Abstract
Introduction
The purpose is to describe the method, safety and efficacy of transperineal gold seed placement for image‐guided radiation therapy.
Methods
An ethics committee approved database was used to review records of consecutive patients from October 2008 through December 2013, who underwent transperineal implantation of three gold markers into the prostate using staged local anaesthesia and transrectal ultrasound. Seeds were counted on radiographs from CT simulation, first treatment and last treatment. Retention and use of at least three markers for kV/kV matching was considered a successful implant. A visual analogue scale (VAS) pain assessment was performed. SAS was used for data analysis.
Results
Fiducial marker placement was successful for kV/kV matching in 556/581 patients (95.7%). The procedure was aborted due to pain in two patients. Additional sedation during the procedure was required in two patients. Complications include urinary infections (2 patients, <0.5%) and transient haematuria (2 patients, <0.5%). There were no recorded calls requesting additional pain medication or delays in radiation due to complications. The number of seeds identified at simulation: 4 (2 patients), 3 (554 patients), 2 (21 patients), 1 (1 patient), 0 (1 patient). One patient with three seeds and two patients with <2 seeds had cone beam CT instead of kV/kV imaging for image guidance. No seeds were lost after simulation. The mean visual analogue pain score associated with transperineal gold seed insertion met patients' expectations (respectively 4.1 vs. 4.4 P = 0.19).
Conclusion
Outpatient transperineal insertion of fiducials avoids the rectum, is effective, convenient, well tolerated and has few side effects.
Keywords: Gold fiducial markers, image‐guided radiation, prostate cancer, transperineal implant
Introduction
Curative therapy of prostate cancer with radiation requires high radiation doses as well as accurate and reproducible delivery of the daily radiation treatments. Since the prostate is a mobile organ, daily variations in prostate position must be accounted for to ensure accurate treatment and avoid irradiating the surrounding sensitive organs that are at risk for damage from the high doses of radiation needed to treat the prostate cancer.1
Older radiation techniques were based on bone anatomy and required large treatment fields to account for prostate motion.2 Newer image‐guided radiation therapy (IGRT) techniques have been developed that accurately locate the prostate and allow the radiation fields to be adjusted daily to correct for prostate motion prior to treatment delivery.1, 2, 3 IGRT allows patients to be treated with smaller conformal fields that reduce the volume of normal tissue exposed to radiation and thereby reduce complications without sacrificing treatment accuracy or tumour control.2, 3
Daily orthogonal kV/kV imaging with adjustments in field position based on the location of previously implanted permanent gold fiducial markers is an accurate and widely used method for delivering IGRT.2, 4, 5 Although the transperineal approach for gold seed insertion was described in 2004,6 and is used for prostate brachytherapy, the transrectal approach is the method most widely used for fiducial placement.3, 6, 7, 8, 9, 10 Wide acceptance of the transrectal approach is due to comfort and ease of placing the patient the foetal rather than lithotomy position. Urologists are comfortable with this approach since it requires the same equipment and set up used for biopsies. This technique gives accurate seed placement and infrequent complications. Disadvantages of the transrectal approach include occasional infection, rectal bleeding and pain.5, 8, 11, 12, 13, 14 Advantages of the transperineal approach include decreased likelihood of infection and rectal bleeding.15 It is uncertain which approach causes less discomfort to the patient.5
We report our evolved technique and experience using a comprehensive pain management strategy including rates of complications and pain assessment with transperineal placement of gold fiducial markers in the prostate for daily kV/kV IGRT.
Methods
A clinical research database approved by the Sheba ethics committee was used to extract data of consecutive patients. This retrospective chart review conformed to the ethical standards of the 2000 Declaration of Helsinki and the 2008 Declaration of Istanbul. All patients signed informed consent for the procedure which included permission for inclusion in the database. Seed placement was done as an outpatient procedure by a single radiation oncologist (ZS) in the brachytherapy suite at Chaim Sheba Medical Center.
