ABSTRACT
Shiga toxin-producing Escherichia coli (STEC) is an important foodborne pathogen that can cause hemorrhagic colitis and hemolytic-uremic syndrome. Cattle are the primary reservoir for STEC, and food or water contaminated with cattle feces is the most common source of infections in humans. Consequently, we conducted a cross-sectional study of 1,096 cattle in six dairy herds (n = 718 animals) and five beef herds (n = 378 animals) in the summers of 2011 and 2012 to identify epidemiological factors associated with shedding. Fecal samples were obtained from each animal and cultured for STEC. Multivariate analyses were performed to identify risk factors associated with STEC positivity. The prevalence of STEC was higher in beef cattle (21%) than dairy cattle (13%) (odds ratio [OR], 1.76; 95% confidence interval [CI], 1.25, 2.47), with considerable variation occurring across herds (range, 6% to 54%). Dairy cattle were significantly more likely to shed STEC when the average temperature was >28.9°C 1 to 5 days prior to sampling (OR, 2.5; 95% CI, 1.25, 4.91), during their first lactation (OR, 1.8; 95% CI, 1.1, 2.8), and when they were <30 days in milk (OR, 3.9; 95% CI, 2.1, 7.2). These data suggest that the stress or the negative energy balance associated with lactation may result in increased STEC shedding frequencies in Michigan during the warm summer months. Future prevention strategies aimed at reducing stress during lactation or isolating high-risk animals could be implemented to reduce herd-level shedding levels and avoid transmission of STEC to susceptible animals and people.
IMPORTANCE STEC shedding frequencies vary considerably across cattle herds in Michigan, and the shedding frequency of strains belonging to non-O157 serotypes far exceeds the shedding frequency of O157 strains, which is congruent with human infections in the state. Dairy cattle sampled at higher temperatures, in their first lactation, and early in the milk production stage were significantly more likely to shed STEC, which could be due to stress or a negative energy balance. Future studies should focus on the isolation of high-risk animals to decrease herd shedding levels and the potential for contamination of the food supply.
INTRODUCTION
Shiga toxin-producing Escherichia coli (STEC) is an important foodborne pathogen in both developed and developing countries. STEC can cause hemorrhagic colitis and hemolytic-uremic syndrome (HUS), which can lead to kidney failure and death, particularly in young children (1). STEC strains belonging to serotype O157:H7 have been reported to cause human infections at the highest frequency, although there has been a steady increase in the detection of cases caused by STEC serotypes other than O157 (non-O157 STEC) (2–4). This increase is due in part to changes in laboratory diagnostic practices targeting non-O157 STEC (5). The incidence of non-O157 STEC infections in the United States increased from 0.12 per 100,000 population in 2000 to 0.95 per 100,000 in 2010, while the incidence of STEC O157 infections decreased from 2.17 to 0.95 per 100,000 over the same time period (4). In 2013, 561 cases (1.17 per 100,000 people) of non-O157 STEC infection were reported, whereas 552 cases (1.15 cases per 100,000) of STEC O157 infection were reported. Similar increases and trends were observed in Michigan patients (6), demonstrating that enteric infections attributable to non-O157 STEC are as important as those attributable to STEC O157 in terms of disease frequency, though STEC O157 has been linked to more severe clinical outcomes (4).
STEC is defined by the presence of genes encoding Shiga toxins (Stx) carried on bacteriophages (7). The two major Stx types are Stx1 and Stx2, but additional subtypes (e.g., Stx2c to Stx2g) have also been described (8). The eaeA gene, which is present on the locus of enterocyte effacement (LEE) pathogenicity island and encodes the intimin protein, is important for intimate attachment to the intestinal mucosa and the formation of attaching and effacing lesions (9). Strains containing the LEE island and at least one stx subtype represent a subset of STEC strains that are classified as enterohemorrhagic E. coli (EHEC) (10). Although some STEC strains of various serotypes are capable of causing hemorrhagic colitis and HUS (11), EHEC typically causes more severe clinical symptoms in humans (10).
Cattle are an important reservoir for STEC, and food or water contaminated with cattle feces are common sources of infection for humans (12). Indeed, one study utilized spatial regression analysis to identify a positive association between cattle density and STEC O157 infections (13). Other sources of potential STEC infection include direct contact with domestic animals, such as swine, dogs, and cats, and wildlife, including wild white-tailed deer (14). The prevalence of STEC has been shown to vary across food animal production systems in the United States and other countries. For example, the prevalence of STEC O157 was 45%, 19%, and 8% in cow-calf operations in Ontario, Canada; feedlots in Scotland; and dairy cattle in Washington State, respectively (15–17). Similarly, the prevalence of non-O157 STEC reported in feedlots and beef cattle on pasture also varies, ranging from 5% to 56% (18).
Factors associated with low or high herd prevalence are not fully understood, particularly for non-O157 STEC. Indeed, most prior studies have attempted to identify risk factors associated with STEC O157 shedding in cattle, though few consistent factors have been identified. For example, season, herd management practices (e.g., manure removal), age, level of animal-to-animal contact, pathogen density, stress, and diet were suggested to be important for STEC O157 shedding in different study populations (15, 19–22). Additional studies are therefore needed to better understand the risk factors associated with shedding at both the herd and animal levels in specific geographical locations, particularly those with a high frequency of human infections. Here, we conducted a cross-sectional study of 1,096 animals from six dairy and five beef herds in Michigan during the summers of 2011 and 2012. Given the detailed epidemiological data collected for each herd, we sought to identify factors associated with STEC shedding in Michigan cattle. The identification of cattle with an enhanced risk of STEC could result in improvements to and the development of intervention practices aimed at reducing the level and frequency at which STEC enters the food supply.
MATERIALS AND METHODS
Herd selection and sampling.
Six dairy farms and five beef feedlots were selected for inclusion in the study on the basis of the availability of records and animal-handling facilities, geographical location, and willingness to participate. The sampling strategy was based on the type of herd and the number of cattle available. In dairy herds with less than 175 animals, all adult cattle were sampled, with the exception of herd D6. For the larger dairy herds, only a subset of animals representing different management groups (e.g., dry versus lactating) was sampled. In the beef feedlots, a herd was defined as cattle managed as one unit during the same time period. Approval to conduct the study was obtained by the Michigan State University Institutional Animal Care and Use Committee (AN12/10-223-00). The farm owners provided written informed consent and were interviewed by one member of the research team. Separate questionnaires were used for the managers of the dairy farms and beef feedlots, though both questionnaires consisted of closed and open-ended questions addressing farm demographics, animal movements, farm management practices, and herd health management strategies.
