Abstract
Background and objective
Airflow limitation in chronic obstructive pulmonary disease (COPD) results in a decrease in oxygen transport to the brain. The aim of the present study was to explore the alteration of spontaneous brain activity induced by hypoxia in patients with COPD.
Patients and methods
Twenty-five stable patients with COPD and 25 matching healthy volunteers were investigated. Amplitude of low-frequency fluctuation (ALFF) of blood oxygenation level-dependent signal at resting state in the brain was analyzed using functional magnetic resonance imaging.
Results
Whole-brain analysis using functional magnetic resonance imaging revealed significant decreases in ALFF in the bilateral posterior cingulate gyri and right lingual gyrus and an increase in ALFF in the left postcentral gyrus of patients with COPD. After controlling for SaO2, patients with COPD only showed an increase in ALFF in the left postcentral gyrus. Region of interest analysis showed a decrease in ALFF in the left precentral gyrus and an increase in ALFF in the left caudate nucleus of patients with COPD. In all subjects, ALFF in the bilateral posterior cingulate gyri and right lingual gyrus showed positive correlations with visual reproduction.
Conclusion
We demonstrated abnormal spontaneous brain activity of patients with COPD, which may have a pathophysiologic meaning.
Keywords: chronic obstructive pulmonary disease, hypoxia, low-frequency fluctuation, neuronal activity, resting-state fMRI
Introduction
The brain maintains a high level of spontaneous neuronal activity, which is relevant for human behavior.1–4 Low-frequency fluctuation (0.01–0.08 Hz) of blood oxygenation level-dependent (BOLD) signal in the brain has been proven to be highly correlated with this spontaneous activity.5 Synchronous low-frequency fluctuation between motor cortices was first observed by Biswal et al.6 Afterward, an analysis of amplitude of low-frequency fluctuation (ALFF) was done by Zang et al7 and ALFF examination has been widely used in studies of various mental disorders, including attention deficit hyperactivity disorder,7 schizophrenia,8,9 posttraumatic stress disorder,10 and mood disorder.11 Recently, abnormal ALFF at resting state has been linked with cognitive impairments.12–15
Chronic obstructive pulmonary disease (COPD) is a syndrome of chronic progressive airflow limitation, which results in decrease in oxygen transport to the brain. Spontaneous neuronal activity is thought to consume the majority of total brain energy,16,17 and thus the spontaneous neuronal activity is inevitably influenced by a reduction in the supply of energy supply caused by hypoxia. Hypoxia has been proven to change the microenvironment around neurons,18,19 inhibit synaptic transmission,20,21 and impair spontaneous and task-stimulated neuronal activity.22–26 Taken together, we hypothesized that hypoxia could suppress spontaneous neuronal activity in the brain of patients with COPD.
In the present study, 25 stable patients with COPD were recruited for examining ALFF in the brain. Changes of resting-state neuronal networks in the brain of patients with COPD were identified by independent component analysis (ICA).27 However, although ICA can measure BOLD signal synchrony, it is difficult to pinpoint which area is responsible for the observed abnormality in connectivity. Useful information about neural process may be present in the oscillatory amplitude envelope.7,28 Therefore, an alternative way of measuring regional brain activity during the resting state is to examine the ALFF of the BOLD signal. Furthermore, ALFF has high levels of reliability and consistency in terms of the spatial pattern generated.28 Such technique may therefore be a useful complement to ICA of interregional coherences between multiple BOLD signals.29
Materials and methods
Subjects
Twenty-five patients were included in this study from Zhongshan Hospital of Xiamen University (Xiamen, People’s Republic of China). Patients received treatment for 30–45 days and were in a stable condition. The controls were 25 healthy volunteers, with matched age, sex, and education. All subjects were free from a history of neurological, cerebrovascular, pulmonary, or metabolic diseases that are known to affect cognition. None of the subjects were current smokers. Patients were provided with therapy, including the inhalation of Bricanyl, Ventolin, ipratropium bromide, or budesonide. All subjects were right handed. Demographic characteristics of subjects are shown in Table 1. The procedure was fully explained to all subjects, and written informed consent obtained. The experimental protocol was approved by the Research Ethics Review Board of Xiamen University.
Table 1.
