Abstract
Lymph node (LN) macrophages play critical roles in anti‐tumor immunity, which develops via the activation of cytotoxic T cells (CTL) and NK cells. The present study aims to determine the prognostic significance of CD169+ LN macrophages in patients with endometrial carcinoma (EC). The number of CD169+ cells or the CD169+‐to‐CD68+ macrophage ratio in regional LN (RLN), and the number of CD8+ CTL or CD57+ NK cells in tumor tissues were investigated by immunohistochemistry in paraffin‐embedded tissue samples from 79 patients with EC. A high density of CD169+ cells in the RLN of patients with EC was correlated with an early clinical stage or no LN metastasis. A high number of CD169+ cells and a high CD169+‐to‐CD68+ macrophage ratio were significantly associated with longer overall survival in EC. We also found that the density of CD169+ macrophages was positively correlated with the number of CD8+ CTL and CD57+ NK cells that infiltrated into tumor tissues. A high density of CD57+ cells in EC tissues was associated with a better prognosis, while a high density of CD8+ cells was not linked to an altered prognosis. The present study showed that the density of CD169+ macrophages in RLN was associated with an improved prognosis in EC patients. CD169+ macrophages in RLN might represent a useful marker for assessing clinical prognoses and monitoring anti‐tumor immunity in patients with EC.
Keywords: CD169, endometrial carcinoma, NK cell, regional lymph node, sinus macrophage
Lymph nodes (LN) are one of the initial organs that dominate immune responses to immunogenic antigens, such as those derived from pathogens or tumor cells. In the case of antitumor immunity, regional lymph nodes (RLN) that drain various malignant tumors are thought to be the first site where the immune system makes contact with tumor cells or their products.1 Fragmented dead tumor cells can flow into the sinus area of the RLN via lymphatic vessels and then can be endocytosed by a specialized type of resident macrophage, known as sinus macrophages.2 Sinus macrophages internalize, process and present antigens on MHC I complexes, and induce activation of tumor antigen‐specific T‐cell and B‐cell responses.3, 4 These findings may indicate that the actions of sinus macrophages in RLN represent a critical component of the antitumor immune response.
Sinus macrophages in the LN specifically express CD169, a transmembrane receptor that is also known as sialoadhesin or sialic acid‐binding lectin (Siglec)‐1.5, 6 CD169 binds sialylated glycoproteins, including CD43 (sialophorin) and MUC1, and is involved in mediating both cell–cell adhesion and cell–pathogen interactions.6, 7, 8 CD169 is also involved in hematopoiesis and the activation of B, T and NK cells.6, 9 Previous studies of CD169‐deficient mice suggested that CD169 might exacerbate disease in experimental autoimmune encephalomyelitis by inhibiting regulatory T‐cell accumulation.10 In other studies, CD169+ liver macrophages have been shown to promote the cluster formation and the subsequent activation of both CD4+ and CD8+ T cells.11 CD169+ sinus macrophages can also activate NK cells by responding to lymph‐borne viral particles.12 These findings suggest that CD169+ macrophages have proinflammatory functions related to T‐cell and NK cell activation.
Endometrial cancer (EC) is the most common malignant gynecological disease, with an incidence of approximately 287 000 new cases per year, and represents the sixth most common cancer in women worldwide.13, 14 Recently, the incidence rates of EC have continually increased. Most patients with early‐stage EC have an excellent outcome because of effective treatment options, including total hysterectomy and bilateral salpingo‐oophorectomy with systematic pelvic/para‐aortic lymphadenectomy. High‐grade EC, however, show a poor prognosis, despite performing adjuvant therapies that include radiotherapy and chemotherapy. Therefore, alternative therapeutic strategies, such as immunotherapy, are thought to be necessary.15 EC with a high density of intratumoral CD8+ T‐cell infiltration have been established to show a favorable prognosis.16, 17, 18 Clinical trials of immunotherapies targeting several tumor antigens, such as Wilms' tumor gene 1 (WT1)19 and survivin,20 have been performed in patients with EC. For example, three out of four Human Leucocyte Antigen‐A2‐positive EC patients showed tumor regression based on vaccination of autologous dendritic cells electroporated with WT1 mRNA.20 Together, these findings indicate that enhancing the anti‐tumor activity of CD8+ cytotoxic T cells and/or NK cells represents an attractive potential therapy for various malignancies.
