Introduction
The human prostate is a walnut sized organ at the base of the urinary bladder. It is the seat of three major causes of morbidity; benign prostatic hyperplasia (BPH), prostate cancer and prostatitis. As such it commands more attention than might be expected from an organ of this size. Anatomical illustrations of the prostate have been published dating at least as far back as the mid-16th century when Andreas Vesalius, in 1543, published his observations of the male accessory glands. 1 The links between testicular and prostatic function have also been known for hundreds of years. John Hunter, writing in 1786 in "Observations on the glands situated between the rectum and the bladder, called vesiculae seminales" said "the prostate and Cowper's glands and those of the urethra which in the perfect male are soft and bulky with a secretion salty to the taste, in the castrated animal are small, flabby, tough and ligermentous and have little secretion". 2
The adult prostate is a compound tubular-alveolar gland found in most mammals. 3 The gross structure differs considerably between species. Much of the descriptive work on the development of the prostate from its origins in the hindgut to descriptions of the adult organ was performed by anatomists and pathologists working in the early to mid-20th century. Subsequent work has outlined the molecular basis for these descriptions. Interest in prostate biology is centered around the human organ and that of the species, notably rats and mice, used to model human diseases. A clear understanding of the differences in the structure of human and rodent prostates is important in assessing the results of animal studies.
Human and rodent prostate embryology and postnatal development
The early mammalian embryo has the potential to develop towards a male or female phenotype. In genetically normal individuals this course is determined at conception and is reflected at the embryonic stage by the interactions of four critical units: the Wolffian and Müllerian ducts, the urogenital sinus (UGS) and the fetal gonad.
In humans the Wolffian ducts start to develop approximately 25-30 days after conception in 2-3mm long embryos. These ducts initially act as excretory canals for the mesonephros, which performs the renal function in the early embryo. The ducts do not become incorporated into the genital system until the excretory function has been taken over by the definitive kidney. The ureters are a diverticulm of the Wolffian duct which becomes separated from the genital tract structures during development and which is the only part of the Wolffian duct derived structures which is preserved in the adult female. In species such as birds and reptiles, where the mesonephros has a prolonged excretory function, the Wolffian ducts are preserved in an ambisexual state, in some cases until birth. In the human, by the time the embryo has reached 4-5mm the ducts have elongated and lumenized to link the hindgut (which caudally becomes the cloaca) with the mesonephros and gonad.
The Müllerian ducts develop later than the Wolffian ducts, at about 6 weeks of gestation. A cleft lined with epithelial cells is formed between the gonadal and mesonephric parts of the urogenital ridge. This closes to form a tube which then extends through the surrounding mesenchyme parallel to the Wolffian ducts. By the eighth week of gestation the Müllerian ducts, which by this time are between the Wolffian ducts, reach (but do not break into) UGS forming the Müllerian tubercule.
The UGS is produced in the 7-9mm embryo by the formation of the uro-rectal septum which divides the cloaca into the rectum and the UGS. The upper part of the UGS forms the urethra, while the part below the Müllerian tubercule forms part of the vagina in the female and the penile urethra in the male.
The process of male sexual differentiation is determined under the influence of androgens produced by the fetal testis. In the absence of either these hormones or appropriate receptors, due to either an absent testis, lack of testicular function, or a mutation in the androgen receptor gene, the fetus will develop a female phenotype. In the male, sexual differentiation is an asymmetric process consisting of the regression of the Müllerian duct system, under the influence of Anti-Müllerian hormone expressed in the testicular Sertoli cells and stabilization, by androgens, of the Wolffian ducts.
The second part of male sexual differentiation occurs under the influence of testosterone produced by the Leydig cells of the fetal testis. This involves changes in the tubules connecting the testis with the mesonephros to form the vasa efferentia, the formation of the convoluted epididymal duct and the vas deferens. The androgenic stimulus also acts to masculinize the UGS and the external genitalia. This process involves the formation of the prostate and the prostatic utricle, the closure of the labial-scrotal lobes and the formation of the penis.
