Abstract
In vivo glucocorticoid (GC) secretion exhibits a distinctive ultradian rhythmicity. The lipophilic hormone can rapidly diffuse into cells, although only the pulse peak is of sufficient amplitude to activate the low affinity glucocorticoid receptor (GR). Discrete pulses readily access brain regions such as the hippocampus where GR expression is enriched and known to regulate neuronal function, including memory and learning processes. In the present study, we have tested the hypothesis that GR brain targets are responsive to ultradian GC rhythmicity. We have used adrenalectomised rats replaced with pulses of corticosterone to determine the transcriptional effects of ultradian pulses in the hippocampus. Confocal microscopy confirmed that each GC pulse results in transient GR nuclear localisation in hippocampal CA1 neurones. Concomitant GR activation and DNA binding was demonstrated by synthetic glucocorticoid response element oligonucleotide binding, and verified for the Clock gene Period 1 promoter region by chromatin immunoprecipitation assays. Strikingly each GC pulse induced a ‘burst’ of transcription of Period 1 measured by heterogeneous nuclear RNA quantitative polymerase chain reaction. The net effect of pulsatile GC exposure on accumulation of the mature transcript was also assessed, revealing a plateau of mRNA levels throughout the time course of pulsatile exposure, indicating the pulse timing works optimally for steady state Per1 expression. The plateau dropped to baseline within 120 min of the final pulse, indicating a relatively short half-life for hippocampal Per1. The significance of this strict temporal control is that any perturbation to the pulse frequency or duration would have rapid quantitative effects on the levels of Per1. This in turn could affect hippocampal function, especially circadian related memory and learning processes.
Keywords: pulsatility, ultradian rhythm, corticosterone, glucocorticoid receptor GR, Period 1, hippocampus
Pulsatile glucocorticoid (GC) secretion has been reported in all mammalian species studied, including man. This ultradian rhythm exhibits remarkable plasticity, changing with the normal physiological parameters of lactation and ageing, as well as with pathophysiological conditions such as long-term stress, chronic disease and affective disorders (1). In chronic inflammatory conditions, the pulse frequency over the 24-h period is significantly increased (2–4) and the phenomenon of altered pulse size or frequency has also been described in patients with long-term depressive illness and obstructive sleep apnoea (5–7). Deregulation of the ultradian GC rhythm has recently been shown to influence transcriptional responses in brain (8) and may also alter the electrophysiological properties of the hippocampus (9). This may then potentially contribute to the functional and structural changes considered to occur in the hippocampus as a result of long-term depressive illness (10).
The discovery of the molecular clock responsible for the generation of circadian rhythms (11–13) provides novel mechanistic insights into how rhythm abnormalities might lead to disrupted behavioural states, as well as timing abnormalities often associated with chronic disease (14) and affective disorders such as depression (15). The finding that the molecular circadian clock is present in many cells in the central nervous system (11, 16–18) and regulates the timing of the expression of at least 10% of the transcripts in many tissues (19, 20) emphasises the potential drastic consequences of circadian deregulation for normal physiological function throughout the body, including the brain. Clock genes also have an important role in regulating the expression of other clock controlled genes (21). They are considered to differentially modulate neurotransmitter systems, such as the release of dopamine, glutamate and γ-amino butyric acid (22). Per and Clock genes have also been shown to modulate dopamine receptor responsiveness (23) and tyrosine hydroxylase activity (24) respectively, thus further highlighting their significance in neuronal function. However, GC regulation of the Clock gene Period 1 in brain has not been addressed.
Excellent and thorough studies, including those of the Schibler group investigating GC regulation of Clock genes, have elucidated the role of clocks in the synchronisation of peripheral tissues (25). Yamamoto et al. (2005) (26) then proposed Per1 as a stress-regulated and GC-regulated gene in peripheral tissues in mouse. This indicated a third pathway of regulation of Per1 transcriptional control, in addition to the clock-regulated feedback loop of CLOCK-BMAL1/E-box and the light-responsive cAMP-responsive element-binding protein/cAMP-responsive element pathways. In 2006, Koyanagi et al.(27) used continuous administration of the long acting synthetic GC analogue prednisolone into mice, and found that this prevented circadian oscillation of clock genes in two peripheral tissues: skeletal muscle and liver. A variety of biological processes were disrupted, resulting in overt rhythms in physiology and behaviour previously attributed to Clock deregulation (14, 15). This finding may point to a mechanism underlying the disturbed 24-h rhythms often observed in patients in chronic glucocorticoid therapy (28–31), as well as the cognitive deficits associated with disrupted circadian rhythms (32, 33). Prednisolone treatment did not affect the mRNA rhythms of clock genes in the suprachiasmatic nucleus, a highly specialised brain nucleus that appears protected from disruption by stress response. Other higher brain regions were not assessed in the study.
We have previously reported, in cell lines and rat liver, that ultradian GC exposure directs a marked gene pulsing effect, which is optimal for physiological expression levels of several GC-regulated genes including the Clock gene Period 1 (34). In the present study, we tested the hypothesis that GC pulses also regulate transcriptional pulsing of Period 1 in rat hippocampus.
Materials and methods
Animals
Adult Sprague-Dawley rats (250–300 g) (Harlan, Bicester, UK) were maintained under standard housing conditions under a 14 : 10 h light/dark cycle (lights on 05.15 h. Food and water (or saline when specified) were available ad lib. All animal procedures were carried out in accordance with the UK Home Office animal welfare regulations.
Surgery
Rats (n = 6 / time / group) were anaesthetised with IM injection of Hypnorm (fentanyl citrate 0.252 mg / kg and fluanisone 8 mg / kg) after i.p. injection of diazepam (4 mg / kg) and then subjected to bilateral adrenalectomy and jugular cannulation. After surgery the rats were let to recover for 5 days, with adrenalectomised rats receiving corticosterone replacement (15 μg / ml) in 0.1% ethanol saline for drinking.
