Abstract
Our objective was to validate a commercially-available ELISA to measure antibody titers against Epstein-Barr virus (EBV) in dried blood spots (DBS) to replace a previously validated assay for DBS that is no longer available. We evaluated the precision, reliability, and stability of the assay for the measurement of EBV antibodies in matched plasma, fingerprick DBS, and venous blood DBS samples from 208 individuals. Effects of hematocrit and DBS sample matrix on EBV antibody determination were also investigated, and the cut-off for seropositivity in DBS was determined. A conversion equation was derived to enable comparison of results generated using this method with the former DBS method. There was a high correlation between plasma and DBS EBV antibody titers (R2 = 0.93) with very little bias (−0.07 based on Bland-Altman analysis). The assay showed good linearity, did not appear to be affected by the DBS matrix, and physiological hematocrit levels had no effect on assay performance. There was reasonable agreement between DBS EBV titer estimates obtained using this assay and the previously-validated assay (R2 = 0.72). The commercially-available ELISA assay for EBV antibody titers that we validated for use with DBS will facilitate continued investigation of EBV antibody titers in DBS.
Keywords: psychosocial stress, Epstein-Barr virus, dried blood spots, ELISA, method validation
Introduction
Epstein-Barr virus (EBV) is a latent herpesvirus currently estimated to infect approximately 95 percent of the world’s adult population (Cohen 2015). Although individuals infected with EBV remain lifelong carriers, EBV titers typically remain at low levels due to control of the virus by the healthy immune system. However, suppression of cell-mediated immunity under conditions of psychosocial stress, such as caregiving for a family member with Alzheimer’s disease (Kiecolt-Glaser et al. 1987), taking medical school exams (Glaser et al. 1994), and space flight (Pierson et al. 2005), has been shown to elevate EBV antibody levels in response to reactivation of latent EBV. In particular, upregulation of the hypothalamic-pituitary-adrenal (HPA) axis and increased production of glucocorticoids and catecholamines are thought to compromise the immune system, leading to reactivation of latent herpesviruses, such as EBV, and an increase in EBV antibody titer as the humoral immune system responds (Glaser and Kiecolt-Glaser 2005). EBV antibody titers therefore serve as an indirect measure of cell-mediated immune function and represent a widely used biomarker of chronic psychosocial stress. Chronic, stress-induced immune dysregulation has numerous adverse health effects, including slow wound healing (Kiecolt-Glaser et al. 1995), aggravation of chronic autoimmune disease (Kemeny and Schedlowski 2007), promotion of tumor growth and metastasis (Powell et al. 2013), and increased risk for infectious disease (Cohen et al. 1991; Sanchez et al. 2015).
A dried blood spot (DBS) assay to assess EBV antibody titers was developed by McDade and colleagues in 2000 to facilitate research on stress and immunity in larger epidemiological, community-based studies than was previously possible using venous blood samples (McDade et al. 2000a). Dried blood spots generally refer to capillary whole blood from fingerprick collected onto filter paper and subsequently dried, although blood collected by venipuncture can also be spotted on DBS cards. The low cost of capillary DBS collection and the ease of DBS sample collection, transport, and storage, especially in field-based settings, have led to the widespread adoption of this sampling technique in biological anthropology, epidemiological, and psychological research, among a variety of other disciplines (McDade et al. 2007).
Using EBV antibody titers in DBS as a biomarker of chronic psychosocial stress, the resulting studies have significantly advanced our understanding of the impact of stress due to economic development on quality of life scores in China (Inoue et al. 2014), highlighted gender differences in the effects of stressful family dynamics in the young urban elite in Kabul, Afghanistan (Panter-Brick et al. 2008), elucidated how shifting social conditions brought on by westernization have impacted adolescent health and well-being in Western Samoan youth (McDade et al. 2000b), revealed how disparities in material wealth and socioeconomic status among indigenous Siberian herders affect immune function (Sorensen et al. 2009), and shown how discrimination-related stress predicts EBV antibody titers in Latin America immigrants in the US (McClure et al. 2010). DBS-based EBV antibody titers are also one of the biomarkers of interest in several large-scale epidemiological studies, including the National Social Life, Health, and Ageing Project (NSHAP) (Williams and McDade 2009), the World Health Organization’s Study on global AGEing and adult health (SAGE) (Kowal et al. 2012), and the National Longitudinal Study of Adolescent to Adult Health (ADDHealth) (Slopen et al. 2013).
