Abstract
Selenium species, particularly the oxyanions selenite (SeO32−) and selenate (SeO42−), are significant pollutants in the environment that leach from rocks and are released by anthropogenic activities. Selenium is also an essential micronutrient for organisms across the tree of life, including microorganisms and human beings, particularly because of its presence in the 21st genetically encoded amino acid, selenocysteine. Environmental microorganisms are known to be capable of a range of transformations of selenium species, including reduction, methylation, oxidation, and demethylation. Assimilatory reduction of selenium species is necessary for the synthesis of selenoproteins. Dissimilatory reduction of selenate is known to support the anaerobic respiration of a number of microorganisms, and the dissimilatory reduction of soluble selenate and selenite to nanoparticulate elemental selenium greatly reduces the toxicity and bioavailability of selenium and has a major role in bioremediation and potentially in the production of selenium nanospheres for technological applications. Also, microbial methylation after reduction of Se oxyanions is another potentially effective detoxification process if limitations with low reaction rates and capture of the volatile methylated selenium species can be overcome. This review discusses microbial transformations of different forms of Se in an environmental context, with special emphasis on bioremediation of Se pollution.
INTRODUCTION
Since the discovery in 1954 by Pinsent that the oxidation of formate by cell suspensions of Escherichia coli requires growth medium containing molybdate and selenite, there has been a growing interest in the biochemical role of selenium in microorganisms (1). Se is an essential component of selenoamino acids, such as selenomethionine and selenocysteine (the 21st proteinogenic amino acid), that occur in certain types of prokaryotic enzymes. Indeed, the requirement for selenite in E. coli growing on formate is linked to the fact that formate dehydrogenase contains selenocysteine. Other prokaryotic enzymes that contain selenocysteine include glycine reductase in several clostridia, formate dehydrogenases in diverse prokaryotes, including Salmonella, Clostridium, and Methanococcus, as well as hydrogenases in Methanococcus and other anaerobes. In addition, other bacterial Se-dependent enzymes, in which the selenium is part of the active site molybdenum-containing cofactor, include nicotinic acid dehydrogenase and xanthine dehydrogenase, which is present in certain clostridial species (2–4).
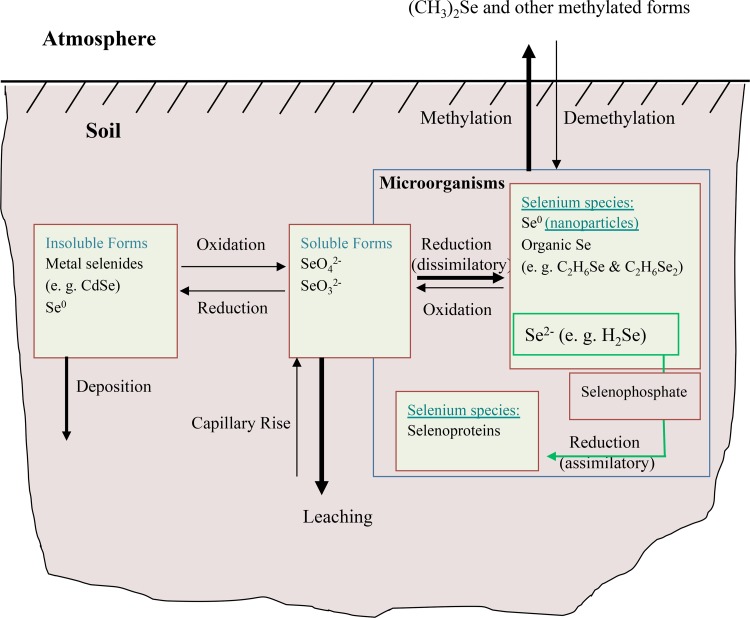
Reactions that are involved in the cycling of Se in soil, including those influenced by microbes, are diagrammatically summarized in Fig. 1. Of the four transformation reactions, dissimilatory reduction and methylation are considered the most important in terms of bioremediation. For example, the microbial reduction of toxic Se oxyanions (SeO42− and SeO32−) to the insoluble and less biologically available elemental selenium (Se0) results in its removal from solution. Microbial transformation of nonvolatile Se forms to volatile compounds is a significant pathway of Se transfer from aquatic and terrestrial environments to the atmosphere. Moreover, the reduction and methylation of SeO42− and SeO32− are effective detoxification processes because the product (dimethyl selenide [DMSe] or dimethyl diselenide [DMDSe]) is 500 to 700 times less toxic than SeO42− or SeO32− (5–8).
FIG 1.
Schematic Se cycle in soil and the influence of microbial processes on the transformation of the element. The bold arrows indicate the dominant direction of the process. Modified from Flury et al. (110) with permission from Elsevier.
Zehr and Oremland (9) tested the assumption that microorganisms involved in the S cycle can also reduce Se oxyanions since Se is adjacent to S in group 16 of the periodic table and both commonly occur in the +6, +4, 0, and −2 oxidation states. Washed cell suspensions of Desulfovibrio desulfuricans (a sulfate-reducing bacterium) were found to be capable of reducing small (nanomolar) amounts of SeO42− to Se2− at the same time as reducing SO42− to S2−. The reduction was dependent on the relative concentrations of SeO42− and SO42−. Increasing concentrations of SO42− inhibited rates of SeO42− reduction but enhanced SO42− reduction rates. Subsequently, however, Oremland et al. (10) reported a novel bacterial dissimilatory reduction of SeO42−, which occurs by pathways different from those for SO42− and was spatially separated from sulfate reduction in the environment despite the presence of substantial concentrations of sulfate where it occurred. Thus, it can be concluded that Se and S have different reductive biogeochemical cycles and appear to involve distinct populations of microorganisms.
With respect to the remediation of seleniferous environments, microbial oxidation and demethylation of Se compounds are not often considered because of the low rates at which these reactions proceed. Microbial demethylation of Se compounds occurs when some microorganisms utilize methylated Se forms as their sole source of carbon and energy (5, 11). The aim of this review is to discuss the reactions involved in the microbial transformation of different forms of selenium and to consider these in an environmental context, with reference to the bioremediation of the element in polluted environments.
MICROBIAL REDUCTION OF SELENIUM SPECIES
During the microbial assimilation of Se oxyanions, selenate (SeO42−) and selenite (SeO32−) are transported into the cells by different permeases. In the cell, the two oxyanions are reduced through assimilatory reduction to selenide (Se2−) (12). In bacteria, selenophosphate is then produced by selenophosphate synthase. Selenocysteine is subsequently synthesized via the enzyme-catalyzed reaction of serine with selenophosphate, while the serine is attached to the tRNASec specific for insertion of selenocysteine into ribosomally synthesized proteins (13). Also, in the presence of excess available Se, cells begin to incorporate Se instead of S into cellular components that normally contain S (5).
In soil, sediment, and water, microbial reduction of SeO32− and SeO42− is known to be important process for removing toxic soluble Se oxyanions. In dissimilatory Se reactions, the reduction of Se oxyanions is a mechanism by which certain microorganisms can obtain metabolic energy (14). Dissimilatory Se-reducing microorganisms are known to use a number of different electron donors, such as alcohols, sugars, organic acids, humic substances, and hydrogen (15–19). In terms of the bioremediation of seleniferous environments, the assimilatory reduction of Se is expected to make only a minor contribution because of the small selenium fluxes involved. In contrast, the dissimilatory reduction of Se is considered to be the more important process for bioremediation. The reduction of selenium oxyanions, including reduction that is apparently not linked to respiration or assimilation, is a highly active reaction among many bacterial isolates and may play an important role in the environment (7). Research into dissimilatory reduction of Se is receiving increased attention, not least because results from these investigations offer a potentially cost-effective means of remediating selenium pollution. In contrast to insoluble Se0, SeO42− and SeO32− are environmentally problematic in aqueous phases because of their high solubility. However, they become immobilized when the selenate and selenite are microbially reduced to Se0 (20). Microbial reduction of Se0 to selenide (Se2−) has received limited attention, but it is noteworthy that insoluble Se0 can be reduced microbiologically to soluble selenide (21, 22).
Certain bacteria are able to grow anaerobically through the dissimilatory reduction of selenium oxyanions. The product from dissimilatory reduction of selenite is generally Se0, which appears in the form of Se nanoparticles. Microbial reduction occurs either in the periplasmic space (intracellularly) (23–25) or extracellularly (26–28). The reduction of Se oxyanions to Se0 nanoparticles can also be mediated aerobically by diverse species of bacteria, namely, selenium-resistant bacteria (29–32). Several investigations have dealt with the mechanisms of microbial formation of Se nanoparticles (33–35). The Se nanoparticles are known to have microbial proteins associated with them, which play a role in the formation and growth of the Se nanoparticles (29) as well as in controlling their size distribution (33). Recently, Jain et al. (34) used biogenic elemental selenium nanoparticles (BioSeNPs), which were produced by anaerobic granular sludge in the treatment of pulp and paper wastewater, in an investigation of the presence of extracellular polymeric substances (EPSs) on the BioSeNPs. Functional group characteristics of proteins and carbohydrates were on the BioSeNPs, suggesting that EPSs form a coating that determines the surface charge on these BioSeNPs. EPSs also contribute to the colloidal properties of the BioSeNPs and thereby influence their fate in the environment and the efficiency of bioremediation technologies (34). Microbial reduction of Se may not only be exploited in Se bioremediation but also in the production of selenium nanoparticles for biotechnological applications (33, 36). However, the mechanisms involved in the formation of the nanoparticles and, more importantly, in their physical and chemical properties are yet to be fully elucidated.