Pulsatile corticosterone treatment
Corticosterone was withdrawn 24 h prior to the experiment. On the day of the experiment, the rats received up to four bolus i.v. injections of corticosterone-HBC, in the form of a preformed water-soluble complex of corticosterone and 2-hydroxypropyl-ß-cyclodextrin (corticosterone-HBC; C-174; Sigma-Aldrich, St Louis, MO, USA) administered at time 0, 60, 120 and 180 min. Each i.v. injection contained the equivalent of 100 μg of corticosterone.
Immunohistochemistry: tissue preparation and processing
Transcardial perfusion-fixation was performed to process the brains for tissue sectioning and immunohistochemistry, as described previously (35). Briefly, animals were deeply anaesthetised by i.v. injection of pentobarbital sodium salt (Nembutal 1 ml/kg bodyweight; A.U.V., Cuijk, The Netherlands) and then perfused i.c. with ice-cold 0.9% physiological saline followed by 4% paraformaldehyde in 0.1 m phosphate buffer (PB) (pH 7.4). Afterwards, brains were removed and cryoprotected by placing in a solution of 30% sucrose in 4% paraformaldehyde (pH 7.4) until complete saturation. Brains were snap-frozen and using a Leica 1900 cryostat (Leica Microsystems, Wetzlar, Germany), sectioned coronally at 30 μm and stored in antifreeze solution at −20 °C until further use.
Free-floating immunohistochemistry
To study changes in subcellular distribution pattern of GR immunoreactivity (IR) in the rat CA1 pyramidal cells as a consequence of corticosterone administration, free-floating immunohistochemistry was performed on brain slices, as described previously (36). Briefly, to reduce nonspecific binding, slices was blocked by incubating with 0.1 m PB (pH 7.4) containing 2% bovine serum albumin and 0.3% TX-100 for 2 h. After rinsing, sections were incubated with rabbit polyclonal GR (dilution 1 : 500, H300; Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted in phosphate-buffered saline (PBS) containing 0.3% TX-100 for 72 h at 4 °C. After washing, sections were incubated with AlexaFluor-488 labelled goatanti-rabbit immunoglobulin (Ig) G (dilution 1 : 1000; Molecular Probes, Carlsbad, CA, USA) in PBS containing 0.3% TX-100 and 2% bovine serum albumin for 2 h. Finally, sections were washed and nuclei were visualised with Hoechst 33242 (dilution 1 : 10000; Molecular Probes) in PBS after which sections were mounted with Aqua Polymount (Polysciences, Inc., Warrington, PA, USA), and stored in the dark until further analysis. Controls sections were incubated with equal amounts of normal rabbit IgG (Santa Cruz Biotechnology), which were used as substitute for the primary antibody. Additionally, sections were incubated without any primary antibodies to check for any nonspecific binding of the secondary antibodies.
Confocal microscopy and image analysis
To examine the subcellular distribution pattern of GR IR in CA1 cells of the rat hippocampus, a Leica Q550IW confocal microscope was used to acquire images (× 630 magnification, 155 × 155 μm, 1 μm focal plane). To relate changes in nuclear IR as a result of experimental conditions and not microscopic parameters, all settings for filters, lasers and images were left unchanged during imaging. Per animal, two frames were imaged with 30–40 cells on average.
To quantify differences in GR subcellular distribution patterns, changes in fluorescence intensity values of nuclear immunoreactivity were measured using ImageJ 1.32j analysis software (NIH, USA; http://rsb.info.nih.gov/ij/) similarly to as described previously (36, 37). Briefly, Hoechst staining was used to identify the boundaries between the nuclear surface and cytoplasm of individual cells and was circled with the analysis software. These circles served as a template and were pasted onto the corresponding GR images to measure the optical density (mean grey levels) within the nucleus. Nonspecific binding (normal rabbit IgG) and background staining of the sections were also measured and subtracted from the total signal to obtain the specific signal. Only cells that had a clear oval-shaped nucleus with a diameter of approximately 5–7 μm and showed IR clearly above background were included for analysis, thereby excluding cells that were not in the plane of focus.
Statistical analysis
Data are presented as the mean ± SEM. Differences in mean optical density were examined by a one-way anova. Tukey’s post-hoc testing was applied to compare individual groups where applicable. P < 0.05 was considered statistically significant.
Hippocampal collection for DNA binding and RNA analysis studies
At defined times throughout the time course, rats were killed by decapitation. The hippocampus was dissected whole, rapidly frozen in liquid nitrogen, and stored at −80 °C until nuclear extracts were prepared, or RNA extracted. The hippocampus to be used for chromatin immunoprecipitation (ChIP) was fixed and processed immediately. Blood was collected from the intravenous cannula, at times 0 and 1 min relative to each injected pulse and from trunk at time of decapitation, into heparinised tubes and the plasma obtained by centrifugation then stored at −20 °C until measurement of corticosterone by radioimmunoassay (RIA).
Corticosterone RIA
Total plasma corticosterone was quantified by RIA, using a specific corticosterone antibody (gift from G. Makara, Institute of Experimental Medicine, Budapest, Hungary) as previously described (38, 39).
Nuclear extract preparation
Nuclear extracts were prepared according to the method of Vallone et al. (40) as described previously for rat tissue (41). All procedures were performed at 4 °C and on ice. Each hippocampus was homogenised in 500 μl of S1 buffer [10 mm HEPES, pH 7.9, 10 mm KCl, 1.5 mm MgCl2, 0.1 mm ethylenediaminetetraacetic acid (EDTA), pH 8, supplemented with 0.5 mm dithiothreitol, 0.2 mm Na orthovanadate, 2 mm NaF, and Complete protease inhibitor (Roche Diagnostics, Basel, Switzerland)] using a Dounce homogeniser. Nuclear proteins were extracted in 1.2 pellet volume of S2 buffer [10 mm hepes, pH 7.9, 400 mm NaCl, 1.5 mm MgCl2, 0.1 mm EDTA, pH 8 and 5% glycerol, supplemented with 0.5 mm dithiothreitol, 0.2 mm Na orthovanadate, 2 mm NaF and Complete protease inhibitor (Roche Diagnostics)] then stored at −80 °C.
