Significance
Experiences are remembered long-term when these memories are formed in a state of arousal and heightened emotion. The arousal-induced release of noradrenaline is critical for modulating consolidation, the process that establishes long-term memory. Although the effects of pharmacological manipulation of adrenergic signaling on memory stability are already being investigated in the clinical setting, how adrenergic receptors mediate long-term memory consolidation remains unclear. This study reports a previously unidentified mechanism with important translational implications: The noradrenergic receptors that in the hippocampus mediate memory consolidation are β2-adrenergic receptors (β2ARs) expressed in astrocytes. These receptors are necessary for the learning-evoked release of lactate from astrocytes, which then is required to support the neuronal molecular changes essential for long-term memory formation.
Keywords: astrocyte, memory, hippocampus, β-adrenergic receptor, lactate
Abstract
Emotionally relevant experiences form strong and long-lasting memories by critically engaging the stress hormone/neurotransmitter noradrenaline, which mediates and modulates the consolidation of these memories. Noradrenaline acts through adrenergic receptors (ARs), of which β2-adrenergic receptors (βARs) are of particular importance. The differential anatomical and cellular distribution of βAR subtypes in the brain suggests that they play distinct roles in memory processing, although much about their specific contributions and mechanisms of action remains to be understood. Here we show that astrocytic rather than neuronal β2ARs in the hippocampus play a key role in the consolidation of a fear-based contextual memory. These hippocampal β2ARs, but not β1ARs, are coupled to the training-dependent release of lactate from astrocytes, which is necessary for long-term memory formation and for underlying molecular changes. This key metabolic role of astrocytic β2ARs may represent a novel target mechanism for stress-related psychopathologies and neurodegeneration.
Emotional arousal and the stress hormone noradrenaline (NA) mediate and modulate memory consolidation, the process required to stabilize long-term memory (1). NA contributes to this effect through the activation of α- and β-adrenergic receptors (βARs) in brain regions such as the amygdala and the hippocampus, which are critical for encoding and consolidating memories (2). Of particular interest is the action of NA through βARs because blockers of these receptors, such as propranolol, have been reported to interfere with memory consolidation and strengthening (3–5) and have been suggested as potential therapeutics to treat anxiety disorders, including panic disorder (6) and posttraumatic stress disorder (7). All three subtypes of βARs—β1, β2, and β3—are present in the central nervous system with distinct distributions (8): In the rat hippocampus, a region required for the consolidation of explicit/episodic memories, β1ARs predominate, and their pattern of distribution is different from that of β2ARs and β3ARs (9–11), suggesting possible distinct roles for these receptor subtypes. Pharmacological investigations using agonists (e.g., NA itself) and antagonists (e.g., propranolol) that bind to both β1ARs and β2ARs have suggested βARs have a similar role in memory encoding, modulation, and retrieval in humans and rodents (3, 5, 12). Studies on animal models lacking β1ARs or treated with selective β1AR agonists or antagonists revealed the critical role of this adrenergic receptor subtype in both synaptic plasticity and memory formation, with particular emphasis on retrieval (12–14). Genetic deletion of β2ARs results in impaired memory modulation by stress or corticosterone and impaired hippocampal plasticity, in agreement with a pharmacologically established role of β2ARs in amygdala-dependent memory modulation and its effect on hippocampal function and in prefrontal cortex (2, 15, 16). However, little is known about the role of hippocampal β2ARs in hippocampus-dependent long-term memory formation and processing.
In general, the functional contributions of βARs to memory processes have been thought to result mainly from their effect on neurons and therefore have been studied largely in neuronal/synaptic models (reviewed in ref. 17). However, in addition to being expressed in pre- and postsynaptic compartments of neurons, βARs are also found in other cell types, particularly in astrocytes (18–21). More specifically, it has been suggested that β2ARs in the nervous system are expressed predominantly in glia (18, 21–23), whereas β1ARs are found primarily in neurons, at synaptic junctions (18, 22, 23). This suggestion raises the question of which βAR subtype (β1AR or β2AR) and which βAR-expressing cells mediate hippocampal memory consolidation.
NA activates glycogenolysis and subsequent lactate release (24), a process known to occur mainly in astrocytes and not in neurons; in fact, glycogen is stored almost exclusively in astrocytes (25, 26). Glycogenolysis and its activation by NA are critical for memory consolidation in chicks (27). Furthermore, in the rat hippocampus, glycogenolysis, which results in lactate increase and astrocyte–neuron lactate transport, mediates memory consolidation (28, 29), in vivo hippocampal long-term potentiation, and the induction of learning-dependent molecular changes, including activity-regulated cytoskeletal protein (Arc) and phosphorylation of cAMP response element-binding protein at serine 133 (pCREB) and cofilin (p-cofilin) (29). Using the hippocampus-dependent task inhibitory avoidance (IA), here we asked which astrocytic and/or neuronal β1ARs and/or β2ARs in the hippocampus mediate memory consolidation.
Results
βARs Mediate Memory Consolidation via Astrocytic Lactate.
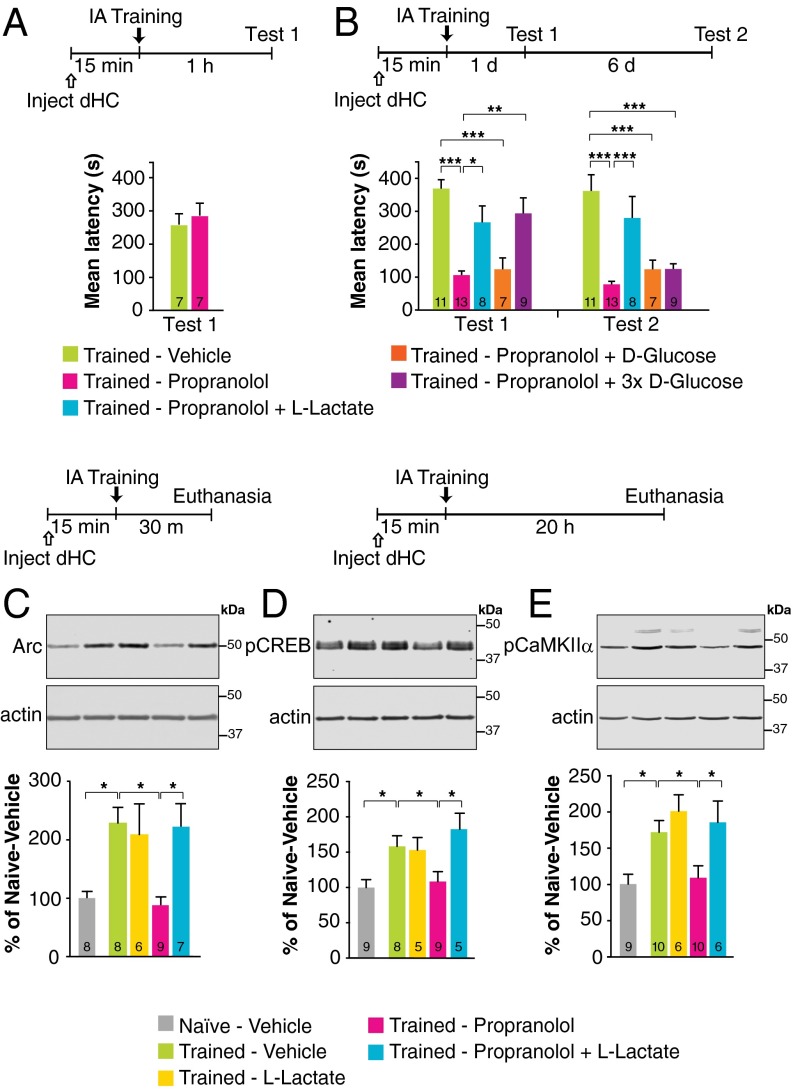
A bilateral injection of the β1+β2AR antagonist propranolol into the rat dorsal hippocampus (dHC) 15 min before training had no effect on short-term memory tested 1 h after training (Fig. 1A) but significantly impaired long-term memory tested 1 d and 7 d after training (Fig. 1B), suggesting that hippocampal β1ARs and/or β2ARs mediate IA memory consolidation. Given that NA promotes glycogenolysis and lactate release from astrocytes (30), which are necessary for memory consolidation (29), we tested whether the effect of propranolol on memory consolidation is linked to astrocytic lactate release. We found that coadministration of l-lactate with propranolol fully and persistently rescued memory impairment. In contrast, an equicaloric concentration of d-glucose had no effect. A threefold higher concentration of glucose rescued memory impairment at 1 d but not at 7 d after training (P < 0.0001; two-way repeated-measures ANOVA followed by Bonferroni’s post hoc tests) (Fig. 1B). Together, these results indicate that the critical role of β1ARs and/or β2ARs in memory consolidation is likely dependent on lactate and hence on astrocytic mechanisms.
Fig. 1.
l-lactate rescues the impairment of long-term IA memory and underlying molecular mechanisms produced by propranolol. Memory retention is expressed as mean latency ± SEM (in seconds). (A) Short-term memory tested 1 h after training following a bilateral dHC injection of propranolol given 15 min before training (n = 7). (B) Long-term memory tested 1 d after training (test 1) and 6 d later (test 2) following a bilateral dHC injection of vehicle or propranolol, in the presence or absence of l-lactate, or of an equicaloric concentration of d-glucose, or of a three times higher concentration (3×) of d-glucose, given 15 min before training (n = 7–13). (C–E) Densitometric Western blot analysis and representative images for Arc (C), pCREB (D), and pCaMKIIα (E) in dHC extracts from untrained (naive) rats injected with vehicle and rats injected 15 min before training with vehicle, l-lactate, propranolol, or propranolol + l-lactate and killed 30 min (for Arc) or 20 h (for pCREB and pCaMKIIα) after training. Data are expressed as mean protein percentage ± SEM of mean values (100%) in vehicle-injected naive rats. Each sample protein densitometric value was normalized to that of relative actin stained on the same blot (n = 5–10). *P < 0.05; **P < 0.01; ***P < 0.001. Numeric values and detailed statistical analyses are reported in Tables S1 and S2.
Table S1.
Mean latencies and statistical analyses related to Fig. 1
| Treatment | n | Mean latency (s) ± SEM | Statistics | |
| Test 1 | Test 2 | |||
| Fig. 1A | ||||
| Vehicle | 7 | 250.9 ± 32.3 | – | t12 = 0.62, P > 0.05 |
| Propranolol | 7 | 284.4 ± 43.0 | – | |
| Fig. 1B | ||||
| Vehicle | 11 | 363.6 ± 26.7 | 356.5 ± 48.8 | Treatment: F4,43 = 12.91, P < 0.0001 |
| Propranolol | 13 | 103.0 ± 12.7 | 75.3 ± 9.0 | Time: F1,43 = 10.37, P = 0.002 |
| Propranolol + l-lactate | 8 | 262.0 ± 49.3 | 275.3 ± 64.3 | Interaction: F4,43 = 7.70, P < 0.0001 |
| Propranolol + d-glucose | 7 | 120.5 ± 34.3 | 120.5 ± 27.5 | |
| Propanolol + 3× d-glucose | 9 | 288.8 ± 46.9 | 121.3 ± 15.8 | |
Table S2.
