Abstract
Moderate temperature hyperthermia (40–450°C for one hour) is emerging as an effective treatment to enhance best available chemotherapy strategies for bladder cancer. A rapidly increasing number of clinical trials have investigated the feasibility and efficacy of treating bladder cancer with combined intravesical chemotherapy and moderate temperature hyperthermia. To date, most studies have concerned treatment of non-muscle invasive bladder cancer (NMIBC) limited to the interior wall of the bladder. Following the promising results of initial clinical trials, investigators are now considering protocols for treatment of muscle invasive bladder cancer (MIBC). This paper provides a brief overview of the devices and techniques used for heating bladder cancer. Systems are described for thermal conduction heating of bladder wall via circulation of hot fluid, intravesical microwave antenna heating, capacitively coupled RF current heating, and radiofrequency phased array deep regional heating of the pelvis. Relative heating characteristics of the available technologies are compared based on published feasibility studies, and the systems correlated with clinical requirements for effective treatment of MIBC and NMIBC.
Keywords: bladder cancer, hyperthermia, intravesical heating, electromagnetic heating, thermochemotherapy
Introduction
The purpose of this paper is to review the capabilities of various heating technologies available for treatment of bladder cancer, with an eye toward correlating typical performance characteristics of each approach with the clinical requirements. Bladder tumors present either as non-muscle invasive (NMIBC), 70%, or as muscle invasive (MIBC), 30%, carcinomas.(1) While most clinical studies to date have concerned treatment of NMIBC superficial disease limited to the interior wall of bladder,(2–7) future clinical trials must address the more aggressive MIBC that extends beyond the bladder wall out into surrounding pelvic tissues. Thus depending on the extent of disease, heating systems are required to accomplish different clinical goals of treatment.
For healthy persons, the mean bladder wall thickness is 3.0±1 mm and 3.3±1.1 mm for female and male respectively with a weak positive correlation between wall thickness and age.(8) According to current staging criteria, NMIBC cancers encompass all Tis, Ta, and T1 bladder tumors that are limited to the mucosa and submucosa, i.e. involving less than 1 mm penetration into the bladder wall. Hence, if adequate transurethral resection of the intrabladder tumor growth has taken place prior to treatment, the therapeutic temperatures (40–43°C) of the hyperthermia treatment should target a depth of about 1 mm in order to produce heat activation of intravesical chemotherapy. For MIBC, i.e. tumor stages T2-T4a, the hyperthermia target extends to much greater depths and a heating system for MIBC must be able to deposit significant power throughout the bladder wall and for some patients out into extravesical pelvic tissues to enhance the delivery and activation of systemically administered chemotherapeutic throughout the muscle invasive component of disease.
The different tumor pathology of NMIBC and MIBC must be reflected in the clinical practice of applying adjuvant hyperthermia and thus the requirements on technology for heating these two diseases are quite different. For NMIBC with Tis, Ta, and T1 tumors, the target to be heated is well defined (~1 mm deep) and the technological demands for adequate heating of such shallow depth tumors are relatively easily met with uncomplicated thermal conduction heating solutions. For MIBC however, the tumor is ill-defined and variable in depth, size, shape and location (especially the advanced cases) demanding much higher flexibility in power deposition capabilities of the hyperthermia system to adequately heat the desired target volume. For this situation, effective use of heating technology is critically dependent on treatment planning, temperature measurement, and feedback control strategies. The modeling and thermal dosimetry approaches used to monitor and guide these complex treatments will be addressed in another paper of this special issue. Similarly, the clinical requirements for treating NMIBC and MIBC will be described in accompanying papers that summarize specific procedures and clinical results of the most common bladder treatment approaches. This special issue also includes specific coverage of the biological effects of hyperthermia, the pharmacologic effects of heat activated drug therapy, and the immunological effects of adjuvant hyperthermia.
In this overview, we describe the currently available methods for heating both NMIBC and MIBC disease, as well as their basic physical principles. Based on the available literature, we compile a semi-quantitative table to compare performance attributes of each technology. Finally, this summary information is organized to provide clinicians with an overview of pros and cons of the alternative approaches to heat NMIBC and MIBC, to aid the selection of hyperthermia method that best matches the tumor target.
Devices and Techniques for Clinical Heating of Bladder Cancer
The available technology for heating bladder cancer differs greatly in principle of heating, technological complexity, labor resources, preparation time, quality assurance demands, and cost. Transfer of energy may occur by direct thermal conduction, electromagnetic (EM) fields, or ultrasound (US) waves. Each of these technologies requires quite specific clinical and technical procedures that produce unique depth of penetration and uniformity of temperature.(9–12) The following paragraphs briefly describe the general characteristics, limitations and benefits of each heating method. To our belief, we have included all currently available clinical approaches. In our attempt to provide meaningful data for comparison of heating techniques in Table 2, we tried carefully to avoid inclusion of duplicate patient data from published feasibility studies. A common challenge is to accurately monitor the temperature distribution of the entire tumor as it invades different depths into the bladder wall, especially in the case of more extensive MIBC disease. Temperature gradients in the target volume occur due to variable local blood perfusion and to heterogeneity of absorbed energy from the heat source. Appropriate thermal dosimetry procedures are covered in another paper of this special issue.
Table 2.
Relative heating characteristics of clinical bladder hyperthermia systems
| Heating Criterion |
External | Intravesical | |||
|---|---|---|---|---|---|
| Deep Regional Electromagnetic |
Deep Focused Ultrasound |
Capacitive Current |
Microwave | Circulating Heated Fluid |
|
| Homogeneity of heating around interior wall of bladder | + | − − | − | − − | + + |
| Depth of penetration of heating through tissue | + + | + + | + + | − | − − |
| Control of maximum temperature | − − | − | − − | − | + + |
| Circulation/mixing of drug in contact with bladder wall | − | − | − | + | + + |
| Heating of tumor tissue outside the bladder | + + | + | + | − | − − |
| Heating of normal tissue outside the bladder | − − | − | − − | + | + + |
| Ease of Heating | − | − − | − | − | + + |
| Patient Tolerance | − | − | − | − | + |
+ + Major Advantage, + Moderate Advantage, −Moderate Shortcoming, − − Major Shortcoming
Radiative EM energy based external heating
Principles
Typical radiofrequency (RF) phased array applicators operating between 70 and 120 MHz produce a large hot spot at the phase focus but also heat skin and a significant portion of pelvic tissue outside the focus. Heating patterns possible with electromagnetic phased array applicators have been studied extensively.(13–17) Located centrally in the pelvis and filled with a mixture of lossy urine and drug that has no blood perfusion cooling, the bladder heats preferentially using an appropriately phased array of RF antennas around the torso to focus power deposition in and around the bladder. A 14-patient Phase I clinical trial was conducted to study the feasibility of treating NMIBC with intravesical Mitomycin C (MMC) combined with external radiofrequency phased array deep hyperthermia.(6) The thermal dosimetry of that 14 patient trial showed that adequate heating, defined as a bladder temperature above 40°C for more than 40 min, was achieved in 96% of the treatments, and 73% of the patients completed the course of 10 treatments per protocol.(18) A subsequent 18 patient trial using the Amsterdam AMC-4 or AMC-8 waveguide system (17) produced similar results, demonstrating feasibility of heating NMIBC with low toxicity using an external electromagnetic phased array.(3) Excellent bladder temperatures were achieved (i.e. an average bladder temperature of 41.6°C for 60–90 min each treatment) with 83% completing the six induction treatments and 50% completing all 10 treatments. Since the diffuse focus produced by 70–120 MHz phased array systems is larger than the bladder dimensions, this heating approach deposits power not only in the bladder contents but also directly in the bladder wall and tissues surrounding the bladder. Thus, these electromagnetic heating approaches can be expected to heat the entire bladder wall and potentially muscle invasive disease extending outside the bladder. To date, no large trials of MIBC have been reported, but the feasibility of heating locally advanced MIBC has been demonstrated in a small study of 5 patients.(19) This study showed that interstitially measured tumor temperatures correlated with intraluminal measured temperatures (average tumor temperature 40.9±1.1°C vs tumor indicative bladder lumen temperature 40.7±1.3°C). These feasibility studies suggest that the RF deep heating approach is suitable for heating bladder cancers extending into the pelvis, especially when combined with MR thermal monitoring to define the 3D temperature distribution throughout the pelvis in real time during treatment.(20–22) Since most centers do not have MR thermal monitoring capability yet, control of the treatment relies more heavily on careful treatment planning whenever the tumor target extends 1 cm or more from the typical bladder and rectal temperature monitoring sites. Software is available for patient specific treatment planning of deep heat treatments, such as Sigma HyperPlan (Dr Sennewald Medizintechnik GmbH, Munich, Germany), a program that was optimized specifically for the BSD2000 Sigma applicators. The utility of this comprehensive planning program looks very promising but overall accuracy in complex tissue regions like the pelvis is still under evaluation.(23–26) Other multi-physics software is available commercially that is flexible for modeling power deposition and corresponding thermal heat transfer in the body from any applicator, such as SEMCAD X (SPEAG, Zurich Switzerland), HFSS (Ansys Corp, Canonsburg PA, USA), COMSOL Multi-Physics (Comsol Inc., Burlington MA, USA), CST Studio Suite (CST, Framingham MA, USA), and others. The value of these powerful electromagnetic and thermal modeling programs is currently under evaluation. See accompanying article in this special issue on thermal dosimetry and treatment planning.(27)
Temperature measurement during radiative electromagnetic heating of bladder cancer is often performed by a single catheter using multiple sensor temperature sensors or by thermal mapping.(3, 6, 18, 19) Recently, Cordeiro et al. (28) introduced a special catheter to improve the quality of temperature measurement during NMIBC hyperthermia. Once in the bladder, the catheter unfolds like an umbrella and places three temperature sensors against the bladder wall equally distributed around the circumference.