Unfortunately, the specific immunoassay kit (Diasorin #7590) that was validated for use with DBS was withdrawn from the market due to a shift in Diasorin’s corporate focus to a high-throughput, automated platform for performing a range of immunoassays (Liaison™).
Given the demonstrated utility of this biomarker for measuring immune dysregulation and chronic psychosocial stress, we initiated the present study to validate an alternative EBV ELISA assay for use with DBS samples.
Methods
Samples
As part of a validation study conducted at the University of Oregon, we collected matched finger-prick DBS (fDBS), venous DBS (vDBS), and plasma samples from a convenience sample of 208 adults (≥18 years) from the Eugene/Springfield, OR area between Nov 2014 and Feb 2015. Age range was 18 to 77 years, and M:F ratio was 120:88. The Institutional Review Board at the University of Oregon approved the study protocol and all participants gave written consent.
Fingerprick DBS collection followed an established protocol (McDade et al. 2007). A finger prick was made using a sterile, single-use lancet and 10 full drops of capillary whole blood were collected per participant onto five Whatman 903 filter paper cards. Each Whatman 903 filter paper card contains five demarcated circles for collection of up to five drops of blood per card. We collected only two drops of blood per card (i.e., only 2/5 demarcated circles were used) but five cards per person for a maximum of 10 full drops of blood per person to minimize the number of freeze-thaw cycles that the cards would be subjected to during assay validation. Each drop corresponds to ~50 μl of blood. DBS cards were dried at room temperature for 4 hours, and then stored at −80°C until analysis. For venous collection, in order to create DBS cards and obtain plasma, participants had ~21 mL of blood (three 7-mL tubes) collected by a certified phlebotomist via venipuncture into EDTA-coated vacutainer tubes. One of the three tubes was used to immediately create venous DBS cards by pipetting five 50 μL drops of whole blood onto 15 Whatman 903 filter paper cards. These were dried and stored as described for fingerprick DBS. The second and third tubes of blood were immediately centrifuged for ~15 min to separate plasma. The plasma was removed and twenty 100 μl aliquots were placed in 2 mL cryotubes that were then immediately frozen at −80°C and stored until analysis.
ELISA protocol
The previously validated ELISA protocol for DBS was the quantitative/qualitative Diasorin ETI-VCA-G kit that targeted IgG antibodies against the viral capsid antigen protein p18 (McDade et al. 2000a). After testing a number of commercially available EBV IgG ELISA kits targeting IgG antibodies to a range of viral capsid antigen proteins and exploring how the EBV titer values compared to those obtained previously using the Diasorin kit, we decided to focus on EBV ELISA kits that target IgG antibodies against the p18 protein of VCA to enable comparison with previous results and for longitudinal consistency in ongoing studies.
The Diamedix EBV ELISA kit (Fisher Scientific, Hanover Park, IL, Cat. #720-600) was developed for the qualitative and semi-quantitative determination of IgG antibodies to Epstein-Barr virus (recombinant) viral capsid antigen (EBV-VCA IgG) in human serum by indirect enzyme immunoassay. Wells of this assay kit are coated with a 47-kDa fusion protein of 53 amino acids from the c-terminal half of p18 of the VCA. To make this assay fully quantitative, we developed a standard curve using the high positive IgG control (stabilized human serum) sourced from the Mikrogen recomWell EBV-VCA IgG kit (Biosell Solutions Inc., Las Vegas, NV).
One 3.2 mm-diameter punch from a DBS card was eluted overnight at 4°C in 250 μl Diamedix sample B diluent. The following day, all reagents and the coated ELISA plate were allowed to equilibrate to room temperature for 1 hr. A standard curve was made by serial dilutions of the Mikrogen high positive IgG control to expected concentration (based on value provided on the label) using the standard diluent buffer to provide 7 calibration points (700 U/mL, 350 U/mL, 175 U/mL, 87.5 U/mL, 43.75 U/mL, 21.88 U/mL, 10.9 U/mL). Note that these values reflect levels of anti-EBV p18, p23, and p54 IgG antibodies measured in the control sera samples provided with the kit, whereas the Diamedix kit will only measure anti-EBV p18. Sample B diluent was used as the zero standard. In addition, one high (EBV antibody titer >300 U/mL) and one low (EBV antibody titer < 10 U/mL) DBS control samples were included on every plate for quality control purposes. One hundred microliters of the standards or the eluted DBS samples were loaded in duplicate wells, and 38 samples were run per 96-well plate.
