Significance
Unicellular species lack the nonreproductive somatic cell types that characterize complex multicellular organisms. We consider two alternative explanations: first, that the costs of lost reproductive potential never exceed the benefits of somatic cells in unicellular organisms; and second, that somatic cells may profit a unicellular population but leave it vulnerable to invasion by common mutants. We test these hypotheses using engineered yeast strains that permit direct comparisons of fitness and evolutionary stability between lifestyles. We find that the benefits of somatic cell production can exceed the costs in unicellular strains. Multicellular, soma-producing strains resist invasion by nondifferentiating mutants that overtake unicellular populations, supporting the theory that somatic differentiation is stabilized by population structure imposed by multicellularity.
Keywords: evolution, multicellularity, differentiation, synthetic biology, yeast
Abstract
Many multicellular organisms produce two cell lineages: germ cells, whose descendants produce the next generation, and somatic cells, which support, protect, and disperse the germ cells. This germ-soma demarcation has evolved independently in dozens of multicellular taxa but is absent in unicellular species. A common explanation holds that in these organisms, inefficient intercellular nutrient exchange compels the fitness cost of producing nonreproductive somatic cells to outweigh any potential benefits. We propose instead that the absence of unicellular, soma-producing populations reflects their susceptibility to invasion by nondifferentiating mutants that ultimately eradicate the soma-producing lineage. We argue that multicellularity can prevent the victory of such mutants by giving germ cells preferential access to the benefits conferred by somatic cells. The absence of natural unicellular, soma-producing species previously prevented these hypotheses from being directly tested in vivo: to overcome this obstacle, we engineered strains of the budding yeast Saccharomyces cerevisiae that differ only in the presence or absence of multicellularity and somatic differentiation, permitting direct comparisons between organisms with different lifestyles. Our strains implement the essential features of irreversible conversion from germ line to soma, reproductive division of labor, and clonal multicellularity while maintaining sufficient generality to permit broad extension of our conclusions. Our somatic cells can provide fitness benefits that exceed the reproductive costs of their production, even in unicellular strains. We find that nondifferentiating mutants overtake unicellular populations but are outcompeted by multicellular, soma-producing strains, suggesting that multicellularity confers evolutionary stability to somatic differentiation.
Somatic differentiation, a permanent change in gene expression inherited by all of a cell’s descendants, produces somatic cells from a totipotent germ line. Although somatic cells may divide indefinitely, they cannot beget the complete organism and are thus considered nonreproductive. Generating such sterile cells has clear fitness costs that must be offset by somatic functions that improve the viability or fecundity of germ cells. The absence of a soma in unicellular species (1), as well as the persistence of undifferentiated multicellular groups among the volvocine algae (2) and cyanobacteria (3), has fueled speculation that multicellularity must arise before somatic differentiation can evolve (4–7). It has been argued that somatic differentiation is not observed in unicellular species because the fitness benefits of somatic cells can never exceed the cost of making them (6–8): although soma can contribute motility and protective structures to multicellular organisms, somatic cells in a unicellular species can only benefit the germ line by secreting useful products into a shared extracellular milieu. However, nutrient exchange between members of microbial consortia (9, 10) demonstrates the potential for productive interactions between cell types in the absence of physical adhesion. Benefits associated with somatic differentiation, including reproductive division of labor (11) and suppression of germ-line mutations through lineage sequestration (12) or reduced oxidative stress (13), are thus likely accessible to unicellular species.
We propose the alternative hypothesis that unicellular somatic differentiation can offer fitness benefits in a population of genetically identical cells but remains rare because it is not an evolutionarily stable strategy (14). Commonly occurring mutants that do not differentiate (“cheats”) could take advantage of somatic cell products in the shared media without paying the reproductive costs of differentiation, thus increasing in frequency until their genotype prevails. We also posit that if multicellularity results from cells of a single lineage failing to disperse (rather than cells aggregating from different lineages), differentiating populations can outcompete cheats: although cheats initially arise through mutation in a group with somatic cells (which the cheats can exploit), lineage-restricted propagation forces the cheat's descendants to be confined to multicellular groups composed entirely of cheats, which thus cannot benefit from the local accumulation of somatic cell products (15–17). This hypothesis invokes the demonstrated ability of population structure to maintain altruistic traits (15, 18, 19).