Demographic characteristics of patients with COPD and healthy controls
| Variables | COPD patients | Controls | P-value |
|---|---|---|---|
| Number of subjects (male:female) | 25 (21:4) | 25 (21:4) | |
| Age (years) (mean ± SD) | 69.2±8.1 (58–84) | 68.0±8.0 (57–86) | 0.59 |
| Education (years) (mean ± SD) | 6.7±3.9 | 7.5±5.0 | 0.53 |
| Family history of COPD (%) | 4 | 0 | |
| Disease duration (years) | 7.0±5.7 | 0 | |
| Smokers (%) | 44 | 40 | 0.86 |
Abbreviations: COPD, chronic obstructive pulmonary disease; SD, standard deviation.
Physiological and neuropsychological tests
Physiological and neuropsychological tests were conducted before a magnetic resonance imaging (MRI) scan. Physiological tests included the arterial blood gas analysis and pulmonary function measure. Neuropsychological tests included the visual reproduction test and figure memory test adopted from the Chinese revised version of Wechsler Memory Scale.30 The detailed test information was described in our previous study.31 An independent t-test measured between-group differences. Statistical significance was set at P<0.05.
MRI data acquisition
Resting-state functional MRI (fMRI) images were acquired on a Siemens Trio Tim 3.0T (Siemens, Erlangen, Germany) at Magnetic Resonance Center, Zhongshan Hospital, Xiamen University, using an echo-planar imaging sequence: TR/TE =3,000 ms/30 ms, flip angle =90°, matrix =64×64, voxel size =3.4×3.4×3.75 mm3, FOV =24×24 cm2, slices =38, slice thickness =3 mm. All subjects lay in the scanner with their eyes closed, but awake. T1 and T2 images were also scanned for any incidental findings. Data analyses were conducted by two researchers who were blind to the status of the subjects.
ALFF analysis
Image preprocessing was performed using data processing assistant for resting-state fMRI (Data Processing Assistant for Resting-State fMRI) implemented in SPM8 (http://www.fil.ion.ucl.ac.uk/spm). The first four time points were discarded for signal equilibrium. Then slice timing correction and realignment for head motion correction were performed for the remainder data. Finally, the images were spatially smoothed using a Gaussian kernel of 6 mm full-width at half-maximum.
ALFF was calculated with RESting-state fMRI data analysis Toolkit (REST) (http://restfmri.net). Before ALFF calculation, the linear trends of the time series were removed. A temporal band-pass filter (0.01–0.08 Hz) was used to remove low-frequency drifts and respiratory and cardiac high-frequency noise. Each filtered voxel’s time series was transformed into the frequency domain by the fast Fourier transform, and then the power spectrum was obtained. The square root was calculated at each frequency of the power spectrum and averaged across 0.01–0.08 Hz at each voxel. This averaged square root was taken as the ALFF. For the purposes of standardization, the ALFF of each voxel was divided by the global mean ALFF. The global mean ALFF was calculated within a brain mask that excluded the background.
In addition, the regions that showed significant differences in the density of gray matter (GM) between patients with COPD and controls were selected for region of interest (ROI) analysis (Zhang et al31), which included the left precentral gyrus, bilateral anterior cingulate gyri, bilateral insula, bilateral thalamus, and head of left caudate nucleus.
Two-sample t-test was performed to assess the ALFF difference between groups, with age, sex, education, and pack-years smoking as covariates. Multiple comparisons were performed using Alphasim program determined by Monte Carlo simulation in REST. Statistical significance was set at P<0.05 (corrected). We further performed an additional group-level analysis that not only regressed out the aforementioned four nuisance variates but also controlled for SaO2 to observe the influence of SaO2 on the regional ALFF.
Correlation analysis
Average ALFF values of all voxels in the clusters that showed significant group differences were extracted using REST. Partial correlation was used to analyze the relationships of ALFF values with neuropsychological measurements. Statistical significance was set at P<0.05 (corrected for Bonferroni multiple comparisons), with sex, age, and education as covariates.
Results
Physiological and neuropsychological tests
Compared with controls, patients with COPD had markedly lower pulmonary measurements in 1 second over forced vital capacity (P<0.001), forced expiratory volume (P<0.001), and forced expiratory volume in 1 second/forced vital capacity (P<0.001), and had lower artery SaO2 (P=0.003) and higher PCO2 (P<0.001). COPD patients had significantly lower scores in visual reproduction (P=0.031) and figure memory (P=0.010).
Regional ALFF values
There was no significant difference in global mean ALFF between patients with COPD and controls across all 76 time points (F(1, 48) =0.654, P=0.457).