Plasma samples were diluted 1:100; however, if they were beyond the range of the standard curve, they were rerun at dilutions ranging from 1:200 to 1:1000. DBS samples with EBV-VCA IgG values beyond the upper range of the standard curve were eluted in 2-4 mL sample B diluent rather than the standard 250 μl of sample B diluent and rerun. After loading 100 μl of DBS eluate or plasma sample dilution per well, the protocol outlined in the Diamedix kit insert was followed. In more detail, the plate was allowed to incubate uncovered at room temperature for 30 ± 5 min. This was followed by a wash step (three washes with 300 μl 1X wash T buffer using a Biotek 405 LS plate washer). After excess wash buffer was tapped out by inverting the plate hard several times on paper towel, 100 μl conjugate was added to all wells using a multichannel pipettor and the plate was allowed to incubate uncovered at room temperature for 30 ± 5 min. The wash step was repeated as described above, and then 100 μl of substrate HRP was added per well, taking care to avoid bubble formation. The plate was placed uncovered in the dark and allowed to incubate for 30 ± 5 min. Stop N solution (100 μl) was then added to each well, and the plate was placed on a plate shaker at ~250 rpm for 1 minute to ensure complete mixing of the well contents. The plate was then read within 15 minutes at 450 nm using a reference wavelength of 630 nm (BioTek EL808 plate reader) and the standards were fit using a four-parameter logistic curve using Gen5 Data Analysis software (BioTek). Concentrations of DBS samples diluted in volumes greater than 250 μl (e.g. 2 mL) were multiplied by the corresponding dilution factors (x4). EBV titers for plasma samples that were diluted more than 1:100 were multiplied by the corresponding dilution factor e.g. (x10 if diluted 1:1000).
Plasma/DBS comparisons
The correlations between DBS VCA IgG titers and plasma VCA IgG titers and between fDBS and vDBS VCA IgG titers were assessed by linear regression analyses. To assess the consistency of estimates based on plasma versus DBS sample types and to assess if there was any bias, we performed Bland-Altman analysis. After converting the fDBS values to plasma equivalent values using the linear regression equation, we plotted the difference in fDBS and plasma values versus the average of these values and evaluated the bias and how many samples fell outside the 95 percent confidence intervals (Bland and Altman 1986).
Linearity
To evaluate the precision of the assay results for different dilutions of sample, three vDBS samples with low, moderate, and high levels of EBV VCA IgG were diluted 1:2, 1:4, and 1:8 in sample diluent, and the measured concentrations were expressed as the percent recovery relative to the undiluted sample (observed concentration/expected concentration *100) after correcting for the dilution factor.
Spike & Recovery
We added low (30 U/mL) and moderate (100 U/mL) amounts of EBV VCA IgG antibodies to the eluate from two vDBS samples and compared the recovered concentrations with those measured for the same amounts of antibodies diluted in Sample diluent B. The results are expressed as the observed concentration (in DBS eluate)/expected concentration (in sample diluent) after correction for the endogenous EBV antibody titer.
Intra- and inter-assay coefficients of variability (CV)
Inter-assay variability was calculated by taking the average concentrations of the high and low DBS controls for 12 plate runs and calculating the CVs. Intra-assay variability was calculated by running 38 samples spanning the range of the assay in duplicate on one plate; the intra-assay CV reported is the average of these individual CVs.
Limit of detection
We calculated the minimum detectable dose by adding two standard deviations to the mean optical density value of 20 zero standard replicates and calculating the corresponding analyte concentration from the standard curve equation.
