Introduction and background
The prevention of hospital acquired pressure ulcers (HAPU) has become a major focus in many institutions. Results from the national Medicare patient safety monitoring system study found that the nationwide hospital acquired pressure ulcer (HAPU) incidence rate was 4.5% with an 11.2 % in-hospital mortality rate (associated with HAPUs). 1 In addition, health care costs related to HAPUs are increasing yearly and are estimated to be between $44K to 128K per PU 2,3. Because of the disease burden, associated risks, and financial burden with HAPUs, it is imperative to implement effective prevention interventions on all hospitalized patients considered to have risk factors.
Research has identified some of the risk factors that contribute to the development of pressure ulcers. Studies suggest that 5% to 53.4% of all HAPUs are associated with prolonged or multiple surgical procedures4-7. During one study, findings suggested that after the first four hours of surgery, the risk of pressure ulcers increased by 33% for every 30 minutes of surgery 5. Surgical patients have multiple factors that contribute to the development of pressure ulcers. Landmark studies indicated that prolonged immobility, lower blood pressures, and increased surface interface pressure may hinder the blood supply delivered to the skin, eventually leading to pressure ulcers6-10. Although multiple factors contribute to the development of pressure ulcers, pressure ulcers have been found to be more prevalent in patients with prolonged high surface interface pressures over time11.
Statement of purpose
Many products exist that are designed to redistribute pressure in the surgical patient during prolonged operating room (OR) procedures. However, limited research exists which evaluates the efficacy of pressure redistribution properties of OR surfaces. The aim of this study was to measure and compare four different OR surfaces to find the most effective pressure redistribution surface for prolonged OR procedures.
Research question
How do the four OR surfaces compare in pressure redistribution properties (decrease interface pressure and increase skin contact area)?
Significance to perioperative nursing
Nurses are responsible for assessing, planning, implementing, communicating and documenting the collaboration of care in the perioperative area. Standards of care for risk assessment in the development of pressure ulcers in the perioperative period do not exist. Yet, nurses have a responsibility to know and act according to standards of care in providing a safe patient environment 12. The American Nurse Association revises the scope of practice for nurses to keep pace with technology13.However, scant research exists which examines the perioperative risk factors that contribute to the development of HAPUs in the perioperative area.
Literature review
Several types of surfaces have been developed to redistribute the interface pressure that occurs during prolonged OR procedures. The interface pressure can be redistributed statically, by molding around the patient and spreading the pressure over a larger surface, or mechanically, by alternating the pressure beneath the patient. This redistributes body weight over a larger surface area14,15.
A literature search found only three studies that tested the pressure redistribution properties of OR surfaces. Keller et al using the XSensor system analyzed the peak pressure and the skin contact area in two different positions of a standard OR mattress (3cm pad filled with polyurethane fibers), a RIK- fluid mattress, and ROHO-inflatable mattress pad, and a 7cm custom made viscoelastic polyurethane foam mattress16. The authors found the fluid-filled surface provided the best pressure-redistribution properties. Kings and Bridges, using XSensor pressure mapping system tested peak pressure in standard OR surface (3- layer pad: 2.25 inch thick slow recovery foam, cushioning foam as a middle layer and high-density foam as top layer), EGGRAGATE (high-density foam overlay) and gel pad overlay 17. This study measured an overlay foam pad and the standard gel pad on top of the standard surface, and found significantly higher interface pressure as compared to the standard surface alone. 17. Defloor and Johan, using the Ergo-check pressure mapping system, evaluated interface average pressures in four different positions on five surfaces: a) a regular 4-cm foam surface, b) a 6-cm foam surface, c) a 1.5-cm gel surface, d) a 6-cm polyether visco-elastic foam surface, and e) 7 cm polyurethane visco-elastic foam surface 18. Results showed that the polyurethane and the polyether surfaces had lower interface pressures when compared to foam and gel surfaces18.
