Abstract
Genomic alterations and protein expression levels have been established as prognostic factors for survival in patients with diffuse large B‐cell lymphoma (DLBCL). In particular, double‐hit DLBCL (DHL), which exhibits translocations in MYC and BCL2 and/or BCL6, is known to be associated with a poor prognosis. However, the clinical significance of gene alterations and protein expression levels for MYC, B‐cell lymphoma (BCL)2, and BCL6 are unclear. In this study, we analyzed 61 adult patients diagnosed with DLBCL without DHL, who were treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone, or similar regimens. There were no differences in the distribution of MYC expression rates among the different MYC gene statuses. In log–rank tests, MYC translocation was a prognostic factor for overall survival (OS; P = 0.011), whereas BCL2 and BCL6 translocation were not prognostic indicators (P = 0.999 and P = 0.925, respectively). Although the expression levels of MYC and BCL6 were not significantly associated with OS, the expression of BCL2 was a prognostic factor for OS (P = 0.027). Furthermore, copy number gains in the MYC, BCL2, and BCL6 genes did not affect OS. MYC translocation (hazard ratio, 4.769; range, 1.518–14.98; P = 0.007) and BCL2 protein expression (hazard ratio, 3.072; range, 1.002–9.413; P = 0.049) were independent prognostic factors for survival in multivariate analyses. In conclusion, MYC translocation and BCL2 expression may need to be investigated at the initial diagnosis to predict prognosis in patients with DLBCL.
Keywords: BCL2, BCL6, diffuse large B‐cell lymphoma, MYC, prognostic factor
Diffuse large B‐cell lymphoma (DLBCL) is a heterogeneous, common type of aggressive B‐cell lymphoma accounting for approximately one‐third of all non‐Hodgkin's lymphomas.1, 2 Rituximab combined with cyclophosphamide, vincristine, adriamycin, and prednisolone (R‐CHOP) therapy has dramatically improved survival rates in patients with DLBCL,3, 4, 5, 6 however, approximately 30–40% of patients with DLBCL die from cancer‐related complications.7 In order to identify patients with DLBCL having poor prognoses, numerous studies have been undertaken to determine related prognostic factors. Clinically, the International Prognostic Index (IPI) is the best indicator for risk stratification.8 Alternatively, according to biological and pathological features, gene expression profiling, immunohistochemistry (IHC) algorithms for detecting overexpression of specific proteins, and FISH for discovering chromosomal translocations have been developed for predicting prognosis.9, 10, 11, 12, 13, 14
MYC, BCL2, and BCL6 gene translocation and/or protein expression have been also intensively studied, with several reports showing the utility of these factors as prognostic markers.15, 16 Alterations in oncogenes such as MYC and anti‐apoptotic genes such as BCL2 are involved in the pathogenesis of DLBCL.17 Deregulation of MYC and BCL2 is thought to be caused by chromosomal translocation, gene copy number gains, and other mechanisms, such as transcriptional upregulation downstream of nuclear factor‐κB signaling.18 Translocation of MYC, BCL2, and BCL6 genes, detected by FISH, has been reported to occur in approximately 10%, 14%, and 20% of patients with DLBCL, respectively.16, 19 Although the clinical impact of BCL2 and BCL6 gene translocations on prognosis is unclear, MYC translocation has been reported to predict prognosis.20, 21, 22, 23, 24 However, the effects of MYC translocation alone on prognosis are still unclear owing to contrasting findings by different research groups. Notably, almost all studies have concluded that double‐hit lymphoma (DHL), which contains translocations of MYC and BCL2 and/or BCL6, is highly aggressive, with a poor prognosis compared with non‐DHL DLBCL.20, 21, 22, 25, 26
Specific protein expression detected by IHC has been reported to predict prognosis in patients with DLBCL. However, data regarding MYC and B‐cell lymphoma (BCL)2 protein expression, and the effects of these proteins on the survival of patients with DLBCL, are controversial.16, 19, 27, 28 Indeed, although some studies have shown that MYC expression by IHC can be used to predict prognosis in patients with DLBCL,16, 29, 30 other studies have reported that there is no correlation between MYC expression by IHC and prognosis. Additionally, some studies have found that the addition of rituximab to standard chemotherapy overcomes the adverse prognostic influence of BCL2 expression,31, 32 whereas others have shown that BCL2 expression remains a marker of poor prognostic in patients undergoing R‐CHOP treatment.33 Furthermore, several reports have indicated that double protein expression of MYC and BCL2 detected by IHC could be a prognostic indicator in patients with DLBCL. However, the combination of protein expression proportions with clinical applicability is still unclear, and no studies have shown the clinical utility of a combination of genomic translocation and protein expression patterns.
Therefore, in this study, we analyzed genomic alterations and protein expression levels of MYC, BCL2, and BCL6 using FISH and IHC in Japanese patients with DLBCL. We surveyed the clinical relationships among genomic alterations and protein expression patterns for MYC, BCL2, and BCL6 in patients with DLBCL. We also investigated whether dual protein expression of MYC and BCL2 and/or BCL6 could be a prognostic factor and analyzed the proportions of MYC and BCL2 and/or BCL6 expression by IHC that could be predictive for survival.
Materials and Methods
Patients and samples
We enrolled 64 adult patients newly diagnosed with DLBCL, not otherwise specified between October 2003 and October 2012 at Niigata University Hospital (Niigata, Japan). Three patients with DHL were excluded, and 61 patients were analyzed. Diagnostic specimens were reviewed by two expert hematopathologists (H.M. and K.O.) according to the 2008 WHO classification. All patients were treated with R‐CHOP or R‐CHOP‐like regimens as an initial standard therapy. Formalin‐fixed, paraffin‐embedded samples were obtained, and FISH analysis for MYC, BCL2, and BCL6 was carried out at initial diagnosis in all patients. Initial treatment responses were evaluated by computed tomography (CT) scanning and/or PET‐CT scanning at the end of the initial treatment. This study was carried out in accordance with recommendation of the Declaration of Helsinki and approved by the ethics review committee of Kurume University (Kurume, Japan).