Herds were sampled between 11 May and 16 August 2011 (n = 5) or between 21 May and 27 August 2012 (n = 6). The date, time, latitude, and longitude were recorded for each farm sampled. Season was defined by the day of the equinoxes and solstices indicated on the Gregorian calendar. The maximum, minimum, and average temperatures from the day of sampling and the preceding 5 days were also recorded using data from the closest weather station (Quality Controlled Local Climatological Data [NOAA]). Fecal samples (i.e., rectal contents) were collected from each animal by rectal palpation using individual obstetrical sleeves. Samples from the first four herds (n = 496 animals) were transported to the laboratory on ice, stored at 4°C, and processed within 48 h. The remaining samples from seven herds were transported in coolers without ice and were processed within 8 h of sample collection.
STEC detection and isolation.
Five grams of feces was inoculated in 2× EC broth (Oxoid Ltd., Waltham, MA) supplemented with novobiocin (8 mg/liter), rifampin (2 mg/liter), and potassium tellurite (1 mg/liter) for 24 h at 42°C (23), followed by subculture on CHROMagar STEC (CHROMagar, Paris, France) and sorbitol MacConkey (SMAC) agar. A portion of the EC culture was also processed by immunomagnetic separation using Dynabeads (Invitrogen Corporation, Carlsbad, CA, USA) specific for E. coli O157, followed by subculture to O157 CHROMagar STEC and SMAC agar. Up to 20 presumptive colonies were selected from each plate, inoculated into Luria-Bertani (LB) broth for growth overnight at 37°C, and confirmed to be STEC by PCR targeting stx1, stx2, and eaeA, as described previously (24). Individual cattle were considered to be positive for STEC if at least one stx-positive colony was recovered from the fecal sample.
Data analysis.
The proportion of cattle with STEC was calculated, and prevalence was compared among herds. For both the univariate and multivariate analyses, the dependent variable was the STEC status (positive or negative) of each animal; the herd variable was included as a random effect to control for differences across herds. Independent variables with nonnormal distributions were converted into binary or categorical variables on the basis of averages or quartiles. Generalized linear mixed models (GLMM) and the chi-square test were used to identify variables yielding significant associations with STEC status. Odds ratios (OR) and 95% confidence intervals (CI) were calculated to estimate the magnitude of these associations and to identify confounders and effect modifiers. Separate analyses were conducted for beef and dairy herds.
A univariate analysis was first conducted to identify associations between the outcome variable and potential risk factors; variables with P values of <0.15 were included in the multivariate analysis. A manual backward elimination procedure was used to build the final multivariable models until only significant covariates and significant and biologically meaningful variables were retained. If potential risk factors were correlated with a correlation coefficient greater than or equal to 0.9, only one of these variables was included in the final multivariable model. Statistical analyses were conducted in SAS (version 9.3) software (SAS Institute, Cary, NC, USA).
RESULTS
Prevalence of stx-positive E. coli in Michigan dairy and beef cattle.
A total of 1,108 animals were sampled during the course of this study; 724 (65%) were dairy cattle, and 384 (35%) were beef cattle. Twelve animals (six beef cattle and six dairy cattle) were excluded due to missing laboratory results, leaving a total of 1,096 individual cattle (378 beef cattle and 718 dairy cattle) in the final analysis. At least one stx-positive E. coli colony (isolate) was detected in 175 (16%) of the animals sampled. The prevalence of STEC varied considerably across herds, with a range of 6% to 54% (Table 1). Among all herds, STEC was significantly more likely to be detected in beef cattle (n = 80; 21.2%) than dairy cattle (n = 95; 13.2%) (OR, 1.8; 95% CI, 1.27, 2.44). There was also a significant difference in the prevalence of STEC by year, with 2012 (n = 118; 23%) having a higher prevalence than 2011 (n = 57; 10%) (OR, 2.8; 95% CI, 1.96, 3.88). Differences in STEC prevalence were not observed between the spring (18%) and summer (15%) months (P < 0.19).
TABLE 1.
Herds sampled for STEC and number of positive cattle per herda
| Herd | Type | Total no. of animals | No. (%) of animals sampled | No. (%) of STEC-positive animals | Yr of sampling |
|---|---|---|---|---|---|
| B1 | Beef | 136 | 136 (100) | 11 (8.2) | 2011 |
| D2 | Dairy | 320 | 154 (48) | 13 (8.7) | 2011 |
| B3 | Beef | 36 | 36 (100) | 3 (9.4) | 2011 |
| D4 | Dairy | 3,000 | 175 (9) | 24 (13.8) | 2011 |
| D6 | Dairy | 98 | 94 (96) | 6 (6.4) | 2011 |
| D7 | Dairy | 12,000 | 100 (1) | 13 (13.0) | 2012 |
| B8 | Beef | 54 | 54 (100) | 29 (53.7) | 2012 |
| D9 | Dairy | 243 | 100 (41) | 28 (28.0) | 2012 |
| D10 | Dairy | 530 | 101 (19) | 11 (10.9) | 2012 |
| B11 | Beef | 83 | 83 (100) | 13 (15.7) | 2012 |
| B12 | Beef | 75 | 75 (100) | 24 (32.0) | 2012 |
Samples were lost from 12 animals from herds B1 (n = 2), D2 (n = 5), B3 (n = 4), and D4 (n = 1).
Among the 522 stx-positive isolates recovered from the 175 STEC-positive animals, O157 was found in only 19 (11%) animals. Sixteen of these 19 animals were from one dairy herd sampled in 2012, and all 16 animals had isolates positive for eae, stx1, and stx2. The remaining three O157 isolates were recovered from animals in two beef herds and one dairy herd. Because of the low frequency of O157 isolates in this cattle population, the remainder of the analyses focused on evaluating all stx-positive isolates together regardless of serotype.
Among the stx-positive isolates recovered, the frequency of specific virulence genes varied across herds as well as among cattle from the same herd. Overall, stx1-positive isolates were detected in 51 (29%) animals, and stx2-positive isolates were found in 74 (42%) animals; a total of 50 (29%) animals had isolates positive for both stx1 and stx2 (Fig. 1). A subset of 17 (10%) cattle were shedding more than one STEC strain type, as multiple isolates with distinct stx profiles were recovered. At the herd level, one beef herd had isolates positive only for stx1, whereas the remaining herds had isolates with multiple stx profiles. In all, beef cattle were significantly more likely to shed stx2-positive isolates than dairy cattle (OR, 2.1; 95% CI, 1.05, 4.08). Characterization of isolates for the presence of EHEC, which was defined as isolates positive for any stx gene as well as eae, identified 108 (62%) animals as shedding EHEC. A total of 60 (75%) beef cattle and 48 (51%) individual dairy cattle were positive for EHEC, while 20 (25%) beef cattle and 47 (49%) dairy cattle were shedding STEC, which were defined as stx-positive isolates lacking eae. No significant difference in EHEC prevalence was observed between the dairy and beef herds (P = 0.9), though differences were observed across herds. For example, STEC predominated in one beef herd, being detected in 24 of the 29 (83%) stx-positive animals, while EHEC was found in 22 of the 24 stx-positive animals in another beef herd. Seventeen (16%) EHEC-positive animals were also positive for STEC.