Effect of hematocrit
To evaluate the effect of hematocrit on assay performance, four 7-mL EDTA-coated vacutainers of whole blood (~ 8 mL of blood per tube) were centrifuged at 1520 g in a Horizon Plasmafuge for 15 min to obtain packed red blood cells (RBCs; equivalent to a hematocrit of 100 percent), and the plasma was reserved. RBCs were then diluted with the reserved plasma to obtain samples with physiologically relevant hematocrit values of 30, 40, 50, and 60 percent and spotted onto filter paper. The sample with 50 percent hematocrit was assumed to be typical of most DBS and the values obtained for the other percentage hematocrit DBS were assayed and expressed as the percent recovery of the concentration in the 50 percent hematocrit sample (100 × observed/expected).
Conversion equation for Diasorin values
To enable direct comparison of EBV antibody titers obtained using the Diamedix kit with those obtained using the Diasorin kit, we determined Diamedix EBV VCA IgG values from 42 fDBS samples (collected as part of the Shuar Health and Life History Project and stored at −20°C for ~5 years at the time of this assay) that had previously determined Diasorin EBV VCA IgG titers.
Seropositivity determination
To establish a DBS-based cutoff value, 18 individuals with low concentrations of EBV were identified, and matched plasma and fDBS samples were run on a single plate. The Diamedix kit classifies samples as having no detectable VCA IgG antibodies, equivocal for VCA IgG antibodies, or positive for VCA IgG antibodies based on a ratio referred to as the index value. The index value is the absorbance of the sample/mean absorbance of cut-off calibrator after subtraction of blank. To evaluate the index value for the plasma samples run on the plate, the cut-off calibrator supplied with the Diamedix kit was included in duplicate on the plate.
Freeze/thaw stability
Venous DBS cards from one individual with a high VCA IgG titer (~516 U/ml) were stored at −80°C after 4 h of drying and then subjected to 1, 2, 4, 8 and 14 cycles of freeze-thaw (22°C for 8 h then −80°C for 16 h) to reflect likely lab conditions; specimens were then stored at −80°C and assayed in a single batch. Percentage recovery was calculated relative to the titer in the sample thawed only once.
Analyte stability
Venous DBS card from one individual with a moderately high VCA IgG titer (~516 U/ml) was stored at 22°C (controlled ambient), 37°C (hot ambient), and −20°C (frozen) for 2, 7, 14, and 21 days after collection and then assayed on a single plate. Percentage recovery was calculated relative to the titer in the sample stored at −80°C after collection and drying and thawed only for the assay.
Results
Plasma/DBS comparisons
There was a very high linear correlation (R2 = 0.93) between fDBS EBV antibody titers and plasma EBV antibody titers (y = 1.5x + 1.9) (Fig.1A). We evaluated the correlation between fDBS and vDBS values for a subset of samples (n=31) and found the expected strong correlation between fDBS and vDBS values (R2 = 0.89) with a slope of 1, indicating good equivalence between the fDBS and vDBS concentrations (Fig.1B). Bland Altman analysis of the difference in fDBS and plasma values versus the average of these values indicated very little bias (−0.07), and only 13 of 208 values (6 percent) fell outside the 95 percent limits of agreement (−178.9 to 178). There was no consistent pattern to the distribution of these outliers. The mean EBV IgG titers and ranges for fDBS, vDBS, and plasma samples were as follows: 174.5 AU/ml [3.15 – 1878 AU/mL, n=208], 391.1 AU/mL [18.4 – 2896 AU/mL, n=31], and 266.4 AU/mL [1 – 2621 AU/mL, n=208].
Figure 1.
A. Plot of fingerprick DBS (fDBS) EBV VCA IgG titers versus plasma values for 208 matched samples. There was a strong linear correlation between values measured in fDBS and plasma. B. Plot of fDBS EBV VCA IgG titers versus venous DBS (vDBS) values. There was a strong linear correlation between values measured in these 31 samples chosen to span the range of assay values and a 1:1 relationship between fDBS titers and vDBS titers. C. Plot of EBV VCA IgG titers for 42 fDBS samples quantitated using both the Diasorin ELISA kit (x-axis) and the Diamedix ELISA kit (y-axis). There was a reasonable correlation between the Diasorin and Diamedix estimates of EBV antibody titer.