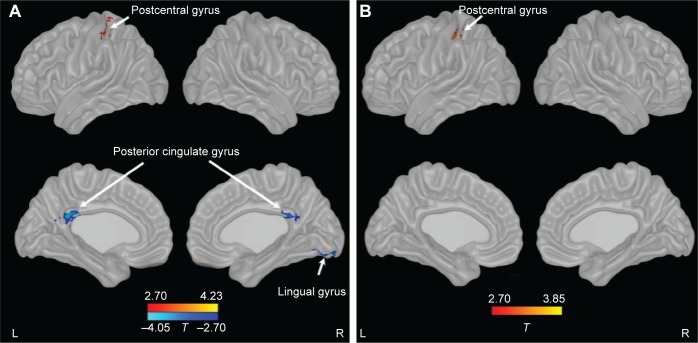
Compared with controls, patients with COPD showed significant decreases in ALFF in bilateral posterior cingulate cortex (PCC) and right lingual gyrus and an increase in ALFF in the left postcentral gyrus. After controlling for SaO2, patients with COPD only showed an increase in ALFF in the left postcentral gyrus (Figure 1; Table 2).
Figure 1.
Changes of ALFF in patients with COPD compared with controls.
Notes: (A) Uncontrolling for SaO2, (B) controlling for SaO2. P<0.05 (corrected). Red to yellow indicates an increase, blue indicates a decrease.
Abbreviations: ALFF, amplitude of low-frequency fluctuation; COPD, chronic obstructive pulmonary disease; L, left; R, right.
Table 2.
Regional information of changed ALFF in patients with COPD
| Areas | Volume (mm3) | Brodmann areas | MNI coordinate
|
P-value (peak) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| No controlling for SaO2 | ||||||
| Bilateral posterior cingulate cortex | 1,755 | 23 | −3 | −39 | 24 | −4.04 |
| Right lingual gyrus | 2,214 | 18 | 18 | −78 | −12 | −4.34 |
| Left postcentral gyrus | 1,161 | 3/40 | −33 | −39 | 63 | 4.28 |
| Controlling for SaO2 | ||||||
| Left postcentral gyrus | 1,539 | 3/40 | −45 | −30 | 54 | 4.18 |
Abbreviations: ALFF, amplitude of low-frequency fluctuation; COPD, chronic obstructive pulmonary disease.
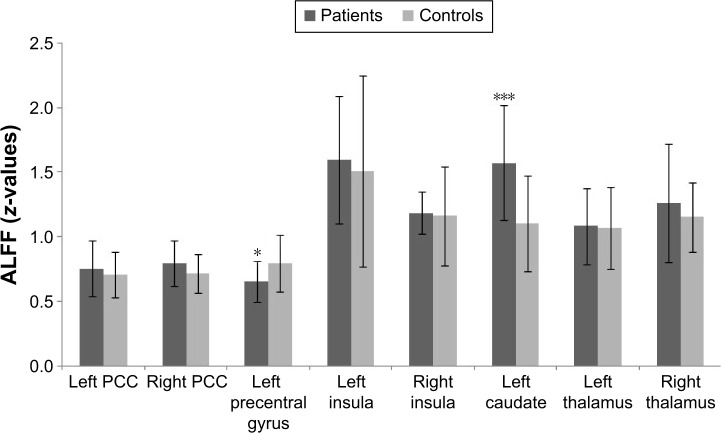
ROI analysis showed that ALFF was decreased in the left precentral gyrus (P=0.021) and increased in the left caudate nucleus (P<0.001) in patients with COPD compared with controls (Figure 2). There were no significant differences between groups in the bilateral anterior cingulate gyri, insula, and thalamus.
Figure 2.
Regional ALFF in the brain of patients with COPD compared with controls by ROI analysis.
Notes: *P<0.05; ***P<0.001. Error bars represent standard deviation.
Abbreviations: ALFF, amplitude of low-frequency fluctuation; COPD, chronic obstructive pulmonary disease; PCC, posterior cingulate cortex; ROI, region of interest.
Correlations of regional ALFF
In patients with COPD and controls, ALFF in the bilateral PCC and right lingual gyrus had positive correlations with visual reproduction score (Figure 3).
Figure 3.
Correlation of regional ALFF with cognitive ability in patients with COPD and controls.
Abbreviations: ALFF, amplitude of low-frequency fluctuation; COPD, chronic obstructive pulmonary disease; PCC, posterior cingulate cortex.
Discussion
In the present study, based on whole-brain and ROI analysis, abnormal spontaneous neuronal activities were detected in several brain regions of patients with COPD. Among these regions, the ALFF in bilateral PCC and right lingual gyrus showed significant correlations with the poor performance in visual reproduction.