FIG 1.
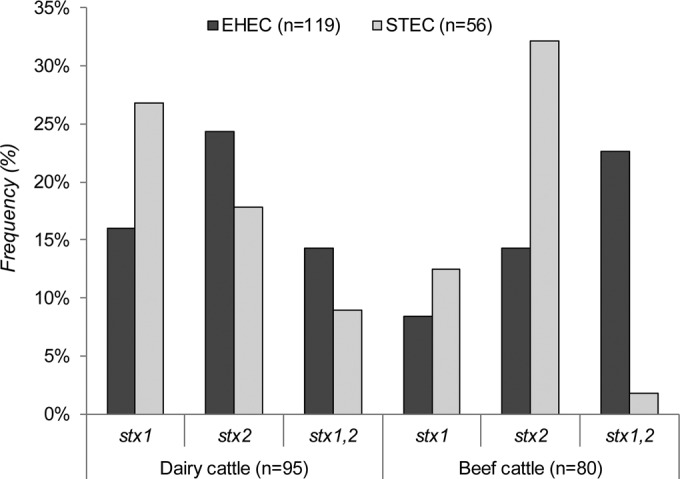
Prevalence of enterohemorrhagic E. coli (EHEC) and Shiga toxin-producing E. coli (STEC) by stx profile and herd type.
Host factors associated with STEC shedding in dairy cattle.
A univariate analysis was first performed to identify host factors associated with STEC (both STEC and EHEC combined) shedding. Notably, cattle in their first lactation were at higher risk for shedding STEC (OR, 1.6; 95% CI, 1.04, 2.58) than animals with more than one lactation period (Table 2). In all, 279 (40%) cows were in their first lactation, and 56% of these animals were sampled during 2011. The mean and standard deviation number of lactations for the cows sampled were 2.12 and 1.29, respectively, with a range of 0 to 10 lactations.
TABLE 2.
Univariate analysis of factors associated with STEC shedding in dairy cattle belonging to six herds in Michigana
| Characteristic | No. (%) with characteristic | No. (%) with STEC | OR (95% CI) | P value |
|---|---|---|---|---|
| Animal-specific factors | ||||
| Lactation status | ||||
| Dry | 53 (7.4) | 4 (7.6) | 0.6 (0.21, 1.88) | 0.41 |
| Lactating | 661 (92.6) | 90 (13.6) | Reference | |
| Lactation no. | ||||
| 1st lactation | 279 (39.6) | 49 (17.6) | 1.6 (1.04, 2.58) | 0.03 |
| ≥2 lactations | 426 (60.4) | 44 (10.3) | Reference | |
| Days in milk | ||||
| 0 days | 54 (7.7) | 5 (9.3) | 1.1 (0.40, 3.00) | <0.0001 |
| 1–30 days | 70 (10.0) | 21 (30.0) | 3.8 (2.07, 6.90) | |
| >30 days | 579 (82.4) | 64 (11.1) | Reference | |
| Herd-specific factors | ||||
| Percent of lactating cattle | ||||
| >50% | 94 (13.1) | 6 (6.4) | 0.4 (0.12, 1.35) | 0.14 |
| ≤50% | 518 (72.1) | 54 (56.8) | Reference | |
| Access to pasture | ||||
| No | 524 (73.0) | 61 (11.6) | 0.7 (0.29, 1.64) | 0.40 |
| Yes | 194 (27.0) | 34 (17.5) | Reference | |
| Culling rate | ||||
| Low (≤31%) | 195 (27.2) | 17 (8.7) | 0.5 (0.22, 1.25) | 0.15 |
| High (>31%) | 523 (72.8) | 78 (15.0) | Reference | |
| Use of anthelmintics | ||||
| Yes | 518 (72.2) | 54 (10.4) | 0.4 (0.23, 0.84) | 0.01 |
| No | 200 (27.9) | 41 (20.5) | Reference | |
| Treatment for respiratory infection | ||||
| Yes | 618 (86.1) | 67 (10.8) | 0.3 (0.19, 0.52) | <0.0001 |
| No | 100 (13.9) | 28 (28.0) | Reference | |
| Use of direct-fed microbials | ||||
| Yes | 344 (47.9) | 30 (8.7) | 0.4 (0.23, 0.83) | 0.01 |
| No | 374 (52.1) | 65 (17.4) | Reference | |
| Exposure to rodents/raccoons | ||||
| Continuous | 200 (27.9) | 41 (20.5) | 2.7 (1.09, 6.52) | 0.03 |
| Frequent | 369 (51.4) | 41 (11.1) | 1.3 (0.53, 3.00) | |
| Rare | 149 (20.8) | 13 (8.7) | Reference | |
| Any exposure to dogs | ||||
| No | 374 (52.1) | 65 (17.4) | 0.4 (0.23, 0.83) | 0.01 |
| Yes | 344 (47.9) | 30 (8.7) | Reference |
Herd was included as a random effect in the univariate analysis. Generalized linear mixed models were used to calculate the P values, odds ratios (OR), and 95% confidence intervals (CI). The number of animals with a given characteristic may differ depending on the variable examined.
STEC shedding was also significantly more common in cows during the first 30 days of lactation (OR, 3.8; 95% CI, 2.07, 6.90) than in animals that had been lactating for more than 30 days. A total of 579 (82.4%) cows had been lactating for more than 30 days, while 70 (10%) cows were sampled during the first 30 days of lactation. The remaining 8% of cattle, which belonged to four of the six dairy herds, were not lactating at the time of sampling. The mean and standard deviation for the number of days in milk were 176.51 and 141.77, respectively, with a range of 0 to 1,212. Although 14 of the 21 (67%) STEC-positive cows in the first lactation were in the first 30-day lactation period and 32 (50%) STEC-positive cows had been lactating for more than 30 days, this difference was not statistically significant. Both lactation number and the number of days in milk were included in the final multivariate model.
Herd-specific factors associated with STEC shedding in dairy cattle.
Several herd-specific variables were associated with STEC shedding in the univariate analysis (Table 2). For example, when the average maximum temperature 1 to 5 days prior to sampling exceeded 28.9°C, there was a higher likelihood of STEC shedding compared to that for animals sampled at lower temperatures (OR, 2.0; 95% CI, 0.99, 4.03). No significant difference in STEC positivity among samples processed on ice and those processed without ice was detected, even after controlling for herd, season, and year.