GR:DNA binding assay
A commercially available enzyme-linked immunosorbent assay-based transcription factor binding assay kit (TransAM GR; catalogue no. 45496; Active Motif, Rixensart, Belgium) was used to measure glucocorticoid response element (GRE) binding activity for each nuclear sample. The protein concentration of each sample was determined with bicinchoninic acid assay (catalogue no. 23225; Thermo Scientific, Rockford, IL, USA), and as an additional normalisation control to more accurately measure the integrity of each sample, an aliquot of nuclear extract from each sample was also processed for nuclear transcription factor Y subunit α (NF-YA) DNA binding activity (catalogue no. 40396; Active Motif). NF-YA is a ubiquitous transcription factor that showed no significant difference between the treatment groups. DNA binding assay kits were used in accordance with the manufacturer’s instructions. Briefly, nuclear extracts (20 μg for GR, 5 μg for NF-Y) were incubated in wells of the 96-well plates coated with GR or NF-Y binding consensus oligonucleotide sequence for 1 h, then incubated with the supplied primary anti-GR or NF-Y antibody (dilution 1 : 1000) for 1 h, then with a peroxidase-conjugated second antibody (dilution 1 : 1000) for 1 h. After the substrate was added, colour development was read at 450 nm, and the optical density of both GR and NF-Y were recorded. The results in optical density obtained from the GR assay were first normalised relative to the results obtained from the NF-Y assay. Data were then expressed as fold induction relative to time zero.
ChIP
ChIP was performed using buffers described in the EZ ChIP kit (Upstate Biotechnology, Lake Placid, NY, USA), although the protocol was modified for use of animal tissue. Fresh whole hippocampus of approximately 100 mg were cut into approximately 3 mm3 cubes on ice, and then fixed in 1% (v/v) formaldehyde PBS (10 min at room temperature). Cross-linking was quenched by addition of glycine (final concentration 125 mm) for 5 min. Fixing solution was removed by aspiration, followed by three washes in ice cold PBS supplemented with 2 mm NaF, 0.2 mm Na orthovanadate and Complete protease inhibitor (Roche Diagnostics). Samples were blotted dry and frozen in S1 buffer (described above) on dry ice and stored at −80 °C before further processing. After defrosting slowly on ice, and dounce homogenised to release the nuclear fraction. Nuclei were recovered by centrifugation and lysed in sodium dodecyl sulphate lysis buffer supplemented with Complete protease inhibitor, NaF and Na orthovanadate. Chromatin was sonicated with a Branson Sonifier 450 (Branson Ultrasonics, Danbury, CT, USA) to an average fragment size of 1.5 kb applying 10-s pulses at 10% output. Fifty micrograms of sheared chromatin was immunoprecipitated at 4 °C overnight with 2 μg of M-20 anti-GR antibody (Santa Cruz Inc., Heidelberg, Germany). Antibody-GR-DNA complexes were collected on 60 μl of protein A agarose beads (2 h, 4 °C) and then washed once with low salt buffer, twice with high salt buffer, once with LiCl buffer, and twice with TE buffer (pH 8.0) to remove nonspecific binding. Following elution of antibody-GR-DNA complexes, RNase (Roche Diagnostics) and proteinase K (Sigma, Poole, UK) digestion removed specific contaminants before samples were extracted once in 25 : 24 : 1 phenol-chloroform-isoamyl alcohol and once in 24 : 1 chloroform-isoamyl alcohol (Sigma). DNA was ethanol precipitated overnight at −20 °C, washed in 70% ethanol, and finally dissolved in nuclease free water (Ambion, Huntingdon, UK) ready for polymerase chain reaction (PCR) amplification.
Unlabelled PCR primers (forward: 5′-CCAAGGCTGAGTGCATGTC-3′; reverse: 5′-GCGGCCAGCGCACTA-3′) and a FAM-labelled probe (5′-CAAGAGAACACGATGTTCC-3′) were designed to amplify across a previously described functional GRE (27) in the rat Period 1 gene promoter (Custom Taqman Gene Expression Assay, Applied Biosystems, Warrington, UK; plate ID 619710). Absolute quantification was achieved by comparing samples to a standard curve generated from known quantities of sonicated rat genomic DNA. Samples and standards were amplified on an ABI PRISM 7500 Detection System (Applied Biosystems) in accordance with the manufacturer’s instructions with Universal Taqman PCR master mix (catalogue no. 4304437; Applied Biosystems).
Quantitative real-time PCR
Total RNA was extracted from each whole hippocampus with the phenol-guanidine isothiocyanate-chloroform extraction method using Trizol reagent (catalogue no. 15596026; Invitrogen, Hopkinton, MA, USA) then further purified through RNeasy columns (catalogue no. 74104; Qiagen, Crawley, UK). Genomic DNA contamination was removed from the samples with DNase (catalogue no. 79254; Qiagen) digestion. Complementary DNA was reverse transcribed from 1 μg of total RNA using the cloned AMV first strand synthesis kit (catalogue no. 12328-040; Invitrogen). Rat Period 1 (NM_001034125.1 GI:86477154) nascent transcript was quantified with a Taqman custom designed expression assay, with unlabelled primers and FAM-dye-labelled probe designed within intron 21; primer sequences ATGTGTGCCTGGTGTCTGT, ATGGTGGCTCAACTCCTTAACTC; FAM probe CCTTCTTCAGATTTCC (ABI assay no. 4331348; plate ID 656011; Applied Biosystems). The expression of Period 1 mRNA was quantified using a Taqman Inventoried Expression Assay. Primers and FAM probe were targeted across the intron boundary in exons 21–22 (ABI assay no. Rn01496761_g1)
Briefly, PCR primer-probe mix was added to 50 ng cDNA and Taqman PCR master mix (catalogue no. 4304437; Applied Biosystems) and a comparative CT method was used to detect relative expression curves on an ABI PRISM 7500 detection system (Applied Biosystems). Data were normalised relative to the expression of the endogenous control, Rat β-actin (ABI assay no. 4352931E; Applied Biosystems).
