Abstract
Vitamin D status is increasingly associated with wide ranging clinical outcomes. There is now a wealth of observational studies reporting on its associations with obstetric complications, including preeclampsia, gestational diabetes and mode and timing of delivery. The findings are inconsistent and currently there is a lack of data from high quality intervention studies to confirm a causal role for vitamin D in these outcomes. This is similarly true with regards to fetal development, including measures of fetal size and skeletal mineralisation. The available data justify the instatement of high-quality randomised placebo controlled trials in a range of populations and health care settings to establish potential efficacy and safety of vitamin D supplementation to improve particular outcomes.
Keywords: Vitamin D, pregnancy, pre-eclampsia, gestational diabetes, osteoporosis, epidemiology
Introduction
The classical role of Vitamin D is in calcium and phosphate homeostasis: it is without doubt that severe vitamin D deficiency (VDD) can result in rickets, osteomalacia and hypocalcaemia. However, there is increasing suggestion that VDD is associated with wide ranging clinical outcomes, including pregnancy complications and adverse fetal development. As a result, a number of national guidelines recommend vitamin D supplementation during pregnancy1–3, although this is not currently supported by the World Health Organisation (WHO)4. Here, we review the evidence basis for antenatal vitamin D supplementation to prevent obstetric complications, and the influence of vitamin D on fetal growth and skeletal development.
Vitamin D physiology and epidemiology in pregnancy
Vitamin D can be derived from the diet, as ergocalciferol (vitamin D2) from plant sources, or cholecalciferol (vitamin D3) from animal sources. However, the majority is formed endogenously within the skin from the action of ultraviolet B (290-315nm wavelength) to convert 7-dehydrocholesterol to pre-vitamin D3. Hydroxylation within the liver produces 25-hydroxyvitamin D [25(OH)D]. This is the main circulating form of vitamin D, found either bound to vitamin D binding protein (VDP), albumin or in the free form. 25(OH)D acts as a reservoir for conversion to 1,25-dihydroxyvitamin D [1,25(OH)2D]), primarily in the renal proximal tubular cells, but also within bone, the parathyroid gland and placenta. Whilst 1,25(OH)2D is the active metabolite, its production is regulated in response to serum calcium and its half life is short at 4-6 hours. Conversely, hepatic 25-hydroxylation is not physiologically regulated and 25(OH)D has a half-life of approximately 2-3 weeks5. Therefore, serum 25(OH)D is currently considered the best marker of vitamin D status6.
The primary function of 1,25(OH)2D is in calcium and phosphate homeostasis, which occurs in conjunction with parathyroid hormone (PTH). Thus, low serum ionised Ca2+ stimulates PTH release, which simultaneously increases renal calcium reabsorption in the distal tubule of the kidney, decreases proximal tubule phosphate reabsorption, and increases 1,25(OH)2D synthesis. The main action of 1,25(OH)2D is to increase uptake of dietary calcium through the intestinal enterocytes, but it also enables PTH induced mobilisation of calcium and phosphate from bone mineral7.
During pregnancy alterations to calcium and phosphate metabolism occur to allow the accretion of calcium within the fetal skeleton, particularly during the last trimester8. This occurs through increased maternal intestinal calcium absorption9, 10 and mobilization of calcium within the maternal skeletal11, but without alteration to maternal serum ionized calcium concentration. Maternal calcitropic hormones, including 1,25(OH)2D, likely have an important role in these adaptations, as total 1,25(OH)2D increases during the second and third trimesters9, 12, although this could also reflect the increase in VDP from early through to late pregnancy10, 13. The effect of pregnancy on 25(OH)D however is less well understood: Zhang et al. observed a reduction in 25(OH)D in late compared with early pregnancy, however as all subjects were recruited in summer months this might reflect seasonal variation13. In contrast, Ritchie et al. reported no significant differences in 25(OH)D measured in 14 women before pregnancy, in each trimester and during lactation10. Nonetheless, biochemically low levels of 25(OH)D are highly prevalent: In a cohort of predominantly Caucasian women in the United Kingdom (UK), 31% had a serum 25(OH)D less than 50nmol/l, which is widely considered to be insufficient, and 18% less than 25nmol/l, often considered deficient14. However in an ethnically more diverse UK population, 36% of women had a 25(OH)D <25nmol/l at pregnancy booking15. Indeed, dark skin pigmentation and extensive skin covering (eg for religious or cultural reasons) are the strongest risk factors for vitamin D deficiency. Obesity is also associated with biochemically low 25(OH)D levels, whereas in pregnancy, use of vitamin D supplements may prevent deficiency14. Maternal 25(OH)D in pregnancy is an important consideration as the fetus is entirely dependent on the mother for 25(OH)D. 25(OH)D readily crosses the placenta, and maternal and umbilical cord venous blood 25(OH)D are moderately well correlated, with randomised controlled trials demonstrating that vitamin D supplementation in pregnancy can increase umbilical cord venous and neonatal serum 25(OH)D compared to placebo16–23.
Obstetric Complications
Observational studies
There are numerous observational studies reporting associations between either vitamin D intake in pregnancy or serum measurement of 25(OH)D and pregnancy complications, including gestational hypertension (GHT) and preeclampsia (PET), gestational diabetes (GDM), timing and mode of delivery. The interpretation and comparison of these studies is limited by the timing of 25(OH)D measurements, ranging from first trimester to delivery, definition used for both VDD and the outcome, covariates adjusted for and study design (eg prospective cohort, case-control).
Gestational hypertension & preeclampsia
Although the aetiology of PET is poorly understood and likely multifactorial, there is some evidence that maternal calcium status might be important, and calcium supplementation can reduce PET risk particularly in women with low calcium intake24. Thus, exploring a role for calcitropic hormones, including vitamin D, is a sensible approach. Several case-control and prospective cohort studies have demonstrated that women who developed PET had lower serum 25(OH)D compared to controls in early25–27, mid28, 29 or late pregnancy25, 30, 31, and that VDD increases the risk of PET25, 30, 32. One case-control study suggested women with serum 25(OH)D<37.5nmol/l measured at less than 22 weeks gestation have a 5-fold higher risk of PET than women with a 25(OH)D>37.5nmol/l, independent of ethnicity, season, gestational age at sampling, pre-pregnancy body mass index (BMI), and educational achievement25. Similarly, in a cohort of 23,425 pregnant women in Norway, lower vitamin D intake estimated from a food frequency questionnaire at 22 weeks gestation was associated with a significantly increased risk of PET33. The lower vitamin D intake in women who developed PET was mostly due to a difference in vitamin D obtained from supplements, suggesting supplementation might prevent PET. However, these findings are not supported by all studies27, 34–41, and indeed in a prospective cohort of 1591 women, for each additional 25nmol/l increment in 25(OH)D in early pregnancy, the risk of GHT (without PET) increased by 30%, but no effect on PET risk was observed38, highlighting possible detrimental effects of higher vitamin D status.
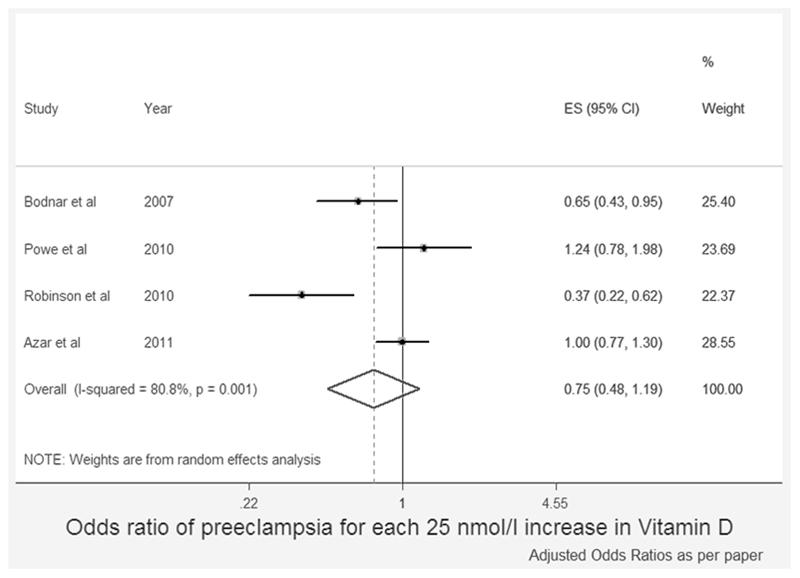
In recent years, there have been several published meta-analyses of the relationship between maternal vitamin D status and PET risk, as shown in table 142–48. Similarly to the observational studies, the conclusions of these are inconsistent. In our own meta-analysis, we found no significant reduction in the risk of PET with higher vitamin D status (Figure 1)47. In contrast, Aghajafari et al. found that the increased risk of PET in VDD was only observed in studies in which blood sampling was later than 16 weeks gestation and when VDD was defined as 25(OH)D<75nmol/l and not <50nmol/l44. However, Tabesh et al., including a larger number of studies defining VDD as less than 50nmol/l, did demonstrate an increased risk of PET, which was not found when deficiency was defined as less than 38nmol/l45. Importantly, the total number of women included in these meta-analyses varied from 610-2485 (excluding those based on intake only and the most recent meta-analyses which included novel data42). However, between January 2013 and July 2014 at least a further 14 case-control or prospective cohort studies with measurement of serum 25(OH)D and assessing PET risk have been published27, 31, 32, 39–42, 48–55. These newer studies include data for a further 21,000 women, considerably more than were included in the meta-analyses.