After controlling for SaO2, the group differences in ALFF in bilateral PCC and right lingual gyrus did not exist, which suggested hypoxia-diminished basal BOLD signal. A number of studies support our finding. For example, acute hypoxia-induced depression of neuronal activity was recorded in a hippocampal brain slice;24,32,33 chronic hypoxia has also been proven to reduce neuronal excitability;26 after exposure to high altitude for 5 weeks, the magnitude of BOLD response to visual stimulation was significantly decreased;22 in rats, after inspiration of low O2 concentration, forepaw-stimulated increase of BOLD signal was significantly smaller.23 Previous studies have shown that cognitive impairments in patients with COPD can be reversed by oxygen therapy.34,35 We therefore suggested that cognitive impairments in patients with COPD may be attributed to hypoxia-reduced neuronal activity.
Patients with COPD in our study developed hypercapnia. In vivo electrophysiological studies have shown that hypercapnia directly increased discharge frequencies and decreased modal interspike intervals for medullary respiratory neurons in decerebrate cats36 and reduced postsynaptic potentials of neocortical and spinal neurons.37 Based on resting-state fMRI, Marshall et al38 found significantly decreased brain functional connectivity in almost all brain lobes induced by mild carbon dioxide. In another aspect, elevated blood CO2 can relax arteriolar smooth muscle, leading to an increase in cerebral blood flow. Increasing blood flow then via vasoactive stimuli increases venous oxygen saturation due to the deoxyhemoglobin removal, causing a concurrent increase in the BOLD signal. For example, hypercapnia was found to be associated with widespread BOLD signal increases, predominantly within the gray matter;39 BOLD signals increased after rats was subjected to 5%–10% CO2;23 BOLD signal response to increasing arterial PaCO2 showed a sigmoidal model.40
Inflammation exists in stable COPD and is enhanced during exacerbations.41 In stable patients with COPD, increased levels of inflammatory factors such as C-reactive protein, fibrinogen, leukocytes, interleukin (IL)-6, IL-8, and tumor necrosis factor-α were found to be associated with reduced lung function.42 Unfortunately, our study did not measure serum inflammatory factors in patients with COPD. Many studies have shown that these proinflammatory cytokines play a key role in the regulation of synaptic transmission and plasticity in the absence and presence of acute hypoxia.21 An fMRI study revealed that IL-β and tumor necrosis factor receptor-II were positively associated with ventral prefrontal activation.43 Endotoxin has been proven to enhance neural activity in a number of regions in response to positive and negative feedbacks.44,45 Taken together, these data suggest changes of functional activity in the brain of patients with COPD may be attributed to inflammatory factors.
Voluntary hyperpnea has been shown to be associated with significant neural activity in a number of regions of the brain, including the primary sensory cortex.46 Consequently, in our study, the increased neuronal activity in the left post-central gyrus and left caudate nucleus of patients with COPD may be driven in large part by hyperpnea.31
Similar to our findings in patients with COPD, abnormal neuronal activities in PCC and lingual gyrus have been found in hypoxic patients. For example, increases in activity of alpha 2 frequency band in bilateral PCC were detected in patients with obstructive sleep apnea;47 increases in regional homogeneity in the PCC and lingual gyrus were found in high-altitude residents;48 impairment of functional connectivity in PCC was detected in patients with asymptomatic carotid stenosis.49 Our previous study had shown a marked decrease in white matter fractional anisotropy in the right lingual gyrus, decreases in the density of GM in the left caudate nucleus and left precentral gyrus, and decrease in the volume of GM in the right PCC of patients with COPD,31,50 suggesting that the impairments of white matter fiber integrity and GM structure could contribute to reduced neuronal activity. Previous multimodal neuroimaging studies have shed light on this function–structure association underlining cognition, aging, disease, and behavior.51
The PCC and lingual gyrus have direct anatomical connectivity with the visual striate cortices.52 The PCC lies in the path of the parieto-medial temporal visual stream, which provides spatial information to the medial temporal lobe.53 It is activated by visual perception, attention, and motion.54–56 In addition, the PCC, connected with the inferior parietal cortex and lateral and medial superior frontal lobes, constitutes the attention network57 and visual search network.58 These two networks were activated by visuoconstructive test59 and visual attention task.60 An animal study showed that a lesion in the PCC produced impairment in visual discrimination learning.61 Decrease of resting-state functional connectivity in the PCC was exhibited in high myopia.62
The lingual gyrus is a part of the occipitotemporal pathway that is engaged in object discrimination63 and drawing.64 The bilateral lingual gyri were activated by visuospatial navigation,65 angle discrimination task,66 and tactile-guided draw.67 Volume of GM in the right lingual cortex has been proven to have a positive correlation with visual reproduction,68 and atrophy of GM in this area was associated with visual hallucination.69 Early clinicopathologic study of superior altitudinal hemianopia revealed the lingual gyrus was related to visual processing.70
The limitation of our present study is that the drugs used for COPD therapy could have neurological effects. However, up to now, there are no definite evidences showing a deleterious effect of these drugs on the brain.71
Conclusion
Our present study revealed abnormal spontaneous functional activity in the brain in patients with COPD, which could be the combined effect of hypoxia, hypercapnia, and inflammation. The changed regional spontaneous neuronal activity may be related to deficit in visual cognition.