Typical EM deep heating systems
The BSD2000 RF Phased Array Hyperthermia System (Pyrexar Corp., Salt Lake City UT, USA) powers 1–3 annular rings of dipole antennas with phase adjustable signals in the range of 80–120 MHz. Several applicator configurations are available for treatment of pelvic disease. A four channel system powers four twin dipole antennas spaced around either a 60 cm diameter annular array (Sigma 60 applicator), or a smaller 57 × 40 cm elliptical array (Sigma Ellipse).(14–16) The BSD2000 3D and 3D/MR Hyperthermia Systems include an elliptically shaped Sigma Eye applicator that is MR compatible to fit inside a 60 cm dia MR magnet.(21, 22) The Sigma Eye applicator includes three coaxial rings of four twin dipole radiators, for a total of 24 antennas driven with the 12 power channels at a frequency of 100 MHz. The system takes advantage of custom software that obtains 3D thermal image data during treatment to provide realtime feedback to the 12 independent phase and amplitude controls.(20, 22, 29, 30) All Sigma applicators have a flexible silicone membrane inside the applicator that inflates like an annular doughnut shape around the torso and fills with circulating temperature controlled deionized water to cool the skin and couple electric field into the patient.
Deep regional heating of large regions in the pelvis may be accomplished similarly with a 70 MHz radiofrequency phased array system (AMC-4 or -8) using either 1 or 2 rings of 4 waveguide antennas, each with an aperture size of 33 × 21 cm and temperature controlled waterbolus coupling to the patient.(3, 17, 31). While the single ring 4 antenna system has been used successfully in a clinical trial to heat bladder disease,(3) recent studies indicate that the 3D SAR steering of the AMC-8 system promises an enlarged SAR focal region as well as improved control and localization of heating.(17, 32) The 70 MHz four antenna system is now available commercially as the Alba 4D (Alba Hyperthermia Systems, Roma Italy).
Capacitively coupled external electric current heating
Principles
Using lower frequency RF such as 8 – 13.56 MHz, electromagnetic energy may be delivered to tissue via electric current that flows between capacitively coupled electrodes on the skin surface. All currently available systems have two electrodes that are generally coupled to the skin with electrically conductive temperature controlled saline pads to conduct and spread electric current into tissue and cool the skin surface.(33–35) Electrical current must flow through the high resistance fat tissue layer before splitting into multiple current paths through the intervening tissue between electrodes. Thus, maximal power deposition normally occurs in the superficial fat layer which must be cooled with temperature regulated saline bolus.(36, 37) At depth, electrical currents distribute such that more current flows through low resistance tissues like muscle and tumor, and less current flows in parallel through high resistance tissues like fat, bone and aerated lung.(33, 38) With power deposition proportional to the current squared, more power is deposited in tissues along the higher current pathways in low resistance tissues. Thus power deposition is possible in low resistance urine filled bladder even though maximum heating rate likely occurs in the high resistance superficial fat layer just under the electrodes. Using different size electrodes, RF currents and tissue heating may be concentrated under the smaller electrode. Ongoing clinical use primarily in Asia has demonstrated the ability to heat into the hyperthermic range in patients with sufficiently thin layers of high resistance fat tissue overlying tumor, using aggressive skin cooling.(5, 37, 39)
Two clinical studies report on the performance of capacitively coupled hyperthermia to heat locally advanced MIBC. Masunaga et al. (40) reports that an average intravesical bladder temperature of 41.5±1.1°C was obtained in their group of 28 patients. They also indicate that significantly higher bladder temperatures were achieved in patients with a subcutaneous fat layer less than 2 cm thickness. The average duration of the heat treatment was 44.4±8 min for patients with an average bladder temperature >41.5°C, and 40.5±5.9 min for patients with bladder Tavg <41.5°C. Uchibayashi et al (41) analyzed the ability to heat locally advanced muscle invasive bladder tumors in 46 patients. They report similar findings: intravesical and tumor temperatures could be raised to 42.5°C or higher for patients with subcutaneous fat layers less than 2 cm thickness. In obese patients, it was more difficult to heat the bladder tumor to the required level. Thermometry was performed using Teflon coated thermocouples placed in the ureter, rectum and tumor. Patient complaints concerned pain at the edge of the electrodes in nearly half of the patients, and was the limiting factor for power elevation in these patients.
Typical heating systems
Capacitively coupled RF heating systems are available with two moveable capacitive plate electrodes around a treatment bed such as the 13.56 MHz 600 W Celsius TCS system (Celsius 42+ GmbH, Cologne Germany), and two electrodes mounted on a parallel opposed rotating arm as in the 8 MHz Thermotron RF-8 (Yamamoto Vinyter, Japan).(35, 36)
Ultrasound energy based external heating
Principles
Several ultrasound array devices have been designed for hyperthermia treatment of large deep tissue targets.(42–44) However the majority of focused ultrasound applications involve smaller target volumes that take advantage of the short wavelength to produce tightly focused hot spots for rapid thermal ablation without effecting surrounding normal tissues.(45, 46) With care to manage reflections and absorption of ultrasound near air and bone, the small hot spot can be shifted to produce successive overlapping regions to treat larger volumes such as human bladder. While ultrasound travels through urine without much loss, power can be focused on the bladder wall itself to produce effective localization of heat in specific tumor target regions. The challenge of this technique is in designing the scanning algorithm that provides homogenous bladder wall heating. Although the feasibility of heating bladder with ultrasound has been demonstrated,(47) large tissue targets in the pelvis are generally heated using electromagnetic phased array sources having longer wavelengths that both penetrate and produce larger bladder-sized focal zones. At present no commercial systems are available that are optimized for bladder treatment and no clinical studies have been reported.
Radiative EM energy based internal heating: Intravesical Microwave Antenna
Principles
As an alternative to localizing heat in bladder from external sources, one can radiate energy from a small diameter intraluminal microwave antenna inserted into the bladder through a special multilumen urethral catheter. The advantage of this method is that energy does not travel through a large volume of normal tissue before impinging on the bladder target, but is instead aimed directly at the adjacent tumor target from inside the bladder. In addition, well localized heating around the intravesical antenna can be achieved with rather simple equipment compared to external phased array sources. Further, the use of microwave radiation increases the penetration of effective heating in perfused tissue of the bladder wall in comparison to thermal conduction only heat sources. Microwave antennas that can be placed inside 1–2 mm diameter catheters have been investigated by many groups and the principles and typical radiation patterns have been reviewed previously.(48) The first system optimized for use inside the bladder applies power to a 915 MHz coaxial cable antenna that is introduced into the bladder inside a special Foley catheter and radiates an EMF into tissue around the antenna tip. Because fluid inside the bladder is circulated around the antenna and through an external temperature controlling heat exchanger, heat is removed from the fluid during treatment enabling use of higher antenna power levels without overheating the fluid. This allows the microwave field to penetrate further through the electrically lossy urine/drug filled bladder to deposit energy directly in the bladder wall.