Synthesis of the evidence from the three studies suggests that only Keller et al and Kings and Bridges studies results can be compared as they used the same pressure mapping instrument and both reported peak interface pressure. Keller at al reported that a fluid-filled operating surface provided the lowest peak interface pressure when compared to other surfaces 16 In contrast to Keller at al., King and Bridges found that standard OR surfaces provided the lowest peak interface pressure when compared to gel or foam overlays17. The differences in the study results are due to fact that studies used different surfaces as a standard OR surface (Keller at al used 3cm thick pad filled with polyurethane fibers and Kings & Bridges used 5.7cm thick pad that consisted of three different types of foam)16,17. Most importantly, Keller et al are the only investigators who measured the skin contact area.16
In addition, much of the literature available on pressure mapping of OR surfaces does not include newer technology surfaces such as the fluid immersion simulation surfaces or the air static overlay. In order to reduce the incidence HAPUs that occur during prolonged OR procedures, it is imperative that operating rooms are equipped with the most effective pressure redistribution surfaces and that perioperative nurses be confident that the product they are using provides the greatest pressure ulcer prevention properties.
Conceptual framework
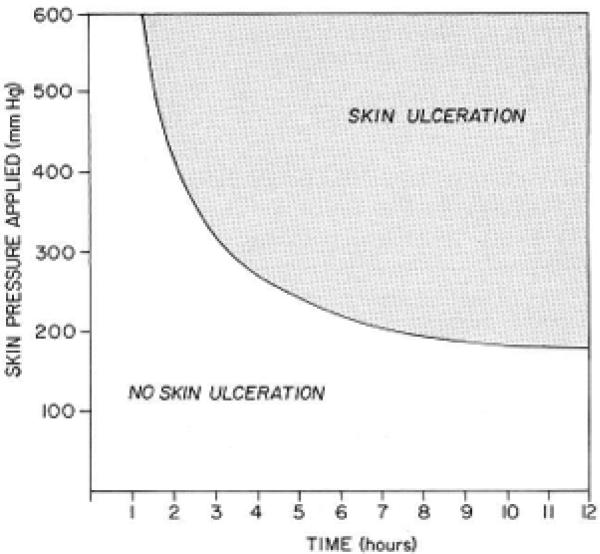
This study was based on a model derived from a classic study conducted by Kosiak 19 and a conceptual schema for the study of the etiology of pressure sores formulated by Braden and Bergstom 20. Kosiak evaluated the interface pressure on skin over time. The model derived from Kosiak's study explained how external pressure in a specific area could lead to vaso-occlusion, resulting in decreased tissue perfusion and possible ischemic injury to both deep and superficial tissues. Kosiak conducted further studies investigating pressure over time and found that the greater the external pressure, the less time is needed for ischemic injury to occur.
Kosiak described a cutoff of 32 millimeters of mercury (mm/Hg) as a guideline for measuring surface interface pressure. While this number is not always predictive of actual perfusion, the interface pressure of 32mm Hg or less is regarded as a useful guideline in determining efficacy of a product in redistributing the interface pressure thus lowering the risk of pressure ulcer development21,22.
Within the Braden and Bergston schema, exposure of the skin to high interface pressures for prolonged periods will indeed lead to tissue damage. At the same time, exposure of skin to low interface pressures for prolonged periods of time may also lead to tissue damage if the tissue tolerance is compromised 20. Therefore, tissue damage that occurs during prolonged OR procedures can be minimized by decreasing the interface pressure.
Operational definitions
Interface pressure-the pressure load between the skin and the support surface
Peak interface pressure-the highest pressure load between the skin and the support surface
Average interface pressure-the average pressure load between the skin and the support surface of a full body or the specific area calculated by XSensor pressure mapping device
Skin contact area-the total contact area between the skin and the support surface
Pressure redistribution-pressure relief to a small concentrated area and the distribution of it over a larger area.
Methods
The design of this study is a descriptive comparative quantitative study that utilized a repeated measures design, whereby the participants served as their own controls. A sample size calculation was conducted prior to recruiting volunteers and yielded a required sample size of 49. Sample size calculations were performed using the SAS® software procedure POWER and were based on the paired t test as an approximation to the repeated measures ANOVA. We used preliminary data from five subjects to estimate both the expected differences between mattress types and the standard deviations of the differences. We powered based on the highest standard deviation of 6.5, to ensure adequate power under the worst-case scenario. Because of the high number of comparisons, we used the Bonferroni correction for multiple comparisons to correct the global type I error at 0.05. We powered at the 90% level.