Acknowledgments
This work was supported by the National Science Foundation of the People’s Republic of China (Project Nos 81171324; 81471630) and national key project (2012CB518200).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sadaghiani S, Kleinschmidt A. Functional interactions between intrinsic brain activity and behavior. Neuroimage. 2013;80:379–386. doi: 10.1016/j.neuroimage.2013.04.100. [DOI] [PubMed] [Google Scholar]
- 2.Romano SA, Pietri T, Pérez-Schuster V, Jouary A, Haudrechy M, Sumbre G. Spontaneous neuronal network dynamics reveal circuit’s functional adaptations for behavior. Neuron. 2015;85(5):1070–1085. doi: 10.1016/j.neuron.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan AY. Spatial diversity of spontaneous activity in the cortex. Front Neural Circuits. 2015;9:48. doi: 10.3389/fncir.2015.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visintin E, De Panfilis C, Antonucci C, Capecci C, Marchesi C, Sambataro F. Parsing the intrinsic networks underlying attention: a resting state study. Behav Brain Res. 2015;278:315–322. doi: 10.1016/j.bbr.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 6.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 7.Zang YF, He Y, Zhu CZ, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29(2):83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Hoptman MJ, Zuo XN, Butler PD, et al. Amplitude of low-frequency oscillations in schizophrenia: a resting state fMRI study. Schizophr Res. 2010;117(1):13–20. doi: 10.1016/j.schres.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang XQ, Lui S, Deng W, et al. Localization of cerebral functional deficits in treatment-naive, first-episode schizophrenia using resting-state fMRI. Neuroimage. 2010;49(4):2901–2906. doi: 10.1016/j.neuroimage.2009.11.072. [DOI] [PubMed] [Google Scholar]
- 10.Yin Y, Li L, Jin C, et al. Abnormal baseline brain activity in posttraumatic stress disorder: a resting-state functional magnetic resonance imaging study. Neurosci Lett. 2011;498(3):185–189. doi: 10.1016/j.neulet.2011.02.069. [DOI] [PubMed] [Google Scholar]
- 11.Qi R, Liu C, Ke J, et al. Intrinsic brain abnormalities in irritable bowel syndrome and effect of anxiety and depression. Brain Imaging Behav. 2015 Nov 10; doi: 10.1007/s11682-015-9478-1. Epub. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y, Wang Z, Zuo XN, et al. Hyper-coupling between working memory task-evoked activations and amplitude of spontaneous fluctuations in first-episode schizophrenia. Schizophr Res. 2014;159(1):80–89. doi: 10.1016/j.schres.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 13.Gao L, Bai L, Zhang Y, et al. Frequency-dependent changes of local resting oscillations in sleep-deprived brain. PLoS One. 2015;10(3):e0120323. doi: 10.1371/journal.pone.0120323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fryer SL, Roach BJ, Ford JM, et al. Relating intrinsic low-frequency BOLD cortical oscillations to cognition in schizophrenia. Neuropsychopharmacology. 2015;40(12):2705–2714. doi: 10.1038/npp.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang J, Bai F, Yang X, Chen C, Bao X, Zhang Y. Identifying brain functional alterations in postmenopausal women with cognitive impairment. Maturitas. 2015;81(3):371–376. doi: 10.1016/j.maturitas.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Raichle ME. Neuroscience. The brain’s dark energy. Science. 2006;314(5803):1249–1250. [PubMed] [Google Scholar]
- 17.Watanabe T, Hirose S, Wada H, et al. Energy landscapes of resting-state brain networks. Front Neuroinform. 2014;8:12. doi: 10.3389/fninf.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teppema LJ, Dahan A. The ventilatory response to hypoxia in mammals: mechanisms, measurement, and analysis. Physiol Rev. 2010;90(2):675–754. doi: 10.1152/physrev.00012.2009. [DOI] [PubMed] [Google Scholar]
