated, to calculate the change in peri-conceptual blood glucose level. Focal females were allowed to give birth naturally and pups were again sexed using anogenital distance. Seven focal females did not conceive, and a further two committed infanticide prior to offspring sexing and were removed from the analysis. The final sample size was 31 (figure 1). The sex ratio of the resultant litter was recorded.
2.3. Sperm collection from focal males
Focal males were sacrificed via cervical dislocation at between 67 and 74 days of age. The left epididymis and vas deferens were dissected into 0.5 ml cryopreservation media (18% raffinose + 3% skim milk). The semen was squeezed from the vas deferens using tweezers and allowed to swim out of the epididymis through lateral incisions. The resultant sperm suspensions were stored in straws and cryopreserved in liquid nitrogen.
2.4. Fluorescence in situ hybridization on sperm
The full methods are described in Edwards et al. [56]. Briefly, the sperm samples were washed and fixed to glass slides, decondensed and treated with pepsin prior to denaturation in 70% formamide. The X-chromosome probes were labelled with Cy3 and Y-chromosome probes with biotin. Denatured probes were added to the slides and hybridizations were performed in a warm, moist chamber for 24–48 h. Slides were washed and detection of the Y-chromosome probe was performed using avidin-fluorescein isothiocyanate (FITC), prior to counterstaining the sperm heads with 4′6-diamidion-2-phenylindole ml−1 (DAPI) and mounting using an anti-fade solution (Vectashield, Vecta Laboratories, CA). Sperm were observed using a Leica DMRXA fluorescence microscope, with Cy3, FITC and DAPI specific filters. A minimum of 500 spermatozoa were counted per individual, from images collected using Leica QFISH with a cooled CCD camera through ×40 or ×63 oil-immersion objectives.
Of the 40 initial litters, four did not produce any males, three sperm samples were destroyed during transportation, and one sample failed to hybridize sufficiently for analysis, resulting in 33 focal males (figure 1).
2.5. Statistics
All analyses were performed in R v. 3.2.2 [57].
2.6. Focal female offspring sex ratio analysis
Binomial generalized linear models with an intercept of 1 were run to determine whether the treatment group or either control group presented with sex ratios different to the predicted 50 : 50 ratios. These results are presented as 95% CIs on the estimate.
A generalized linear model with binomial error was run to determine whether peri-conceptual change in glucose, treatment or body condition influenced the sex ratio of offspring. This model also included an interaction effect between peri-conceptual glucose and treatment. While a multivariate analysis of variance (MANOVA) was run to determine whether the treatment had any effect on the physical body measurement of focal animals. An analysis of variance (ANOVA) was also run to determine whether litter size varied with treatment.
2.7. Focal male sperm sex ratio analysis
A full generalized linear model with binomial error was run to determine whether treatment or body condition influences the sex ratio of sperm. While a MANOVA was run to determine whether the treatment had any effect on the physical body measurement of focal animals.
3. Results
3.1. Litter sex ratios
The treatment group produced sex ratios that were significantly lower than the predicted 50 : 50 ratio (generalized linear model (GLM): −0.943, −0.161; figure 2), whereas neither control group differed from parity (GLM negative control: −0.798, 0.274; GLM vehicle control: −0.922, 0.738).
Figure 2.
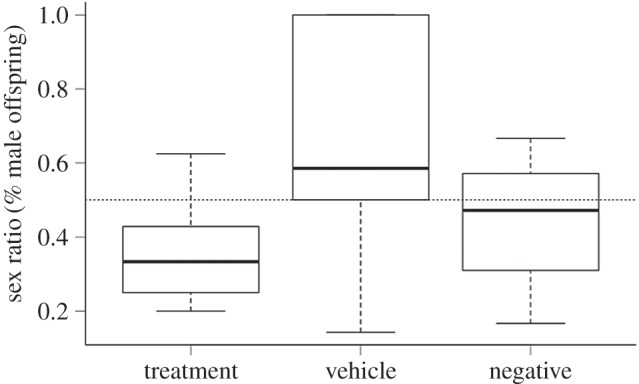
Female mice that receive dexamethasone treatment in utero produce litters with sex ratios that are lower than the expected 50 : 50 ratio (GLM: −0.943, −0.161), but females who received the vehicle or untreated water did not (GLM vehicle control: −0.922, 0.738; GLM negative control: −0.798, 0.274). The dotted line indicates the expected 50 : 50 ratio.