The rudimentary prostate starts to appear in 50mm human embryos as epithelial buds growing laterally from the walls of the UGS at the site of the Müllerian tubercle. Under local mesenchymal control, the buds form solid branching cords which start to develop a lumen giving rise, by birth, to a network of tubules and alveoli. As the lumen forms, some of the apical cells become structurally polarized and appear to start some secretory activity. The organ develops a stroma containing a large proportion of smooth muscle while the ducts and acini are lined with a layer of flat basal epithelium and a luminal layer of tall columnar secretory epithelium. 4 The basal and luminal epithelial cells are distinguishable on the basis not only of morphology but also functionally and by their expression of different cytokeratin classes (keratins 5 and 14 in basal cells, 8 and 18 in luminal). 5
Details of prostatic development, in particular molecular details have been largely established using animal models, in particular the rat and mouse. The availability of tissues from these animals and, more recently, the development of transgenic and gene knock-out models makes them amenable to such studies. Historically, a number of workers in the field, notably including Dorothy Price, established the basic developmental profile of the rodent prostate. 3,6,7 Rodent prostatic embryogenesis mirrors the processes seen in humans, although the timing reflects the much faster development of these species; for example, an UGS is present in the mouse by embryonic day 16 and in the rat at embryonic day 18 with early prostatic buds being seen a day or so later. Richly illustrated descriptions of the gross 8 and molecular 9 phenotypes of the developing rodent urogenital tract have been published recently which vastly expand the details available in the historic documents. The GenitoUrinary Development Molecular Anatomy Project (GUDMAP) consortium maintains an updated database of gene expression at its website: http://www.gudmap.org
Growth and development of the prostate begins with formation of prostatic buds from the fetal UGS and are complete at sexual maturity. 10 In the mouse this begins at 17 days gestation 11,12, at 19 days in the rat 6 and approximately at 10 weeks in the human fetus. 13,14 The initial event in morphogenesis of the prostate is the outgrowth of solid epithelial buds from the urogenital sinus epithelium (UGE) into the surrounding urogenital sinus mesenchyme (UGM). 10 The prostatic buds proliferate under the influence of testicular androgens to form solid cords of epithelial cells which grow into the UGM in a particular spatial arrangement to establish the lobar divisions of the prostate. 11,12,14,15 At birth in rodents the prostate is small with a limited number of undeveloped buds, postnatally these cells proliferate, predominantly at the tips 16, and undergo a process of canalization in a proximal to distal direction (from the urethra towards the tips). Concurrent with this, the epithelial cells differentiate to luminal and basal phenotypes. 17 The prostatic basal cells, at least in rodents, are complex structures with processes that wrap around the ducts; this phenotype is not obvious from traditional histologic sections. 18,19 Concurrent with epithelial differentiation, the UGM proliferates and differentiates into interfasicular fibroblasts and prostatic smooth muscle. 20 Postnatally, under the influence of androgens, the epithelial cells undergo differentiation, including the expression of androgen receptors, and begin to synthesize a variety of lobe- and species-specific secretory products. 21
In the mouse the majority of prostatic branch points develop before 15 days of age 22 and most of the growth and development of the prostate is complete by 60 days of age. 23 In contrast, the human prostate does not grow significantly between birth and puberty, when growth commences in response to rising androgen levels. The prostate then slowly increases in size over several years. It should be noted that, while androgens drive the development and growth of the prostate, they also play a key role in maintaining a growth quiescent adult organ. It is noteworthy that young adult males, in whom androgen levels are at their lifetime peak, do not suffer from prostatic enlargement or cancer, rather these are diseases associated with aging and a decrease in serum androgen titers.
Anatomy of the human and rodent prostate
In 1912, Lowsley 14 utilized serial sections as anatomical models to describe the lobes of the fetal human prostate in an attempt to clarify the origin of the middle and posterior lobes as described by earlier investigators. 25 Using tissue from a 3-month gestation fetus, Lowsley identified five separate groups of prostatic ducts originating from the UGS and used the term lobes to describe them. These were designated the middle lobe, two lateral, posterior, and ventral lobes. Lowsley described the ventral lobe as being formed by the glands arising from the anterior or ventral wall of the prostatic urethra and consisting of four pairs of epithelial buds. The middle lobe was formed by roughly twelve tubules associated with the posterior urethra and was situated between the bladder and the ejaculatory ducts under the floor of the urethra. The paired left and right lateral tubules, the largest group of tubules, originated from the sides of the urethra and followed the prostatic furrows. The tubules grew laterally and posteriorly, were distal to the ejaculatory ducts, and located on the caudal portion of the urethra, giving rise to the posterior lobe. Although their direction of growth was predominantly toward the bladder, a small number of ducts followed the anterior course of growth as seen in the lateral lobe.
Lowsley’s work started a debate over the nomenclature used to describe the prostate anatomy that continued for 70 years or so. In the adult human the lobes that he described are fused and cannot be separated or defined by dissection, giving rise to a number of different views on the anatomic division of the human prostate. 24,26-28 The situation is further confused by the fact that, in most other animals, including some other primates, the various prostatic lobes are separable in varying degrees on an anatomical, histological and physiological basis.
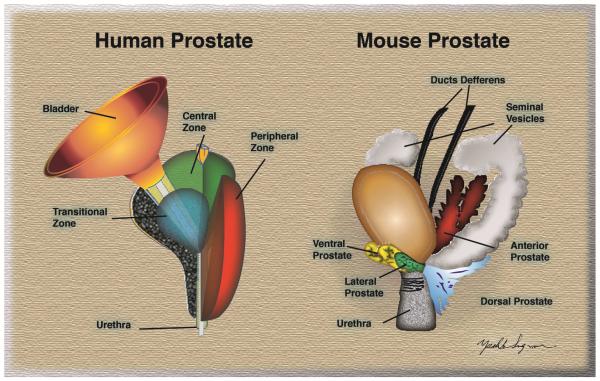
The nomenclature that is now most commonly used to describe the structure of the human prostate is that of John McNeal. 24 McNeal divided the prostate into three major areas that are histologically distinct and anatomically separate (Figure 1). These areas are, the non-glandular fibromuscular stroma that surrounds the organ and the two glandular regions termed peripheral and central zones which contain a complex, yet histologically distinct ductal system. The central zone was described as a wedge of glandular tissue which constitutes most of the base of the prostate and surrounds the ejaculatory ducts. The peripheral zone made up the remainder of the gland. It surrounded most of the central zone and extended caudally to partially surround the distal portion of the urethra. McNeal’s classification of the central zone included the middle lobe and part of the posterior lobe described in Lowsley’s earlier studies, while the peripheral zone included Lowsley’s lateral lobes and a portion of the posterior lobe. McNeal also identified an additional, smaller, glandular region which surrounded the prostatic urethra, referred to as the transition zone.
Figure 1. Structure of human and mouse prostate.
Left. Diagram of an adult human prostate showing the urethra and bladder in relation to the three major glandular regions of the prostate as described by McNeal. 24 Central zone (CZ), peripheral zone (PZ), and transitional zone (TZ). Right. Diagram depicting the four major prostatic lobes of the mouse prostate, the rat has a similar organization. Lateral prostate (LP), dorsal prostate (DP), ventral prostate (VP), anterior prostate (AP).