Asano and colleagues found that CD169+ LN macrophages could engulf tumor cell antigens and provoke the proliferation of antigen‐specific CD8+ cytotoxic T cells in a mouse tumor transplantation model.21 Bernhard and colleagues show that CD169+ LN macrophages could induce antigen‐specific cytotoxic T‐cell responses in dendritic cell‐depleted mice.22 Together, these findings suggest that CD169+ macrophages play an important role in tumor antigen‐targeted immunotherapy by presenting tumor antigens to cytotoxic lymphocytes. However, interactions between CD169+ macrophages and anti‐tumor immune responses in human EC patients have not yet been fully addressed. Therefore, we investigated potential correlations between CD169+ RLN macrophages and infiltrating immune cells by immunohistochemistry using pathological specimens that we obtained from patients with EC.
Materials and Methods
Patients
In this study, paraffin‐embedded specimens of primary lesions and RLN resected from 79 patients with EC who had undergone surgery at Kumamoto University Hospital between 2003 and 2007 were used. Patient characteristics are indicated in Table S1. The right obturator or internal iliac lymph nodes were dissected as RLN. All patients provided informed written consent for participation in this study, in accordance with protocols approved by the Kumamoto University Hospital Review Board.
Immunostaining and double immunostaining
Tissue samples from EC lesions or RLN were routinely fixed in 10% neutral buffered formalin and embedded in paraffin. All 3‐μm serial sections were stored in a deep freezer until immunostaining was performed. Antigen retrieval was performed as follows: sections were immersed in 1‐mM ethylenediamine tetra‐acetic acid solution (pH 8.0), and samples were heated in a microwave (95°C, 5 min) for CD169 staining (clone HSn 7D2; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or using a pressure cooker for staining CD68 (clone PG‐M1; Dako, Glostrup, Denmark), CD57 (clone NK‐1; Leica, Wetzlar, Germany) and CD8 (clone C8/144B; Nichirei, Tokyo, Japan). An isotype‐matched mouse IgG (Dako) antibody was used as a negative control. Following incubation with primary antibodies, samples were incubated with an HRP‐labeled goat anti‐mouse antibody (Nichirei). Immune reactivity was visualized using a diaminobenzidine substrate system (Nichirei). Double immunostaining of CD169 and CD57 in RLN was performed as described previously.23 Briefly, sections were incubated with an anti‐CD57 antibody, staining was visualized using DAB, and then slides were washed with citrate buffer (pH 2.2). Sections were then incubated with anti‐CD169 antibodies and visualized using HistoGreen (Linaris, Dossenheim, Germany).
Macrophages counts in regional lymph nodes and assessments of CD8+ T‐cell and CD57+ NK cell infiltration in primary lesions
It was possible to identify RLN sinus areas visually on H&E‐stained slides because sinus areas are clearly demarcated by reticular fibers. The numbers of CD68+ and CD169+ cells per mm2 in sinus areas in RLN without metastatic cancer cells and the numbers of CD8+ and CD57+ cells per mm2 in intra‐tumor areas were counted in high power fields (0.028 mm2 per field) by two independent pathologists (K.O. and Y.K.). Both pathologists were blinded to the identities of the samples. To calculate the number of positive cells per unit area, we measured the areas (mm2) using the ImageJ software program (US National Institute of Health, Bethesda, MD, USA). Count data assessed by K.O. or Y.K. were averaged as described previously.23
Statistical analysis
Statistical analyses were carried out using JMP 10 (SAS Institute, Chicago, IL, USA). Associations between different categorical variables were assessed using multivariate analysis. The cumulative survival rate was compared between two groups via the log‐rank test and generalized using the Wilcoxon test. Simultaneous relationships between multiple prognostic factors for survival were assessed using the Cox proportional hazards model with stepwise backwards reductions. Multivariate analysis included adjustments for age, clinical stages, histological grades, LN metastasis, the ratio of CD169+‐to‐CD68+ cells in RLN, and the number of CD57+ cells in tumor tissue. A P‐value < 0.05 was considered to represent a statistically significant difference.
Results
Fewer CD169+ sinus macrophages in regional lymph node from endometrial carcinoma patients with advanced‐stage or lymph node metastasis
We first performed immunostaining to investigate CD68 and CD169 expression in RLN obtained from all EC patients. In patients with LN metastasis, RLN that contained no metastatic carcinoma cells were used for these evaluations. The RLN sinus could be clearly identified as a demarcated area filled with macrophages by both H&E staining and immunostaining. All sinus macrophages were positive for the pan‐macrophage marker CD68, while the number of CD169+ sinus macrophages varied in each patient (Fig. 1). In addition, the sinus macrophages around metastatic carcinoma cells were completely negative for CD169 in RLN of patients with LN metastasis. Similar results were found in other pelvic RLN such as iliac, inguinal or sacral LN (Table S2).
Figure 1.