The sex ratio of offspring was significantly influenced by peri-conceptual change in glucose (Pr (>χ)1,29 = 0.033; figure 3), but not by treatment (Pr (>χ)2,27 = 0.676) or body condition (Pr (>χ)1,26 = 0.915). There was also no interaction effect between the change in peri-conceptual glucose and treatment (Pr (>χ)2,24 = 0.554). The treatment did not result in a change in litter size (F2,28 = 3.174, p = 0.057); however, there was a slight trend for the vehicle control group to have smaller litters. The treatment also did not influence the physical and physiological body measurements of the focal animals (F10,48 = 0.955, p = 0.493).
Figure 3.
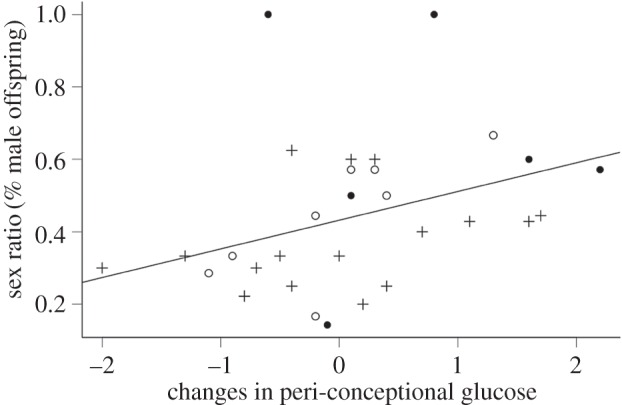
The linear relationship between sex ratio (as percentage of male offspring) and peri-conceptional blood glucose changes from day 0 to day 3 after confirmed copulation in laboratory mice (GLM, Pr (>χ) = 0.03). Crosses represent the sex ratios of females who received dexamethasone treatment during late development (in utero). Filled circles represent females who received the vehicle control and open circles represent females that did not receive any treatments.
3.2. Sperm sex ratios
The sperm sex ratio was not significantly influenced by treatment (Pr (>χ)2,30 = 0.192) or body condition (Pr (>χ)1,29 = 0.488). There was also no effect of treatment on any physical or physiological body measurement of the focal males (F8,56 = 0.975, p = 0.477).
4. Discussion
Maternal effects altered focal female sex ratios, but not the X- and Y-chromosome ratio in focal male sperm. Females that received the dexamethasone treatment during late-gestational development gave birth to litters with sex ratios lower than the predicted 50 : 50 ratio, with no change to litter size. However, increases in blood glucose were more strongly associated with an increase in male offspring than treatment per se, suggesting that environmental interactions with glucose metabolism may be more influential than maternal effects.
The developmental impacts of late-gestational maternal stress manipulation influence stress responses and glucose metabolism in later life [22]. Embryonic female guinea pigs exposed to dexamethasone in utero have increases in glucocorticoid receptor and mineralocorticoid receptor mRNA in all regions of their hippocampus and altered GC levels, which are lower in the luteal phase but higher during oestrous [22]. However, increases in cortisol are associated with hepatic gluconeogenesis [42] and an overall increase in glucose [58]. Therefore, the lowering of cortisol levels during the luteal phase and the observed increase in female offspring might be better explained through the glucose hypothesis [5], through associated low levels of gluconeogenesis, and therefore, an overall decrease in free glucose.
In this study, the focal females that had an increase in blood glucose levels over the time of conception and early gestation give birth to more sons. This provides further evidence in support of the glucose hypothesis [5], where early blastocyst females survive better in low glucose environments, and males in high glucose environments [45]. Change in blood glucose levels significantly influence sex ratios while treatment only did so indirectly through an interaction with glucose levels, probably due to the delivery method, because drinking water results in variable dosages [52]. However, as dexamethasone was used to simulate low stress, variable dosage was preferable to negating the treatment from injection-induced stress [52,53].