Linearity
Average percentage recovery of three venous DBS samples serially diluted in sample diluent B was 97.9 percent, with a range of 85-107 percent, indicating good assay linearity.
Spike and Recovery
In the spike and recovery experiment, percentage recovery ranged from 78 percent – 107 percent, indicating that the DBS matrix is valid for the assay procedure.
Intra- and inter-assay CV
The intra-assay CV was 3.6 percent. The inter-assay CV for the high control was 6.6 percent, while that for the low control was 20.9 percent, yielding an average inter-assay CV of 13.7 percent, which is an acceptable inter-assay CV for an ELISA assay.
Lower Limit of detection
The lower limit of detection of the assay was 5.17 AU/mL.
Effect of hematocrit
Hematocrit had a negligible effect on sample recovery, with percent recovery (relative to the level in the 50 percent hematocrit sample) ranging from 94 to 123 percent (mean, 107 percent).
Conversion equation for Diasorin values
There was a fairly good correlation between the Diasorin EBV VCA IgG values and the Diamedix values (R2 = 0.72), with a slope of 1.1 (Fig. 1C), though there was some scatter for those samples with high EBV VCA IgG levels.
Seropositivity determination
By ranking the low EBV antibody samples according to their index values, and then examining the corresponding fDBS VCA IgG titers, we found that a value of 21 U/mL resulted in zero false positives, exclusion of two individuals with equivocal levels of VCA IgG, and one false negative. Use of this conservative value may mean that a few false-negative individuals may be excluded from the analysis.
Analyte stability
The percentage recovery of EBV antibodies relative to the sample stored immediately at −80°C after drying for 4 hours was 96, 103, 106, and 88 percent after 2, 4, 8, and 14 freeze-thaw cycles, respectively.
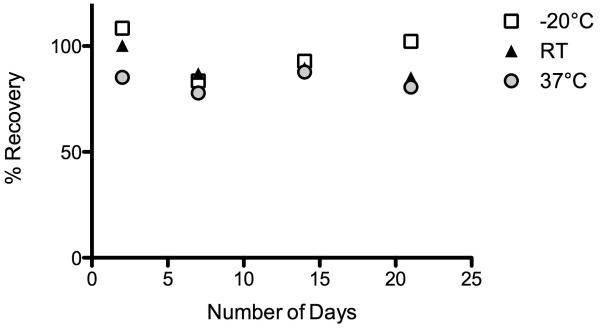
DBS cards stored at room temperature or −20°C showed no clear pattern of increase or decrease over time, whereas the EBV titer of the sample stored at 37°C was consistently lower than that of the sample stored at −80°C (Fig. 2). Number of freeze-thaw cycles and storage at temperatures higher than −80°C therefore do not appear to have a large effect on EBV antibody titer, though exposure to high temperatures (>37°C) is not recommended.
Figure 2.
Effect of different temperature storage conditions on EBV antibody titer. Data are expressed as the percent recovery relative to the titer measured for the DBS card stored at −80°C after collection and 4 hours drying at room temperature (RT). DBS cards were kept at −20°C (open squares), room temperature (RT, ~ 22°C, black triangles), or 37°C (gray circles) for 2, 7, 14, and 21 days.
Discussion
By allowing reliable indirect measurement of cell-mediated immune function, the analysis of EBV antibody titers has become a widely utilized tool for exploring the relationship between human psychosocial stress and immunity (Inoue et al. 2014; Kiecolt-Glaser et al. 1987; McClure et al. 2010; McDade et al. 2000b; Panter-Brick et al. 2008; Pierson et al. 2005; Powell et al. 2013; Sorensen et al. 2009), and has become a focus of several large-scale epidemiological studies including SAGE (Kowal et al. 2012) and ADDHealth (Slopen et al. 2013). Much of this work has arisen following initial validation of an ELISA for the quantitative assessment of EBV antibodies in fingerprick DBS (McDade et al. 2000a). As for any analyte, the analysis of EBV antibodies in DBS offers numerous advantages over the traditional collection and assessment of venous blood samples, including reduced burden to participants and increased convenience to researchers owing to simplified sample collection procedures, storage, and transportation (Li and Lee 2014; McDade et al. 2007; Mei et al. 2001). These characteristics have facilitated the recent and rapid expansion of EBV antibody assessment within new areas of research among traditionally understudied groups (e.g., children and the elderly) and within large community-based studies carried out across diverse ecological contexts (Kowal et al. 2012; Slopen et al. 2013).