Results
GR localisation in rat hippocampal CA1 neurones throughout the time course of one corticosterone pulse
At time 0 in the adrenalectomised rats, GR immunoreactivity was predominantly cytoplasmic, with very little nuclear GR detectable in the CA1 neurones (Fig. 1A). At 15 min after the pulse was delivered peripherally, significant nuclear translocation of GR was detected (Fig. 1B). This time (15 min) was the earliest time point possible with the perfusion protocol. It is likely that maximal nuclear localisation occurred even more rapidly than this because we have previously reported a significant increase in hippocampal nuclear GR levels as early as 10 min after a pulse is delivered peripherally, albeit using a different method of detection (western blotting of nuclear and cytoplasmic fractions) (41). The remarkable finding, obtained with both methodologies, is not so much the rapidity of nuclear localisation, but rather its transience. By 60 min post GC pulse, nuclear GR immunofluorescence had returned to baseline levels (Fig. 1B). The circulating corticosterone levels measured in the collected plasma samples is shown in Fig. 1(C).
Fig. 1.
GR immunofluorescence throughout the time course of one pulse. (A) Visualisation of GR (top panel) and nuclei stained with Hoechst 33242 (bottom panel). (B) Quantification of GR subcellular localization in pyramidal CA1 cells after one corticosterone pulse with immunohistochemistry and confocal imaging. A clear transient increase of approximately 2-fold was observed in nuclear GR IR already 15 min after corticosterone injection. This effect was not observed in the cytoplasmic compartment. (C) Plasma corticosterone levels were monitored before and at different time points after injection in ADX rats. Data is expressed as mean ± SEM. One-way anova and Tukey’s post-hoc test, *P < 0.01, n = 3–4.
GR activation profiles and GR:GRE association kinetics during two pulses
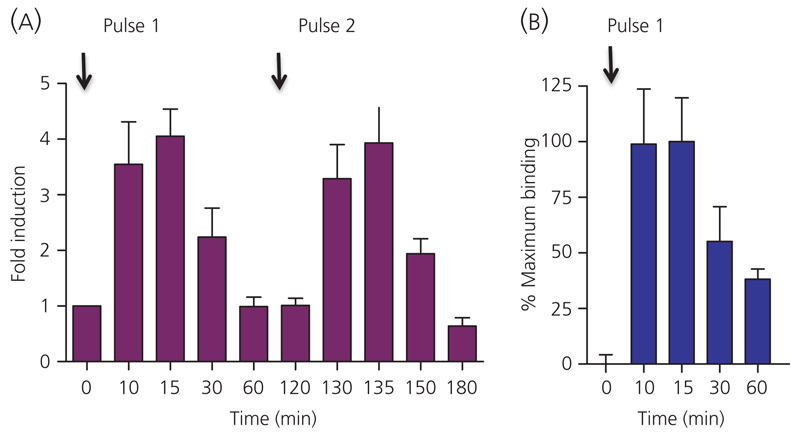
To determine the temporal effect on GR activation of pulsatile GC treatment, rats were injected with two pulses of corticosterone at time 0 and 120 min, as described previously (41). The time course of GR activation resulting from these exogenusly applied pulses is shown in Fig. 2(A). As expected, each corticosterone pulse resulted in a rapid induction of nuclear activated GR, measured by GRE binding ability of the nuclear extracts. Here, a significant induction in GRE binding is evident as early as 10 min after the first pulse is delivered peripherally to the rats (3.6 ± 0.8-fold change relative to time 0; P < 0.05, one-way anova). Maximal nuclear levels of activated GR are detected rapidly after each pulse, and induction at 15 min after the first pulse (4.1 ± 0.5-fold change relative to time 0; P < 0.05, one-way anova) is not significantly higher than the 10-min induction (P > 0.05, one-way anova). Furthermore, the second pulse elicits an effect of similar magnitude to the first (3.3 ± 0.6-fold and 3.9 ± 0.8-fold at 10 and 15 min, respectively, after the pulse is delivered peripherally; all comparisons P < 0.05, one-way anova).
Fig. 2.
GR activation and chromatin association kinetics at the Period 1 gene promoter throughout the time course. (A) GR binding to GRE containing synthetic oligonucleotides was maximal at 10 and 15 min after the first IV corticosterone pulse (fold induction relative to time 0 was 3.6 ± 0.8 at 10 min and 4.1 ± 0.5 at 15 min). Response to the second pulse was of a similar magnitude (fold induction relative to time 0 was 3.3 ± 0.6 at 10 min and 3.9 ± 0.8 at 15 min). This induction declined rapidly and in parallel with ligand clearance from the circulation. At 60 min after each pulse GRE binding had decreased to baseline levels. As well as a similar magnitude of response, each pulse resulted in a similar time course of induction then decline of activated GR levels. (B) The TransAM GRE binding results were validated, for the time course of the first pulse up to 60 min, with chromatin immunoprecipitation (ChIP) assays for GR association with a GRE containing promoter region of the rat Period 1 gene. The time course of GR chromatin association and dissociation detected with the ChIP assay was quantified with qPCR using a custom designed Taqman assay with primer/probe sets flanking the described GRE in the promoter region of the Period 1 gene.