The possibility of maternal effects constraining a father's sperm production has not been previously investigated. No significant shift in sperm sex ratios of the focal males is unsurprising, as we do not anticipate that stress or changes to HPA axis functioning should affect sperm production. Unlike mothers, mammalian fathers do not require large energetic investment in the production of gametes [59], or even in the offspring themselves [59], and therefore, changes to stress pathways are unlikely to influence paternal sex allocation. However, research into paternal sex allocation and the possibility of adaptive control by fathers is limited ([9], but see [60–62]), and it is unknown under what circumstances paternal sex allocation could occur [9,56], although James [63] has suggested a role for pre-mating androgens in fathers.
There were no changes to the physical appearance of either sex offspring, even though previous studies on gestational dexamethasone have shown variation in physical characteristics (reviewed in [64]). Many of the studies that have presented offspring with physical changes have used much larger intravenous or subcutaneous dosages, and even multiple dosages, which leads to greatly exaggerated effects [64]. In comparison, our dosage was high enough to have physiological effects on subsequent sex ratios (suggesting changes to underlying physiology) but not enough to have deleterious effects on offspring morphological development. In addition, we found no evidence that testosterone was linked to sex allocation. We measured both the digit ratio and the anogenital distance of the mice, which are indicative of the female's prenatal androgen exposure [65], but neither of these were correlated with sex ratio. There is contention regarding the use of digit ratios as androgen exposure indicators [66], and, therefore, although our data show no support for a role of testosterone, we cannot rule out a role for testosterone influencing sex ratios.
We have shown that the gestational environment results in female offspring whose physiology is altered in a way that affects her reproductive functioning as an adult, which could influence the success of management and captive breeding programmes. Changes to female physiological pathways due to maternal effects can constrain maternal sex allocation in subsequent generations, producing females that respond differently to the same environmental conditions, despite appearing otherwise similar.
Acknowledgements
We would like to thank Lauren Richards and Dr Joanne McEvoy for assistance with mouse husbandry, and Dr Joanne McEvoy for assistance with animal dissections. We also thank Paul Scowen for his assistance with cryopreservation techniques and storing of samples.
Ethics
All experiments were performed under permits granted from the University of Tasmania Animal Ethics Committee (permit nos. A12366 and A13748).
Data accessibility
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.b18gv.
Authors' contributions
A.M.E. designed and coordinated the study, maintained the animals and carried out the experimental breeding procedures, all dissections and molecular work, completed the statistical analysis and drafted the manuscript. E.Z.C. conceived the study, participated in the design of the study, assisted with statistical analysis and helped draft the manuscript. J.C.P. and M.A.F.-S. prepared the paint probes, assisted with the molecular work and helped draft the manuscript. E.W. participated in the design of the study and helped draft the manuscript. S.R.H. and K.T. assisted with ideas, and undertook animal physical body measurements. All authors gave final approval for publication. The authors are listed in order of contribution.
Competing interests
We have no competing interests.
Funding
This study was funded by the Australian Research Council (DP140103227) to E.Z.C. and E.W.