Microorganisms that reduce the Se oxyanions SeO32− and SeO42− are not confined to any particular group of prokaryotes and are widely distributed throughout the bacterial and archaeal domains (37–49). However, compared to the SeO32−-reducing microorganisms that have been isolated, the number of known SeO42− reducers is relatively small. The reduction of SeO42− to Se0 is generally a two-step process in which SeO32− is an intermediate product. Some bacteria are capable of reducing SeO42− and SeO32− to Se0 (50–52), while other bacterial species can only reduce SeO32− to Se0 (53, 54). In some instances, dissimilatory reduction of SeO42− supports growth via anaerobic respiration. In other cases, reduction of selenium oxyanions may serve a detoxifying function or be an adventitious reaction of enzymes with different functions. The reductions of SeO42− and SeO32− are considered in detail below. Major cultured selenium-reducing prokaryotes and their properties are summarized in Table 1.
TABLE 1.
Cultured SeO42−- and SeO32−-reducing microorganisms and observed Se transformation reactions
| Microorganism(s) | Se transformation | Reference |
|---|---|---|
| Bacteria with dissimilatory Se reduction supporting anaerobic respiration | ||
| Thauera selenatis | Respiration via reduction of SeO42− to SeO32− in the absence of NO3−, minor reduction of SeO32− to Se0; in the presence of NO3−, SeO42− is completely reduced to Se0 | 40 |
| Chrysiogenetes S5 | Respiration via reduction of SeO42− to Se0 | 111 |
| Deferribacteres S7 | ||
| Deltaproteobacteria KM | ||
| Sulfurospirillum barnesii SES-3 | Respiration via reduction of SeO42− and SeO32− to Se0 | 37 |
| Bacillus arseniciselenatis E-1H | Respiration via reduction of SeO42− to SeO32− | 55 |
| Bacillus selenitireducens MLS10 | Respiration via reduction of SeO32− to Se0 | 55 |
| Selenihalanaerobacter shriftii DSSe-1 | Respiration via reduction of SeO42− to Se0 | 112 |
| Archaea with dissimilatory Se reduction supporting anaerobic respiration | ||
| Pyrobaculum arsenaticum and Pyrobaculum aerophilum | Anaerobic chemolithotrophs that also grow organotrophically with SeO42− as electron acceptor; hyperthermophiles | 113 |
| Pyrobaculum ferrireducens | Anaerobic organotrophic growth on SeO42− and SeO32−; produces Se0; hyperthermophile | 49 |
| Bacteria with dissimilatory Se reduction not clearly supporting respiration | ||
| Rhodospirillum rubrum | Extracellular reduction of SeO32− to Se0; reduction under anoxic conditions is greater than that under oxic conditions | 64 |
| Rhodobacter sphaeroides | Reduction of SeO32− to Se0 with intracellular accumulation under aerobic and anaerobic conditions | 53 |
| Shewanella oneidensis MR-1 | Extracellular reduction of SeO32− to Se0 under aerobic or anaerobic conditions | 114 |
| Clostridium pasteurianum | Enzymatic reduction of SeO32− using hydrogenase I | 42 |
| Enterobacter cloacae SLD1a-1 | Reduction of SeO42− to Se0 through SeO32− as intermediate in the presence of NO3− | 44 |
| Azospira oryzae | Reduction of SeO42− and SeO32− to Se0 under anaerobic and microaerobic conditions using O2 or NO3 as terminal electron acceptors for growth | 45 |
| Veillonella atypica | Reduction of SeO32− to Se0 and then to Se2− under anaerobic conditions | 22 |
| Desulfovibrio desulfuricans | Reduction of SeO42− and SeO32− to Se0 with formate as the electron donor and fumarate or sulfate as the electron acceptor | 41 |
| Enterobacter cloacae SLD1a-1 | Reduction of SeO42− to Se0 through SeO32− as an intermediate in the presence of NO3− | 44 |
| Pseudomonas stutzeri NT-1 | Aerobic reduction of SeO42− and SeO32− to Se0 | 46 |
| Rhodopseudomonas palustris N | Aerobic reduction of SeO42− and SeO32− to Se0 | 115 |
| Wolinella succinogenes | Aerobic reduction of SeO42− and SeO32− to Se0 | 116 |
| Salmonella enterica serovar Heidleberg | Reduction of SeO32− to intracellular granules Se0 | 38 |
| Ralstonia metallidurans CH34 | Aerobic reduction of SeO32− to Se0 | 54 |
| Salmonella Heidelberg | Aerobic reduction of SeO32− to Se0 | 38 |
| Azospirillum brasilense | Reduction of SeO32− to Se0 nanoparticles | 117 |
| Pseudomonas sp. strain CA-5 | Reduction of SeO32− to Se0 under aerobic conditions | 70 |
| Bacillus cereus CM100B | Reduction of SeO32− to Se0 under aerobic conditions | 31 |
| Bacillus megaterium BSB6 and BSB12 | Aerobic reduction of SeO32− to Se0 at high salt concentrations | 118 |
| Duganella sp. strains C1 and C4 | Reduction of SeO32− to Se0 nanoparticles | 30 |
| Agrobacterium sp. strains C 6 and C 7 | ||
| Pseudomonas sp. strain RB | Reduction of SeO32− in the presence of cadmium producing CdSe nanoparticles | 119 |
| Archaea with dissimilatory Se reduction not clearly supporting respiration | ||
| Halorubrum xinjiangense | Aerobic reduction of SeO32− to Se0; halophile | 120 |
| Well-studied example of assimilatory Se reduction | ||
| Escherichia coli | Reduction of SeO42− and SeO32− to Se0; incorporation of Se into proteins | 51 |
Reduction of selenate.
The mechanism of selenate reduction varies among the cultured microorganisms studied to date. Several selenate-respiring bacterial species (i.e., bacteria that can use selenate as the terminal electron acceptor to support growth), including Thauera selenatis, Sulfurospirillum barnesii, and Bacillus arseniciselenatis, have been well-characterized and shown to respire anaerobically by using SeO42− as the terminal electron acceptor (55–57). Membrane-bound nitrate reductase (Nar), periplasmic nitrate reductase (Nap), and selenate reductase (Ser) have all been shown to be able to catalyze the reduction of SeO42− to SeO32−. Current evidence from Enterobacter cloacae (58) and other organisms indicates that selenate reductases have evolved specifically for the reduction of selenate and are more important in cultures of specific strains and, by implication, environmentally than the adventitious capacity of nitrate reductases to reduce selenate. Selenate reductase (Ser) has been purified and characterized from T. selenatis (20). It is a heterotrimer that is located in the periplasm, forming a complex of approximately 180 kDa containing the subunits SerA (96 kDa), SerB (40 kDa), and SerC (23 kDa). It contains molybdenum, iron, and acid-labile sulfur as prosthetic groups (20). Ser has been demonstrated to be specific for SeO42− reduction to SeO32− and does not use nitrate, nitrite, chlorate, or sulfate as electron acceptors. In contrast, the selenate reductase complex in S. barnesii is found in the membrane. It is a heterotetramer with subunits of 82, 53, 34, and 21 kDa and also contains molybdenum at the active site (59–61).
In the facultative anaerobe Enterobacter cloacae SLD1a-1, which can reduce selenate under aerobic conditions, the selenate reductase is located in the membrane fraction. It discriminates between SeO42− and NO3− and is expressed under aerobic and anaerobic conditions. It is located in the cytoplasmic membrane, with its active site facing the periplasmic compartment (58). The enzyme is a heterotrimeric (αβγ) complex with an apparent molecular mass of approximately 600 kDa. The individual subunit masses are 100 kDa (α), 55 kDa (β), and 36 kDa (γ). It contains molybdenum, heme, and nonheme iron in its prosthetic groups and displays activity on chlorate and bromate but none on nitrate (39, 62). It is noteworthy that the reductase of E. cloacae SLD1a-1 is similar to periplasmic Ser from T. selenatis. Both have active sites located in the periplasm, both are molybdoenzymes with catalytic α subunits of similar sizes (SerA is ∼96 kDa), and both possess b-type cytochromes. Yee et al. (63) investigated the mechanisms of SeO42− reduction using the Se-reducing bacterium E. cloacae SLD1a-1 in order to identify the gene(s) required for SeO42− reduction. They demonstrated that the selenate reductase of the bacterium is controlled at the genetic level by the global anaerobic fumarate nitrate reduction (FNR) regulator and is induced under suboxic conditions.
Reduction of selenite.
Microorganisms can carry out the conversion of SeO32− to Se0 via a number of different mechanisms (64–66). SeO32− reduction can be catalyzed by reductases, including the periplasmic nitrite reductase, sulfite reductase, and dimethyl sulfoxide (DMSO) reductase (67–69). A number of thiol-mediated reactions have also been observed to reduce selenite to elemental selenium (14).