To test the evolutionary stability of germ-soma differentiation, we designed strains of the budding yeast Saccharomyces cerevisiae that produce soma, are multicellular, or combine both traits: one strain is a multicellular, differentiating organism and the other two represent both possible intermediates in its evolution from a nondifferentiating, unicellular ancestor (Fig. 1A). Using synthetic strains that differ from one another at only a few, well-defined loci ensures that no undesired variables confound the direct comparison of fitness and evolutionary stability and permits the study of a unicellular, differentiating lifestyle not found in nature. Experiments on living organisms also avoid the potential pitfall of biologically unrealistic parameter regimes in simulations or purely analytical models. Our results show that production of soma can be advantageous even in unicellular populations, although these are susceptible to invasion by nondifferentiating cheats, which multicellular populations effectively repel. We therefore speculate that multicellularity facilitates the evolution of somatic differentiation principally by providing stability against common mutants that prevent differentiation.
Fig. 1.
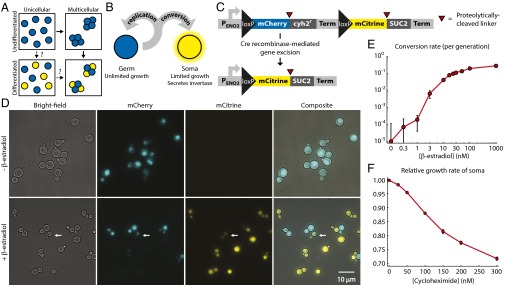
Engineering yeast as a model for somatic differentiation. (A) Alternative evolutionary trajectories for the evolution of development. Multicellularity and somatic differentiation have separate biological bases and thus probably evolved sequentially. In some clades, the persistence of likely evolutionary intermediates suggests that multicellularity arose first (solid arrows); no examples of evolution through a unicellular differentiating intermediate (dashed arrows) are known. Somatic cells are colored yellow. (B) Schematic of germ-soma division of labor model engineered in our differentiating strains. (C) Cell type-defining locus in the differentiating strains. Each pair of cell type-specific proteins (mCherry and Cyh2r for germ cells, mCitrine and Suc2 for somatic cells) is initially expressed as a single polypeptide with a ubiquitin linker (red triangles). Cellular deubiquitinating enzymes cleave this linker posttranslationally at its C terminus (34), allowing the two resulting peptides to localize and function independently: for example, Suc2 enters the secretory pathway, whereas mCitrine remains in the cytoplasm. A transcriptional terminator (Term) blocks expression of the somatic cell proteins in germ cells. Cre recombinase-mediated gene excision between loxP sites removes the terminator, as well as the germ cell-specific genes. (D) The unicellular differentiating strain (yMEW192) during growth in yeast extract-peptone-dextrose media (YPD) before (Top) or 5 h after addition of 1 μM β-estradiol (Bottom). The white arrow indicates a budded cell that has recently undergone conversion and contains both mCherry and mCitrine proteins and thus fluoresces in both channels (cf Fig. S1). (E) Conversion rates estimated by flow cytometry during growth in YPD containing β-estradiol. Error bars represent 95% confidence intervals (CIs) of the mean, determined using data obtained from three biological replicates. (F) The growth disadvantage of somatic cells relative to germ cells was measured by growing them in coculture in YPD plus cycloheximide and determining the change in the ratio between cell types over multiple rounds of cell division. Error bars represent 95% CIs of the mean, determined using data obtained from three biological replicates.
Construction of a Synthetic Differentiation System
We mimicked somatic differentiation by engineering fast-growing “germ” cells that can give rise to slower dividing, differentiated “somatic” cells that secrete invertase (Suc2), an enzyme that hydrolyzes sucrose (which laboratory yeast strains cannot take up directly) into the monosaccharides glucose and fructose, which any cell in the shared medium can then import (20, 21) (Fig. 1B). The somatic cells thus perform a digestive function: liberating monosaccharides, the sole importable carbon source during growth in sucrose minimal media. In nature, differentiation is often achieved through multiply redundant gene regulatory networks that stabilize cell fate (22): to simplify our system, we instead made differentiation permanent and heritable by making the expression of somatic cell-specific genes depend on a site-specific recombinase that excises a gene needed for rapid cell proliferation (Fig. 1C).
Germ and somatic cells must be present at a suitable ratio for growth on sucrose: germ cells have the higher maximum growth rate, but monosaccharides become limiting when somatic cells are rare. We predicted that the ratio between cell types would reach a steady-state value reflecting the balance between unidirectional conversion of germ cells into somatic cells and the restricted division of somatic cells. We designed tunable differentiation and division rates to allow us to regulate the ratio between cell types and thus control the growth rate of the culture as a whole.