Western blot relative densitometry and statistical analyses related to Fig. 1
| Treatment | n | % of naive ± SEM | Statistics |
| Fig. 1C: Arc | |||
| Naive–vehicle | 8 | 100 ± 11.3 | F4,33 = 5.34, P = 0.002 |
| Trained–vehicle | 8 | 227.6 ± 27.3 | |
| Trained–l-lactate | 6 | 207.7 ± 53.4 | |
| Trained–propranolol | 9 | 87.3 ± 14.3 | |
| Trained–propranolol + l-lactate | 7 | 221.5 ± 39.7 | |
| Fig. 1D: pCREB | |||
| Naive–vehicle | 9 | 100 ± 11.1 | F4,31 = 6.15, P = 0.001 |
| Trained–vehicle | 8 | 158.2 ± 15.0 | |
| Trained–l-lactate | 5 | 153.1 ± 17.8 | |
| Trained-propranolol | 9 | 108.4 ± 14.1 | |
| Trained–propranolol + l-lactate | 5 | 182.7 ± 22.5 | |
| Fig. 1E: pCaMKIIα | |||
| Naive–vehicle | 9 | 100 ± 13.6 | F4,36 = 4.91, P = 0.003 |
| Trained–vehicle | 10 | 171.7 ± 16.0 | |
| Trained–l-lactate | 6 | 200.5 ± 22.6 | |
| Trained–propranolol | 10 | 108.8 ± 16.5 | |
| Trained–propranolol + l-lactate | 6 | 185.1 ± 29.4 | |
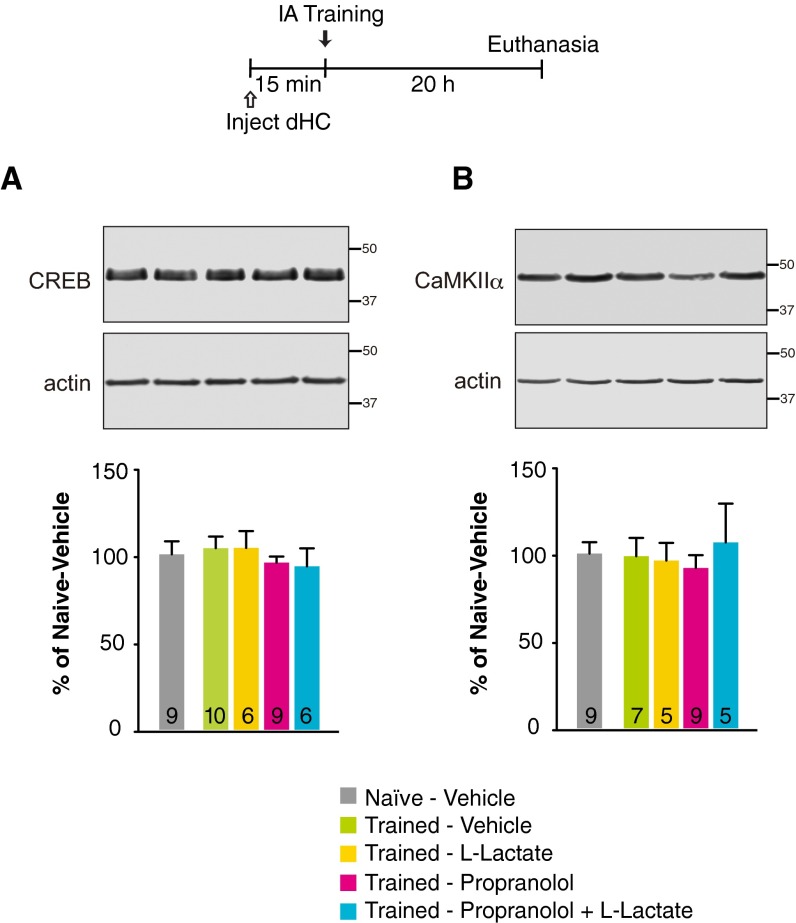
Propranolol injected bilaterally into the dHC 15 min before training also blocked the learning-dependent increase of molecular changes known to underlie synaptic plasticity and memory formation; these included the immediate early gene Arc 30 min after training (Fig. 1C), and phosphorylation of CREB (Fig. 1D) and of calcium calmodulin kinase II α (CaMKIIα) (Fig. 1E) 20 h after training, time points at which these molecular changes have been previously established as learning dependent (29, 31). Lactate coadministration rescued all these molecular impairments (P < 0.01; one-way ANOVA followed by Newman–Keuls post hoc test). The levels of total CREB and CaMKIIα remained unchanged (P > 0.05) (Fig. S1 and Table S3). Hence, βARs in the hippocampus engage astrocytic mechanisms to support memory formation.
Fig. S1.
No change in CREB and CaMKIIα concentration was seen with training in the presence or absence of propranolol and/or lactate treatments. (A and B) Densitometric analyses of Western blots and representative images for CREB (A) and CaMKIIα (B) obtained with dHC extracts from untrained (naive) rats injected with vehicle and rats injected 15 min before training with vehicle, l-lactate, propranolol, or propranolol + l-lactate and killed 20 h after training. Data are expressed as the percentage (mean ± SEM) of mean values (100%) in vehicle-injected naive rats (n = 5–10 per group). Numeric values and detailed statistical analyses are reported in Table S3.
Table S3.
Western blot relative densitometry and statistical analyses related to Fig. S1
| Treatment | n | % of naive ± SEM | Statistics |
| Fig. S1A | |||
| Naive–vehicle | 9 | 100 ± 7.4 | F4,35 = 0.40, P > 0.05 |
| Trained–vehicle | 10 | 103.6 ± 6.6 | |
| Trained–l-lactate | 6 | 103.7 ± 9.6 | |
| Trained–propranolol | 9 | 95.3 ± 3.5 | |
| Trained–propranolol + l-lactate | 6 | 93.2 ± 10.3 | |
| Fig. S1B | |||
| Naive–vehicle | 9 | 100 ± 6.6 | F4,30 = 0.24, P > 0.05 |
| Trained–vehicle | 7 | 98.6 ± 10.5 | |
| Trained–l-lactate | 5 | 96.1 ± 10.1 | |
| Trained–propranolol | 9 | 91.9 ± 7.3 | |
| Trained–propranolol + l-lactate | 5 | 106.5 ± 22.1 | |
β2ARs, but Not β1ARs, Mediate the Learning-Dependent Lactate Increase and Long-Term Memory Formation.
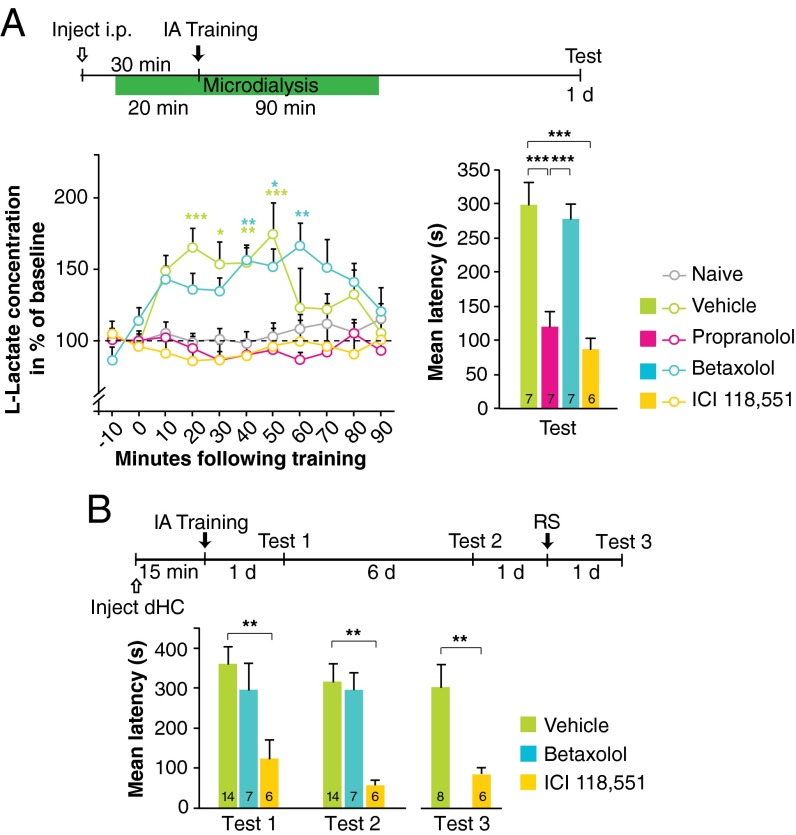
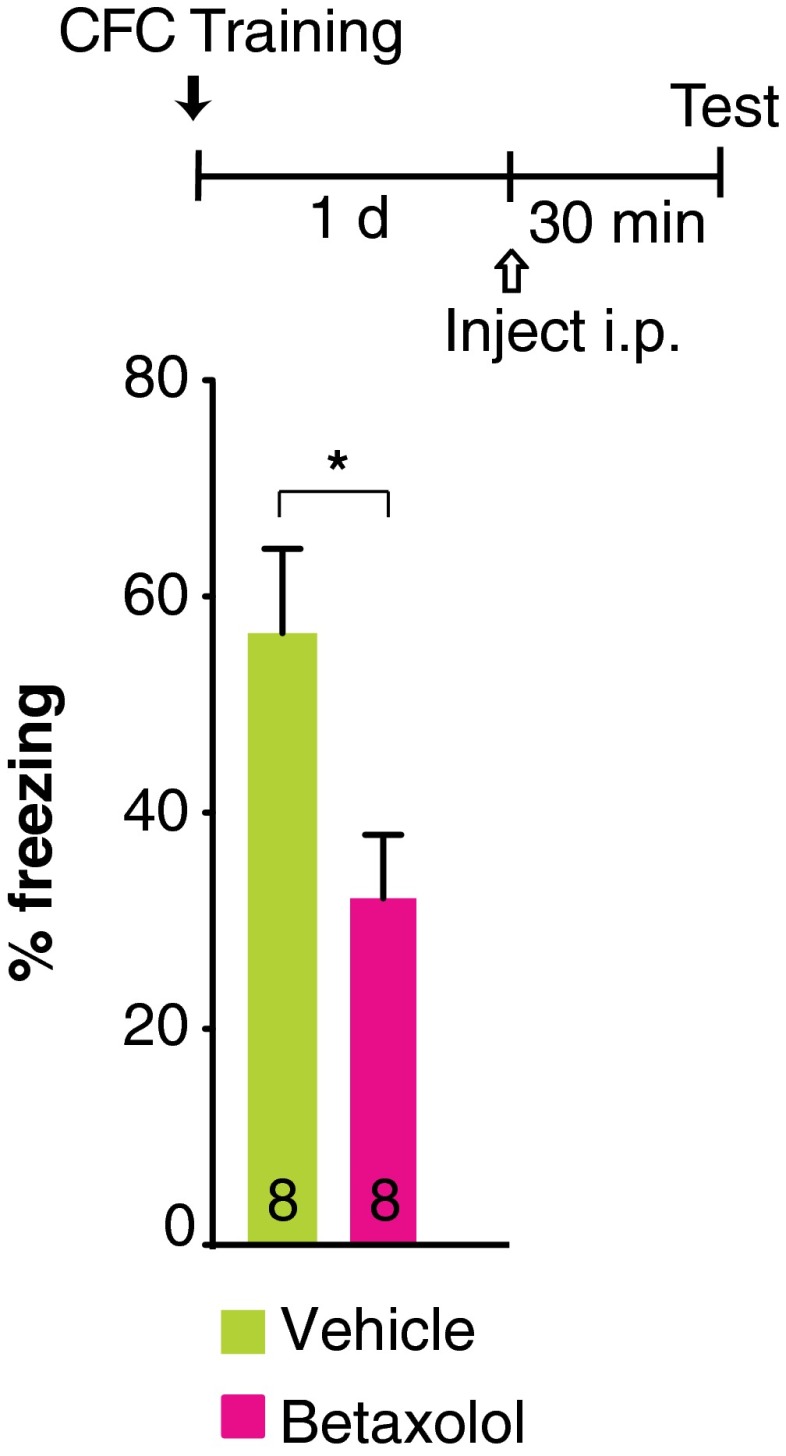
Next we sought to dissociate the roles of the βAR subtypes, β1AR and β2AR, in regulating training-induced astrocytic lactate release and memory consolidation. In agreement with our previous finding (29), we found that training leads to a significant increase in lactate levels in the dHC as measured by microdialysis; this increase lasted for more than 60 min and returned to baseline by ∼90 min. However, this lactate increase was completely abolished by a systemic (i.p.) injection of propranolol or of the β2AR-selective antagonist ICI 118,551, but not the β1AR-selective antagonist betaxolol, given 30 min before training (P < 0.0001; two-way ANOVA followed by Bonferroni post hoc tests) (Fig. 2A). Compared with vehicle-injected control rats, propranolol- and ICI 118,551-injected rats, but not betaxolol-injected rats, had significantly impaired long-term memory when tested 1 d after training (P < 0.0001; one-way ANOVA followed by Bonferroni post hoc tests) (Fig. 2A). The efficacy of betaxolol was confirmed in parallel by reproducing the previously established effect of this compound on retrieval of contextual fear conditioning (CFC) (Fig. S2 and Table S4) (12). In addition, as with systemic injections, bilateral injection of ICI 118,551 into the dHC 15 min before training impaired long-term memory when tested 1 d and 7 d after training, whereas injection of betaxolol had no effect (P < 0.001; two-way repeated-measures ANOVA followed by Bonferroni post hoc tests) (Fig. 2B). Following the 7-d test, rats received a reminder footshock (RS)—the unconditioned stimulus in a different context—and testing 1 d later showed that the memory deficit persisted, suggesting that the amnesia was likely caused by impaired consolidation rather than by inhibition of memory expression (P < 0.01; Student’s t test) (Fig. 2B). Hence, both the IA-induced increase in hippocampal lactate and long-term memory consolidation require β2ARs but not β1ARs. We therefore focused our subsequent investigations on β2ARs.