Daily cleaning of cattle feeders was also associated with a lower risk of STEC shedding than less frequent cleaning (OR, 2.0; 95% CI, 0.96, 4.11); however, the environmental cleanliness scores, which represented a visual subjective evaluation of farm cleanliness by the interviewer, was not significant (P = 0.14). Other variables, such as housing transition cows separately, were reported at the same frequency as other variables (e.g., cleaning of cattle feeders) across herds and resulted in similar univariate associations. Although access to pasture or a dry lot was not significantly associated with STEC shedding (P = 0.4), there was a tendency for cattle with access to pasture to have higher STEC frequencies. Specifically, 18% of cattle with pasture access were positive for STEC, whereas 12% of cattle without pasture access were positive for STEC.
In the five herds with a history of antimicrobial use for the treatment of respiratory diseases, the odds of STEC shedding was significantly lower (OR, 0.3; 95% CI, 0.19, 0.52) than that in the six herds in which antimicrobials were not used. Among the herds in which antimicrobials were used, ceftiofur, florfenicol, and tulathromycin were the most common agents used for treatment. In contrast, antimicrobial use for the treatment of foot infections or metritis was not associated with STEC positivity (P = 0.14). The most common treatment for foot infections was copper sulfate, whereas ceftiofur and oxytetracycline were used for metritis. Similar to respiratory disease treatments, the prophylactic use of anthelmintics, a control measure applied in four of the six dairy herds, was also significantly associated with decreased STEC shedding. The four herds in which the use of anthelmintics was reported had a lower likelihood of STEC shedding (OR, 0.4; 95% CI, 0.23, 0.84) than the remaining two herds.
Several dietary variables were also examined, including the percentage of corn silage, distiller's grains, and cottonseed as well as the use of monensin (Rumensin) and direct-fed microbials. Among these variables, only direct-fed microbial use yielded a significant association with STEC shedding; 9% of cows that were given a direct-fed microbial product were positive for STEC, whereas 17% that were not given a direct-fed microbial product were positive for STEC (OR, 0.4; 95% CI, 0.23, 0.83). Because dairy farms had different diets for dry and lactating cows as well as cows with different levels of milk production, comparisons among herds was difficult for the diet-associated variables.
Associations were also observed for reported contact with domestic animals and wildlife. Two herds with continuous (OR, 2.7; 95% CI, 1.09, 6.52) and frequent (OR, 1.3; 95% CI, 0.53, 3.00) exposure to rodents and raccoons, for instance, had a higher risk for STEC shedding than herds with rare exposures. On the other hand, cows without frequent exposure to dogs had a lower likelihood of STEC shedding than cows with regular contact with dogs (OR, 0.4; 95% CI, 0.23, 0.83). Interestingly, herds for which managers reported raccoon exposure were less likely to have frequent dog exposures (P ≤ 0.0001). No association was identified for regular contact with birds, horses, opossum, deer, or cats.
Multivariate analysis of factors associated with STEC shedding in dairy cattle.
On the basis of the strength of the associations identified in the univariate analyses, several variables were included in the final model as fixed effects, with herd being included as a random effect. These variables were the average maximum temperature 1 to 5 days before sampling, lactation number, and number of days in milk. In all, a total of 692 animals were included in the final model; 26 animals had missing values for one or more of the variables examined.
A higher average temperature (>28.9°C) increased the likelihood of STEC shedding 2.5 times compared to that for lower sampling temperatures (Table 3). Adjusting for temperature in the model also strengthened the associations between shedding of STEC and lactation and milk production. Cattle in their first lactation, for example, were 1.8 times more likely to shed STEC than cattle with two or more lactations. Additionally, cows that were in their first 31 days of milk production were 3.9 times more likely to shed STEC than those cows that had been producing milk for longer periods of time.
TABLE 3.
Multivariate analysis highlighting factors associated with STEC shedding in dairy cattle from six herds in Michigana
| Variable | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| Maximum temp (>28.9°C) 1–5 days before sampling | 2.5 | 1.25, 4.91 | 0.009 |
| First lactation | 1.8 | 1.09, 2.83 | 0.02 |
| Days in milk (range, 1–30) | 3.9 | 2.12, 7.18 | <0.0001 |
| Access to pasture | 0.6 | 0.35, 0.95 | 0.03 |
| Anthelmintic treatment | 0.5 | 0.29, 0.76 | 0.003 |
| Treatment for respiratory infections | 0.4 | 0.21, 0.70 | 0.002 |
Herd was included as a random effect. Temperature, lactation status, and days in milk were included in the final model, and the values presented for these three variables are adjusted for the other two variables. All additional variables were added separately to the final model and were adjusted for each of these three variables.
Controlling for temperature, lactation number, and number of days in milk production also yielded protective associations between STEC and access to pasture as well as anthelmintic use and use of antimicrobials to treat respiratory disease. No significant associations were observed for direct-fed microbial use, monensin treatment, the culling rate, or contact with deer, dogs, and raccoons, all of which yielded noteworthy associations in the univariate analysis.
Factors associated with STEC shedding in beef herds.
A univariate analysis was also conducted to identify factors associated with STEC shedding in the five feedlots. The two beef herds sampled during 2011 had a decreased likelihood of STEC shedding compared to that for the three herds sampled during 2012 (OR, 0.19; 95% CI, 0.06, 0.65) (Table 4). Because there was a significant difference in the maximum temperature 1 to 5 days prior to sampling between the two years (P < 0.0001), it is possible that year-to-year variation in STEC prevalence was completely confounded by the average maximum temperature. Similar to the findings for cattle from dairy herds, cattle from beef herds sampled during high temperatures (>28.9°C) were six times more likely to shed STEC than cattle from herds sampled during lower temperatures (P < 0.0001).
TABLE 4.
Univariate analysis of factors associated with STEC shedding in beef cattle belonging to five herds in Michigana
| Characteristic | No. (%) with characteristic | No. (%) with STEC | OR (95% CI) | P value |
|---|---|---|---|---|
| Maximum temp 1–5 days prior to sampling | ||||
| >28.9°C | 129 (34.1) | 53 (41.1) | 6.0 (2.79, 12.70) | <0.0001 |
| ≤28.9°C | 249 (65.9) | 27 (10.8) | Reference | |
| Treatment for respiratory disease | ||||
| Several antibiotics | 129 (34.1) | 53 (41.1) | 5.9 (2.79, 12.70) | <0.0001 |
| Only one antibiotic type | 249 (65.9) | 76 (59.0) | Reference | |
| Anthelmintic/monensin treatment | ||||
| Yes | 324 (85.7) | 51 (15.7) | 0.2 (0.04, 0.57) | 0.005 |
| No | 54 (14.3) | 29 (53.7) | Reference | |
| Pasture access/unique diet | ||||
| Yes | 54 (14.3) | 29 (53.7) | 6.6 (1.77, 24.31) | 0.005 |
| No | 324 (85.7) | 51 (15.7) | Reference | |
| Breed | ||||
| Holstein | 83 (22.0) | 13 (15.7) | 1.0 (0.26, 4.14) | 0.019 |
| Angus | 54 (14.3) | 29 (53.7) | 6.6 (1.69, 25.89) | |
| Crossbreed | 241 (63.8) | 38 (15.8) | Reference |
Herd was included as a random effect in the univariate analysis. Generalized linear mixed models were used to calculate the P values, odds ratios (OR), and 95% confidence intervals (CI). The number of animals with a given characteristic may differ depending on the variable examined. Pasture access, corn silage diet, high-forage diet (100%), and 0% total mixed ration all yielded the same association, as these characteristics were unique to one herd with the highest STEC prevalence.