In T. selenatis, which is able to grow anaerobically with SeO42− as the electron acceptor, little of the SeO32− produced is reduced to Se0 when SeO42− is supplied as the sole electron acceptor. In contrast, SeO32− formed during SeO42− respiration is completely reduced to Se0 by the same bacterium when NO3− and SeO42− are available as electron acceptors. Mutants of T. selenatis that lack periplasmic NO3− reductase activity are unable to reduce either SeO32− or NO3−, while mutants with increased nitrate reductase activity show rapid reduction of NO3− and SeO32−. Together, these observations suggest that nitrate reductase is required for the reduction of SeO32− to Se0 by T. selenatis (67). Pseudomonas seleniipraecipitans strain CA-5 is capable of reducing SeO32− and SeO42− to Se0. The strain is resistant to selenite at high concentrations (>150 mM). Two activities capable of reducing selenate were detected by zymography, one of which may correspond to nitrate reductase (70). Analyses of fractions from this strain indicate the presence of two reductases that can reduce SeO32− to Se0 in the presence of NADPH and that (based upon proteomics analysis of mixed protein samples) may correspond to glutathione reductase and thioredoxin reductase, both of which are able to reduce SeO32− to Se0 when derived from other sources (71). Similar zymography and proteomic analysis of fractions from Rhizobium selenitireducens suggest that a protein belonging to the old yellow enzyme (OYE) family of flavoproteins is capable of reducing SeO32− to Se0 using NADH as the electron donor (72). In a study by Li et al. (23), Shewanella oneidensis MR-1, an organism that shows substantial metabolic versatility and is known for its ability to perform biological electron transfer to solid minerals, is also able to reduce SeO32− to Se0. Specific mutants of S. oneidensis MR-1 have been used to investigate the contribution of the anaerobic respiration system to the microbial reduction of SeO32−. Deletions of the genes that encode nitrate reductase (napA), nitrite reductase (nrfA), and two other periplasmic mediators of electron transfer for anaerobic respiration (mtrA and dmsE) were not impaired in their ability to reduce SeO32−, which indicated that neither nitrate reductase nor nitrite reductase was essential for selenite reduction. In contrast, in the fumarate reductase (fccA) mutant of S. oneidensis MR-1, selenite reduction was decreased by 60% compared to that of the wild-type strain. This suggests that FccA contributes substantially to selenite reduction in the organism. Deletion of cymA, which encodes a membrane-anchored c-type cytochrome that transfers electrons from the quinol pool in the cell membrane to various reductases (including fumarate reductase) that are involved in anaerobic respiration, resulted in a strain that exhibited only 9.6% of the selenite-reducing rate of the wild-type strain. While this indicates that a respiratory electron transport chain is involved in supplying electrons for the reduction of selenite, it is unclear whether this can support growth in S. oneidensis MR-1. In these experiments, the culture actually lost biomass when it reduced selenite anaerobically, using lactate as an electron donor. Thus, the culture may have employed fumarate reductase to reduce the selenite and used it as a means of detoxifying selenite in the periplasm to prevent it from entering the cytoplasm, where it would be toxic (14, 23).
Reduction of selenite to elemental selenium has also been observed in living systems via a reaction that appears to be partly abiotic. Here, the selenite reacted chemically with biological thiol compounds, such as glutathione, via Painter-type reactions to produce molecules containing an S-Se-S bridge moiety known as a selenotrisulfide. It may break down spontaneously with the generation of reactive oxygen species, but it may also be reduced enzymatically by thioredoxin reductase or glutathione reductase, whose natural principal function is to regenerate the thiols in glutathione and thioredoxin through the oxidation of S-S bridges. When the substrate is a selenotrisulfide, the selenium is liberated as Se0 (73). This may, of course, be the reaction via which glutathione and thioredoxin reductases are involved in the reduction of selenite to elemental selenium in Pseudomonas seleniipraecipitans (71) detailed above. Other reports of the reduction of SeO32− to Se0 by bacterial cultures include a detoxification mechanism in Salmonella (38).
The reduction of selenite to elemental selenium is clearly of pivotal importance to the bioremediation of selenium species, so further work is needed to provide information about the role and mechanisms of selenite reductases.
Reduction of selenium species to selenide.
Dissimilatory reduction of selenium species to selenide (Se2−) has been observed to at least a limited extent in environmental microorganisms. The obligate acidophile, Thiobacillus ferrooxidans can convert Se0 to hydrogen selenide (H2Se) under anaerobic conditions (74). The selenite-respiring bacterium Bacillus selenitireducens produces significant amounts of selenide when supplemented with Se0. The strain is also able to reduce SeO32− through Se0 to Se2− (21, 22).
OXIDATION OF SELENIUM COMPOUNDS
The oxidation of reduced selenium species may be relevant with respect to the availability of selenium as a trace nutrient for crop plants. It is not, however, considered to be of major relevance to the environmental toxicity of selenium species because of the low rates of transformation involved. Various studies indicate that microorganisms are capable of aerobic oxidation of Se0 and SeO32− in soil (75–77). A photosynthetic purple sulfur bacterium has been reported to use the oxidation of Se0 to selenic acid (H2SeO4) as a sole source of energy (50), and Acidithiobacillus ferrooxidans has been shown to use copper selenide oxidation as a source of energy (76). Oxidation of Se0 by an aerobic heterotrophic bacterium, a strain of Bacillus megaterium, that was isolated from soil via an enrichment procedure using elemental selenium has also been found to be capable of oxidizing Se0 to SeO32− and a trace of SeO42− (<1% of SeO32−) (77). The genes and enzymes and the pathways involved in the biological oxidation of selenium species have not yet been reported.
Studies with bulk soil have indicated that the oxidation of Se0 in soils is largely biotic in nature, occurs at relatively low rates, and produces SeO32− and SeO42− (78). In a study of the oxidation of Se0 in oxic soil slurries, SeO32− was the predominant product, with small amounts of SeO42− produced also. The oxidation rate constants were found to be between 0.0009 and 0.0117 day−1 in unamended soil slurries. Oxidation of Se0 may have been carried out by heterotrophic bacteria, sulfur-oxidizing bacteria, and possibly fungi (79). These rates indicate that the removal of Se0 from soil via biological oxidation would take hundreds of days. In contrast, field studies have shown that the SeO42− pool of contaminated anoxic sediments can have turnover times of less than 1 h due to the reductive processes that are much more rapid (80). Oxidation, as well as reduction, of the selenium species also occurs during the methylation of the selenium species, which is considered in the next section.
METHYLATION OF SELENIUM SPECIES
Environmental microorganisms can use the Se methylation process as a mechanism to remove SeO32− and SeO42− by converting them to volatile compounds, such as dimethyl selenide (DMSe, CH3SeCH3) and dimethyl diselenide (DMDSe, CH3SeSeCH3). They may also be important in the natural cycling of Se to the atmosphere and may play a role as a detoxification mechanism, too (81).
A number of studies have shown microbial production of DMSe and DMDSe in various environmental samples, including soil, sewage sludge, and water, from selenium sources, including SeO42−, SeO32−, selenocysteine, and selenomethionine (82). A substantial number of cultured microorganisms, both bacteria and fungi, are now recognized as being able to produce methylated forms of selenium. Methylated forms of selenium produced by microorganisms also include dimethyl selenone [(CH3)2SeO2, also known as methyl methylselenite] (83), dimethyl triselenide (DMTSe, CH3SeSeSeCH3), and mixed selenium/sulfur-methylated species, dimethyl selenyl sulfide (DMSeS, CH3SeSCH3,), dimethyl selenyl disulfide (DMSeDS, CH3SeSSCH3,), and dimethyl diselenenyl sulfide (DMDSeS, CH3SeSeSCH3) (84). Known cultured microorganisms that are capable of producing methylated selenium species are summarized in Table 2. The predominant groups of Se-methylating organisms that can be found in soils and sediments are bacteria and fungi, while bacteria are the active Se-methylating organisms in the aquatic environments (5, 50).
TABLE 2.
Se methylating bacteria and fungi, with indications of selenium-containing substrate and methylated products
| Organism(s) | Substrate(s) | Product(s) | Reference |
|---|---|---|---|
| Bacteria | |||
| Corynebacterium spp. | SeO42−, SeO32−, Se0 | DMSe | 11 |
| Aeromonas spp. | SeO42− | DMSe, DMDSe | 121 |
| Rhodocyclus tenuis | SeO42−, SeO32− | DMSe, DMDSe | 122 |
| Aeromonas veronii | SeO42−, SeO32−, Se0, SeS2, H2SeO3, NaSeH | DMSe, DMDSe, methylselenol, DMSeS | 123 |
| Bacillus spp. | SeO32−, SeO42−, selenocyanate | DMSe, DMSeS, DMDSe, DMSeDS, DMDSeS, DMTSe | 84 |
| Rhodospirillum rubrum S1 | SeO32−, Se0 | DMSe, DMDSe | 124 |
| Desulfovibrio gigas | SeO32− | DMSe, DMDSe | 125 |
| Methanobacterium formicicum | SeO32− | DMSe, DMDSe | 125 |
| Pseudomonas fluorescens K27 | SeO42− | DMSe, DMDSe, DMSeS | 126 |
| Citrobacter freundii KS8 | SeO42− | DMSe, DMDSe, DMSeS | 126 |
| Pseudomonas sp. strain Hsa.28 | SeO42−, SeO32− | DMSe, DMDSe | 126 |
| Stenotrophomonas maltophilia | SeO42−, SeO32− | DMSe, DMDSe, DMSeS | 127 |
| Pseudomonas stutzeri NT-I | SeO42−, SeO32−, Bio-Se0 | DMSe, DMDSe | 93 |
| Fungi | |||
| Scopulariopsis brevicaulis | SeO42−, SeO32 | DMSe | 128 |
| Penicillium notatum/Penicillium chrysogenum | SeO42−, SeO32− | DMSe | 129 |
| Penicillium spp. | SeO42− | DMSe | 130 |
| Cephalosporium spp. | SeO42−, SeO32− | DMSe | 131 |
| Fusarium spp. | SeO42−, SeO32− | DMSe | 131 |
| Candida humicola | SeO42−, SeO32− | DMSe | 132 |
| Alternaria alternata | SeO42−, SeO32− | DMSe | 133 |
| Penicillium citrinum | SeO32− | DMSe, DMDSe | 134 |
| Acremonium falciforme | SeO32− | DMSe, DMDSe | 134 |
Selenium methylation pathways.