Immunochemical staining
Tissue samples were processed as formalin‐fixed, paraffin‐embedded tissues according to standard institutional procedures. We created tissue microarrays from samples from 61 patients and undertook evaluations of IHC with antibodies using these microarrays. Antibodies (clones) used for IHC included anti‐CD20 (L‐26; DakoCytomation, Glostrup, Denmark), anti‐BCL2 (clone124; DakoCytomation), anti‐BCL6 (P1F6; Leica Microsystems, Wetzlar, Germany), anti‐Multiple myeloma oncogene ‐1 (MUM ‐1) (MUM1p; DakoCytomation), anti‐CD10 (56C6; Leica Microsystems), and anti‐c‐MYC (Y69) antibodies (Abcam, Cambridge, UK). Immunohistochemistry results were reviewed by two expert hematopathologists (H.M. and K.O.). Cut‐off points for MYC, BCL2, and BCL6 protein expression were defined as DLBCL with 30% or more, 1% or more, and 30% or more positive cells, respectively, as recommended in previous studies.12, 15, 34
Fluorescence in situ hybridization analysis
The FISH analysis was carried out using specimens collected at the time of the initial diagnosis to detect chromosomal translocations and copy number gains. We used Vysis LSI MYC (Cat. No. 05J91‐001), Vysis LSI BCL2 (Cat. No. 07J75‐001), and Vysis LSI BCL6 (Cat. No. 05J68‐001) dual‐color break‐apart rearrangement probes (Vysis/Abbott Molecular Diagnostics, Wiesbaden‐Delkenheim, Germany). We used an Axio Imager M2 (Zeiss, Oberkochen, Germany) for microscopic evaluations. Cut‐off levels for the break‐apart probes were established by evaluating the split‐signal distributions in samples of reactive lymphoid tissues, calculating the mean number of split signals. Cut‐off levels were the same as those in previous studies.19, 35 If three or more gene copies were detected in tumor cells, the tumor was categorized as having copy number gains, as described in previous studies.35, 36
Statistical evaluation
Clinicopathological characteristics of the patients were compared by χ2‐tests, Fisher's exact tests, and Mann–Whitney U‐tests. Overall survival (OS) was defined as the time from diagnosis to the death or the last follow‐up. Progression‐free survival (PFS) was defined as the time from the first day of treatment to the day at which the disease progressed or the day of death from any cause. Kaplan–Meier estimates were used to predict the OS and PFS, as compared using log–rank tests. The effects of the study variables were assessed by multivariate analysis according to a Cox regression model for OS and PFS. All calculated P‐values were two‐sided, and results with P‐values of less than 0.05 were considered statistically significant. All statistical analyses were carried out with EZR software.37
Results
Patient characteristics
The median age was 62 years (range, 17–85 years), and the median follow‐up period was 40 months (range, 2–127 months). The clinical features of this study cohort are shown in Table 1. In this cohort, 26 (42.3%) patients were men, and 35 (57.7%) were women. Thirty‐nine patients (63.9%) showed high lactate dehydrogenase levels, and 30 patients (49.2%) had IPI scores of 3–5. Moreover, 34 patients (55.7%) had late‐stage (III–IV) cancer according to the Ann Arbor classification. Fifty cases (82.0%) achieved complete response.
Table 1.
Characteristics of patients with diffuse large B‐cell lymphoma (n = 61)
| Patient characteristic | n | % |
|---|---|---|
| Sex | ||
| Male | 26 | 42.3 |
| Female | 35 | 57.3 |
| Age, median (range), years | 62 (17–85) | |
| ≥61 years | 39 | 63.9 |
| <61 years | 22 | 36.1 |
| Stage | ||
| I/II | 27 | 44.3 |
| III/IV | 34 | 55.7 |
| Serum LDH | ||
| Normal | 22 | 36.1 |
| Elevated | 39 | 63.9 |
| ECOG performance status | ||
| 0–2 | 44 | 72.1 |
| 3–5 | 17 | 27.9 |
| Extranodal sites | ||
| ≥2 | 9 | 14.8 |
| <2 | 52 | 85.2 |
| IPI score | ||
| 0 | 6 | 9.8 |
| 1 | 15 | 24.6 |
| 2 | 10 | 16.4 |
| 3 | 20 | 32.8 |
| 4 | 8 | 13.1 |
| 5 | 2 | 3.3 |
| IPI 0–2 | 31 | 50.8 |
| IPI 3–5 | 30 | 49.2 |
| Initial therapy response | ||
| Complete response | 50 | 82.0 |
| Partial response | 5 | 8.2 |
| Stable disease | 2 | 3.3 |
| Progressive disease | 4 | 6.5 |
ECOG, Eastern Cooperative Oncology Group; IPI, International Prognostic Index; LDH, lactate dehydrogenase.
Hans classification, FISH, and IHC status for each MYC status
Among the 61 patients, 45 cases of DLBCL (73.8%) were of germinal center B‐cell type, and 16 (26.2%) were of non‐ germinal center B‐cell type. Six patients (8.8%) exhibited MYC translocations, seven patients (11.5%) had BCL2 translocations, and eight patients (13.1%) harbored BCL6 translocations.
We subsequently compared the cells of origin, FISH results, and IHC results among the three groups (MYC translocation group, MYC copy number gains group, and normal MYC group). Hans classification, FISH, and IHC status in these three groups are shown in Table 2. There were no significant differences between the MYC translocation group (n = 6) and the normal MYC group (n = 42), or between the MYC copy number gains group (n = 13) and the normal MYC group (n = 42).
Table 2.