GR is recruited to the Period 1 gene promoter region in hippocampus
Because the TransAM assay measures GR binding to naked DNA, it does not address the question of GR accessibility to specific regulatory regions of target genes in vivo. It certainly cannot answer whether GR will be able to access a specific GRE in native chromatin in a particular cell type because the chromatin structure (and hence GRE availability) is strictly cell specific (42). Under the basal nonstressed conditions in the brain, it appears that regulatory regions may often be less accessible than in other cell types (43). Figure 2(B) shows that the described GRE (27) containing promoter region of Period 1 is accessible for GR binding. The association kinetics are again seen to be extremely rapid, occurring maximally within 10 min of the generation of the pulse (98.9 ± 24.8% of maximum binding at 10 min, and 100.0 ± 19.8% of maximum binding at 15 min; both significantly different (P < 0.05 compared to time 0, one-way anova) and both not significantly different compared to each other (P < 0.05, one-way anova)].
The net association measured by ChIP is relatively short-lived, decreasing to half of maximal association 30 min after the pulse (P < 0.05 compared to both 10 and 15 min) and approaching baseline levels by 60 min (P < 0.05 compared to 10, 15 and 30 min).
Transcriptional pulsing of the Period 1 gene in the hippocampus tracks the ultradian GC rhythm
To determine the effect of pulsatile corticosterone treatment on transcription of the Period 1 gene, four concurrent pulses were applied at hourly intervals. Regular time points (at 30-min intervals) were taken to precisely track the transcriptional temporal kinetics because 30 min was found to be the time of maximal induction of heterogeneous nuclear RNA (hnRNA) after an applied pulse (times analysed 0, 10, 15, 30, 60 min; results not shown). Strikingly, each pulse of intravenous corticosterone resulted in a ‘burst’ of transcription from the Period 1 gene in the hippocampus, which was maximal at 30 min after each pulse was applied (Fig. 3) (fold inductions relative to time 0 were 2.0 ± 0.2 at 30 min, 2.0 ± 0.2 at 90 min, 2.0 ± 0.3 at 150 min, 2.0 ± 0.1 at 210 min; all significantly different from Per1 hnRNA expression at time 0; all P < 0.01, one-way anova). By 60 min after each pulse was applied, the Per1 hnRNA expression levels had decreased such that they were not significantly different from baseline at time 0 (fold inductions relative to time 0 were all P > 0.05, one-way anova).
Fig. 3.
Transcription of Per1 (hnRNA) in the HC during ultradian GC treatment. (A) Circulating corticosterone levels were detected maximally in plasma at 1 min after each pulsed IV injection and then subsequently cleared according to the characterized half-life of corticosterone in blood (t1/2 < 10 min). (B) Nascent transcript production from the Period 1 gene occurred rapidly, reaching a maximum at 30 min after each injection (fold inductions relative to time 0 were 2.0 ± 0.2 at 30 min, 2.0 ± 0.2 at 90 min, 2.0 ± 0.3 at 150 min, 2.0 ± 0.1 at 210 min, for pulses 1, 2, 3 and 4 respectively; all P < 0.01 relative to time 0, one way anova). Consistent with the pattern of GR dissociation from the DNA as ligand was cleared from the circulation, nascent transcript production also decreased in parallel to declining corticosterone levels (albeit delayed) with diminished levels at 60 min after each pulse (all P > 0.05 relative to time 0, one way anova).
Net effect of pulsatile GC exposure on Per1 mRNA accumulation in the hippocampus
The net effect of pulsatile GC exposure on accumulation of the mature Per1 transcript was also assessed over a 5-h period to model the onset and maintenance of the diurnal GC secretory peak. These pulses were, however, delivered in the morning at the time of their natural diurnal nadir. This time course revealed a significant induction of Per1 levels that reached a maximum at 60 min (Fig. 4) (1.6 ± 0.2-fold change relative to time 0; P < 0.001, one-way anova) after the first pulse, and then remained at similar amplitude throughout the time that the pulses were applied (all P < 0.01, except for 30 min and 300 min, P > 0.05). The steady-state expression of Per1 mRNA levels continued throughout the duration of pulsatile GC exposure. After the last pulse was applied, the time course was continued for 2 h to experimentally simulate the approach of the animal’s daily rest phase, and the mRNA levels dropped rapidly to baseline (1.0 ± 0.1-fold change relative to time 0; P > 0.05, one-way anova), indicating a relatively short half-life for hippocampal Per1.
Fig. 4.
Accumulation of Per1 mRNA in the HC during 4 pulses. Accumulation of the mature Per1 transcript reached maximal values at 60 min after the start of the pulses (1.6 ± 0.2 fold change relative to time 0; P < 0.001, one way anova) and then remained elevated throughout the time that the pulses were applied (all P < 0.01, except for time 30 min and 300 min, P > 0.05). The plateau of Per1 mRNA levels continued throughout the duration of pulsatile GC exposure. After the last pulse was applied, the time course was continued for 2 hours to experimentally simulate the approach of the animal’s daily rest phase, and the mRNA levels dropped to baseline in this time (1.0 ± 0.1 fold change at 300 min, relative to time 0; P > 0.05, one way anova).
Discussion
We have shown for the first time that, in the hippocampus, in vivo, rapid responsiveness to changes in hormone levels occurs via interaction of episodically activated GR with GREs in the DNA template. Our immunofluorescent confocal imaging of GR in hippocampal CA1 neurones indicates a rapid yet transient increase in nuclear GR levels after a pulse of ligand is applied peripherally. This finding is in agreement with our previous finding that pulsatile GC addition causes phasic nuclear localisation of GR, but not MR, in hippocampus nuclear extracts. It is, however, in contrast to our finding in cell lines, where GR is retained in the nucleus for several hours after a GC pulse (34, 44). Whether this divergence is due to cell culture/in vivo differences or brain/peripheral tissue differences is unclear at present. However, it is clear that there is defined cell-type specificity in GR nuclear retention kinetics.
The transcriptional regulation of the Clock gene Period 1 by ultradian corticosterone rhythmicity in the hippocampus is rapid and temporally distinctive. Furthermore, the pattern of ‘transcriptional bursting’ was similar to that observed previously in the liver (34). This dramatic gene pulsing effect for Period 1 is the first evidence demonstrating that GCs can regulate Period 1 in the hippocampus, and the first evidence for regulation of a Clock regulator gene in brain by ultradian GC treatment.