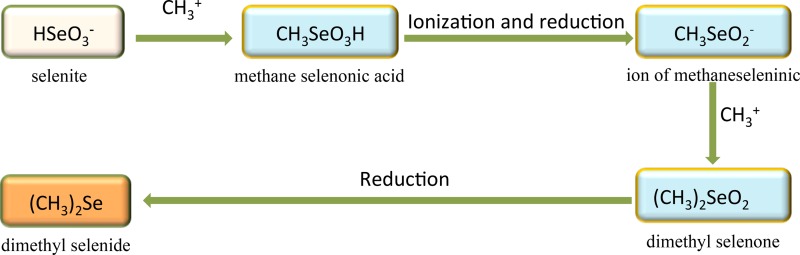
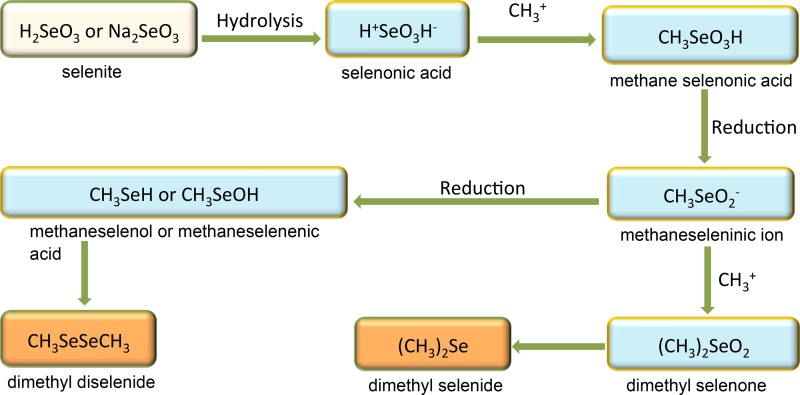
If the initial form of selenium is one of the selenium oxyanions or elemental selenium, Se methylation must involve both reduction and methylation reactions. To date, a number of pathways have been suggested for the biomethylation of selenium, with evidence from proposed intermediates. Methyltransferases capable of methylating selenium species have been identified. The original pathway proposed by Challenger (85) suggested that methylation of SeO32− by fungi involved the methylation and reduction of the Se atom in four steps to form DMSe as the final product (Fig. 2). Reamer and Zoller (83) subsequently reported that inorganic selenium compounds (SeO32− or Se0) are converted into DMDSe, DMSe, and dimethyl selenone (or possibly DMSeS [86]) by microorganisms in soil and sewage sludge. Challenger's proposed scheme was modified to introduce a branch that yielded DMDSe (Fig. 3). In this pathway, the methaneseleninic ion intermediate can form either methaneselenol or methaneseleninic acid, which would then be reduced to DMDSe. It was found that at low concentrations of SeO32− (1 to 10 mg/liter Se), DMSe was the predominant product, while DMDSe or dimethyl selenone was produced at high concentrations of SeO32− (10 to 1,000 mg/liter). In contrast, when Se0 was added to sewage sludge, DMSe was the only product. There was a direct dependence of the production of DMDSe on the concentration of added SeO32−, as at high concentrations of Se, DMSe production was inhibited. During the 30-day period of the experiment, the maximum proportion of selenium across the tested concentration range that was volatilized was 7.9% (83).
FIG 2.
Challenger's pathway (85) for the microbial transformations of selenium.
FIG 3.
Reamer and Zoller's pathway (83) for the microbial transformations of selenium.
Zhang and Chasteen (87) observed that the amounts of DMSe and DMDSe released from cultures of the Se-resistant bacterium Pseudomonas fluorescens K27 amended with dimethyl selenone were more than those formed from SeO42−. This finding suggested that dimethyl selenone may be an intermediate in the reduction and methylation of selenium oxyanions (87), which is consistent with the proposed pathway for the production of DMSe (Fig. 2).
In the scheme proposed by Doran (50), the methylation of inorganic Se by soil Corynebacterium involved the reduction of SeO32− to Se0 and then a reduction to the selenide. The selenide was then methylated to form DMSe (Fig. 4). Although hydrogen selenide and methane selenol were not identified as intermediates, the roles of selenide and methane selenol as intermediates have been suggested in other investigations (88–90).
FIG 4.
Doran's pathway (50) for the microbial transformations of selenium.
The bacterial thiopurine methyltransferase (bTPMT) from Pseudomonas syringae, which catalyzes methyl transfer reactions using S-adenosylmethionine (SAM) as the methyl donor, confers upon Escherichia coli the ability to transform selenite into DMSe and selenomethionine or (methyl)selenocysteine into DMSe and DMDSe (91). Production of methylated selenium species was also observed with an E. coli isolate that was transformed with a methyltransferase gene (amtA) from a freshwater isolate of Hydrogenophaga sp. that produced DMSe and DMDSe (92).
While rates for biological production of methylated selenium species are generally low, applications of selenium-methylating microorganisms in bioremediation and biotechnology have been suggested, such as for the recovery of selenium from seleniferous water via biovolatilization. A fermenter culture of Pseudomonas stutzeri NT-I under aerobic conditions was able to produce methylated selenium species at rate of 14 mol liter−1 h−1. The selenium could be recovered from the gas phase via a simple gas trap containing nitric acid (93).
DEMETHYLATION OF SELENIUM COMPOUNDS
Doran and Alexander (11) isolated from seleniferous clay a pseudomonad able to grow on DMSe as well as strains of Xanthomonas and Corynebacterium that were able to grow on DMDSe as the sole carbon and energy sources. The pathways for breakdown of methylated selenium compounds, which presumably involve demethylation in such organisms, are currently unknown. In anoxic sediments, DMSe undergoes rapid demethylation. It has been suggested that DMSe could be anaerobically transformed to methane (CH4), carbon dioxide (CO2), and hydrogen selenide (H2Se) by sediment organisms (methanogens and sulfate-reducing bacteria) in a pathway similar to dimethyl sulfide (DMS) degradation in freshwater and estuarine sediments (94).
SELENIUM BIOREMEDIATION
As its industrial and agricultural usage increases, increasing amounts of selenium (particularly in the forms of SeO32− and SeO42−) will be discharged into the environment, posing a threat to aquatic and terrestrial environments. Indeed, of the 2,700 tons of selenium that is produced annually, only about 15% is recycled (95). Therefore, there is a need to develop efficient, eco-friendly, and cost-effective methods for the remediation of Se pollution and also, where possible, for the recovery of this valuable element. As more stringent regulations come into force in order to limit the discharge of Se-containing waste, the use of bioremediation technologies are preferable because they will offer more cost-effective approaches for the removal of the pollutant. There has been a growing interest in the use of microorganisms in remediating Se-contaminated environments (96–99). In this context, a number of studies have been carried out in order to exploit the use of Se-oxyanion-reducing microorganisms in small/large-scale remediation schemes. These studies have demonstrated that many microorganisms may be used in remediation approaches designed for the treatment of Se-contaminated soil, sediments, and wastewater. Selenium is to a large extent immobilized and can be recovered in solid form after the biological reduction of selenium oxyanions to Se0. Alternatively, if limitations due to low reaction rates can be overcome, the biological conversion of Se0 to volatile methylated forms potentially permits remediation and subsequent removal and collection in a controlled manner.
A range of carbon and energy sources have been tested as electron donors for the microbial reduction of selenium species. These included inexpensive algal biomass, which has been explored as an electron donor and carbon source for bacterial reduction of SeO42− to Se0 as well as reduction of NO3− to N2 in agricultural drainage (100). In another study, the SeO42−-respiring bacterium Thauera selenatis was used to treat Se-oxyanion-containing oil refinery wastewater in a laboratory-scale bioreactor. A reduction of 95% of the soluble element was achieved from an initial concentration of 3.7 mg liter−1 (101). The SeO42−-reducing bacterium, Bacillus sp. strain SF-1, has been tested in an anoxic continuous flow bioreactor under steady-state conditions for removing SeO42− from a model wastewater containing 41.8 mg/liter SeO42−, with lactate as the electron donor. The system effectively removed SeO42− at short cell retention times (2.9 h), but there was accumulation of SeO32− under these conditions. As the retention time was increased, more of the selenium was reduced to Se0. Conversion of Se0 was ≥99% at a cell retention time of 92.5 h and an Se0 production rate of 0.45 mg liter−1 h−1 (102).
T. selenatis has been employed on a pilot scale for the remediation of Se-containing drainage water from the San Joaquin Valley, CA. The inflow to the reactor had a Se oxyanion (SeO32− plus SeO42−) concentration of 0.237 mg liter−1. The reactor effected 97.9% conversion to recoverable insoluble Se0 and left the treated water with only 5 μg liter−1 of selenium. This high removal of Se0 was achieved via polymer coagulation with Nalmet 8072, which helped to overcome the general technical challenge of recovering Se0 due to small particle size (103). The Se-reducing bacterium Pseudomonas stutzeri NT-I has also been effectively employed for the bioremediation of Se-containing refinery wastewater in 256-liter pilot-scale bioreactors via reduction to elemental selenium (96). In a high-throughput sequencing study to investigate the effect of an electron acceptor on community structure during respiration of an activated-sludge-derived microbial population using hydrogen as the electron donor, principal-component analysis revealed a substantial shift in the composition of the microbial population upon the first addition of nitrate as an alternative to selenate as the electron acceptor (104). This gives additional evidence for the presence of environmental communities of microorganisms that utilize selenite as an electron acceptor and that these are, to a significant extent, distinct from nitrate-reducing microorganisms.
Since some algae can volatilize substantial quantities of inorganic Se compounds (105–107), algal methylation of selenium compounds offers a possible way to remove selenium from the aqueous phase. The inclusion of an algal pretreatment unit into a constructed wetland system was investigated in order to remove Se from river water entering the Salton Sea in California. The alga Chlorella vulgaris removed 96% of Se supplied as selenium oxyanions (1.58 mg liter−1) from the microcosm water column within 72 h. With this arrangement, up to 61% of the selenium was removed by volatilization to the atmosphere, suggesting that an algal pretreatment stage can be included for selenium bioremediation into constructed wetland systems (108).