Results of Hans classifier, FISH, and immunohistochemistry (IHC) analyses in patients with diffuse large B‐cell lymphoma with MYC translocation (A), MYC copy number gains (B), or normal MYC (C)
| All patients (n = 61) | (A) MYC translocation (n = 6) | (B) MYC amplification (n = 13) | (C) MYC normal (n = 42) | (A)–(C) | (B)–(C) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | P‐value | P‐value | |
| Hans classification | ||||||||||
| GCB type | 45 | 73.8 | 5 | 83.3 | 12 | 92.3 | 28 | 66.7 | 0.650 | 0.086 |
| Non‐GCB type | 16 | 26.2 | 1 | 16.7 | 1 | 7.7 | 14 | 33.3 | ||
| FISH results | ||||||||||
| MYC translocation | 6 | 8.8 | 6 | 100 | 0 | 0.0 | 0 | 0.0 | N.A. | N.A. |
| BCL2 translocation | 7 | 11.5 | 0 | 0.0 | 0 | 0.0 | 7 | 100.0 | 0.573 | 0.179 |
| BCL6 translocation | 8 | 13.1 | 0 | 0.0 | 0 | 0.0 | 8 | 100.0 | 0.571 | 0.176 |
| IHC results | ||||||||||
| MYC ≥30% | 32 | 52.3 | 4 | 66.7 | 8 | 61.5 | 20 | 47.6 | 0.666 | 0.528 |
| BCL2 ≥1% | 36 | 59.0 | 3 | 50.0 | 7 | 53.8 | 26 | 61.9 | 0.669 | 0.748 |
| BCL6 ≥30% | 42 | 68.9 | 2 | 33.3 | 12 | 92.3 | 28 | 66.7 | 0.200 | 0.087 |
| MYC ≥30% and BCL2 ≥1% | 22 | 36.1 | 2 | 33.3 | 4 | 30.8 | 16 | 38.1 | 1.000 | 0.749 |
| MYC ≥30% and BCL6 ≥30% | 24 | 39.3 | 1 | 16.7 | 8 | 61.5 | 15 | 35.7 | 0.648 | 0.119 |
N.A., not available; BCL, B‐cell lymphoma; GCB, germinal center B‐cell.
Association between genomic alterations and protein expression for MYC, BCL2, and BCL6
Next, we analyzed the associations between gene alterations detected by FISH and protein expression measured by IHC. We statistically compared the differences in the distributions of positive MYC staining rates by IHC between the MYC translocation group and normal MYC group and between the MYC copy number gains group and normal MYC group. There were no significant differences between the two comparisons (Fig. 1a,b). Similar results were observed for BCL2 and BCL6 (Fig. 1c–f). Examples of pathological images of DLBCL cases in this study are shown in Fig. S1.
Figure 1.
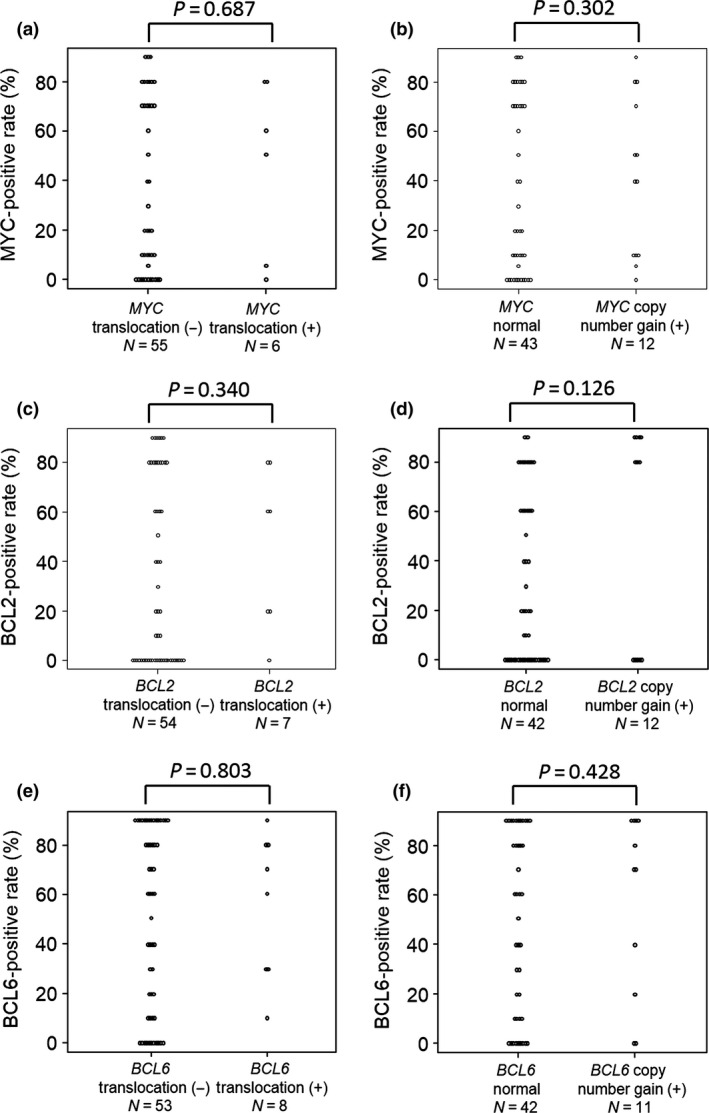
Distributions of positive rates of MYC, B‐cell lymphoma (BCL)2, and BCL6 immunohistochemical staining in different groups of patients with diffuse large B‐cell lymphoma. (a) Correlation between MYC staining and MYC translocation. (b) Correlation between MYC copy number gains and normal MYC. (c,d) Correlations between BCL2 staining and BCL2 translocation (c) and between BCL2 staining and BCL2 copy number gains (d). (e,f) Correlations between BCL6 staining and BCL6 translocation (e) and between BCL6 staining and BCL6 copy number gains (f).
Survival analysis
In the analysis by log–rank tests, high IPI score (IPI 3–5) was a significant prognostic factor for poor PFS (P = 0.006) and OS (P = 0.0045; Fig. 2a,b). In contrast, cell of origin by Hans classifier was not a prognostic factor for PFS (P = 0.207) or OS (P = 0.093; Fig. 2c,d).
Figure 2.