The two beef herds for which frequent or continuous contact with opossums, dogs, deer, and skunks was reported had a higher likelihood of STEC shedding than the three herds for which no such contact was reported. Because unique treatment and cleanliness practices were also found for these two herds, it was not possible to identify which factors were most important for shedding in this analysis. For example, the use of both anthelmintics and monensin was also reported for these two beef herds, and they were 0.2 times less likely to shed STEC (95% CI, 0.04, 0.57) than cattle from herds in which these two products were not used. The use of more than one type of antibiotic for treatment of respiratory infections was also reported for these herds, and they had a higher odds of STEC shedding than animals on farms using only one type of antibiotic (OR, 5.9; 95% CI, 2.79, 12.70). The same association was observed for the treatment of foot infections, as cattle from the same set of farms using oxytetracycline rather than other agents were significantly more likely to shed STEC. Contrary to the findings for dairy cattle, direct-fed microbial use was not significantly associated with decreased STEC shedding in the univariate analysis (P = 0.45), though the use of these products was reported for only one herd.
The diet was also very similar across herds with the exception of one (n = 54 cows), the cattle of which were raised on a pasture; importantly, this herd had the highest prevalence of STEC (54%). Consequently, pasture access was significantly associated with STEC shedding, as were Angus breed and a corn silage diet, high-forage diet (100%), and 0% total mixed ration (TMR), which were also unique to this herd. Because of the high prevalence of STEC in this single herd and the uniqueness of the animals and management practices relative to those on the remaining feedlots, a multivariate analysis could not be conducted for the beef herds. Indeed, there was not enough variability to use herd as a random effect, and hence, our ability to identify herd-specific factors important for STEC shedding in beef cattle was limited.
DISCUSSION
An evaluation of 1,096 cattle in five dairy and six beef herds in Michigan demonstrated significant variation in the prevalence of STEC. Although all 11 herds had at least one STEC-positive animal, the prevalence ranged considerably across herds, with significant variation being detected between the two sampling periods and herd types. Contrary to our finding that beef cattle had a greater frequency of STEC than dairy cattle, a prior study from Washington State reported a higher prevalence in dairy farms than in feedlots (25). Given the challenges associated with STEC detection and the use of different methodologies, however, it is not surprising to observe variation in the prevalence of STEC across studies. In this Michigan study, one beef herd had the highest overall prevalence of STEC relative to all other herds and, hence, may have been responsible for several of the observed associations. Indeed, when data for this beef herd were omitted from the STEC prevalence estimates, there was no significant difference between dairy and beef herds (P = 0.28). Because of this bias and a difference in cow-level and management risk factors, the multivariate analysis solely focused on identifying predictors of STEC shedding in dairy cattle.
On the basis of the findings of prior studies, it is evident that several factors are likely to be innate contributors of STEC prevalence and persistence in a given environment or host. For example, geographical location, particularly those areas with high cattle densities, has previously been shown to impact the incidence of human STEC O157 infections in Canada and The Netherlands (13, 26). Another study conducted in Germany observed a 68% increased risk of human STEC infections for 100 additional cattle per km2, though the risk differed depending on the serotype (27). In the United States, considerable variation in the number of human STEC infections was also observed across the 10 Foodborne Disease Surveillance Network (FoodNet) sites (4), further highlighting the importance of geographical or region-specific risk factors. Variations in the serotype and stx gene distribution have also been observed. In 2012, the number of human non-O157 STEC infections surpassed the number of STEC O157 infections in Michigan (6), which is consistent with the increasing incidence of non-O157 infections observed nationwide (4). Among the 11 cattle herds sampled in 2011 and 2012, non-O157 STEC predominated in animals from all but one herd. Importantly, the majority (71%) of the isolates recovered from these animals were stx2 positive regardless of the serotype. This finding is in agreement with the findings of previous studies from other locations (28, 29) and is of importance, given that stx2-based infections have been linked to more severe disease outcomes in humans (30–32). Likewise, 68% of the animals were positive for EHEC, which has also been linked to enhanced virulence (10). Given that specific serotypes have been linked to more severe infections, the isolates recovered in this study will need to be further characterized in the future to assess shedding frequencies by serotype.
Similar to the epidemiology of STEC in humans, variations in its prevalence have been observed in cattle populations in those studies that utilize the same diagnostic tools; comparisons cannot be made among studies that use different methods. The United States Department of Agriculture (USDA), for instance, has reported regional variation in prevalence rates in dairy herds (33), which has been observed in other countries as well (29, 34, 35). In our study, significant variation was observed across farms, though additional geographical studies are needed to identify specific ecological and landscape factors that may impact prevalence and persistence. We did note, however, that higher temperatures 1 to 5 days prior to sampling were significantly associated with STEC shedding in both the beef and dairy herds. This finding is consistent with other reports demonstrating higher shedding frequencies in the summer months regardless of the geographical location (20, 25, 36–38). It is clear that the higher temperatures promote bacterial growth and survival in the environment as well as in food and water sources, thereby increasing the likelihood of transmission and prevalence in a herd (39). Access to deer and other wildlife reservoirs as well as an increased prevalence of Stx bacteriophage populations in the warm summer months may also play a role in transmission (24). While contact with various wildlife and domestic animals was not identified by the multivariate analysis to be a risk factor for STEC shedding in dairy cattle, additional studies are needed to identify the importance of these factors for the persistence, shedding, and interherd transmission of Stx bacteriophages. The negative correlation between dogs and exposure to raccoons in the dairy herds, for instance, is noteworthy and may indicate that dogs can decrease the risk of STEC acquisition in cattle by limiting wildlife exposure.