References
- 1.Trivers RL, Willard DE. 1973. Natural selection of parental ability to vary the sex ratio of offspring. Science 179, 90–92. (doi:10.1126/science.179.4068.90) [DOI] [PubMed] [Google Scholar]
- 2.Silk JB. 1984. Local resource competition and the evolution of male-biased sex-ratios. J. Theor. Biol. 108, 203–213. (doi:10.1016/s0022-5193(84)80066-1) [DOI] [PubMed] [Google Scholar]
- 3.Clark AB. 1978. Sex-ratio and local resource competition in a prosimian primate. Science 201, 163–165. (doi:10.1126/science.201.4351.163) [DOI] [PubMed] [Google Scholar]
- 4.Hamilton WD. 1967. Extraordinary sex ratios. Science 156, 477–488. (doi:10.1126/science.156.3774.477) [DOI] [PubMed] [Google Scholar]
- 5.Cameron EZ. 2004. Facultative adjustment of mammalian sex ratios in support of the Trivers–Willard hypothesis: evidence for a mechanism. Proc. R. Soc. Lond. B 271, 1723–1728. (doi:10.1098/rspb.2004.2773) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clutton-Brock TH, Iason GR. 1986. Sex-ratio variation in mammals. Q. Rev. Biol. 61, 339–374. (doi:10.1086/415033) [DOI] [PubMed] [Google Scholar]
- 7.Sheldon BC, West SA. 2004. Maternal dominance, maternal condition, and offspring sex ratio in ungulate mammals. Am. Nat. 163, 40–54. (doi:10.1086/381003) [DOI] [PubMed] [Google Scholar]
- 8.West SA. 2009. Sex allocation. Princeton, NJ: Princeton University Press. [Google Scholar]
- 9.Edwards AM, Cameron EZ. 2014. Forgotten fathers: paternal influences on mammalian sex allocation. Trends Ecol. Evol. 29, 158–164. (doi:10.1016/j.tree.2013.12.003) [DOI] [PubMed] [Google Scholar]
- 10.Edwards AM, Cameron EZ, Wapstra E. 2016. Are there physiological constraints on maternal ability to adjust sex ratios in mammals? J. Zool. 299, 1–9. (doi:10.1111/jzo.12327) [Google Scholar]
- 11.Lucas A. 1991. Programming by early nutrition in man. In The childhood environment and adult disease, CIBA Foundation Symposium 156, pp. 38–55. Chichester, UK: Wiley. [Google Scholar]
- 12.Lucas A. 1998. Programming by early nutrition: an experimental approach. J. Nutr. 128, 401S–406S. [DOI] [PubMed] [Google Scholar]
- 13.Marshall DJ, Uller T. 2007. When is a maternal effect adaptive? Oikos 116, 1957–1963. (doi:10.1111/j.2007.0030-1299.16203.x) [Google Scholar]
- 14.Maestripieri D, Mateo JM. 2009. Maternal effects in mammals. Chicago, IL: University of Chicago Press. [Google Scholar]
- 15.Wolf JB, Wade MJ. 2009. What are maternal effects (and what are they not)? Phil. Trans. R. Soc. B 362, 1107–1115. (doi:10.1098/rstb.2008.0238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grant VJ. 2007. Could maternal testosterone levels govern mammalian sex ratio deviations? J. Theor. Biol. 246, 708–719. (doi:10.1016/j.jtbi.2007.02.005) [DOI] [PubMed] [Google Scholar]
- 17.Grant VJ, Chamley LW. 2010. Can mammalian mothers influence the sex of their offspring peri-conceptually? Reproduction 140, 425–433. (doi:10.1530/REP-10-0137) [DOI] [PubMed] [Google Scholar]
- 18.James WH. 2013. Evolution and the variation of mammalian sex ratios at birth: reflections on Trivers and Willard (1973). J. Theor. Biol. 334, 141–148. (doi:10.1016/j.jtbi.2013.06.023) [DOI] [PubMed] [Google Scholar]
- 19.Navara KJ. 2010. Programming of offspring sex ratios by maternal stress in humans: assessment of physiological mechanisms using a comparative approach. J. Comp. Physiol. B 180, 785–796. (doi:10.1007/s00360-010-0483-9) [DOI] [PubMed] [Google Scholar]