Table 1. Meta-analyses of maternal vitamin D status (intake and serum 25-hydroxyvitamin D level) and risk of pre-eclampsia.
| Author | Publication cut-off | Number of studies included | Number of women included | Comparison | Risk of preeclampsia with low vitamin D status | |
|---|---|---|---|---|---|---|
| Direction of effect | Reported odds ratio (95%CI) | |||||
| Vitamin D intake | ||||||
| Thorne-Lyman, 2012 43 | June 2011 | 2 | 25141 | Highest vs lowest category of vitamin D intake | ↔ | 0.95 (0.86,1.06) |
| Hypponen, 2013 42 | March 2013 + inclusion of novel data | 2 | 77165 | Self-supplementation vs unsupplemented | ↑ | 1.23 (1.15, 1.33) |
| Serum 25(OH)D | ||||||
| Aghajafari, 2013 44 | August 2012 | 2 | 697 | Serum 25(OH)D ≥50nmol/l vs <50nmol/l | ↔ | 1.27 (0.67, 2.42) |
| 5 | 1165 | Serum 25(OH)D ≥75nmol/l vs <75nmol/l | ↑ | 2.11 (1.36, 3.27) | ||
| 7 | 1862 | Higher serum 25(OH)D as defined by each study vs lower serum 25(OH)D | ↑ | 1.79 (1.25, 2.58) | ||
| 7 | 1862 | Higher serum 25(OH)D as defined by each study vs lower serum 25(OH)D, adjusted for “critical confounders” | ↔ | 1.51 (0.89, 2.57) | ||
| Hypponen, 2013 42 | March 2013 + inclusion of novel data | 6 | 6864 | Higher serum 25(OH)D as defined by each study vs lower serum 25(OH)D | ↑ | 1.92 (1.12, 3.33) |
| Tabesh, 2013 45 | December 2012 | 4 | 931 | Serum 25(OH)D ≥38nmol/l vs <38nmol/l | ↔ | Actual odds ratios not reported |
| 5 | 1775 | Serum 25(OH)D ≥50nmol/l vs <50nmol/l | ↑ | |||
| 8 | 2485 | Higher serum 25(OH)D as defined by each study vs lower serum 25(OH)D | ↑ | |||
| Wei, 2013 46 | October 2012 | 6 | 610 | Serum 25(OH)D ≥50nmol/l vs <50nmol/l | ↑ | 2.09 (1.50, 2.90) |
| 5 | 802 | Serum 25(OH)D ≥75nmol/l vs <75nmol/l | ↑ | 1.78 (1.23, 2.56) | ||
| Harvey, 2014 47 | June 2012 | 4 | 628 | Each 25nmol/l increase in serum 25(OH)D | ↔ | 0.78 (0.59-1.05) |
Figure 1.
Forest plot of the association between maternal vitamin D status and risk of preeclampsia (observational studies)
Reproduced from Harvey N, Holroyd C, Ntani G, Javaid M, Cooper P, Moon R, Cole Z, Tinati T, Godfrey K, Dennison E, Bishop N, Baird J & Cooper C. Vitamin D supplementation in pregnancy: a systematic review. Health Technol Assess 2014 18.
Gestational Diabetes
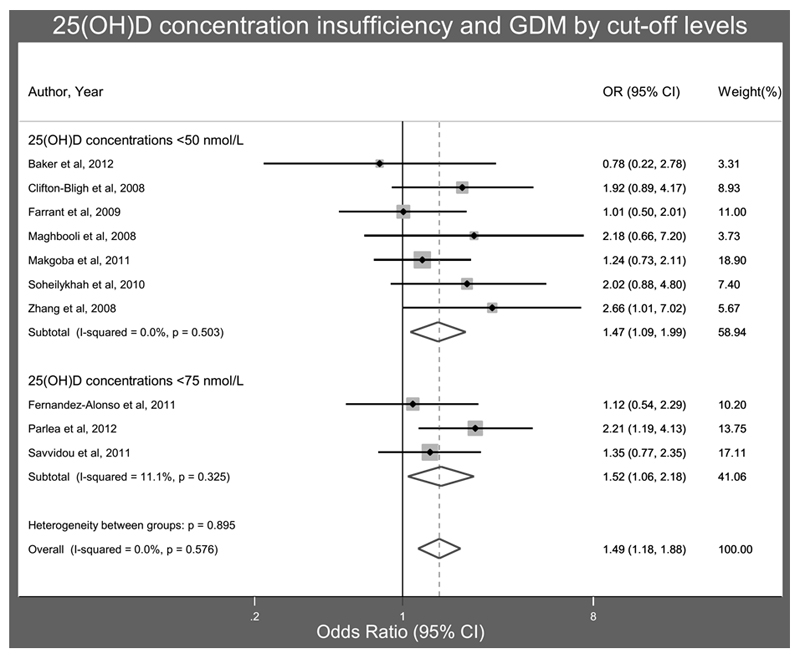
Similarly to PET, conflicting findings have been reported for 25(OH)D status in case-control and prospective cohort studies of GDM risk: both lower48, 56–61 and similar serum 25(OH)D62, 63 during pregnancy in women with and without GDM have been reported. One study of women referred for GDM screening did not find a difference in the prevalence of GDM in women with 25(OH)D above and below 50nmol/l, but the women with 25(OH)D<50nmol/l did have higher fasting blood glucose, HBA1C and insulin resistance. However these women also had higher BMI, lower physical activity and were less likely to be Caucasian, which might have confounded the findings64. Three separate meta-analyses of published studies all concluded that women with GDM had significantly lower mean 25(OH)D than normoglycaemic women44, 46, 65 with the mean difference in 25(OH)D ranging from 3.9 to 7.4nmol/l. Furthermore, these meta-analyses suggested that the risk of GDM was increased by 40-60% in women with VDD44, 46, 65, as shown in Figure 144. However, similarly to studies assessing PET risk, there is now substantially more data available than was used for these meta-analyses39, 48, 58–61, 63, 66 and whilst many of the smaller studies would support the previous conclusions, a large prospective cohort of women in Australia, including 5109 women, of whom 7.4% developed GDM, first trimester VDD (defined either as <25nmol/l or <37.5nmol/l) was not associated with increased risk of GDM compared to 25(OH)D 50-75nmol/l after adjustment for age, parity, smoking during pregnancy, maternal weight, previously diagnosed hypertension, diabetes, season at sampling, country of birth, or socioeconomic disadvantage48. Furthermore in 1953 women in Southern China vitamin D sufficiency (25(OH)D>75nmol/l) at 16-20 weeks gestation was associated with a small, but statistically significant, increased risk of GDM (OR 1.02, 95%CI 1.00, 1.04)39.
Caesarean Delivery
Unsurprisingly, in recent years, there has also been an increase in studies reporting maternal vitamin D status in relation to mode and timing of delivery. Again, these are inconsistent. After adjustment for potential confounding factors three studies which assessed 25(OH)D in early pregnancy, when attending for GDM screening, and at delivery, reported an increased risk of Caesarean delivery64, 67, 68. Conversely, two studies, which measured 25(OH)D in the first trimester demonstrated no increased risk37, 39. Assessment of the influence of VDD on mode of delivery is further complicated by the underlying cause for intervention; however Savvidou et al. additionally categorised women requiring emergency caesarean delivery due to failure to progress and for fetal distress. Neither group had significantly different serum 25(OH)D in early pregnancy compared to women who delivered vaginally69.