Through our multivariate analysis, we also identified several cattle-specific risk factors for STEC shedding in dairy cattle. The association with first lactation is notable, as no difference in prevalence between lactating and dry cows or in the percentage of lactating cattle on the farms was observed. This finding suggests that the stage of lactation is most important for shedding in this dairy cattle population. Although other studies have observed opposite trends (29), this finding is in agreement with the findings of earlier studies that reported associations between STEC shedding and animal age and/or lactation number. Indeed, several prior studies have reported differences in STEC shedding in young versus old animals (40–43). A prior study from Minnesota found that dairy calves were significantly more likely than adult cows to be stx positive (42), while a Dutch study found that dairy calves between 4 and 12 months of age were more frequently positive for STEC O157 (40). With regard to age at the time of calving, a German study found that animals undergoing first lactation had a higher risk of STEC shedding than older cows (44), while a longitudinal study in the United Kingdom reported the prevalence of STEC O157 to be the highest in cows 2 years of age, the typical age at first calving (43). Although the multitude of factors that could contribute to the association between lactation number and STEC are not completely understood, they could be related to stress and the energy requirements needed by cows in the first lactation (45), in addition to decreased immune function. A negative energy balance, which is common during the first lactation, has been suggested to alter the digestive microbiota composition (45), which could potentially favor STEC colonization and shedding.
In Michigan dairy cattle, we also observed STEC shedding frequencies to be the highest in animals at the beginning of their lactation cycle. A similar finding was observed in dairy cattle from the United Kingdom, as STEC O157 shedding peaked in the first month of lactation, followed by lower levels of shedding and then a less intense increase at 7 months postpartum (43). It was also suggested that modifications to the diet may explain changes in STEC shedding frequencies, since dietary changes could alter the intestinal microbiota, thereby favoring STEC growth (43). During early lactation, significant physiological and metabolic changes occur with high metabolic demand, which may favor intestinal STEC colonization and shedding (46). Two additional studies, however, found no association between STEC and stage of lactation, yet these studies also focused only on STEC O157 and were conducted in the southwestern United States (45, 47).
Importantly, most of the associations identified through this study may contribute in some way to animal stress, though little is known about the short-term impact of stress on bovine immune function (48). Heat stress has been suggested to impact STEC O157 shedding and was found to be important for Salmonella shedding as well (45). Not all studies, however, have observed differences associated with ambient temperature (45, 49, 50), which varies considerably by geographic location. The lack of positive associations between STEC shedding and the degree of animal contact and the number and proportion of lactating cattle provides support for the hypothesis that specific stressors may enhance host susceptibility and contribute to shedding. This is in contrast to the hypothesis that specific stressors impact STEC transmission, acquisition, and subsequent shedding. Indeed, protective effects were observed for access to pasture and both anthelmintic and respiratory disease treatments, which could impact STEC acquisition frequencies. Additional studies are greatly needed to characterize the strain types that are shed during lactation to better understand the epidemiology of STEC in dairy cattle. On the basis of the findings of this study, it is possible that control strategies might be considered for dairy cattle in their first lactation and/or within the first 30 days of milking. Such strategies could potentially decrease STEC shedding frequencies and the potential for transmission to other animals on the farm and in the environment.
ACKNOWLEDGMENTS
We thank the farm owners and personnel for their support of the project and the many individuals who participated in the field sampling (Justine Zingsheim, Tonya Newman, Marion Tseng, Yi-An Yang, Jacquelyn Del Valle, Aaron Balogh, and Erin Jagodzinski) and the laboratory work (Davis Thomas and Katherine Jernigan).
This work was supported by USDA NIFA grant number 2011-67005-30004, and salary support was provided by the National Institutes of Health Enterics Research Investigational Network (ERIN) Cooperative Research Center at Michigan State University (grant number U19AI090872 to S.D.M.). We also thank the Michigan State University Food Safety Graduate Assistantship, the National Institutes of Health Merial Scholars Program, and Vilma Yuzsbasyian-Gurkan of the College of Veterinary Medicine at Michigan State University for funding a subset of students to participate in the field samplings.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Karmali MA, Petric M, Lim C, Fleming PC, Arbus GS, Lior H. 2004. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J Infect Dis 189:556–563. doi: 10.1086/jid/189.3.566. [DOI] [PubMed] [Google Scholar]
- 2.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crim S, Iwamoto M, Huang J, Griffin P, Gilliss D, Cronquist A, Cartter M, Tobin-D'Angelo M, Blythe D, Smith K, Lathrop S, Zansky S, Cieslak P, Dunn J, Holt K, Lance S, Tauxe R, Henao O, Centers for Disease Control and Prevention (CDC). 2014. Incidence and trends of infection with pathogens transmitted commonly through food—Foodborne Diseases Active Surveillance Network, 10 U.S. sites, 2006-2013. MMWR Morb Mortal Wkly Rep 63:328–332. [PMC free article] [PubMed] [Google Scholar]
- 4.Gould LH, Mody RK, Ong KL, Clogher P, Cronquist AB, Garman KN, Lathrop S, Medus C, Spina NL, Webb TH, White PL, Wymore K, Gierke RE, Mahon BE, Griffin PM. 2013. Increased recognition of non-O157 Shiga toxin-producing Escherichia coli infections in the United States during 2000-2010: epidemiologic features and comparison with E. coli O157 infections. Foodborne Pathog Dis 10:453–460. doi: 10.1089/fpd.2012.1401. [DOI] [PubMed] [Google Scholar]
