Abstract
[Purpose] The effects of vitamin D on the circulating levels of IL-17 and IL-13 were investigated in patients with diabetic peripheral neuropathy, patients with diabetes mellitus type 2 without neuropathy, and healthy controls. [Subjects and Methods] A single-blind controlled clinical study was performed, including70 type 2 diabetic patients with or without diabetic peripheral neuropathy and 33 healthy volunteer controls. The 25(OH)D levels were evaluated using ultra-performance liquid chromatography, and IL-17 and IL-13 levels were assessed using enzyme-linked immunosorbent assays. [Results] The 25(OH) vitamin D concentration was lower in diabetic peripheral neuropathy patients than in diabetes mellitus patients without neuropathy and healthy controls. Similarly, 25(OH)D levels were lower in diabetes mellitus patients than healthy controls. IL-17 and IL-13 levels were higher in diabetes mellitus patients than in controls. Additionally, IL-13 levels were higher in diabetic peripheral neuropathy patients than in diabetes mellitus patients without neuropathy. These differences were statistically significant. There was a significant positive correlation between 25(OH)D and IL-13,and a negative correlation between 25(OH)D andIL-17 in the diabetic and diabetic neuropathy groups. [Conclusion] Vitamin D is a potential modifiable risk factor for diabetic peripheral neuropathy and may regulate inflammatory mediators, e.g., IL-17 and IL-13.
Key words: Vitamin D, Cytokines, Diabetic peripheral neuropathy
INTRODUCTION
The global epidemic of diabetes mellitus (DM) and related complications are increasing worldwide. Diabetic peripheral neuropathy (DPN) is a microvascular complication that affects up to 50% of these diabetic patients and is a major cause of mortality and morbidity in this population. The complex etiology of DPN is still not clear1, 2). However, hyperglycemia, decreased blood flow, hypoxia, hypoxia-induced proangiogenesis, and pro-inflammatory responses may play a role in the pathogenesis. Moreover, proinflammatory cytokines, such as interleukins (IL), affect neurons and glial cells and are involved in the pathology of diabetic neuropathy3).
Vitamin D deficiency is a suspected risk factor for DPN because it is related to inflammation and hyperglycemia. Accordingly, deficiencies in vitamin D are associated with an altered incidence of infections. Several studies have evaluated this relationship4, 5).
IL-13 is an immunoregulatory cytokine secreted mainly by activated Th (T helper)2 cells. Additionally, IL-13 suppresses the production of pro-inflammatory cytokines and prostaglandins by monocytes and macrophages6). IL-17 is a proinflammatory cytokine produced by activated Th17;it has an important role in the regulation of immune responses. Moreover, the pathogenic effects of Th17 cells are regulated by Th2 cytokines, such as IL-4, IL-5, IL-10, and IL-137).
Proinflammatory cytokines and vitamin D deficiencies are thought to play a role in DPN pathogenesis. The identification of the pathogenesis of DPN and its relationships with modifiable risk factors may facilitate the development of novel therapies. Since vitamin D deficiency is a potential modifiable risk factor for DPN, the aim of the present study was to investigate the effects of vitamin D on the circulating levels of IL-17 and IL-13 in patients with DPN, DM Type 2 patients without DPN, and healthy control individuals.
SUBJECTS AND METHODS
The procedures conformed to the ethical standards of the Responsible Committee on Human Experimentation and with the Declaration of Helsinki. This study was approved by the local ethics committee of Namik Kemal University. Informed consent was obtained from all individuals before inclusion in the study.
Demographic data, HbA1c, fasting blood glucose, serum lipid profiles, arterial blood pressure, and medical data were recorded for all participants.
The diagnosis of DPN included evaluations of the clinical symptom history, a neurological examination, electrophysiological tests, quantitative sensory testing, and autonomic function tests. The diagnoses were based on clinical symptoms and the results of the entire electromyography8,9,10). Medical data were documented. Patients with type 1 DM, clinical evidence of cardiovascular or cerebrovascular disease, hepatic or renal failure, malignancy, autoimmune diseases, acute or chronic infections, a history of trauma or surgery, or pregnancy were excluded.