Preterm Delivery
More studies have concluded that maternal 25(OH)D status is not related to preterm birth34, 37, 48, 70–74, than have shown VDD increases this risk64, 75, 76. Furthermore, Zhou et al reported women with higher vitamin D status at 16-20 weeks gestation had a higher odds of preterm delivery39, and similarly Hossain et al. found that cord blood 25(OH)D was higher in preterm (<37 weeks gestation) deliveries (mean 55nmol/l) compared to term pregnancies (mean 40nmol/l, p=0.009) in women in Pakistan77. Interestingly, two of the studies which suggest VDD increased the risk of preterm delivery used a definition of less than 35 weeks gestation for preterm75, 76, whereas all, but one74, of the studies reporting either no relationship or VDD reduced the risk considered preterm delivery to be at less than 37 weeks gestation. Whilst this might suggest that VDD is particularly associated with an increased risk of very preterm birth, Schneuer et al, who prospectively studied first trimester 25(OH)D status in over 5000 women, found VDD did not increase the risk of either, all, or spontaneous, preterm birth <34 weeks gestation, before or after adjustment for potential confounding factors48. However, differences in timing of 25(OH)D assessment, and one study showing increased risk including only twin pregnancies75, could account for these different findings. Furthermore, Bodnar et al. observed that only non-white mothers had an increased risk of preterm birth with low 25(OH)D at 26 weeks gestation76, suggesting stratification of women by ethnicity in future intervention studies might be necessary.
Intervention studies of vitamin D supplementation to reduce obstetric complications
Observational data cannot confirm a causal effect of vitamin D or justification for population wide supplementation, particularly as some studies have suggested possible detrimental effects of higher 25(OH)D38, 39, 77. As 25(OH)D status is primarily determined by environmental factors, confounding and reverse causality need to be considered, and differences in covariates included in multivariate models might explain the inconsistent findings. For example, obese individuals have lower 25(OH)D status, and a higher incidence of GDM, GHT, PET, caesarean section and preterm delivery78, 79. Similarly African-American women are more likely to require delivery by Caesarean section and to experience pre-eclampsia and preterm labour80. Whether these outcomes can truly be attributed to lower 25(OH)D compared to Caucasian women and therefore prevented by vitamin D supplementation must be established through intervention studies.
Despite the expanse of observational data, there are currently few trials of antenatal vitamin D supplementation reporting on maternal outcomes other than maternal/neonatal vitamin D and calcium status81. In three of the five studies, the interventional product contained only vitamin D21, 82, 83, whereas a further two assessed the effects of combined vitamin D and calcium supplementation84, 85 (Table 2). The interpretation of these two studies with regards to GHT and PET is limited as calcium supplementation is known to reduce the risk of PET24. Nonetheless, high dose vitamin D supplementation, with or without calcium supplementation, did not improve the incidence of GHT, PET, GDM, or preterm delivery compared to either usual care or low dose supplementation21, 82–85. However these studies were most likely underpowered to detect a difference in these outcomes. GDM complicates approximately 4.5% of pregnancies in the UK86. Thus, to detect a 50% reduction in this incidence with 80% power at the 5% significance level, 1010 women would be needed in each study arm. As PET occurs in 2-3% of pregnancies, even larger study numbers are needed.
Table 2. Intervention studies of vitamin D supplementation (alone, and in combination with calcium supplementation) in pregnancy to reduce obstetric complications.
| Study | Population | Gestation at randomisation | Interventional medicinal product (IMP) | Control | Effect of IMP vs control on incidence of obstetric events | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hypertensive disorders | GDM | Preterm delivery | Caesarean section | Intrauterine death/stillbirth | ||||||
| GHT | PET | |||||||||
| Vitamin D supplementation | ||||||||||
| Hossain, 2014 82 (Karachi, Pakistan) | N=178 | 20 weeks | 4000 IU/day oral cholecalciferol | Usual care | ↔ | ↔ | ↔ | ↔ |
↓ (0 vs 1 case, p=0.05) |
|
| Wagner, 2013 21 (South Carolina, USA)1 | N=504 | 12-16 weeks | 2000 IU/day oral cholecalciferol (n=201) | 400 IU/day oral cholecalciferol (n=111) | ↔ | ↔ | ↔ | |||
| 4000 IU/day oral cholecalciferol (n=193) | ↔ | ↔ | ↔ | |||||||
| Yap, 2014 83 (Sydney, Australia) | N=179 25(OH)D<80nmol/l at baseline | < 20 weeks | 5000 IU/day oral cholecalciferol | 400 IU/day oral cholecalciferol | ↔ | ↔ | ↔ | ↔ | ||
| Vitamin D + Calcium supplementation | ||||||||||
| Kalra, 2011 84 (Lucknow, India) | N=140 | 12-24 weeks | Group 1: 60,000 IU single dose oral cholecalciferol at recruitment + 1g elemental Ca/day until delivery (n=48) Group 2: 120,000 IU oral cholecalciferol at recruitment and 28 weeks gestation + 1g elemental Ca/day until delivery (n=49) |
Usual care (n=43) | ↔ | ↔ | ↔ | |||
| Marya, 1987 85 (Rothak, India) | N=400 | 20-24 weeks | 1200 IU/day vitamin D + 375mg calcium | Usual care | ↔ | |||||
Although trials of vitamin D supplementation have not yet demonstrated a reduction in the incidence of PET or GDM, there is some evidence to support effects on blood pressure and glucose metabolism when considered as continuous outcomes. For example, Marya et al. demonstrated a reduction in both systolic and diastolic BP in women randomised to vitamin D and calcium supplementation compared to those who received usual care85. Confirmation of this finding using vitamin D alone is now needed. Three studies have assessed the effects of vitamin D supplementation on insulin resistance. In an unblinded study of 113 Iranian women randomised to one of three treatment groups (200 IU/day, 50,000 IU/month, 50,000 IU/fortnight) from 12 weeks gestation until delivery, insulin resistance, assessed by HOMA-IR, increased significantly from baseline to delivery in all three groups, but the rise was significantly less in women randomised to 50,000 IU/fortnight than in women who received 200 IU/day87. In contrast, Yap et al found no difference in either fasting blood glucose or that measured two hours post glucose load in women randomised to either 400 IU/day or 5000 IU/day cholecalciferol, with similar results for HOMA-IR83. Finally, in a small study of 54 women with a diagnosis of GDM, two doses of 50,000IU cholecalciferol 3 weeks apart did improve fasting blood glucose and insulin resistance compared to placebo. However the women randomised to vitamin D supplementation had significantly higher insulin resistance at baseline making these results difficult to interpret88. Nonetheless, these findings support the need for further high quality large randomised controlled trials, and to concurrently determine if any effects on maternal physiology might also have beneficial effects on maternal and/or fetal morbidity, for example macrosomia or neonatal hypoglycaemia.
Fetal Development
Early rickets and symptomatic neonatal hypocalcaemia have been reported in infants born to mothers with VDD89–91. However, these outcomes are rarely reported in infants of white mothers, and most commonly occur in those born to mothers with dark skin pigmentation, extensive skin covering and profound VDD. The fetus is dependent on the mother for accretion of approximately 30g of calcium to enable skeletal development. As such, a subclinical role for vitamin D and/or calcium in fetal growth and bone development has been considered, yet maternal supplementation with calcium alone does not appear to have beneficial effects on fetal bone mineral accrual81.
Size at birth
There are now a number of intervention studies assessing the effect of vitamin D supplementation on birth anthropometry, although the dose and timing of introduction of vitamin D varied widely (Table 3). Most studies trialled supplementation with vitamin D alone and did not find a significant effect on birth weight, length or head circumference (Table 1) However, interestingly, vitamin D in combination with calcium did increase birth weight in three studies despite women in the control group also receiving calcium supplementation in two of these studies84, 92, 93. Indeed the prevalence of VDD at baseline and mean 25(OH)D achieved was similar in a study of women in Bangladesh, who received 35,000 IU/day cholecalciferol from 26-30 weeks gestation19, to women participating in a study of 50,000 IU cholecalciferol per week in addition to 200mg elemental calcium supplementation in Iran93. Both studies included a similar number of women. However, in the former study birth weight was similar in both intervention and control groups, whereas in the latter study mean birth weight in the intervention group was 170g greater than that in the control group. These differing findings might suggest that the effect of vitamin D is be dependent on the availability of calcium, or could result from genetic/racial variation in response to vitamin D supplementation, but nonetheless highlight the importance of using data obtained from an appropriate population in the development of antenatal supplementation policies.