The purpose of the present study was to describe and validate a commercially available ELISA assay for the quantitative measurement of EBV VCA IgG titers in DBS, providing a replacement for the original McDade EBV antibody DBS assay whose commercial components are no longer available. Through a rigorous series of validation tests, we determined that this new assay, based on modification of a commercially available kit, provides sensitive, reliable, and stable measurement of EBV IgG antibodies against the viral capsid antigen protein p18 in DBS prepared with capillary or venous blood. We note that we have evaluated assay performance in a large matched plasma-DBS sample set drawn from over 200 individuals. Using this sample size, we easily met the recommendation of The Clinical and Laboratory Standards Institute (Method Comparison and Bias Estimation using Patient Samples; Approved Guideline, 2nd edition) that at least 40 samples with analyte concentrations distributed over the analytical measurement range be included in the validation (Method Comparison and Bias Estimation using Patient Samples; Approved Guideline, 2nd edition).
Importantly, we have also demonstrated that DBS EBV antibody titers determined using the assay presented here compare closely to those obtained using the original protocol (McDade et al. 2000a), and we have provided a conversion equation to allow for the conversion of values for between-protocol comparison. However, it should be kept in mind that the discrepancy between EBV antibody titers obtained using the Diasorin kit and those obtained using our new approach tends to increase at higher concentrations. This may reflect the larger measurement range of the new assay (10.2 – 700 U/mL vs. 10 – 400 U/mL), slight differences in plate coating antigens, and/or the use of DBS samples that have been in long-term storage at −20°C for several years and exposed to multiple freeze-thaw cycles. We therefore encourage caution when interpreting these results. Furthermore, we discourage the comparison of values obtained with the present assay to those obtained using protocols other than that of McDade and colleagues (McDade et al. 2000a), particularly those measuring populations of antibodies other than VCA-p18.
Plasma EBV antibody titers were higher than matched DBS titers. This was not an unexpected finding, because DBS collection is a microsampling process. Mei and colleagues estimated that a 3.2 mm punch from a 100-μl dried blood spot contains only ~1.5 μl of serum at a hematocrit of 55 percent (Mei et al. 2001). Elution of a DBS punch in sample diluent therefore results in dilution of the analyte proportional to the volume of eluent and the initial amount of plasma in the punch. Because it is neither feasible nor practical to quantitate the exact amount of plasma in each punch, this dilution factor is unknown, in contrast to serum or plasma samples, and cannot be corrected for when comparing plasma and finger-prick values. The lower DBS values than plasma values likely indicate that EBV antibody titers in the DBS samples were diluted to a greater extent than the plasma samples. Conversion of DBS values to plasma equivalent values using the reported linear regression equation addresses this issue, but it should be kept in mind that the plasma equivalent values are more of a relative than an absolute indicator of circulating levels of EBV antibodies, given that we converted a semi-quantitative assay to a fully quantitative assay.
This study had several limitations. Most notably, we did not use a gold standard method of EBV antibody determination such as indirect immunofluorescence for comparison of values obtained through the new DBS assay. Thus, the accuracy of the assay was not fully evaluated. A second potential limitation of the present study is measurement of a specific population of IgG antibodies (i.e. IgG antibodies that recognize epitopes of the viral structural capsid antigen p18). Although multiple studies have demonstrated the value of measuring these VCA-p18 (Farber et al. 2001; McDade et al. 2000a; van Grunsven et al. 1994), this may not accurately reflect overall concentrations of diverse EBV antibodies. In addition, the absolute absorbance value of the high Mikrogen control used to make the standard curve dilution series is based on quantitation of p18, p23, and p54 EBV antibodies in human sera, whereas the Diamedix kit is specific for p18 EBV antibodies. The absolute values assigned to samples are therefore not likely to be strictly accurate, but will be entirely internally consistent in that the same EBV antibody population is being measured and compared among all samples and controls.