A convenience sample of eleven men and forty women (n=51) participated in the study. Study participants were recruited from hospital staff with various body mass indexes. Eligibility criteria included volunteers who a) work at the hospital, b) agreed to self-report height and weight, c) had 30 minutes to participate, and, d) agreed to have the pressure mapping performed on four different surfaces while lying flat for over seven minutes per surface. The study was approved by hospital Institutional Review Board.
We tested the following OR table surfaces: a) a standard three layers visco-elastic memory foam surgical table surface, b) an air-inflated static seat cushion that was used under the sacral area placed over standard surgical table surface, c) a two layers OR surface consisting of a top layer of non-powered self-contouring copolymer gel and the bottom of high density foam, and d) a fluid immersion simulation surgical surface.
To evaluate pressure redistribution properties of OR surfaces, we used full-body interface pressure testing. This method has been found to be valid and reliable in measuring interface pressure 23. The instrument used for measuring the interface pressure for this study was the XSENSOR X3 PX100 system (XSENSOR Technology Corporation; Calgary, Canada). Pressure-mapping systems are composed of a pressure-sensing device that sends data to a computer program. The data are displayed as, a color-coded map, a three-dimensional grid, and a numerical pressure value for each area. Numerical pressure values are typically expressed in millimeters of mercury (mmHg) and reflect the pressure between the body and the surface used 24. The XSENSOR X3 PX 100 system consists of thin, 99.06cm×220.98cm full body pressure-mapping pad with 1664 sensing points. The sensors in the pad have 3.175 spatial resolutions. The pad was placed between the volunteer and the support surface and connected to the XSENSOR X3 display for real-time pressure mapping recording.
All participants were instructed to lie flat on the surface for five minutes before XSENSOR measurements were collected. During the pilot study, no difference was found in the interface pressure readings that were recorded between three minutes and 30 minutes for acclimation. However, five minutes was used as an acclimation period as was recommended in a previous study 25. The data were displayed on the screen for a minimum of 1200 frames per participant, and were recorded on each surface. The XSENSOR data collected was then downloaded into a computer using the X3-medical V6.0 software. The peak pressures and surface contact areas recorded were then transcribed into excel spreadsheets by two investigators and all measurements were validated by two investigators.
A repeated measures ANOVA was fitted to test for differences in average sacral pressure, peak sacral pressure, and sacral surface area between the four surfaces using the SAS® software procedure GLM. Pair-wise paired t tests were then performed to test for significant differences between each surface pair using the SAS® software procedure TTEST with the paired option. We used the Bonferroni correction for multiple comparisons. A p-value of 0.05 was considered statistically significant for the omnibus tests. For the pair-wise comparisons, the Bonferroni correction indicated that a p-value of 0.008 should be used for each comparison to control the global type 1 error at 0.05. Distributional assumptions were validated using residual diagnostic plots to assess normality.
Results
Residual diagnostics plots revealed no skew or irregularity in the distribution of the residuals, indicating that the normal assumption was validated. All ANOVAs were significant at the 0.05 level (sacral average pressure p-value = 0.0004, sacral peak pressure and sacral area p-value <0.0001 and sacral area skin contact area p-value <00001). The ANOVAs results are shown in Table 1.
Table 1.