Both features depend on a single, genetically engineered locus (Fig. 1C). In germ cells, this locus expresses the fluorescent protein mCherry and a gene that accelerates cell division, the cycloheximide resistant (cyh2r) allele of ribosomal protein L28 (23); in somatic cells, the locus expresses a different fluorescent protein (mCitrine) and invertase. The germ-line form is converted to the somatic form by a version of Cre recombinase engineered by Lindstrom et al. (24) to be active only in the presence of β-estradiol. Adding β-estradiol to a growing culture induced conversion of germ to somatic cells, apparent as the onset of mCitrine expression and slow loss of mCherry fluorescence by dilution (Fig. 1D, Fig. S1, and Movie S1). As the β-estradiol concentration increased, conversion rates ranged from undetectable levels (<10−3 conversions per cell per generation) to ∼0.3 conversions per cell per generation (Fig. 1E and Fig. S2A). Expression of the codominant, cycloheximide-sensitive WT allele of CYH2 from its native locus permitted continued growth following cyh2r excision, but at a reduced rate that depended on cycloheximide concentration. The growth rate deficit of somatic cells ranged from undetectable (<1%) to nearly 30% as the cycloheximide concentration increased (Fig. 1F and Fig. S2B).
Fig. S1.
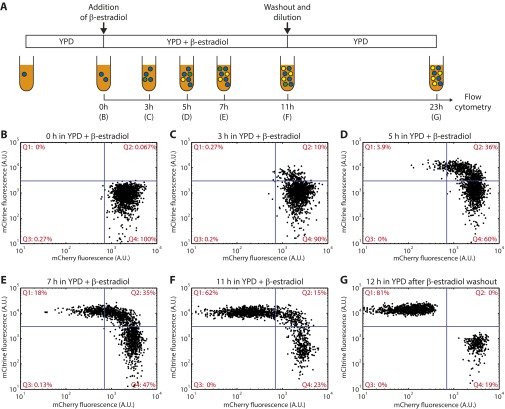
Flow cytometry time course of conversion in a cyh2r excision strain. (A) Schematic of the experiment. A culture of germ cells (blue) of the cyh2r excision strain (yMEW192) was pregrown in log phase in YPD media. Immediately after addition of 1 μM β-estradiol (B), and at the indicated time points during subsequent growth (C–F), samples were collected for flow cytometry. Converted cells began to express mCitrine and lost mCherry fluorescence gradually by dilution through growth. After 11 h in 1 μM β-estradiol, cells were washed and transferred to fresh YPD media: 12 h of further growth and division diluted remaining mCherry from mCitrine+ somatic cells, permitting the two cell types to be unambiguously distinguished (G). Reversion to the germ cell state, as defined by recovery of mCherry fluorescence, was never observed by flow cytometry or time-lapse microscopy in flow chambers.
Fig. S2.
Measurement of conversion and relative growth rates. (A) Measurement of conversion rates by flow cytometry. Samples were collected at various time points after induction of conversion to determine the number of generations elapsed since β-estradiol addition, as well as the fraction of mCherry+ mCitrine− (germ) cells within the culture. β-Estradiol washout and continued growth permits unambiguous discrimination of germ and somatic cells by flow cytometry (cf Fig. S1G). Representative data are shown at right. In the absence of cycloheximide, the fraction of germ cells is predicted to fall exponentially according to the conversion rate. Slopes from best-fit linear regression lines were used to calculate conversion rates (Fig. 1E). (B) Measurement of relative growth rate differences through competition fitness assays. Pure cultures of germ and somatic cells were combined at a 1:1 ratio and passaged in log phase in YPD + cycloheximide. Samples were collected at each dilution to determine the ratio between cell types by flow cytometry. Representative data are shown at right. Slopes calculated from best-fit regression lines were used to determine differences in relative growth rates (Fig. 1F).
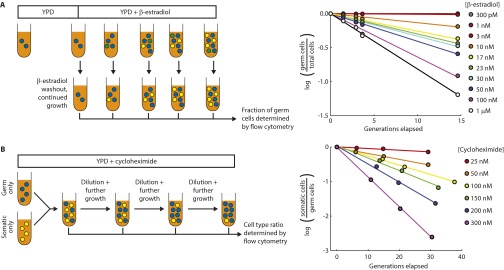
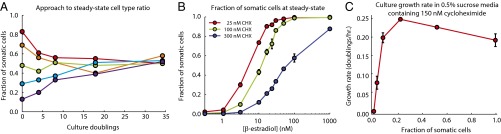
The combination of irreversible differentiation and restricted somatic cell division caused cultures to approach a steady-state ratio between the two cell types over time (Fig. 2A). The steady-state fraction of somatic cells increased with the conversion rate and decreased with the somatic cells’ growth disadvantage, as expected (Fig. 2B). The steady-state ratio between cell types could be tuned over four orders of magnitude by altering the cycloheximide and β-estradiol concentrations (Fig. 2B).
Fig. 2.