Fig. 2.
Propranolol and ICI 118,551, but not betaxolol, block both training-evoked l-lactate increase in the dHC and long-term IA memory formation. (A, Left) The relative concentration of l-lactate in the dHC was measured by microdialysis in freely moving rats injected i.p. with vehicle, propranolol, betaxolol, or ICI 118,551 30 min before training. Untrained (naive) rats injected with vehicle were used as controls. Baseline data were collected for 20 min before training, and data collection continued for 90 min after training. Data are expressed as the percent of baseline l-lactate concentration ± SEM (mean of the first two samples set at 100%; n = 4–7). (Right) Long-term memory retention of the rats tested in microdialysis expressed as mean latency (in seconds) ± SEM (n = 6–7). (B) Long-term memory tested 1 d (test 1) and 7 d (test 2) after training and following an RS (test 3) in rats that received a bilateral dHC injection of vehicle, ICI 118,551, or betaxolol 15 min before training. Data are expressed as mean latency (in seconds) ± SEM (n = 6–14). **P < 0.01; ***P < 0.001. Numeric values and detailed statistical analyses are reported in Tables S5, S6, and S7.
Fig. S2.
Betaxolol impairs CFC retrieval. Memory retention is expressed as percentage of freezing (± SEM). Betaxolol was injected i.p. 30 min before CFC retrieval that occurred 1 d after training (n = 8 per group). *P < 0.05. Numeric values and detailed statistical analyses are reported in Table S4.
Table S4.
Percent freezing and statistical analyses related to Fig. S2
| Treatment | n | % freezing ± SEM |
| Vehicle | 8 | 57.7 ± 7.7 |
| Betaxolol | 8 | 32.1 ± 5.9 |
Statistics: t14 = 2.53, P = 0.024.
Table S5.
Lactate concentration and statistical analyses related to Fig. 2
| Time, min | Naive | Vehicle | Propranolol | Betaxolol | ICI 118,551 | ||||||||||
| Mean | SEM | n | Mean | SEM | n | Mean | SEM | n | Mean | SEM | n | Mean | SEM | n | |
| −10 | 100.9 | 7.5 | 7 | 104.0 | 3.9 | 7 | 100.3 | 3.2 | 7 | 86.2 | 9.1 | 7 | 104.4 | 9.0 | 6 |
| 0 | 99.1 | 7.5 | 7 | 96.0 | 3.9 | 7 | 99.7 | 3.2 | 7 | 113.8 | 9.1 | 7 | 95.6 | 9.0 | 6 |
| 10 | 104.9 | 8.0 | 7 | 148.6 | 11.0 | 7 | 102.0 | 5.0 | 7 | 142.8 | 9.2 | 7 | 91.1 | 9.3 | 6 |
| 20 | 98.7 | 6.2 | 7 | 165.3 | 13.3 | 7 | 94.4 | 8.4 | 7 | 135.9 | 11.1 | 7 | 85.7 | 12.0 | 6 |
| 30 | 100.8 | 7.4 | 7 | 153.2 | 15.8 | 7 | 86.3 | 5.8 | 7 | 134.4 | 9.4 | 7 | 86.4 | 11.2 | 6 |
| 40 | 98.0 | 8.1 | 7 | 154.3 | 10.7 | 7 | 89.8 | 5.2 | 7 | 156.1 | 10.7 | 7 | 89.1 | 10.0 | 6 |
| 50 | 103.1 | 9.1 | 7 | 174.3 | 23.9 | 6 | 93.2 | 9.0 | 7 | 151.8 | 12.3 | 7 | 96.0 | 12.4 | 6 |
| 60 | 108.3 | 9.9 | 7 | 122.8 | 27.7 | 7 | 86.0 | 5.5 | 7 | 166.2 | 16.1 | 7 | 99.6 | 14.4 | 6 |
| 70 | 111.8 | 14.1 | 7 | 121.7 | 10.9 | 7 | 91.2 | 8.0 | 7 | 150.9 | 19.9 | 7 | 96.1 | 15.0 | 6 |
| 80 | 105.3 | 6.9 | 7 | 131.9 | 17.4 | 4 | 104.7 | 9.9 | 4 | 141.3 | 12.7 | 7 | 90.5 | 12.7 | 5 |
| 90 | 115.0 | 10.7 | 7 | 104.9 | 15.5 | 4 | 92.5 | 9.8 | 4 | 120.4 | 16.6 | 7 | 100.6 | 12.6 | 5 |
Statistics: Treatment: F4,304 = 35.88, P < 0.0001. Time: F10,304 = 2.08, P = 0.03. Interaction: F40,304 = 1.92, P = 0.001.
Table S6.
Mean latencies and statistical analyses related to Fig. 2A
| Treatment | n | Mean latency(s) ± SEM |
| Vehicle | 7 | 297.1 ± 32.8 |
| Propranolol | 7 | 119.3 ± 21.9 |
| Betaxolol | 7 | 276.5 ± 21.6 |
| ICI 118,551 | 6 | 86.2 ± 16.1 |
Statistics: F3,23 = 19.97, P < 0.0001.
Table S7.
Mean latencies and statistical analyses related to Fig. 2B
| Treatment | n | Mean latency(s) ± SEM | Statistics | n | Mean latency(s) ± SEM | Statistics | |
| Test 1 | Test 2 | Test 3 | |||||
| Vehicle | 14 | 358.4 ± 45.4 | 313.9 ± 45.8 | Treatment: F2,24 = 6.97, P = 0.0002 | 8 | 300.2 ± 57.9 | t12 = 3.18, P = 0.008 |
| Betaxolol | 7 | 292.6 ± 67.7 | 291.6 ± 43.7 | Time: F1,24 = 2.01, P > 0.05 | – | – | |
| ICI 118,551 | 6 | 121.9 ± 49.6 | 54.6 ± 10.0 | Interaction: F2,24 = 0.46, P > 0.05 | 6 | 81.1 ± 16.1 | |
Astrocytic, Not Neuronal, Hippocampal β2ARs Mediate Long-Term Memory Formation.
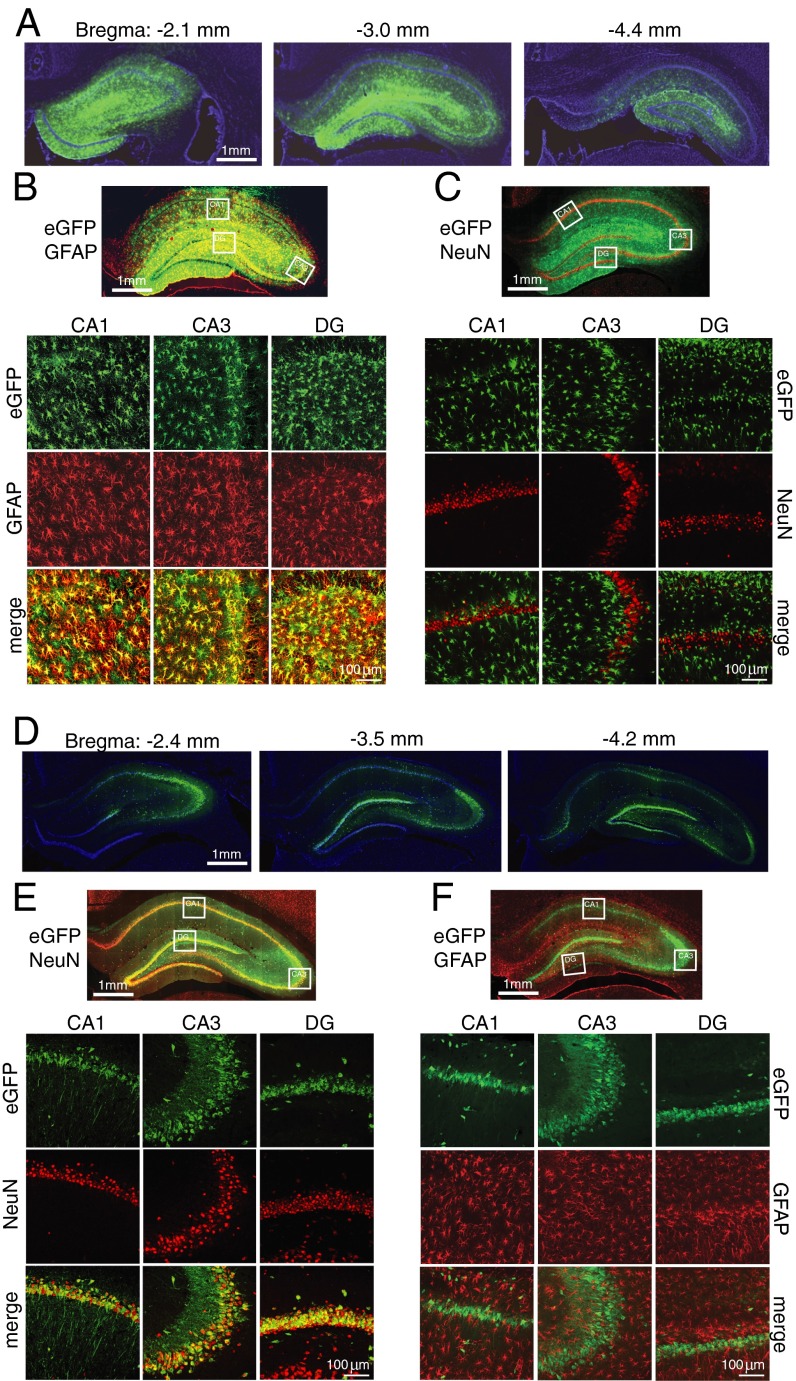
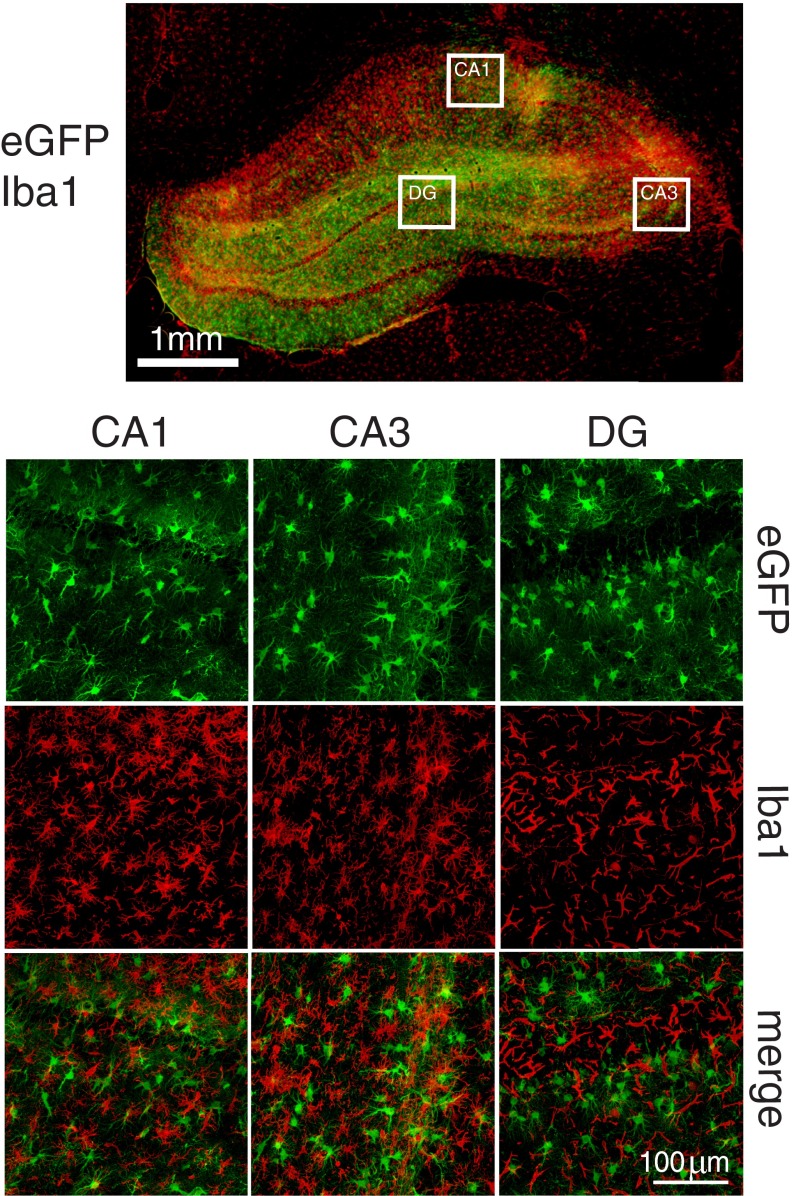
The expression and distribution of β2ARs on different cell types is not clear; although some studies suggest that β2ARs are expressed mainly in glia (18, 21–23), others report that they are found on both astrocytes and neurons (20). To determine the selective functional contribution of astrocytic and/or neuronal β2AR in long-term memory formation, we used cell-specific virus-mediated knockdown of the receptors. The astrocyte-specific promoter glial fibrillary acidic protein (short gfaABC1D) was packaged into adeno-associated virus 9 (AAV9), and the neuron-specific human synapsin (hSyn) promoter was packaged in AAVDJ; both were tagged with eGFP and injected into the dHC. Two weeks after injection, histochemical staining revealed that the expressed fluorescence of eGFP was distributed throughout the dHC (Fig. 3A). The AAV9-short gfaABC1D-eGFP expression was selectively astrocytic, as revealed by its colocalization with the endogenous astrocytic marker GFAP but not with the neuronal marker NeuN (Fig. 3 B and C) or with the microglial marker ionized calcium-binding adaptor molecule 1 (Iba1) (Fig. S3). Conversely, AAVDJ-hSyn-eGFP expression was found selectively in neurons and not astrocytes (Fig. 3 D–F).