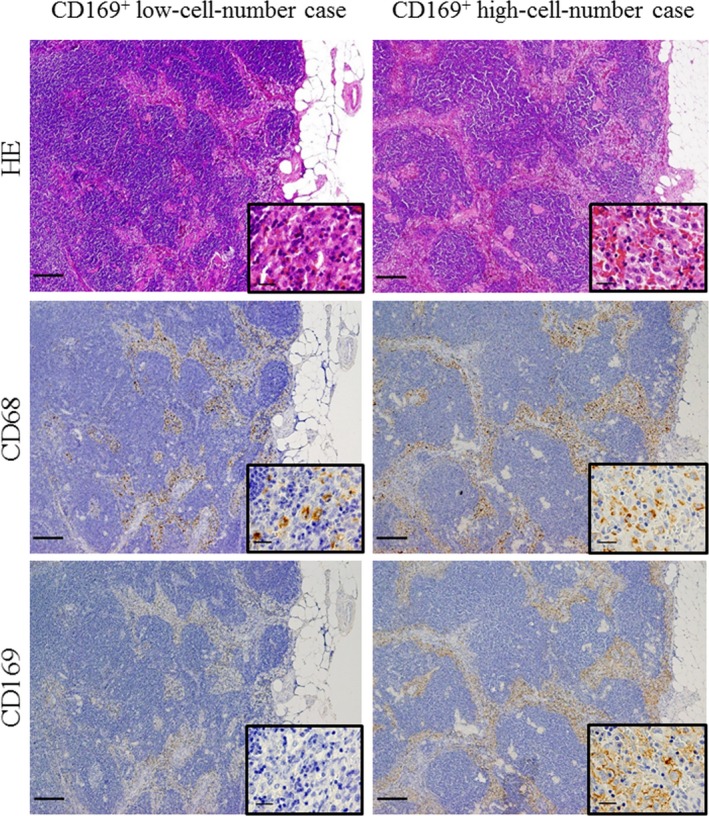
Immunostaining of sinus macrophages in the regional lymph node (RLN) of the endometrial carcinoma (EC) patients. HE staining of sinus areas in the RLN and immunohistochemical analyses of CD68+ and CD169+ macrophages in the RLN. Representative results are shown for cases with high or low numbers of CD169+ cells. Higher magnification images of the squared area are inserted in the lower right inset. Scale bar, 50 μm.
We then analyzed correlations between the clinicopathological features and the number of sinus macrophages in patients with EC. We counted CD68+ and CD169+ cells in the sinus areas, and subsequently classified patients into two groups (those with a low or high cell number) based on the adequate cut‐off value, which was selected by using a comparable categorization while referring to the median count. Statistical analyses showed that fewer CD169+ cells and a lower ratio of CD169+‐to‐CD68+ macrophages were strongly correlated with an advanced clinical stage or LN metastasis; however, the number of CD169+ cells per mm2 and ratio of CD169+‐to‐CD68+ macrophages were not associated with age, grading, depth of muscle invasion, vascular invasion or menstruation (Table 1).
Table 1.
Clinicopathological features and the number of macrophages in regional lymph nodes (RLN) from 79 patients with endometrial carcinoma (EC)
| Clinicopathological feature | n | CD169+ cells/mm2 in RLN | CD68+ cells/mm2 in RLN | ||||
|---|---|---|---|---|---|---|---|
| <350 | ≥350 | P‐value | <750 | ≥750 | P‐value | ||
| Age (years) | |||||||
| <60 | 41 | 20 | 21 | NS | 17 | 24 | NS |
| ≥60 | 38 | 19 | 19 | 22 | 16 | ||
| Stage | |||||||
| I | 41 | 21 | 30 | 0.049a | 25 | 26 | NS |
| II–IV | 38 | 18 | 10 | 14 | 14 | ||
| Grading | |||||||
| G1 | 27 | 12 | 15 | NS | 11 | 16 | NS |
| G2, 3 | 44 | 24 | 20 | 25 | 19 | ||
| Depth of muscle invasion | |||||||
| <50% | 46 | 21 | 25 | NS | 21 | 25 | NS |
| ≥50% | 28 | 13 | 15 | 13 | 15 | ||
| Vascular invasion | |||||||
| Negative | 40 | 18 | 22 | NS | 20 | 20 | NS |
| Positive | 33 | 17 | 16 | 18 | 15 | ||
| Lymph node metastasis | |||||||
| Negative | 66 | 29 | 37 | 0.029a | 32 | 34 | NS |
| Positive | 13 | 10 | 3 | 7 | 6 | ||
| Menstruation | |||||||
| Premenopausal | 23 | 8 | 15 | NS | 5 | 18 | NS |
| Postmenopausal | 56 | 26 | 30 | 25 | 31 | ||
| CD8+ cells/mm2 in tumor | |||||||
| <120 | 36 | 20 | 16 | NS | 21 | 15 | NS |
| ≥120 | 39 | 17 | 22 | 17 | 22 | ||
| CD57+ cells/mm2 in tumor | |||||||
| <50 | 38 | 25 | 13 | 0.004a | 21 | 17 | NS |
| ≥50 | 37 | 12 | 25 | 17 | 20 | ||
Statistically significant results. NS, not significant.
