Abstract
Age-related hearing impairment (ARHI) is a progressive and a common sensory disorder in the elderly and will become an increasingly important clinical problem given the growing elderly population. Apoptosis of cochlear cells is an important factor in animal models of ARHI. As these cells cannot regenerate, their loss leads to irreversible hearing impairment. Identification of molecular mechanisms can facilitate disease prevention and effective treatment. In this study, we compared the expression of the genes BAK1 and BCL2 as two arms of the intrinsic apoptosis pathway between patients with ARHI and healthy subjects. ARHI and healthy subjects were selected after an ear nose throat examination, otoscopic investigation, and pure tone audiometry. RNA was extracted from peripheral blood samples, and relative gene expression levels were measured using quantitative real-time polymerase chain reaction. BAK1 and the BAK1/BCL2 ratio were statistically significantly upregulated in the ARHI subjects. The BAK1/BCL2 ratio was positively correlated with the results of the audiometric tests. Our results indicate that BAK-mediated apoptosis may be a core mechanism in the progression of ARHI in humans, similar to finding in animal models. Moreover, the gene expression changes in peripheral blood samples could be used as a rapid and simple biomarker for early detection of ARHI.
Keywords: age-related hearing impairment (ARHI), presbycusis, biomarker, treatment
Introduction
Age-related hearing impairment (ARHI), or presbycusis, is the most common multifactorial sensory disease in the elderly.1 ARHI has a huge societal impact as a result of its effects on quality of life and is a public health concern.2 It is a bilateral, symmetrical, and sensorineural high-frequency impairment. Damage or loss of the inner ear hair cells and spiral ganglion cells is an important cause of ARHI,3 and a study on the auditory organ of aging mice demonstrated that apoptosis is a major form of cell death.4 As these cells are not able to regenerate, their loss causes irreversible hearing impairment.
There are two pathways for cell signal transmission in apoptosis: intrinsic and extrinsic. The BCL-2 protein family is responsible for the intrinsic pathway. Genes of this family are divided into two categories that include proapoptotic genes (BAK1, BAX, BAD) and antiapoptotic genes (BCL2, BCLXL).5 An investigation of early-onset hearing loss (HL) in a mouse model demonstrated upregulation of the apoptosis-related genes Bak1 and Casp3 during the development of ARHI.6 In addition, the MCAT transgenic mice exhibit delayed onset of age-related HL, and investigation of the underlying molecular mechanisms revealed a decreased in the mRNA expression level of Bak1 in the cochlea and a reduction in cell death.7 Moreover, C57BL/6J mice with a deletion of the Bak1 gene exhibited a reduction in age-related apoptotic cell death of hair cells in the cochlea and spiral ganglion neurons, resulting in the postponement of ARHI.7
High expression of Bcl2 and Bcl-xL was identified as a key factor in inner ear aging in the mouse cochlea by Tadros et al.8 Given the role of apoptosis in ARHI, Yang et al6 reported an antiapoptotic treatment that resulted in the preservation of hearing and reduced outer hair cell loss at the cochleae of DBA/J2 mice. Understanding the mechanism of cell apoptosis in ARHI will not only shed light on the intracellular mechanisms underlying aging of the cochlear but also make it possible to manipulate these mechanisms for treatment and prevention of this common human impairment.
The aim of this study was to investigate the role of expression of two genes, representing different arms of the BCL-2 protein family, BAK1 (proapoptotic) and BCL2 (antiapoptotic), in peripheral blood samples from ARHI patients in comparison to healthy subjects.
Materials and methods
A total of 52 subjects with ARHI and 29 healthy subjects were selected by otolaryngologist at the ENT and Head & Neck Research Center of Iran University of Medical Science. The participants signed informed consent letters prior to inclusion in the study. This study was approved by the Ethics Committee of Iran University of Medical Sciences.