Surface comparison summary
| Surface Type | |||||
|---|---|---|---|---|---|
| Mean (std dev) | |||||
| Variable | Standard | Air-inflated static seat cushion | Self-contouring gel/foam | Fluid immersion simulation | p-value |
| Sacral area average interface pressure (mmHg) | 23.6 (3.2) | 23.9 (3.1) | 23.4 (4.2) | 22.1 (2.0) | 0.0004* |
| Sacral area peak interface interface pressure (mmHg) | 43.4 (7.3) | 35.8 (4.4) | 40.6 (6.0) | 35.9 (4.7) | <0.0001* |
| Sacral area skin contact area (in2) | 214.5 (52.9) | 250.2 (47.8) | 225.4 (50.1) | 213.7 (47.9) | <0.0001* |
A P-value <0.05 is considered significant and is represented by a *
As depicted, the average sacral interface pressure between the surfaces was statistically significant with the air-inflated static seat cushion having the highest measured average pressure (23.9 mmHg) and the fluid immersion simulation surgical surface having the lowest average sacral pressure (22.1 mmHg). The peak sacral interface differences were also found to be significant. The sacral peak pressure was lowest in the air-inflated static seat cushion (35.8mmHg). When comparing the skin contact area, it was found that the air-inflated static seat cushion had statistically significant results for the greatest skin contact area (250.2 cm2). These results suggest that the sacral interface pressure is better distributed with the use of the air-inflated static seat cushion than any of the other surface types used in this study.
The sacral average interface pressures were pair-wise compared. As depicted in Table 2, the air-inflated static seat cushion and the fluid immersion simulation surgical surface where statistically significantly different. Table 3 shows the results for the sacral peak interface pressure pair-wise comparisons where p-value of < 0.08 is regarded as significant. There is statistical significance among all surfaces, except the fluid immersion simulation surgical surface and the air-inflated static seat cushion, which are not significantly different from each other with the measured difference of −0.09 mm Hg between the surfaces (p=0.9). The test showed there is no difference in sacral peak pressures between the fluid immersion simulation surgical surface and the air-inflated static seat cushion.
Table 2.
Sacral average interface pressure pair-wise comparisons
| Comparison | Difference (mm Hg) | p-value | 95% Confidence Interval |
|---|---|---|---|
| Regular - Air-inflated static seat cushion | −0.3 | 0.4 | (−0.9, 0.3) |
| Regular – Self-contouring gel/foam | 0.2 | 0.5 | (−0.3, 0.6) |
| Regular – Fluid immersion simulation | 1.5 | 0.006* | (0.4, 2.5) |
| Air-inflated static seat cushion – Self-contouring gel/foam | 0.5 | 0.3 | (−0.4, 1.4) |
| Air-inflated static seat cushion – Fluid immersion simulation | 1.8 | <0.0001* | (0.9, 2.6) |
| Self-contouring gel/foam – Fluid immersion simulation | 1.3 | 0.05 | (0.02, 2.6) |
A p value <0.008 is considered significant and is represented by a *
Table 3.
Sacral peak interface pressure pair-wise comparisons
| Comparison | Difference (mm Hg) | p-value | 95% Confidence Interval |
|---|---|---|---|
| Regular - Air-inflated static seat cushion | 7.56 | <0.0001* | (5.7, 9.4) |
| Regular – Self-contouring gel/foam | 2.78 | 0.0011* | (1.2, 4.4) |
| Regular – Fluid immersion simulation | 7.46 | <0.0001* | (5.3,9.7) |
| Air-inflated static seat cushion – Self-contouring gel/foam | −4.77 | <0.0001* | (−6.3, −3.2) |
| Air-inflated static seat cushion – Fluid immersion simulation | −0.09 | 0.9 | (−1.3, 6.3) |
| Self-contouring gel/foam – Fluid immersion simulation | 4.68 | <0.0001* | (3.0,6.3) |
A p value <0.008 is considered significant and is represented by a *
The results for the sacral skin contact area pair-wise comparisons are summarized in Table 4. The sacral contact area pair-wise comparisons found that the difference between comparisons was the greatest in the air-inflated static seat cushion and the fluid immersion simulation surgical surface with contact area at 36.6 (95% CI 25.9- 47.2).
Table 4.