- 19.Fukushi I, Takeda K, Yokota S, et al. Effects of arundic acid, an astrocytic modulator, on the cerebral and respiratory functions in severe hypoxia. Respir Physiol Neurobiol. 2016;226:24–29. doi: 10.1016/j.resp.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Pena F, Ramirez JM. Hypoxia-induced changes in neuronal network properties. Mol Neurobiol. 2005;32(3):251–283. doi: 10.1385/MN:32:3:251. [DOI] [PubMed] [Google Scholar]
- 21.Mukandala G, Tynan R, Lanigan S, O’Connor JJ. The effects of hypoxia and inflammation on synaptic signaling in the CNS. Brain Sci. 2016;6(1):E6. doi: 10.3390/brainsci6010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rostrup E, Larsson HB, Born AP, Knudsen GM, Paulson OB. Changes in BOLD and ADC weighted imaging in acute hypoxia during sea-level and altitude adapted states. Neuroimage. 2005;28(4):947–955. doi: 10.1016/j.neuroimage.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 23.Sicard KM, Duong TQ. Effects of hypoxia, hyperoxia, and hypercapnia on baseline and stimulus-evoked BOLD, CBF, and CMRO2 in spontaneously breathing animals. Neuroimage. 2005;25(3):850–858. doi: 10.1016/j.neuroimage.2004.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gavello D, Rojo-Ruiz J, Marcantoni A, Franchino C, Carbone E, Carabelli V. Leptin counteracts the hypoxia-induced inhibition of spontaneously firing hippocampal neurons: a microelectrode array study. PLoS One. 2012;7(7):e41530. doi: 10.1371/journal.pone.0041530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sumiyoshi A, Suzuki H, Shimokawa H, Kawashima R. Neurovascular uncoupling under mild hypoxic hypoxia: an EEG-fMRI study in rats. J Cereb Blood Flow Metab. 2012;32(10):1853–1858. doi: 10.1038/jcbfm.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodall S, Twomey R, Amann M. Acute and chronic hypoxia: implications for cerebral function and exercise tolerance. Fatigue. 2014;2(2):73–92. doi: 10.1080/21641846.2014.909963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dodd JW, Chung AW, van den Broek MD, Barrick TR, Charlton RA, Jones PW. Brain structure and function in chronic obstructive pulmonary disease: a multimodal cranial magnetic resonance imaging study. Am J Respir Crit Care Med. 2012;186(3):240–245. doi: 10.1164/rccm.201202-0355OC. [DOI] [PubMed] [Google Scholar]
- 28.Zuo XN, Di Martino A, Kelly C, et al. The oscillating brain: complex and reliable. Neuroimage. 2010;49(2):1432–1445. doi: 10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cole DM, Smith SM, Beckmann CF. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci. 2010;4:8. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong YX. Manual for the Wechsler Memory Scale-Revised. Changsha, Hunan, China: Hunan Medical University; 1989. [Google Scholar]
- 31.Zhang H, Wang X, Lin J, et al. Grey and white matter abnormalities in chronic obstructive pulmonary disease: a case-control study. BMJ Open. 2012;2(2):e000844. doi: 10.1136/bmjopen-2012-000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fowler JC. Adenosine antagonists delay hypoxia-induced depression of neuronal activity in hippocampal brain slice. Brain Res. 1989;490(2):378–384. doi: 10.1016/0006-8993(89)90258-8. [DOI] [PubMed] [Google Scholar]
- 33.Noh J, Koh YH, Chung JM. Attenuated effects of Neu2000 on hypoxia-induced synaptic activities in a rat hippocampus. Arch Pharm Res. 2014;37(2):232–238. doi: 10.1007/s12272-013-0170-y. [DOI] [PubMed] [Google Scholar]
- 34.Thakur N, Blanc PD, Julian LJ, et al. COPD and cognitive impairment: the role of hypoxemia and oxygen therapy. Int J Chron Obstruct Pulmon Dis. 2010;5:263–269. doi: 10.2147/copd.s10684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dal Negro RW, Bonadiman L, Bricolo FP, Tognella S, Turco P. Cognitive dysfunction in severe chronic obstructive pulmonary disease (COPD) with or without Long-Term Oxygen Therapy (LTOT) Multidiscip Respir Med. 2015;10(1):17. doi: 10.1186/s40248-015-0013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.John WM, Wang SC. Response of medullary respiratory neurons to hypercapnia and isocapnic hypoxia. J Appl Physiol Respir Environ Exerc Physiol. 1977;43(5):812–821. doi: 10.1152/jappl.1977.43.5.812. [DOI] [PubMed] [Google Scholar]