All subjects completed a questionnaire to determine their medical history and environmental factors that may have affected their ability to hear. Subjects who had experienced factors known to affect hearing sensitivity were excluded from the study.9,10 The relevant factors included exposure to ototoxic drugs, early-onset hearing impairment, history of any known cause of sensorineural HL, external or middle ear disease, cognitive dysfunction, neurological or psychiatric disease, brain tumor or vestibular schwannoma, liver cirrhosis, renal failure, cardiovascular disease, history of stroke, and cancer.
Otoscopic examination, tympanometry, pure tone audiometry, and measurement of speech reception thresholds (SRTs) and speech discrimination scores (SDSs) were performed for all subjects at the Audiology Department. Tympanometry was performed using an Amplaid 728 (Amplaid Inc., Milan, Italy) multifrequency admittance meter. Pure tone audiometry was performed using an Amplaid 319 audiometer (Amplaid Inc.). Calibration was performed according to American National Standards Institute standards (ANSI S3.6-2004, ANSI, 2004a). Air conduction thresholds were measured at 250, 500, 1,000, 2,000, 4,000, and 8,000 Hz, and bone conduction thresholds were measured at 500, 1,000, 2,000, and 4,000 Hz with the same conditions for all subjects. SRT was performed by presenting four spondaic words to each patient, according to the Farsi-language version of the staggered spondaic word test, at different intensities.11 The SRT was defined at the lowest intensity that a patient could repeat 50% of spondees correctly. SDS was performed by presenting 25 unfamiliar one-syllable words at 40 dB above the SRT for the patient. The percentage of words which the patient understood and repeated correctly was defined as the SDS. Audiological exclusion criteria were conductive HL (mean air–bone differences at 500, 1,000, and 2,000 Hz of more than 15 dB); acoustic trauma (differences between air thresholds at 4,000 and 8,000 Hz exceeding 20 dB); or experience of extensive noise exposure (self-report).
Subjects with ARHI had symmetric, bilateral hearing impairment with pure tone average (PTA) values at medium frequencies (1, 2, 4, and 8 kHz) of ≥30 dB HL. The healthy subjects had normal ear examinations and PTA values at medium frequencies of <25 dB HL (Figure 1).
Figure 1.
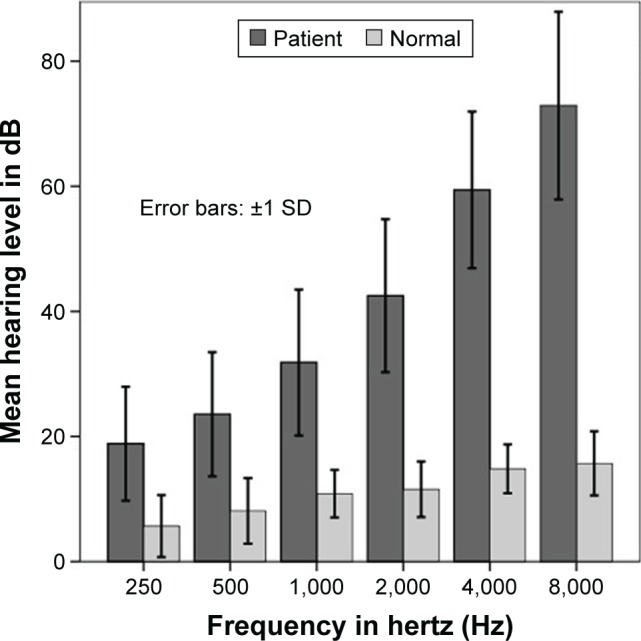
Mean air conduction hearing threshold.
Note: Error bars represent standard deviations (SDs).
Total RNA samples were extracted from 1 mL of peripheral blood using a QIAamp RNA Blood Mini Kit (Cat No 52304; QIAGEN, Hilden, Germany), according to the manufacturer’s instructions. cDNA was synthesized from 1 μg total RNA using a QuantiTect Reverse Transcription Kit (Cat No 205311; QIAGEN) according to the manufacturer’s instructions.