Furthermore, the Diamedix assay was validated by its manufacturer for serum samples rather than plasma samples; this may have introduced matrix-specific bias, but the excellent correlation between plasma and fingerprick DBS samples argues against this. Last, two separate kits have to be sourced to run this assay – a Diamedix kit, from which all reagents and the coated plate are used, and a Mikrogen kit, from which only the high positive calibrator is used.
In summary, we adapted a commercially-available ELISA assay for EBV antibody titers and validated it for use with dried blood spots. There was a strong positive correlation between DBS and plasma EBV antibody titers, and the assay showed good performance with DBS eluates. Results obtained using the approach outlined here can be compared to those obtained previously for DBS. Last, but not least, this method facilitates continued investigation of EBV antibody titers in DBS.
Acknowledgements
We would like to acknowledge the help and enthusiasm of the Eugene200 team of volunteers who made this study possible (Melissa Liebert, Theresa Gildner, Elisabeth Goldman, Lauren Moore, Zach Clayton, Tyler Barrett, Josh Schrock, Tyler Fording, Devan Compton, Brian McCree, Micaela Burns, Caroline Porter, Anna Hanson, Molly Turner, Haley Brown, Robyn Brigham, Blanche Blumenthal, Sophie McGinley, Colin Lipps, Oliver Wald) and all the Eugene200 participants for contributing samples. Julia Ridgeway-Diaz generated all the unpublished Diasorin EBV values for the Colono samples. Funding was provided by a Richard Bray Faculty Fellowship (JJS), NIH NIA Interagency Agreement YA1323-08-CN-0020, and NIH R01-AG034479.
References
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10. [PubMed] [Google Scholar]
- Cohen JI. Epstein-barr virus vaccines. Clin Transl Immunology. 2015;4(1):e32. doi: 10.1038/cti.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Tyrrell DA, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991;325(9):606–12. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- Farber I, Hinderer W, Rothe M, Lang D, Sonneborn HH, Wutzler P. Serological diagnosis of Epstein-Barr virus infection by novel ELISAs based on recombinant capsid antigens p23 and p18. J Med Virol. 2001;63(4):271–76. doi: 10.1002/1096-9071(200104)63:4<271::aid-jmv1001>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5(3):243–51. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Glaser R, Pearl DK, Kiecolt-Glaser JK, Malarkey WB. Plasma cortisol levels and reactivation of latent Epstein-Barr virus in response to examination stress. Psychoneuroendocrinology. 1994;19(8):765–72. doi: 10.1016/0306-4530(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Inoue Yosuke, Yazawa Aki, Li Dandan, Du Jianwei, Jin Yuming, Chen Yan, Watanabe Chiho, Umezaki Masahiro. Epstein-Barr virus antibody titer and its association with the domain scores from the World Health Organization's Quality of Life questionnaire: Findings from Rural Hainan Province, China. American Journal of Human Biology. 2014;26(1):51–55. doi: 10.1002/ajhb.22478. [DOI] [PubMed] [Google Scholar]
- Kemeny ME, Schedlowski M. Understanding the interaction between psychosocial stress and immune-related diseases: a stepwise progression. Brain Behav Immun. 2007;21(8):1009–18. doi: 10.1016/j.bbi.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R, Shuttleworth EC, Dyer CS, Ogrocki P, Speicher CE. Chronic stress and immunity in family caregivers of Alzheimer's disease victims. Psychosom Med. 1987;49(5):523–35. doi: 10.1097/00006842-198709000-00008. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Marucha PT, Malarkey WB, Mercado AM, Glaser R. Slowing of wound healing by psychological stress. Lancet. 1995;346(8984):1194–96. doi: 10.1016/s0140-6736(95)92899-5. [DOI] [PubMed] [Google Scholar]