Group I (n=33) was composed of type 2 DM patients without DPN and group II (n=37) was composed of type 2 DM patients with DPN. Group III (n=33) was composed of healthy control subjects who were age-, gender-, and body mass index-matched with patients in the DM groups.
Blood samples were collected after a 30-minute resting period between 8:30 and 9:30 a.m. Serum was centrifuged at 2,000 × g for 15 minutes at 4 °C. All samples were stored at −80 °C until the analysis.
Fasting plasma glucose, total cholesterol, HDL-cholesterol, and triglycerides were analyzed using an automated analyzer (Cobas e6000-e501; Roche Diagnostics, Tokyo, Japan). LDL-cholesterol was calculated using the Friedewald formula11).
Serum 25(OH)D levels were evaluated using ultra-performance liquid chromatography with a ClinRep commercial kit (Germany) and an UPLC analyzer (Thermo Scientific, Waltham, MA, USA). The results are reported in ng/ml. Serum IL-17 concentrations were measured using the RayBio Human IL-17 ELISA Kit (Atlanta, GA, USA).The sensitivity of the IL-17 commercial kits was 80 pg/ml, and the intra- and inter-assay coefficients of variation were <10% and <12%, respectively. Serum IL-13 concentrations were measured using the RayBio Human IL-13 ELISA Kit. The sensitivity of the IL-13 commercial kit was 0.15 pg/ml. The intra- and inter-assay coefficients of variation were <10% and <12%, respectively.
Statistical analyses were performed with SPSS version 17 (Chicago, IL, USA). The homogeneity of data within groups was analyzed with the Shapiro-Wilk test. Results are expressed as means ± standard deviation or medians and ranges, depending on the distribution of data. Normally distributed data were analyzed by independent t-tests and non-normally distributed data were analyzed by Mann-Whitney U tests. In normally distributed multiple group comparisons, one-way ANOVA with the Bonferroni correction was used. The Pearson’s test was used for the correlation analysis. The alpha significance level was set at <0.05.
RESULTS
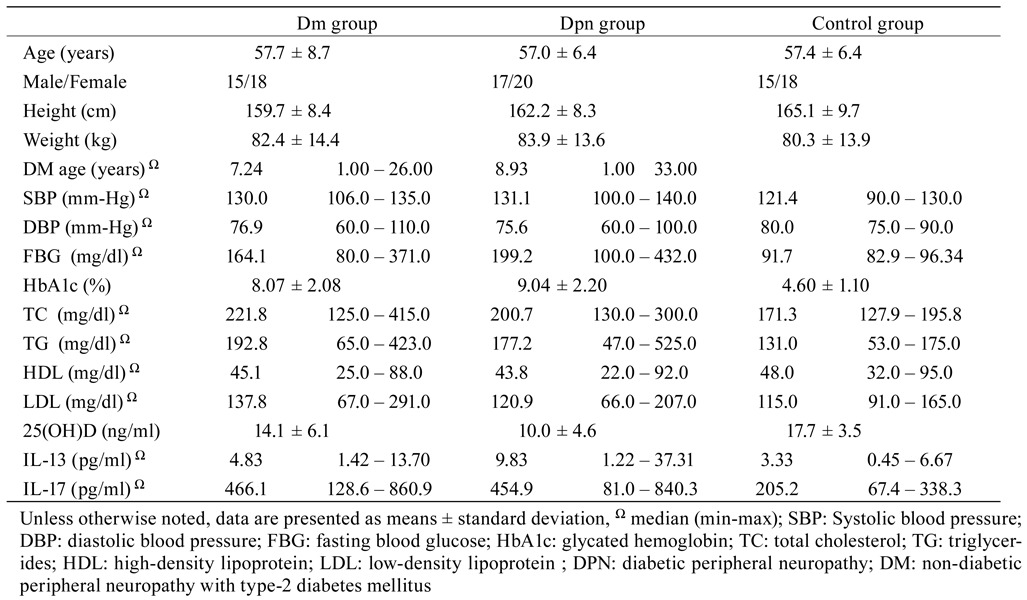
DM patients without DPN (Group I: 15 males and 18 females), diabetic patients with DPN (Group II: 17 males and 20 females), and healthy volunteers (Group III: 15 males and 18 females) were enrolled in the study. Demographic data for each group were recorded. No significant difference was observed in terms of age, gender, and body mass index among groups (p>0.05).