Table 3. Intervention studies of the effect of vitamin D supplementation in pregnancy on offspring anthropometry at birth.
| Study | Population | Gestation at Allocation/Randomisation | Interventional medicinal product (IMP) | Control | Effect of vitamin D supplementation | ||
|---|---|---|---|---|---|---|---|
| Birth Weight | Birth Length | Head Circumference | |||||
| Vitamin D only | |||||||
| Brooke 1980 16 (London, UK) |
126 Asian women | 28-32 weeks | 1000 IU/day oral vitamin D | Placebo | ↔ | ↔ | ↔ |
| Mallet 1986 18 (France) |
68 women | Last trimester | Group A: 1000 IU/day oral vitamin D Group B: 200,000 IU single dose in 7th month of pregnancy |
Usual care | ↔ | ||
| Marya 1988 110 (Rohtak, India) |
200 Indian women | 7 months | Single dose of 600000IU cholecalciferol in months 7 and 8 of pregnancy | Usual care | ↑ | ↑ | ↑ |
| Yu 2009 20 (London, UK) |
180 women | 27 weeks | Group A: 800 IU/day oral cholecalciferol Group B: 200000IU oral cholecalciferol single dose at 27 weeks gestation |
Usual care | ↔ | ||
| Dawodu 2013 23 (Al Ain, UAE) |
192 Arab women | 12-16 weeks | Group A: 4000 IU/day oral cholecalciferol Group B: 2000 IU/day oral cholecalciferol |
400 IU/day oral cholecalciferol | ↔ | ↔ | ↔ |
| Grant 2013 17 (Auckland, New Zealand) |
260 women | 26-30 weeks | Group A: 1000IU/day oral cholecaclciferol Group B: 2000IU/day oral cholecalciferol |
Placebo | ↔ | ||
| Wagner 2013 21 (USA) |
Combined analysis of two trials including a total of 513 women | 12-16 weeks | Group A: 2000IU/day oral cholecalciferol Group B: 4000IU/day oral cholecalciferol |
400 IU/day oral cholecalciferol | ↔ | ||
| Roth, 2013 19 (Dhaka, Bangladesh) |
148 | 26-30 weeks | 35000 IU/week oral cholecalcfierol | Placebo | ↔ | ↔ | ↔ |
|
Vitamin D + calcium Marya 1981 92 (Rohtak, India) |
120 Hindu women |
Last trimester |
Group A: 1200IU/day vitamin D + 375mg calcium during third trimester Group B: 600000IU vitamin D orally in the 7th and 8th months of pregnancy (n=20) |
Usual care | ↑ | ||
| Kalra 2011 84 (Lucknow, India) |
140 women | 12-24 weeks | Group A: 60,000IU oral cholecalciferol single dose at randomisation + 1g/day calcium carbonate Group B: 120,000IU oral cholecalciferol at randomisation and at 28 weeks gestation + 1g/day calcium carbonate |
1g calcium carbonate/day | ↑ | ↑ | ↑ |
| Hashemipour 2014 93 (Qazin, Iran) |
109 women, 25(OH)D<75nmol/l | 24-26 weeks | 50,000 IU/week cholecalciferol for 8 weeks in addition to the supplement received by control group | 400IU/day oral cholecalciferol; 200mg elemental calcium | ↑ | ↑ | ↑ |
| Hossain 2014 82 (Karachi, Pakistan) |
198 | 20 weeks | 4000IU/day oral cholecalciferol, 600mg calcium lactate & 200mg ferrous sulphate | 600mg calcium lactate & 200mg ferrous sulphate | ↔ | ↔ | ↔ |
no effect shown,
vitamin D supplementation increased the outcome,
vitamin D supplementation the outcome
Skeletal Development
Currently, the data relating maternal 25(OH)D status to offspring bone development is largely observational in nature, but does span antenatal measurements to peak bone mass. Indeed, using gestational ultrasound, smaller femoral volumes94 and widening of the distal femoral metaphysis relative to femur length has been demonstrated in fetuses of mothers with low levels of serum 25(OH)D95.
A number of studies have demonstrated associations between maternal 25(OH)D status in pregnancy and offspring bone mineralisation in the neonatal period. In 71 Korean neonates, those born in summer (July-September) had 6% higher whole body bone mineral content (BMC) than infants born in winter (January-March), and neonatal 25(OH)D at delivery was correlated with whole body BMC in all children (r=0.24, p=0.05)96. However, in three similar studies by the same author in North America a reversed pattern was observed with whole body BMC 8-12% lower in infants born in summer97. The authors suggest that this difference reflects low uptake of vitamin D supplementation throughout pregnancy in Korea, but only during the first trimester in North America, thereby suggesting early pregnancy during winter might impact on skeletal development97. However, Weiler et al. studied 50 Canadian infants born between August and April, with the majority of mothers taking vitamin D supplementation in pregnancy. Infants with a cord blood 25(OH)D<37.5nmol/l (n=18) were heavier and longer than those with a cord blood 25(OH)D above this cut-point, but skeletal size was not relatively increased, such that whole body and femur BMC relative to body weight were significantly lower98. In a Finnish study, peripheral quantitative computed tomography (pQCT) was used to assess both BMC and bone geometry of the tibia in 98 neonates. In this analysis, the mean of two maternal 25(OH)D measurements in early pregnancy and 2 days postpartum was used to define maternal vitamin D status, and the median for the cohort used to establish two groups. BMC and bone cross-sectional area (CSA) were 13.9% and 16.3% higher, respectively, in infants of mothers with higher 25(OH)D 99. When these children were reassessed at 14 months of age, the difference in tibial BMC was no longer present, but the greater CSA persisted100. Conversely, in 125 Gambian mother-offspring pairs, no significant relationships were observed between maternal 25(OH)D at either 20 or 36 weeks gestation and offspring whole body BMC or bone area at 2, 13 or 52 weeks of age101. However, in contrast to the other studies, no mother had a 25(OH)D less than 50nmol/l, consistent with the notion that poorer skeletal mineralisation might only occur in fetuses of mothers with the lowest vitamin D levels.
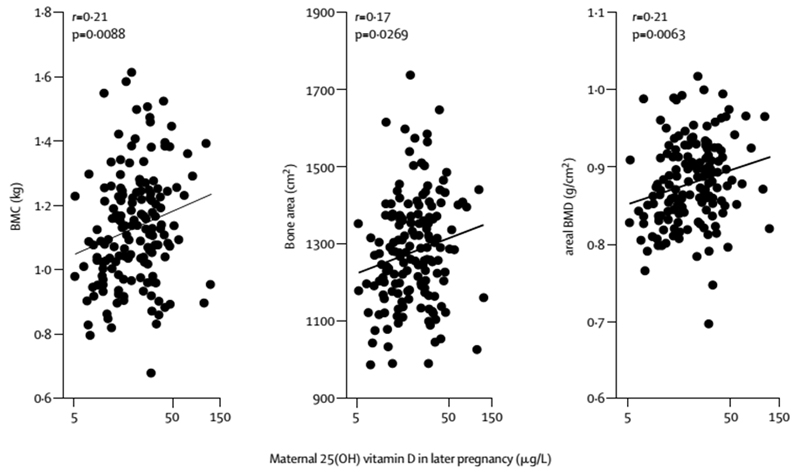
There is evidence to support the persistence of these relationships outside of the neonatal period, although the data are less consistent. In the first study to report on the relationship between maternal 25(OH)D status and offspring bone mineralisation in childhood, Javaid et al demonstrated positive associations between late pregnancy 25(OH)D and offspring whole body and lumbar spine BMC, bone area and areal bone mineral density (aBMD) measured at 9 years (Figure 2)14. Positive relationships with umbilical venous calcium concentration were also observed, suggesting that the effect of vitamin D on skeletal development might be mediated through placental calcium transport14. This was initially supported by data from the Avon Longitudinal Study of Parents and Children (ALSPAC), in which maternal estimated ultraviolet B exposure in late pregnancy, used as a proxy measure of vitamin D status, was positively associated with offspring whole body less head (WBLH) BMC and bone area at 9-10 years of age in 6955 children102. However, subsequent re-analysis in a more limited subset of the ALSPAC cohort using serum 25(OH)D measured in pregnancy demonstrated no association with WBLH BMC or bone area103. Interestingly there was strong collinearly between maternal gestational UVB exposure and offspring age at bone assessment, which limits the interpretation of these studies104. Finally, data from the Raine cohort in Western Australia provide support for a positive relationship between maternal gestational vitamin D status and offspring bone development to peak bone mass: In this study, whole body BMC and aBMD were 2.7% and 1.7% lower, respectively, at 20 years of age in offspring of mothers with 25(OH)D<50nmol/l (compared with offspring of mothers >50nmol/l) at 18 weeks gestation after adjustment for sex, age, height and body composition at 20 years, maternal height and prepregnancy weight, age at delivery, parity, education, ethnicity, smoking during pregnancy, and season of maternal blood sampling105.
Figure 2.
Meta-analysis of maternal serum 25(OH)D in pregnancy and gestational diabetes.
Reproduced from Association between maternal serum25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies, Aghajafari F et al, BMJ 2013;346:f1169 doi: 10.1136/bmj.f1169 with permission from BMJ Publishing Group Ltd.