In addition to the problems that it causes as an environmental pollutant, selenium is an essential micronutrient and a valuable metalloid for which there are a dearth of high-yielding geological sources. Hence, the most advantageous systems for remediation of selenium pollution would put the recovered selenium to good nutritional or technological use. Elemental selenium is used in semiconductors. In this connection, it must be noted that a great diversity of prokaryotes are able to reduce selenium oxyanions to elemental selenium in the form of nanoparticles, which have properties that are difficult to mimic by chemical technologies. The microbially produced nanoparticles may have application in semiconductor and other technologies (14, 26). In effective selenium bioremediation, the selenium may have several acceptable fates. The likely fates of selenate in the presence of a variety of organisms have been demonstrated in an engineered aquatic ecosystem designed for brine shrimp production. In this investigation, selenate was taken up and metabolized differently by microalgae, bacteria, and diatoms to selenite, selenide, or elemental Se. Some of the biotransformed selenium species were incorporated and bioaccumulated as organic selenium compounds, as they were transferred between the different trophic levels. Organic selenium-enriched invertebrates suitable for human and animal consumption were produced as a result of these metabolic biotransformations (109).
Microbial methylation of inorganic Se oxyanions to volatile species offers a possible approach to bioremediation of selenium compounds in Se-polluted soils and aquatic environments. This has the attraction that the selenium may be completely removed in the vapor phase, although the limitation of low reaction rates would have to be overcome. In principle, organisms that demethylate selenium species may be used to recover vapor-phase selenium, provided that the reaction rate limitations and the possible production of toxic and volatile H2Se can be overcome. Genetic characterizations of the pathways of selenium methylation and demethylation may enable their modification by overexpressing the necessary enzymes, resulting in acceleration of these processes.
CONCLUSIONS
Selenium species may be transformed in a diversity of metabolic reactions. Interest in the microorganisms capable of transforming selenium compounds involved in environmental pollution and in making selenium nutritionally available will increase as the activities of these organisms become better understood. Further characterizations of the mechanisms of selenite reduction to elemental selenium and of selenium methylation and demethylation are needed. Culture-independent analysis will be useful in studying the diversity and distribution of selenium-transforming organisms in a range of environments using a combination of functional gene analysis and metagenomics. Sequencing with 16S rRNA gene analysis should be fruitful in unraveling the role of microorganisms in the global selenium cycle. Their ability to produce selenium nanoparticles will be industrially exploited. Their ability to transform different selenium species by reduction, methylation, and demethylation will be harnessed further in the remediation of selenium-containing wastewater.
ACKNOWLEDGMENTS
We thank Nicola Woodroofe, Head of the Biomolecular Sciences Research Centre, for encouragement and support.
A.S.E. gratefully acknowledges financial support from the Libyan Government for a Ph.D. scholarship.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Pinsent J. 1954. The need for selenite and molybdate in the formation of formic dehydrogenase by members of the coli-aerogenes group of bacteria. Biochem J 57:10–16. doi: 10.1042/bj0570010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stadtman TC. 1991. Biosynthesis and function of selenocysteine-containing enzymes. J Biol Chem 266:16257–16260. [PubMed] [Google Scholar]
- 3.Heider J, Bock A. 1993. Selenium metabolism in micro-organisms. Adv Microb Physiol 35:71–109. doi: 10.1016/S0065-2911(08)60097-1. [DOI] [PubMed] [Google Scholar]
- 4.Johansson L, Gafvelin G, Arnér ES. 2005. Selenocysteine in proteins—properties and biotechnological use. Biochim Biophys Acta 1726:1–13. doi: 10.1016/j.bbagen.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Dungan RS, Frankenberger WT. 1999. Microbial transformations of selenium and the bioremediation of seleniferous environments. Biorem J 3:171–188. doi: 10.1080/10889869991219299. [DOI] [Google Scholar]
- 6.Franke KW, Moxon AL. 1936. A comparison of the minimum fatal doses of selenium, tellurium, arsenic and vanadium. J Pharmacol Exp Ther 58:454–459. [Google Scholar]
- 7.Wilber CG. 1980. Toxicology of selenium: a review. Clin Toxicol 17:171–230. doi: 10.3109/15563658008985076. [DOI] [PubMed] [Google Scholar]
- 8.Ranjard L, Nazaret S, Cournoyer B. 2003. Freshwater bacteria can methylate selenium through the thiopurine methyltransferase pathway. Appl Environ Microbiol 69:3784–3790. doi: 10.1128/AEM.69.7.3784-3790.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zehr JP, Oremland RS. 1987. Reduction of selenate to selenide by sulfate-respiring bacteria: experiments with cell suspensions and estuarine sediments. Appl Environ Microbiol 53:1365–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oremland RS, Hollibaugh JT, Maest AS, Presser TS, Miller LG, Culbertson CW. 1989. Selenate reduction to elemental selenium by anaerobic bacteria in sediments and culture: biogeochemical significance of a novel, sulfate-independent respiration. Appl Environ Microbiol 55:2333–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doran JW, Alexander M. 1977. Microbial transformations of selenium. Appl Environ Microbiol 33:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karle JA, Shrift A. 1986. Use of selenite, selenide, and selenocysteine for the synthesis of formate dehydrogenase by a cysteine-requiring mutant of Escherichia coli K-12. Biol Trace Elem Res 11:27–35. doi: 10.1007/BF02795520. [DOI] [PubMed] [Google Scholar]
- 13.Forchhammer K, Bock A. 1991. Selenocysteine synthase from Escherichia coli. Analysis of the reaction sequence. J Biol Chem 266:6324–6328. [PubMed] [Google Scholar]
- 14.Nancharaiah YV, Lens PN. 2015. Ecology and biotechnology of selenium-respiring bacteria. Microbiol Mol Biol Rev 79:61–80. doi: 10.1128/MMBR.00037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Astratinei V, Lens PNL, Van Hullebusch ED. 2006. Bioconversion of selenate in methanogenic anaerobic granular sludge. J Environ Qual 35:1873–1883. doi: 10.2134/jeq2005.0443. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Okeke BC, Frankenberger WT Jr. 2008. Bacterial reduction of selenate to elemental selenium utilizing molasses as a carbon source. Bioresour Technol 99:1267–1273. doi: 10.1016/j.biortech.2007.02.048. [DOI] [PubMed] [Google Scholar]
- 17.Kashiwa M, Nishimoto S, Takahashi K, Ike M, Fujita M. 2000. Factors affecting soluble selenium removal by a selenate-reducing bacterium Bacillus sp. SF-1. J Biosci Bioeng 89:528–533. doi: 10.1016/S1389-1723(00)80051-1. [DOI] [PubMed] [Google Scholar]
- 18.Chung J, Nerenberg R, Rittmann BE. 2006. Bioreduction of selenate using a hydrogen-based membrane biofilm reactor. Environ Sci Technol 40:1664–1671. doi: 10.1021/es051251g. [DOI] [PubMed] [Google Scholar]
- 19.Lovley DR, Fraga JL, Coates JD, Blunt-Harris EL. 1999. Humics as an electron donor for anaerobic respiration. Environ Microbiol 1:89–98. doi: 10.1046/j.1462-2920.1999.00009.x. [DOI] [PubMed] [Google Scholar]
- 20.Schröder I, Rech S, Krafft T, Macy JM. 1997. Purification and characterization of the selenate reductase from Thauera selenatis. J Biol Chem 272:23765–23768. doi: 10.1074/jbc.272.38.23765. [DOI] [PubMed] [Google Scholar]
- 21.Herbel MJ, Blum JS, Oremland RS, Borglin SE. 2003. Reduction of elemental selenium to selenide: experiments with anoxic sediments and bacteria that respire Se-oxyanions. Geomicrobiol J 20:587–602. doi: 10.1080/713851163. [DOI] [Google Scholar]
- 22.Pearce CI, Pattrick RA, Law N, Charnock JM, Coker VS, Fellowes JW, Oremland RS, Lloyd JR. 2009. Investigating different mechanisms for biogenic selenite transformations: Geobacter sulfurreducens, Shewanella oneidensis and Veillonella atypica. Environ Technol 30:1313–1326. doi: 10.1080/09593330902984751. [DOI] [PubMed] [Google Scholar]
- 23.Li D, Cheng Y, Wu C, Li W, Li N, Yang Z, Tong Z, Yu H. 2014. Selenite reduction by Shewanella oneidensis MR-1 is mediated by fumarate reductase in periplasm. Sci Reports 4:3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sonkusre P, Nanduri R, Gupta P, Cameotra SS. 2014. Improved extraction of intracellular biogenic selenium nanoparticles and their specificity for cancer chemoprevention. J Nanomed Nanotechnol 5:194. [Google Scholar]