No statistically significant difference was found between Group I and Group II in terms of height, weight, FBG, systolic and diastolic blood pressure, HbA1C, TC, TG, and HDL and LDL levels.
The 25(OH)D levels of Group I and Group II were lower than those of Group III (p=0.036 and p<0.001, respectively). The 25(OH)D levels of Group II were significantly lower than those of Group I (p=0.005).
IL-17 levels of Group I and Group II were higher than those of Group III (p<0.001 and p<0.001, respectively). The difference in IL-17 levels between Group II and Group I was not statistically significant (p=0.897).
The IL-13 levels of Group I and Group II were higher than those of group III (p=0.038 and p<0.001, respectively). The IL-13 levels of Group II were significantly higher than those of Group I (p<0.001). Biochemical test results are described in Table 1 .
Table 1. Demographic data and inter-group comparisons of biochemical parameters.
A significant positive correlation was detected between IL-13 levels and 25(OH)D levels (r=0.301, p=0.013). There was a significant negative correlation betweenIL-17and 25(OH)D levels (r=−0.354, p=0.003).
DISCUSSION
There is increasing evidence that nerve tissues in DM undergo a pro-inflammatory process that causes symptoms and precipitates the development of neuropathy. Certainly, macrophages and lymphocytes invade the diabetic nerves and release IL or TNF-α12,13,14).
Moreover, there is considerable evidence that pro-inflammatory cytokines play a role in the pathogenesis of diabetic neuropathy. New data regarding the relationship between neuropathy and inflammatory cytokines have resulted in new approaches to suppress neuropathy via specific targets of cytokines15,16,17,18,19). Nevertheless, very few studies have examined DPN and cytokines.
Doupis et al. evaluated the relationship between inflammation and microvascular reactivity as well as the development of DPN. They suggested that DPN is associated with altered levels of biochemical indicators of endothelial dysfunction andinflammation16).
Hang et al. evaluated the roles of plasma cytokines, including IL-13 and IL-17, in another kind of microvascular complication of DM, diabetic retinopathy (DR). DPN and DR have a similar pathogenetic basis. Hang et al. assessed cytokine levels and their relationship with the severity of DR and found significantly elevated cytokine levels, including IL-13 and IL-17, in patients with DM. TNF-α plasma levels were higher in proliferative-DR compared with the levels in patients with non-proliferative DR and patients with no apparent DR20).
In this study, IL-13 and IL-17 levels of diabetes patients with and without DPN were higher than the levels observed in the control group. While the IL-13 levels of DPN patients were statistically significantly higher than those of diabetes patients without DPN, the difference in IL-17 levels between the groups was not statistically significant. These differences in cytokine levels might reflect known inflammatory processes in diabetes and its complications. Moreover, IL-13 levels might indicate DPN development in type 2 DM.
Additionally, the status of vitamin D deficiency affects the immune system and tends to increase infections4, 5). Since IL-13 is an immunoregulatory factor and IL-17 is a proinflammatory cytokine, the effects of vitamin D levels on these cytokines may provide insight into the pathogenesis of DPN. The role of vitamin D in the prevention and treatment of various neurological diseases has been evaluated for several years. The effect of vitamin D is confirmed by VDR and CYP27B1 expression in glial cells in the nervous system21). Despite many studies on the relationship between diabetes and its complications and vitamin D deficiency, there is little data concerning the association between neuropathy and vitamin D deficiency.