Sacral skin contact area pair-wise comparisons
| Comparison | Difference (in^2) | p-value | 95% Confidence Interval |
|---|---|---|---|
| Regular - Air-inflated static seat cushion | −35.7 | <0.0001* | (−43.2, −28.2) |
| Regular – Self-contouring gel/foam | −10.9 | <0.0001* | (15.7879, −5.9) |
| Regular – Fluid immersion simulation | 0.9 | 0.9 | (−7.6, 9.3) |
| Air-inflated static seat cushion – Self-contouring gel/foam | 24.8 | <0.0001* | (17.1, 32.6) |
| Air-inflated static seat cushion – Fluid immersion simulation | 36.6 | <0.0001* | (25,9, 47.2) |
| Self-contouring gel/foam – Fluid immersion simulation | 11.7 | 0.003 | (4.3, 19.2) |
A p value <0.008 is considered significant and is represented by a *
The ANOVA results for the sacral average interface pressure indicate that the fluid immersion simulation surgical surface provides the lowest average pressure (22.1 mmHg).There is strong evidence to support that among the surfaces measured in this study, the fluid immersion simulation surgical surface and the air-inflated static seat cushion have the lowest sacral peak interface pressure (the air-inflated static seat cushion =35.8 mmHg, the fluid immersion simulation surgical surface = 35.9). The peak interface pressure pair-wise comparison indicated no statistical significance between the air-inflated static seat cushion and the fluid immersion simulation surgical surface measurements.
In relation to surface contact area, the ANOVA provides statistically significant results indicating the air-inflated static seat cushion as the surface that provides the largest skin contact area (250.21 in2) over the sacrum. Moreover, the pair-wise comparisons test show that there is a statistically significant difference between the air-inflated static seat cushion and all surfaces measured in this study. The air-inflated static seat cushion outperformed all surfaces in increasing the measured skin contact area of the sacral region (difference ranging 24.8 −36.6 in 2) as measured by the XSensor device.
Discussion
While many OR surfaces are available on the market, the best perioperative positioning techniques cannot protect the patient from tissue damage if the products used do not provide pressure redistribution. When identifying the concept of pressure and how it contributes to the development of pressure ulcers, several measurements of pressure contribute to pressure redistribution: the average pressure, the peak pressure, and the skin contact area 19,20. Therefore, the best surface attributes that provide efficient pressure redistribution should have the following properties, the lowest average interface pressure, the lowest peak interface pressure, and the highest skin contact area.
From previous studies of OR surfaces for pressure ulcer prevention properties, only Keller at al reported both peak interface pressure and surface contact area. The study indicated that a fluid-filled mattress had the lowest interface peak pressure (68 mmHg), air inflated had the second lowest (75 mmHg), polyurethane foam mattress had the third lowest (112 mmHg) and a standard foam mattress had the highest peak interface pressure (181 mmHg). When skin contact area was measured, it was found that the fluid-filled mattress had the largest skin contact area (5226 cm2) the polyurethane foam mattress had the second largest skin contact area (5067 cm2), the air inflated mattress had the third largest skin contact area (4391 cm2) and the standard foam surface had the lowest measured skin contact area (4249 cm2) 16. However, within the Keller at al study, air in the air-filled mattress was not adjusted for each participant as is indicated by the manufacture. Therefore, this result cannot be regarded as accurate.
The outcomes from our study show that the fluid immersion simulation surgical surface outperformed all tested surfaces in providing the lowest average interface pressure in the sacral area. However, these results are not clinically relevant as all surfaces had average interface pressures below 32mmHg and the differences between all the average interface pressures is less than 2 mmHg. In addition, results of the pair-wise comparison show a statistical significance between only the air-inflated static seat cushion and the fluid immersion simulation surgical surface. Therefore, no surface can be identified as the best surface in providing lowest average interface pressures.
In relation to the peak interface pressure, we found that both the fluid immersion simulation surgical surface and the air-inflated static seat cushion provided lower measured peak interface pressures in the sacral area. The pair-wise comparison test indicated no difference between the air-inflated static seat cushion and the fluid immersion simulation surgical surface in providing the lowest measured interface peak pressure. Consequently, the fluid immersion simulation surgical surface and the air-inflated static seat cushion are equally identified as the best surfaces for providing low peak interface pressures in the sacral area. However, the low peak interface pressure alone does not provide the full pressure redistribution properties. A surface with the properties of a lower measured peak interface pressure, while increasing the area of skin contact over a larger surface area, is required for minimizing risk for pressure ulcer development.