Adapted from Sugimura Y, Cunha GR, Donjacour AA. Morphogenesis of ductal networks in the mouse prostate. Biol Reprod 1986;34:961-71; with permission.
The peripheral zone ducts exit directly laterally from the postero-lateral recesses of the urethral wall. The system consists of small, simple round to oval acinar structures emptying into long narrow ducts surrounded by a stroma of loosely arranged and randomly interwoven muscle bundles. Ducts and acini are lined with simple columnar epithelium. This area is the principal site of prostatitis and carcinoma of the prostate (CaP), although not of BPH. The peripheral zone includes the proximal urethral segment of the prostate. This comprises the region of the prostate between the base of the urinary bladder and the verumontanum (the area where the ejactulatory ducts feed into the urethra). The principal feature of this region, which comprises about 5% of the total prostate mass, is the preprostatic sphincter. The sphincter is a cylindrical sleeve of smooth muscle which stretches from the base of the bladder to the verumontanum.
The central zone ducts run predominantly proximally, closely following the ejaculatory ducts. These ducts and acini are much larger and of irregular contour. The acini are polyhedral in cross section. The muscular stroma is much more compact than in the peripheral zone. The central zone has a low incidence of disease.
The transitional zone surrounds the urethra between the bladder and the verumontanum. This is a small volume of the prostate, perhaps 5% in the normal organ, but is the principal site of BPH pathogenesis. Nodular expansion of this region of the prostate results in compression of the urethra and the partial bladder outlet obstruction associated with BPH.
Unlike the human, the rodent prostate is not merged into one compact anatomical structure. The rodent prostate is composed of four distinct lobular structures (Figure 1); the anterior lobe, also known as the coagulating gland, the dorsal, ventral and lateral lobes.16 These lobes exist as pairs on the left and right sides. Due to differences in lobe-specific branching morphogenesis, the final shape of each lobe is distinct. 10
In both rats and mice, the ventral lobes are located immediately below the urinary bladder on the ventral aspect of the urethra. The lateral lobes lie just below the coagulating glands and seminal vesicles, partially overlapping the ventral lobes and dorsally blend with the dorsal lobe. 29,30 The dorsal lobes are found inferior and posterior to the urinary bladder, behind and below the coagulating glands and seminal vesicles. The anterior lobes, or coagulating glands, are directly adjacent to the seminal vesicles.
Lobe/zone homology between the rodent and human prostates has been suggested by various authors. However, the 2001 Bar Harbor Consensus meeting concluded that “there is no existing supporting evidence for a direct relationship between the specific mouse prostate lobes and the specific zones in the human prostate”. 31
Etiology of BPH
There are three well studied conditions which affect the prostate; BPH, prostate cancer and prostatitis. BPH is a nonmalignant enlargement of the prostate gland and refers to the stromal and glandular epithelial hyperplasia that occurs in the transition zone of the prostate (Figure 2). 32 Clinically, the condition manifests with lower urinary tract symptoms (LUTS) consisting of obstructive (weak urination stream, incomplete bladder emptying, hesitancy) and irritative symptoms (frequency, urgency, nocturia). 33 It is important to note that LUTS can result from a variety of conditions including problems relating to bladder innervation and aging as well as the outflow obstruction caused by BPH. LUTS due to BPH increases with age, and nearly all men develop histologic BPH by 90 years of age. 34 It is also important to note that BPH is not generally considered to be a precursor lesion to prostate cancer.
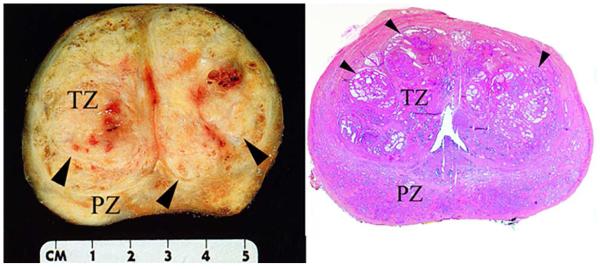
Figure 2. Appearance of BPH in human prostate.
Left. Gross anatomy of a human prostate affected by benign prostatic hyperplasia (BPH) in the transitional zone. Hyperplastic nodules (arrowheads) are clearly visible in the transitional zone (TZ) but not the peripheral zone (PZ) of the gross sample. Right. Hematoxylin and eosin (H&E) stained wholemount cross-section of a human prostate affected by BPH in the transitional zone. The architectural organization of the glandular structures within the nodules (arrowheads) is evident in this low magnification figure. Courtesy of Scott B. Shappell, MD, PhD, Dallas, TX.
BPH is a common condition linked to both aging and the presence of functional testes. McNeal proposed the idea that BPH results from a “reawakening” of inductive potential in adult prostatic stroma35-37 resulting in focalized formation of new ductal architecture in the transition zone of the prostate (Figure 2). He described pure stromal nodules and, more commonly, nodules that had been invaded by epithelium to form new glandular architecture. McNeal also made the point that the glands themselves appear normal (Figure 3), it is the overall focal organization that is definitive of BPH (Figure 2). This is in contrast to the epithelial or stromal hyperplasia seen in many rodent models. These focal nodules adjacent to the urethra give rise to urethral compression and obstruction. One of the central tenets of McNeal’s hypothesis was that adult prostatic epithelium should retain an ability to respond to inductive signaling with proliferation and new ductal branching morphogenesis. We, and others, verified this concept for adult rat, mouse and human prostatic epithelium. 38-40
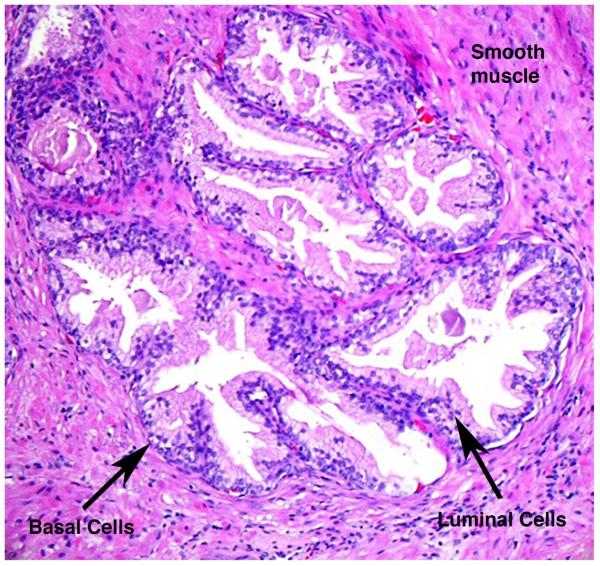
Figure 3. Structure of individual glands within a focus of BPH.