Currently there is only one intervention study of the effect of vitamin D supplementation in pregnancy on offspring bone mineralisation. Congdon et al. assessed forearm BMC using single photon absorptiometry in 64 infants of Asian mothers living in the UK who participated in a non-randomised study of vitamin D and calcium supplementation in pregnancy106. 19 women received 1000IU vitamin D and a calcium supplement (of unknown strength) during the last trimester, and were compared to 45 women who did not receive any supplement. No significant differences were identified between these two groups, but interpretation of the study findings is limited by the small study size, lack of randomisation and technique used to assess BMC. The ongoing Maternal Vitamin D Osteoporosis Study (MAVIDOS) in which over 1000 women were randomised to 1000 IU cholecalciferol or placebo daily from 14 weeks gestation till delivery, with assessment of offspring bone mineralisation at birth and 4 years of age by dual energy X-ray absorptiometry (DXA)107, will provide much needed high quality evidence on the role of vitamin D supplementation in pregnancy in fetal skeletal development108.
Conclusions
There is now a wealth of observational data relating vitamin D status in pregnancy to obstetric complications, fetal growth and offspring bone development. The findings of these studies are inconsistent and whilst justifying the need for assessment of vitamin D supplementation in high quality randomised controlled trials, observational data alone should not be used as a basis for population wide vitamin D supplementation in pregnancy. Indeed it is possible that the variability in findings of both observational and the few intervention studies reflects the wide heterogeneity in the populations studied (including prevalence of VDD and calcium status, ethnic diversity), dose of vitamin D and timing of initiation or assessment of 25(OH)D status and definition used for the outcomes considered. Thus any public health recommendations need to be based on an appropriate population. Furthermore, whilst currently available data does not suggest any short term detrimental effects for the mother or fetus, the long term safety of vitamin D supplementation, particularly at supra-physiological doses remains to be established.
Figure 3.
Maternal 25(OH)D concentration in late pregnancy and childhood bone mass at age 9 years
Reprinted from The Lancet, Vol 367, Javaid MK et al, Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study, Pages 36–43, Copyright (2014), with permission from Elsevier
Footnotes
Disclosures: NH has received consultancy, lecture fees and honoraria from Alliance for Better Bone Health, AMGEN, MSD, Eli Lilly, Servier, Shire, Consilient Healthcare and Internis Pharma; CC has received consultancy, lecture fees and honoraria from AMGEN, GSK, Alliance for Better Bone Health, MSD, Eli Lilly, Pfizer, Novartis, Servier, Medtronic and Roche.
References
- 1.National Institute for Health and Clincial Excellence. Antenatal care (NICE Clinical Guideline 62) 2010 www.guidance.nice.org.uk/cg62 [PubMed]
- 2.Paxton GA, Teale GR, Nowson CA, Mason RS, McGrath JJ, Thompson MJ, Siafarikas A, Rodda CP, Munns CF. Vitamin D and health in pregnancy, infants, children and adolescents in Australia and New Zealand: a position statement. Med J Aust. 2013;198:142–143. doi: 10.5694/mja11.11592. [DOI] [PubMed] [Google Scholar]
- 3.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organisation. Guideline: Vitamin D supplementation in pregnant women. Geneva: 2012. [PubMed] [Google Scholar]
- 5.Jones KS, Assar S, Harnpanich D, Bouillon R, Lambrechts D, Prentice A, Schoenmakers I. 25(OH)D2 Half-Life Is Shorter Than 25(OH)D3 Half-Life and Is Influenced by DBP Concentration and Genotype. J Clin Endocrinol Metab. 2014;99:3373–3381. doi: 10.1210/jc.2014-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hollis BW, Wagner CL. Clinical review: The role of the parent compound vitamin D with respect to metabolism and function: Why clinical dose intervals can affect clinical outcomes. J Clin Endocrinol Metab. 2013;98:4619–4628. doi: 10.1210/jc.2013-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bikle DS, Adams J, Christakos S. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. John Wiley & Sons, Inc; 2013. Vitamin D: Production, Metabolism, Mechanism of Action, and Clinical Requirements; pp. 235–248. [Google Scholar]
- 8.Kovacs CS. Calcium and Bone Metabolism in Pregnancy and Lactation*. The Journal of Clinical Endocrinology & Metabolism. 2001;86:2344–2348. doi: 10.1210/jcem.86.6.7575. [DOI] [PubMed] [Google Scholar]
- 9.Cross NA, Hillman LS, Allen SH, Krause GF, Vieira NE. Calcium homeostasis and bone metabolism during pregnancy, lactation, and postweaning: a longitudinal study. Am J Clin Nutr. 1995;61:514–523. doi: 10.1093/ajcn/61.3.514. [DOI] [PubMed] [Google Scholar]
- 10.Ritchie LD, Fung EB, Halloran BP, Turnlund JR, Van Loan MD, Cann CE, King JC. A longitudinal study of calcium homeostasis during human pregnancy and lactation and after resumption of menses. Am J Clin Nutr. 1998;67:693–701. doi: 10.1093/ajcn/67.4.693. [DOI] [PubMed] [Google Scholar]
- 11.More C, Bhattoa HP, Bettembuk P, Balogh A. The effects of pregnancy and lactation on hormonal status and biochemical markers of bone turnover. Eur J Obstet Gynecol Reprod Biol. 2003;106:209–213. doi: 10.1016/s0301-2115(02)00237-3. [DOI] [PubMed] [Google Scholar]
- 12.Ardawi MS, Nasrat HA, HS BAA. Calcium-regulating hormones and parathyroid hormone-related peptide in normal human pregnancy and postpartum: a longitudinal study. Eur J Endocrinol. 1997;137:402–409. doi: 10.1530/eje.0.1370402. [DOI] [PubMed] [Google Scholar]
- 13.Zhang JY, Lucey AJ, Horgan R, Kenny LC, Kiely M. Impact of pregnancy on vitamin D status: a longitudinal study. Br J Nutr. 2014:1–7. doi: 10.1017/S0007114514001883. [DOI] [PubMed] [Google Scholar]
- 14.Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, Arden NK, Godfrey KM, Cooper C. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. The Lancet. 2006;367:36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- 15.McAree T, Jacobs B, Manickavasagar T, Sivalokanathan S, Brennan L, Bassett P, Rainbow S, Blair M. Vitamin D deficiency in pregnancy - still a public health issue. Matern Child Nutr. 2013;9:23–30. doi: 10.1111/mcn.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooke OG, Brown IR, Bone CD, Carter ND, Cleeve HJ, Maxwell JD, Robinson VP, Winder SM. Vitamin D supplements in pregnant Asian women: effects on calcium status and fetal growth. Br Med J. 1980;280:751–754. doi: 10.1136/bmj.280.6216.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grant CC, Stewart AW, Scragg R, Milne T, Rowden J, Ekeroma A, Wall C, Mitchell EA, Crengle S, Trenholme A, Crane J, et al. Vitamin D During Pregnancy and Infancy and Infant Serum 25-Hydroxyvitamin D Concentration. Pediatrics. 2013 doi: 10.1542/peds.2013-2602. [DOI] [PubMed] [Google Scholar]
- 18.Mallet E, Gugi B, Brunelle P, Henocq A, Basuyau JP, Lemeur H. Vitamin D supplementation in pregnancy: a controlled trial of two methods. Obstet Gynecol. 1986;68:300–304. doi: 10.1097/00006250-198609000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Roth DE, Al Mahmud A, Raqib R, Akhtar E, Perumal N, Pezzack B, Baqui AH. Randomized placebo-controlled trial of high-dose prenatal third-trimester vitamin D3 supplementation in Bangladesh: the AViDD trial. Nutr J. 2013;12:47. doi: 10.1186/1475-2891-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu CK, Sykes L, Sethi M, Teoh TG, Robinson S. Vitamin D deficiency and supplementation during pregnancy. Clin Endocrinol (Oxf) 2009;70:685–690. doi: 10.1111/j.1365-2265.2008.03403.x. [DOI] [PubMed] [Google Scholar]