Prior to the procedure, patients underwent a standard history and physical evaluation. Patient education prior to the procedure included oral and written instructions for preparation and a description of the procedure. Anticoagulant and anti‐platelet therapy were discontinued 10 days prior to fiducial placement. Patients were able to resume anticoagulant and anti‐platelet therapy three days post procedure, provided they had no bleeding complications. Prophylactic antibiotic therapy with oral Ofloxacin 200 mg was started the night prior and continued the morning of the procedure and then twice daily until 3 days after the procedure. Patients did not eat after midnight, and in the morning of the procedure had fleet enema until clear. Patients were given a 30‐g tube of lidocaine/prilocaine (EMLA cream, AstraZeneca Pharmaceuticals LP, Wilmington, DE) and instructed to liberally apply to their perineum the morning of the procedure before leaving home. No other analgesia or premedication was used.
The evolved technique is described: patients are prepared and draped in the lithotomy position. Lidocaine spray 10% and gel are applied to the anus and rectum. A trans‐rectal ultrasound probe is inserted into the rectum for guidance and the prostate is imaged. Patients are coached throughout the procedure to relax the pelvic muscles and distracted to minimise pain perception. Multi‐layer anaesthesia using 20 cc of 2% lidocaine is given through the perineum adapted from Wallner et al. who used local anaesthesia for prostate brachytherapy.16, 17 First, a subcutaneous and then a deeper injection into the muscular perineum using a 21 gauge needle is given using a free‐hand approach. A 19 gauge spinal needle is introduced 1–1.5 cm lateral to the linea alba bilaterally under ultrasound guidance to infiltrate the nerves located at the angle of the seminal vesicles and prostate, avoiding the midline to prevent urethral puncture. A few minutes following lidocaine injection, three gold seeds are inserted into different areas of the prostate under ultrasound guidance using 17 gauge 20 cm needles. A configuration that avoids seed overlap on orthogonal kV imaging is chosen, for example one at the right anterior apex, one at the right base slightly posterior and one central mid‐gland on the left. We observed that posteriorly placed seeds close to the rectal wall are likely to migrate and should be avoided. Prior to leaving the brachytherapy suite, seed placement is reviewed by ultrasound and if not satisfactory an additional seed is placed.
Patients were transferred to the recovery room and discharged home soon after the procedure. Patients were given written and verbal instructions to contact the department if they have persistent bleeding, pain, fever or any other concerns or questions. All patients returned to the radiation department in 1 week for CT simulation (Big Bore CT, Phillips, Netherlands). Patients were informally surveyed by the simulation therapist. A formal questionnaire was not used. Symptoms if present were reported at the day of the simulation to the treating physician or nurse. Urine analysis and urine culture and sensitivity were taken only for patients complaining of bleeding or urinary symptoms suggesting infection. Urine analysis or cultures were not done on asymptomatic patients. Patients were not screened for micro‐haematuria.
The planning CT was transferred to the treatment planning station (Varian Eclipse, Palo Alto, CA). The gold seeds were contoured by the physician for use with daily kV/kV imaging or cone beam CT scan. If all the seeds could not be identified or were not placed in a position suitable for imaging, image guidance would proceed with either the reduced number of seeds or with cone beam imaging alone. The implant procedure was considered unsuccessful if it was aborted prior to seed placement due to patient discomfort, if a minimum of three gold seeds could not be identified at time of treatment planning CT for kV/kV matching, or if seeds were placed in a position deemed unsatisfactory for matching.
Seed placement was assessed for each patient by retrospectively reviewing: the number of seeds initially placed, the number of seeds present at time of treatment planning CT scan and the number of seeds present on first and last kV/kV image or cone beam CT scan. All images were reviewed using the offline imaging record from the Eclipse treatment planning system (Varian Eclipse, Palo Alto, CA).