Association of a high density of CD169+ sinus macrophages with a good prognosis in endometrial carcinoma patients
The number of CD169+ cells per mm2 and the ratio of CD169+‐to‐CD68+ macrophages were significantly correlated with more favorable overall survival in a univariate analysis (log‐rank test, P = 0.0139 and P = 0.0042, respectively), but our multivariate analysis indicated that there was no significant correlation (Table 2, Fig. 2). In addition, no correlation existed between the number of CD68+ RLN macrophages and overall survival (Fig. 2). A younger age (<60 years), a lower clinical stage (stage 1), a low histological grading (grade 1) and the absence of LN metastasis were also associated with longer overall survival in the univariate analysis (Table 2). Multivariate analysis showed a significant correlation between overall survival and age, grading, and LN status (Table 2).
Table 2.
Univariate and multivariate Cox regression analyses of potential prognostic factors for overall survival in patients with endometrial carcinoma (EC) (n = 79)
| Clinicopathological feature | n | Univariate analysis P‐value | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| Log‐rank | Wilcoxon | HR | 95% CI | P‐value | ||
| Age (years) | ||||||
| <60 | 41 | 0.0393a | 0.0431a | 8.08 | 0.01‐0.12 | <0.001a |
| ≥60 | 38 | |||||
| Stage | ||||||
| I | 41 | 0.0016a | 0.0012a | ND | ND | ND |
| II–IV | 38 | |||||
| Grading | ||||||
| G1 | 27 | 0.0235a | 0.0229a | 4.46 | 1.59–14.42 | 0.0036a |
| G2, 3 | 44 | |||||
| Depth of muscle invasion | ||||||
| <50% | 46 | 0.1435 | 0.1185 | ND | ND | ND |
| ≥50% | 28 | |||||
| Vascular invasion | ||||||
| Negative | 40 | 0.2808 | 0.3001 | ND | ND | ND |
| Positive | 33 | |||||
| Lymph node metastasis | ||||||
| Negative | 66 | 0.0028a | 0.0029a | 3.99 | 1.52–10.8 | 0.0054a |
| Positive | 13 | |||||
| Menstruation | ||||||
| Premenopausal | 23 | 0.7004 | 0.6791 | ND | ND | ND |
| Postmenopausal | 56 | |||||
| CD169+ cells/mm2 in RLNs | ||||||
| <350 | 40 | 0.0139a | 0.0098a | ND | ND | ND |
| ≥350 | 39 | |||||
| CD68+ cells/mm2 in RLNs | ||||||
| <750 | 40 | 0.6403 | 0.5173 | ND | ND | ND |
| ≥750 | 39 | |||||
| CD169+ cells/CD68+ cells in RLNs | ||||||
| <0.7 | 41 | 0.0042a | 0.0029a | 1.29 | 0.72‐2.39 | 0.395 |
| ≥0.7 | 38 | |||||
| CD8+ cells/mm2 in tumor | ||||||
| <120 | 36 | 0.0959 | 0.1098 | ND | ND | ND |
| ≥120 | 39 | |||||
| CD57+ cells/mm2 in tumor | ||||||
| <50 | 38 | 0.0114a | 0.0177a | 0.32 | 0.11–0.83 | 0.018a |
| ≥50 | 37 | |||||
Statistically significant results. CI, confidence interval; HR, hazard ratio; ND, not done.
Figure 2.
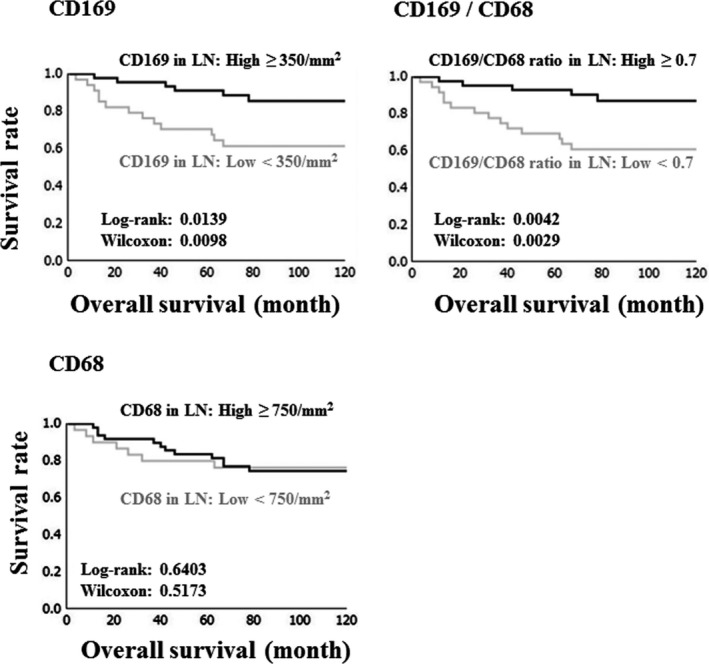
Overall, Kaplan–Meier survival curves for 79 endometrial carcinoma (EC) patients as related to the number of CD169+ macrophages, CD169+‐to‐CD68+ macrophage ratio, and number of CD68+ macrophages in regional lymph node (RLN). LN, lymph node.