Quantitative real-time polymerase chain reaction (qPCR) analysis was performed for BAK1, BCL2, and the housekeeping gene ACTB, which was selected as a reference gene. qPCR was performed using specific primers (Table 1) and RealQ-PCR Master Mix with Green DNA dye (Cat No 250507; Ampliqon, Odense, Denmark) in a Rotor-Gene6000 instrument (Corbett life Science, Sydney, Australia). All reactions were performed in duplicate in a total volume of 25 μL, containing 12.5 μL of RealQ-PCR 2× Master Mix, 0.4 μL of each primer (10 pmol), 1 μL cDNA, and 10.7 μL RNase-free water. A two-step PCR program was used, with an initial activation step for 15 minutes at 95°C, followed by 35 cycles of denaturation at 95°C for 20 seconds, and annealing and extension at 60°C for 60 seconds. The threshold cycle (Ct) was determined for BAK1, BCL2, and ACTB in each sample. Gene expression levels were calculated using the following equation:
Table 1.
Sequences of the PCR primers for BAK1, BCL2, and ACTB
| Gene | GenBank accession number | Amplicon size (bp) | Reverse primer | Forward primer |
|---|---|---|---|---|
| BAK1 | NM_001188.3 | 120 | CAA ACA GGC TGG TGG CAA TC | TCA TCG GGG ACG ACA TCA AC |
| BCL2 | NM_000633.2 | 129 | CAG CCA GGA GAA ATC AAA CAG AGG | ATC GCC CTG TGG ATG ACT GAG |
| ACTB | NM_001101.3 | 131 | GTA GTT TCG TGG ATG CCA CA | TCC CTG GAG AAG AGC TAC G |
Abbreviation: PCR, polymerase chain reaction.
Statistical analysis was performed using SPSS 22 for Windows (IBM Corporation, Armonk, NY, USA). Statistical significance was defined as P<0.05. All numerical variables were checked for normal distribution using the one-sample Kolmogorov–Smirnov test. The Mann–Whitney U-test was used for the comparison of gene expression values between ARHI and healthy subjects. The Spear-man’s rank-order correlation was used to evaluate potential correlations between PTA, SRT, and SDS, and gene expression.
Results
The mean ages of ARHI and healthy subjects were 66.7±9 years and 64±6.4 years, respectively. The sex balance was 54.3% male and 45.7% female, with no statistically significant differences in age (t [79] =−1.5; P=0.13) and sex (χ2 [1, N=81] =1.6; P=0.2) between ARHI and healthy subjects.
BAK1 and BCL2 gene expression levels were determined in ARHI and healthy subjects, relative to those of the ACTB gene. The difference between the BAK1 expression level in ARHI subjects (median =1) compared to healthy subjects (median =0.34) was statistically significant (U=484, P=0.008); hence, the ARHI subjects exhibited elevated levels of expression of the proapoptotic gene, BAK1, compared with healthy subjects (Figure 2).
Figure 2.
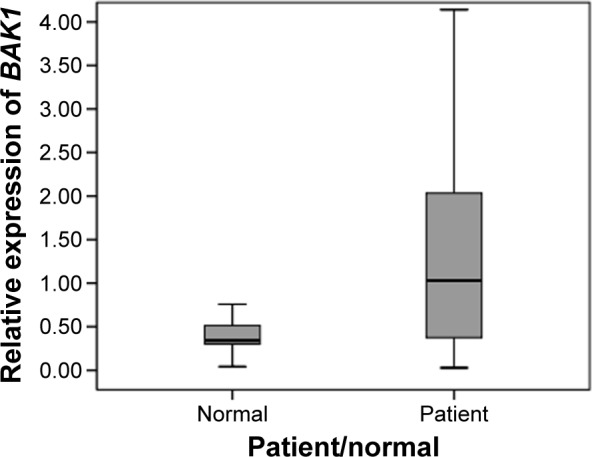
Gene expression of BAK1 in peripheral blood samples from Iranian patients with ARHI and healthy subjects.
Notes: The difference between the two groups is statistically significant (U=484, P=0.008). The box plots show the median (solid line across the box).