These data indicate that corticosterone pulses are sufficient to drive transcription of the Period 1 gene independent of the two previously described regulation pathways of a clock-regulated feedback loop and light/dark cycle cues. We propose that this system may exist to provide a mechanism to ensure optimal interaction between GC ultradian rhythmicity and circadian activity in discrete brain nuclei, in addition to potential regulation by suprachiasmatic nucleus projections. This has significant implications for the interaction of Clock gene products with higher cognitive function.
The present study allows us to suggest a simple mechanistic flow-chart of ultradian glucocorticoid action in the rat hippocampus: (i) there are rapidly fluctuating levels of circulating GC hormone, that result in (ii) rapid and transient nuclear localisation of GR in individual pyramidal neurones of the CA1 region of the hippocampal formation. This is associated with (iii) concomitant ‘global’ GR activation kinetics and in vivo real-time GR cycling on the GRE containing promoter region of the Period 1 gene. This GR activity at the chromatin template leads to (iv) temporally defined transcriptional ‘bursts’ of nascent Per1 hnRNA and (v) accumulation of Per1 mRNA during several hours of ultradian corticosterone treatment, reaching a plateau that is maintained by the hourly pulses. Finally, by artificially mimicking the natural onset of the animal’s inactive or diurnal trough, we can show the rapidity of the effect of declining corticosterone pulses on Per1 mRNA levels in the hippocampus.
Hence, the circadian change in cortisol pulse amplitude provides a highly dynamic system enabling the brain to detect and rapidly respond to the active demands at the start of the day and, equally importantly, to act as a molecular timer for the approach of the rest phase. Recently, using an animal model in which the effect of hourly pulses of corticosterone was compared with that of constant levels of the steroid, it was reported that corticosteroid pulsatility is crucial in maintaining genomic responsiveness to glucocorticoids (45). It appeared that the pulsatile nature of GC stimulation prevents desensitisation of responsivity of GC targets. Such desensensitisation may occur under conditions characteristic for stress-related diseases (10).
There are numerous examples of altered GC secretory patterns. Intriguingly, we have recently found normal pulse frequency but increased pulse mass (assessed by area under curve analysis) in patients with obstructive sleep apnoea (46). After interventive treatment of the obstructive sleep apnoea with continuous positive airways pressure treatment, both pulse characteristics and cardiovascular and metabolic parameters improve (47). suggesting a functional link between altered pulse profile and the associated health disorders. Apart from direct changes in secreted GC patterns, other factors can also alter temporal profiles of GC action. Most notably, synthetic glucocorticoid analogues, which are widely used therapeutically for the treatment of inflammatory disorders from asthma to ulcerative colitis, have much longer half-lives in the circulation, as well as increased GR affinity and prolonged GR activation time (prednisolone t1/2 = 90 min; dexamethasone t1/2 = 120 min). Therefore, treatment with these drugs will effectively change the temporal profile of GR activity. Presently, it is unknown what effect any of these temporal alterations to the GC ultradian rhythm may have on brain function. Clinical reports of mood disorder, mania and anxiety are abundant in patients treated long term with these drugs, although the underlying mechanisms or relationship with temporal deregulation of GR activity in discrete brain regions has never been investigated.
In conclusion, we report the novel finding that ultradian GC exposure regulates transcription of the Period 1 gene in hippocampus in a precise and timely manner. Because this Clock regulator gene then acts to regulate a significant percentage of the genome, it is reasonable to predict that any perturbation in the fine balance of it expression would lead to significant changes in hippocampal function.
Acknowledgements
This work was funded by Wellcome Trust Programme Grant 089647/Z/09/Z to S.L.L. and B.C.C. The support by NWO Mozaïek (R.A.S) and the Royal Academy of Arts and Sciences (KNAW) (E.R.dK) is gratefully acknowledged. We gratefully acknowledge Ms Jennie Douthwaite for her advice and technical assistance in qPCR. The authors declare that there are no conflicts of interest.
References
- 1.Lightman SL, Wiles CC, Atkinson HC, Henley DE, Russell GM, Leendertz JA, McKenna MA, Spiga F, Wood SA, Conway-Campbell BL. The significance of glucocorticoid pulsatility. Eur J Pharmacol. 2008;583:255–262. doi: 10.1016/j.ejphar.2007.11.073. [DOI] [PubMed] [Google Scholar]
- 2.Harbuz MS, Windle RJ, Jessop DS, Renshaw D, Ingram CD, Lightman SL. Differential effects of psychological and immunological challenge on the hypothalamo-pituitary-adrenal axis function in adjuvant-induced arthritis. Ann NY Acad Sci. 1999;876:43–52. doi: 10.1111/j.1749-6632.1999.tb07621.x. [DOI] [PubMed] [Google Scholar]