Of all hyperthermia systems used for heating NMIBC, intravesical microwave heating has the highest number of clinical studies performed. The eight studies reported in Table 1 are not exhaustive; however they cover 287 patients treated by four different institutions and hence provide strong endorsement for the clinical viability of intravesical microwave antenna heating.(2, 49–54) The largest reported study of this approach by Nativ et al. (55) is a retrospective analysis of 111 NMIBC patients. Overall the clinical experience with intravesical microwave heating demonstrates that therapeutic temperatures (42±2°C) are routinely achieved inside the bladder, though the median temperature and temperature range vary substantially between institutes. In general, the studies indicate that heat treatments are well-tolerated with a high proportion of patients completing the entire treatment course. Reported adverse events are mostly local and transient such as pain and bladder spasm during treatment sessions, followed by hematuria, dysuria and transient incontinence. It should be noted that typical intravesical microwave systems measure temperature at three different points along the inner wall of bladder, providing a small but representative sampling of achieved intra-bladder temperatures.(28, 51) The relatively large temperature range reported can be explained by the non-uniform irradiation pattern of an interstitial microwave antenna in an irregular shaped fluid-filled bladder. This non-uniform power deposition pattern is also associated with the relatively high incidence of thermal reactions seen in the posterior bladder wall.
Table 1.
Comparison chart of clinical studies of bladder hyperthermia
| Equipment | Reference | Frequency (MHz) |
Study Size | Prescribed # Tx |
Treatment Schedule |
Ave Power [W] or Temp [°C] |
Goal/Achieved Temperature |
HT Duration |
Goal Treatment Completed |
Max Toxicity (CTCAE score) |
Most Common Toxicity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| External Deep Regional RF | |||||||||||
| BSD-2000 RF Phased Array HT System | Inman 2014 (6) Juang 2014 (24) |
80–120 | 14 | 10 | 6×1/wk + 4×1/ mon |
740 ± 162W | 96% > 40 min at Tmin>40°C |
Ave Time >40°C 53.1 ± 8.8 min |
73% | Grade 2 | 40% Foley Cath Pain 33% Abdomenal Discomfort |
| BSD-2000 RF Phased Array HT System | Fatehi 2007 (25) | 75–80 | 5 | NR | 1/wk | NR | 40.9°C tumor 40.7 °C lumen |
60–90 min | NR | NR | NR |
| AMC-4, AMC-8 Waveguide Phased Array System | Geijsen 2015 (3) | 70 | 18 | 10 | 6×1/wk + 4×1/mon |
553W | T50 = 41.6°C | 60 – 90 min | 83% Induction 50% Full |
Grade 2 | 38% Local Pain and Bladder Spasms |
| Capacitive Plate Electrodes | |||||||||||
| Yamamoto Vinyter - RF-8 Capacitive Heating System | Masunaga 1994 (41) | 8 | 28 | 4 | 2/wk + RT | 578 ± 86W | 42.1 + 8.0°C | 35 – 60 min | 43% Pain Under Electrodes | ||
| Yamamoto Vinyter - RF-8 Capacitive Heating System | Uchibayashi 1995 (42) | 8 | 46 | 6 – 10 | 2/wk + RT | 42.5°C | 60 | Grade 2 | 28% Burn, 24% Anorexia 20% Sub-Q Induration |
||
| Intravesical Microwave Antenna | |||||||||||
| Synergo SB-TS 101 Transurethral MW System | Colombo 1995 (44) | 915 | 44 | 8 | 2/wk in <6 wks | < 60 W | 42.5 – 44.5°C | 60 min Goal >40 min Minimum |
97% | NR | Mild Urge and Urethral Burning |
| Synergo SB-TS 101 Transurethral MW System | Colombo 1996 (45) | 915 | 29 | 6 – 8 | 1–2/wk in <6 wks |
NR | 42.5 – 46°C | 60 min (2 × 30 min to Refresh Drug) |
93% | Grade 3 | Mild Urgency |
| Synergo SB-TS 101 Transurethral MW System | Colombo 1998 (46) | 915 | 19 | 8 | 1/wk | NR | 42.5 – 46°C | 60 min Goal >40 min Minimum |
96% | NR | Moderate/Severe Urgency, Mild Urethral Burning |
| Synergo SB-TS 101 Transurethral MW System | Colombo 2003 (2) | 915 | 42 | 12 | 8×1/wk + 4×1/ mon |
NR | 42 ± 2°C | 60 min (2 × 30 min to Refresh Drug) |
88% | NR | Pelvic Pain and Posterior Wall Thermal Reaction |
| Synergo SB-TS 101 Transurethral MW System | Kiss 2015 (47) | 915 | 21 | 6 – 12 | NR | 42 ± 2°C | 60 min (2 × 30 min to Refresh Drug) |
62% | Grade 4 CTC Score - NCIC |
57% Pain and Bladder Spasms | |
| Synergo SB-TS 101 Transurethral MW System | Maffezzini 2014 (48) | 915 | 42 | 14 | 4×1/wk + 6×2/mon + 4×1/mon |
NR | 42.5 ± 1.5°C | 60 min Goal >40 min Minimum |
76% | Grade 2 | 100% Thermal Reaction Posterior Bladder Wall |
| Synergo SB-TS 101 Transurethral MW System | van der Heijden 2004 (49) |
915 | 90 | 10 – 14 | 6–8×1/wk + 4–6×1/mon |
NR | 41 – 44°C | 60 min (2 × 30 min to Refresh Drug) |
10 ± 2 | Toxicity All Local Healed Spontaneously |
72% ≥ 1 Toxicity 100% Thermal Reaction Posterior Bladder Wall |
| Synergo SB-TS 101 Transurethral MW System | Nativ 2009 (50) | 915 | 111 | 12 | 6×1/wk + every 4–6 wks |
NR | 42 ± 2°C | 60 min (2 × 30 min to Refresh Drug) |
95% | Grade 3 | 31% Pain and Bladder Spasms |
| Intravesical Thermal Conduction | |||||||||||
| Combat Medical Ltd – Combat BRS System | Sousa 2014 (54) | - | 15 | 8 | 8×1/wk | 43 ± 1°C | Bladder Temp Not Measured |
96% – 60 min 99% > 40 min |
99% | Grade 2 | 33% Irritative Lower Urinary Tract |
| Elmedical Ltd - Unithermia System | Ekin 2015 (55) | - | 43 | 12 | 6×1/wk + 3×1/wk in months 3 and 6 |
NR | Bladder Temp Not Measured |
60 min | 93% | Grade 5 | 37% Noninfective Cystitis |
| Elmedical Ltd - Unithermia System | Soria 2015 (56) | - | 34 | 6 | 6×1/wk | 42.5 ± 1°C 44.5°C Peak |
Bladder Temp Not Measured |
45 min | 88% | Institutional CTC Score | 24% Bladder Spasms |
Typical intravesical microwave antenna system
The Synergo SB-TS 101 System (Medical Enterprises Europe B.V., Amstelveen, The Netherlands) consists of a 915 MHz microwave applicator that is inserted into the bladder via a special multilumen 19.5F or 20F catheter. A peristaltic pump slowly circulates MMC through the catheter into the bladder through a temperature controlled heat exchanger that cools the drug and helps homogenize intrabladder temperature during application of microwave power. Temperatures are measured in the urethra and with three thermocouples pressed against the bladder wall by the inflated catheter balloon.(4, 51)
Intravesical Thermal Conduction Heating
Principles
The most straightforward approach to heating tissue is to apply a heated surface in intimate contact with the target tissue to enable heat transfer across a thermal gradient. To uniformly heat the interior wall of a complex shaped bladder cavity, this simple heat transfer approach is best accomplished by vigorously circulating externally heated fluid (and drug) at a controlled temperature throughout the bladder. Due to the dynamics of turbulent flow, there is a non-uniform velocity profile across the inner bladder surface but with sufficient circulation the entire target tissue on the inner wall of bladder will come in contact with rapidly moving near equi-temperature fluid. With no power deposition directly in tissue, the resulting temperature distribution is dependent only on bladder tissue thermal parameters rather than heterogeneous electrical or acoustic tissue properties. Using thermal conduction only heating, maximum tissue temperature is readily determined with confidence to be the measured input temperature of the circulating fluid, and thus thermal toxicity is easily avoided. The constant temperature interface should provide relatively uniform heating of the inner surface of bladder even with heterogeneous thermal properties that effect heat penetration into the wall. While temperature of the bladder lumen may be nearly constant, there is a steep temperature gradient within the bladder wall as heat dissipates rapidly into surrounding cooler tissues. Especially because of heat losses to normothermic blood perfusing the bladder wall tissues, the penetration depth of effective heating is limited when the driving thermal gradient is only 7°C (44 - 37°C). The accompanying modeling and thermal dosimetry paper in this special issue specifically addresses penetration of heat into the bladder wall with various heating technologies. Previous studies of temperature gradients in perfused tissue adjacent to a hot (or cold) surface show that the effective heating potential of a 7°C thermal gradient extend no more than 2–3 mm deep.(56, 57). For NMIBC cancers that extend <2 mm into the bladder wall, this thermal gradient induced heating should be sufficient for effective treatment. Clinical experience at three institutes using two different intravesical thermal conduction heating systems indicates excellent performance with a very high percentage of patients completing the prescribed treatment course (see Table 1).