Density of CD169+ sinus macrophages correlated positively with CD8+ T‐cell or CD57+ NK cell infiltration in tumor tissues
We next analyzed potential associations between CD169+ RLN macrophages and tumor‐infiltrating CD8+ T cells or CD57+ NK cells in tissues from EC patients. The number of both CD8+ T cells and CD57+ NK cells in tumor nests and tumor stroma increased significantly when CD169+ RLN sinus macrophages were abundant (Fig. 3a). The number of CD169+ cells and the ratio of CD169+‐to‐CD68+ cells correlated positively with the number of CD8+ T cells or CD57+ NK cells in tumor nests and tumor stroma (Fig. 3b). EC patients with abundant CD57+ NK cell infiltration had a more favorable overall survival; however, the number of CD8+ T cells in tumor tissue was not associated with overall survival in EC patients (Table 2, Fig. 3c).
Figure 3.
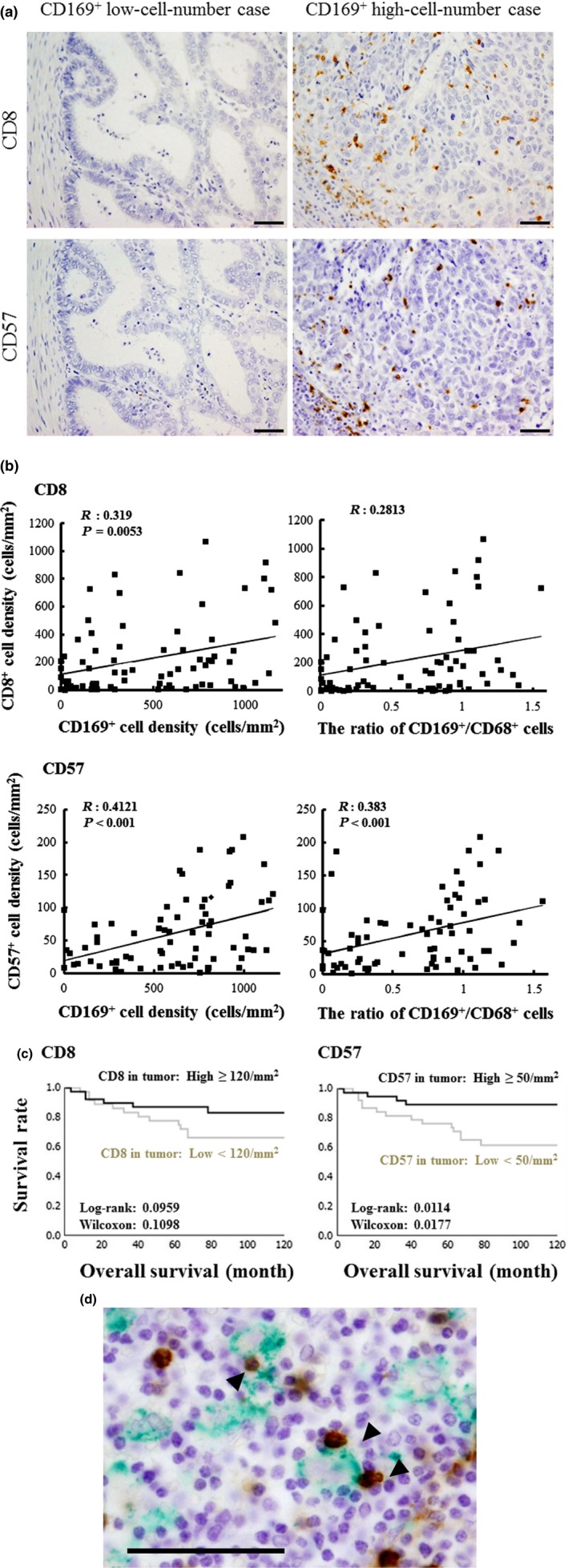
Interactions between CD169+ macrophages in the regional lymph node (RLN) and CD8+ T cells or CD57+ NK cells in endometrial carcinoma (EC). (a) Immunohistochemical analyses of CD8+ T cells and CD57+ NK cells in tumor tissues. Scale bar, 50 μm. (b) Correlation between the number of CD8+ T cells or CD57+ NK cells in tumor tissues and the number of CD169+ macrophages or the CD169+‐to‐CD68+ macrophage ratio in RLN. (c) Overall survival curves for patients showing CD8+ T cells or CD57+ NK cells in tumor tissues. (d) Double immunostaining of CD169 and CD57 in RLN. Arrowheads indicate direct contacts between CD169+ cells and CD57+ cells. Scale bar, 50 μm.
