BACKGROUND
Lower extremity deep venous thromboses (DVT) are frequently diagnosed in the emergency department (ED). The estimated annual rates of ED visits by patients with a primary diagnosis of DVT nearly doubled from 22 (95% CI 14–29) per 100,000 population in 1998–2000 to 41 (95% CI 30–52) in 2006–2009. [1] A concerted effort was made in the early 2000s, when low molecular weight heparin (LMWH) was approved by the United States Food and Drug Administration, to treat patients with uncomplicated DVT as outpatients. [2, 3] Prior to the approval of LMWH, DVT treatment typically involved hospitalization and unfractionated heparin anticoagulation until the patient was therapeutic on a vitamin K antagonist (eg, warfarin). [2] Our institutional experience found that discharging ED patients on LMWH therapy for uncomplicated DVT was frequently complicated by a number of obstacles including: medication cost, insufficient social resources (eg, homeless, no social support system to assist with medication administration), and patient level barriers, including low health literacy and/or numeracy, and unwillingness to perform self-injection.
METHODS
In May 2015, Vanderbilt University Medical Center convened a multidisciplinary team of physicians, pharmacists, and patient safety consultants to develop outpatient management protocols for adult ED patients with conditions that were frequently resulting in short stay admissions. Vanderbilt University Medical Center is an urban, university-affiliated, tertiary care, referral center with an estimated annual ED census of 70,000 adult ED visits. Due to the hospital’s status as the region’s largest tertiary care referral center, Level 1 trauma center, and burn center, the percentage of ED visits resulting in hospitalization approaches 35%. In an effort to reduce medically-unnecessary admissions, our institution made a concerted effort to develop protocols for conditions that commonly resulted in short stay admissions that could be managed safely and effectively in the outpatient setting. The introduction of the direct oral anticoagulant (DOAC) medications as treatment options for newly diagnosed DVT, as recommended by the recent American College of Chest Physicians guidelines for managing DVT [4], made DVT an ideal candidate for our multidisciplinary team’s focus.
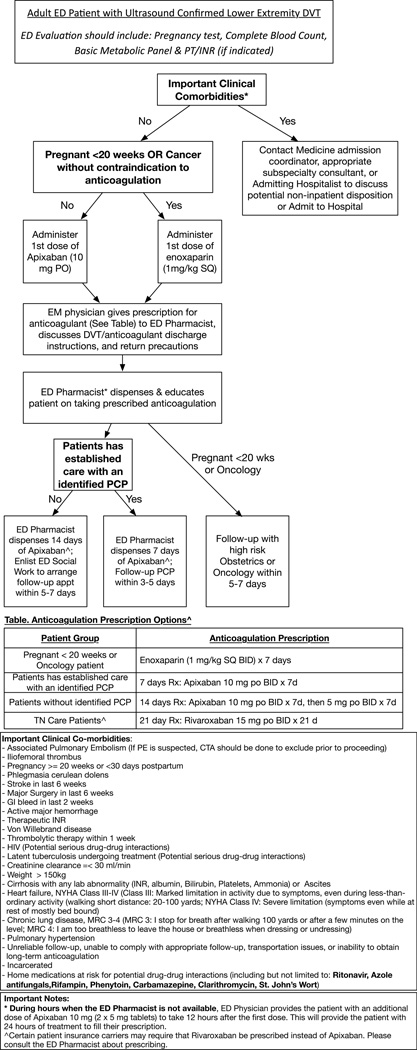
Our team reviewed the available literature and most recent guidelines for the treatment of hemodynamically stable, newly diagnosed DVT with neither medical nor social contraindication to outpatient management. The group developed multiple draft protocols in an iterative process that were vetted by team members and numerous ED and inpatient clinicians. The multidisciplinary team included physician representatives from emergency medicine, clinical pharmacy, and cardiovascular medicine including an expert in anticoagulation. Representatives from our Quality, Safety, and Risk Prevention department assisted with the development of the protocol and monitoring of the protocol adherence following initiation of the new treatment recommendations. In addition to the protocol flowchart (Figure 1), we revised our discharge instructions to include specific directions for patients discharged on apixaban. Apixaban was selected as our preferred DOAC agent after detailed review of the literature and discussion amongst our institutional anticoagulation experts. The advantages of apixaban included: no heparin needed at initiation of therapy, extended dosing scheme tested, lower bleeding rates compared to warfarin and other DOACs, and cost neutral with pharmacy benefit costs. The Tennessee Medicaid (ie, TennCare) preferred medication for DVT is rivaroxaban. Therefore, we developed detailed patient instructions for apixaban, rivaroxaban, and low molecular weight-heparin (Appendix).