Alternatively, thermal conduction heating from inside the bladder may be accomplished without the need to circulate expensive chemotherapeutic through multi-lumen connecting tubes to an external temperature regulating heat exchanger. Efforts are underway to investigate the use of ferromagnetic nanoparticle (MNP) solutions that can be mixed with drug and injected into the bladder through a standard Foley catheter. With this approach, the MNP are heated via magnetic induction coupling from an externally applied alternating magnetic field, usually at a frequency of 50–100 kHz to minimize direct eddy current heating in the pelvis. As demonstrated in preclinical studies, the MNP can produce sufficient heating to raise the temperature of the chemotherapeutic mixture in bladder to the desired treatment temperature.(58) Inductively coupled heating is distributed throughout the MNP solution producing relatively homogeneous internal bladder temperature, and convection current mixing of bladder contents further minimizes temperature gradients during treatment. For magnetic coupled nanoparticle solutions, temperature must be monitored with a probe inserted into the bladder through the Foley catheter. Unlike the rapidly circulated heated fluid approach, the maximum bladder temperature is not known precisely as the intraluminal probe will generally underestimate the peak temperature due to small gradients within the unstirred magnetic fluid/chemotherapeutic mixture.
Typical intravesical thermal conduction systems
There are two commercial systems that heat bladder by circulating heated drug through the bladder. The Combat BRS System (Combat Medical Ltd – Wheathampstead, Herts UK) heats chemotherapeutic (e.g. MMC) to the desired intrabladder temperature in the range of 40–44°C and circulates the heated drug through the bladder via a soft and flexible 16F 3-way catheter with coude tip to facilitate insertion into the bladder.(59) The device consists of a temperature controlled waterbath heat source and peristaltic pump to circulate drug through the bladder via a low volume high efficiency heat exchanger. Temperature of the circulating fluid is monitored with an inline probe and all treatment parameters are controlled via a simple touch screen user interface with graphical temperature display and safety alarms for high and low temperature, and overpressure.
The Unithermia System (Elmedical Ltd, Hod-Hasharon Israel) for Bladder Wall Thermo-chemotherapy uses a similar design concept to heat chemotherapeutic to about 46.5°C in order to obtain 44–44.5°C drug temperature inside the bladder using a circulation rate that achieves approximately 4 exchanges of bladder fluid contents per minute.(60, 61) This rapid circulation of drug within the bladder improves the uniformity of drug temperature contacting the inner surface of bladder and provides fresh drug to the bladder wall surface continuously for the typical 50 min thermochemotherapy treatment.
Devices and Techniques for Pre-Clinical Bladder Heating
Preclinical studies are often used in the development and optimization of new cancer treatment strategies. Rodent studies are most common though larger animals are also used to model the human treatment situation more closely. Since human bladder capacity is approximately 500 ml,(62) the small size of bladder in mice (~ 0.15 ml) and rats (~ 1 – 1.5 ml) requires different heating systems(58, 63). Farm pigs are also used for preclinical studies, with bladder volumes ranging from 250 ml to larger than human bladders. Thus preclinical studies require unique heating technology that can localize heat within the desired bladder target which may be buried over 3 to 12 mm from the skin surface. Hyperthermia studies are often performed in mice using simple thermal conduction heating via waterbath, but that approach generally produces regional if not systemic temperature rise that would complicate interpretation of biologic response to specific bladder therapy. In recent years, a number of approaches have been reported for improved localization of heat in animal bladders.
Radiative external microwave antenna heating
In order to elevate temperature in a urine filled bladder at depths of 3–8 mm below the skin of small animals like mice and rats, microwave antenna systems have been developed at 915 MHz and 2450 MHz. These antennas are generally 1–2 cm in diameter and coupled to tissue with a temperature controlled waterbolus to cool the superficial tissues and allow localization of heat in underlying bladder. Salahi et al. describe the heating performance of a 1 cm diameter water filled circular waveguide microwave antenna designed specifically for heating small rodent bladders.(63) Another microwave antenna designed for small volume heat treatments has been reported previously (64, 65) with heating patterns that demonstrate feasibility of treating small animal bladders. Snow et al. report the feasibility of heating in vivo pig bladder with no acute toxicity using a small array of 915 MHz square slot dual concentric conductor antennas.(66, 67)
Inductively coupled ferromagnetic nanoparticle heating
An increasing body of literature describes the use of ferromagnetic nanoparticles (MNP) injected into a tumor directly or administered systemically with the goal of concentrating sufficient magnetic material in the target to heat tissue from coupling to an externally applied magnetic field. For NMIBC disease where the target tissue is located ≤ 2 mm deep into the bladder wall, the injection of magnetic fluid into the bladder intermixed with chemotherapeutic and subsequent heating of bladder via external magnetic field seems straightforward. Oliveira et al. (58) report a feasibility study of introducing a concentrated magnetic fluid into rat bladder via urethral catheter and heating the bladder by magnetic field coupling to the ferrofluid. With no forced stirring, thermal gradients will occur inside the bladder. However, homogenization of intrabladder temperatures is anticipated due to natural convective stirring of bladder contents driven by the small thermal gradients.
Gold nanoparticle enhanced photothermal ablation
Another area of development involves laser or electric field coupled heating of gold nanoparticles.(68) Cho et al.(69) describe a treatment approach involving photothermal ablation of superficial bladder cancer. They propose to fill the bladder with a solution of gold nanorods conjugated to anti-EGFR antibodies that will bind to EGFR-expressing bladder cancer cells. Then by introducing near infrared radiation to the interior of the bladder via a modified cystoscope, power may be deposited preferentially in the gold nanorods sufficient to thermally ablate adjacent tumor cells.
Correlation of Heating Technologies with Clinical Requirements
General characteristics of the clinical problem
As explained earlier, there are primarily two types of disease to be addressed in this review: NMIBC and MIBC. These two diseases are quite different biologically and anatomically, and require significantly different treatment approaches. The corresponding requirements for heating these two diseases are also quite different. Superficial bladder cancers involve only the mucosa and sub-mucosa layers along the inner bladder surface, generally with a thickness ≤2 mm. On the other hand, MIBC by definition extends into the muscle layers of the bladder wall and even into extravesical structures outside the bladder; this deeper disease presents a wide range of technical challenges to effective heating. Thus optimal heating of NMIBC requires homogeneous heating of the bladder interior extending < 2–3 mm into the bladder wall, and ideally there would be minimal heating of normal tissues outside the bladder. Optimal treatment of MIBC requires heating not only the bladder wall but also a variable amount of tissue outside the bladder. Of all the available heating technology, some systems are better suited to limiting the extent of heating to just a thin rim of target disease on the interior of the bladder, and other devices are better suited to heating large regions of pelvis including bladder and surrounding tissues.