Although CD169+ sinus macrophages exhibited direct contact with CD8+ T cells that expressed CD43, a major ligand of CD169, in the sinus area of RLN in colorectal cancer patients,23 whether interactions occur between CD169+ sinus macrophages and CD57+ NK cells in RLN has remained unclear. To investigate the details of interactions between macrophages and NK cells in RLN, we performed double immunostaining using monoclonal antibodies for CD169 and CD57. We found that approximately 10% of CD169+ cells made direct contact with CD57+ NK cells in the sinus area of the RLN (Fig. 3d).
Discussion
In this present study, we observed greater numbers of CD169+ cells and ratios of CD169+‐to‐CD68+ macrophages in cases of EC with an early clinical stage or no LN metastasis; these findings were significantly correlated with a more favorable overall survival of EC patients. The significant correlation between the number of CD169+ sinus macrophages and density of infiltrating CD8+ T cells and CD57+ NK cells in the primary lesions suggests that CD169+ RLN macrophages are closely associated with the activation of cytotoxic lymphocyte‐mediated antitumor immunity.
Although many previous studies of CD169+ macrophages had led to advances in understanding the distribution of these cells among various macrophage subsets in rodents, only a few have described the regulation of CD169 expression in human macrophages.5, 6 Previously, we showed that a high number of CD169+ sinus macrophages were closely associated with a better prognosis and a higher density of infiltrating CD8+ T cells in tumors of patients with colorectal carcinomas or melanomas.23, 24 Although the density of CD169+ macrophages in RLN was not an independent risk factor for overall survival by multivariate analysis, a higher number of CD169+ macrophages and an increased ratio of CD169+‐to‐CD68+ macrophages in RLN were both significantly associated with a better clinical prognosis in patients with EC. Moreover, we found that the density of CD169+ macrophages was positively correlated with the number of CD8+ T cells and CD57+ NK cells in tumor tissues. These findings suggest that CD169+ sinus macrophages might activate cytotoxic lymphocyte‐mediated anti‐tumor immunity in EC.
Generally, NK cells are considered to be important for immune responses to malignant tumors in humans. Previous studies have examined associations between NK cell infiltration into tumors in patients with breast cancer,25 vulvar cancer26 and colorectal cancer,27, 28 and renal cell carcinoma;29 however, the significance of NK cells in patients with EC had not yet been reported. The present study reveals that EC cases with abundant NK cell infiltration showed better overall survival. Moreover, the number of CD57+ NK cells in tumor tissues was an independent risk factor for overall survival. Garcia and colleagues showed that CD169+ macrophages promoted the accumulation and activation of NK cells after they engulfed viral particles.12 Coombes and colleagues demonstrated that CD169+ sinus macrophages which have inflammatory functions are necessary to NK cell activation in the lymph node sinus area.30 Therefore, we decided to investigate the relationship between CD169+ sinus macrophages and NK cells. By immunohistochemistry, we demonstrated that CD169+ macrophages made direct contact with CD57+ NK cells in the sinus areas of RLN, and that a higher density of CD169+ RLN sinus macrophages was correlated with increased numbers of infiltrating NK cells in tumor tissues. Because all infiltrating macrophages in tumor tissue were negative for CD169 (data not shown), the interaction between CD169+ macrophages and NK cells was shown not in local tumor tissue but in lymph nodes. Although the full mechanism underlying CD169+ macrophage‐mediated NK cell activation remains unclear, our findings suggest that CD169+ macrophages play important roles in anti‐tumor immunity in patients with EC by activating NK cells.