Fig. 3.
AAV under the short gfaABC1D or the hSyn promoter selectively targets astrocytes or neurons, respectively. dHC infection of AAV9-short gfaABC1D-eGFP (A–C) or AAVDJ-hSyn-eGFP (D–F) 2 wk after bilateral injection (A, D). Shown are representative images of rostral-to-caudal brain sections [bregma −2.4 to −4.4 mm (45)]. All images of whole dHC are composite tile scans. In AAV9-short gfaABC1D-eGFP–infected cells, eGFP expression colocalizes with immunostaining of GFAP (B) but not NeuN (C) in subregions CA1 and CA3 and the dentate gyrus (DG). In AAVDJ-hSyn-eGFP–infected cells, eGFP expression colocalizes with NeuN (E) but not GFAP (F) in subregions CA1 and CA3 and the dentate gyrus.
Fig. S3.
AAV under short gfaABC1D does not infect microglia. dHC infection of AAV9-short gfaABC1D-eGFP 2 wk after bilateral injection. In cells infected with AAV9-short gfaABC1D-eGFP, eGFP expression does not colocalize with immunostaining of Iba1 in subregions CA1, CA3, and dentate gyrus (DG).
To knock down β2ARs, we used two methods: shRNA miR (Sh) or antisense (AS) sequences against β2ARs. shRNA were designed by Vector Biolabs (SI Materials and Methods). The Sh and AS sequences were engineered into the astrocyte-specific virus, resulting in AAV9-short gfaABC1D-eGFP-β2AR-Sh and AAV9-short gfaABC1D-β2AR-AS (henceforth referred to as “gfa-Sh” and “gfa-AS,” respectively) or into the neuron-specific virus, resulting in AAVDJ-hSyn-eGFP-β2AR-Sh and AAVDJ-hSyn-β2AR-AS (henceforth referred to as “hSyn-Sh” and “hSyn-AS,” respectively). Relative scrambled (Scr) or sense (S) sequences were used as controls.
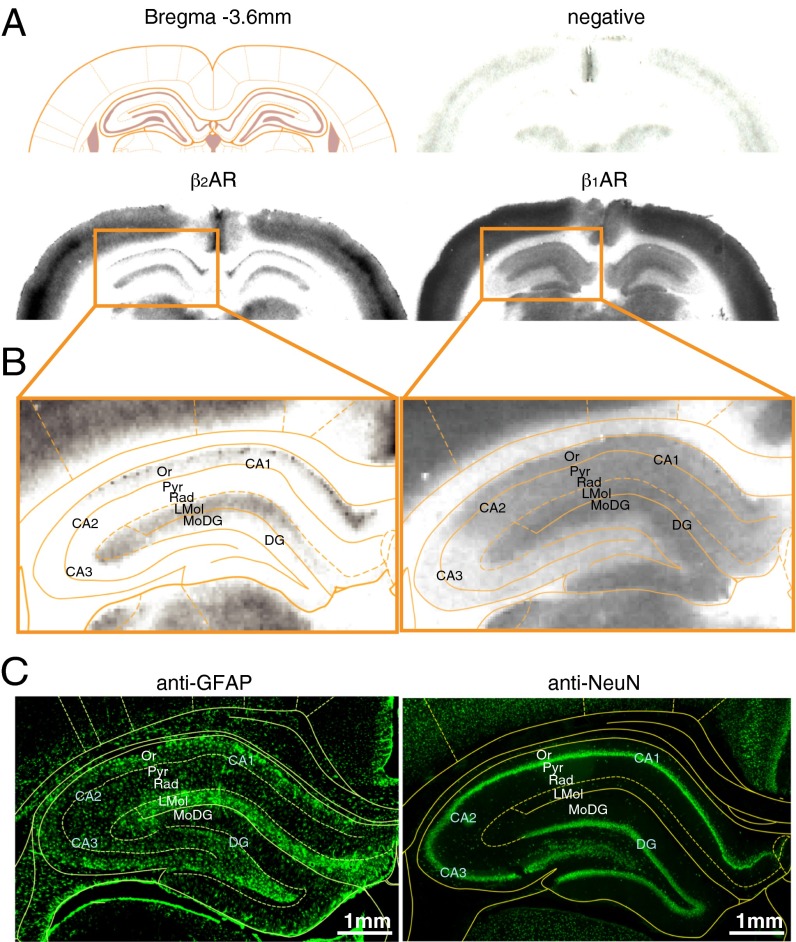
First, we measured the ability of the Sh and AS viruses to affect the selective expression of the β2AR protein. Using β1AR/β2AR-knockout mouse brains (32) we used Western blot analyses and/or immunohistochemistry to conduct specificity testing of several available antibodies indicated to bind β2ARs specifically, but we failed to identify any specific reactivity (Table S8). Thus, to quantify β2AR and β1AR protein levels, we used receptor autoradiography binding assays. As previously established (10), this method is based on the quantification of iodo-(-)-cyanopindolol ([125I] CYP) binding in the presence of serotonergic inhibitors (SB 224289 and WAY 100,635) and of the β1AR inhibitor CGP-20712A to assess the expression of β2AR or in the presence of the β2AR inhibitor ICI 118,551 to assess the expression of β1AR. Using this method, we quantified the distribution of β1AR and β2AR binding and confirmed the previously described differential distributions of these receptors in the hippocampus (Fig. 4A) (10). We observed that β2AR binding occurs in the lacunosum moleculare and the stratum oriens subregions (Fig. 4B), which are enriched in astrocytes rather than neurons (Fig. 4C), whereas β1AR binding was diffused throughout the hippocampus (Fig. 4B).
Table S8.
β2AR antibodies tested in Western blot and/or immunohistochemistry
| Source or reference | Catalog no. | Type | Target region | Application |
| Abcam | ab 36956 | Polyclonal | Hippocampus | Western blot |
| Immunohistochemistry | ||||
| ab 182136 | Monoclonal | Hippocampus | Western blot | |
| Cerebellum | ||||
| Amygdala | ||||
| Liver | ||||
| Hippocampus | Immunohistochemistry | |||
| ab 61778 | Polyclonal | Hippocampus | Western blot | |
| Cerebellum | ||||
| Prefrontal cortex | ||||
| Liver | ||||
| Hippocampus | Immunohistochemistry | |||
| Prefrontal cortex | ||||
| ab 176490 | Polyclonal | Hippocampus | Immunohistochemistry | |
| Santa Cruz | sc 569 | Polyclonal | Hippocampus | Western blot |
| Cerebellum | ||||
| Amygdala | ||||
| Liver | ||||
| Hippocampus | Immunohistochemistry | |||
| Cerebellum | ||||
| Cell Signaling Technologies | #8513 | Monoclonal | Hippocampus | Western blot |
| Cerebellum | ||||
| Prefrontal cortex | ||||
| Liver | ||||
| Hippocampus | Immunohistochemistry | |||
| Proteintech Group | 13096-1-AP | Polyclonal | Hippocampus | Western blot |
| Immunohistochemistry | ||||
| Strader et al. (48, 49) | BAR 248 | Polyclonal | Hippocampus | Western blot |
| BAR 404 |
Western blot and immunohistochemistry images are available upon request.
Fig. 4.
β2AR and β1AR autoradiography. (A) Anatomical schematic representation of coronal brain sections at bregma −3.6 mm (45) relative to representative examples of receptor autoradiography: negative control and [125I] CYP binding to β2AR or β1AR. (B) dHC autoradiography for β2AR or β1AR binding. (C) Composite tile scan images of immunostaining with anti-GFAP (astrocytes) or anti-NeuN (neurons) antibodies of a dHC section shown at the same bregma coordinates. DG, dentate gyrus; LMol, stratum lacunosum moleculare; MoDG, stratum moleculare of the dentate gyrus; Or, stratum oriens; Pyr, stratum pyramidale; Rad, stratum radiatum.
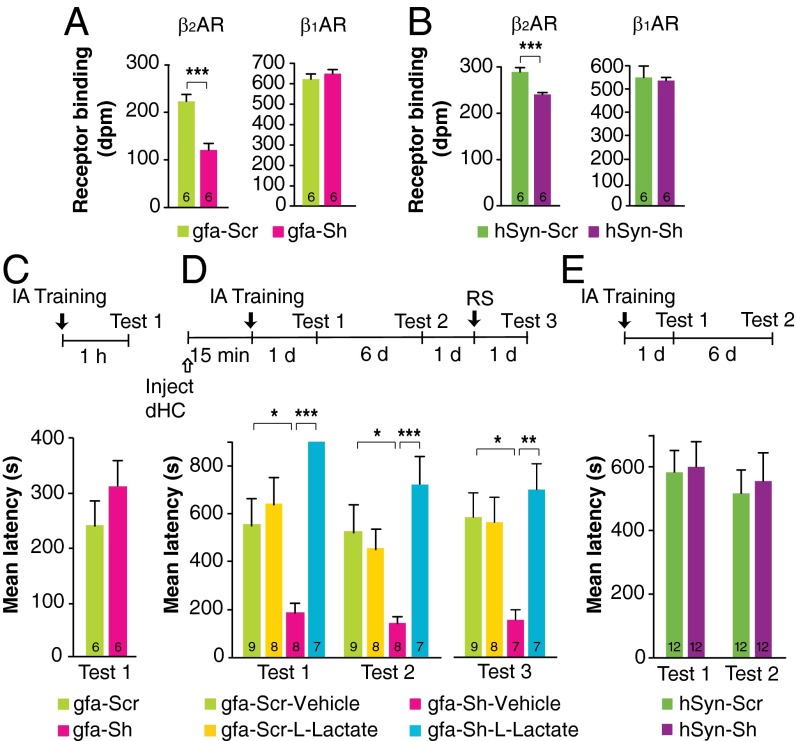
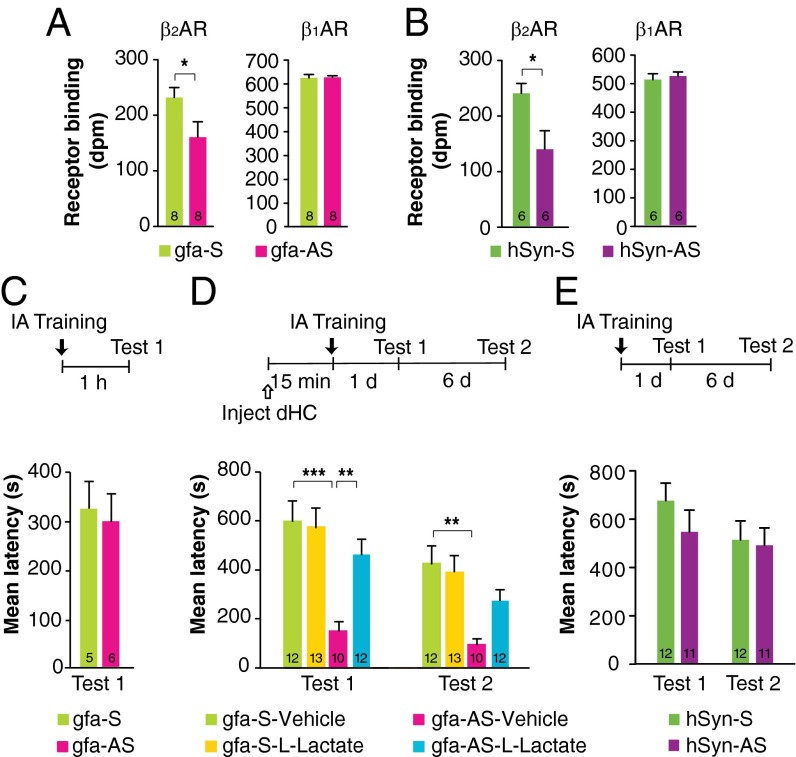
Compared with relative Scr controls, the infection of both gfa-Sh and hSyn-Sh resulted in a significant decrease in β2AR binding without affecting the binding levels of β1AR (P < 0.001 Student’s t test) (Fig. 5 A and B), providing evidence for the specific knockdown of β2AR in astrocytes or neurons, respectively. Notably, the knockdown of the astrocyte-selective virus was more robust (about 50%) than that of the neuron-selective virus (about 20%), as is consistent with a more abundant β2AR binding codistribution with astrocytes than with neurons and with previous literature suggesting higher expression of β2AR in glia (18, 21–23).