Prostatic glands are composed of columnar luminal epithelial cells and more flattened basal cells surrounded by well differentiated smooth muscle. Occasional capillaries can be seen spaced around the ducts adjacent to the basal epithelium.
Work on canine BPH showed that the condition can be induced with androstanediol and with combinations of androstanediol and estradiol. A combined dose of DHT and estradiol was also found to induce the disease. 41,42 In men, levels of serum testosterone decrease by about 35% between the ages of 21 and 85 against a constant level of estradiol. Thus, there is a change in the androgen/estrogen ratio, which has been suggested to be sufficient to promote the growth of BPH. However, since these changes are not significant until after the first initiation of the disease, their relationship to its induction can be questioned. 43
McNeal’s hypothesis does not address the underlying issue of why “mesenchymal reawakening” may occur or whether there are other etiologic factors in play. Common co-morbidities suggest a role for inflammation and possibly metabolic anomalies in the pathogenesis of BPH. Obesity is an epidemic in many developed countries. Low levels of physical activity compound this situation, giving rise to many diseases and patterns of comorbidity including cardiovascular disease, increased insulin resistance and type II diabetes. Insulin resistance may well be an underlying factor resulting in “metabolic syndrome”, a condition affecting around 50 million Americans. Metabolic syndrome includes; impaired glucose metabolism, elevated weight, altered fat distribution, and hypertension, along with elevated C-reactive protein (which is associated with chronic intraprostatic inflammation in BPH). 44,45 While causal links to BPH remain unproven, there appears to be a link between metabolic syndrome and LUTS. 46 Diabetes and increased LUTS severity are significantly correlated, even when other co-variables, such as age, are factored out. 47
Obesity is a well-recognized pro-inflammatory condition, and increased inflammation is closely associated BPH severity, progression and increased urinary retention. 48,49 In a mouse model of chronic prostatitis, regions of epithelial hyperplasia and dysplasia were found adjacent to areas of inflammation.50 In addition inflammation, activation of NF-κB signaling and subsequent and expression of constitutively active androgen receptor variant 7 have been shown to correlate with both BPH progression and prostate volume. 51 Stromal nodules of BPH contain increased T and B lymphocytes. 52 Elevated levels of inflammatory cells have also been detected in the interstitium and surrounding epithelial glands of human BPH. 53 Infiltration of inflammatory cells in BPH is accompanied by increases in pro-inflammatory cytokines. Elevated levels of interleukins IL-2, IL-8, IL-17, and IFNγ have been shown in BPH samples. 54-56 Cytokine expression is seen in early development where the cytokines have direct mitogenic effects on the prostate. 57,58
The mechanistic basis for the initiation and progression of BPH from asymptomatic to symptomatic remains unclear. A number of potential causes have been attributed to the overgrowth of smooth muscle tissue and glandular epithelial tissue in the prostate. These include aging, genetic factors, hormonal changes, and lifestyle. 33,59,60 Although there is still much work to be done to fully understand the basis of BPH progression, resources, such as animal models, to study BPH are limited and there is a pressing need for new approaches.
BPH was primarily treated surgically for many years, however, in the 1990s this was superseded by the medical approaches that are now the front line therapy. 5α-reductase inhibitors such as finasteride and dutasteride and α-adrenergic blockers such as doxazosin and tamsulosin are used to shrink and relax the organ, respectively. The MTOPS study demonstrated that a combination of the α-blocker Doxazosin and the 5α-reductase inhibitor Finasteride are more effective at reducing LUTS progression than either drug given alone. 48 While these approaches are effective in many patients, a significant proportion, in the range of 35%, showed progressive disease even in the face of the two drug combination 48,49. A detailed understanding of the pathways that lead to the genesis of BPH nodules would assist in the design of better or complementary therapies.
Animal models in the study of BPH
Animal models are necessary for systematic and mechanistic studies of human prostate diseases. The dog and the chimpanzee are the only animals other than man known to suffer from BPH. As might be expected in a closely related species, the anatomy of the chimpanzee prostate is a close match for the human organ. However, chimpanzees are not a useful experimental model, and reports of BPH in this species demonstrate that, as in humans, the disease is sporadic and associated with aging.61 Historically a number of studies were performed on spontaneously arising BPH in the canine prostate. Canine BPH, like its human counterpart, arises with increasing frequency with age and requires functional testes. The diseases differ, in that human BPH is strongly focal with distinct nodules of hyperplasia within the gland, whereas the canine disease is diffuse, occurring throughout the gland. 42,62 In the dog, there is therefore a general expansion of the gland, which is less anatomically fixed than in man, resulting in compression of the rectum, producing constipation as a symptom as opposed to the urinary retention found in humans. Practical considerations, notably the fact that the disease occurs in older animals (generally greater than eight years) and the costs associated with maintaining colonies of large mammals, have severely limited work in this model.