Our previous and present studies together show that the number of CD169+ sinus macrophages in human LN varies considerably, although the number of total sinus macrophages remains approximately constant. However, the reason for this variation in CD169 expression has remained obscure. In the present study, we chose to but examine LN sinus macrophages that were limited to the right obturator or internal iliac LN, we note that we obtained similar data from other RLN, such as the iliac or sacral LN (Table S2), suggesting that CD169 expression varied systemically rather than focally. We propose the following two potential mechanisms for the regulation of CD169 expression: (i) CD169 expression is different between individuals; and (ii) yet to be characterized lymph‐borne effector molecules from tumors suppress CD169 expression. As one potential mechanism, an IFN‐α secretion by cells in RLN might be a strong regulator of CD169 expression, as IFN‐α‐secreting cells are in close proximity to CD169+ sinus macrophages in the RLN and IFN‐α can induce robust CD169 expression in human macrophages.23, 24 Further experiments will be needed to more completely elucidate the mechanisms that regulate CD169 expression in human LN. With a previous paper describing that an activation of NK cells could be enhanced by an IFN‐α secretion in the LN sinus area,12 the CD169+ macrophage‐mediated NK cell activation also might be regulated by IFN‐α‐secreting cells.
In conclusion, we have demonstrated the prognostic significance of CD169+ sinus macrophages in the RLN of patients with EC. A greater number of CD169+ macrophages and an increased ratio of CD169+‐to‐CD68+ macrophages in the RLN were significantly associated with a better clinical prognosis. CD169+ sinus macrophages found in RLN may, therefore, be involved in cytotoxic T‐cell‐mediated and NK cell‐mediated antitumor immunity. Evaluation of CD169 protein expression in RLN may aid estimations of clinical prognoses and improve the monitoring of anti‐tumor immune responses in patients with EC.
Disclosure Statement
The authors have no conflict of interest to declare.
Supporting information
Table S1. Summary of patient characteristics.
Table S2. Distribution of CD169+ cells in dissected regional lymph nodes.
Acknowledgments
We sincerely thank Ms Yui Hayashida, Mr Osamu Nakamura and Mr Takenobu Nakagawa for providing valuable technical assistance. The present study was supported in part by Grants‐in‐Aid for Scientific Research (25293089 and 26460454) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Cancer Sci 107 (2016) 846–852
Funding Information
Grants‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (25293089 and 26460454).
References
- 1. Lores B, García‐Estevez JM, Arias C. Lymph nodes and human tumors (review). Int J Mol Med 1998; 1: 729–33. [DOI] [PubMed] [Google Scholar]
- 2. Swartz MA. Immunomodulatory roles of lymphatic vessels in cancer progression. Cancer Immunol Res 2014; 2: 701–7. [DOI] [PubMed] [Google Scholar]
- 3. Hickok DF, Miller L, Harris L. Regional hyperplastic lymph nodes in breast cancer: the role of lymphocytes and nodal macrophages. An immunological study with a five‐year follow‐up. Surgery 1977; 82: 710–5. [PubMed] [Google Scholar]
- 4. Gray EE, Cyster JG. Lymph node macrophages. J Innate Immun 2012; 4: 424–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martens JH, Kzhyshkowska J, Falkowski‐Hansen M, et al Differential expression of a gene signature for scavenger/lectin receptors by endothelial cells and macrophages in human lymph node sinuses, the primary sites of regional metastasis. J Pathol 2006; 208: 574–89. [DOI] [PubMed] [Google Scholar]
- 6. Martinez‐Pomares L, Gordon S. CD169+ macrophages at the crossroads of antigen presentation. Trends Immunol 2012; 33: 66–70. [DOI] [PubMed] [Google Scholar]
- 7. Nath D, Hartnell A, Happerfield L, et al Macrophage‐tumour cell interactions: identification of MUC1 on breast cancer cells as a potential counter‐receptor for the macrophage‐restricted receptor, sialoadhesin. Immunology 1999; 98: 213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van den Berg TK, Nath D, Ziltener HJ et al Cutting edge: CD43 functions as a T cell counterreceptor for the macrophage adhesion receptor sialoadhesin (Siglec‐1). J Immunol 2001; 166: 3637–40. [DOI] [PubMed] [Google Scholar]
- 9. Chow A, Lucas D, Hidalgo A, et al Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med 2011; 208: 261–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu C, Rauch U, Korpos E et al Sialoadhesin‐positive macrophages bind regulatory T cells, negatively controlling their expansion and autoimmune disease progression. J Immunol 2009; 182: 6508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Müerköster S, Rocha M, Crocker PR, Schirrmacher V, Umansky V. Sialoadhesin‐positive host macrophages play an essential role in graft‐versus‐leukemia reactivity in mice. Blood 1999; 93: 4375–86. [PubMed] [Google Scholar]