Several studies suggest a possible relationship between DPN and vitamin D deficiency in type 2 DM patients, but the general pattern is in conclusive. Therefore, it is still unclear whether serum 25(OH) D levels are related to DPN risk in type 2 DM patients22).
Shebab et al. evaluated the incidence of vitamin D deficiency in 210 type 2 DM patients with and without DPN. In total, 81.5% of diabetic neuropathy patients had a vitamin D deficiency, compared with 60.4% of patients without diabetic neuropathy. Accordingly, the authors suggested that vitamin D deficiency is a risk factor of DPN5).
Ahmadieh et al. investigated 25(OH)D levels in diabetic neuropathy patients compared to those without neuropathy. They found lower vitamin D levels in DPN patients and suggested that 25(OH)D vitamin levels are significant predictors of diabetic neuropathy23).
Skalli et al. evaluated serum 25(OH)D levels in 111 DPN patients. They found significantly lower 25(OH) D levels in the group with DPN than without DPN24).
The results of this study were consistent with the limited literature on this issue. Vitamin D levels in diabetic patients were lower than those in the control group. Meanwhile, vitamin D levels of the DPN group were lower than those of non-neuropathic diabetes patients. Vitamin D levels had a positive correlation with IL-13 and a negative correlation with IL-17. These results suggested that vitamin D impacts DPN development via inflammatory pathogenesis.
Highlighting the pathogenesis of DPN and relationships with modifiable risk factors as well as methods for the early diagnosis of DPN could help inhibit disease progression and facilitate the development of new treatment strategies with a molecular basis. Cytokines like IL-13 and IL-17 may reflect low-grade inflammation in DM. Moreover, IL-13 might indicate DPN in type2 DM patients. Additionally, vitamin D deficiency seems to play a role in the development of DPN, and it is highly likely to be a modifiable risk factor for DPN in type 2 diabetes patients.
This study had a few limitations that should be considered. The number of patients included was relatively small. The study design was cross-sectional, and it was a single-center study. Additional studies with larger sample sizes and multi-center designs are needed to confirm the results.
REFERENCES
- 1.Boulton AJ, Vinik AI, Arezzo JC, et al. American Diabetes Association: Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care, 2005, 28: 956–962. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y, Liu SX, Cai Y, et al. : Effects of combined aerobic and resistance training on the glycolipid metabolism and inflammation levels in type 2 diabetes mellitus. J Phys Ther Sci, 2015, 27: 2365–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu LN, Yang XS, Hua Z, et al. : [Serum levels of pro-inflammatory cytokines in diabetic patients with peripheral neuropathic pain and the correlation among them]. Zhonghua Yi Xue Za Zhi, 2009, 89: 469–471. [PubMed] [Google Scholar]
- 4.Soderstrom LH, Johnson SP, Diaz VA, et al. : Association between vitamin D and diabetic neuropathy in a nationally representative sample: results from 2001–2004 NHANES. Diabet Med, 2012, 29: 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shehab D, Al-Jarallah K, Mojiminiyi OA, et al. : Does Vitamin D deficiency play a role in peripheral neuropathy in Type 2 diabetes? Diabet Med, 2012, 29: 43–49. [DOI] [PubMed] [Google Scholar]
- 6.Hershey GK: IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol, 2003, 111: 677–690, quiz 691. [DOI] [PubMed] [Google Scholar]
- 7.Kumar P, Subramaniyam G: Molecular underpinnings of Th17 immune-regulation and their implications in autoimmune diabetes. Cytokine, 2015, 71: 366–376. [DOI] [PubMed] [Google Scholar]