- 20.Mousseau TA, Fox CW. 1998. The adaptive significance of maternal effects. Trends Ecol. Evol. 13, 403–407. (doi:10.1016/s0169-5347(98)01472-4) [DOI] [PubMed] [Google Scholar]
- 21.Moore EPB, Hayward M, Robert K. 2015. High density, maternal condition and stress are associated with male-biased sex allocation in a marsupial. J. Mamm. 96, 1203–1213. (doi:10.1093/jmammal/gyv129) [Google Scholar]
- 22.Dunn E, Kapoor A, Leen J, Matthews SG. 2010. Prenatal synthetic glucocorticoid exposure alters hypothalamic–pituitary–adrenal regulation and pregnancy outcomes in mature female guinea pigs. J. Physiol. 588, 887–899. (doi:10.1113/jphysiol.2009.182139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. 2006. Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J. Physiol. 572, 31–44. (doi:10.1113/jphysiol.2006.105254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Welch WJ. 1992. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol. Rev. 72, 1063–1081. [DOI] [PubMed] [Google Scholar]
- 25.Charmandari E, Kino T, Chrousos G. 2004. Glucocorticoids and their actions: an introduction. Ann. NY Acad. Sci. 1024, 1–8. (doi:10.1196/annals.1321.001) [DOI] [PubMed] [Google Scholar]
- 26.Welberg L, Seckl JR, Holmes M. 2001. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: possible implications for behaviour. Neuroscience 104, 71–79. (doi:10.1016/S0306-4522(01)00065-3) [DOI] [PubMed] [Google Scholar]
- 27.Nyirenda MJ, Welberg L. 2001. Programming hyperglycemia in the rat through prenatal exposure to glucocorticoids-fetal effect or maternal influence? J. Endocrinol. 170, 653–660. (doi:10.1677/joe.0.1700653) [DOI] [PubMed] [Google Scholar]
- 28.Diaz R, Brown RW, Seckl JR. 1998. Distinct ontogeny of glucocorticoid and mineralocorticoid receptor and 11 beta-hydroxysteroid dehydrogenase types I and II mRNAs in the fetal rat brain suggest a complex control of glucocorticoid actions. J. Neurosci. 18, 2570–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myatt L. 2006. Placental adaptive responses and fetal programming. J. Physiol. 572, 25–30. (doi:10.1113/jphysiol.2006.104968) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris A, Seckl JR. 2011. Glucocorticoids, prenatal stress and the programming of disease. Horm. Behav. 59, 279–289. (doi:10.1016/j.yhbeh.2010.06.007) [DOI] [PubMed] [Google Scholar]
- 31.Seckl JR. 2004. Prenatal glucocorticoids and long-term programming. Eur. J. Endocrinol. 151, U49–U62. (doi:10.1530/eje.0.151U049) [DOI] [PubMed] [Google Scholar]
- 32.Sheriff MJ, Krebs CJ, Boonstra R.. 2009. The sensitive hare: sublethal effects of predator stress on reproduction in snowshoe hares. J. Anim. Ecol. 78, 1249–1258. (doi:10.1111/j.1365-2656.2009.01552.x) [DOI] [PubMed] [Google Scholar]
- 33.Mech LD, Nelson ME, McRoberts RE. 1991. Effects of maternal and grandmaternal nutrition on deer mass and vulnerability to wolf predation. J. Mamm. 72, 146–151. (doi:10.2307/1381989) [Google Scholar]
- 34.Monteith KL, Schmitz LE, Jenks JA, Delger JA, Bowyer RT. 2009. Growth of male white-tailed deer: consequences of maternal effects. J. Mamm. 90, 651–660. (doi:10.1644/08-mamm-a-191r1.1) [Google Scholar]
- 35.Love OP, Williams TD. 2008. The adaptive value of stress-induced phenotypes: effects of maternally derived corticosterone on sex-biased investment, cost of reproduction, and maternal fitness. Am. Nat. 172, E135–E149. (doi:10.1086/590959) [DOI] [PubMed] [Google Scholar]