Abbreviation: ARHI, age-related hearing impairment.
A Mann–Whitney U-test indicated that levels of expression of the antiapoptotic gene, BCL2, were also greater for ARHI (median =1.06) compared with healthy subjects (median =0.58; U=548, P=0.04) (Figure 3).
Figure 3.
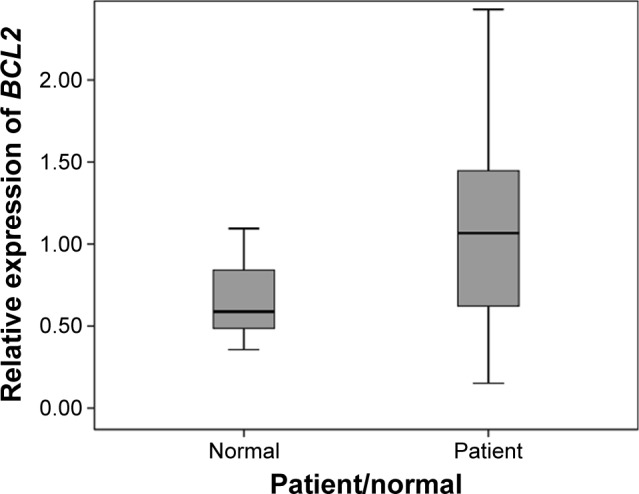
Gene expression of BCL2 in peripheral blood samples from Iranian patients with ARHI and healthy subjects.
Notes: Difference between the two groups is statistically significant (U=548, P=0.04).
Abbreviation: ARHI, age-related hearing impairment.
Since the balance between pro- and antiapoptotic members of the BCL-2 protein family is a key factor in apoptosis, we evaluated the BAK1/BCL2 expression ratio, revealing a statistically significant difference between ARHI and healthy subjects (U=544, P=0.039). The BAK1/BCL2 median ratios for ARHI and healthy subjects were 1.46 and 0.8, respectively (Figure 4).
Figure 4.
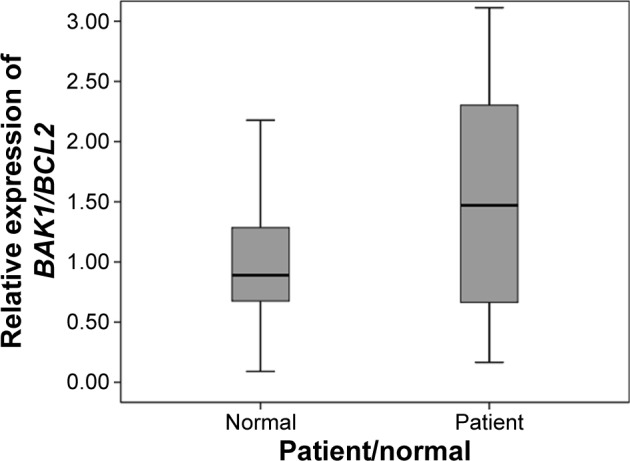
The BAK1/BCL2 gene expression ratios of Iranian patients with ARHI and healthy subjects. The difference between the two groups is statistically significant (U=544, P=0.039).
Abbreviation: ARHI, age-related hearing impairment.
Next, correlations between the BAK1/BCL2 ratio and differences in auditory perception were evaluated. Auditory perception measures included PTA, SRT, and SDS. As the data were not normally distributed, we performed Spearman’s rank-order correlation analysis. The results revealed a significant and positive correlation between BAK1/BCL2 ratio and PTA (rs[81] =0.3, P=0.015) and SRT (rs[81] =0.3, P=0.007). In addition, there was a significant negative correlation between SDS and the BAK1/BCL2 ratio (rs[81] =0.25, P=0.03).