BPH in humans has a number of common components; the essential element is the focal nodular growth that usually occurs close to the urethra. There is also commonly inflammation and the activation of associated transcription factors such as NF-κB and upregulation of the androgen receptor and constitutively active variants. This process is associated with compression of the urethra and LUTS due to partial bladder outlet obstruction. The anatomy of the rodent prostate largely precludes the recapitulation of all of these characteristics in a single model. For this reason it is important to assess which particular aspects of the human disease are present in any given model. Most of the models that are available develop some form of hyperplasia, either of the epithelium, stroma or both, however, the glandular structures are generally histologically abnormal, and appropriate caution must be exercised in interpreting the data. Focalized glandular expansion with normal appearing new glands, as seen in human BPH nodules is not evident in the vast majority of animal models. For these reasons, most of these should not be considered models of BPH, but, rather, of whichever process(es) of the disease they most accurately reflect.
Manipulating the hormonal environment has been used in rats to induce prostate cancer.63 Similar manipulations can also be applied to induce bladder outflow obstruction and inflammation.64 Applying such a regimen to mice to mimic the changes in the testosterone to estrogen ratio seen in the aging human male results in bladder outflow obstruction and urination patterns that in some ways mirror human LUTS. 65 In this model there are increases in glandular ducts surrounding the proximal urethra with the potential to compress this structure and give rise to a partial bladder outlet obstruction.
A number of transgenic, knock-out and knock-in mouse models have been described with various prostatic phenotypes. These include a prostate-specific 15-LOX-2 transgenic mouse generated using the ARR2PB promoter allowing for targeted expression of 15-LOX-2 or 15-LOX-2sv-b, a splice variant lacking arachidonic acid-metabolizing activity. These manipulations both resulted in age-dependent increases in prostatic wet weight with predominantly-epithelial hyperplasia. 66 A conditional knockout of PPARγ in the mouse prostate also resulted in increases in prostate size with epithelial hyperplasia that progressed to prostatic intraepithelial neoplasia and occasional cancer. 67 This observation makes the point that epithelial hyperplasia in the mouse might be an early pre-malignant change, clearly differentiating it from human BPH.
Non-obese diabetic (NOD) mice represent a model of immune dysregulation and type 1 diabetes. First reported in 1980, these mice exhibit spontaneous development of autoimmune insulin dependent diabetes mellitus (IDDM). 68 These mice are important because the autoimmune response is not fully penetrant; as a result, sub-groups of mice exhibit diabetes, with or without prostatic inflammation. This allows for independent assessment of the effects of these two human BPH-relevant variables. Histochemical analysis reveals a reduction of the epithelium and increased stroma. As a result, muscular and collagen hypertrophy in the prostatic gland when inflammation occurs. 69 In non-inflamed but diabetic NOD/SCID mice, a strong epithelial hyperplastic phenotype in the anterior and ventral prostate has been noted. 70
Summary
Many researchers have studied the embryology, development and anatomy of the human prostate. Debates over the nomenclature to describe the gland have been settled and standardized nomenclature exists. BPH is a complex disease that probably has multiple causes often occurring in patients with complex co-morbidities, prominently including obesity and diabetes.
Animal models have proven to be useful in understanding the underlying mechanisms of many human diseases. However, the structure of the human and rodent prostates are very different. Extrapolation of data between species requires an understanding of these differences and of the limitations of specific models.
Key Points.
Development of the prostate in humans and laboratory animals follows similar principles but the details vary.
The anatomy of the human prostate is significantly different from that seen in laboratory animals.
The disease profile of the human and rodent prostate is very different.
Animal models describe certain aspects of human BPH but not the whole disease profile.
Care should be taken in extrapolating observations made in rodents and applying them to humans.
Synopsis.
The prostate is a secretory organ of the male reproductive tract found in most mammals. The development of the prostate follows a common pattern between species and is dependent upon the actions of androgens to induce and support ductal branching morphogenesis of buds emerging from the urogenital sinus. The human prostate has a compact zonal anatomy immediately surrounding the urethra and below the urinary bladder. Rodents in contrast have a lobular prostate with the lobes radiating away from the urethra. The human prostate is the site of three important conditions, benign hyperplasia (BPH), prostate cancer and prostatitis. In contrast, the rodent prostate has little naturally occurring disease. Rodents can be used to model aspects of human BPH, however care should be taken in the interpretation of such data and their extrapolation to the human condition.