- 36.Brunton PJ. 2010. Resetting the dynamic range of hypothalamic-pituitary-adrenal axis stress responses through pregnancy. J. Neuroendocrinol. 22, 1198–1213. (doi:10.1111/j.1365-2826.2010.02067.x) [DOI] [PubMed] [Google Scholar]
- 37.Takahashi LK, Kalin NH. 1991. Early developmental and temporal characteristics of stress-induced secretion of pituitary-adrenal hormones in prenatally stressed rat pups. Brain Res. 558, 75–78. (doi:10.1016/0006-8993(91)90715-8) [DOI] [PubMed] [Google Scholar]
- 38.Weinstock M. 2005. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav. Immun. 19, 296–308. (doi:10.1016/j.bbi.2004.09.006) [DOI] [PubMed] [Google Scholar]
- 39.Hauser J, et al. 2008. Effects of prenatal dexamethasone treatment on physical growth, pituitary-adrenal hormones, and performance of motor, motivational, and cognitive tasks in juvenile and adolescent common marmoset monkeys. Endocrinology 149, 6343–6355. (doi:10.1210/en.2008-0615) [DOI] [PubMed] [Google Scholar]
- 40.Bock J, Murmu MS, Biala Y, Weinstock M, Braun K.. 2011. Prenatal stress and neonatal handling induce sex-specific changes in dendritic complexity and dendritic spine density in hippocampal subregions of prepubertal rats. Neuroscience 193, 34–43. (doi:10.1016/j.neuroscience.2011.07.048) [DOI] [PubMed] [Google Scholar]
- 41.Kraemer S. 2000. The fragile male. Br. Med. J. 321, 1609–1612. (doi:10.1136/bmj.321.7276.1609) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haynes RC, Lu YS. 1969. Measurement of cortisol-stimulated gluconeogenesis in the rat. Endocrinology 85, 811–814. (doi:10.1210/endo-85-5-811) [DOI] [PubMed] [Google Scholar]
- 43.Maniam J, Antoniadis C, Morris MJ. 2014. Early-life stress, HPA axis adaptation, and mechanisms contributing to later health outcomes. Front. Endocrinol. 5, 1–17. (doi:10.3389/fendo.2014.00073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brunton PJ, Sullivan KM, Kerrigan D, Russell JA, Seckl JR, Drake AJ. 2013. Sex-specific effects of prenatal stress on glucose homeostasis and peripheral metabolism in rats. J. Endocrinol. 217, 161–173. (doi:10.1530/JOE-12-0540) [DOI] [PubMed] [Google Scholar]
- 45.Larson MA, Kimura K, Kubisch HM, Roberts RM. 2001. Sexual dimorphism among bovine embryos in their ability to make the transition to expanded blastocyst and in the expression of the signalling molecule IFN-t. Proc. Natl Acad. Sci. USA 98, 9677–9682. (doi:10.1073/pnas.171305398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gutierrez-Adan A, Granados J, Pintado B, De La Fuente J. 2001. Influence of glucose on the sex ratio of bovine IVM/IVF embryos cultured in vitro. Reprod. Fertil. Dev. 13, 361–365. (doi:10.1071/RD00039) [DOI] [PubMed] [Google Scholar]
- 47.Kimura K, Spate LD, Green MP, Roberts RM. 2005. Effects of D-glucose concentration, D-fructose, and inhibitors of enzymes of the pentose phosphate pathway on the development and sex ratio of bovine blastocysts. Mol. Reprod. Dev. 72, 201–207. (doi:10.1002/mrd.20342) [DOI] [PubMed] [Google Scholar]
- 48.Grant VJ. 1996. Sex determination and the maternal dominance hypothesis. Human Reprod. 11, 2371–2375. (doi:10.1093/oxfordjournals.humrep.a019117) [DOI] [PubMed] [Google Scholar]
- 49.Helle S, Laaksonen T, Adamsson A, Paranko J, Huitu O.. 2008. Female field voles with high testosterone and glucose levels produce male-biased litters. Anim. Behav. 75, 1031–1039. (doi:10.1016/j.anbehav.2007.08.015) [Google Scholar]
- 50.Grant VJ, Irwin RJ, Standley AN, Shelling AN, Chamley LW. 2008. Sex of bovine embryos may be related to mothers’ preovulatory follicular testosterone. Biol. Reprod. 78, 812–815. (doi:10.1095/biolreprod.107.066050) [DOI] [PubMed] [Google Scholar]