The air-inflated static seat cushion provided the combined properties of lower peak interface pressure with redistributing the pressure, by increasing the area of skin contact over a larger surface area, thus reducing the concentration of pressure over the sacrum area. The results from our study identified the air-inflated static seat cushion as having better pressure redistribution properties in the sacral region as compared to the other surfaces tested. Although the manufacturer of the air-inflated static seat cushion produces a full-body OR air-inflated static overlay, the seat cushion was used under the sacrum for this study. The air-inflated products are not radiolucent, therefore, not always practical for use in procedures which require x-rays or fluoroscopy of the pelvis. At the same time, the air-inflated static seat cushion can be easily removed for X-rays or fluoroscopy and is easy and economical for use in the OR for sacral pressure ulcer prevention.
Limitations of the study
One limitation of this study was that all of the participants were healthy volunteers. The average pressure, peak pressure and skin contact area may be different when a person is fully sedated, has a history of low blood pressure or is hemo-dynamically unstable. The full body pressure mapping device used in this study was used to measure pressures and contact areas, however, only the sacral area was evaluated for this study. In addition, all the surfaces we tested were from different manufactures. Pressure redistribution properties of the same type of surface might vary from manufacture to manufacture.
Recommendation for clinical practice
Perioperative nurses are responsible for maintaining patient safety, selecting effective protective equipment, and minimizing HAPUs. The best perioperative positioning techniques cannot protect a patient from tissue damage if the products used to redistribute pressure in the surgical patient during prolonged OR procedures are inadequate. Pressure over time is a concept that nurses can understand and help to alleviate with the use of pressure redistribution surfaces. Perioperative nurses should have a voice regarding what is needed and purchased for use in the OR for the best patient safety outcomes to decrease HAPU. Perioperative nurses already advocate for patients. This advocacy improves safety outcomes, minimizes the likelihood of litigation related to HAPUs, and optimizes Medicare and Medicaid reimbursement. After completion of this study, perioperative nurses working at this institution are promoting practice changes by using the air-inflated static seat cushion in combination with the recommended five-layered soft silicone bordered dressings to the buttocks and sacrum26 prior to prolonged OR procedures.
Recommendation for education
The results of the study indicate the need for perioperative nursing education about the most current information related to pressure ulcer prevention. Perioperative nurses need to understand that attention to perioperative positioning techniques alone cannot protect the patient from tissue damage if the products used lack efficacy. The findings from the study demonstrate that there are differences among products and perioperative nurses should be educated about positioning devices that provide both stability and pressure redistribution. Through knowledge and implementation of the most current methods available, perioperative nurses can help reduce HAPUs.
Recommendation for future research
Because a wide range of body positions (lateral, supine, prone, jackknife, lithotomy) are used in the OR, additional studies are needed which evaluate pressure redistribution products for each position. Additional research is needed on patients who have low blood pressure, are immobile, have low hemoglobin or low serum albumin, are undergoing long surgeries, are hemodynamically unstable, and have known risk factors. Future studies should evaluate the same type of surfaces from different manufactures as pressure redistribution properties might vary from manufacture to manufacture. To develop a body of literature capable of sustaining evidence based practice, it is important to build on existing research, to use consistent methodologies for evaluating pressure redistribution surfaces, and to continually compare and contrast newly developed products. Randomized trials are needed to ensure that results are representative of a broad spectrum of patients that are cared for by perioperative nurses. Studies that include cost effectiveness analysis and studies that quantify the loss of reimbursement associated with HAPUs are also needed.
Conclusions
It is important to identify OR surfaces that provide the lowest peak pressure and the largest skin contact area in order to minimize the risk of pressure ulcer development during prolonged OR procedures. All of the surfaces measured in this study had peak interface pressures higher than 32 mmHg. Since all of the surfaces measured have high peak interface pressures, skin contact area was used for further identification of the surface for pressure redistribution properties. The skin contact over a larger area reduces the concentrated pressure at the sacrum. Only one of the tested surfaces significantly increased skin contact area in the sacral area. Further studies should be conducted to identify the best OR surfaces available for pressure redistribution and preventing pressure ulcers which occur during prolonged OR procedures.