Criteria for determining effectiveness of treatment include:
Homogeneity of heating around the interior surface of bladder
Depth of penetration of heating through bladder tissue
Circulation/mixing of drug in contact with inner surface of bladder wall
Heating of normal tissue outside the bladder
Heating of tumor tissue outside the bladder
Ease of heating
Patient tolerance
Clinical infrastructure requirements
In addition to comparing technological performance of the above mentioned systems by characterizing their ability to localize and uniformly heat tumor, other important considerations for selecting a hyperthermia system include the initial capital investment, space, supplies, and personnel and time required to prepare for and apply each treatment.
Of the intravesical systems, the circulating fluid thermal conduction method is the most straightforward to apply. Due to the known maximum temperature of the circulating fluid, this approach has the lowest risk of side effects from uncontrolled excessive temperature. It also is the least demanding of resources for calibration and quality assurance validation. The intravesical microwave antenna system is similarly compact, but includes a power generator that produces non-uniform power deposition around the antenna and unknown temperature peaks in tissue. Thus this technology requires stepwise more quality assurance and personnel training to obtain safe and reproducible performance. In either case, the intravesical systems should be applied only for heating superficial NMIBC.
External RF hyperthermia systems require a more substantial infrastructure to purchase and maintain the equipment, and to provide appropriate RF shielding for non-ISM band EMF. The technology for capacitively coupled electric current systems is relatively straight forward and maintenance with minimal QA verification measurements should be sufficient for safe operational use. A consequence of the relatively simple capacitive plate technology is a lack of flexibility to adjust power deposition pattern for optimal tumor heating and to effectively accommodate patient complaints with minor adjustments of heating parameters. In comparison, the external RF radiative systems use the most complex technology and require the most extensive support structure. The advantage is that they are the only systems that can heat both NMIBC and MIBC disease. And with carefully designed treatment protocols, heating of NMIBC has been demonstrated with acceptable personnel resources, i.e. with only one person to operate the system. If the bladder is filled with a high absorbing liquid, preferential heating can be obtained with predefined antenna settings and without extensive hyperthermia treatment planning. Investing in a radiative EMF method should be considered when there is a need to apply hyperthermia to locally advanced muscle invasive bladder tumors. In that application, heating of MIBC should be performed using optimal antenna settings obtained by patient specific hyperthermia treatment planning, and with the deep hyperthermia system operated following appropriate quality assurance guidelines.(70–73)
Pros and Cons of Bladder Heating Technologies
A summary of advantages and disadvantages is provided in Table 2 for each of the heating technologies described above. For treatment of NMIBC limited to the interior of bladder, intravesical heating approaches appear most appropriate. As illustrated before, the simplest way to heat bladder wall tumors is via the circulating, heated fluid approach. This has the advantage of knowing both the maximum temperature anywhere in tissue and effective extent of heating is limited to about 2–3 mm maximum penetration through the bladder wall, which is sufficient for NMIBC. Thus, circulating fluid thermal conduction heating may be expected to produce fewer toxicities compared to more complicated microwave heating approach that is known to produce non-homogeneous power deposition with "hot spots” and potentially burns in the posterior bladder wall and "cold spots” or places of ineffective heating at the dome and near the trigone. Potential advantages of the intravesical microwave thermo-chemotherapy approach stem from the increased penetration of microwave energy into somewhat thicker tumors (3–6 mm) and the use of circulating cooled drug to limit indiscriminant heating particularly in the bladder neck and urethra. This claim may be overstated however, as the precooled drug solution is heated during circulation both in the multilumen catheter where drug flows slowly through the same catheter with a hot radiating microwave antenna and in the bladder where the drug surrounds the radiating antenna. Thus, although improved penetration of heating may be possible using the intravesical microwave antenna, significant thermal gradients are produced within the bladder as well as the tumor due to non-uniform power deposition from the antenna and slow circulation of fluid in the multilumen catheter.
Although external electromagnetic heating systems are more complex than intravesical devices, there are potential advantages that result from the more complete heating of bladder, bladder wall, and tissues surrounding the bladder, which may prove important for long term clinical response. Obvious weaknesses of external regional heating approaches stem from the lack of knowledge of peak temperatures and hot spots in tissue, and inclusion of significant volumes of normal tissue within the heated region. Current pilot studies have demonstrated the feasibility of using external RF systems to effectively heat NMIBC with minimal toxicity,(3, 6, 18) but the determination of any advantage in terms of clinical outcome awaits larger clinical trials.
Summary
This review outlines the clinical requirements for heating bladder as a component of thermochemotherapy for bladder cancer. Devices and techniques currently available to heat bladder are described briefly and the equipment correlated with most appropriate clinical disease, either muscle invasive bladder cancer or non-muscle invasive bladder cancer. Representative commercially available clinical equipment systems are identified for each type of energy delivery. And several systems are described within the context of pre-clinical heating equipment currently available for use in animal studies. This article is intended to supplement the detailed descriptions of clinical disease, biological goals of treatment, clinical trial results, thermal dosimetry and treatment planning descriptions that are contained in other articles of this special issue on bladder cancer.
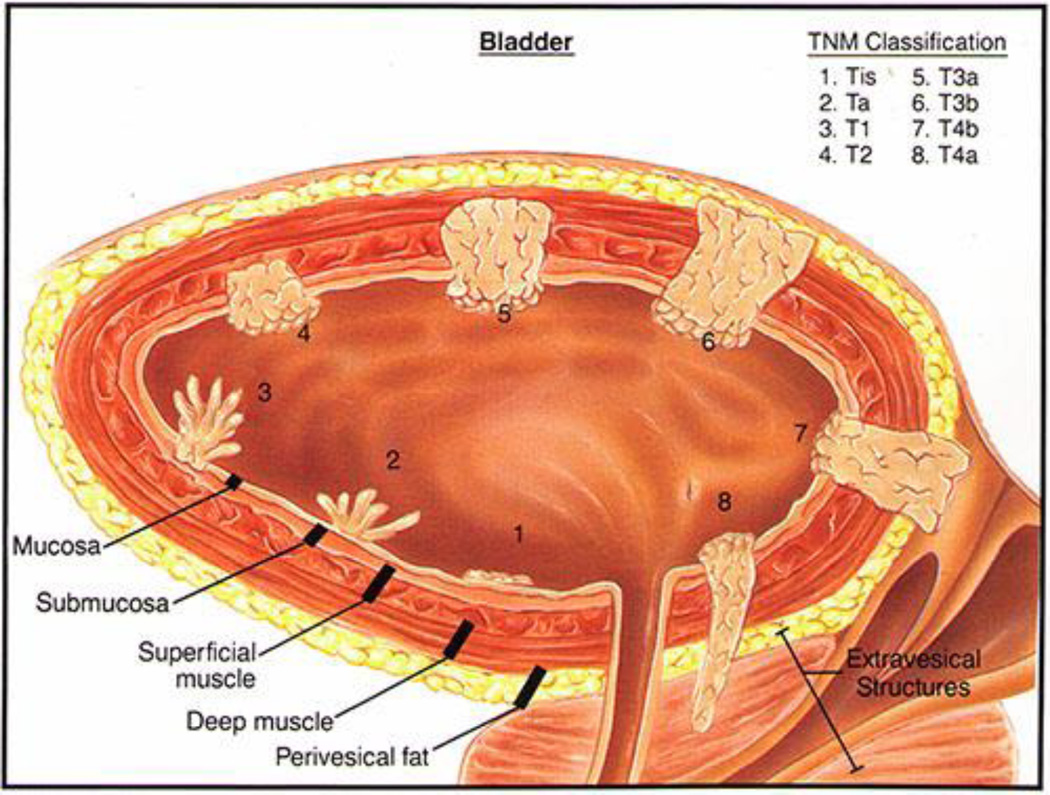
Figure 1.