- 5.Gould LH. 2012. Recommendations for diagnosis of Shiga toxin-producing Escherichia coli infections by clinical laboratories. Clin Microbiol News 34:75–83. doi: 10.1016/j.clinmicnews.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Tseng M, Sha Q, Rudrik JT, Collins J, Henderson T, Funk JA, Manning SD. 2016. Increasing incidence of non-O157 Shiga toxin-producing Escherichia coli (STEC) in Michigan and association with clinical illness. Epidemiol Infect 144:1394–1405. doi: 10.1017/S0950268815002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Brien AD, Newland JW, Miller SF, Holmes RK, Smith HW, Formal SB. 1984. Shiga-like toxin-converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science 226:694–696. doi: 10.1126/science.6387911. [DOI] [PubMed] [Google Scholar]
- 8.Scheutz F, Teel LD, Beutin L, Pierard D, Buvens G, Karch H, Mellmann A, Caprioli A, Tozzoli R, Morabito S, Strockbine NA, Melton-Celsa AR, Sanchez M, Persson S, O'Brien AD. 2012. Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing Stx nomenclature. J Clin Microbiol 50:2951–2963. doi: 10.1128/JCM.00860-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDaniel TK, Jarvis KG, Donnenberg MS, Kaper JB. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci U S A 92:1664–1668. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nataro JP, Kaper JB. 1998. Diarrheagenic Escherichia coli. Clin Microbiol Rev 11:142–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Croxen MA, Law RJ, Scholz R, Keeney KM, Wlodarska M, Finlay BB. 2013. Recent advances in understanding enteric pathogenic Escherichia coli. Clin Microbiol Rev 26:822–880. doi: 10.1128/CMR.00022-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armstrong GL, Hollingsworth J, Morris JG Jr. 1996. Emerging foodborne pathogens: Escherichia coli O157:H7 as a model of entry of a new pathogen into the food supply of the developed world. Epidemiol Rev 18:29–51. doi: 10.1093/oxfordjournals.epirev.a017914. [DOI] [PubMed] [Google Scholar]
- 13.Friesema IH, Van De Kassteele J, De Jager CM, Heuvelink AE, Van Pelt W. 2011. Geographical association between livestock density and human Shiga toxin-producing Escherichia coli O157 infections. Epidemiol Infect 139:1081–1087. doi: 10.1017/S0950268810002050. [DOI] [PubMed] [Google Scholar]
- 14.Beutin L, Geier D, Steinruck H, Zimmermann S, Scheutz F. 1993. Prevalence and some properties of verotoxin (Shiga-like toxin)-producing Escherichia coli in seven different species of healthy domestic animals. J Clin Microbiol 31:2483–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cernicchiaro N, Pearl DL, Ghimire S, Gyles CL, Johnson RP, LeJeune JT, Ziebell K, McEwen SA. 2009. Risk factors associated with Escherichia coli O157:H7 in Ontario beef cow-calf operations. Prev Vet Med 92:106–115. doi: 10.1016/j.prevetmed.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Chase-Topping ME, McKendrick IJ, Pearce MC, MacDonald P, Matthews L, Halliday J, Allison L, Fenlon D, Low JC, Gunn G, Woolhouse MEJ. 2007. Risk factors for the presence of high-level shedders of Escherichia coli O157 on Scottish farms. J Clin Microbiol 45:1594–1603. doi: 10.1128/JCM.01690-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hancock DD, Besser TE, Kinsel ML, Tarr PI, Rice DH, Paros MG. 1994. The prevalence of Escherichia coli O157.H7 in dairy and beef cattle in Washington state. Epidemiol Infect 113:199–207. doi: 10.1017/S0950268800051633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hussein HS. 2007. Prevalence and pathogenicity of Shiga toxin-producing Escherichia coli in beef cattle and their products. J Anim Sci 85:E63–E72. doi: 10.2527/jas.2006-421. [DOI] [PubMed] [Google Scholar]
- 19.Cho S, Fossler CP, Diez-Gonzalez F, Wells SJ, Hedberg CW, Kaneene JB, Ruegg PL, Warnick LD, Bender JB. 2013. Herd-level risk factors associated with fecal shedding of Shiga toxin-encoding bacteria on dairy farms in Minnesota, USA. Can Vet J 54:693–697. [PMC free article] [PubMed] [Google Scholar]
- 20.Dunn JR, Keen JE, Thompson RA. 2004. Prevalence of Shiga-toxigenic Escherichia coli O157:H7 in adult dairy cattle. J Am Vet Med Assoc 224:1151–1158. doi: 10.2460/javma.2004.224.1151. [DOI] [PubMed] [Google Scholar]
- 21.Garber L. 1999. Factors associated with fecal shedding of verotoxin-producing Escherichia coli O157 on dairy farms. J Food Prot 62:307–312. [DOI] [PubMed] [Google Scholar]
- 22.Matthews L, Low JC, Gally DL, Pearce MC, Mellor DJ, Heesterbeek JA, Chase-Topping M, Naylor SW, Shaw DJ, Reid SW, Gunn GJ, Woolhouse ME. 2006. Heterogeneous shedding of Escherichia coli O157 in cattle and its implications for control. Proc Natl Acad Sci U S A 103:547–552. doi: 10.1073/pnas.0503776103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jasson V, Rajkovic A, Baert L, Debevere J, Uyttendale M. 2009. Comparison of enrichment conditions for rapid detection of low numbers of sublethally injured Escherichia coli O157 in food. J Food Prot 72:1862–1868. [DOI] [PubMed] [Google Scholar]
- 24.Singh P, Sha Q, Lacher DW, Del Valle J, Mosci RE, Moore JA, Scribner KT, Manning SD. 2015. Characterization of enteropathogenic and Shiga toxin-producing Escherichia coli in cattle and deer in a shared agroecosystem. Front Cell Infect Microbiol 5:29. doi: 10.3389/fcimb.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cobbold RN, Rice DH, Szymanski M, Call DR, Hancock DD. 2004. Comparison of Shiga-toxigenic Escherichia coli prevalences among dairy, feedlot, and cow-calf herds in Washington State. Appl Environ Microbiol 70:4375–4378. doi: 10.1128/AEM.70.7.4375-4378.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michel P, Wilson JB, Martin SW, Clarke RC, McEwen SA, Gyles CL. 1999. Temporal and geographical distributions of reported cases of Escherichia coli O157:H7 infection in Ontario. Epidemiol Infect 122:193–200. doi: 10.1017/S0950268899002083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frank C, Kapfhammer S, Werber D, Stark K, Held L. 2008. Cattle density and Shiga toxin-producing Escherichia coli infection in Germany: increased risk for most but not all serogroups. Vector Borne Zoonotic Dis 8:635–643. doi: 10.1089/vbz.2007.0237. [DOI] [PubMed] [Google Scholar]
- 28.Polifroni R, Etcheverria AI, Sanz ME, Cepeda RE, Kruger A, Lucchesi PM, Fernandez D, Parma AE, Padola NL. 2012. Molecular characterization of Shiga toxin-producing Escherichia coli isolated from the environment of a dairy farm. Curr Microbiol 65:337–343. doi: 10.1007/s00284-012-0161-0. [DOI] [PubMed] [Google Scholar]