- 37.Lehmenkühler A, Bingmann D, Speckmann EJ. Neuronal and glial responses to hypoxia and hypercapnia. Biomed Biochim Acta. 1989;48(2–3):S155–S160. [PubMed] [Google Scholar]
- 38.Marshall O, Uh J, Lurie D, Lu H, Milham MP, Ge Y. The influence of mild carbon dioxide on brain functional homotopy using resting-state fMRI. Hum Brain Mapp. 2015;36(10):3912–3921. doi: 10.1002/hbm.22886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corfield DR, Murphy K, Josephs O, Adams L, Turner R. Does hypercapnia-induced cerebral vasodilation modulate the hemodynamic response to neural activation? NeuroImage. 2001;13(6 Pt 1):1207–1211. doi: 10.1006/nimg.2001.0760. [DOI] [PubMed] [Google Scholar]
- 40.Bhogal AA, Siero JC, Fisher JA, et al. Investigating the non-linearity of the BOLD cerebrovascular reactivity response to targeted hypo/hypercapnia at 7T. Neuroimage. 2014;98:296–305. doi: 10.1016/j.neuroimage.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 41.Sethi S, Mahler DA, Marcus P, Owen CA, Yawn B, Rennard S. Inflammation in COPD: implications for management. Am J Med. 2012;125(12):1162–1170. doi: 10.1016/j.amjmed.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 42.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59(7):574–580. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Connor MF, Irwin MR, Wellisch DK. When grief heats up: pro-inflammatory cytokines predict regional brain activation. Neuroimage. 2009;47(3):891–896. doi: 10.1016/j.neuroimage.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inagaki TK, Muscatell KA, Irwin MR, Cole SW, Eisenberger NI. Inflammation selectively enhances amygdala activity to socially threatening images. Neuroimage. 2012;59(4):3222–3226. doi: 10.1016/j.neuroimage.2011.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muscatell KA, Moieni M, Inagaki TK, et al. Exposure to an inflammatory challenge enhances neural sensitivity to negative and positive social feedback. Brain Behav Immun. 2016 Mar 28; doi: 10.1016/j.bbi.2016.03.022. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McKay LC, Evans KC, Frackowiak RS, Corfield DR. Neural correlates of voluntary breathing in humans. J Appl Physiol (1985) 2003;95(3):1170–1178. doi: 10.1152/japplphysiol.00641.2002. [DOI] [PubMed] [Google Scholar]
- 47.Toth M, Faludi B, Wackermann J, Czopf J, Kondakor I. Characteristic changes in brain electrical activity due to chronic hypoxia in patients with obstructive sleep apnea syndrome (OSAS): a combined EEG study using LORETA and omega complexity. Brain Topogr. 2009;22(3):185–190. doi: 10.1007/s10548-009-0110-9. [DOI] [PubMed] [Google Scholar]
- 48.Yan X, Zhang J, Gong Q, Weng X. Prolonged high-altitude residence impacts verbal working memory: an fMRI study. Exp Brain Res. 2011;208(3):437–445. doi: 10.1007/s00221-010-2494-x. [DOI] [PubMed] [Google Scholar]
- 49.Cheng HL, Lin CJ, Soong BW, et al. Impairments in cognitive function and brain connectivity in severe asymptomatic carotid stenosis. Stroke. 2012;43(10):2567–2573. doi: 10.1161/STROKEAHA.111.645614. [DOI] [PubMed] [Google Scholar]
- 50.Zhang H, Wang X, Lin J, et al. Reduced regional gray matter volume in patients with chronic obstructive pulmonary disease: a voxel-based morphometry study. AJNR Am J Neuroradiol. 2013;34(2):334–339. doi: 10.3174/ajnr.A3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sui J, Huster R, Yu Q, Segall JM, Calhoun VD. Function-structure associations of the brain: evidence from multimodal connectivity and covariance studies. Neuroimage. 2014;102(Pt 1):11–23. doi: 10.1016/j.neuroimage.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whittingstall K, Bernier M, Houde JC, Fortin D, Descoteaux M. Structural network underlying visuospatial imagery in humans. Cortex. 2014;56:85–98. doi: 10.1016/j.cortex.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 53.Kravitz DJ, Saleem KS, Baker CI, Mishkin M. A new neural framework for visuospatial processing. Nat Rev Neurosci. 2011;12(4):217–230. doi: 10.1038/nrn3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Antal A, Baudewig J, Paulus W, Dechent P. The posterior cingulate cortex and planum temporale/parietal operculum are activated by coherent visual motion. Vis Neurosci. 2008;25(1):17–26. doi: 10.1017/S0952523808080024. [DOI] [PubMed] [Google Scholar]