Pictorial of bladder cancer staging classification as regards penetration of disease into the bladder wall. Reprinted with permission from Katelaris Urology, http://www.katelarisurology.com.au/about-bladder-cancer/.
Acknowledgments
The authors would like to acknowledge support from NIH R21 GM111560 (Stauffer) and the Koningin Wilhelmina Foundation (Dutch Cancer Society) grant DDHK 2013-6072 (van Rhoon).
Footnotes
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46(4):765–781. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Colombo R, Da Pozzo LF, Salonia A, Rigatti P, Leib Z, Baniel J, et al. Multicentric study comparing intravesical chemotherapy alone and with local microwave hyperthermia for prophylaxis of recurrence of superficial transitional cell carcinoma. Journal of Clinical Oncology. 2003;21(23):4270–4276. doi: 10.1200/JCO.2003.01.089. [see comment] [DOI] [PubMed] [Google Scholar]
- 3.Geijsen ED, de Reijke TM, Koning CC, Zum Vorde Sive Vording PJ, de la Rosette JJ, Rasch CR, et al. Combining Mitomycin C and Regional 70 MHz Hyperthermia in Patients with Nonmuscle Invasive Bladder Cancer: A Pilot Study. Journal of Urology. 2015;194:1202–1208. doi: 10.1016/j.juro.2015.05.102. [DOI] [PubMed] [Google Scholar]
- 4.Gofrit ON, Shapiro A, Pode D, Sidi A, Nativ O, Leib Z, et al. Combined local bladder hyperthermia and intravesical chemotherapy for the treatment of high-grade superficial bladder cancer. Urology. 2004;63(3):466–471. doi: 10.1016/j.urology.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 5.Hashimoto T, Hisazumi H, Nakajima K, Matsubara F. Studies on endocrine changes induced by 8 MHz local radiofrequency hyperthermia in patients with bladder cancer. Int J Hyperthermia. 1991;7(4):551–557. doi: 10.3109/02656739109034967. [DOI] [PubMed] [Google Scholar]
- 6.Inman BA, Stauffer PR, Craciunescu OA, Maccarini PF, Dewhirst MW, Vujaskovic Z. A pilot clinical trial of intravesical mitomycin-C and external deep pelvic hyperthermia for non-muscle-invasive bladder cancer. Int J Hyperthermia. 2014;30(3):171–175. doi: 10.3109/02656736.2014.882021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lammers RJ, Witjes JA, Inman BA, Leibovitch I, Laufer M, Nativ O, et al. The role of a combined regimen with intravesical chemotherapy and hyperthermia in the management of non-muscle-invasive bladder cancer: a systematic review. Eur Urol. 2011;60(1):81–93. doi: 10.1016/j.eururo.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 8.Hakenberg OW, Linne C, Manseck A, Wirth MP. Bladder wall thickness in normal adults and men with mild lower urinary tract symptoms and benign prostatic enlargement. Neurourol Urodyn. 2000;19(5):585–593. doi: 10.1002/1520-6777(2000)19:5<585::aid-nau5>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 9.Hynynen K. Ultrasound heating technology. In: Seegenschmiedt MH, Fessenden P, Vernon CC, editors. Thermoradiotherapy and thermochemotherapy. 1, Chapter 12. Berlin, Heidelberg: Springer-Verlag; 1995. pp. 253–277. [Google Scholar]
- 10.Lee ER. Electromagnetic superficial heating technology. In: Seegenschmiedt MH, Fessenden P, Vernon CC, editors. Thermoradiotherapy and Thermochemotherapy. 1, Chapter 10. Berlin, Heidelberg: Springer-Verlag; 1995. pp. 193–217. [Google Scholar]
- 11.van Rhoon GC. External electromagnetic methods and devices. In: Moros EG, editor. Physics of thermal therapy - Fundamentals and clinical applications. Boca Raton CRC Press, Taylor and Francis Group; 2013. pp. 139–158. [Google Scholar]
- 12.Stauffer PR. Evolving technology for thermal therapy of cancer. Int J Hyperthermia. 2005;21(8):731–744. doi: 10.1080/02656730500331868. [DOI] [PubMed] [Google Scholar]
- 13.Canters RA, Wust P, Bakker JF, Van Rhoon GC. A literature survey on indicators for characterisation and optimisation of SAR distributions in deep hyperthermia, a plea for standardisation. Int J Hyperthermia. 2009;25(7):593–608. doi: 10.3109/02656730903110539. [DOI] [PubMed] [Google Scholar]
- 14.Fatehi D, van Rhoon GC. SAR characteristics of the Sigma-60-Ellipse applicator. Int J Hyperther. 2008;24(4):347–356. doi: 10.1080/02656730701832326. [DOI] [PubMed] [Google Scholar]
- 15.Paulsen KD, Geimer S, Tang J, Boyse WE. Optimization of pelvic heating rate distributions with electromagnetic phased arrays. Int J Hyperthermia. 1999;15(3):157–186. doi: 10.1080/026567399285701. [DOI] [PubMed] [Google Scholar]
- 16.Van Rhoon GC, Van Der Heuvel DJ, Ameziane A, Rietveld PJ, Volenec K, Van Der Zee J. Characterization of the SAR-distribution of the Sigma-60 applicator for regional hyperthermia using a Schottky diode sheet. Int J Hyperther. 2003;19(6):642–654. doi: 10.1080/0265673031000140813. [DOI] [PubMed] [Google Scholar]
- 17.Crezee J, Van Haaren PM, Westendorp H, De Greef M, Kok HP, Wiersma J, et al. Improving locoregional hyperthermia delivery using the 3-D controlled AMC-8 phased array hyperthermia system: a preclinical study. Int J Hyperthermia. 2009;25(7):581–592. doi: 10.3109/02656730903213374. [DOI] [PubMed] [Google Scholar]
- 18.Juang T, Stauffer PR, Craciunescu OA, Maccarini PF, Yuan Y, Das SK, et al. Thermal dosimetry characteristics of deep regional heating of non-muscle invasive bladder cancer. Int J Hyperthermia. 2014;30(3):176–183. doi: 10.3109/02656736.2014.898338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fatehi D, van der Zee J, Notenboom A, van Rhoon GC. Comparison of intratumor and intraluminal temperatures during locoregional deep hyperthermia of pelvic tumors. Strahlenther Onkol. 2007;183(9):479–486. doi: 10.1007/s00066-007-1768-0. [DOI] [PubMed] [Google Scholar]
- 20.Craciunescu OI, Stauffer PR, Soher BJ, Wyatt CR, Arabe O, Maccarini P, et al. Accuracy of real time noninvasive temperature measurements using magnetic resonance thermal imaging in patients treated for high grade extremity soft tissue sarcomas. Med Phys. 2009;36(11):4848–4858. doi: 10.1118/1.3227506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gellermann J, Wlodarczyk W, Ganter H, Nadobny J, Fahling H, Seebass M, et al. A practical approach to thermography in a hyperthermia/magnetic resonance hybrid system: validation in a heterogeneous phantom. International Journal of Radiation Oncology, Biology, Physics. 2005;61(1):267–277. doi: 10.1016/j.ijrobp.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Gellermann J, Wlodarczyk W, Feussner A, Fahling H, Nadobny J, Hildebrandt B, et al. Methods and potentials of magnetic resonance imaging for monitoring radiofrequency hyperthermia in a hybrid system. Int J Hyperther. 2005;21(6):497–513. doi: 10.1080/02656730500070102. [DOI] [PubMed] [Google Scholar]