Acknowledgements
This work was supported in part by NIH grants 1R01 DK103483 and 2 R25 GM059994-13
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
The authors have nothing to disclose
Literature Cited
- 1.Vesalius A, O'Malley CD, Calcar JSv, Saunders JBdCM. The illustrations from the works of Andreas Vesalius of Brussels : with annotations and translations, a discussion of the plates and their background, authorship and influence, and a biographical sketch of Vesalius. World Publishing Company; 1950. [Google Scholar]
- 2.Geller J. Pathogenesis and medical treatment of benign prostatic hyperplasia. Prostate Suppliment. 1989;2:95–104. doi: 10.1002/pros.2990150510. [DOI] [PubMed] [Google Scholar]
- 3.Price D, Williams-Ashman H. The accessory reproductive glands of mammals. In: Young W, editor. Sex and internal secretions. 3 Vol. 1. Williams and Wilkins; Baltimore: 1961. pp. 366–448. [Google Scholar]
- 4.Shapiro E, Hartanto V, Lepor H. Quantifying the smooth muscle content of the prostate using double-immunoenzymatic staining and color assisted image analysis. J. Urol. 1992;147:1167–1170. doi: 10.1016/s0022-5347(17)37508-0. [DOI] [PubMed] [Google Scholar]
- 5.Josso N. Physiology of Sex Differentiation. Pediat. Adolesc. Endocr. 1981;8:1–13. [Google Scholar]
- 6.Price D. Normal development of the prostate and seminal vesicles of the rat with a study of experimental postnatal modifications. Am. J. Anat. 1936;60(j):79–127. [Google Scholar]
- 7.Price Comparative aspects of development and structure in the prostate. Natl. Cancer Inst. Monogr. 1963;12:1–27. [PubMed] [Google Scholar]
- 8.Staack A, Donjacour AA, Brody J, Cunha GR, Carroll P. Mouse urogenital development: a practical approach. Differentiation. 2003 Sep;71(7):402–413. doi: 10.1046/j.1432-0436.2003.7107004.x. [DOI] [PubMed] [Google Scholar]
- 9.Georgas KM, Armstrong J, Keast JR, et al. An illustrated anatomical ontology of the developing mouse lower urogenital tract. Development. 2015 May 15;142(10):1893–1908. doi: 10.1242/dev.117903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marker PC, Donjacour AA, Dahiya R, Cunha GR. Hormonal, cellular, and molecular control of prostatic development. Dev Biol. 2003 Jan 15;253(2):165–174. doi: 10.1016/s0012-1606(02)00031-3. [DOI] [PubMed] [Google Scholar]
- 11.Cunha GR, Donjacour AA, Cooke PS, et al. The endocrinology and developmental biology of the prostate. Endocr Rev. 1987 Aug;8(3):338–362. doi: 10.1210/edrv-8-3-338. [DOI] [PubMed] [Google Scholar]
- 12.Timms BG, Mohs TJ, Didio LJ. Ductal budding and branching patterns in the developing prostate. J Urol. 1994 May;151(5):1427–1432. doi: 10.1016/s0022-5347(17)35273-4. [DOI] [PubMed] [Google Scholar]
- 13.Kellokumpu-Lehtinen P, Santti R, Pelliniemi LJ. Correlation of early cytodifferentiation of the human fetal prostate and Leydig cells. Anat Rec. 1980 Mar;196(3):263–273. doi: 10.1002/ar.1091960302. [DOI] [PubMed] [Google Scholar]
- 14.Lowsley OS. The development of the human prostate gland with reference to the development of other structures at the neck of the urinary bladder. American Journal of Anatomy. 1912;13(3):299–349. [Google Scholar]
- 15.Kellokumpu-Lehtinen P. Development of sexual dimorphism in human urogenital sinus complex. Biol Neonate. 1985;48(3):157–167. doi: 10.1159/000242167. [DOI] [PubMed] [Google Scholar]
- 16.Sugimura Y, Cunha GR, Donjacour AA, Bigsby RM, Brody JR. Whole-mount autoradiography study of DNA synthetic activity during postnatal development and androgen-induced regeneration in the mouse prostate. Biol. Reprod. 1986;34(j):985–995. doi: 10.1095/biolreprod34.5.985. [DOI] [PubMed] [Google Scholar]
- 17.Hayward SW, Baskin LS, Haughney PC, et al. Epithelial development in the rat ventral prostate, anterior prostate and seminal vesicle. Acta Anatomica. 1996;155:81–93. doi: 10.1159/000147793. [DOI] [PubMed] [Google Scholar]
- 18.Hayward SW, Brody JR, Cunha GR. An edgewise look at basal cells: Three-dimensional views of the rat prostate, mammary gland and salivary gland. Differentiation. 1996;60:219–227. doi: 10.1046/j.1432-0436.1996.6040219.x. [DOI] [PubMed] [Google Scholar]
- 19.Soeffing WJ, Timms BG. Localization of androgen receptor and cell-specific cytokeratins in basal cells of rat ventral prostate. Journal of andrology. 1995 May-Jun;16(3):197–208. [PubMed] [Google Scholar]
- 20.Hayward SW, Baskin LS, Haughney PC, et al. Stromal development in the ventral prostate, anterior prostate and seminal vesicle of the rat. Acta Anat (Basel) 1996;155(2):94–103. doi: 10.1159/000147794. [DOI] [PubMed] [Google Scholar]
- 21.Timms BG. Prostate development: a historical perspective. Differentiation. 2008 Jul;76(6):565–577. doi: 10.1111/j.1432-0436.2008.00278.x. [DOI] [PubMed] [Google Scholar]
- 22.Sugimura Y, Cunha GR, Donjacour AA. Morphogenesis of ductal networks in the mouse prostate. Biol. Reprod. 1986;34:961–971. doi: 10.1095/biolreprod34.5.961. [DOI] [PubMed] [Google Scholar]
- 23.Donjacour AA, Cunha GR. The effect of androgen deprivation on branching morphogenesis in the mouse prostate. Dev Biol. 1988 Jul;128(1):1–14. doi: 10.1016/0012-1606(88)90260-6. [DOI] [PubMed] [Google Scholar]