- 21.Wagner CL, McNeil RB, Johnson DD, Hulsey TC, Ebeling M, Robinson C, Hamilton SA, Hollis BW. Health characteristics and outcomes of two randomized vitamin D supplementation trials during pregnancy: a combined analysis. J Steroid Biochem Mol Biol. 2013;136:313–320. doi: 10.1016/j.jsbmb.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res. 2011;26:2341–2357. doi: 10.1002/jbmr.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dawodu A, Saadi HF, Bekdache G, Javed Y, Altaye M, Hollis BW. Randomized controlled trial (RCT) of vitamin D supplementation in pregnancy in a population with endemic vitamin D deficiency. J Clin Endocrinol Metab. 2013;98:2337–2346. doi: 10.1210/jc.2013-1154. [DOI] [PubMed] [Google Scholar]
- 24.Hofmeyr GJ, Lawrie TA, Atallah AN, Duley L, Torloni MR. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst Rev. 2014;6:Cd001059. doi: 10.1002/14651858.CD001059.pub4. [DOI] [PubMed] [Google Scholar]
- 25.Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. 2007;92:3517–3522. doi: 10.1210/jc.2007-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker AM, Haeri S, Camargo CA, Jr, Espinola JA, Stuebe AM. A nested case-control study of midgestation vitamin D deficiency and risk of severe preeclampsia. J Clin Endocrinol Metab. 2010;95:5105–5109. doi: 10.1210/jc.2010-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bodnar LM, Simhan HN, Catov JM, Roberts JM, Platt RW, Diesel JC, Klebanoff MA. Maternal vitamin D status and the risk of mild and severe preeclampsia. Epidemiology. 2014;25:207–214. doi: 10.1097/EDE.0000000000000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei SQ, Audibert F, Hidiroglou N, Sarafin K, Julien P, Wu Y, Luo ZC, Fraser WD. Longitudinal vitamin D status in pregnancy and the risk of pre-eclampsia. Bjog. 2012;119:832–839. doi: 10.1111/j.1471-0528.2012.03307.x. [DOI] [PubMed] [Google Scholar]
- 29.Robinson CJ, Alanis MC, Wagner CL, Hollis BW, Johnson DD. Plasma 25-hydroxyvitamin D levels in early-onset severe preeclampsia. Am J Obstet Gynecol. 2010;203:366.e361–366. doi: 10.1016/j.ajog.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu L, Lee M, Jeyabalan A, Roberts JM. The relationship of hypovitaminosis D and IL-6 in preeclampsia. Am J Obstet Gynecol. 2014;210:149.e141–147. doi: 10.1016/j.ajog.2013.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abedi P, Mohaghegh Z, Afshary P, Latifi M. The relationship of serum vitamin D with pre-eclampsia in the Iranian women. Matern Child Nutr. 2014;10:206–212. doi: 10.1111/mcn.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scholl TO, Chen X, Stein TP. Vitamin D, secondary hyperparathyroidism, and preeclampsia. Am J Clin Nutr. 2013;98:787–793. doi: 10.3945/ajcn.112.055871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haugen M, Brantsaeter AL, Trogstad L, Alexander J, Roth C, Magnus P, Meltzer HM. Vitamin D supplementation and reduced risk of preeclampsia in nulliparous women. Epidemiology. 2009;20:720–726. doi: 10.1097/EDE.0b013e3181a70f08. [DOI] [PubMed] [Google Scholar]
- 34.Shand AW, Nassar N, Von Dadelszen P, Innis SM, Green TJ. Maternal vitamin D status in pregnancy and adverse pregnancy outcomes in a group at high risk for pre-eclampsia. Bjog. 2010;117:1593–1598. doi: 10.1111/j.1471-0528.2010.02742.x. [DOI] [PubMed] [Google Scholar]
- 35.Powe CE, Seely EW, Rana S, Bhan I, Ecker J, Karumanchi SA, Thadhani R. First trimester vitamin D, vitamin D binding protein, and subsequent preeclampsia. Hypertension. 2010;56:758–763. doi: 10.1161/HYPERTENSIONAHA.110.158238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seely EW, Wood RJ, Brown EM, Graves SW. Lower serum ionized calcium and abnormal calciotropic hormone levels in preeclampsia. J Clin Endocrinol Metab. 1992;74:1436–1440. doi: 10.1210/jcem.74.6.1592891. [DOI] [PubMed] [Google Scholar]
- 37.Fernandez-Alonso AM, Dionis-Sanchez EC, Chedraui P, Gonzalez-Salmeron MD, Perez-Lopez FR. First-trimester maternal serum 25-hydroxyvitamin D(3) status and pregnancy outcome. Int J Gynaecol Obstet. 2012;116:6–9. doi: 10.1016/j.ijgo.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 38.Burris HH, Rifas-Shiman SL, Huh SY, Kleinman K, Litonjua AA, Oken E, Rich-Edwards JW, Camargo CA, Jr, Gillman MW. Vitamin D status and hypertensive disorders in pregnancy. Ann Epidemiol. 2014;24:399–403. doi: 10.1016/j.annepidem.2014.02.001. e391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou J, Su L, Liu M, Liu Y, Cao X, Wang Z, Xiao H. Associations between 25-hydroxyvitamin D levels and pregnancy outcomes: a prospective observational study in southern China. Eur J Clin Nutr. 2014 doi: 10.1038/ejcn.2014.99. [DOI] [PubMed] [Google Scholar]
- 40.Dalmar A, Raff H, Chauhan SP, Singh M, Siddiqui DS. Serum 25-hydroxyvitamin D, calcium, and calcium-regulating hormones in preeclamptics and controls during first day postpartum. Endocrine. 2014 doi: 10.1007/s12020-014-0296-9. [DOI] [PubMed] [Google Scholar]
- 41.Yu CK, Ertl R, Skyfta E, Akolekar R, Nicolaides KH. Maternal serum vitamin D levels at 11-13 weeks of gestation in preeclampsia. J Hum Hypertens. 2013;27:115–118. doi: 10.1038/jhh.2012.1. [DOI] [PubMed] [Google Scholar]
- 42.Hypponen E, Cavadino A, Williams D, Fraser A, Vereczkey A, Fraser WD, Banhidy F, Lawlor D, Czeizel AE. Vitamin D and pre-eclampsia: original data, systematic review and meta-analysis. Ann Nutr Metab. 2013;63:331–340. doi: 10.1159/000358338. [DOI] [PubMed] [Google Scholar]
- 43.Thorne-Lyman A, Fawzi WW. Vitamin D during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis. Paediatr Perinat Epidemiol. 2012;26 Suppl 1:75–90. doi: 10.1111/j.1365-3016.2012.01283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O'Beirne M, Rabi DM. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ. 2013;346:f1169. doi: 10.1136/bmj.f1169. [DOI] [PubMed] [Google Scholar]
- 45.Tabesh M, Salehi-Abargouei A, Tabesh M, Esmaillzadeh A. Maternal vitamin D status and risk of pre-eclampsia: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98:3165–3173. doi: 10.1210/jc.2013-1257. [DOI] [PubMed] [Google Scholar]
- 46.Wei SQ, Qi HP, Luo ZC, Fraser WD. Maternal vitamin D status and adverse pregnancy outcomes: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2013;26:889–899. doi: 10.3109/14767058.2013.765849. [DOI] [PubMed] [Google Scholar]
- 47.Harvey N, Holroyd C, Ntani G, Javaid M, Cooper P, Moon R, Cole Z, Tinati T, Godfrey K, Dennison E, Bishop N, et al. Vitamin D supplementation in pregnancy: a systematic review. Health Technol Assess. 2014;18 doi: 10.3310/hta18450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schneuer FJ, Roberts CL, Guilbert C, Simpson JM, Algert CS, Khambalia AZ, Tasevski V, Ashton AW, Morris JM, Nassar N. Effects of maternal serum 25-hydroxyvitamin D concentrations in the first trimester on subsequent pregnancy outcomes in an Australian population. Am J Clin Nutr. 2014;99:287–295. doi: 10.3945/ajcn.113.065672. [DOI] [PubMed] [Google Scholar]
- 49.Reeves IV, Bamji ZD, Rosario GB, Lewis KM, Young MA, Washington KN. Vitamin D deficiency in pregnant women of ethnic minority: a potential contributor to preeclampsia. J Perinatol. 2014 doi: 10.1038/jp.2014.91. [DOI] [PubMed] [Google Scholar]
- 50.Xu L, Nicholson P, Wang Q, Alen M, Cheng S. Bone and muscle development during puberty in girls: a seven-year longitudinal study. J Bone Miner Res. 2009;24:1693–1698. doi: 10.1359/jbmr.090405. [DOI] [PubMed] [Google Scholar]
- 51.Bener A, Al-Hamaq AO, Saleh NM. Association between vitamin D insufficiency and adverse pregnancy outcome: global comparisons. Int J Womens Health. 2013;5:523–531. doi: 10.2147/IJWH.S51403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wetta LA, Biggio JR, Cliver S, Abramovici A, Barnes S, Tita AT. Is midtrimester vitamin D status associated with spontaneous preterm birth and preeclampsia? Am J Perinatol. 2014;31:541–546. doi: 10.1055/s-0033-1356483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ullah MI, Koch CA, Tamanna S, Rouf S, Shamsuddin L. Vitamin D deficiency and the risk of preeclampsia and eclampsia in Bangladesh. Horm Metab Res. 2013;45:682–687. doi: 10.1055/s-0033-1345199. [DOI] [PubMed] [Google Scholar]