In 2013, as part of our quality of care assessment programme, a pain assessment study using a visual analogue scale (VAS) from 1–10 was initiated to compare anticipatory and experienced perception of pain from the implant. Scores of 1–3 were little or no pain, 4–7 were moderate pain and 8–10 were severe pain. Patients were asked to fill out a questionnaire before the implant and rate their expectations of pain from the implant. Following the implant, the patients were asked to record the experienced pain compared to their expectation. Patients were excluded from the pain analysis if they were sedated during the fiducial placement.
Statistical analysis
Patient characteristics are reported as absolute figures. Questionnaire responses were analysed according to the box plot analysis (Excel, Microsoft Corporation, Redmond, WA). Tests for normality were done using the Shapiro–Wilk test. Comparisons of responses were done using a Wilcoxon signed rank test. Confidence intervals were considered significant if greater than P = 0.05. SAS software (Cary, NC) was used for all statistical calculations.
Results
Between October 2008 and December 2013, 581 patients scheduled to receive definitive radiation for prostate cancer underwent transperineal implantation of gold seeds into the prostate for IGRT. Patients were characterised as T1 or T2 (68%), T3 (25%) and T4 (7%), Gleason 6 or less (22%), Gleason 7 (43%) and Gleason 8–10 (35%). Median pretreatment PSA was 9 ng/mL (SD ± 6 ng/mL).
Placement of three seeds was planned for all patients. Initial gold seed placement succeeded in 556 patients (95.7%) and was confirmed by ultrasound review after the procedure. Gold seed placement was aborted in two patients due to discomfort during the procedure. Two additional patients required sedation to complete the procedure without discomfort. Two patients had placement of an additional seed because of difficulty locating the seed on ultrasound during the procedure. There were no calls to the clinic requesting additional pain medication.
Transperineal seed placement was associated with a very low incidence of infection and side effects. Two patients suffered symptoms consistent with urinary infections. Urine cultures in both patients showed E‐Coli infection. One culture was resistant to Ofloxacin but both cultures were sensitive to Ampicillin. Both patients were treated initially with Ampicillin and did well. Two patients experienced transient gross haematuria. One of these patients visited the emergency room, was reassured and discharged home. Haematuria resolved in both patients without intervention. Radiation therapy commenced 4–6 weeks following gold seed placement and apart from the four patients reported above no additional toxicity was reported in this interval.
At the time of planning CT scan, the number of seeds present in each patient was: 4 seeds (n = 2), 3 seeds (n = 554), 2 seeds (n = 21), 1 seed (n = 1) and 0 seeds (n = 1). Review of each patient's kV images at the beginning and end of therapy showed no further change in seed number.
Seed placement was considered unsatisfactory on CT simulation in one patient with three seeds. This patient had a prior suprapubic prostatectomy, the ultrasound image was distorted and the seeds were placed posteriorly close to the rectum. Patients (n = 2) with less than two seeds identified on CT simulation were also considered to have unsatisfactory implants. One patient had one seed present and one patient had 0 seeds present.
Although these implants were considered unsuccessful, patients with two seeds (n = 21) had cone beam CT scans during the first week of treatment and if there was good correlation between the cone beam CT scan and the kV/kV image, treatment was continued with kV/kV imaging alone. Patients (n = 5) with unsatisfactory or aborted seed placement received image guidance using daily cone beam CT verification. No patients were brought back for a second attempt at seed placement.
Almost all patient started radiation therapy as planned. One patient reconsidered his decision and selected surgery and nine patients decided not to receive treatment.
Eighty‐four patients were enrolled into the pain assessment study and 79 completed the VAS questionnaire. Five patients were excluded from the study because they had no memory of the previous transrectal prostate biopsy due to sedation during the biopsy procedure or fiducial placement and were unable to complete the full questionnaire.
Figure 1 shows the results of the pain assessment survey. Patients experienced moderate discomfort with the transperineal implants. Shapiro–Wilk test showed that data did not conform to a normal distribution. A Wilcoxson signed rank test showed no significant difference between patient's expected and actual discomfort (mean: 4.4 vs. 4.1 P = 0.19) associated with the gold seed insertion, thus reflecting that realistic expectations of discomfort were generated by the pretreatment education procedure.