- 24.McNeal JE. Anatomy of the prostate and morphogenesis of BPH. Prog Clin Biol Res. 1984;145:27–53. [PubMed] [Google Scholar]
- 25.Evatt EJ. A Contribution to the Development of the Prostate in Man. J Anat Physiol. 1909 Jul;43(Pt 4):314–321. [PMC free article] [PubMed] [Google Scholar]
- 26.Franks LM. Benign nodular hyperplasia of the prostate: A review. Ann. R. Coll. Surg. 1954;14:92–106. [PMC free article] [PubMed] [Google Scholar]
- 27.Hutch JA, Rambo ON. A study of the anatomy of the prostate, prostatic urethra and the urinary sphincter system. J. Urol. 1970;105:443–452. doi: 10.1016/s0022-5347(17)61756-7. [DOI] [PubMed] [Google Scholar]
- 28.Tissell LE, Salander H. Anatomy of the human prostate and its three paired lobes. Prog. Clin. Biol. Res. 1984;145:55–66. [PubMed] [Google Scholar]
- 29.Hayashi N, Sugimura Y, Kawamura J, Donjacour AA, Cunha GR. Morphological and functional heterogeneity in the rat prostatic gland. Biol Reprod. 1991 Aug;45(2):308–321. doi: 10.1095/biolreprod45.2.308. [DOI] [PubMed] [Google Scholar]
- 30.Roy-Burman P, Wu H, Powell WC, Hagenkord J, Cohen MB. Genetically defined mouse models that mimic natural aspects of human prostate cancer development. Endocr Relat Cancer. 2004 Jun;11(2):225–254. doi: 10.1677/erc.0.0110225. [DOI] [PubMed] [Google Scholar]
- 31.Shappell SB, Thomas GV, Roberts RL, et al. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer research. 2004 Mar 15;64(6):2270–2305. doi: 10.1158/0008-5472.can-03-0946. [DOI] [PubMed] [Google Scholar]
- 32.Dhingra N, Bhagwat D. Benign prostatic hyperplasia: An overview of existing treatment. Indian J. Pharmacol. 2011 Feb;43(1):6–12. doi: 10.4103/0253-7613.75657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller J, Tarter TH. Combination therapy with dutasteride and tamsulosin for the treatment of symptomatic enlarged prostate. Clin Interv Aging. 2009;4:251–258. doi: 10.2147/cia.s4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984 Sep;132(3):474–479. doi: 10.1016/s0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- 35.McNeal JE. The prostate gland: morphology and pathobiology. Monogr. Urology. 1983;4:3–37. [Google Scholar]
- 36.McNeal JE. Origin and evolution of benign prostatic enlargement. Invest. Urol. 1978;15(j):340–345. [PubMed] [Google Scholar]
- 37.McNeal JE. Morphology and biology of benign prostatic hyperplasia. In: Bruchovsky N, Chapdellaine A, Neumann F, editors. Regulation of androgen action. Congressdruck R. Bruckner; Berlin: 1985. pp. 191–197. [Google Scholar]
- 38.Norman JT, Cunha GR, Sugimura Y. The induction of new ductal growth in adult prostatic epithelium in response to an embryonic prostatic inductor. Prostate. 1986;8:209–220. doi: 10.1002/pros.2990080302. [DOI] [PubMed] [Google Scholar]
- 39.Hayashi N, Cunha GR, Parker M. Permissive and instructive induction of adult rodent prostatic epithelium by heterotypic urogenital sinus mesenchyme. Epithelial cell biology. 1993 Apr;2(2):66–78. [PubMed] [Google Scholar]
- 40.Hayward SW, Haughney PC, Rosen MA, et al. Interactions between adult human prostatic epithelium and rat urogenital sinus mesenchyme in a tissue recombination model. Differentiation. 1998;63(3):131–140. doi: 10.1046/j.1432-0436.1998.6330131.x. [DOI] [PubMed] [Google Scholar]
- 41.Walsh PC, Wilson JD. The induction of prostatic hypertrophy in the dog with androstanediol. J. Clin. Invest. 1976;57:1093–1097. doi: 10.1172/JCI108353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeKlerk DP, Coffey DS, Ewing LL, et al. Comparison of spontaneous and experimentally induced canine prostatic hyperplasia. J Clin Invest. 1979;64(3):842–849. doi: 10.1172/JCI109532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson JD. The pathogenesis of benign prostatic hyperplasia. Amer. J. Med. 1980;68:745–756. doi: 10.1016/0002-9343(80)90267-3. [DOI] [PubMed] [Google Scholar]
- 44.Kasturi S, Russell S, McVary KT. Metabolic syndrome and lower urinary tract symptoms secondary to benign prostatic hyperplasia. Current urology reports. 2006;7(4):288–292. doi: 10.1007/s11934-996-0008-y. [DOI] [PubMed] [Google Scholar]
- 45.Rohrmann S, De Marzo AM, Smit E, Giovannucci E, Platz EA. Serum C-reactive protein concentration and lower urinary tract symptoms in older men in the Third National Health and Nutrition Examination Survey (NHANES III) Prostate. 2005;62(1):27–33. doi: 10.1002/pros.20110. [DOI] [PubMed] [Google Scholar]
- 46.Hammarsten J, Hogstedt B. Clinical, haemodynamic, anthropometric, metabolic and insulin profile of men with high-stage and high-grade clinical prostate cancer. Blood Press. 2004;13(1):47–55. doi: 10.1080/08037050310025735. [DOI] [PubMed] [Google Scholar]
- 47.Michel MC, Mehlburger L, Schumacher H, Bressel HU, Goepel M. Effect of diabetes on lower urinary tract symptoms in patients with benign prostatic hyperplasia. J Urol. 2000 Jun;163(6):1725–1729. [PubMed] [Google Scholar]
- 48.McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003 Dec 18;349(25):2387–2398. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 49.McVary KT. A review of combination therapy in patients with benign prostatic hyperplasia. Clinical therapeutics. 2007 Mar;29(3):387–398. doi: 10.1016/s0149-2918(07)80077-4. [DOI] [PubMed] [Google Scholar]