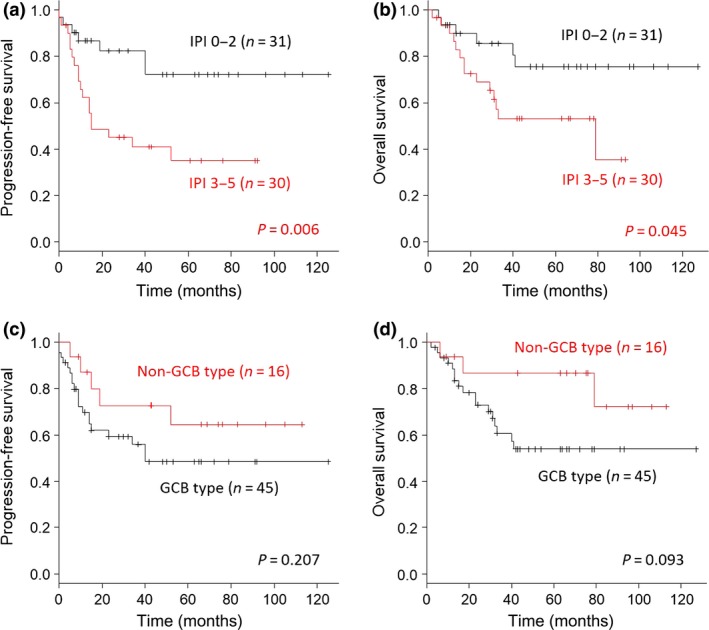
Progression‐free survival (PFS) and overall survival (OS) according to the International Prognostic Index (IPI) and cells of origin in patients with diffuse large B‐cell lymphoma. (a,b) Analysis of IPI score as a prognostic factor for PFS (a) and OS (b). (c,d) Analysis of cells of origin (Hans classifier) as a prognostic factor for PFS (c) and OS (b). GCB, germinal center B‐cell.
MYC translocation was a prognostic factor for PFS (P = 0.015) and OS (P = 0.006; Fig. 3a,b), whereas copy number gain in the MYC gene had no effect on survival (Fig. 3c,d). Although copy number gain in the BCL2 gene was not a prognostic factor for OS, it was a prognostic factor for PFS (log–rank test). Translocation and copy number gain in the BCL6 gene were not prognostic factors for PFS or OS (Figs S2,S3).
Figure 3.
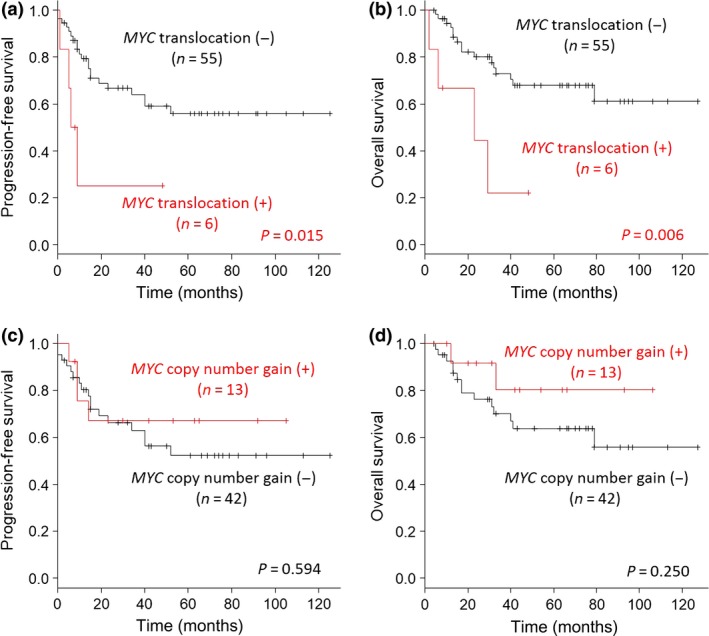
Progression‐free survival (PFS) and overall survival (OS) according to MYC translocation and copy number gains in patients with diffuse large B‐cell lymphoma. (a,b) Analysis of MYC translocation as a prognostic factor for PFS (a) and OS (b). (c,d) Analysis of MYC copy number gains as a prognostic factor for PFS (c) and OS (d).
We subsequently investigated whether protein expression patterns of MYC, BCL2, and BCL6 by IHC affected survival. There was no significant association between MYC expression and survival in patients with DLBCL (Fig. 4a,b). However, patients with DLBCL with 1% or more BCL2 expression showed poorer prognoses than those without BCL2 expression (Fig. 4c,d). There was no significant association between BCL6 expression and survival in patients with DLBCL (Fig. 4e,f).
Figure 4.
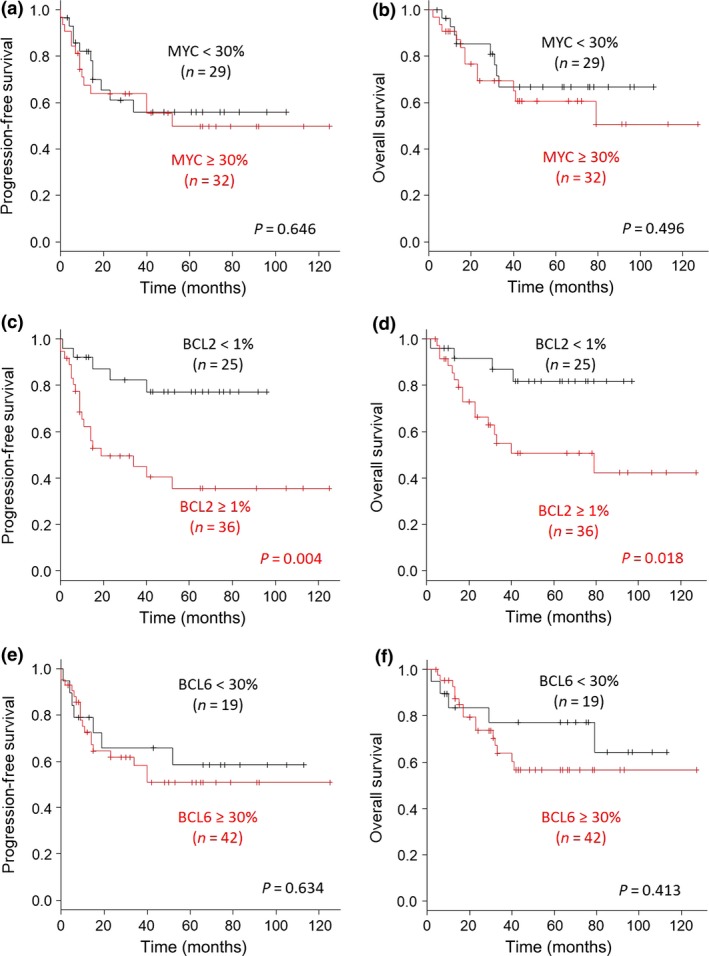
Progression‐free survival (PFS) and overall survival (OS) according to MYC, B‐cell lymphoma (BCL)2, and BCL6 protein expression in patients with diffuse large B‐cell lymphoma. (a,b) Analysis of clinical differences in PFS (a) and OS (b) between the ≥30% MYC expression group and the <30% expression group. (c,d) Analysis of ≥1% BCL2 expression as a prognostic factor for PFS (c) and OS (d). (e,f) Analysis of <30% BCL6 expression as a prognostic factor for PFS (e) and OS (f).