- Kowal P, Chatterji S, Naidoo N, Biritwum R, Fan W, Lopez Ridaura R, Maximova T, Arokiasamy P, Phaswana-Mafuya N, Williams S, Snodgrass JJ, Minicuci N, D'Este C, Peltzer K, Boerma JT. Data resource profile: the World Health Organization Study on global AGEing and adult health (SAGE) Int J Epidemiol. 2012;41(6):1639–49. doi: 10.1093/ije/dys210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Wenkui, Lee Mike S. Dried Blood Spots: Applications and Techniques. John Wiley & Sons; 2014. [Google Scholar]
- McClure HH, Martinez CR, Snodgrass JJ, Eddy JM, Jimenez RA, Isiordia LE, McDade TW. Discrimination-related stress, blood pressure and epstein-barr virus antibodies among latin american immigrants in Oregon, us. J Biosoc Sci. 2010;42(4):433–61. doi: 10.1017/S0021932010000039. [DOI] [PubMed] [Google Scholar]
- McDade TW, Stallings JF, Angold A, Costello EJ, Burleson M, Cacioppo JT, Glaser R, Worthman CM. Epstein-Barr virus antibodies in whole blood spots: a minimally invasive method for assessing an aspect of cell-mediated immunity. Psychosom Med. 2000a;62(4):560–67. doi: 10.1097/00006842-200007000-00015. [DOI] [PubMed] [Google Scholar]
- McDade TW, Stallings JF, Worthman CM. Culture change and stress in Western Samoan youth: Methodological issues in the cross-cultural study of stress and immune function. Am J Hum Biol. 2000b;12(6):792–802. doi: 10.1002/1520-6300(200011/12)12:6<792::AID-AJHB7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- McDade TW, Williams S, Snodgrass JJ. What a drop can do: dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography. 2007;44(4):899–925. doi: 10.1353/dem.2007.0038. [DOI] [PubMed] [Google Scholar]
- Mei JV, Alexander JR, Adam BW, Hannon WH. Use of filter paper for the collection and analysis of human whole blood specimens. J Nutr. 2001;131(5):1631S–6S. doi: 10.1093/jn/131.5.1631S. [DOI] [PubMed] [Google Scholar]
- Panter-Brick C, Eggerman M, Mojadidi A, McDade TW. Social stressors, mental health, and physiological stress in an urban elite of young Afghans in Kabul. Am J Hum Biol. 2008;20(6):627–41. doi: 10.1002/ajhb.20797. [DOI] [PubMed] [Google Scholar]
- Pierson DL, Stowe RP, Phillips TM, Lugg DJ, Mehta SK. Epstein-Barr virus shedding by astronauts during space flight. Brain Behav Immun. 2005;19(3):235–42. doi: 10.1016/j.bbi.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Powell ND, Tarr AJ, Sheridan JF. Psychosocial stress and inflammation in cancer. Brain Behav Immun. 2013;30(Suppl):S41–7. doi: 10.1016/j.bbi.2012.06.015. [DOI] [PubMed] [Google Scholar]
- Sanchez JL, Cooper MJ, Myers CA, Cummings JF, Vest KG, Russell KL, Sanchez JL, Hiser MJ, Gaydos CA. Respiratory Infections in the U.S. Military: Recent Experience and Control. Clin Microbiol Rev. 2015;28(3):743–800. doi: 10.1128/CMR.00039-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, McLaughlin KA, Dunn EC, Koenen KC. Childhood adversity and cell-mediated immunity in young adulthood: does type and timing matter? Brain Behav Immun. 2013;28:63–71. doi: 10.1016/j.bbi.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen Mark V, Snodgrass James J, Leonard William R, McDade Thomas W, Tarskaya Larissa A, Ivanov Kiundiul I, Krivoshapkin Vadim G, Alekseev Vladimir P. Lifestyle incongruity, stress and immune function in indigenous Siberians: the health impacts of rapid social and economic change. American journal of physical anthropology. 2009;138(1):62–69. doi: 10.1002/ajpa.20899. [DOI] [PubMed] [Google Scholar]
- van Grunsven WM, Spaan WJ, Middeldorp JM. Localization and diagnostic application of immunodominant domains of the BFRF3-encoded Epstein-Barr virus capsid protein. J Infect Dis. 1994;170(1):13–19. doi: 10.1093/infdis/170.1.13. [DOI] [PubMed] [Google Scholar]
- Williams SR, McDade TW. The use of dried blood spot sampling in the national social life, health, and aging project. J Gerontol B Psychol Sci Soc Sci. 2009;64(Suppl 1):i131–6. doi: 10.1093/geronb/gbn022. [DOI] [PMC free article] [PubMed] [Google Scholar]