- 51.Seckl JR, Meaney MJ. 2004. Glucorticoid programming. Ann. NY Acad. Sci. 1032, 63–84. (doi:10.1196/annals.1314.006) [DOI] [PubMed] [Google Scholar]
- 52.Cameron EZ, Lemons PR, Bateman PW, Bennett NC. 2008. Experimental alteration of litter sex ratios in a mammal. Proc. R. Soc. B 275, 323–327. (doi:10.1098/rspb.2007.1401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pratt NC, Lisk RD. 1990. Dexamethasone can prevent stress-related litter deficits in the golden hamster. Behav. Neural Biol. 54, 1–12. (doi:10.1016/0163-1047(90)91201-L) [DOI] [PubMed] [Google Scholar]
- 54.Drake AJ, Walker BR, Seckl JR. 2005. Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R34–R38. (doi:10.1152/ajpregu.00106.2004) [DOI] [PubMed] [Google Scholar]
- 55.Schulte-Hostedde AI, Zinner B, Millar JS, Hickling GJ. 2005. Restitution of mass-size residuals: validating body condition indices. Ecology 86, 155–163. (doi:10.1890/04-0232) [Google Scholar]
- 56.Edwards AM, Cameron EZ, Pereira JC, Ferguson-Smith MA. 2016. Paternal sex allocation: how variable is the sperm sex ratio? J. Zool. 299, 37–41. (doi:10.1111/jzo.12317) [Google Scholar]
- 57.R Core Team. 2015. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org/ [Google Scholar]
- 58.Goldstein RE, Wasserman DH, McGuinness OP, Lacy DB, Cherrington AD, Abumrad NN. 1993. Effects of chronic elevation in plasma cortisol on hepatic carbohydrate metabolism. Am. J. Physiol. 264, 119–127. [DOI] [PubMed] [Google Scholar]
- 59.Trivers RL. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man 1871–1971 (ed. Campbell B.), pp. 136–179. Los Angeles, CA: University of California, Aldine Publishing Company. [Google Scholar]
- 60.Gomendio M, Malo AF, Soler AJ, Fernandez-Santos MR, Esteso MC, Garcia AJ, Roldan ERS, Garde J.. 2006. Male fertility and sex ratio at birth in red deer. Science 314, 1445–1447. (doi:10.1126/science.1133064) [DOI] [PubMed] [Google Scholar]
- 61.Roed KH, Holand O, Mysterud A, Tverdal A, Kumpula J, Nieminen M. 2007. Male phenotypic quality influences offspring sex ratio in a polygynous ungulate. Proc. R. Soc. B 274, 727–733. (doi:10.1098/rspb.2006.0214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Douhard M, Festa-Bianchet M, Coltman DW, Pelletier F. 2014. Paternal reproductive success drives sex allocation in a wild mammal. Evolution 70, 358–368. (doi:10.1111/evo.12860) [DOI] [PubMed] [Google Scholar]
- 63.James WH. 2008. Evidence that mammalian sex ratios at birth are partially controlled by parental hormone levels around the time of conception. J. Endocrinol. 198, 3–15. (doi:10.1677/JOE-07-0446) [DOI] [PubMed] [Google Scholar]
- 64.McKinlay CJD, Dalziel SR, Harding JE. 2015. Antenatal glucocorticoids: where are we after forty years? J. Dev. Orig. Health Dis. 6, 127–142. (doi:10.1017/S2040174414000579) [DOI] [PubMed] [Google Scholar]
- 65.Hurd PL, Bailey AA, Gongal PA, Yan RH, Greer JJ, Pagliardini S.. 2008. Intrauterine position effects on anogenital distance and digit ratio in male and female mice. Arch. Sex. Behav. 37, 9–18. (doi:10.1007/s10508-007-9259-z) [DOI] [PubMed] [Google Scholar]
- 66.Dressler SG, Voracek M. 2011. No association between two candidate markers of prenatal sex hormones: digit ratios (2D:4D and other) and finger-ridge counts. Dev. Psychobiol. 53, 69–78. (doi:10.1002/dev.20488) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.b18gv.