- 25.Debieux CM, Dridge EJ, Mueller CM, Splatt P, Paszkiewicz K, Knight I, Florance H, Love J, Titball RW, Lewis RJ, Richardson DJ, Butler CS. 2011. A bacterial process for selenium nanosphere assembly. Proc Natl Acad Sci U S A 108:13480–13485. doi: 10.1073/pnas.1105959108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oremland RS, Herbel MJ, Blum JS, Langley S, Beveridge TJ, Ajayan PM, Sutto T, Ellis AV, Curran S. 2004. Structural and spectral features of selenium nanospheres produced by Se-respiring bacteria. Appl Environ Microbiol 70:52–60. doi: 10.1128/AEM.70.1.52-60.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Li D, Gao P. 2012. Expulsion of selenium/protein nanoparticles through vesicle-like structures by Saccharomyces cerevisiae under microaerophilic environment. World J Microbiol Biotechnol 28:3381–3386. doi: 10.1007/s11274-012-1150-y. [DOI] [PubMed] [Google Scholar]
- 28.Jiang S, Ho CT, Lee J, Van Duong H, Han S, Hur H. 2012. Mercury capture into biogenic amorphous selenium nanospheres produced by mercury resistant Shewanella putrefaciens 200. Chemosphere 87:621–624. doi: 10.1016/j.chemosphere.2011.12.083. [DOI] [PubMed] [Google Scholar]
- 29.Wang T, Yang L, Zhang B, Liu J. 2010. Extracellular biosynthesis and transformation of selenium nanoparticles and application in H2O2 biosensor. Colloids Surf B Biointerfaces 80:94–102. doi: 10.1016/j.colsurfb.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 30.Bajaj M, Schmidt S, Winter J. 2012. Formation of Se (0) nanoparticles by Duganella sp. and Agrobacterium sp. isolated from Se-laden soil of North-East Punjab, India. Microb Cell Fact 11:64. doi: 10.1186/1475-2859-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhanjal S, Cameotra SS. 2010. Aerobic biogenesis of selenium nanospheres by Bacillus cereus isolated from coalmine soil. Microb Cell Fact 9:52. doi: 10.1186/1475-2859-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prakash NT, Sharma N, Prakash R, Raina KK, Fellowes J, Pearce CI, Lloyd JR, Pattrick RA. 2009. Aerobic microbial manufacture of nanoscale selenium: exploiting nature's bio-nanomineralization potential. Biotechnol Lett 31:1857–1862. doi: 10.1007/s10529-009-0096-0. [DOI] [PubMed] [Google Scholar]
- 33.Dobias J, Suvorova EI, Bernier-Latmani R. 2011. Role of proteins in controlling selenium nanoparticle size. Nanotechnology 22:195605. doi: 10.1088/0957-4484/22/19/195605. [DOI] [PubMed] [Google Scholar]
- 34.Jain R, Jordan N, Weiss S, Foerstendorf H, Heim K, Kacker R, Hübner R, Kramer H, van Hullebusch ED, Farges F. 2015. Extracellular polymeric substances govern the surface charge of biogenic elemental selenium nanoparticles. Environ Sci Technol 49:1713–1720. doi: 10.1021/es5043063. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Gao X, Zhang L, Bao Y. 2001. Biological effects of a nano red elemental selenium. Biofactors 15:27–38. doi: 10.1002/biof.5520150103. [DOI] [PubMed] [Google Scholar]
- 36.Jain R, Gonzalez-Gil G, Singh V, Van Hullebusch ED, Farges F, Lens PN. 2014. Biogenic selenium nanoparticles: production, characterization and challenges, p 361–390. In Kumar A. (ed), Nanobiotechnology. Studium Press LLC, New Delhi, India. [Google Scholar]
- 37.Oremland RS, Blum JS, Culbertson CW, Visscher PT, Miller LG, Dowdle P, Strohmaier FE. 1994. Isolation, growth, and metabolism of an obligately anaerobic, selenate-respiring bacterium, strain SES-3. Appl Environ Microbiol 60:3011–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCready R, Campbell J, Payne J. 1966. Selenite reduction by Salmonella heidelberg. Can J Microbiol 12:703–714. doi: 10.1139/m66-097. [DOI] [PubMed] [Google Scholar]
- 39.Han X, Gu J. 2010. Sorption and transformation of toxic metals by microorganisms, p 153–176. In Mitchell R, Gu J-D (ed), Environmental Microbiology, 2nd ed John Wiley & Sons, Hoboken, NJ. [Google Scholar]
- 40.Macy J. 1994. Biochemistry of selenium metabolism by Thauera selenatis gen. nov. sp. nov. and use of the organism for bioremediation of selenium oxyanions in San Joaquin Valley drainage water, p 421–444. In Frankenberger WT Jr, Benso S (ed), Selenium in the environment. Marcel Dekker, New York, NY. [Google Scholar]
- 41.Tomei FA, Barton LL, Lemanski CL, Zocco TG, Fink NH, Sillerud LO. 1995. Transformation of selenate and selenite to elemental selenium by Desulfovibrio desulfuricans. J Ind Microbiol 14:329–336. doi: 10.1007/BF01569947. [DOI] [Google Scholar]
- 42.Yanke LJ, Bryant RD, Laishley EJ. 1995. Hydrogenase I of Clostridium pasteurianum functions as a novel selenite reductase. Anaerobe 1:61–67. doi: 10.1016/S1075-9964(95)80457-9. [DOI] [PubMed] [Google Scholar]
- 43.Fujita M, Ike M, Nishimoto S, Takahashi K, Kashiwa M. 1997. Isolation and characterization of a novel selenate-reducing bacterium, Bacillus sp. SF-1. J Ferment Bioeng 83:517–522. doi: 10.1016/S0922-338X(97)81130-0. [DOI] [Google Scholar]
- 44.Losi ME, Frankenberger WT. 1997. Reduction of selenium oxyanions by Enterobacter cloacae SLD1a-1: isolation and growth of the bacterium and its expulsion of selenium particles. Appl Environ Microbiol 63:3079–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hunter WJ. 2007. An Azospira oryzae (syn Dechlorosoma suillum) strain that reduces selenate and selenite to elemental red selenium. Curr Microbiol 54:376–381. doi: 10.1007/s00284-006-0474-y. [DOI] [PubMed] [Google Scholar]
- 46.Kuroda M, Notaguchi E, Sato A, Yoshioka M, Hasegawa A, Kagami T, Narita T, Yamashita M, Sei K, Soda S, Ike M. 2011. Characterization of Pseudomonas stutzeri NT-I capable of removing soluble selenium from the aqueous phase under aerobic conditions. J Biosci Bioeng 112:259–264. doi: 10.1016/j.jbiosc.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 47.Ike M, Takahashi K, Fujita T, Fujita M, Kashiwa M. 2000. Selenate reduction by bacteria isolated from aquatic environment free from selenium contamination. Water Res 34:3019–3025. doi: 10.1016/S0043-1354(00)00041-5. [DOI] [Google Scholar]
- 48.Siddique T, Zhang Y, Okeke BC, Frankenberger WT Jr. 2006. Characterization of sediment bacteria involved in selenium reduction. Bioresour Technol 97:1041–1049. doi: 10.1016/j.biortech.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 49.Slobodkina GB, Lebedinsky AV, Chernyh NA, Bonch-Osmolovskaya EA, Slobodkin AI. 2015. Pyrobaculum ferrireducens sp. nov., a hyperthermophilic Fe(III)-, selenate- and arsenate-reducing crenarchaeon isolated from a hot spring. Int J Syst Evol Microbiol 65:851–856. doi: 10.1099/ijs.0.000027. [DOI] [PubMed] [Google Scholar]
- 50.Doran JW. 1982. Microorganisms and the biological cycling of selenium, p 1–32. In Marshall KC. (ed), Advances in microbial ecology. Springer, New York, NY. [Google Scholar]
- 51.Turner RJ, Weiner JH, Taylor DE. 1998. Selenium metabolism in Escherichia coli. Biometals 11:223–227. doi: 10.1023/A:1009290213301. [DOI] [PubMed] [Google Scholar]
- 52.Lortie L, Gould WD, Rajan S, McCready RG, Cheng K. 1992. Reduction of selenate and selenite to elemental selenium by a Pseudomonas stutzeri isolate. Appl Environ Microbiol 58:4042–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bebien M, Chauvin J, Adriano J, Grosse S, Verméglio A. 2001. Effect of selenite on growth and protein synthesis in the phototrophic bacterium Rhodobacter sphaeroides. Appl Environ Microbiol 67:4440–4447. doi: 10.1128/AEM.67.10.4440-4447.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roux M, Sarret G, Pignot-Paintrand I, Fontecave M, Coves J. 2001. Mobilization of selenite by Ralstonia metallidurans CH34. Appl Environ Microbiol 67:769–773. doi: 10.1128/AEM.67.2.769-773.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Switzer Blum J, Burns Bindi A, Buzzelli J, Stolz JF, Oremland RS. 1998. Bacillus arsenicoselenatis, sp. nov., and Bacillus selenitireducens, sp. nov.: two haloalkaliphiles from Mono Lake, California that respire oxyanions of selenium and arsenic. Arch Microbiol 171:19–30. doi: 10.1007/s002030050673. [DOI] [PubMed] [Google Scholar]
- 56.Macy JM, Lawson S, DeMoll-Decker H. 1993. Bioremediation of selenium oxyanions in San Joaquin drainage water using Thauera selenatis in a biological reactor system. Appl Microbiol Biotechnol 40:588–594. [Google Scholar]
- 57.Stolz J, Ellis D, Blum J, Ahmann D, Lovley D, Oremland R. 1999. Sulfurospirillum barnesii sp. nov. and Sulfurospirillum arsenophilum sp. nov., new members of the Sulfurospirillum clade of the Epsilonproteobacteria. Int J Syst Bacteriol 49:1177–1180. doi: 10.1099/00207713-49-3-1177. [DOI] [PubMed] [Google Scholar]
- 58.Watts CA, Ridley H, Condie KL, Leaver JT, Richardson DJ, Butler CS. 2003. Selenate reduction by Enterobacter cloacae SLD1a-1 is catalysed by a molybdenum-dependent membrane-bound enzyme that is distinct from the membrane-bound nitrate reductase. FEMS Microbiol Lett 228:273–279. doi: 10.1016/S0378-1097(03)00782-1. [DOI] [PubMed] [Google Scholar]