- 23.Yuan Y, Cheng KS, Craciunescu OI, Stauffer PR, Maccarini PF, Arunachalam K, et al. Utility of treatment planning for thermochemotherapy treatment of nonmuscle invasive bladder carcinoma. Med Phys. 2012;39(3):1170–1181. doi: 10.1118/1.3679839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gellermann J, Wust P, Stalling D, Seebass M, Nadobny J, Beck R, et al. Clinical evaluation and verification of the hyperthermia treatment planning system hyperplan. International Journal of Radiation Oncology, Biology, Physics. 2000;47(4):1145–1156. doi: 10.1016/s0360-3016(00)00425-9. [DOI] [PubMed] [Google Scholar]
- 25.Sreenivasa G, Gellermann J, Rau B, Nadobny J, Schlag P, Deuflhard P, et al. Clinical use of the hyperthermia treatment planning system HyperPlan to predict effectiveness and toxicity. Int J Radiat Oncol Biol Phys. 2003;55(2):407–419. doi: 10.1016/s0360-3016(02)04144-5. [DOI] [PubMed] [Google Scholar]
- 26.van der Wal E, Franckena M, Wielheesen DH, van der Zee J, van Rhoon GC. Steering in locoregional deep hyperthermia: evaluation of common practice with 3D-planning. Int J Hyperthermia. 2008;24(8):682–693. doi: 10.1080/02656730802256359. [DOI] [PubMed] [Google Scholar]
- 27.Schooneveldt G, Bakker A, Balidemaj E, Chopra R, Crezee J, Geijsen E, et al. Thermal dosimetry for bladder hyperthermia. An overview. Int J Hyperthermia. doi: 10.3109/02656736.2016.1156170. Submitted. [DOI] [PubMed] [Google Scholar]
- 28.Cordeiro ER, Geijsen DE, Zum Vorde Sive Vording PJ, Schooneveldt G, Sijbrands J, Hulshof MC, et al. Novel multisensor probe for monitoring bladder temperature during locoregional chemohyperthermia for nonmuscle-invasive bladder cancer: technical feasibility study. J Endourol. 2013;27(12):1504–1509. doi: 10.1089/end.2013.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gellermann J, Hildebrandt B, Issels R, Ganter H, Wlodarczyk W, Budach V, et al. Noninvasive magnetic resonance thermography of soft tissue sarcomas during regional hyperthermia: correlation with response and direct thermometry. Cancer. 2006;107(6):1373–1382. doi: 10.1002/cncr.22114. [DOI] [PubMed] [Google Scholar]
- 30.Stauffer P, Craciunescu O, Maccarini P, Wyatt C, Arunachalam K, Arabe O, et al. Clinical utility of magnetic resonance thermal imaging (MRTI) for realtime guidance of deep hyperthermia. Proceedings of SPIE. 2009;7181:OI-1–OI-12. doi: 10.1117/12.812188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kok HP, de Greef M, Wiersma J, Bel A, Crezee J. The impact of the waveguide aperture size of the 3D 70 MHz AMC-8 locoregional hyperthermia system on tumour coverage. Phys Med Biol. 2010;55(17):4899–4916. doi: 10.1088/0031-9155/55/17/002. [DOI] [PubMed] [Google Scholar]
- 32.Kok HP, de Greef M, Borsboom PP, Bel A, Crezee J. Improved power steering with double and triple ring waveguide systems: the impact of the operating frequency. Int J Hyperthermia. 2011;27(3):224–239. doi: 10.3109/02656736.2011.561270. [DOI] [PubMed] [Google Scholar]
- 33.Brezovich IA, Lilly MB, Durant JR, Richards DB. A practical system for clinical radiofrequency hyperthermia. Int J Radiat Oncol Biol Phys. 1981;7(3):423–430. doi: 10.1016/0360-3016(81)90120-6. [DOI] [PubMed] [Google Scholar]
- 34.Kato H, Hiraoka M, Nakajima T, Ishida T. Deep-heating characteristics of an RF capacitive heating device. Int J Hyperther. 1985;1(1):15–28. doi: 10.3109/02656738509029271. [DOI] [PubMed] [Google Scholar]
- 35.Kikuchi M, Amemiya Y, Egawa S, Onoyama Y, Kato H, Kanai H, et al. Guide to the use of hyperthermic equipment 1. Capacitively-coupled heating. Int J Hyperther. 1993;9(2):187–203. doi: 10.3109/02656739309022534. [DOI] [PubMed] [Google Scholar]
- 36.Hiraoka M, Jo S, Akuta K, Nishimura Y, Takahashi M, Abe M. Radiofrequency capacitive hyperthermia for deep-seated tumors. I. Studies on thermometry. Cancer. 1987;60(1):121–127. doi: 10.1002/1097-0142(19870701)60:1<121::aid-cncr2820600123>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 37.van Rhoon GC, van der Zee J, Broekmeyer-Reurink MP, Visser AG, Reinhold HS. Radiofrequency capacitive heating of deep-seated tumours using pre-cooling of the subcutaneous tissues: results on thermometry in Dutch patients. Int J Hyperther. 1992;8(6):843–854. doi: 10.3109/02656739209005031. [DOI] [PubMed] [Google Scholar]
- 38.Orcutt N, Gandhi OP. Use of the impedance method to calculate 3-D power deposition patterns for hyperthermia with capacitive plate electrodes. Ieee T Bio-Med Eng. 1990;37:36–43. doi: 10.1109/10.43611. [DOI] [PubMed] [Google Scholar]
- 39.Uchibayashi T, Nakajima K, Hisazumi H, Mihara S, Yamamoto H, Koshida K. Studies of temperature rise in bladder cancer and surrounding tissues during radiofrequency hyperthermia. Eur Urol. 1992;21(4):299–303. doi: 10.1159/000474861. [DOI] [PubMed] [Google Scholar]
- 40.Masunaga SI, Hiraoka M, Akuta K, Nishimura Y, Nagata Y, Jo S, et al. Phase I/II trial of preoperative thermoradiotherapy in the treatment of urinary bladder cancer. Int J Hyperthermia. 1994;10(1):31–40. doi: 10.3109/02656739409009329. [DOI] [PubMed] [Google Scholar]
- 41.Uchibayashi T, Yamamoto H, Kunimi K, Koshida K, Nakajima K. Radiofrequency capacitive hyperthermia combined with irradiation or chemotherapy for patients with invasive bladder cancers. Int Urol Nephrol. 1995;27(6):735–741. doi: 10.1007/BF02552139. [DOI] [PubMed] [Google Scholar]
- 42.Hynynen K, Roemer R, Anhalt D, Johnson C, Xu ZX, Swindell W, et al. A scanned, focused, multiple transducer ultrasonic system for localized hyperthermia treatments. Int J Hyperthermia. 2010;26(1):1–11. doi: 10.3109/02656730903492916. [DOI] [PubMed] [Google Scholar]
- 43.Lindsley K, Stauffer PR, Sneed P, Chin R, Phillips TL, Seppi E, et al. Heating patterns of the Helios ultrasound hyperthermia system. Int J Hyperthermia. 1993;9(5):675–684. doi: 10.3109/02656739309032055. [DOI] [PubMed] [Google Scholar]
- 44.Lu XQ, Burdette EC, Bornstein BA, Hansen JL, Svensson GK. Design of an ultrasonic therapy system for breast cancer treatment. Int J Hyperther. 1996;12(3):375–399. doi: 10.3109/02656739609022526. [DOI] [PubMed] [Google Scholar]
- 45.Hynynen K. MRI-guided focused ultrasound treatments. Ultrasonics. 2010;50(2):221–229. doi: 10.1016/j.ultras.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 46.McDannold N, Tempany CM, Fennessy FM, So MJ, Rybicki FJ, Stewart EA, et al. Uterine leiomyomas: MR imaging-based thermometry and thermal dosimetry during focused ultrasound thermal ablation. Radiology. 2006;240(1):263–272. doi: 10.1148/radiol.2401050717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watkin NA, Morris SB, Rivens IH, Woodhouse CR, ter Haar GR. A feasibility study for the non-invasive treatment of superficial bladder tumours with focused ultrasound. Br J Urol. 1996;78(5):715–721. doi: 10.1046/j.1464-410x.1996.02189.x. [DOI] [PubMed] [Google Scholar]
- 48.Stauffer PR, Diederich CJ, Seegenschmiedt MH. Interstitial heating technologies. In: Seegenschmiedt MH, Fessenden P, Vernon CC, editors. Thermoradiotherapy and Thermochemotherapy: Volume 1, Biology, Physiology and Physics. Berlin, New York: Springer-Verlag; 1995. pp. 279–320. [Google Scholar]