- 3.Shanks N, Windle RJ, Perks PA, Harbuz MS, Jessop DS, Ingram CD, Lightman SL. Early-life exposure to endotoxin alters hypothalamic-pituitary-adrenal function and predisposition to inflammation. Proc Natl Acad Sci USA. 2000;97:5645–5650. doi: 10.1073/pnas.090571897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Windle RJ, Wood SA, Kershaw YM, Lightman SL, Ingram CD, Harbuz MS. Increased corticosterone pulse frequency during adjuvant-induced arthritis and its relationship to alterations in stress responsiveness. J Neuroendocrinol. 2001;13:905–911. doi: 10.1046/j.1365-2826.2001.00715.x. [DOI] [PubMed] [Google Scholar]
- 5.Young EA, Abelson J, Lightman SL. Cortisol pulsatility and its role in stress regulation and health. Front Neuroendocrinol. 2004;25:69–76. doi: 10.1016/j.yfrne.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Young EA, Carlson NE, Brown MB. Twenty-four-hour ACTH and cortisol pulsatility in depressed women. Neuropsychopharmacology. 2001;25:267–276. doi: 10.1016/S0893-133X(00)00236-0. [DOI] [PubMed] [Google Scholar]
- 7.Deuschle M, Schweiger U, Weber B, Gotthardt U, Korner A, Schmider J, Standhardt H, Lammers CH, Heuser I. Diurnal activity and pulsatility of the hypothalamus-pituitary-adrenal system in male depressed patients and healthy controls. J Clin Endocrinol Metab. 1997;82:234–238. doi: 10.1210/jcem.82.1.3689. [DOI] [PubMed] [Google Scholar]
- 8.Datson NA, van der Perk J, de Kloet ER, Vreugdenhil E. Identification of corticosteroid-responsive genes in rat hippocampus using serial analysis of gene expression. Eur J Neurosci. 2001;14:675–689. doi: 10.1046/j.0953-816x.2001.01685.x. [DOI] [PubMed] [Google Scholar]
- 9.Joels M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 11.Hastings MH, Reddy AB, Garabette M, King VM, Chahad-Ehlers S, O'Brien J, Maywood ES. Expression of clock gene products in the suprachiasmatic nucleus in relation to circadian behaviour. Novartis Found Symp. 2003;253:203–217. doi: 10.1002/0470090839.ch15. [DOI] [PubMed] [Google Scholar]
- 12.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- 13.Meyer-Bernstein EL, Jetton AE, Matsumoto SI, Markuns JF, Lehman MN, Bittman EL. Effects of suprachiasmatic transplants on circadian rhythms of neuroendocrine function in golden hamsters. Endocrinology. 1999;140:207–218. doi: 10.1210/endo.140.1.6428. [DOI] [PubMed] [Google Scholar]
- 14.Maywood ES, O'Neill J, Wong GK, Reddy AB, Hastings MH. Circadian timing in health and disease. Prog Brain Res. 2006;153:253–269. doi: 10.1016/S0079-6123(06)53015-8. [DOI] [PubMed] [Google Scholar]
- 15.Winget CM, DeRoshia CW, Markley CL, Holley DC. A review of human physiological and performance changes associated with desynchronosis of biological rhythms. Aviat Space Environ Med. 1984;55:1085–1096. [PubMed] [Google Scholar]
- 16.Yamamoto S, Shigeyoshi Y, Ishida Y, Fukuyama T, Yamaguchi S, Yagita K, Moriya T, Shibata S, Takashima N, Okamura H. Expression of the Per1 gene in the hamster: brain atlas and circadian characteristics in the suprachiasmatic nucleus. J Comp Neurol. 2001;430:518–532. [PubMed] [Google Scholar]
- 17.Reddy AB, Wong GK, O'Neill J, Maywood ES, Hastings MH. Circadian clocks: neural and peripheral pacemakers that impact upon the cell division cycle. Mutat Res. 2005;574:76–91. doi: 10.1016/j.mrfmmm.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 18.Hastings MH. Central clocking. Trends Neurosci. 1997;20:459–464. doi: 10.1016/s0166-2236(97)01087-4. [DOI] [PubMed] [Google Scholar]
- 19.Liu D, Peddada SD, Li L, Weinberg CR. Phase analysis of circadian-related genes in two tissues. BMC Bioinformatics. 2006;7:87. doi: 10.1186/1471-2105-7-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akhtar RA, Reddy AB, Maywood ES, Clayton JD, King VM, Smith AG, Gant TW, Hastings MH, Kyriacou CP. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr Biol. 2002;12:540–550. doi: 10.1016/s0960-9822(02)00759-5. [DOI] [PubMed] [Google Scholar]
- 21.Delaunay F, Laudet V. Circadian clock and microarrays: mammalian genome gets rhythm. Trends Genet. 2002;18:595–597. doi: 10.1016/s0168-9525(02)02794-4. [DOI] [PubMed] [Google Scholar]
- 22.Castaneda TR, de Prado BM, Prieto D, Mora F. Circadian rhythms of dopamine, glutamate and GABA in the striatum and nucleus accumbens of the awake rat: modulation by light. J Pineal Res. 2004;36:177–185. doi: 10.1046/j.1600-079x.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- 23.Andretic R, Hirsh J. Circadian modulation of dopamine receptor responsiveness in Drosophila melanogaster. Proc Natl Acad Sci USA. 2000;97:1873–1878. doi: 10.1073/pnas.97.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, Nestler EJ. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci USA. 2005;102:9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto T, Nakahata Y, Tanaka M, Yoshida M, Soma H, Shinohara K, Yasuda A, Mamine T, Takumi T. Acute physical stress elevates mouse period1 mRNA expression in mouse peripheral tissues via a glucocorticoid-responsive element. J Biol Chem. 2005;280:42036–42043. doi: 10.1074/jbc.M509600200. [DOI] [PubMed] [Google Scholar]
- 27.Koyanagi S, Okazawa S, Kuramoto Y, Ushijima K, Shimeno H, Soeda S, Okamura H, Ohdo S. Chronic treatment with prednisolone represses the circadian oscillation of clock gene expression in mouse peripheral tissues. Mol Endocrinol. 2006;20:573–583. doi: 10.1210/me.2005-0165. [DOI] [PubMed] [Google Scholar]
- 28.Nishiyama M, Makino S, Suemaru S, Nanamiya W, Asaba K, Kaneda T, Mimoto T, Nishioka T, Takao T, Hashimoto K. Glucocorticoid effects on the diurnal rhythm of circulating leptin levels. Horm Res. 2000;54:69–73. doi: 10.1159/000053234. [DOI] [PubMed] [Google Scholar]