Discussion
BCL-2 protein family members regulate mitochondrial membrane permeabilization during apoptosis, with BAK1 and BAX proteins forming an apoptotic pore within the mitochondrial outer membrane.12 A recent study demonstrated that the role of the carboxyl terminus of BAK1 in lining the apoptotic pore or aggregating on the membrane surface is key to the destabilization of the mitochondrial outer membrane by BAK1 to generate pores and consequent initiation of the apoptotic cascade.13 Various different tissues in a BAK1-deficient mouse model showed a marked reduction in apoptosis,14 and BAK1 played a central role in apoptosis-mediated murine cerebral cortical neuron degeneration after DNA damage.15 Furthermore, C57BL/6J mice with a deletion in Bak1 have reduced apoptotic cell death and exhibit a delay in onset of ARHI.7 Oxidative stress, an important factor in age-related diseases, induces Bak1 expression in primary cochlear cells, leading to apoptosis; however, apoptotic cell death is prevented in Bak1-deficient mice, even under conditions of oxidative stress.7
This study demonstrated a statistically significant increase in the BAK1 gene expression level in ARHI compared to healthy subjects (Figure 2). A possible explanation for this could be a general increase in apoptosis among ARHI subjects. This result is in agreement with that reported by Dong et al,16 demonstrating an association between immune-and apoptosis-related genes and ARHI.
In this study, we found that BCL2 was significantly upregulated in ARHI subjects (Figure 3). Tadros et al8 reported an increase in expression of the apoptosis-related genes, Bcl2 and Bcl-xL, in the cochlea of the mice with age-related HL. They hypothesized that higher expression of Bcl2 in the age-related HL model, the CBA mouse, may be a protective effect induced during the cochlear aging process.8 A previous study of the mechanism of BCL-2 family regulation of the onset of apoptosis in cells demonstrated that BCL2 inhibits the activation of BAK1 by binding to this protein in healthy cells.17 Neomycin, an aminoglycoside antibiotic, induces cell death of inner hair cells; however, over expression of Bcl2 prevents neomycin-induced HL in adult mice.18 The significantly higher expression of BCL2 in our ARHI subjects provides further support for the role of BCL2 in a defense response mechanism in age-related HL in humans after the upregulation of BAK1.
Experimental evidence indicates that the ratio of anti- and proapoptotic BCL-2 protein family members is a key factor in determining cell death or survival.19,20 The BAK1/BCL2 median ratio was significantly elevated in ARHI compared with healthy subjects, indicating the potential importance of the role of BAK1 gene expression, which may be indicative of increased apoptosis among ARHI subjects (Figure 4). These results were consistent with data obtained from humans and mouse models, demonstrating a reduced neuron size and a decline in the medial olivocochlear efferent system during ARHI.21–23
This study demonstrated positive correlations between the BAK1/BCL2 ratio and PTA and SRT values. Hence, our results indicate an association between gene expression levels related to elevated apoptosis and increased PTA and SRT scores. SDS values were significantly negatively correlated with the BAK1/BCL2 ratio. This finding is consistent with the reduced speech understanding exhibited by ARHI patients;3 however, the speech in noise test may be helpful to improving understanding of the relationship between BAK1/BCL2 ratios and speech understanding in people with ARHI.
Our findings are in agreement with previous studies and support the hypothesis that elevated BAK1 expression induces the apoptotic pathway in hair cells, initiating the molecular mechanism of impairment. Manipulation of the BAK1 gene pathway is a treatment target of apoptosis-related diseases.24 Supplementation of mouse food, by increasing coenzyme Q10 and lipoic acid content, reduced Bak1 expression in the cochlea and prevented age-related HL.7
ARHI has profound economic and social health impacts, and the increasing elderly population makes ARHI an important issue for future generations. There is a long delay between the start of ARHI-related bodily changes and signs that are measurable using standard diagnostic tests.25 Therefore, there is a pressing need for a suitable diagnostic biomarker for ARHI, and this is actively being sought by a number of research groups. Observation of a decline in the auditory brainstem gap from middle age in human and animal models was one interesting result in this area.23,26 BAK1 has previously been identified as a predictive and prognostic biomarker in cancer therapy.27 The significantly elevated expression of BAK1 in peripheral blood samples from subjects with ARHI and the correlation of its expression with increased PTA and SRT values indicate that it may be a suitable and easy-to-use early detection diagnostic biomarker for this impairment. Further studies using a larger study population are required to confirm the present findings and establish their application in clinical practice.