- 54.Robinson CJ, Wagner CL, Hollis BW, Baatz JE, Johnson DD. Association of maternal vitamin D and placenta growth factor with the diagnosis of early onset severe preeclampsia. Am J Perinatol. 2013;30:167–172. doi: 10.1055/s-0032-1322514. [DOI] [PubMed] [Google Scholar]
- 55.Anderson CM, Ralph J, Johnson L, Scheett A, Wright ML, Taylor JY, Ohm JE, Uthus E. First trimester vitamin D status and placental epigenomics in preeclampsia among Northern Plains primiparas. Life Sci. 2014 doi: 10.1016/j.lfs.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 56.Maghbooli Z, Hossein-Nezhad A, Karimi F, Shafaei AR, Larijani B. Correlation between vitamin D3 deficiency and insulin resistance in pregnancy. Diabetes Metab Res Rev. 2008;24:27–32. doi: 10.1002/dmrr.737. [DOI] [PubMed] [Google Scholar]
- 57.Clifton-Bligh RJ, McElduff P, McElduff A. Maternal vitamin D deficiency, ethnicity and gestational diabetes. Diabet Med. 2008;25:678–684. doi: 10.1111/j.1464-5491.2008.02422.x. [DOI] [PubMed] [Google Scholar]
- 58.Lacroix M, Battista MC, Doyon M, Houde G, Menard J, Ardilouze JL, Hivert MF, Perron P. Lower vitamin D levels at first trimester are associated with higher risk of developing gestational diabetes mellitus. Acta Diabetol. 2014 doi: 10.1007/s00592-014-0564-4. [DOI] [PubMed] [Google Scholar]
- 59.McManus R, Summers K, de Vrijer B, Cohen N, Thompson A, Giroux I. Maternal, umbilical arterial and umbilical venous 25-hydroxyvitamin D and adipocytokine concentrations in pregnancies with and without gestational diabetes. Clin Endocrinol (Oxf) 2014;80:635–641. doi: 10.1111/cen.12325. [DOI] [PubMed] [Google Scholar]
- 60.Cho GJ, Hong SC, Oh MJ, Kim HJ. Vitamin D deficiency in gestational diabetes mellitus and the role of the placenta. Am J Obstet Gynecol. 2013;209:560.e561–568. doi: 10.1016/j.ajog.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 61.Wang O, Nie M, Hu YY, Zhang K, Li W, Ping F, Liu JT, Chen LM, Xing XP. Association between vitamin D insufficiency and the risk for gestational diabetes mellitus in pregnant Chinese women. Biomed Environ Sci. 2012;25:399–406. doi: 10.3967/0895-3988.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 62.Farrant HJ, Krishnaveni GV, Hill JC, Boucher BJ, Fisher DJ, Noonan K, Osmond C, Veena SR, Fall CH. Vitamin D insufficiency is common in Indian mothers but is not associated with gestational diabetes or variation in newborn size. Eur J Clin Nutr. 2009;63:646–652. doi: 10.1038/ejcn.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whitelaw DC, Scally AJ, Tuffnell DJ, Davies TJ, Fraser WD, Bhopal RS, Wright J, Lawlor DA. Associations of circulating calcium and 25-hydroxyvitamin D with glucose metabolism in pregnancy: a cross-sectional study in European and South Asian women. J Clin Endocrinol Metab. 2014;99:938–946. doi: 10.1210/jc.2013-2896. [DOI] [PubMed] [Google Scholar]
- 64.Perez-Ferre N, Torrejon MJ, Fuentes M, Fernandez MD, Ramos A, Bordiu E, del Valle L, Rubio MA, Bedia AR, Montanez C, Calle-Pascual AL. Association of low serum 25-hydroxyvitamin D levels in pregnancy with glucose homeostasis and obstetric and newborn outcomes. Endocr Pract. 2012;18:676–684. doi: 10.4158/EP12025.OR. [DOI] [PubMed] [Google Scholar]
- 65.Poel YH, Hummel P, Lips P, Stam F, van der Ploeg T, Simsek S. Vitamin D and gestational diabetes: a systematic review and meta-analysis. Eur J Intern Med. 2012;23:465–469. doi: 10.1016/j.ejim.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 66.Parildar H, Dogruk Unal A, Aksan Desteli G, Cigerli O, Guvener Demirag N. Frequency of Vitamin D deficiency in pregnant diabetics at Baskent University Hospital, Istanbul. Pak J Med Sci. 2013;29:15–20. doi: 10.12669/pjms.291.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Merewood A, Mehta SD, Chen TC, Bauchner H, Holick MF. Association between vitamin D deficiency and primary cesarean section. J Clin Endocrinol Metab. 2009;94:940–945. doi: 10.1210/jc.2008-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scholl TO, Chen X, Stein P. Maternal vitamin D status and delivery by cesarean. Nutrients. 2012;4:319–330. doi: 10.3390/nu4040319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Savvidou MD, Makgoba M, Castro PT, Akolekar R, Nicolaides KH. First-trimester maternal serum vitamin D and mode of delivery. Br J Nutr. 2012;108:1972–1975. doi: 10.1017/S0007114512000207. [DOI] [PubMed] [Google Scholar]
- 70.Dunlop AL, Taylor RN, Tangpricha V, Fortunato S, Menon R. Maternal micronutrient status and preterm versus term birth for black and white US women. Reprod Sci. 2012;19:939–948. doi: 10.1177/1933719112438442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mehta S, Hunter DJ, Mugusi FM, Spiegelman D, Manji KP, Giovannucci EL, Hertzmark E, Msamanga GI, Fawzi WW. Perinatal outcomes, including mother-to-child transmission of HIV, and child mortality and their association with maternal vitamin D status in Tanzania. J Infect Dis. 2009;200:1022–1030. doi: 10.1086/605699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Delmas PD, Glorieux FH, Delvin EE, Salle BL, Melki I. Perinatal serum bone Gla-protein and vitamin D metabolites in preterm and fullterm neonates. J Clin Endocrinol Metab. 1987;65:588–591. doi: 10.1210/jcem-65-3-588. [DOI] [PubMed] [Google Scholar]
- 73.Thorp JM, Camargo CA, McGee PL, Harper M, Klebanoff MA, Sorokin Y, Varner MW, Wapner RJ, Caritis SN, Iams JD, Carpenter MW, et al. Vitamin D status and recurrent preterm birth: a nested case-control study in high-risk women. Bjog. 2012;119:1617–1623. doi: 10.1111/j.1471-0528.2012.03495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baker AM, Haeri S, Camargo CA, Jr, Stuebe AM, Boggess KA. A nested case-control study of first-trimester maternal vitamin D status and risk for spontaneous preterm birth. Am J Perinatol. 2011;28:667–672. doi: 10.1055/s-0031-1276731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bodnar LM, Rouse DJ, Momirova V, Peaceman AM, Sciscione A, Spong CY, Varner MW, Malone FD, Iams JD, Mercer BM, Thorp JM, Jr, et al. Maternal 25-hydroxyvitamin d and preterm birth in twin gestations. Obstet Gynecol. 2013;122:91–98. doi: 10.1097/AOG.0b013e3182941d9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bodnar LM, Klebanoff MA, Gernand AD, Platt RW, Parks WT, Catov JM, Simhan HN. Maternal vitamin D status and spontaneous preterm birth by placental histology in the US Collaborative Perinatal Project. Am J Epidemiol. 2014;179:168–176. doi: 10.1093/aje/kwt237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hossain N, Khanani R, Hussain-Kanani F, Shah T, Arif S, Pal L. High prevalence of vitamin D deficiency in Pakistani mothers and their newborns. Int J Gynaecol Obstet. 2011;112:229–233. doi: 10.1016/j.ijgo.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 78.Li N, Liu E, Guo J, Pan L, Li B, Wang P, Liu J, Wang Y, Liu G, Baccarelli AA, Hou L, et al. Maternal prepregnancy body mass index and gestational weight gain on pregnancy outcomes. PLoS One. 2013;8:e82310. doi: 10.1371/journal.pone.0082310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bautista-Castano I, Henriquez-Sanchez P, Aleman-Perez N, Garcia-Salvador JJ, Gonzalez-Quesada A, Garcia-Hernandez JA, Serra-Majem L. Maternal obesity in early pregnancy and risk of adverse outcomes. PLoS One. 2013;8:e80410. doi: 10.1371/journal.pone.0080410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang S, Cardarelli K, Shim R, Ye J, Booker KL, Rust G. Racial disparities in economic and clinical outcomes of pregnancy among Medicaid recipients. Matern Child Health J. 2013;17:1518–1525. doi: 10.1007/s10995-012-1162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Curtis EM, Moon RJ, Dennison EM, Harvey NC. Prenatal calcium and vitamin d intake, and bone mass in later life. Curr Osteoporos Rep. 2014;12:194–204. doi: 10.1007/s11914-014-0210-7. [DOI] [PubMed] [Google Scholar]