Univariate and multivariate analysis
Univariate and multivariate analyses showed that MYC translocation (hazard ratio [HR], 4.227 [range, 1.385–12.90] for univariate analysis; HR, 4.769 [range, 1.518–14.98] for multivariate analysis) and 1% or more BCL2 expression (HR, 3.481 [range, 1.158–10.46] for univariate analysis; HR, 3.072 [range, 1.002–9.413] for multivariate analysis) were independent prognostic factors for OS. Furthermore, MYC translocation (HR, 3.353 [range, 1.089–10.32] for univariate analysis; HR, 5.645 [range, 1.725–18.47] for multivariate analysis) and 1% or more BCL2 expression (HR, 3.838 [range, 1.433–10.28] for univariate analysis; HR, 3.776 [range, 1.389–10.27] for multivariate analysis) were independent prognostic factors for PFS (Table 3). Although IPI 3–5 was a prognostic factor for PFS in univariate and multivariate analyses, it was not a prognostic factor for OS in univariate or multivariate analyses.
Table 3.
Univariate and multivariate analysis for predicting prognosis of diffuse large B‐cell lymphoma
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| Overall survival | ||||
| IPI 3–5 | 2.576 (0.985–6.735) | 0.054 | 2.453 (0.926–6.498) | 0.071 |
| MYC translocation | 4.227 (1.385–12.90) | 0.011 | 4.769 (1.518–14.98) | 0.007 |
| BCL2 translocation | 1.001 (0.231–4.333) | 0.999 | ||
| BCL6 translocation | 1.061 (0.310–3.627) | 0.925 | ||
| MYC copy number gain | 0.906 (0.329–2.497) | 0.849 | ||
| BCL2 copy number gain | 1.620 (0.582–4.506) | 0.356 | ||
| BCL6 copy number gain | 0.890 (0.297–2.667) | 0.835 | ||
| MYC expression ≥30% | 1.361 (0.556–3.334) | 0.499 | ||
| BCL2 expression ≥1% | 3.481 (1.158–10.46) | 0.027 | 3.072 (1.002–9.413) | 0.049 |
| BCL6 expression ≥30% | 1.526 (0.548–4.249) | 0.418 | ||
| Progression–free survival | ||||
| IPI 3–5 | 3.169 (1.321–7.600) | 0.009 | 3.248 (1.331–7.924) | 0.009 |
| MYC translocation | 3.353 (1.089–10.32) | 0.035 | 5.645 (1.725–18.47) | 0.004 |
| BCL2 translocation | 1.885 (0.636–5.583) | 0.253 | ||
| BCL6 translocation | 0.740 (0.221–2.475) | 0.625 | ||
| MYC copy number gain | 1.129 (0.470–2.709) | 0.786 | ||
| BCL2 copy number gain | 2.163 (0.896–5.218) | 0.086 | ||
| BCL6 copy number gain | 0.681 (0.234–1.987) | 0.482 | ||
| MYC expression ≥30% | 1.182 (0.536–2.605) | 0.679 | ||
| BCL2 expression ≥1% | 3.838 (1.433–10.28) | 0.007 | 3.776 (1.389–10.27) | 0.009 |
| BCL6 expression ≥30% | 1.235 (0.514–2.965) | 0.637 | ||
BCL, B‐cell lymphoma; CI, confidence interval; HR, hazard ratio; IPI, International Prognostic Index.
From the above results, MYC translocation detected by FISH and BCL2 expression measured by IHC were prognostic factors for poor OS and PFS. Patients who had MYC translocation and/or BCL2 expression showed markedly poorer clinical outcomes than other patients (OS, P = 0.003; PFS, P = 0.009; Fig. 5).
Figure 5.
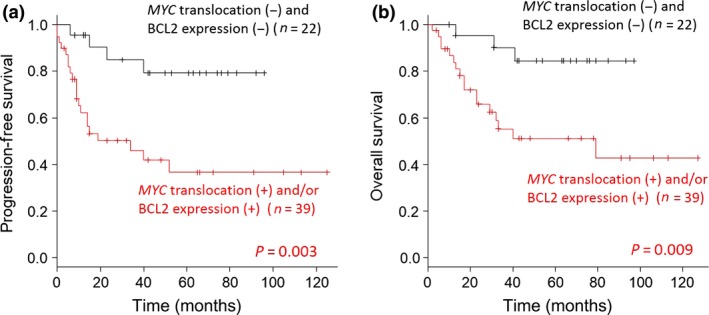
Progression‐free survival (a) and overall survival (OS) (b) according to MYC translocation detected by FISH or B‐cell lymphoma (BCL)2 expression detected by immunohistochemistry in patients with diffuse large B‐cell lymphoma.
Discussion
In this study, we found that MYC translocation detected by FISH and BCL2 expression detected by IHC were important factors for predicting the prognosis of patients with DLBCL. Additionally, copy number gains in MYC, BCL2, and BCL6 were not prognostic factors for OS.
Our results showed that IPI, but not cells of origin by Hans classifier, was a prognostic factor for OS and PFS. Interestingly, before the use of rituximab (an anti‐CD20 mAb), IPI and cells of origin were representative prognostic indicators in patients with DLBCL.8, 12 However, rituximab has been reported to overcome the impairments caused by cell of origin,38 and IPI is currently the primary clinical tool used to predict outcomes in patients with DLBCL, even in the post‐rituximab era.39 Therefore, based on these previous works, our study cohort was assumed to be adequate for analysis.