Fig. 5.
ShRNA-mediated knockdown of β2AR in astrocytes but not in neurons impairs IA long-term memory. This impairment is rescued by l-lactate. (A and B) β2AR and β1AR binding, expressed in disintegrations per minute (dpm), (n = 6) measured 2 wk after injection of gfa-Scr or gfa-Sh (A) or hSyn-Scr or hSyn-Sh (B). (C) Short-term memory tested 1 h after training and expressed as mean latency ± SEM (in seconds) in rats injected with either gfa-Scr or gfa-Sh 2 wk before training (n = 6). (D) Long-term memory tested 1 d (test 1) and 7 d (test 2) after training and after an RS (test 3), expressed as mean latency ± SEM (in seconds), in rats injected with either gfa-Scr or gfa-Sh 2 wk before training (n = 7–9) and with a bilateral dHC injection of vehicle or l-lactate administered 15 min before training. (E) Long-term memory tested 1 d (test 1) and 7 d (test 2) after training, expressed as mean latency ± SEM (in seconds), in rats injected with either hSyn-Scr or hSyn-Sh 2 wk before training (n = 12). *P < 0.05; **P < 0.01; ***P < 0.001. Numeric values and detailed statistical analyses are reported in Tables S10 and S11.
Table S10.
Receptor binding (dpm) and statistical analyses related to Fig. 5
| Treatment | n | dpm ± SEM | Statistics |
| Fig. 5A: β2 AR | |||
| gfa-Scr | 6 | 222.7 ± 14.46 | t10 = 5.10, P = 0.0005 |
| gfa-Sh | 6 | 120.9 ± 13.77 | |
| Fig. 5A: β1AR | |||
| gfa-Scr | 6 | 618.1 ± 25.29 | t10 = 0.84, P > 0.05 |
| gfa-Sh | 6 | 645.0 ± 19.82 | |
| Fig. 5B: β2AR | |||
| hSyn-Scr | 6 | 285.9 ± 9.190 | t10 = 4.72, P = 0.0008 |
| hSyn-Sh | 6 | 238.6 ± 4.024 | |
| Fig. 5B: β1AR | |||
| hSyn-Scr | 6 | 551.4 ± 49.40 | t10 = 0.25, P > 0.05 |
| hSyn-Sh | 6 | 538.4 ± 13.38 | |
Table S11.
Mean latencies and statistical analyses related to Fig. 5
| Treatment | n | Mean latency(s) ± SEM | Statistics | |||
| Test 1 | Test 2 | n | Test 3 | |||
| Fig. 5C | t10 = 1.03, P > 0.05 | |||||
| gfa-Scr | 6 | 239.2 ± 44.66 | – | – | – | |
| gfa-Sh | 6 | 308.1 ± 49.60 | – | – | – | |
| Fig. 5D | Tests 1 and 2: Treatment: F3,28 = 11.54, P < 0.0001 | |||||
| gfa-Scr–vehicle | 9 | 549.7 ± 114.5 | 522.4 ± 112.0 | 9 | 579.1 ± 100.2 | Time: F1,28 = 5.86, P = 0.02 |
| gfa-Scr–l-lactate | 8 | 641.4 ± 109.0 | 457.5 ± 75.3 | 8 | 558.3 ± 103.3 | Interaction: F3,28= 0.85, P > 0.05 |
| gfa-Sh–vehicle | 8 | 185.4 ± 37.9 | 135.6 ± 32.3 | 7 | 154.9 ± 41.6 | |
| gfa-Sh–l-lactate | 7 | 900 ± 0 | 718.1 ± 117.7 | 7 | 692.9 ± 106.5 | Test 3: F3,27 = 5.67, P = 0.004 |
| Fig. 5E | Treatment: F1,22 = 0.07, P > 0.05 | |||||
| hSyn-Scr | 12 | 583.3 ± 69.0 | 516.8 ± 73.6 | – | – | Time: F1,22 = 2.03, P > 0.05 |
| hSyn-Sh | 12 | 600.2 ± 79.0 | 555.4 ± 88.7 | – | – | Interaction: F1,22 = 0.08, P > 0.05 |
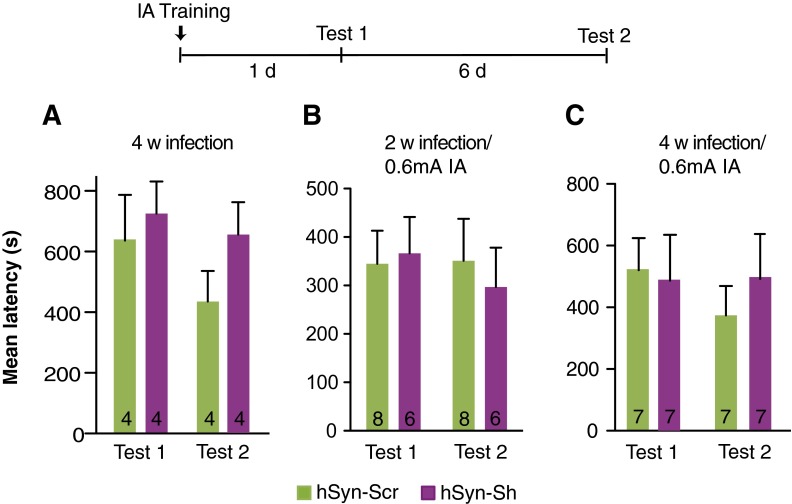
Next we determined the effect of virus-mediated knockdown of β2ARs on memory retention. gfa-Sh bilaterally injected into the dHC 2 wk before training had no effect on short-term memory tested 1 h after training (Fig. 5C) but impaired long-term memory 1 d and 7 d later (P < 0.0001; two-way repeated-measures ANOVA followed by Bonferroni post hoc tests) and following an RS (P < 0.01; one-way ANOVA followed by Bonferroni post hoc tests) (Fig. 5D). Lactate injected bilaterally into the dHC 15 min before training fully and persistently rescued the memory impairment (Fig. 5D). In contrast, 2 wk after hippocampal injection, hSyn-Sh elicited no change in long-term memory retention compared with Scr controls (P > 0.05; two-way repeated-measures ANOVA) (Fig. 5E). To rule out the contribution of behavioral ceiling retentions and/or suboptimal time of infection to the lack of an effect of neuronal β2AR knockdown on memory, we also tested the effect of a longer, 4-wk infection of hSyn-Sh and of a weaker memory evoked by milder-intensity training (0.6 mA footshock) given either 2 or 4 wk after infection. Memory tested 1 d and 7 d after training showed no differences in any of these conditions (P > 0.05; two-way repeated-measures ANOVA) (Fig. S4 and Table S9), indicating that neuronal β2ARs are not critical for IA memory consolidation.
Fig. S4.
Prolonged hSyn-Sh hippocampal infection and/or footshock training (0.6-mA intensity) has no effect on memory retention. Memory retention is expressed as mean latency ± SEM (in seconds). Rats were trained with a 0.9-mA footshock 4 wk after viral infection (n = 4 per group) (A), 0.6-mA footshock 2 wk after viral infection (n = 6–8 per group) (B), or 0.6-mA footshock 4 wk after viral infection (n = 7 per group) (C). All animals were tested 1 d (test 1) and 7 d (test 2) after training. Numeric values and detailed statistical analyses are reported in Table S9.
Table S9.
Mean latencies and statistical analyses related to Fig. S4
| Treatment | n | Mean latency (s) ± SEM | Statistics | |
| Test 1 | Test 2 | |||
| Fig. S4A | Treatment: F1,6 = 1.11, P > 0.05 | |||
| hSyn-Scr | 4 | 638.1 ± 150.5 | 432.2 ± 100.4 | Time: F1,6 = 2.86, P > 0.05 |
| hSyn-Sh | 4 | 722.2 ± 105.6 | 654.3 ± 106.7 | Interaction: F1,6 = 0.73, P > 0.05 |
| Fig. S4B | Treatment: F1,12 = 0.02, P > 0.05 | |||
| hSyn-Scr | 8 | 344.9 ± 68.1 | 350.9 ± 86.8 | Time: F1,12 = 0.48, P > 0.05 |
| hSyn-Sh | 6 | 366.7 ± 74.8 | 296.7 ± 81.6 | Interaction: F1,12 = 0.68, P > 0.05 |
| Fig. S4C | Treatment: F1,12 = 0.08, P > 0.05 | |||
| hSyn-Scr | 7 | 523.6 ± 104.1 | 371.1 ± 95.5 | Time: F1,12 = 1.10, P > 0.05 |
| hSyn-Sh | 7 | 489.8 ± 148.4 | 495.0 ± 145.9 | Interaction: F1,12 = 1.26, P > 0.05 |
Similar results were obtained using gfa-AS and hSyn-AS. Four weeks after infection, both gfa-AS and hSyn-AS significantly reduced β2AR binding compared with S controls (P < 0.05; Student’s t test), whereas β1AR binding remained unchanged (Fig. 6 A and B). Gfa-AS had no effect on short-term memory (Fig. 6C) but significantly impaired long-term memory tested at 1 d and 7 d after training (Fig. 6D). The memory impairment was rescued by a bilateral injection of lactate into the dHC 15 min before training (P < 0.0001; two-way repeated measures ANOVA followed by Bonferroni post hoc tests) (Fig. 6D). In contrast, hSyn-AS had no effect on long-term memory (P > 0.05; one-way ANOVA) (Fig. 6E).
Fig. 6.
Antisense-mediated knockdown of β2AR in astrocytes but not in neurons impairs IA long-term memory. The impairment is rescued by l-lactate. (A and B) β2AR and β1AR binding expressed in disintegration per minute (n = 6) measured 4 wk after injection of gfa-S or gfa-AS (A) or hSyn-S or hSyn-AS (B). (C) Short-term memory tested 1 h after training and expressed as mean latency ± SEM (in seconds) of rats injected with either gfa-S or gfa-AS 4 wk before training (n = 5 or 6). (D) Long-term memory tested 1 d (test 1) and 7 d (test 2) after training, expressed as mean latency ± SEM (in seconds) of rats injected with either gfa-S or gfa-AS 4 wk before training (n = 10–13). A bilateral dHC injection of vehicle or l-lactate was administered 15 min before training. (E) Long-term memory tested 1 d (test 1) and 7 d (test 2) after training, expressed as mean latency ± SEM (in seconds) of rats injected with either hSyn-S or hSyn-AS 4 wk before training (n = 11 or 12). *P < 0.05; **P < 0.01; ***P < 0.001. Numeric values and detailed statistical analyses are reported in Tables S12 and S13.