Figure 1.
The DVT treatment protocol was officially approved by the executive leadership of emergency medicine and internal medicine. In January 2016, all emergency medicine faculty received formal training on the new protocol and the new management strategy was initiated. During the first 4 weeks following the protocol initiation, three women who were initially treated with apixaban for newly diagnosed DVT returned to the ED with vaginal bleeding. None of these women received a blood transfusion. One woman had two return ED visits and her anticoagulant was changed from apixaban to low molecular weight heparin and warfarin after her second return visit. Vaginal bleeding is a known risk associated with initiation of DOAC therapy. [5, 6] Based on these cases, we did revise the discharge instructions to specifically review return precautions for vaginal bleeding. We also recommended that the emergency physician specifically discuss this potential adverse effect. We also revised the pathway to inform emergency physicians regarding the most common home medications that might interact with the DOAC therapy. Patients whose home medications may interact with the chosen DOAC are evaluated by our ED clinical pharmacist and emergency physician to determine whether the potential interaction should exclude them from the outpatient treatment pathway. Since initiation of the DVT treatment protocol, we have successfully treated three of the six patients with newly diagnosed DVT as outpatients.
CONCLUSIONS
Current guidelines recommend outpatient management for patients with uncomplicated DVT and the introduction of DOAC medications has improved access to outpatient therapy at our institution. [4] Our multidisciplinary team developed an outpatient DVT treatment protocol that facilitates appropriate patient selection, reduces concern for medication noncompliance through an ED clinical pharmacist educating and dispensing drug prior to ED discharge, and includes detailed follow-up within 7–14 days. The development of similar protocols for appropriate outpatient management will be even more important given the increasing number of ED visits and the limited availability of inpatient beds.
Supplementary Material
Acknowledgments
Funding Sources: Dr. Self is supported in part by NIH K23GM110469. Dr. Ward is supported in part by NIH K23 HL127130. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Conflicts of Interests/Disclosures: There are no conflicts of interest in connection with this submission or are there any copyright constraints. No industry financial support or compensation has been or will be received for conducting this study. Barrett: Consultant, Red Bull GmbH, Fuschl am See, Salzburg and Boehringer Ingelheim Pharmaceuticals, Inc. Ridgefield, Connecticut; Research Support as Site Principal Investigator: Janssen Pharmaceuticals, Raritan, NJ; Alere, San Diego, CA; Beckman: Merck, Astra Zeneca, Bristol Myers Squibb, and Sanofi. Others: None
Role of the Sponsors: The funding organizations had no role in the design and conduct of the study; the collection, analysis, and interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
References
- 1.Yusuf HR, Tsai J, Siddiqi AE, Boulet SL, Soucie M. Emergency department visits by patients with venous thromboembolism, 1998–2009. J Hosp Adm. 2012;1:1–8. [Google Scholar]
- 2.Bates SM, Ginsburg JS. Treatment of Deep-Vein Thrombosis. N Engl J Med. 2004;351:268–277. doi: 10.1056/NEJMcp031676. [DOI] [PubMed] [Google Scholar]
- 3.Vinson DR, Berman DA. Outpatient Treatment of Deep Venous Thrombosis: A Clinical Care Pathway Managed by the Emergency Department. Ann Emerg Med. 2001;37:251–258. doi: 10.1067/mem.2001.113703. [DOI] [PubMed] [Google Scholar]
- 4.Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris T, Sood N, Stevens SM, Vintch JRE, Wells P, Woller SC, Moores CL. Antithrombotic Therapy for VTE Disease: CHEST Guideline. CHEST. 2016 doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 5.Martinelli I, Lensing AW, Middeldorp S, Levi M, Beyer-Westendorf J, van Bellen B, Bounameaux H, Brighton TA, Cohen AT, Trajanovic M, Gebel M, Lam P, Wells PS, Prins MH. Recurrent venous thromboembolism and abnormal uterine bleeding with anticoagulant and hormone therapy use. Blood. 2016;127(11):1417–1425. doi: 10.1182/blood-2015-08-665927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Crem N, Peerlinck K, Vanassche T, Vanheule K, Debaveye B, Middeldorp S, Verhamme P, Peetermans M. Abnormal uterine bleeding in VTE patients treated with rivaroxaban compared to vitamin K antagonists. Thromb Res. 2015;136(4):749–753. doi: 10.1016/j.thromres.2015.07.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.