Conclusion
In summary, we demonstrate an upregulation of BAK1 gene expression and the BAK1/BCL2 ratio in peripheral blood samples from Iranian ARHI subjects. Both BAK1 gene expression and the BAK1/BCL2 ratio were significantly correlated with the result of audiological tests routinely used for the diagnosis of presbycusis. Furthermore, the observation of significant changes in the expression of the apoptosis-related gene, BAK1, in peripheral blood samples suggests that it may be a useful biomarker for rapid diagnosis of ARHI.
Acknowledgments
We gratefully appreciate all the volunteers for participating in this scientific research. We thank the department and audiometry clinic of Rasoul Akram Hospital of Iran University of Medical Science for their kind cooperation during this research. We would like to acknowledge the Iran University of Medical Sciences for supporting this research.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Yang CH, Schrepfer T, Schacht J. Age-related hearing impairment and the triad of acquired hearing loss. Front Cell Neurosci. 2015;9:276. doi: 10.3389/fncel.2015.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciorba A, Hatzopoulos S, Bianchini C, Aimoni C, Skarzynski H, Skarzynski PH. Genetics of presbycusis and presbystasis. Int J Immunopathol Pharmacol. 2015;28(1):29–35. doi: 10.1177/0394632015570819. [DOI] [PubMed] [Google Scholar]
- 3.Huang Q, Tang J. Age-related hearing loss or presbycusis. Eur Arch Otorhinolaryngol. 2010;267(8):1179–1191. doi: 10.1007/s00405-010-1270-7. [DOI] [PubMed] [Google Scholar]
- 4.Sha SH, Chen FQ, Schacht J. Activation of cell death pathways in the inner ear of the aging CBA/J mouse. Hear Res. 2009;254(1–2):92–99. doi: 10.1016/j.heares.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9(1):47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 6.Yang L, Zhang H, Han X, et al. Attenuation of hearing loss in DBA/2J mice by anti-apoptotic treatment. Hear Res. 2015;327:109–116. doi: 10.1016/j.heares.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Someya S, Xu J, Kondo K, et al. Age-related hearing loss in C57BL/6J mice is mediated by Bak-dependent mitochondrial apoptosis. Proc Natl Acad Sci U S A. 2009;106(46):19432–19437. doi: 10.1073/pnas.0908786106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tadros SF, D’Souza M, Zhu X, Frisina RD. Apoptosis-related genes change their expression with age and hearing loss in the mouse cochlea. Apoptosis. 2008;13(11):1303–1321. doi: 10.1007/s10495-008-0266-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Eyken E, Van Laer L, Fransen E, et al. KCNQ4: a gene for age-related hearing impairment? Hum Mutat. 2006;27(10):1007–1016. doi: 10.1002/humu.20375. [DOI] [PubMed] [Google Scholar]
- 10.Van Laer L, Huyghe JR, Hannula S, et al. A genome-wide association study for age-related hearing impairment in the Saami. Eur J Hum Genet. 2010;18(6):685–693. doi: 10.1038/ejhg.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hajiabolhassan F, Lotfi Y, Azordegan F. Introducing and evaluating a Farsi – language version of the staggered spondaic word test in normal hearing subject. Audiol. 2006;15(1):39–46. [Google Scholar]