Table S12.
Receptor binding (dpm) and statistical analyses related to Fig. 6
| Treatment | n | dpm ± SEM | Statistics |
| Fig. 6A: β2AR | |||
| gfa-S | 8 | 230.0 ± 17.98 | t14 = 2.16, P = 0.049 |
| gfa-AS | 8 | 158.5 ± 27.80 | |
| Fig. 6A: β1AR | |||
| gfa-S | 8 | 622.4 ± 13.93 | t14 = 0.22, P > 0.05 |
| gfa-AS | 8 | 625.8 ± 6.519 | |
| Fig. 6B: β2AR | |||
| hSyn-S | 6 | 238.8 ± 17.88 | t10 = 2.67, P = 0.023 |
| hSyn-AS | 6 | 138.3 ± 33.07 | |
| Fig. 6B: β1AR | |||
| hSyn-S | 6 | 509.4 ± 20.55 | t10 = 0.48, P > 0.05 |
| hSyn-AS | 6 | 521.6 ± 14.90 | |
Table S13.
Mean latencies and statistical analyses related to Fig. 6
| Treatment | n | Mean latency(s) ± SEM | Statistics | |
| Test 1 | Test 2 | |||
| Fig. 6C | t9 = 0.31, P > 0.05 | |||
| gfa-S | 5 | 326.8 ± 54.5 | – | |
| gfa-AS | 6 | 301.6 ± 58.0 | – | |
| Fig. 6D | Treatment: F3,43 = 11.52, P < 0.0001 | |||
| gfa-S–vehicle | 12 | 590.6 ± 82.8 | 419.9 ± 70.5 | Time: F1,43 = 13.25, P = 0.0007 |
| gfa-S–l-lactate | 13 | 566.4 ± 76.8 | 385.8 ± 68.0 | Interaction: F3,43 = 0.51, P > 0.05 |
| gfa-AS–vehicle | 10 | 150.2 ± 37.2 | 93.2 ± 19.9 | |
| gfa-AS–l-lactate | 12 | 455.3 ± 60.8 | 269.93 ± 43.5 | |
| Fig. 6E | Treatment: F1,21 = 0.54, P > 0.05 | |||
| hSyn-S | 12 | 677.9 ± 72.7 | 514.8 ± 79.0 | Time: F1,21 = 7.31, P = 0.01 |
| hSyn-AS | 11 | 548.1 ± 90.5 | 492.4 ± 72.0 | Interaction: F1,21 = 1.76 P > 0.05 |
Together, these data show that β2ARs expressed by hippocampal astrocytes rather than neurons are the critical effectors of the adrenergic-mediated effect on long-term memory formation. Specifically, astrocytic β2ARs play a critical role in memory consolidation by activating lactate release that supports long-term memory formation and its underlying molecular changes.
SI Materials and Methods
Animals.
Adult male Long–Evans rats weighing between 200 and 250 g were used. Animals were individually housed and maintained on a 12-h light/dark cycle. Experiments were performed during the light cycle. All rats were allowed ad libitum access to food and water and were handled for 3 min/d for 5 d prior any procedure. For all experiments, rats were randomly assigned to different groups. All protocols complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (44) and were approved by the Icahn School of Medicine at Mount Sinai Institutional Animal Care and Use Committee or by the New York University Animal Welfare Committee.
Cannula Implants.
Bilateral hippocampal surgeries were performed as previously described (31). Rats were anesthetized with ketamine (75 mg/kg) and xylazine (10 mg/kg), and stainless steel cannulas (22 gauge; Plastics One) were stereotaxically implanted (4 mm posterior to bregma, 2.6 mm lateral from midline, and 2 mm ventral). After surgery, rats recovered for 7 d before undergoing any procedure. After completion of an experiment, brains were fixed in 10% formalin, and 50-μm sections were cut on a Leica vibratome to verify cannula placements. Rats with incorrect placement were discarded from the study.
IA.
IA was carried out as described previously (31). The IA chamber (Med Associates) consisted of a rectangular Perspex box divided into a safe compartment and a shock compartment. The safe compartment was white and illuminated by a light fixture fastened to the compartment wall. The shock compartment was black and unilluminated. Foot shocks were delivered to the grid floor of this chamber via a constant current scrambler circuit. The two compartments were separated by an automatically operated sliding door. During training sessions, each rat was placed in the safe compartment with its head facing away from the door. After 10 s the door was automatically opened, allowing the rat access to the shock chamber. The door closed 1 s after the rat entered the shock chamber, and a brief foot shock (0.6 or 0.9 mA for 2 s) was administered. Latency to enter the shock compartment was taken as a measure of acquisition. The rat then was returned to its home cage. Retention tests were performed at the indicated times by placing the rat back into the safe compartment and measuring the latency to enter the shock compartment. Foot shock was not administered on the retention test, and testing was terminated at 540 or 900 s. The duration and intensity of the RS were identical to that given during training, but the RS was carried out in a novel chamber with transparent walls that was placed in a separate, well-lit room. Training and testing procedures were performed blind to treatment conditions.
CFC.
Rats were conditioned in a fear-conditioning chamber, which consisted of a rectangular Plexiglas box (30.5 × 24.1 × 21.0 cm) with a metal grid floor (Model ENV-008; Med Associates). Rats were placed in the chamber for 120 s and then received a 1.5-mA footshock for 2 s. One minute later, the rats were removed. Testing occurred 1 d later; rats were placed in the same chamber for 5 min. No footshock was delivered during testing. All experiments were video recorded, and freezing, defined as lack of movement except for breathing, was scored by an experimenter blind to the treatment conditions.
Drug Injections.
Propranolol (Sigma-Aldrich) was dissolved in PBS containing 10% DMSO. Sodium l-lactate, d-glucose, and ICI 118,551 (Sigma-Aldrich) and betaxolol (Tocris Bioscience) were dissolved in PBS (pH 7.4), or in 10% DMSO when delivered in the same experiment as propranolol. Systemic injections were given i.p. at 10 mg/kg (propranolol, betaxolol, and ICI 118,551) in a 0.5-mL volume at the indicated time points. Intrahippocampal injections (1 μL per hippocampal side) were performed with the infusion needles extending 1.5 mm beyond the cannula. At the indicated time points before training, the rats received bilateral hippocampal injections at a rate of 0.333 μL/min via an infusion pump. The injection needle was left in place for 3 min following the injection to allow complete dispersion of the solution. Intrahippocampal concentrations were propranolol: 5 μg/μL; betaxolol: 2.2 μg/μL; ICI 118,551: 5 μg/μL; l-lactate: 100 mM; d-glucose: 50 mM or 150 mM.
Western Blot Analysis.
Ten to fifty micrograms of total protein extract per lane were resolved using 7.5 or 10% SDS/PAGE and analyzed by Western blot as described previously (31). Primary antibodies were rabbit anti-pCREB (1:2,000; Cell Signaling), rabbit anti-CREB (1:1,000; Millipore), rabbit anti-Arc (1:1,000; Synaptic Systems), rabbit anti-pCaMKII (1:5,000; Cell Signaling), and mouse anti-CaMKII (1:4,000; Millipore). We also tested the following primary anti-β2AR antibodies (Table S8) according to the manufacturer’s recommendation or as previously described: ab36956 and ab182136, ab61778 (46) and ab176490 (all from Abcam); sc-569 (47) (Santa Cruz Biotechnology); 8513 (Cell Signaling Technologies); and 13096-1-AP (Proteintech Group). BAR248 and BAR404 (48, 49) were a generous gift from Chiye Aoki, Center for Neural Science, New York University, New York. Actin (1:5,000; Santa Cruz Biotechnology) was used for loading normalization. HRP-coupled specific secondary antibodies (1:4,000; Santa Cruz Biotechnology) were incubated in Tris-buffered saline (TBS) containing 5% (wt/vol) skim milk, or LI-COR secondary antibodies, anti-rabbit IRDye800CW and anti-mouse IRDye680 (1:10,000), were incubated in TBS containing 0.1% Tween and 0.01% SDS, for 1 h at room temperature. Membranes were scanned on the LI-COR Odyssey imager under nonsaturating conditions. Data were quantified using pixel intensities with the Odyssey software according to the manufacturer’s protocols (LI-COR). Alternately, ECL detection (GE Healthcare Bio-Sciences) was used, and relative densitometric analysis was done using ImageJ software (NIH).
Immunohistochemistry.
Rats were killed with pentobarbital (100 mg/kg) or chloral hydrate (750 mg/kg) and then perfused transcardially with ice-cold 0.1-M PBS for 3 min followed by 4% (wt/vol) paraformaldehyde (PFA) for 15 min at a flow rate of 9 mL/min. Brains were postfixed overnight in 4% (wt/vol) PFA, followed by a 2- to 3-d cryoprotection in 30% (wt/vol) sucrose/PBS at 4 °C. Serial sets of 30-μm coronal sections from fixed brains were collected on a cryostat. Fixed sections were incubated in blocking solution [3% (vol/vol) serum, 0.3% Triton X-100], followed by incubation with primary antibody overnight in 3% serum, 0.3% Tween 20 [mouse anti-GFAP (1:400; Millipore), mouse anti-NeuN (1:200; Millipore), and rabbit anti-Iba1 (1:400; Wako)]. We also tested the following primary anti-β2AR antibodies (Table S8) according to the manufacturer’s recommendation or as previously described: ab36956, ab182136, ab61778 (46), and ab176490 (all from Abcam); sc-569 (47) (Santa Cruz Biotechnology); 8513 (Cell Signaling Technologies); and 13096-1-AP (Proteintech Group). Sections were washed and subsequently incubated with Alexa-Fluor 488–conjugated secondary antibody (1:400; Fisher Scientific) for 2 h at room temperatures. Sections were mounted on gelatin-coated slides and coverslipped with Vectashield mounting medium containing DAPI (Vector Laboratories). Images were captured on a Leica SP5 or SP8 confocal microscope or an Olympus VS120 fluorescent microscope.
Microdialysis.
Microdialysis was carried out as previously described (29). Cannulae (CMA 12), probes (CMA 12 elite, 2 mm long), and fluorinated ethylene propylene (FEP) tubing were from CMA Microdialysis, Inc. Rats underwent surgery as described for cannula implants but were implanted with microdialysis cannulae unilaterally targeting the left dHC. Rats were handled for 10 min/d for 5 d before microdialysis. On the day of microdialysis, the probe was inserted into the guide cannula and perfused with artificial cerebrospinal fluid containing 119 mM NaCl, 26 mM NaHCO3, 2.5 mM KCl, 1 mM NaH2PO4, 1.25 mM Na2HPO4, 2 mM CaCl2, and 4 mM MgCl2 at a rate of 3 μL/min, collected every 10 min. The first two samples were discarded, and then, after 20 min of baseline collection, rats were trained in IA with a 0.9-mA footshock; sample collection was continued for 90 min after training. Samples were subsequently analyzed using the Abcam Lactate Fluorescence Assay Kit. Because baseline lactate concentrations showed interindividual differences, data from each animal were expressed as percentages of baseline, and the individual values were calculated accordingly for each animal.
Viral Constructs and Injections.