- 82.Hossain N, Kanani FH, Ramzan S, Kausar R, Ayaz S, Khanani R, Pal L. Obstetric and neonatal outcomes of maternal vitamin D supplementation: Results of an open label randomized controlled trial of antenatal vitamin D supplementation in Pakistani women. J Clin Endocrinol Metab. 2014:jc20133491. doi: 10.1210/jc.2013-3491. [DOI] [PubMed] [Google Scholar]
- 83.Yap C, Cheung NW, Gunton JE, Athayde N, Munns CF, Duke A, McLean M. Vitamin d supplementation and the effects on glucose metabolism during pregnancy: a randomized controlled trial. Diabetes Care. 2014;37:1837–1844. doi: 10.2337/dc14-0155. [DOI] [PubMed] [Google Scholar]
- 84.Kalra P, Das V, Agarwal A, Kumar M, Ramesh V, Bhatia E, Gupta S, Singh S, Saxena P, Bhatia V. Effect of vitamin D supplementation during pregnancy on neonatal mineral homeostasis and anthropometry of the newborn and infant. Br J Nutr. 2012;108:1052–1058. doi: 10.1017/S0007114511006246. [DOI] [PubMed] [Google Scholar]
- 85.Marya RK, Rathee S, Manrow M. Effect of Calcium and Vitamin D Supplementation on Toxaemia of Pregnancy. Gynecol Obstet Invest. 1987;24:38–42. doi: 10.1159/000298772. [DOI] [PubMed] [Google Scholar]
- 86.NICE Clinical Guideline 63. Diabetes in Pregnancy. 2008 [Google Scholar]
- 87.Soheilykhah S, Mojibian M, Moghadam MJ, Shojaoddiny-Ardekani A. The effect of different doses of vitamin D supplementation on insulin resistance during pregnancy. Gynecol Endocrinol. 2013;29:396–399. doi: 10.3109/09513590.2012.752456. [DOI] [PubMed] [Google Scholar]
- 88.Asemi Z, Hashemi T, Karamali M, Samimi M, Esmaillzadeh A. Effects of vitamin D supplementation on glucose metabolism, lipid concentrations, inflammation, and oxidative stress in gestational diabetes: a double-blind randomized controlled clinical trial. Am J Clin Nutr. 2013;98:1425–1432. doi: 10.3945/ajcn.113.072785. [DOI] [PubMed] [Google Scholar]
- 89.Innes AM, Seshia MM, Prasad C, Al SS, Friesen FR, Chudley AE, Reed M, Dilling LA, Haworth JC, Greenberg CR. Congenital rickets caused by maternal vitamin D deficiency. Paediatr Child Health. 2002;7:455–458. doi: 10.1093/pch/7.7.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Anatoliotaki M, Tsilimigaki A, Tsekoura T, Schinaki A, Stefanaki S, Nicolaidou P. Congenital rickets due to maternal vitamin D deficiency in a sunny island of Greece. Acta Paediatr. 2003;92:389–391. doi: 10.1080/08035250310009347. [DOI] [PubMed] [Google Scholar]
- 91.Orbak Z, Karacan M, Doneray H, Karakelleoglu C. Congenital rickets presenting with hypocalcaemic seizures. West Indian Med J. 2007;56:364–367. [PubMed] [Google Scholar]
- 92.Marya RK, Rathee S, Lata V, Mudgil S. Effects of vitamin D supplementation in pregnancy. Gynecol Obstet Invest. 1981;12:155–161. doi: 10.1159/000299597. [DOI] [PubMed] [Google Scholar]
- 93.Hashemipour S, Ziaee A, Javadi A, Movahed F, Elmizadeh K, Javadi EH, Lalooha F. Effect of treatment of vitamin D deficiency and insufficiency during pregnancy on fetal growth indices and maternal weight gain: a randomized clinical trial. Eur J Obstet Gynecol Reprod Biol. 2014;172:15–19. doi: 10.1016/j.ejogrb.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 94.Ioannou C, Javaid MK, Mahon P, Yaqub MK, Harvey NC, Godfrey KM, Noble JA, Cooper C, Papageorghiou AT. The effect of maternal vitamin D concentration on fetal bone. J Clin Endocrinol Metab. 2012;97:E2070–2077. doi: 10.1210/jc.2012-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mahon P, Harvey N, Crozier S, Inskip H, Robinson S, Arden N, Swaminathan R, Cooper C, Godfrey K, Group SWSS. Low maternal vitamin D status and fetal bone development: cohort study. J Bone Miner Res. 2010;25:14–19. doi: 10.1359/jbmr.090701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Namgung R, Tsang RC, Lee C, Han DG, Ho ML, Sierra RI. Low total body bone mineral content and high bone resorption in Korean winter-born versus summer-born newborn infants. J Pediatr. 1998;132:421–425. doi: 10.1016/s0022-3476(98)70013-7. [DOI] [PubMed] [Google Scholar]
- 97.Namgung R, Tsang RC. Factors affecting newborn bone mineral content: in utero effects on newborn bone mineralization. Proc Nutr Soc. 2000;59:55–63. doi: 10.1017/s0029665100000070. [DOI] [PubMed] [Google Scholar]
- 98.Weiler H, Fitzpatrick-Wong S, Veitch R, Kovacs H, Schellenberg J, McCloy U, Yuen CK. Vitamin D deficiency and whole-body and femur bone mass relative to weight in healthy newborns. CMAJ. 2005;172:757–761. doi: 10.1503/cmaj.1040508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Viljakainen HT, Saarnio E, Hytinantti T, Miettinen M, Surcel H, Makitie O, Andersson S, Laitinen K, Lamberg-Allardt C. Maternal vitamin D status determines bone variables in the newborn. J Clin Endocrinol Metab. 2010;95:1749–1757. doi: 10.1210/jc.2009-1391. [DOI] [PubMed] [Google Scholar]
- 100.Viljakainen HT, Korhonen T, Hytinantti T, Laitinen EK, Andersson S, Makitie O, Lamberg-Allardt C. Maternal vitamin D status affects bone growth in early childhood--a prospective cohort study. Osteoporos Int. 2011;22:883–891. doi: 10.1007/s00198-010-1499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Prentice A, Jarjou LM, Goldberg GR, Bennett J, Cole TJ, Schoenmakers I. Maternal plasma 25-hydroxyvitamin D concentration and birthweight, growth and bone mineral accretion of Gambian infants. Acta Paediatr. 2009;98:1360–1362. doi: 10.1111/j.1651-2227.2009.01352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sayers A, Tobias JH. Estimated maternal ultraviolet B exposure levels in pregnancy influence skeletal development of the child. J Clin Endocrinol Metab. 2009;94:765–771. doi: 10.1210/jc.2008-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lawlor DA, Wills AK, Fraser A, Sayers A, Fraser WD, Tobias JH. Association of maternal vitamin D status during pregnancy with bone-mineral content in offspring: a prospective cohort study. The Lancet. 2013;381:2176–2183. doi: 10.1016/S0140-6736(12)62203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harvey NC, Javaid MK, Inskip HM, Godfrey KM, Cooper C. Maternal vitamin D status during pregnancy and bone-mineral content in offspring. Lancet. 2013;382:766. doi: 10.1016/S0140-6736(13)61827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhu K, Whitehouse AJ, Hart P, Kusel M, Mountain J, Lye S, Pennell C, Walsh JP. Maternal Vitamin D Status During Pregnancy and Bone Mass in Offspring at 20 Years of Age: A Prospective Cohort Study. J Bone Miner Res. 2013 doi: 10.1002/jbmr.2138. [DOI] [PubMed] [Google Scholar]
- 106.Congdon P, Horsman A, Kirby PA, Dibble J, Bashir T. Mineral content of the forearms of babies born to Asian and white mothers. Br Med J (Clin Res Ed) 1983;286:1233–1235. doi: 10.1136/bmj.286.6373.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Harvey NC, Javaid K, Bishop N, Kennedy S, Papageorghiou AT, Fraser R, Gandhi SV, Schoenmakers I, Prentice A, Cooper C. MAVIDOS Maternal Vitamin D Osteoporosis Study: study protocol for a randomized controlled trial. The MAVIDOS Study Group. Trials. 2012;13:13. doi: 10.1186/1745-6215-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Harvey NC, Cooper C. Vitamin D: some perspective please. BMJ. 2012;345:e4695. doi: 10.1136/bmj.e4695. [DOI] [PubMed] [Google Scholar]
- 109.Wagner CL, McNeil R, Hamilton SA, Winkler J, Rodriguez Cook C, Warner G, Bivens B, Davis DJ, Smith PG, Murphy M, Shary JR, et al. A randomized trial of vitamin D supplementation in 2 community health center networks in South Carolina. Am J Obstet Gynecol. 2013;208:137.e131–113. doi: 10.1016/j.ajog.2012.10.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Marya RK, Rathee S, Dua V, Sangwan K. Effect of vitamin D supplementation during pregnancy on foetal growth. Indian J Med Res. 1988;88:488–492. [PubMed] [Google Scholar]