Figure 1.
Kosiak's Model: the relationship between applied pressure and time for pressure ulcer development
Acknowledgements
The project described was supported in part by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH), through grant #UL1 TR000002 and the University of California Davis Medical Center, Center for Professional Practice of Nursing Research Mentorship Program.
Biography
Holly Kirkland-Walsh, MSN, FNP, CWCN, is a wound care team director for UC Davis Medical Center, Sacramento, CA. Ms. Kirkland-Walsh has no declared affiliation that could be perceived as posing a potential conflict of interest in the publication of this article.
Oleg Teleten, MS, RN, CWCN, is a clinical resource nurse and wound care team quality improvement champion for the UC Davis Medical Center, Sacramento, CA. Mr. Teleten has no declared affiliation that could be perceived as posing a potential conflict of interest in the publication of this article.
Machelle Wilson, PhD, is a senior statistician for the Clinical and Translational Science Center, Department of Public Health Sciences, Division of Biostatistics at the University of California, Davis, Sacramento, CA. Dr. Wilson has no declared affiliation that could be perceived as posing a potential conflict of interest in the publication of this article.
Bonnie Raingruber, PhD, RN, is in the nursing research department at the UC Davis Medical Center, Sacramento, CA. Dr. Raingruber has no declared affiliation that could be perceived as posing a potential conflict of interest in the publication of this article.
References
- 1.Lyder CH, Wang Y, Metersky M, et al. Hospital-acquired pressure ulcers: results from the national Medicare Patient Safety Monitoring System study. Journal of the American Geriatrics Society. 2012 Sep;60(9):1603–1608. doi: 10.1111/j.1532-5415.2012.04106.x. [DOI] [PubMed] [Google Scholar]
- 2.Chan B, Ieraci L, Mitsakakis N, Pham B, Krahn M. Net costs of hospital-acquired and pre-admission PUs among older people hospitalised in Ontario. J Wound Care. 2013 Jul;22(7):341–342, 344-346. doi: 10.12968/jowc.2013.22.7.341. [DOI] [PubMed] [Google Scholar]
- 3.Brem H, Maggi J, Nierman D, et al. High cost of stage IV pressure ulcers. Am J Surg. 2010 Oct;200(4):473–477. doi: 10.1016/j.amjsurg.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganos D, Siddiqui A. Pressure ulcers: prevalence, incidence and implications for the future. NPUAP; Washington: 2013. Pressure ulcer in the operating room. pp. 57–63. [Google Scholar]
- 5.Tschannen D, Bates O, Talsma A, Guo Y. Patient-specific and surgical characteristics in the development of pressure ulcers. Am J Crit Care. 2012 Mar;21(2):116–125. doi: 10.4037/ajcc2012716. [DOI] [PubMed] [Google Scholar]
- 6.Schoonhoven L, Defloor T, Grypdonck MH. Incidence of pressure ulcers due to surgery. J Clin Nurs. 2002 Jul;11(4):479–487. doi: 10.1046/j.1365-2702.2002.00621.x. [DOI] [PubMed] [Google Scholar]
- 7.Fu Shaw L, Chang P-C, Lee J-F, Kung H-Y, Tung T-H. Incidence and Predicted Risk Factors of Pressure Ulcers in Surgical Patients: Experience at a Medical Center in Taipei, Taiwan. BioMed Research International. 2014;2014:9. doi: 10.1155/2014/416896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kemp MG, Keithley JK, Smith DW, Morreale B. Factors that contribute to pressure sores in surgical patients. Res Nurs Health. 1990 Oct;13(5):293–301. doi: 10.1002/nur.4770130505. [DOI] [PubMed] [Google Scholar]