- 59.Stolz JF, Oremland RS. 1999. Bacterial respiration of arsenic and selenium. FEMS Microbiol Rev 23:615–627. doi: 10.1111/j.1574-6976.1999.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 60.Stolz JF, Gugliuzza T, Blum JS, Oremland R, Murillo FM. 1997. Differential cytochrome content and reductase activity in Geospirillum barnesii strain SeS3. Arch Microbiol 167:1–5. doi: 10.1007/s002030050408. [DOI] [PubMed] [Google Scholar]
- 61.Barton L. 2005. Structural and functional relationships in prokaryotes. Springer, New York, NY. [Google Scholar]
- 62.Ridley H, Watts CA, Richardson DJ, Butler CS. 2006. Resolution of distinct membrane-bound enzymes from Enterobacter cloacae SLD1a-1 that are responsible for selective reduction of nitrate and selenate oxyanions. Appl Environ Microbiol 72:5173–5180. doi: 10.1128/AEM.00568-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yee N, Ma J, Dalia A, Boonfueng T, Kobayashi DY. 2007. Se(VI) Reduction and the precipitation of Se(0) by the facultative bacterium Enterobacter cloacae SLD1a-1 are regulated by FNR. Appl Environ Microbiol 73:1914–1920. doi: 10.1128/AEM.02542-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kessi J, Ramuz M, Wehrli E, Spycher M, Bachofen R. 1999. Reduction of selenite and detoxification of elemental selenium by the phototrophic bacterium Rhodospirillum rubrum. Appl Environ Microbiol 65:4734–4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kessi J. 2006. Enzymic systems proposed to be involved in the dissimilatory reduction of selenite in the purple non-sulfur bacteria Rhodospirillum rubrum and Rhodobacter capsulatus. Microbiology 152:731–743. doi: 10.1099/mic.0.28240-0. [DOI] [PubMed] [Google Scholar]
- 66.Kessi J, Hanselmann KW. 2004. Similarities between the abiotic reduction of selenite with glutathione and the dissimilatory reaction mediated by Rhodospirillum rubrum and Escherichia coli. J Biol Chem 279:50662–50669. doi: 10.1074/jbc.M405887200. [DOI] [PubMed] [Google Scholar]
- 67.DeMoll-Decker H, Macy JM. 1993. The periplasmic nitrite reductase of Thauera selenatis may catalyze the reduction of selenite to elemental selenium. Arch Microbiol 160:241–247. [Google Scholar]
- 68.Harrison G, Curle C, Laishley EJ. 1984. Purification and characterization of an inducible dissimilatory type sulfite reductase from Clostridium pasteurianum. Arch Microbiol 138:72–78. doi: 10.1007/BF00425411. [DOI] [PubMed] [Google Scholar]
- 69.Afkar E, Lisak J, Saltikov C, Basu P, Oremland RS, Stolz JF. 2003. The respiratory arsenate reductase from Bacillus selenitireducens strain MLS10. FEMS Microbiol Lett 226:107–112. doi: 10.1016/S0378-1097(03)00609-8. [DOI] [PubMed] [Google Scholar]
- 70.Hunter WJ, Manter DK. 2009. Reduction of selenite to elemental red selenium by Pseudomonas sp. strain CA5. Curr Microbiol 58:493–498. doi: 10.1007/s00284-009-9358-2. [DOI] [PubMed] [Google Scholar]
- 71.Hunter WJ. 2014. Pseudomonas seleniipraecipitans proteins potentially involved in selenite reduction. Curr Microbiol 69:69–74. doi: 10.1007/s00284-014-0555-2. [DOI] [PubMed] [Google Scholar]
- 72.Hunter WJ. 2014. A Rhizobium selenitireducens protein showing selenite reductase activity. Curr Microbiol 68:311–316. doi: 10.1007/s00284-013-0474-7. [DOI] [PubMed] [Google Scholar]
- 73.Zannoni D, Borsetti F, Harrison JJ, Turner RJ. 2008. The bacterial response to the chalcogen metalloids Se and Te. Adv Microb Physiol 53:1–72. doi: 10.1016/S0065-2911(07)53001-8. [DOI] [PubMed] [Google Scholar]
- 74.Bacon M, Ingledew WJ. 1989. The reductive reactions of Thiobacillus ferrooxidans on sulphur and selenium. FEMS Microbiol Lett 58:189–194. doi: 10.1111/j.1574-6968.1989.tb03042.x. [DOI] [Google Scholar]
- 75.Lipman JG, Waksman SA. 1923. The oxidation of elemental Se by a new group of autotrophic microorganisms. Science 57:60. doi: 10.1126/science.57.1463.60. [DOI] [PubMed] [Google Scholar]
- 76.Torma AE, Habashi F. 1972. Oxidation of copper (II) selenide by Thiobacillus ferrooxidans. Can J Microbiol 18:1780–1781. doi: 10.1139/m72-278. [DOI] [PubMed] [Google Scholar]
- 77.Sarathchandra SU, Watkinson JH. 1981. Oxidation of elemental selenium to selenite by Bacillus megaterium. Science 211:600–601. doi: 10.1126/science.6779378. [DOI] [PubMed] [Google Scholar]
- 78.Losi ME, Frankenberger WT Jr. 1998. Microbial oxidation and solubilization of precipitated elemental selenium in soil. J Environ Qual 27:836–843. doi: 10.2134/jeq1998.00472425002700040018x. [DOI] [Google Scholar]
- 79.Dowdle PR, Oremland RS. 1998. Microbial oxidation of elemental selenium in soil slurries and bacterial cultures. Environ Sci Technol 32:3749–3755. doi: 10.1021/es970940s. [DOI] [Google Scholar]
- 80.Oremland RS, Steinberg NA, Presser TS, Miller LG. 1991. In situ bacterial selenate reduction in the agricultural drainage systems of western Nevada. Appl Environ Microbiol 57:615–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McConnell KP, Portman OW. 1952. Toxicity of dimethyl selenide in the rat and mouse. Proc Soc Exp Biol Med 79:230–231. doi: 10.3181/00379727-79-19333. [DOI] [PubMed] [Google Scholar]
- 82.Francis AJ, Duxbury JM, Alexander M. 1974. Evolution of dimethylselenide from soils. Appl Microbiol 28:248–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reamer DC, Zoller WH. 1980. Selenium biomethylation products from soil and sewage sludge. Science 208:500–502. doi: 10.1126/science.208.4443.500. [DOI] [PubMed] [Google Scholar]
- 84.Burra R, Pradenas GA, Montes RA, Vásquez CC, Chasteen TG. 2010. Production of dimethyl triselenide and dimethyl diselenenyl sulfide in the headspace of metalloid-resistant Bacillus species grown in the presence of selenium oxyanions. Anal Biochem 396:217–222. doi: 10.1016/j.ab.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 85.Challenger F. 1945. Biological methylation. Chem Rev 36:315–361. doi: 10.1021/cr60115a003. [DOI] [Google Scholar]
- 86.Chasteen TG. 1993. Confusion between dimethyl selenenyl sulfide and dimethyl selenone released by bacteria. Appl Organometal Chem 7:335–342. doi: 10.1002/aoc.590070507. [DOI] [Google Scholar]
- 87.Zhang L, Chasteen TG. 1994. Amending cultures of selenium-resistant bacteria with dimethyl selenone. Appl Organometal Chem 8:501–508. doi: 10.1002/aoc.590080602. [DOI] [Google Scholar]
- 88.Bird ML, Challenger F. 1942. Studies in biological methylation. Part IX. The action of Scopulariopsis brevicaulis and certain penicillia on salts of aliphatic seleninic and selenonic acids. J Chem Soc 1942:574–577. [Google Scholar]
- 89.Bremer J, Natori Y. 1960. Behavior of some selenium compounds in transmethylation. Biochim Biophys Acta 44:367–370. doi: 10.1016/0006-3002(60)91580-8. [DOI] [Google Scholar]
- 90.Hsieh HS, Ganther HE. 1975. Acid-volatile selenium formation catalyzed by glutathione reductase. Biochemistry 14:1632–1636. doi: 10.1021/bi00679a014. [DOI] [PubMed] [Google Scholar]
- 91.Ranjard L, Prigent-Combaret C, Nazaret S, Cournoyer B. 2002. Methylation of inorganic and organic selenium by the bacterial thiopurine methyltransferase. J Bacteriol 184:3146–3149. doi: 10.1128/JB.184.11.3146-3149.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ranjard L, Prigent-Combaret C, Favre-Bonté S, Monnez C, Nazaret S, Cournoyer B. 2004. Characterization of a novel selenium methyltransferase from freshwater bacteria showing strong similarities with the calicheamicin methyltransferase. Biochim Biophys Acta 1679:80–85. doi: 10.1016/j.bbaexp.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 93.Kagami T, Narita T, Kuroda M, Notaguchi E, Yamashita M, Sei K, Soda S, Ike M. 2013. Effective selenium volatilization under aerobic conditions and recovery from the aqueous phase by Pseudomonas stutzeri NT-I. Water Res 47:1361–1368. doi: 10.1016/j.watres.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 94.Oremland RS, Zehr JP. 1986. Formation of methane and carbon dioxide from dimethylselenide in anoxic sediments and by a methanogenic bacterium. Appl Environ Microbiol 52:1031–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Haug A, Graham RD, Christophersen OA, Lyons GH. 2007. How to use the world's scarce selenium resources efficiently to increase the selenium concentration in food. Microb Ecol Health Dis 19:209–228. doi: 10.1080/08910600701698986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Soda S, Takahashi H, Kagami T, Miyake M, Notaguchi E, Sei K, Iwasaki N, Ike M. 2012. Biotreatment of selenium refinery wastewater using pilot-scale granular sludge and swim-bed bioreactors augmented with a selenium-reducing bacterium Pseudomonas stutzeri NT-I. Japan J Water Treatment Biol 48:63–71. [Google Scholar]
- 97.Williams KH, Wilkins MJ, N′Guessan A, Arey B, Dodova E, Dohnalkova A, Holmes D, Lovley DR, Long PE. 2013. Field evidence of selenium bioreduction in a uranium-contaminated aquifer. Environ Microbiol Rep 5:444–452. doi: 10.1111/1758-2229.12032. [DOI] [PubMed] [Google Scholar]