- 49.Colombo R, Lev A, Da Pozzo LF, Freschi M, Gallus G, Rigatti P. A new approach using local combined microwave hyperthermia and chemotherapy in superficial transitional bladder carcinoma treatment. The Journal of urology. 1995;153(3 Pt 2):959–963. [PubMed] [Google Scholar]
- 50.Colombo R, Da Pozzo LF, Lev A, Freschi M, Gallus G, Rigatti P. Neoadjuvant combined microwave induced local hyperthermia and topical chemotherapy versus chemotherapy alone for superficial bladder cancer. The Journal of urology. 1996;155(4):1227–1232. [PubMed] [Google Scholar]
- 51.Colombo R, Da Pozzo LF, Lev A, Salonia A, Rigatti P, Leib Z, et al. Local microwave hyperthermia and intravesical chemotherapy as bladder sparing treatment for select multifocal and unresectable superficial bladder tumors. The Journal of urology. 1998;159(3):783–787. [PubMed] [Google Scholar]
- 52.Kiss B, Schneider S, Thalmann GN, Roth B. Is thermochemotherapy with the Synergo system a viable treatment option in patients with recurrent non-muscle-invasive bladder cancer? Int J Urol. 2015;22(2):158–162. doi: 10.1111/iju.12639. [DOI] [PubMed] [Google Scholar]
- 53.Maffezzini M, Campodonico F, Canepa G, Manuputty EE, Tamagno S, Puntoni M. Intravesical mitomycin C combined with local microwave hyperthermia in non-muscle-invasive bladder cancer with increased European Organization for Research and Treatment of Cancer (EORTC) score risk of recurrence and progression. Cancer Chemother Pharmacol. 2014;73(5):925–930. doi: 10.1007/s00280-014-2423-y. [DOI] [PubMed] [Google Scholar]
- 54.van der Heijden AG, Kiemeney LA, Gofrit ON, Nativ O, Sidi A, Leib Z, et al. Preliminary European results of local microwave hyperthermia and chemotherapy treatment in intermediate or high risk superficial transitional cell carcinoma of the bladder. Eur Urol. 2004;46(1):65–71. doi: 10.1016/j.eururo.2004.01.019. discussion-2. [DOI] [PubMed] [Google Scholar]
- 55.Nativ O, Witjes JA, Hendricksen K, Cohen M, Kedar D, Sidi A, et al. Combined thermo-chemotherapy for recurrent bladder cancer after bacillus Calmette-Guerin. The Journal of urology. 2009;182(4):1313–1317. doi: 10.1016/j.juro.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 56.Arunachalam K, Maccarini PF, Craciunescu OI, Schlorff JL, Stauffer PR. Thermal characteristics of thermobrachytherapy surface applicators for treating chest wall recurrence. Phys Med Biol. 2010;55(7):1949–1969. doi: 10.1088/0031-9155/55/7/011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee ER, Kapp DS, Lohrbach AW, Sokol JL. Influence of water bolus temperature on measured skin surface and intradermal temperatures. Int J Hyperther. 1994;10(1):59–72. doi: 10.3109/02656739409009332. [DOI] [PubMed] [Google Scholar]
- 58.Oliveira TR, Stauffer PR, Lee CT, Landon CD, Etienne W, Ashcraft KA, et al. Magnetic fluid hyperthermia for bladder cancer: a preclinical dosimetry study. Int J Hyperthermia. 2013;29(8):835–844. doi: 10.3109/02656736.2013.834384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sousa A, Inman BA, Pineiro I, Monserrat V, Perez A, Aparici V, et al. A clinical trial of neoadjuvant hyperthermic intravesical chemotherapy (HIVEC) for treating intermediate and high-risk non-muscle invasive bladder cancer. Int J Hyperthermia. 2014;30(3):166–170. doi: 10.3109/02656736.2014.900194. [DOI] [PubMed] [Google Scholar]
- 60.Ekin RG, Akarken I, Cakmak O, Tarhan H, Celik O, Ilbey YO, et al. Results of intravesical chemo-hyperthermia in high-risk non-muscle invasive bladder cancer. Asian Pac J Cancer Prev. 2015;16(8):3241–3245. doi: 10.7314/apjcp.2015.16.8.3241. [DOI] [PubMed] [Google Scholar]
- 61.Soria F, Milla P, Fiorito C, Pisano F, Sogni F, Di Marco M, et al. Efficacy and safety of a new device for intravesical thermochemotherapy in non-grade 3 BCG recurrent NMIBC: a phase I-II study. World J Urol. 2015 doi: 10.1007/s00345-015-1595-3. [DOI] [PubMed] [Google Scholar]
- 62.Watanabe H, Azuma Y. Periodical measurement of urine volume in the bladder during sleep: Temporary volume reduction suggestive of absorption. Int J Urol. 2015 doi: 10.1111/iju.12999. [DOI] [PubMed] [Google Scholar]
- 63.Salahi S, Maccarini PF, Rodrigues DB, Etienne W, Landon CD, Inman BA, et al. Miniature microwave applicator for murine bladder hyperthermia studies. Int J Hyperthermia. 2012;28(5):456–465. doi: 10.3109/02656736.2012.677931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stauffer PR, Swift PS, Sneed PK, Char DH, Phillips TL. Temperature Controlled Microwave Ring Radiator for Hyperthermia Therapy. IEEE Engr in Med Biol Symp Digest. 1988:1273–1274. [Google Scholar]
- 65.Swift PS, Stauffer PR, Fries PD, Kaleta-Michaels S, Murray T, Sneed PK, et al. Microwave hyperthermia for choroidal melanoma in rabbits. Invest Ophthalmol Vis Sci. 1990;31(9):1754–1760. [PubMed] [Google Scholar]
- 66.Snow BW, Arunachalam K, De Luca V, Maccarini PF, Klemetsen O, Birkelund Y, et al. Non-invasive vesicoureteral reflux detection: Heating risk studies for a new device. Journal of Pediatric Urology. 2011;7(6):624–630. doi: 10.1016/j.jpurol.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stauffer PR, Maccarini PF, Arunachalam K, De Luca V, Salahi S, Boico A, et al. Microwave radiometry for non-invasive detection of vesicoureteral reflux (VUR) following bladder warming. Proceedings of SPIE. 2011;7901 doi: 10.1117/12.875636. 7901OV-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sperling RA, Rivera Gil P, Zhang F, Zanella M, Parak WJ. Biological applications of gold nanoparticles. Chem Soc Rev. 2008;37(9):1896–1908. doi: 10.1039/b712170a. [DOI] [PubMed] [Google Scholar]
- 69.Cho SK, Emoto K, Su LJ, Yang X, Flaig TW, Park W. Functionalized gold nanorods for thermal ablation treatment of bladder cancer. J Biomed Nanotechnol. 2014;10(7):1267–1276. doi: 10.1166/jbn.2014.1838. [DOI] [PubMed] [Google Scholar]
- 70.Bruggmoser G, Bauchowitz S, Canters R, Crezee H, Ehmann M, Gellermann J, et al. Quality assurance for clinical studies in regional deep hyperthermia qualitatssicherung in der regionalen tiefenhyperthermie. Strahlenther Onkol. 2011;187(10):605–610. doi: 10.1007/s00066-011-1145-x. [DOI] [PubMed] [Google Scholar]
- 71.Bruggmoser G, Bauchowitz S, Canters R, Crezee H, Ehmann M, Gellermann J, et al. Guideline for the clinical application, documentation and analysis of clinical studies for regional deep hyperthermia: quality management in regional deep hyperthermia. Strahlenther Onkol. 2012;188(Suppl 2):198–211. doi: 10.1007/s00066-012-0176-2. [DOI] [PubMed] [Google Scholar]
- 72.Bruggmoser G. Some aspects of quality management in deep regional hyperthermia. Int J Hyperthermia. 2012;28(6):562–569. doi: 10.3109/02656736.2012.714049. [DOI] [PubMed] [Google Scholar]
- 73.Sapozink MD, Corry PM, Kapp DS, Myerson RJ, Dewhirst MW, Emami B, et al. RTOG quality assurance guidelines for clinical trials using hyperthermia for deep-seated malignancy. Int J Radiat Oncol. 1991;20(5):1109–1115. doi: 10.1016/0360-3016(91)90212-m. [DOI] [PubMed] [Google Scholar]