Sh sequences were identified by Vector Biolabs using second-generation prediction algorithms; the candidates were embedded in miR arms (sequence proprietary) and screened using A549 cells. Plasmids overexpressing rat β2AR were cotransfected with the plasmids containing Sh or Scr sequences driven by a CMV promoter. A luciferase assay was used to select the sequence with the highest β2AR knockdown; Sh (GCTG-TAAGGAGGATGTAAACTTCCT-GTTTTGGCCACTGACTGAC-AGGAAGTTCATCCTCCTTA) and Scr (CCGGCAACAAGATGAAGAGCACCAACTCGAGTTGGTGCTCTTCATCTTGTTGTTTTT) sequences were engineered into plasmids pAAV-short gfaABC1D-eGFP, a generous gift from Bernard Schneider, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, and pAAV-hSyn-eGFP, a generous gift from Karl Deisseroth, Stanford University, Stanford, CA. Packaging of AAV9 and AAVDJ (50) viruses was carried out by Stanford’s Gene Vector and Virus Core. Mark Kay, Stanford University, Stanford, CA provided the pRC-DJ plasmid used to produce AAVDJ. AS and S sequences targeting the first 770 bp of the rat β2AR coding sequence were generated using rat hippocampal cDNA. RNA was extracted with TRIzol (Invitrogen) and reverse transcribed using the Bio-Rad iScript first-strand synthesis kit (Bio-Rad). PCR (Bio-Rad) was performed using Pfu DNA polymerase (Invitrogen) using the following primers: AS forward: 5′-TCCGTGCCCGCTCCTC-3′ and reverse: 5′CCGGCCATGGAGCCAC-3′; and S forward: 5′-CCGGCCATGGAGCCAC-3′ and reverse: 5′-TCCGTGCCCGCTCCTC-3′. Resulting sequences were engineered into pAAV-short gfaABC1D-eGFP and pAAV-hSyn-eGFP in place of the eGFP sequence; they were packaged into AAV9 [generated at the EPFL; design, production, and titration of the AAV9 vector for antisense expression in astrocytes have been described previously (51)] and AAVDJ (generated at Stanford’s Gene Vector and Virus Core), respectively. All constructs were verified by sequencing. All viral genomic copies (GC) were determined by TaqMan (Applied Biosystems) quantitative PCR. Viruses were produced at titers of 1013 GC/μL. Two microliters of virus were injected per hippocampal side at a rate of 0.2μL/min. The injection needle was left in place for 10 min following injection to allow complete dispersion of the solution.
Receptor Autoradiography.
Receptor autoradiography was carried out as previously described (10). Brains were flash-frozen for autoradiography analysis to assess βAR binding. Sections were cut at 20 μm on a cryostat, mounted on Superfrost Plus Slides (Fisher Scientific), and stored at −80 °C. Slides were fixed for 2 min in 0.1% PFA and washed in Tris buffer (50 mM Tris⋅HCl, 120 mM NaCl, 5 mM KCl; pH 7.4). To determine β1AR- and β2AR-specific binding, slides were incubated for 150 min at room temperature in a 150-pM [125I] CYP (Perkin-Elmer) solution containing 1 μM SB 224289 hydrochloride, 1 μM WAY 100635 maleate (Tocris), and 100 nM CGP 20712 dihydrochloride (Tocris) to assess β2AR-specific binding or 50 nM ICI 118,551 hydrochloride (Sigma) to assess β1AR-specific binding in Tris buffer with 10 mM MgCl2 and 0.1% BSA. To assess nonspecific binding, a separate set of slides was incubated in 150-pM [125I] CYP with all the above inhibitors. All slides then were washed in 4 °C Tris buffer with MgCl2 and then in ice-cold water. Slides were dried and then exposed to Carestream Kodak Biomax MR film for 1 d. Films were developed with Kodak GBX Developer and Fixer. Regions of interest were quantified as previously described (52). Optical densities for βAR binding (throughout the hippocampus for β1AR and in the lacunosum moleculare and stratum oriens for β2AR) were measured using ImageJ, and [125I] CYP standards were used to convert the uncalibrated optical densities to disintegrations per minute. Background was subtracted using binding measurements from the corpus callosum. Brain sections from β1AR/β2AR-knockout mice (32) in pilot experiments confirmed the specificity of the assay.
Statistical Analyses.
For sample-size estimation using power analyses, we used a power analysis calculator (G*Power3). For biochemical studies, power calculation of one-way ANOVA indicated that a minimum sample size of four or five rats per group was necessary to achieve power of 0.8 and an error probability of 0.05. For behavioral experiments, a similar power analysis calculated the requirement of a minimum sample size of six for two-way ANOVA to achieve power of 0.8 and an error probability of 0.05. Statistical analyses were designed using the assumption of normal distribution and similar variance among groups. Data were analyzed using Prism 6 (GraphPad Software, Inc.) using one-way or two-way ANOVA or two-way repeated-measures ANOVA followed by Bonferroni or Newman–Keuls post hoc tests. When two groups were compared, a Student’s t test was used. All tests were two-sided.
Discussion
Using two distinct, virus-mediated, cell-specific knockdown approaches, we showed that hippocampal β2ARs, but not β1ARs, and specifically β2ARs expressed on astrocytes but not on neurons, are required for IA memory consolidation. The critical action of the hippocampal β2ARs is coupled to the training-evoked lactate release.
These results together with the previously established roles for β1ARs in synaptic plasticity and CFC memory retrieval of both rats and mice (12, 14) show that β1ARs and β2ARs contribute differentially to hippocampal memory function and processes. We speculate that in the hippocampus β1ARs may activate neuronal responses during retrieval and that β2ARs activate astrocytic metabolic coupling to neurons to mediate memory consolidation. This differential role of β1ARs and β2ARs in memory processes seems to be part of a broader picture of distinct, and in some cases opposite, roles of the β1ARs and β2ARs in memory processes such as consolidation and retrieval which involve different brain regions in different memory systems (13, 33). Our findings of a cell-specific role of β2ARs in hippocampal astrocytes may contribute to explaining controversial data on systemic or genetic ablation vs. intraregional inhibition of receptor subtypes (12, 15). Understanding these differential roles and contributions of glial vs. neuronal βARs, perhaps in different neuronal populations (excitatory vs. inhibitory neurons) as well as in different brain regions, is key to unraveling the mechanisms of NA-mediated responses. This understanding also should help the clinical use of βAR antagonists or agonists, and the understanding of the relative behavioral outcomes.
Similar to the results described in chicks (34), our data showed that astrocytic β2ARs in the hippocampus play a critical role in memory consolidation. Although the abundance of βAR subtypes does not correlate in chick and mammalian brains (34), it seems that β2ARs expressed by astrocytes have conserved roles in memory formation by controlling glycogenolysis (27). In addition, lactate production from aerobic glycolysis coupled to β2AR stimulation does not seem to occur exclusively in the brain; in fact, it has also been described as occurring in muscle during shock states associated with a reduced or maintained blood flow (35).
Our results are consistent with several previous findings concerning functional regulation, mechanisms of action, and anatomical targeting of NA in the mammalian brain, where NA, produced by the locus coeruleus (LC), projects diffusely to a variety of brain areas, including the neocortex (36) and the hippocampus (37, 38). First, because NA is released in the extracellular space from junctional varicosities along its fibers, it can act on extrasynaptic receptors, which are localized on astrocytes in particular (39). Second, in the hippocampus the highest noradrenergic innervation is found in the dentate gyrus and in the stratum lacunosum moleculare, where we found an enrichment of β2ARs (Fig. 4B). Third, one cellular consequence of NA release during LC firing in particular behavioral states or evoked by glutamatergic inputs is glycogenolysis (30). Fourth, NA signaling is necessary for long-term potentiation (17), memory consolidation (2), and the induction of plasticity and memory genes such as Arc, pCREB, and pCaMKIIα in the hippocampus in vivo (Fig. 1).
Our findings that the training-dependent lactate increase in the hippocampus requires β2ARs and not β1ARs and that supplying lactate rescues the IA memory impairment caused by hippocampal astrocytic β2AR knockdown strengthens the conclusion that astrocytically generated lactate is a key mediator of hippocampus-dependent memory formation under arousing conditions (29). Our results do not dissect whether this lactate originates solely from glycogenolysis or also from glutamate-mediated glycolysis, as proposed by the astrocytic–neuronal lactate shuttle hypothesis (40). As suggested by Hertz and Gibbs (41), the level of stress/arousal evoked by the task may dictate whether glycogenolysis and/or glycolysis are recruited to provide the lactate necessary for memory consolidation. It is possible that the relevance of the experience, and thus the experience-evoked stress level, determines whether astrocytic β2ARs are recruited as essential mechanisms of memory consolidation.
Furthermore, similar to previous observations with the glycogenolysis inhibitor 1,4-dideoxy-1,4-imino-d-arabinitol hydrochloride (DAB) (29), a direct supply of glucose into the hippocampus was unable to replicate the effect of lactate, and only at higher concentrations were the memory impairments caused by β2ARs disruption transiently rescued. Although further experiments are needed to dissect this issue, one possible explanation is that activity-dependent processes promote glucose entry into astrocytes rather than directly into neurons. Glucose then would be metabolized into lactate and finally transported from astrocytes to neurons (42). If this is the case, direct hippocampal delivery of lactate therefore might be more efficient.
Because the dysregulation of β2ARs in astrocytes is associated with multiple sclerosis, Parkinson’s disease, and Alzheimer’s disease (43), we suggest that the disruption of the metabolic role of astrocytic β2ARs in regions supporting cognitive functions may contribute to the pathological features of those diseases. Hence, targeting astrocytic β2ARs mechanisms may help prevent or repair these disorders and/or their precipitation by stress.
Materials and Methods
All animal protocols complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (44) and were approved by the Icahn School of Medicine at Mount Sinai Institutional Animal Care and Use Committee or by the New York University Animal Welfare Committee. Adult male Long–Evans rats were implanted with cannulae and/or injected with AAV bilaterally into the dHC (from bregma: anteroposterior, −4 mm; mediolateral, ±2.6 mm; and dorsoventral, −3.5 mm) as described in ref. 31. IA training (0.9 mA for 2 s) and testing were performed as described in ref. 31. Drug injections (i.p.) of propranolol (Sigma-Aldrich), betaxolol (Tocris Bioscience), or ICI 118,551 (Sigma) were done at 10 mg/kg. Intra-dHC injections (1 μL per side) of propranolol (5 μg/μL), betaxolol (2.2 μg/μL), ICI 118,551 (5 μg/μL), l-lactate (100 mM), or d-glucose (50 mM or 150 mM, as indicated) were performed as described previously (29, 31). For Western blot analysis, dHC protein extracts were done as described in ref. 31; primary antibodies were rabbit anti-pCREB (1:2,000; Cell Signaling), rabbit anti-CREB (1:1,000; Millipore), rabbit anti-Arc (1:1,000; Synaptic Systems), rabbit anti-pCaMKII (1:5,000; Cell Signaling), and mouse anti-CaMKII (1:4,000; Millipore). Immunohistochemistry was performed using mouse anti-GFAP (1:400; Millipore), mouse anti-NeuN (1:200; Millipore), or rabbit anti-Iba1 (1:400; Wako). Microdialysis samples, collected every 10 min, were analyzed using the Abcam Lactate Fluorescence Assay Kit as described in ref. 29. Receptor autoradiography was performed as described in ref. 10; [125I] CYP (Perkin-Elmer) containing 1 μM SB 224289 (Tocris), 1 μM WAY 100635 (Tocris), and 100 nM CGP 20712 (Tocris) or 50 nM ICI 118,551 (Sigma) was used. Data were analyzed using one-way or two-way ANOVA or two-way repeated-measures ANOVA followed by Bonferroni or Newman–Keuls post hoc tests or Student’s t test.
Acknowledgments
We thank Dr. Karl Deisseroth (Stanford University) for generously providing pAAV-hSyn plasmids; Dr. Mark Kay (Stanford University) for generously providing the pRC-DJ plasmid used to produce AAVDJ; Dr. Bernard Schneider (École Polytechnique Fédérale de Lausanne) for generously providing the pAAV-short gfaABC1D-eGFP plasmid used to produce AAV9; and Dr. Alberto Julio Kaumann (University of Murcia) for helpful advice concerning βAR radiolabeling. This work was supported by NIH Grant R01 MH100822 and the McKnight Memory and Cognitive Disorder Award (to C.M.A.) and NIH Grant F30 MH098570 (to V.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1605063113/-/DCSupplemental.