Our study showed that MYC translocation was an independent prognostic factor for PFS and OS, although BCL2 and BCL6 translocations were not prognostic factors. Individually, BCL2 and BCL6 translocations are not thought to be prognostic factors in patients undergoing rituximab‐based therapy.16, 19 However, MYC translocation has been reported to be a prognostic factor in patients with DLBCL treated with R‐CHOP therapy,20, 21, 22, 23, 24 although these results are controversial.19 According to a previous report examining MYC single translocation cases in DLBCL,40 fusion of the MYC gene and immunoglobulin (IG) gene (IG heavy chain gene [IGH] or light chain genes κ [IGK] or λ [IGL]) was reported to be associated with a poorer prognosis than that of cases without fusion of the MYC gene and immunoglobulin gene (non‐IG). In this study, we confirmed the detection MYC/IGH fusion by FISH in three of six cases (50%). Although there was no significant difference in prognosis between MYC/IGH fusion‐positive cases (n = 3) and non‐MYC/IGH fusion cases (n = 3), there may be some cases with MYC and IGK or IGL in non‐MYC/IGH cases. Fusion of the MYC gene and IGK or IGL should also be investigated among cases without MYC/IGH fusion. Future studies are needed to identify and characterize MYC translocation partners.
The clinical significance of MYC, BCL2, and BCL6 expression as evaluated by IHC has recently been extensively studied. However, the specific effects of MYC, BCL2, and BCL6 expression in patients with DLBCL treated with R‐CHOP therapy remain unclear. These conflicting results may be explained by the differences in cut‐off values for each study. For example, 40% or more MYC protein expression detected by IHC has been reported to be a prognostic factor in several studies;15, 16, 19, 35 however, our results showed that overexpression of MYC at any cut‐off level had no effect on survival in patients with DLBCL (Fig. S4). Similarly, BCL6 protein expression at any cut‐off level had no effect on survival (data not shown). In contrast, 1% or more BCL2 protein expression as detected by IHC was a prognostic factor for survival in this study. The reasons for setting the cut‐off value of BCL2 expression to ≥1% were: to extract as many patients with poor prognosis as possible, to be able to judge clearly and easily whether there was expression of BCL2 for applications by pathologists in daily clinical practice, and to suggest the possibility that a BCL2 inhibitor could be used for as many patients with BCL2‐positive DLBCL as possible. Recently, BCL2 inhibitor has been suggested to be effective for DLBCL.41 Based on these considerations, the cut‐off value of BCL2 was set to ≥1% in this study. A previous report also showed that 1% or more BCL2 protein expression was a prognostic factor in patients with DLBCL.19 Moreover, BCL2 protein expression at any cut‐off value was found to affect OS (Fig. S5). Several studies have shown that R‐CHOP therapy overcomes the clinical effects of BCL2 expression.15, 31, 32 In contrast, some researchers concluded that BCL2 expression remains an adverse prognostic marker in patients treated with R‐CHOP.16, 19, 33 Based on our current results, we suggest that BCL2 staining may be important for predicting prognosis in patients with DLBCL.
Coexpression of MYC and BCL2 protein in DLBCL, termed double‐expresser lymphoma, has been suggested to be a prognostic factor. However, the cut‐off values for MYC and BCL2 expression have not been consistent among published reports.16, 19, 27, 28, 42, 43 In addition, because these studies have included DHL, which has been shown to be associated with a poor prognosis, the results may reflect the heterogeneous nature of DLBCL.44 Although we investigated whether coexpression of MYC and BCL2 at any cut‐off level, only coexpression of MYC ≥ 30% and BCL2 ≥ 30% was a prognostic factor (data not shown; OS, P = 0.0024; PFS, P = 0.0017). Namely, excluding coexpression of MYC ≥ 30% and BCL2 ≥ 30%, the coexpression of MYC and BCL2 at any cut‐off level including MYC ≥ 30% and BCL2 ≥ 1%, was not a prognostic factor (Fig. S6; OS, P = 0.140; PFS, P = 0.176). Patients with coexpression of MYC ≥ 30% and BCL2 1–29% (n = 3) showed comparatively good prognosis in this study. However, if we compared the respective prognosis curves, the clinical significance of coexpression of MYC and BCL2 was considered to be small because, in this study, the expression of BCL2 had stronger effects than MYC expression did on prognosis. In addition, double expression of MYC and BCL6 was not a prognostic factor for survival. However, survival of lymphoma cells in the context of coexpression of BCL2 and c‐MYC has been shown to depend on BCL2 function, and inhibition of BCL2 function by ABT‐737 (a selective inhibitor of BCL‐2, BCL–extra large [BCL‐xL], and BCL‐w) could can also induce cell death in a mouse model of MYC‐driven lymphoma.45 This report also suggested that BCL2 expression may be more important for prognosis than overexpression of MYC in double‐expresser lymphoma.
This study had several limitations. First, we included relatively few cases. Additionally, the study was carried out at a single center with a Japanese cohort. The number of patients in this study may have been too small to reach strong conclusions. Therefore, further studies are needed to establish the validity of our results in a large cohort.
In conclusion, MYC translocation as detected by FISH and BCL2 expression as measured by IHC may be important for predicting prognosis. Patients with DLBCL harboring MYC translocation as detected by FISH and BCL2 expression as detected by IHC may achieve improved outcomes using a therapeutic strategy including intensive chemotherapy rather than conventional R‐CHOP therapy.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Fig. S1. MYC, B‐cell lymphoma (BCL)2, and BCL6 detected by immunohistochemistry in patients with diffuse large B‐cell lymphoma.
Fig. S2. Progression‐free survival (a,c) and overall survival (b,d) according to translocation (a,b) and copy number gain (c,d) of BCL2 in patients with diffuse large B‐cell lymphoma.
Fig. S3. Progression‐free survival (a,c) and overall survival (b,d) according to translocation (a,b) and copy number gain (c,d) of BCL6 in patients with diffuse large B‐cell lymphoma.
Fig. S4. Overall survival according to MYC protein expression in patients with diffuse large B‐cell lymphoma.
Fig. S5. Overall survival according to B‐cell lymphoma (BCL)2 protein expression in patients with diffuse large B‐cell lymphoma.