- 98.Santos S, Ungureanu G, Boaventura R, Botelho C. 2015. Selenium contaminated waters: an overview of analytical methods, treatment options and recent advances in sorption methods. Sci Total Environ 521–522:246–260. [DOI] [PubMed] [Google Scholar]
- 99.Higashi RM, Cassel TA, Skorupa JP, Fan TW. 2005. Remediation and bioremediation of selenium-contaminated waters, p 355–360. In Lehr JH, Keeley J (ed), Water encyclopedia: water quality and resource development. Wiley, Hoboken, NJ. [Google Scholar]
- 100.Gerhardt MB, Green FB, Newman RD, Lundquist TJ, Tresan RB, Oswald WJ. 1991. Removal of selenium using a novel algal-bacterial process. Res J Water Pollut Control Fed 63:799–805. [Google Scholar]
- 101.Lawson S, Macy J. 1995. Bioremediation of selenite in oil refinery wastewater. Appl Microbiol Biotechnol 43:762–765. doi: 10.1007/BF00164785. [DOI] [Google Scholar]
- 102.Fujita M, Ike M, Kashiwa M, Hashimoto R, Soda S. 2002. Laboratory-scale continuous reactor for soluble selenium removal using selenate-reducing bacterium, Bacillus sp. SF-1. Biotechnol Bioeng 80:755–761. doi: 10.1002/bit.10425. [DOI] [PubMed] [Google Scholar]
- 103.Cantafio AW, Hagen KD, Lewis GE, Bledsoe TL, Nunan KM, Macy JM. 1996. Pilot-scale selenium bioremediation of San Joaquin drainage water with Thauera selenatis. Appl Environ Microbiol 62:3298–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lai C, Yang X, Tang Y, Rittmann BE, Zhao H. 2014. Nitrate shaped the selenate-reducing microbial community in a hydrogen-based biofilm reactor. Environ Sci Technol 48:3395–3402. doi: 10.1021/es4053939. [DOI] [PubMed] [Google Scholar]
- 105.Oyamada N, Takahashi G, Ishizaki M. 1991. Methylation of inorganic selenium compounds by freshwater green algae, Ankistrodesmus sp., Chlorella vulgaris and Selenastrum sp. Eisei Kagaku 37:83–88. doi: 10.1248/jhs1956.37.83. [DOI] [Google Scholar]
- 106.Fan TWM, Lane AN, Higashi RM. 1997. Selenium biotransformations by a euryhaline microalga isolated from a saline evaporation pond. Environ Sci Technol 31:569–576. doi: 10.1021/es960471e. [DOI] [Google Scholar]
- 107.Neumann PM, De Souza MP, Pickering IJ, Terry N. 2003. Rapid microalgal metabolism of selenate to volatile dimethylselenide. Plant Cell Environ 26:897–905. doi: 10.1046/j.1365-3040.2003.01022.x. [DOI] [PubMed] [Google Scholar]
- 108.Huang J, Suárez MC, Yang SI, Lin Z, Terry N. 2013. Development of a constructed wetland water treatment system for selenium removal: incorporation of an algal treatment component. Environ Sci Technol 47:10518–10525. [DOI] [PubMed] [Google Scholar]
- 109.Schmidt R, Tantoyotai P, Fakra SC, Marcus MA, Yang SI, Pickering IJ, Bañuelos GS, Hristova KR, Freeman JL. 2013. Selenium biotransformations in an engineered aquatic ecosystem for bioremediation of agricultural wastewater via brine shrimp production. Environ Sci Technol 47:5057–5065. doi: 10.1021/es305001n. [DOI] [PubMed] [Google Scholar]
- 110.Flury M, Frankenberger WT, Jury WA. 1997. Long-term depletion of selenium from Kesterson dewatered sediments. Sci Total Environ 198:259–270. doi: 10.1016/S0048-9697(97)05460-0. [DOI] [Google Scholar]
- 111.Narasingarao P, Häggblom MM. 2007. Identification of anaerobic selenate-respiring bacteria from aquatic sediments. Appl Environ Microbiol 73:3519–3527. doi: 10.1128/AEM.02737-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Blum JS, Stolz JF, Oren A, Oremland RS. 2001. Selenihalanaerobacter shriftii gen. nov., sp. nov., a halophilic anaerobe from Dead Sea sediments that respires selenate. Arch Microbiol 175:208–219. doi: 10.1007/s002030100257. [DOI] [PubMed] [Google Scholar]
- 113.Huber R, Sacher M, Vollmann A, Huber H, Rose D. 2000. Respiration of arsenate and selenate by hyperthermophilic archaea. Syst Appl Microbiol 23:305–314. doi: 10.1016/S0723-2020(00)80058-2. [DOI] [PubMed] [Google Scholar]
- 114.Klonowska A, Heulin T, Vermeglio A. 2005. Selenite and tellurite reduction by Shewanella oneidensis. Appl Environ Microbiol 71:5607–5609. doi: 10.1128/AEM.71.9.5607-5609.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li B, Liu N, Li Y, Jing W, Fan J, Li D, Zhang L, Zhang X, Zhang Z, Wang L. 2014. Reduction of selenite to red elemental selenium by Rhodopseudomonas palustris strain N. PLoS One 9(4):e95955. doi: 10.1371/journal.pone.0095955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tomei FA, Barton LL, Lemanski CL, Zocco TG. 1992. Reduction of selenate and selenite to elemental selenium by Wolinella succinogenes. Can J Microbiol 38:1328–1333. doi: 10.1139/m92-219. [DOI] [Google Scholar]
- 117.Tugarova AV, Vetchinkina EP, Loshchinina EA, Burov AM, Nikitina VE, Kamnev AA. 2014. Reduction of selenite by Azospirillum brasilense with the formation of selenium nanoparticles. Microb Ecol 68:495–503. doi: 10.1007/s00248-014-0429-y. [DOI] [PubMed] [Google Scholar]
- 118.Mishra RR, Prajapati S, Das N, Das J, Dangar TK, Thatoi H. 2011. Reduction of selenite to red elemental selenium by moderately halotolerant Bacillus megaterium strains isolated from Bhitarkanika mangrove soil and characterization of reduced product. Chemosphere 84:1231–1237. doi: 10.1016/j.chemosphere.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 119.Ayano H, Miyake M, Terasawa K, Kuroda M, Soda S, Sakaguchi T, Ike M. 2014. Isolation of a selenite-reducing and cadmium-resistant bacterium Pseudomonas sp. strain RB for microbial synthesis of CdSe nanoparticles. J Biosci Bioeng 117:576–581. doi: 10.1016/j.jbiosc.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 120.Güven K, Mutlu MB, Çırpan C, Kutlu HM. 2013. Isolation and identification of selenite reducing archaea from Tuz (salt) Lake in Turkey. J Basic Microbiol 53:397–401. doi: 10.1002/jobm.201200008. [DOI] [PubMed] [Google Scholar]
- 121.Chau YK, Wong PT, Silverberg BA, Luxon PL, Bengert GA. 1976. Methylation of selenium in the aquatic environment. Science 192:1130–1131. doi: 10.1126/science.192.4244.1130. [DOI] [PubMed] [Google Scholar]
- 122.McCarthy S, Chasteen T, Marshall M, Fall R, Bachofen R. 1993. Phototrophic bacteria produce volatile, methylated sulfur and selenium compounds. FEMS Microbiol Lett 112:93–97. doi: 10.1111/j.1574-6968.1993.tb06429.x. [DOI] [Google Scholar]
- 123.Rael RM, Frankenberger WT. 1996. Influence of pH, salinity, and selenium on the growth of Aeromonas veronii in evaporation agricultural drainage water. Water Res 30:422–430. doi: 10.1016/0043-1354(95)00160-3. [DOI] [Google Scholar]
- 124.Van Fleet-Stalder V, Chasteen TG. 1998. Using fluorine-induced chemiluminescence to detect organo-metalloids in the headspace of phototrophic bacterial cultures amended with selenium and tellurium. J Photochem Photobiol B 43:193–203. doi: 10.1016/S1011-1344(98)00108-0. [DOI] [Google Scholar]
- 125.Michalke K, Wickenheiser EB, Mehring M, Hirner AV, Hensel R. 2000. Production of volatile derivatives of metal(loid)s by microflora involved in anaerobic digestion of sewage sludge. Appl Environ Microbiol 66:2791–2796. doi: 10.1128/AEM.66.7.2791-2796.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chasteen TG, Bentley R. 2003. Biomethylation of selenium and tellurium: microorganisms and plants. Chem Rev 103:1–25. doi: 10.1021/cr010210+. [DOI] [PubMed] [Google Scholar]
- 127.Dungan RS, Yates SR, Frankenberger WT Jr. 2003. Transformations of selenate and selenite by Stenotrophomonas maltophilia isolated from a seleniferous agricultural drainage pond sediment. Environ Microbiol 5:287–295. doi: 10.1046/j.1462-2920.2003.00410.x. [DOI] [PubMed] [Google Scholar]
- 128.Challenger F, North HE. 1934. The production of organo-metalloidal compounds by micro-organisms. Part II. Dimethyl selenide. J Chem Soc 1934:68–71. [Google Scholar]
- 129.Bird ML, Challenger F. 1939. The formation of organo-metalloidal and similar compounds by microorganisms. Part VII. Dimethyl telluride. J Chem Soc 1939:163–168. [Google Scholar]
- 130.Fleming RW, Alexander M. 1972. Dimethylselenide and dimethyltelluride formation by a Strain of Penicillium. Appl Microbiol 24:424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Barkes L, Fleming RW. 1974. Production of dimethylselenide gas from inorganic selenium by eleven soil fungi. Bull Environ Contam Toxicol 12:308–311. doi: 10.1007/BF01709124. [DOI] [PubMed] [Google Scholar]
- 132.Cox D, Alexander M. 1974. Factors affecting trimethylarsine and dimethylselenide formation by Candida humicola. Microb Ecol 1:136–144. doi: 10.1007/BF02512385. [DOI] [PubMed] [Google Scholar]
- 133.Thompson-Eagle ET, Frankenberger WT Jr, Karlson U. 1989. Volatilization of selenium by Alternaria alternata. Appl Environ Microbiol 55:1406–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chasteen T, Silver G, Birks J, Fall R. 1990. Fluorine-induced chemiluminescence detection of biologically methylated tellurium, selenium, and sulfur compounds. Chromatographia 30:181–185. doi: 10.1007/BF02274543. [DOI] [Google Scholar]