- 12. Garcia Z, Lemaître F, van Rooijen N, et al Subcapsular sinus macrophages promote NK cell accumulation and activation in response to lymph‐borne viral particles. Blood 2012; 120: 4744–50. [DOI] [PubMed] [Google Scholar]
- 13. Murali R, Soslow RA, Weigelt B. Classification of endometrial carcinoma: more than two types. Lancet Oncol 2014; 15: e268–78. [DOI] [PubMed] [Google Scholar]
- 14. Kübler K, Ayub TH, Weber SK, et al Prognostic significance of tumor‐associated macrophages in endometrial adenocarcinoma. Gynecol Oncol 2014; 135: 176–83. [DOI] [PubMed] [Google Scholar]
- 15. Elit L, Hirte H. Current status and future innovations of hormonal agents, chemotherapy and investigational agents in endometrial cancer. Curr Opin Obstet Gynecol 2002; 14: 67–73. [DOI] [PubMed] [Google Scholar]
- 16. Kondratiev S, Sabo E, Yakirevich E, Lavie O, Resnick MB. Intratumoral CD8+ T lymphocytes as a prognostic factor of survival in endometrial carcinoma. Clin Cancer Res 2004; 10: 4450–6. [DOI] [PubMed] [Google Scholar]
- 17. de Jong RA, Leffers N, Boezen HM, et al Presence of tumor‐infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol 2009; 114: 105–10. [DOI] [PubMed] [Google Scholar]
- 18. de Jong RA, Boerma A, Boezen HM, Mourits MJ, Hollema H, Nijman HW. Loss of HLA class I and mismatch repair protein expression in sporadic endometrioid endometrial carcinomas. Int J Cancer 2012; 131: 1828–36. [DOI] [PubMed] [Google Scholar]
- 19. Coosemans A, Vanderstraeten A, Tuyaerts S, et al Wilms' Tumor Gene 1 (WT1)–loaded dendritic cell immunotherapy in patients with uterine tumors: a phase I/II clinical trial. Anticancer Res 2013; 33: 5495–500. [PubMed] [Google Scholar]
- 20. Vanderstraeten A, Everaert T, Van Bree R, et al In vitro validation of survivin as target tumor‐associated antigen for immunotherapy in uterine cancer. J Immunother 2015; 38: 239–49. [DOI] [PubMed] [Google Scholar]
- 21. Asano K, Nabeyama A, Miyake Y, et al CD169‐positive macrophages dominate antitumor immunity by crosspresenting dead cell‐associated antigens. Immunity 2011; 34: 85–95. [DOI] [PubMed] [Google Scholar]
- 22. Bernhard CA, Ried C, Kochanek S, Brocker T. CD169+ macrophages are sufficient for priming of CTLs with specificities left out by cross‐priming dendritic cells. Proc Natl Acad Sci USA 2015; 112: 5461–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ohnishi K, Komohara Y, Saito Y, et al CD169‐positive macrophages in regional lymph nodes are associated with a favorable prognosis in patients with colorectal carcinoma. Cancer Sci 2013; 104: 1237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saito Y, Ohnishi K, Miyashita A, et al Prognostic significance of CD169+ lymph node sinus macrophages in patients with malignant melanoma. Cancer Immunol Res 2015; 3: 1356–63. [DOI] [PubMed] [Google Scholar]
- 25. Rathore AS, Goel MM, Makker A, Kumar S, Srivastava AN. Is the tumor infiltrating natural killer cell (NK‐TILs) count in infiltrating ductal carcinoma of breast prognostically significant? Asian Pac J Cancer Prev 2014; 15: 3757–61. [DOI] [PubMed] [Google Scholar]
- 26. Sznurkowski JJ, Zawrocki A, Biernat W. Subtypes of cytotoxic lymphocytes and natural killer cells infiltrating cancer nests correlate with prognosis in patients with vulvar squamous cell carcinoma. Cancer Immunol Immunother 2014; 63: 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qiu H, Xiao‐Jun W, Zhi‐Wei Z, et al The prognostic significance of peripheral T‐lymphocyte subsets and natural killer cells in patients with colorectal cancer. Hepatogastroenterology 2009; 56: 1310–5. [PubMed] [Google Scholar]
- 28. Sconocchia G, Eppenberger S, Spagnoli GC, et al NK cells and T cells cooperate during the clinical course of colorectal cancer. Oncoimmunology 2014; 3: e952197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Geissler K, Fornara P, Lautenschläger C, Holzhausen HJ, Seliger B, Riemann D. Immune signature of tumor infiltrating immune cells in renal cancer. Oncoimmunology 2015; 4: e985082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Coombes JL, Han SJ, van Rooijen N, Raulet DH, Robey EA. Infection‐induced regulation of natural killer cells by macrophages and collagen at the lymph node subcapsular sinus. Cell Rep 2012; 2: 124–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of patient characteristics.
Table S2. Distribution of CD169+ cells in dissected regional lymph nodes.