Fig. S6. Progression‐free survival (a,c) and overall survival (b,d) according to levels of MYC and B‐cell lymphoma (BCL)2 or BCL6 expression in patients with diffuse large B‐cell lymphoma.
Cancer Sci 107 (2016) 853–861
Funding Information
No sources of funding were declared for this study.
References
- 1. Smith A, Roman E, Howell D, et al The Haematological Malignancy Research Network (HMRN): a new information strategy for population based epidemiology and health service research. Br J Haematol 2010; 148: 739–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sabattini E, Bacci F, Sagramoso C, Pileri SA. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica 2010; 102: 83–7. [PubMed] [Google Scholar]
- 3. Coiffier B, Lepage E, Briere J, et al CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large‐B‐cell lymphoma. N Engl J Med 2002; 346: 235–42. [DOI] [PubMed] [Google Scholar]
- 4. Sehn LH, Donaldson J, Chhanabhai M, et al Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B‐cell lymphoma in British Columbia. J Clin Oncol 2005; 23: 5027–33. [DOI] [PubMed] [Google Scholar]
- 5. Fu K, Weisenburger DD, Choi WW, et al Addition of rituximab to standard chemotherapy improves the survival of both the germinal center B‐cell‐like and non‐germinal center B‐cell‐like subtypes of diffuse large B‐cell lymphoma. J Clin Oncol 2008; 26: 4587–94. [DOI] [PubMed] [Google Scholar]
- 6. Jacobs SA, Swerdlow SH, Kant J, et al Phase II trial of short‐course CHOP‐R followed by 90Y‐ibritumomab tiuxetan and extended rituximab in previously untreated follicular lymphoma. Clin Cancer Res 2008; 14: 7088–94. [DOI] [PubMed] [Google Scholar]
- 7. Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood 2011; 117: 5019–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shipp MA, Harrington DP, Anderson JR, et al A predictive model for aggressive non‐Hodgkin's lymphoma. The International Non‐Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med 1993; 329: 987–94. [DOI] [PubMed] [Google Scholar]
- 9. Lenz G, Staudt LM. Aggressive lymphomas. N Engl J Med 2010; 362: 1417–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ninan MJ, Wadhwa PD, Gupta P. Prognostication of diffuse large B‐cell lymphoma in the rituximab era. Leuk Lymphoma 2011; 52: 360–73. [DOI] [PubMed] [Google Scholar]
- 11. Nogai H, Dorken B, Lenz G. Pathogenesis of non‐Hodgkin's lymphoma. J Clin Oncol 2011; 29: 1803–11. [DOI] [PubMed] [Google Scholar]
- 12. Hans CP, Weisenburger DD, Greiner TC, et al Confirmation of the molecular classification of diffuse large B‐cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004; 103: 275–82. [DOI] [PubMed] [Google Scholar]
- 13. Choi WW, Weisenburger DD, Greiner TC, et al A new immunostain algorithm classifies diffuse large B‐cell lymphoma into molecular subtypes with high accuracy. Clin Cancer Res 2009; 15: 5494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martelli M, Ferreri AJ, Agostinelli C, Di Rocco A, Pfreundschuh M, Pileri SA. Diffuse large B‐cell lymphoma. Crit Rev Oncol/Hematol 2013; 87: 146–71. [DOI] [PubMed] [Google Scholar]
- 15. Yan LX, Liu YH, Luo DL, et al MYC expression in concert with BCL2 and BCL6 expression predicts outcome in Chinese patients with diffuse large B‐cell lymphoma, not otherwise specified. PLoS ONE 2014; 9: e104068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perry AM, Alvarado‐Bernal Y, Laurini JA, et al MYC and BCL2 protein expression predicts survival in patients with diffuse large B‐cell lymphoma treated with rituximab. Br J Haematol 2014; 165: 382–91. [DOI] [PubMed] [Google Scholar]
- 17. Plati J, Bucur O, Khosravi‐Far R. Apoptotic cell signaling in cancer progression and therapy. Integr Biol 2011; 3: 279–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Young RM, Shaffer AL 3rd, Phelan JD, Staudt LM. B‐cell receptor signaling in diffuse large B‐cell lymphoma. Semin Hematol 2015; 52: 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Horn H, Ziepert M, Becher C, et al MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B‐cell lymphoma. Blood 2013; 121: 2253–63. [DOI] [PubMed] [Google Scholar]
- 20. Barrans S, Crouch S, Smith A, et al Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B‐cell lymphoma treated in the era of rituximab. J Clin Oncol 2010; 28: 3360–5. [DOI] [PubMed] [Google Scholar]
- 21. Klapper W, Stoecklein H, Zeynalova S, et al Structural aberrations affecting the MYC locus indicate a poor prognosis independent of clinical risk factors in diffuse large B‐cell lymphomas treated within randomized trials of the German High‐Grade Non‐Hodgkin's Lymphoma Study Group (DSHNHL). Leukemia 2008; 22: 2226–9. [DOI] [PubMed] [Google Scholar]
- 22. Kojima M, Nishikii H, Takizawa J, et al MYC rearrangements are useful for predicting outcomes following rituximab and chemotherapy: multicenter analysis of Japanese patients with diffuse large B‐cell lymphoma. Leuk Lymphoma 2013; 54: 2149–54. [DOI] [PubMed] [Google Scholar]
- 23. Yoon SO, Jeon YK, Paik JH, et al MYC translocation and an increased copy number predict poor prognosis in adult diffuse large B‐cell lymphoma (DLBCL), especially in germinal centre‐like B cell (GCB) type. Histopathology 2008; 53: 205–17. [DOI] [PubMed] [Google Scholar]
- 24. Savage KJ, Johnson NA, Ben‐Neriah S, et al MYC gene rearrangements are associated with a poor prognosis in diffuse large B‐cell lymphoma patients treated with R‐CHOP chemotherapy. Blood 2009; 114: 3533–7. [DOI] [PubMed] [Google Scholar]
- 25. Niitsu N, Okamoto M, Miura I, Hirano M. Clinical features and prognosis of de novo diffuse large B‐cell lymphoma with t(14;18) and 8q24/c‐MYC translocations. Leukemia 2009; 23: 777–83. [DOI] [PubMed] [Google Scholar]