- 50.Elkahwaji JE, Zhong W, Hopkins WJ, Bushman W. Chronic bacterial infection and inflammation incite reactive hyperplasia in a mouse model of chronic prostatitis. Prostate. 2007 Jan 1;67(1):14–21. doi: 10.1002/pros.20445. [DOI] [PubMed] [Google Scholar]
- 51.Austin DC, Strand DW, Love HL, et al. NF-kappaB and androgen receptor variant expression correlate with human BPH progression. Prostate. 2015 Dec 28; doi: 10.1002/pros.23140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bierhoff E, Vogel J, Benz M, Giefer T, Wernert N, Pfeifer U. Stromal nodules in benign prostatic hyperplasia. Eur Urol. 1996;29(3):345–354. doi: 10.1159/000473774. [DOI] [PubMed] [Google Scholar]
- 53.Theyer G, Kramer G, Assmann I, et al. Phenotypic characterization of infiltrating leukocytes in benign prostatic hyperplasia. Lab Invest. 1992 Jan;66(1):96–107. [PubMed] [Google Scholar]
- 54.Kramer G, Steiner GE, Handisurya A, et al. Increased expression of lymphocyte-derived cytokines in benign hyperplastic prostate tissue, identification of the producing cell types, and effect of differentially expressed cytokines on stromal cell proliferation. Prostate. 2002 Jun 1;52(1):43–58. doi: 10.1002/pros.10084. [DOI] [PubMed] [Google Scholar]
- 55.Steiner GE, Stix U, Handisurya A, et al. Cytokine expression pattern in benign prostatic hyperplasia infiltrating T cells and impact of lymphocytic infiltration on cytokine mRNA profile in prostatic tissue. Lab Invest. 2003 Aug;83(8):1131–1146. doi: 10.1097/01.lab.0000081388.40145.65. [DOI] [PubMed] [Google Scholar]
- 56.Giri D, Ittmann M. Interleukin-8 is a paracrine inducer of fibroblast growth factor 2, a stromal and epithelial growth factor in benign prostatic hyperplasia. Am J Pathol. 2001 Jul;159(1):139–147. doi: 10.1016/S0002-9440(10)61681-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jerde TJ, Bushman W. IL-1 induces IGF-dependent epithelial proliferation in prostate development and reactive hyperplasia. Sci Signal. 2009;2(86):ra49. doi: 10.1126/scisignal.2000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang L, Zoetemelk M, Chitteti BR, et al. Expansion of prostate epithelial progenitor cells after inflammation of the mouse prostate. Am J Physiol Renal Physiol. 15 2015 Jun;308(12):F1421–1430. doi: 10.1152/ajprenal.00488.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Culig Z, Hobisch A, Cronauer MV, et al. Regulation of prostatic growth and function by peptide growth factors. Prostate. 1996 Jun;28(6):392–405. doi: 10.1002/(SICI)1097-0045(199606)28:6<392::AID-PROS9>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 60.Jenkins EP, Andersson S, Imperato-McGinley J, Wilson JD, Russell DW. Genetic and pharmacological evidence for more than one human steroid 5 alpha-reductase. J Clin Invest. 1992 Jan;89(1):293–300. doi: 10.1172/JCI115574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steiner MS, Couch RC, Raghow S, Stauffer D. The chimpanzee as a model of human benign prostatic hyperplasia. J Urol. 1999 Oct;162(4):1454–1461. [PubMed] [Google Scholar]
- 62.Berry SJ, Strandberg JD, Saunders WJ, Coffey DS. Development of canine benign prostatic hyperplasia with age. Prostate. 1986;9(4):363–373. doi: 10.1002/pros.2990090406. [DOI] [PubMed] [Google Scholar]
- 63.Noble RL. The development of prostatic adenocarcinoma in Nb rats following prolonged sex hormone administration. Cancer Res. 1977;37(6):1929–1933. [PubMed] [Google Scholar]
- 64.Bernoulli J, Yatkin E, Konkol Y, Talvitie EM, Santti R, Streng T. Prostatic inflammation and obstructive voiding in the adult Noble rat: impact of the testosterone to estradiol ratio in serum. Prostate. 2008 Sep 1;68(12):1296–1306. doi: 10.1002/pros.20791. [DOI] [PubMed] [Google Scholar]
- 65.Nicholson TM, Ricke EA, Marker PC, et al. Testosterone and 17beta-estradiol induce glandular prostatic growth, bladder outlet obstruction, and voiding dysfunction in male mice. Endocrinology. 2012 Nov;153(11):5556–5565. doi: 10.1210/en.2012-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suraneni MV, Schneider-Broussard R, Moore JR, et al. Transgenic expression of 15-lipoxygenase 2 (15-LOX2) in mouse prostate leads to hyperplasia and cell senescence. Oncogene. 2010 Jul 29;29(30):4261–4275. doi: 10.1038/onc.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang M, Fernandez S, Jerome WG, et al. Disruption of PPARgamma signaling results in mouse prostatic intraepithelial neoplasia involving active autophagy. Cell Death Differ. 2010 Mar;17(3):469–481. doi: 10.1038/cdd.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Makino S, Kunimoto K, Muraoka Y, Mizushima Y, Katagiri K, Tochino Y. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu. 1980 Jan;29(1):1–13. doi: 10.1538/expanim1978.29.1_1. [DOI] [PubMed] [Google Scholar]
- 69.Ribeiro DL, Caldeira EJ, Candido EM, Manzato AJ, Taboga SR, Cagnon VH. Prostatic stromal microenvironment and experimental diabetes. Eur J Histochem. 2006 Jan-Mar;50(1):51–60. [PubMed] [Google Scholar]
- 70.Jiang M, Strand DW, Franco OE, Clark PE, Hayward SW. PPARgamma: a molecular link between systemic metabolic disease and benign prostate hyperplasia. Differentiation. 2011 Nov-Dec;82(4-5):220–236. doi: 10.1016/j.diff.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]