References
- 1.McGaugh JL. Consolidating memories. Annu Rev Psychol. 2015;66:1–24. doi: 10.1146/annurev-psych-010814-014954. [DOI] [PubMed] [Google Scholar]
- 2.Roozendaal B, McGaugh JL. Memory modulation. Behav Neurosci. 2011;125(6):797–824. doi: 10.1037/a0026187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Przybyslawski J, Roullet P, Sara SJ. Attenuation of emotional and nonemotional memories after their reactivation: Role of beta adrenergic receptors. J Neurosci. 1999;19(15):6623–6628. doi: 10.1523/JNEUROSCI.19-15-06623.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dornelles A, et al. Adrenergic enhancement of consolidation of object recognition memory. Neurobiol Learn Mem. 2007;88(1):137–142. doi: 10.1016/j.nlm.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Cahill L, Prins B, Weber M, McGaugh JL. Beta-adrenergic activation and memory for emotional events. Nature. 1994;371(6499):702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- 6.Ravaris CL, Friedman MJ, Hauri PJ, McHugo GJ. A controlled study of alprazolam and propranolol in panic-disordered and agoraphobic outpatients. J Clin Psychopharmacol. 1991;11(6):344–350. [PubMed] [Google Scholar]
- 7.Vaiva G, et al. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol Psychiatry. 2003;54(9):947–949. doi: 10.1016/s0006-3223(03)00412-8. [DOI] [PubMed] [Google Scholar]
- 8.Insel PA. Adrenergic receptors, G proteins, and cell regulation: Implications for aging research. Exp Gerontol. 1993;28(4-5):341–348. doi: 10.1016/0531-5565(93)90061-h. [DOI] [PubMed] [Google Scholar]
- 9.Milner TA, Shah P, Pierce JP. beta-adrenergic receptors primarily are located on the dendrites of granule cells and interneurons but also are found on astrocytes and a few presynaptic profiles in the rat dentate gyrus. Synapse. 2000;36(3):178–193. doi: 10.1002/(SICI)1098-2396(20000601)36:3<178::AID-SYN3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Rainbow TC, Parsons B, Wolfe BB. Quantitative autoradiography of beta 1- and beta 2-adrenergic receptors in rat brain. Proc Natl Acad Sci USA. 1984;81(5):1585–1589. doi: 10.1073/pnas.81.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Summers RJ, Papaioannou M, Harris S, Evans BA. Expression of beta 3-adrenoceptor mRNA in rat brain. Br J Pharmacol. 1995;116(6):2547–2548. doi: 10.1111/j.1476-5381.1995.tb17205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murchison CF, et al. A distinct role for norepinephrine in memory retrieval. Cell. 2004;117(1):131–143. doi: 10.1016/s0092-8674(04)00259-4. [DOI] [PubMed] [Google Scholar]
- 13.Ramos BP, et al. The beta-1 adrenergic antagonist, betaxolol, improves working memory performance in rats and monkeys. Biol Psychiatry. 2005;58(11):894–900. doi: 10.1016/j.biopsych.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 14.Winder DG, et al. ERK plays a regulatory role in induction of LTP by theta frequency stimulation and its modulation by beta-adrenergic receptors. Neuron. 1999;24(3):715–726. doi: 10.1016/s0896-6273(00)81124-1. [DOI] [PubMed] [Google Scholar]
- 15.Schutsky K, Ouyang M, Castelino CB, Zhang L, Thomas SA. Stress and glucocorticoids impair memory retrieval via β2-adrenergic, Gi/o-coupled suppression of cAMP signaling. J Neurosci. 2011;31(40):14172–14181. doi: 10.1523/JNEUROSCI.2122-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou HC, et al. Activation of β2-adrenoceptor enhances synaptic potentiation and behavioral memory via cAMP-PKA signaling in the medial prefrontal cortex of rats. Learn Mem. 2013;20(5):274–284. doi: 10.1101/lm.030411.113. [DOI] [PubMed] [Google Scholar]
- 17.O’Dell TJ, Connor SA, Guglietta R, Nguyen PV. β-Adrenergic receptor signaling and modulation of long-term potentiation in the mammalian hippocampus. Learn Mem. 2015;22(9):461–471. doi: 10.1101/lm.031088.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mantyh PW, et al. Beta 2-adrenergic receptors are expressed by glia in vivo in the normal and injured central nervous system in the rat, rabbit, and human. J Neurosci. 1995;15(1 Pt 1):152–164. doi: 10.1523/JNEUROSCI.15-01-00152.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shao Y, Sutin J. Expression of adrenergic receptors in individual astrocytes and motor neurons isolated from the adult rat brain. Glia. 1992;6(2):108–117. doi: 10.1002/glia.440060205. [DOI] [PubMed] [Google Scholar]
- 20.Zhu Y, Kimelberg HK. Cellular expression of P2Y and beta-AR receptor mRNAs and proteins in freshly isolated astrocytes and tissue sections from the CA1 region of P8-12 rat hippocampus. Brain Res Dev Brain Res. 2004;148(1):77–87. doi: 10.1016/j.devbrainres.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Catus SL, Gibbs ME, Sato M, Summers RJ, Hutchinson DS. Role of β-adrenoceptors in glucose uptake in astrocytes using β-adrenoceptor knockout mice. Br J Pharmacol. 2011;162(8):1700–1715. doi: 10.1111/j.1476-5381.2010.01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cash R, Raisman R, Lanfumey L, Ploska A, Agid Y. Cellular localization of adrenergic receptors in rat and human brain. Brain Res. 1986;370(1):127–135. doi: 10.1016/0006-8993(86)91112-1. [DOI] [PubMed] [Google Scholar]
- 23.Waeber C, Rigo M, Chinaglia G, Probst A, Palacios JM. Beta-adrenergic receptor subtypes in the basal ganglia of patients with Huntington’s chorea and Parkinson’s disease. Synapse. 1991;8(4):270–280. doi: 10.1002/syn.890080405. [DOI] [PubMed] [Google Scholar]
- 24.Magistretti PJ, Morrison JH. Noradrenaline- and vasoactive intestinal peptide-containing neuronal systems in neocortex: Functional convergence with contrasting morphology. Neuroscience. 1988;24(2):367–378. doi: 10.1016/0306-4522(88)90338-7. [DOI] [PubMed] [Google Scholar]
- 25.Brown AM. Brain glycogen re-awakened. J Neurochem. 2004;89(3):537–552. doi: 10.1111/j.1471-4159.2004.02421.x. [DOI] [PubMed] [Google Scholar]
- 26.Vilchez D, et al. Mechanism suppressing glycogen synthesis in neurons and its demise in progressive myoclonus epilepsy. Nat Neurosci. 2007;10(11):1407–1413. doi: 10.1038/nn1998. [DOI] [PubMed] [Google Scholar]
- 27.Gibbs ME, Hutchinson D, Hertz L. Astrocytic involvement in learning and memory consolidation. Neurosci Biobehav Rev. 2008;32(5):927–944. doi: 10.1016/j.neubiorev.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Newman LA, Korol DL, Gold PE. Lactate produced by glycogenolysis in astrocytes regulates memory processing. PLoS One. 2011;6(12):e28427. doi: 10.1371/journal.pone.0028427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki A, et al. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144(5):810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorg O, Magistretti PJ. Characterization of the glycogenolysis elicited by vasoactive intestinal peptide, noradrenaline and adenosine in primary cultures of mouse cerebral cortical astrocytes. Brain Res. 1991;563(1-2):227–233. doi: 10.1016/0006-8993(91)91538-c. [DOI] [PubMed] [Google Scholar]
- 31.Chen DY, Bambah-Mukku D, Pollonini G, Alberini CM. Glucocorticoid receptors recruit the CaMKIIα-BDNF-CREB pathways to mediate memory consolidation. Nat Neurosci. 2012;15(12):1707–1714. doi: 10.1038/nn.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rohrer DK, Chruscinski A, Schauble EH, Bernstein D, Kobilka BK. Cardiovascular and metabolic alterations in mice lacking both beta1- and beta2-adrenergic receptors. J Biol Chem. 1999;274(24):16701–16708. doi: 10.1074/jbc.274.24.16701. [DOI] [PubMed] [Google Scholar]
- 33.Gibbs ME, Summers RJ. Contrasting roles for beta1, beta2 and beta3-adrenoceptors in memory formation in the chick. Neuroscience. 2005;131(1):31–42. doi: 10.1016/j.neuroscience.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 34.Hutchinson DS, Summers RJ, Gibbs ME. Beta2- and beta3-adrenoceptors activate glucose uptake in chick astrocytes by distinct mechanisms: A mechanism for memory enhancement? J Neurochem. 2007;103(3):997–1008. doi: 10.1111/j.1471-4159.2007.04789.x. [DOI] [PubMed] [Google Scholar]
- 35.Levy B, Desebbe O, Montemont C, Gibot S. Increased aerobic glycolysis through beta2 stimulation is a common mechanism involved in lactate formation during shock states. Shock. 2008;30(4):417–421. doi: 10.1097/SHK.0b013e318167378f. [DOI] [PubMed] [Google Scholar]
- 36.Morrison JH, Grzanna R, Molliver ME, Coyle JT. The distribution and orientation of noradrenergic fibers in neocortex of the rat: An immunofluorescence study. J Comp Neurol. 1978;181(1):17–39. doi: 10.1002/cne.901810103. [DOI] [PubMed] [Google Scholar]
- 37.Loy R, Koziell DA, Lindsey JD, Moore RY. Noradrenergic innervation of the adult rat hippocampal formation. J Comp Neurol. 1980;189(4):699–710. doi: 10.1002/cne.901890406. [DOI] [PubMed] [Google Scholar]
- 38.Swanson LW, Hartman BK. The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat utilizing dopamine-beta-hydroxylase as a marker. J Comp Neurol. 1975;163(4):467–505. doi: 10.1002/cne.901630406. [DOI] [PubMed] [Google Scholar]
- 39.Porter JT, McCarthy KD. Astrocytic neurotransmitter receptors in situ and in vivo. Prog Neurobiol. 1997;51(4):439–455. doi: 10.1016/s0301-0082(96)00068-8. [DOI] [PubMed] [Google Scholar]
- 40.Magistretti PJ, Allaman I. A cellular perspective on brain energy metabolism and functional imaging. Neuron. 2015;86(4):883–901. doi: 10.1016/j.neuron.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 41.Hertz L, Gibbs ME. What learning in day-old chickens can teach a neurochemist: Focus on astrocyte metabolism. J Neurochem. 2009;109(Suppl 1):10–16. doi: 10.1111/j.1471-4159.2009.05939.x. [DOI] [PubMed] [Google Scholar]
- 42.Magistretti PJ, Pellerin L. Astrocytes Couple Synaptic Activity to Glucose Utilization in the Brain. News Physiol Sci. 1999;14:177–182. doi: 10.1152/physiologyonline.1999.14.5.177. [DOI] [PubMed] [Google Scholar]
- 43.Dong JH, et al. β2-adrenergic receptor and astrocyte glucose metabolism. J Mol Neurosci. 2012;48(2):456–463. doi: 10.1007/s12031-012-9742-4. [DOI] [PubMed] [Google Scholar]
- 44.Committee on Care and Use of Laboratory Animals 1996. Guide for the Care and Use of Laboratory Animals (Natl Inst Health, Bethesda), DHHS Publ No (NIH) 85-23.
- 45.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th Ed Academic/Elsevier; Cambridge, MA: 2007. [Google Scholar]
- 46.Liu Y, Liang X, Ren WW, Li BM. Expression of β1- and β2-adrenoceptors in different subtypes of interneurons in the medial prefrontal cortex of mice. Neuroscience. 2014;257:149–157. doi: 10.1016/j.neuroscience.2013.10.078. [DOI] [PubMed] [Google Scholar]
- 47.Cox DJ, Racca C, LeBeau FE. Beta-adrenergic receptors are differentially expressed in distinct interneuron subtypes in the rat hippocampus. J Comp Neurol. 2008;509(6):551–565. doi: 10.1002/cne.21758. [DOI] [PubMed] [Google Scholar]
- 48.Strader CD, et al. The carboxyl terminus of the hamster beta-adrenergic receptor expressed in mouse L cells is not required for receptor sequestration. Cell. 1987;49(6):855–863. doi: 10.1016/0092-8674(87)90623-4. [DOI] [PubMed] [Google Scholar]
- 49.Strader CD, et al. Identification of residues required for ligand binding to the beta-adrenergic receptor. Proc Natl Acad Sci USA. 1987;84(13):4384–4388. doi: 10.1073/pnas.84.13.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grimm D, et al. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. J Virol. 2008;82(12):5887–5911. doi: 10.1128/JVI.00254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dirren E, et al. Intracerebroventricular injection of adeno-associated virus 6 and 9 vectors for cell type-specific transgene expression in the spinal cord. Hum Gene Ther. 2014;25(2):109–120. doi: 10.1089/hum.2013.021. [DOI] [PubMed] [Google Scholar]
- 52.Bales KL, et al. Neonatal oxytocin manipulations have long-lasting, sexually dimorphic effects on vasopressin receptors. Neuroscience. 2007;144(1):38–45. doi: 10.1016/j.neuroscience.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]