- 12.Westphal D, Dewson G, Czabotar PE, Kluck RM. Molecular biology of Bax and Bak activation and action. Biochim Biophys Acta. 2011;1813(4):521–531. doi: 10.1016/j.bbamcr.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 13.Iyer S, Bell F, Westphal D, et al. Bak apoptotic pores involve a flexible C-terminal region and juxtaposition of the C-terminal transmembrane domains. Cell Death Differ. 2015;22(10):1665–1675. doi: 10.1038/cdd.2015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooks C, Wei Q, Feng L, et al. Bak regulates mitochondrial morphology and pathology during apoptosis by interacting with mitofusins. Proc Natl Acad Sci U S A. 2007;104(28):11649–11654. doi: 10.1073/pnas.0703976104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin LJ, Liu Z, Pipino J, Chestnut B, Landek MA. Molecular regulation of DNA damage-induced apoptosis in neurons of cerebral cortex. Cereb Cortex. 2009;19(6):1273–1293. doi: 10.1093/cercor/bhn167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong Y, Li M, Liu P, Song H, Zhao Y, Shi J. Genes involved in immunity and apoptosis are associated with human presbycusis based on microarray analysis. Acta Otolaryngol. 2014;134(6):601–608. doi: 10.3109/00016489.2014.880795. [DOI] [PubMed] [Google Scholar]
- 17.van Delft MF, Huang DC. How the Bcl-2 family of proteins interact to regulate apoptosis. Cell Res. 2006;16(2):203–213. doi: 10.1038/sj.cr.7310028. [DOI] [PubMed] [Google Scholar]
- 18.Cunningham LL, Matsui JI, Warchol ME, Rubel EW. Overexpression of Bcl-2 prevents neomycin-induced hair cell death and caspase-9 activation in the adult mouse utricle in vitro. J Neurobiol. 2004;60(1):89–100. doi: 10.1002/neu.20006. [DOI] [PubMed] [Google Scholar]
- 19.Khodapasand E, Jafarzadeh N, Farrokhi F, Kamalidehghan B, Houshmand M. Is Bax/Bcl-2 ratio considered as a prognostic marker with age and tumor location in colorectal cancer? Iran Biomed J. 2015;19(2):69–75. doi: 10.6091/ibj.1366.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golestani Eimani B, Sanati MH, Houshmand M, Ataei M, Akbarian F, Shakhssalim N. Expression and prognostic significance of bcl-2 and bax in the progression and clinical outcome of transitional bladder cell carcinoma. Cell J. 2014;15(4):356–363. [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobson M, Kim S, Romney J, Zhu X, Frisina RD. Contralateral suppression of distortion-product otoacoustic emissions declines with age: a comparison of findings in CBA mice with human listeners. Laryngoscope. 2003;113(10):1707–1713. doi: 10.1097/00005537-200310000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Kim S, Frisina DR, Frisina RD. Effects of age on contralateral suppression of distortion product otoacoustic emissions in human listeners with normal hearing. Audiol Neurootol. 2002;7(6):348–357. doi: 10.1159/000066159. [DOI] [PubMed] [Google Scholar]
- 23.Zhu X, Vasilyeva ON, Kim S, et al. Auditory efferent feedback system deficits precede age-related hearing loss: contralateral suppression of otoacoustic emissions in mice. J Comp Neurol. 2007;503(5):593–604. doi: 10.1002/cne.21402. [DOI] [PubMed] [Google Scholar]
- 24.Karasawa S, Azuma M, Kasama T, et al. Vitamin K2 covalently binds to Bak and induces Bak-mediated apoptosis. Mol Pharmacol. 2013;83(3):613–620. doi: 10.1124/mol.112.082602. [DOI] [PubMed] [Google Scholar]
- 25.Carson AJ. “What brings you here today?” The role of self-assessment in help-seeking for age-related hearing loss. J Aging Stud. 2005;19(2):185–200. [Google Scholar]
- 26.Williamson TT, Zhu X, Walton JP, Frisina RD. Auditory brainstem gap responses start to decline in mice in middle age: a novel physiological biomarker for age-related hearing loss. Cell Tissue Res. 2015;361(1):359–369. doi: 10.1007/s00441-014-2003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kubo T, Kawano Y, Himuro N, et al. BAK is a predictive and prognostic biomarker for the therapeutic effect of docetaxel treatment in patients with advanced gastric cancer. Gastric Cancer. 2016;19(3):827–838. doi: 10.1007/s10120-015-0557-1. [DOI] [PubMed] [Google Scholar]