- 26. Li S, Desai P, Lin P, et al MYC/BCL6 double hit lymphoma (DHL): a tumor associated with an aggressive clinical course and poor prognosis. Histopathology 2015; doi: 10.1111/his.12884 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 27. Hu S, Xu‐Monette ZY, Tzankov A, et al MYC/BCL2 protein coexpression contributes to the inferior survival of activated B‐cell subtype of diffuse large B‐cell lymphoma and demonstrates high‐risk gene expression signatures: a report from The International DLBCL Rituximab‐CHOP Consortium Program. Blood 2013; 121: 4021–31; quiz 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johnson NA, Slack GW, Savage KJ, et al Concurrent expression of MYC and BCL2 in diffuse large B‐cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 2012; 30: 3452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou M, Wang J, Ouyang J, et al MYC protein expression is associated with poor prognosis in diffuse large B cell lymphoma patients treated with RCHOP chemotherapy. Tumour Biol 2014; 35: 6757–62. [DOI] [PubMed] [Google Scholar]
- 30. Kluk MJ, Chapuy B, Sinha P, et al Immunohistochemical detection of MYC‐driven diffuse large B‐cell lymphomas. PLoS ONE 2012; 7: e33813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mounier N, Briere J, Gisselbrecht C, et al Rituximab plus CHOP (R‐CHOP) overcomes bcl‐2–associated resistance to chemotherapy in elderly patients with diffuse large B‐cell lymphoma (DLBCL). Blood 2003; 101: 4279–84. [DOI] [PubMed] [Google Scholar]
- 32. Wilson KS, Sehn LH, Berry B, et al CHOP‐R therapy overcomes the adverse prognostic influence of BCL‐2 expression in diffuse large B‐cell lymphoma. Leuk Lymphoma 2007; 48: 1102–9. [DOI] [PubMed] [Google Scholar]
- 33. Iqbal J, Meyer PN, Smith LM, et al BCL2 predicts survival in germinal center B‐cell‐like diffuse large B‐cell lymphoma treated with CHOP‐like therapy and rituximab. Clin Cancer Res 2011; 17: 7785–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Agarwal R, Lade S, Liew D, et al Role of immunohistochemistry in the era of genetic testing in MYC‐positive aggressive B‐cell lymphomas: a study of 209 cases. J Clin Pathol 2016; 69: 266–70. [DOI] [PubMed] [Google Scholar]
- 35. Valera A, Lopez‐Guillermo A, Cardesa‐Salzmann T, et al MYC protein expression and genetic alterations have prognostic impact in patients with diffuse large B‐cell lymphoma treated with immunochemotherapy. Haematologica 2013; 98: 1554–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ventura RA, Martin‐Subero JI, Jones M, et al FISH analysis for the detection of lymphoma‐associated chromosomal abnormalities in routine paraffin‐embedded tissue. J Mol Diagn 2006; 8: 141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013; 48: 452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nyman H, Adde M, Karjalainen‐Lindsberg ML, et al Prognostic impact of immunohistochemically defined germinal center phenotype in diffuse large B‐cell lymphoma patients treated with immunochemotherapy. Blood 2007; 109: 4930–5. [DOI] [PubMed] [Google Scholar]
- 39. Tay K, Tai D, Tao M, Quek R, Ha TC, Lim ST. Relevance of the International Prognostic Index in the rituximab era. J Clin Oncol 2011; 29: e14; author reply e5. [DOI] [PubMed] [Google Scholar]
- 40. Copie‐Bergman C, Cuilliere‐Dartigues P, Baia M, et al MYC‐IG rearrangements are negative predictors of survival in DLBCL patients treated with immunochemotherapy: a GELA/LYSA study. Blood 2015; 126: 2466–74. [DOI] [PubMed] [Google Scholar]
- 41. Klanova M, Andera L, Brazina J, et al Targeting of BCL2 Family Proteins with ABT‐199 and Homoharringtonine Reveals BCL2‐ and MCL1‐Dependent Subgroups of Diffuse Large B‐Cell Lymphoma. Clin Cancer Res 2016; 22: 1138–49. [DOI] [PubMed] [Google Scholar]
- 42. Green TM, Young KH, Visco C, et al Immunohistochemical double‐hit score is a strong predictor of outcome in patients with diffuse large B‐cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 2012; 30: 3460–7. [DOI] [PubMed] [Google Scholar]
- 43. Dunleavy K, Grant C, Wilson WH. Using biologic predictive factors to direct therapy of diffuse large B‐cell lymphoma. Ther Adv Hematol 2013; 4: 43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sarkozy C, Traverse‐Glehen A, Coiffier B. Double‐hit and double‐protein‐expression lymphomas: aggressive and refractory lymphomas. Lancet Oncol 2015; 16: e555–67. [DOI] [PubMed] [Google Scholar]
- 45. Mason KD, Vandenberg CJ, Scott CL, et al In vivo efficacy of the Bcl‐2 antagonist ABT‐737 against aggressive Myc‐driven lymphomas. Proc Natl Acad Sci USA 2008; 105: 17961–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. MYC, B‐cell lymphoma (BCL)2, and BCL6 detected by immunohistochemistry in patients with diffuse large B‐cell lymphoma.
Fig. S2. Progression‐free survival (a,c) and overall survival (b,d) according to translocation (a,b) and copy number gain (c,d) of BCL2 in patients with diffuse large B‐cell lymphoma.
Fig. S3. Progression‐free survival (a,c) and overall survival (b,d) according to translocation (a,b) and copy number gain (c,d) of BCL6 in patients with diffuse large B‐cell lymphoma.
Fig. S4. Overall survival according to MYC protein expression in patients with diffuse large B‐cell lymphoma.
Fig. S5. Overall survival according to B‐cell lymphoma (BCL)2 protein expression in patients with diffuse large B‐cell lymphoma.
Fig. S6. Progression‐free survival (a,c) and overall survival (b,d) according to levels of MYC and B‐cell lymphoma (BCL)2 or BCL6 expression in patients with diffuse large B‐cell lymphoma.