- 29.Motson RW, Glass DN, Smith DA, Daly JR. The effect of short-and long-term corticosteroid treatment on sleep-associated growth hormone secretion. Clin Endocrinol (Oxf) 1978;8:315–326. doi: 10.1111/j.1365-2265.1978.tb02774.x. [DOI] [PubMed] [Google Scholar]
- 30.Tornatore KM, Logue G, Venuto RC, Davis PJ. Cortisol pharmacodynamics after methylprednisolone administration in young and elderly males. J Clin Pharmacol. 1997;37:304–311. doi: 10.1002/j.1552-4604.1997.tb04307.x. [DOI] [PubMed] [Google Scholar]
- 31.Ivarsen P, Jensen LW, Pedersen EB. Circadian blood pressure rhythm and atrial natriuretic peptide in prednisolone-induced blood pressure elevation. Scand J Clin Lab Invest. 1995;55:655–662. doi: 10.3109/00365519509075395. [DOI] [PubMed] [Google Scholar]
- 32.Cho K, Ennaceur A, Cole JC, Suh CK. Chronic jet lag produces cognitive deficits. J Neurosci. 2000;20:RC66. doi: 10.1523/JNEUROSCI.20-06-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilpin H, Whitcomb D, Cho K. Atypical evening cortisol profile induces visual recognition memory deficit in healthy human subjects. Mol Brain. 2008;1:4. doi: 10.1186/1756-6606-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stavreva DA, Wiench M, John S, Conway-Campbell BL, McKenna MA, Pooley JR, Johnson TA, Voss TC, Lightman SL, Hager GL. Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat Cell Biol. 2009;11:1093–1102. doi: 10.1038/ncb1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heine VM, Maslam S, Zareno J, Joels M, Lucassen PJ. Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. Eur J Neurosci. 2004;19:131–144. doi: 10.1046/j.1460-9568.2003.03100.x. [DOI] [PubMed] [Google Scholar]
- 36.Sarabdjitsingh RA, Meijer OC, Schaaf MJ, de Kloet ER. Subregion-specific differences in translocation patterns of mineralocorticoid and glucocorticoid receptors in rat hippocampus. Brain Res. 2009;1249:43–53. doi: 10.1016/j.brainres.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 37.van der Laan S, Lachize SB, Schouten TG, Vreugdenhil E, de Kloet ER, Meijer OC. Neuroanatomical distribution and colocalisation of nuclear receptor corepressor (N-CoR) and silencing mediator of retinoid and thyroid receptors (SMRT) in rat brain. Brain Res. 2005;1059:113–121. doi: 10.1016/j.brainres.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 38.Atkinson HC, Wood SA, Kershaw YM, Bate E, Lightman SL. Diurnal variation in the responsiveness of the hypothalamic-pituitary-adrenal axis of the male rat to noise stress. J Neuroendocrinol. 2006;18:526–533. doi: 10.1111/j.1365-2826.2006.01444.x. [DOI] [PubMed] [Google Scholar]
- 39.Windle RJ, Wood SA, Shanks N, Lightman SL, Ingram CD. Ultradian rhythm of basal corticosterone release in the female rat: dynamic interaction with the response to acute stress. Endocrinology. 1998;139:443–450. doi: 10.1210/endo.139.2.5721. [DOI] [PubMed] [Google Scholar]
- 40.Vallone D, Battista S, Pierantoni GM, Fedele M, Casalino L, Santoro M, Viglietto G, Fusco A, Verde P. Neoplastic transformation of rat thyroid cells requires the junB and fra-1 gene induction which is dependent on the HMGI-C gene product. EMBO J. 1997;16:5310–5321. doi: 10.1093/emboj/16.17.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conway-Campbell BL, McKenna MA, Wiles CC, Atkinson HC, de Kloet ER, Lightman SL. Proteasome-dependent down-regulation of activated nuclear hippocampal glucocorticoid receptors determines dynamic responses to corticosterone. Endocrinology. 2007;148:5470–5477. doi: 10.1210/en.2007-0585. [DOI] [PubMed] [Google Scholar]
- 42.John S, Sabo PJ, Johnson TA, Sung MH, Biddie SC, Lightman SL, Voss TC, Davis SR, Meltzer PS, Stamatoyannopoulos JA, Hager GL. Interaction of the glucocorticoid receptor with the chromatin landscape. Mol Cell. 2008;29:611–624. doi: 10.1016/j.molcel.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 43.Reul JM, Chandramohan Y. Epigenetic mechanisms in stress-related memory formation. Psychoneuroendocrinology. 2007;32(Suppl 1):S21–S25. doi: 10.1016/j.psyneuen.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 44.Conway-Campbell BL, Knight DM, Pooley JR, Lightman SL. Intranuclear activation of glucocorticoid receptors defines a novel mechanism for continuous cellular responsiveness during glucocorticoid pulsatility. The 90th annual meeting of the Endocrine Society; San Francisco, US. 2008. [Google Scholar]
- 45.Sarabdjitsingh RA, Isenia S, Polman A, Mijalkovic J, Lachize S, Datson N, de Kloet ER, Meijer OC. Disrupted corticosterone pulsatile patterns attenuate responsiveness to glucocorticoid signaling in rat brain. Endocrinology. 2010;151:1177–1186. doi: 10.1210/en.2009-1119. [DOI] [PubMed] [Google Scholar]
- 46.Henley DE, Russell GM, Douthwaite JA, Wood SA, Buchanan F, Gibson R, Woltersdorf WW, Catterall JR, Lightman SL. Hypothalamic-pituitary-adrenal axis activation in obstructive sleep apnea: the effect of continuous positive airway pressure therapy. J Clin Endocrinol Metab. 2009;94:4234–4242. doi: 10.1210/jc.2009-1174. [DOI] [PubMed] [Google Scholar]
- 47.Henley DE, Buchanan F, Gibson R, Douthwaite JA, Wood SA, Woltersdorf WW, Catterall JR, Lightman SL. Plasma apelin levels in obstructive sleep apnea and the effect of continuous positive airway pressure therapy. J Endocrinol. 2009;203:181–188. doi: 10.1677/JOE-09-0245. [DOI] [PubMed] [Google Scholar]