- 9.Bliss M, Simini B. When are the seeds of postoperative pressure sores sown?. Often during surgery. Bmj. 1999 Oct 2;319(7214):863–864. doi: 10.1136/bmj.319.7214.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aronovitch SA. Intraoperatively acquired pressure ulcer prevalence: a national study. J Wound Ostomy Continence Nurs. 1999 May;26(3):130–136. doi: 10.1016/s1071-5754(99)90030-x. [DOI] [PubMed] [Google Scholar]
- 11.Reswick J, Rogers J. Experience at Rancho Los Amigos Hospital with devices and techniques to prevent pressure sores. In: Kenedi R, Coweden J,JS, editors. Bedsore biomechanics. Macmillan; London: 1976. pp. 301–310. [Google Scholar]
- 12.American Nurse Association . Code of Ethics for Nurses With Interpretive Statement. Washington, DC: 2001. Nursesbooks.org. [Google Scholar]
- 13.American Nurses Association . Nursing: Scope and standards of practice. Silver Spring; MD: 2010. Nursesbooks.org. [Google Scholar]
- 14.McInnes E, Jammali-Blasi A, Bell-Syer SE, Dumville JC, Cullum N. Support surfaces for pressure ulcer prevention. Cochrane Database Syst Rev. 2011;(4):Cd001735. doi: 10.1002/14651858.CD001735.pub4. [DOI] [PubMed] [Google Scholar]
- 15.Matsuo J, Sugama J, Sanada H, et al. Development and validity of a new model for assessing pressure redistribution properties of support surfaces. J Tissue Viability. 2011 May;20(2):55–66. doi: 10.1016/j.jtv.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Keller BP, van Overbeeke J, van der Werken C. Interface pressure measurement during surgery: a comparison of four operating table surfaces. J Wound Care. 2006 Jan;15(1):5–9. doi: 10.12968/jowc.2006.15.1.26858. [DOI] [PubMed] [Google Scholar]
- 17.King C, Bridges E. Comparison of Pressure Relief Properties of Operating Room Surfaces. Perioperative Nursing Clinics. 2006;1:261–265. [Google Scholar]
- 18.Defloor T, De Schuijmer JD. Preventing pressure ulcers: an evaluation of four operating-table mattresses. Appl Nurs Res. 2000 Aug;13(3):134–141. doi: 10.1053/apnr.2000.7653. [DOI] [PubMed] [Google Scholar]
- 19.Kosiak M. Etiology and pathology of ischemic ulcers. Arch Phys Med Rehabil. 1959 Feb;40(2):62–69. [PubMed] [Google Scholar]
- 20.Braden B, Bergstrom N. A conceptual schema for the study of the etiology of pressure sores. Rehabil Nurs. 1987 Jan-Feb;12(1):8–12. doi: 10.1002/j.2048-7940.1987.tb00541.x. [DOI] [PubMed] [Google Scholar]
- 21.Hanson DS, Langemo D, Anderson J, Thompson P, Hunter S. Can pressure mapping prevent ulcers? Nursing. 2009 Jun;39(6):50–51. doi: 10.1097/01.NURSE.0000352337.67771.e0. [DOI] [PubMed] [Google Scholar]
- 22.Krouskop TA, Garber SL. Interface pressure measurements. J Enterostomal Ther. 1990 Jul-Aug;17(4):182. [PubMed] [Google Scholar]
- 23.Shelton F, Barnett R, Meyer E. Full-body interface pressure testing as a method for performance evaluation of clinical support surfaces. Appl Ergon. 1998 Dec;29(6):491–497. doi: 10.1016/s0003-6870(97)00069-0. [DOI] [PubMed] [Google Scholar]
- 24.Stinson MD, Porter-Armstrong AP, Eakin PA. Pressure mapping systems: reliability of pressure map interpretation. Clin Rehabil. 2003 Aug;17(5):504–511. doi: 10.1191/0269215503cr643oa. [DOI] [PubMed] [Google Scholar]
- 25.Yuen HK, Garrett D. Comparison of three wheelchair cushions for effectiveness of pressure relief. Am J Occup Ther. 2001 Jul-Aug;55(4):470–475. doi: 10.5014/ajot.55.4.470. [DOI] [PubMed] [Google Scholar]
- 26.Black J, Clark M, Dealey C, et al. Dressings as an adjunct to pressure ulcer prevention: consensus panel recommendations. Int Wound J. 2014 Mar 3; doi: 10.1111/iwj.12197. [DOI] [PMC free article] [PubMed] [Google Scholar]