Figure 1.
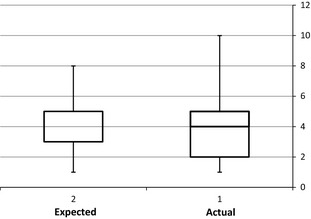
Expected and actual pain from transperineal fiducial implant (visual analogue scale) (n = 79). Expected versus actual pain P = 0.18.
Discussion
Transperineal gold seed placement is an effective, safe, well‐tolerated technique for placement of gold fiducial markers used in IGRT of the prostate.5, 18 An advantage of the transperineal technique is that it avoids puncturing the rectum and reduces the possibility of infections.5, 15, 18, 19 Our modification of Wallner's technique for transperineal local anaesthesia contributed to the good patient tolerance of the gold seed implants.16, 17
Rates of infection and rectal bleeding associated with the transrectal approach are low but can be bothersome for patients and can delay the onset of treatment.5, 11 In addition, the development of antibiotic‐resistant infections is a growing concern and is being reported in increasing frequency in patients having transrectal biopsies.14, 15 In comparison, the transperineal approach offers few complications and allows patients to start their prescribed radiation treatment soon after the fiducial marker placement.5, 18
Our results confirm that the fiducial implant is associated with very few symptoms as all patients were informally surveyed at CT simulation 1 week after procedure and there were no additional reports of side effects in the 4‐week interval prior to commencement of radiotherapy. This is similar to other studies that report few side effects or infections with the transperineal approach.5, 18 However, comparison to studies that surveyed patients about their experience with either transrectal or transperineal implant after completion of a course radiation therapy treatments reports much higher rates of side effects associated with gold seed placements (Table 1).4, 5, 11, 12, 19 Patients surveyed about side effects of the gold seed implant after completion of their radiation therapy are probably reporting symptoms associated with the external beam radiation therapy rather than the seed implant since the symptoms reported resemble complaints commonly experienced by patients after completing a course of radiation therapy.4, 20 For example, Gill reported results in 234 patients who responded to a questionnaire mailed to them after completing treatment and reports that 32% of the patients had at least one new symptom after the procedure: frequency 16%, haematuria, rectal bleeding, dysuria and hematospermia, 9–13%, pain obstruction and shivers 3–4% and 9% had symptoms lasting more than 2 weeks. 4 In contrast, studies that surveyed patients prior to starting radiation reported fewer complications. 19
Table 1.
Summary of results from implants
| Authors | Year | Patients | Technique | Time of data collection | Outcome | Complications |
|---|---|---|---|---|---|---|
| Saada | 2015 | 581 | TP | Pre RT | 95.7% |
Urinary infections 0.4% Haematuria 0.4% |
| Fawaz et al.19 | 2014 | 169 | TR | Pre RT | 99% | Antibiotic‐resistant UTI‐2 patients |
| Moman et al.5 | 2010 |
512 402 |
TP TR |
Pre RT |
99% 213 pts – 2 markers |
TP‐No grade 3 or 4 toxicity TR‐Grade 3 urinary sepsis 0.5% |
| Langenhuijsen et al.12 | 2007 | 177 | TR | Pre RT | No data |
Fever 6%, Haematuria 3.8%, Rectal bleeding 9.1% |
| Gill et al.4 | 2012 | 234 | TR | Post RT | No data |
Urinary frequency 16% Haematuria or rectal bleeding 9–13% Pain obstruction fever 3–4% Grade 3 symptoms .5–1.5% Grade 4 sepsis 1 patient 32% of patients reported at least one symptom. |
| Igdem et al.11 | 2009 | 135 | TR | Post RT | 98% |
Fever 2% Antibiotics 2.2% Haematuria 15% Rectal bleeding 4% |
TP, transperineal; TR, transrectal; RT, radiation therapy.
Current paper.
We expect that the initial costs of both transrectal and transperineal gold seed placement to be similar since both are outpatient procedures done without general anaesthesia. However, since the rates of infection with transperineal implants are low and all infections in our series were treated on an outpatient basis, costs due to infectious complications are avoided.