- 55.Fischer E, Bülthoff HH, Logothetis NK, Bartels A. Visual motion responses in the posterior cingulate sulcus: a comparison to V5/MT and MST. Cereb Cortex. 2012;22(4):865–876. doi: 10.1093/cercor/bhr154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137(Pt 1):12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3(3):284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- 58.Pollmann S, Eštočinová J, Sommer S, Chelazzi L, Zinke W. Neural structures involved in visual search guidance by reward-enhanced contextual cueing of the target location. Neuroimage. 2016;124(Pt A):887–897. doi: 10.1016/j.neuroimage.2015.09.040. [DOI] [PubMed] [Google Scholar]
- 59.Förster S, Teipel S, Zach C, et al. FDG-PET mapping the brain substrates of visuo-constructive processing in Alzheimer’s disease. J Psychiatr Res. 2010;44(7):462–469. doi: 10.1016/j.jpsychires.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 60.Vossel S, Weidner R, Moos K, Fink GR. Individual attentional selection capacities are reflected in interhemispheric connectivity of the parietal cortex. Neuroimage. 2016;129:148–158. doi: 10.1016/j.neuroimage.2016.01.054. [DOI] [PubMed] [Google Scholar]
- 61.Bussey TJ, Muir JL, Everitt BJ, Robbins TW. Dissociable effects of anterior and posterior cingulate cortex lesions on the acquisition of a conditional visual discrimination: facilitation of early learning vs impairment of late learning. Behav Brain Res. 1996;82(1):45–56. doi: 10.1016/s0166-4328(97)81107-2. [DOI] [PubMed] [Google Scholar]
- 62.Zhai L, Li Q, Wang T, et al. Altered functional connectivity density in high myopia. Behav Brain Res. 2016;303:85–92. doi: 10.1016/j.bbr.2016.01.046. [DOI] [PubMed] [Google Scholar]
- 63.Mishkin M, Ungerleider LG, Macko KA. Object vision and spatial vision: two cortical pathways. Trends Neurosci. 1983;6(10):414–417. [Google Scholar]
- 64.Ogawa K, Inui T. The role of the posterior parietal cortex in drawing by copying. Neuropsychologia. 2009;47(4):1013–1022. doi: 10.1016/j.neuropsychologia.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 65.Grön G, Wunderlich AP, Spitzer M, Tomczak R, Riepe MW. Brain activation during human navigation: gender-different neural networks as substrate of performance. Nat Neurosci. 2000;3(4):404–408. doi: 10.1038/73980. [DOI] [PubMed] [Google Scholar]
- 66.Prvulovic D, Hubl D, Sack AT, et al. Functional imaging of visuospatial processing in Alzheimer’s disease. Neuroimage. 2002;17(3):1403–1414. doi: 10.1006/nimg.2002.1271. [DOI] [PubMed] [Google Scholar]
- 67.Likova LT. Drawing in the blind and the sighted as a probe of cortical reorganization. In: Rogowitz BE, Pappas TN, editors. Human Vision and Electronic Imaging Proc SPIE. 2010. pp. 8–14. [Google Scholar]
- 68.Chee MW, Chen KH, Zheng H, et al. Cognitive function and brain structure correlations in healthy elderly East Asians. Neuroimage. 2009;46(1):257–269. doi: 10.1016/j.neuroimage.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 69.Goldman JG, Stebbins GT, Dinh V, et al. Visuoperceptive region atrophy independent of cognitive status in patients with Parkinson’s disease with hallucinations. Brain. 2014;137(Pt 3):849–859. doi: 10.1093/brain/awt360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bogousslavsky J, Miklossy J, Deruaz JP, Assal G, Regli F. Lingual and fusiform gyri in visual processing: a clinico-pathologic study of superior altitudinal hemianopia. J Neurol Neurosurg Psychiatry. 1987;50(5):607–614. doi: 10.1136/jnnp.50.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Owens MY, Wallace KL, Mamoon N, Wyatt-Ashmead J, Bennett WA. Absence of neurotoxicity with medicinal grade terbutaline in the rat model. Reprod Toxicol. 2011;31(4):447–453. doi: 10.1016/j.reprotox.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]