- 8.Kang JH, Lee YS: Sensory nerve conduction studies in the diagnosis of diabetic sensorimotor polyneuropathy: electrophysiologicl features. J Phys Ther Sci, 2012, 24: 139–142. [Google Scholar]
- 9.Callaghan BC, Price RS, Chen KS, et al. : The importance of rare subtypes in diagnosis and treatment of peripheral neuropathy: a review. JAMA Neurol, 2015, 72: 1510–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han D: Retrogression of nervous fibers according to the age of patients with Diabetes Mellitus (DM). J Phys Ther Sci, 2013, 25: 1063–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedewald WT, Levy RI, Fredrickson DS: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem, 1972, 18: 499–502. [PubMed] [Google Scholar]
- 12.Younger DS, Rosoklija G, Hays AP, et al. : Diabetic peripheral neuropathy: a clinicopathologic and immunohistochemical analysis of sural nerve biopsies. Muscle Nerve, 1996, 19: 722–727. [DOI] [PubMed] [Google Scholar]
- 13.Conti G, Scarpini E, Baron P, et al. : Macrophage infiltration and death in the nerve during the early phases of experimental diabetic neuropathy: a process concomitant with endoneurial induction of IL-1beta and p75NTR. J Neurol Sci, 2002, 195: 35–40. [DOI] [PubMed] [Google Scholar]
- 14.Yagihashi S, Yamagishi S, Wada R: Pathology and pathogenetic mechanisms of diabetic neuropathy: correlation with clinical signs and symptoms. Diabetes Res Clin Pract, 2007, 77: S184–S189. [DOI] [PubMed] [Google Scholar]
- 15.Ramos KM, Jiang Y, Svensson CI, et al. : Pathogenesis of spinally mediated hyperalgesia in diabetes. Diabetes, 2007, 56: 1569–1576. [DOI] [PubMed] [Google Scholar]
- 16.Doupis J, Lyons TE, Wu S, et al. : Microvascular reactivity and inflammatory cytokines in painful and painless peripheral diabetic neuropathy. J Clin Endocrinol Metab, 2009, 94: 2157–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saini AK, Kumar H S A, Sharma SS: Preventive and curative effect of edaravone on nerve functions and oxidative stress in experimental diabetic neuropathy. Eur J Pharmacol, 2007, 568: 164–172. [DOI] [PubMed] [Google Scholar]
- 18.Chun J, Hong J, ATC: Relationships between presynaptic inhibition and static postural sway in subjects with and without diabetic neuropathy. J Phys Ther Sci, 2015, 27: 2697–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grbovic V, Jurisic-Skevin A, Djukic S, et al. : Comparative analysis of the effects combined physical procedures and alpha-lipoic acid on the electroneurographic parameters of patients with distal sensorimotor diabetic polyneuropathy. J Phys Ther Sci, 2016, 28: 432–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hang H, Yuan S, Yang Q, et al. : Multiplex bead array assay of plasma cytokines in type 2 diabetes mellitus with diabetic retinopathy. Mol Vis, 2014, 20: 1137–1145. [PMC free article] [PubMed] [Google Scholar]
- 21.Hayes CE, Nelson CD, Spanier JA: 11 Vitamin D and Autoimmune Disease. Vitamin D: Oxidative Stress, Immunity, and Aging, 2012, p 239. [Google Scholar]
- 22.Putz Z, Martos T, Németh N, et al. : Is there an association between diabetic neuropathy and low vitamin D levels? Curr Diab Rep, 2014, 14: 537. [DOI] [PubMed] [Google Scholar]
- 23.Ahmadieh H, Azar ST, Lakkis N, et al. : Hypovitaminosis d in patients with type 2 diabetes mellitus: a relation to disease control and complications. ISRN Endocrinol, 2013, 2013: 641098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skalli S, Muller M, Pradines S, et al. : Vitamin D deficiency and peripheral diabetic neuropathy. Eur J Intern Med, 2012, 23: e67–e68. [DOI] [PubMed] [Google Scholar]