In contrast, the transrectal biopsy route has recently been reported to have high rates of antibiotic‐resistant infections which if true for transrectal gold seed placement, may be associated with costly inpatient treatment of infection.14, 15 Because of increasing concern over the risk of infections, the transperineal route has been described as a viable route for prostate biopsy and suggested as an alternative to the transrectal route to avoid infections.15 The transperineal approach is also suitable for placement of radiofrequency transponders (Calypso Varian Palo Alto California USA)
Although were able to use kV/kV imaging with cone beam CT verification as an image guidance technique for most of our patients with only two identifiable gold seeds, we considered these implants as suboptimal since three seeds were not available for daily image guidance. Our rate of lost seeds seems higher than other studies. We think that this may be due to our implanting only three seeds for most patients. Although most studies report using only three markers, studies that implant four or more seeds may have three seeds found at simulation and record a successful implant. 21 In addition, other studies may not consider two seeds as an unsuccessful implant. 5 We think that routinely implanting four seeds would improve our yield of satisfactory implants.
The relationship between anxiety and pain has been well characterised. 22 To reduce perception of pain from this procedure, we employed a comprehensive strategy of patient education, suggestion, reassurance prior to the procedure and distraction in addition to the multi‐layered local anaesthesia. As we hypothesised, there was no significant difference between expectations and actual pain experience suggesting that the pain management strategy was indeed effective. While numbers are small and a prospective comparison was not performed, it seems that transperineal gold seed placement is well tolerated and does not cause patients substantial discomfort. Our results are consistent with others that report little discomfort from this procedure. 18
Conclusion
Transperineal insertion of gold fiducial markers for IGRT of the prostate is effective, convenient, and associated with low risk of complications such as rectal bleeding or infection. This well‐tolerated approach avoids the rectum and can be done in an outpatient setting by a radiation oncologist or urologist experienced in brachytherapy. 15
Conflict of Interest
The authors declare no conflict of interest.
J Med Radiat Sci 62 (2015) 261–266
References
- 1. Martin JM, Bayley A, Bristow R, et al. Image guided dose escalated prostate radiotherapy: Still room to improve. Radiat Oncol 2009; 4: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chung HT, Xia P, Chan LW, Park‐Somers E, Roach M 3rd. Does image‐guided radiotherapy improve toxicity profile in whole pelvic‐treated high‐risk prostate cancer? Comparison between IG‐IMRT and IMRT. Int J Radiat Oncol Biol Phys 2009; 73: 53–60. [DOI] [PubMed] [Google Scholar]
- 3. Brown S, Lehman M, Ferrari‐Anderson J, Glyde A, Burmeister E, Nicol D. Assessment of prostatic fiducial marker introduction: Patient morbidity, staff satisfaction and improved treatment field placement. J Med Imaging Radiat Oncol 2011; 55: 417–24. [DOI] [PubMed] [Google Scholar]
- 4. Gill S, Li J, Thomas J, et al. Patient‐reported complications from fiducial marker implantation for prostate image‐guided radiotherapy. Br J Radiol 2012; 85: 1011–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moman MR, van der Heide UA, Kotte AN, et al. Long‐term experience with transrectal and transperineal implantations of fiducial gold markers in the prostate for position verification in external beam radiotherapy; feasibility, toxicity and quality of life. Radiother Oncol 2010; 96: 38–42. [DOI] [PubMed] [Google Scholar]