- 29.Kuhnert P, Dubosson CR, Roesch M, Homfeld E, Doherr MG, Blum JW. 2005. Prevalence and risk-factor analysis of Shiga toxigenic Escherichia coli in faecal samples of organically and conventionally farmed dairy cattle. Vet Microbiol 109:37–45. doi: 10.1016/j.vetmic.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Friedrich AW, Bielaszewska M, Zhang WL, Pulz M, Kuczius T, Ammon A, Karch H. 2002. Escherichia coli harboring Shiga toxin 2 gene variants: frequency and association with clinical symptoms. J Infect Dis 185:74–84. doi: 10.1086/338115. [DOI] [PubMed] [Google Scholar]
- 31.Persson S, Olsen KE, Ethelberg S, Scheutz F. 2007. Subtyping method for Escherichia coli Shiga toxin (verocytotoxin) 2 variants and correlations to clinical manifestations. J Clin Microbiol 45:2020–2024. doi: 10.1128/JCM.02591-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manning SD, Motiwala AS, Springman AC, Qi W, Lacher DW, Ouellette LM, Mladonicky JM, Somsel P, Rudrik JT, Dietrich SE, Zhang W, Swaminathan B, Alland D, Whittam TS. 2008. Variation in virulence among clades of Escherichia coli O157:H7 associated with disease outbreaks. Proc Natl Acad Sci U S A 105:4868–4873. doi: 10.1073/pnas.0710834105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.United States Department of Agriculture. 2003. Escherichia coli O157 on U.S. dairy operations. United States Department of Agriculture, Washington, DC. [Google Scholar]
- 34.Kistemann T, Zimmer S, Vagsholm I, Andersson Y. 2004. GIS-supported investigation of human EHEC and cattle VTEC O157 infections in Sweden: geographical distribution, spatial variation and possible risk factors. Epidemiol Infect 132:495–505. doi: 10.1017/S0950268803001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang E, Hwang SY, Kwon KH, Kim KY, Kim JH, Park YH. 2014. Prevalence and characteristics of Shiga toxin-producing Escherichia coli (STEC) from cattle in Korea between 2010 and 2011. J Vet Sci 15:369–379. doi: 10.4142/jvs.2014.15.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kondo S, Hoar BR, Villanueva V, Mandrell RE, Atwill ER. 2010. Longitudinal prevalence and molecular typing of Escherichia coli O157:H7 by use of multiple-locus variable-number tandem-repeat analysis and pulsed-field gel electrophoresis in fecal samples collected from a range-based herd of beef cattle in California. Am J Vet Res 71:1339–1347. doi: 10.2460/ajvr.71.11.1339. [DOI] [PubMed] [Google Scholar]
- 37.Smith DR, Moxley RA, Clowser SL, Folmer JD, Hinkley S, Erickson GE, Klopfenstein TJ. 2005. Use of rope devices to describe and explain the feedlot ecology of Escherichia coli O157:H7 by time and place. Foodborne Pathog Dis 2:50–60. doi: 10.1089/fpd.2005.2.50. [DOI] [PubMed] [Google Scholar]
- 38.Dewsbury DM, Renter DG, Shridhar PB, Noll LW, Shi X, Nagaraja TG, Cernicchiaro N. 2015. Summer and winter prevalence of Shiga toxin-producing Escherichia coli (STEC) O26, O45, O103, O111, O121, O145, and O157 in feces of feedlot cattle. Foodborne Pathog Dis 12:726–732. doi: 10.1089/fpd.2015.1987. [DOI] [PubMed] [Google Scholar]
- 39.Gautam R, Bani-Yaghoub M, Neill WH, Dopfer D, Kaspar C, Ivanek R. 2011. Modeling the effect of seasonal variation in ambient temperature on the transmission dynamics of a pathogen with a free-living stage: example of Escherichia coli O157:H7 in a dairy herd. Prev Vet Med 102:10–21. doi: 10.1016/j.prevetmed.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Heuvelink AE, van den Biggelaar FLAM, Zwartkruis-Nahuis JTM, Herbes RG, Huyben R, Nagelkerke N, Melchers WJG, Monnens LAH, de Boer E. 1998. Occurrence of verocytotoxin-producing Escherichia coli O157 on Dutch dairy farms. J Clin Microbiol 36:3480–3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cobbold R, Desmarchelier P. 2000. A longitudinal study of Shiga-toxigenic Escherichia coli (STEC) prevalence in three Australian diary herds. Vet Microbiol 71:125–137. doi: 10.1016/S0378-1135(99)00173-X. [DOI] [PubMed] [Google Scholar]
- 42.Cho S, Fossler CP, Diez-Gonzalez F, Wells SJ, Hedberg CW, Kaneene JB, Ruegg PL, Warnick LD, Bender JB. 2009. Cattle-level risk factors associated with fecal shedding of Shiga toxin-encoding bacteria on dairy farms, Minnesota, USA. Can J Vet Res 73:151–156. [PMC free article] [PubMed] [Google Scholar]
- 43.Mechie SC, Chapman PA, Siddons CA. 1997. A fifteen month study of Escherichia coli O157:H7 in a dairy herd. Epidemiol Infect 118:17–25. doi: 10.1017/S0950268896007194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menrath A, Wieler LH, Heidemanns K, Semmler T, Fruth A, Kemper N. 2010. Shiga toxin producing Escherichia coli: identification of non-O157:H7-super-shedding cows and related risk factors. Gut Pathog 2:7. doi: 10.1186/1757-4749-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edrington TS, Schultz CL, Genovese KJ, Callaway TR, Looper ML, Bischoff KM, McReynolds JL, Anderson RC, Nisbet DJ. 2004. Examination of heat stress and stage of lactation (early versus late) on fecal shedding of E. coli O157:H7 and Salmonella in dairy cattle. Foodborne Pathog Dis 1:114–119. doi: 10.1089/153531404323143639. [DOI] [PubMed] [Google Scholar]
- 46.Dunn J. 2003. The epidemiology of STEC O157:H7 in Louisiana dairy cattle, beef cattle, and white-tailed deer. Ph.D. thesis. Louisiana State University, Baton Rouge, LA. [Google Scholar]
- 47.Fitzgerald AC, Edrington TS, Looper ML, Callaway TR, Genovese KJ, Bischoff KM, McReynolds JL, Thomas JD, Anderson RC, Nisbet DJ. 2003. Antimicrobial susceptibility and factors affecting the shedding of E. coli O157:H7 and Salmonella in dairy cattle. Lett Appl Microbiol 37:392–398. doi: 10.1046/j.1472-765X.2003.01417.x. [DOI] [PubMed] [Google Scholar]
- 48.Callaway TR, Edrington TS, Loneragan GH, Carr MA, Nisbet DJ. 2013. Shiga toxin-producing Escherichia coli (STEC) ecology in cattle and management based options for reducing fecal shedding. Agric Food Anal Bacteriol 3:39–69. [Google Scholar]
- 49.Morrow JL, Mitloehner FM, Johnson AK, Galyean ML, Dailey JW, Edrington TS, Anderson RC, Genovese KJ, Poole TL, Duke SE, Callaway TR. 2005. Effect of water sprinkling on incidence of zoonotic pathogens in feedlot cattle. J Anim Sci 83:1959–1966. [DOI] [PubMed] [Google Scholar]
- 50.Brown-Brandl TM, Berry ED, Wells JE, Arthur TM, Nienaber JA. 2009. Impacts of individual animal response to heat and handling stresses on Escherichia coli and E. coli O157:H7 fecal shedding by feedlot cattle. Foodborne Pathog Dis 6:855–864. doi: 10.1089/fpd.2008.0222. [DOI] [PubMed] [Google Scholar]