- 6. Henry AM, Wilkinson C, Wylie JP, Logue JP, Price P, Khoo VS. Trans‐perineal implantation of radio‐opaque treatment verification markers into the prostate: An assessment of procedure related morbidity, patient acceptability and accuracy. Radiother Oncol 2004; 73: 57–9. [DOI] [PubMed] [Google Scholar]
- 7. Ohashi T, Yorozu A, Saito S, et al. Combined brachytherapy and external beam radiotherapy without adjuvant androgen deprivation therapy for high‐risk prostate cancer. Radiat Oncol 2014; 9: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zelefsky MJ, Nedelka MA, Arican ZL, et al. Combined brachytherapy with external beam radiotherapy for localized prostate cancer: Reduced morbidity with an intraoperative brachytherapy planning technique and supplemental intensity‐modulated radiation therapy. Brachytherapy 2008; 7: 1–6. [DOI] [PubMed] [Google Scholar]
- 9. Shinohara K, Roach M 3rd. Technique for implantation of fiducial markers in the prostate. Urology 2008; 71: 196–200. [DOI] [PubMed] [Google Scholar]
- 10. Linden RA, Weiner PR, Gomella LG, et al. Technique of outpatient placement of intraprostatic fiducial markers before external beam radiotherapy. Urology 2009; 73: 881–6. [DOI] [PubMed] [Google Scholar]
- 11. Igdem S, Akpinar H, Alco G, Agacayak F, Turkan S, Okkan S. Implantation of fiducial markers for image guidance in prostate radiotherapy: Patient‐reported toxicity. Br J Radiol 2009; 82: 941–5. [DOI] [PubMed] [Google Scholar]
- 12. Langenhuijsen JF, van Lin EN, Kiemeney LA, et al. Ultrasound‐guided transrectal implantation of gold markers for prostate localization during external beam radiotherapy: Complication rate and risk factors. Int J Radiat Oncol Biol Phys 2007; 69: 671–6. [DOI] [PubMed] [Google Scholar]
- 13. Lee G, Attar K, Laniado M, Karim O. Safety and detailed patterns of morbidity of transrectal ultrasound guided needle biopsy of prostate in a urologist‐led unit. Int Urol Nephrol 2006; 38: 281–5. [DOI] [PubMed] [Google Scholar]
- 14. Berglund RK, Zaytoun O, Thousand R, et al. Early infectious complications with transponder placement for external beam radiation therapy for prostate cancer. BJU Int 2012; 110: 834–9. [DOI] [PubMed] [Google Scholar]
- 15. Chang DT, Challacombe B, Lawrentschuk N. Transperineal biopsy of the prostate–is this the future? Nat Rev Urol 2013; 10: 690–702. [DOI] [PubMed] [Google Scholar]
- 16. Wallner K. Prostate brachytherapy under local anesthesia; lessons from the first 600 patients. Brachytherapy 2002; 1: 145–8. [DOI] [PubMed] [Google Scholar]
- 17. Mueller A, Wallner K, Corriveau J, Arthurs S, Gwinn M, Sutlief S. A reappraisal of local anesthesia for prostate brachytherapy. Radiother Oncol 2003; 67: 309–12. [DOI] [PubMed] [Google Scholar]
- 18. Tang CI, Sethukavalan P, Cheung P, Morton G, Pang G, Loblaw DA. A prospective study on pain score with transperineal prostatic gold seed fiducial implantation under local anesthetic alone. Can Urolo Assoc J 2013; 7: E202–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fawaz ZS, Yassa M, Nguyen DH, Vavassis P. Fiducial marker implantation in prostate radiation therapy: Complication rates and technique. Cancer Radiother 2014; 18: 736–9. [DOI] [PubMed] [Google Scholar]
- 20. Lips IM, Dehnad H, van Gils CH, Boeken Kruger AE, van der Heide UA, van Vulpen M. High‐dose intensity‐modulated radiotherapy for prostate cancer using daily fiducial marker‐based position verification: Acute and late toxicity in 331 patients. Radiat Oncol 2008; 3: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Poggi MM, Gant DA, Sewchand W, Warlick WB. Marker seed migration in prostate localization. Int J Radiat Oncol Biol Phys 2003; 56: 1248–51. [DOI] [PubMed] [Google Scholar]
- 22. Tang J, Gibson SJ. A psychophysical evaluation of the relationship between trait anxiety, pain perception, and induced state anxiety. J Pain 2005; 6: 612–19. [DOI] [PubMed] [Google Scholar]