Stable maintenance of both cell types facilitates growth in sucrose + cycloheximide media. (A) Representative time courses of the fraction of somatic cells in cultures of the unicellular differentiating strain (yMEW192 and yMEW192 convertant) initiated at various cell type ratios and passaged in YPD containing 10 nM β-estradiol and 600 nM cycloheximide. (B) The steady-state fraction of somatic cells was determined as in A for various cycloheximide and β-estradiol concentrations. Error bars represent 2 standard deviations, calculated using data obtained from three biological replicates. (C) Dependence of growth rate on fraction of somatic cells in cultures of the unicellular differentiating strain growing in 0.5% sucrose minimal media containing 150 nM cycloheximide and β-estradiol concentrations of 0, 0.3,1, 3, 10, and 50 nM, respectively. Error bars represent 95% CIs of the mean, determined using data obtained from three biological replicates.
To investigate the ability of invertase secretion from somatic cells to support germ cell proliferation, we determined how the culture's growth rate on sucrose depended on the ratio between cell types. In the presence of cycloheximide, cultures containing both cell types at an intermediate ratio grew more quickly in sucrose media than cultures of either cell type alone (Fig. 2C), confirming that somatic cells benefit their germ line through invertase secretion.
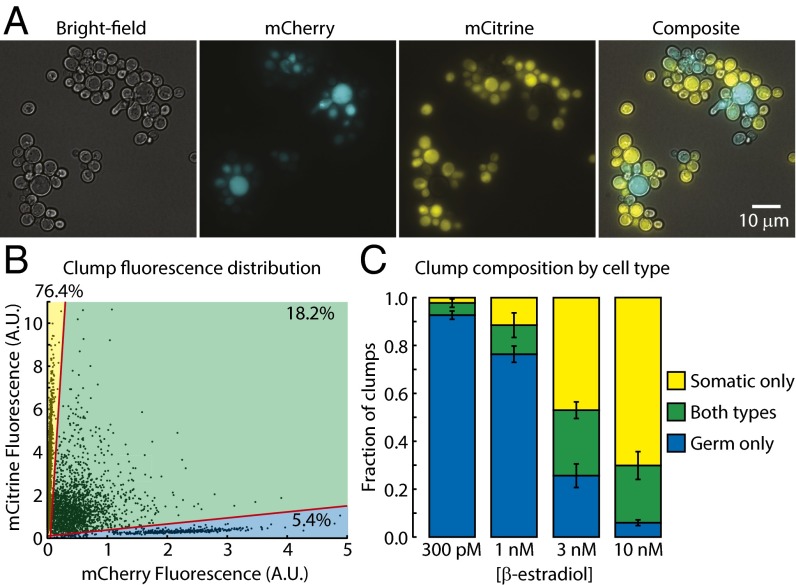
Clonal multicellularity arises through the maintenance of contact between daughter cells following cytokinesis (25). In budding yeast, clonal multicellularity can be produced, either through engineering (26) or evolution (16, 27), by mutations that prevent degradation of the septum, the specialized part of the cell wall that holds mother and daughter cells together after their cytoplasms have been separated by cytokinesis (28). Deletion of CTS1, a chitinase gene required for septum degradation, causes formation of “clumps” (groups of daughter cells attached through persistent septa) that typically contain 4–30 cells during growth in well-mixed liquid medium (Fig. 3A) (29). In the presence of β-estradiol, differentiating strains that lack CTS1 (Δcts1) produced clumps that frequently contained both germ and somatic cells, as shown by fluorescence microscopy (Fig. 3A) and flow cytometry (Fig. 3 B and C). Combining our gene excision-based differentiation system with CTS1 deletion thus allowed us to produce strains exhibiting all of the life strategies needed to compare the evolutionary stability of unicellular and multicellular differentiation.
Fig. 3.
Δcts1 strains form multicellular clumps containing both cell types. (A) Representative image of clump size and cell type composition in a well-mixed liquid culture of the Δcts1 multicellular differentiating strain (yMEW208) at a steady-state cell type ratio in sucrose minimal media containing 10 nM β-estradiol and 150 nM cycloheximide. (B) Representative distribution of clump mCherry and mCitrine fluorescence for the multicellular differentiating strain (yMEW208) at a steady-state cell type ratio in sucrose minimal media containing 10 nM β-estradiol and 150 nM cycloheximide. Gating of clumps by cell type composition (lower sector: germ cells only; middle sector: both cell types; top sector: somatic cells only) is shown in red. (C) The fraction of clumps containing one or both cell types was determined as in B for cultures of the multicellular differentiating strain (yMEW208) at a steady-state cell type ratio in sucrose minimal media containing 150 nM cycloheximide at the indicated β-estradiol concentrations. Error bars represent 95% CIs of the mean, determined with data obtained from six biological replicates.
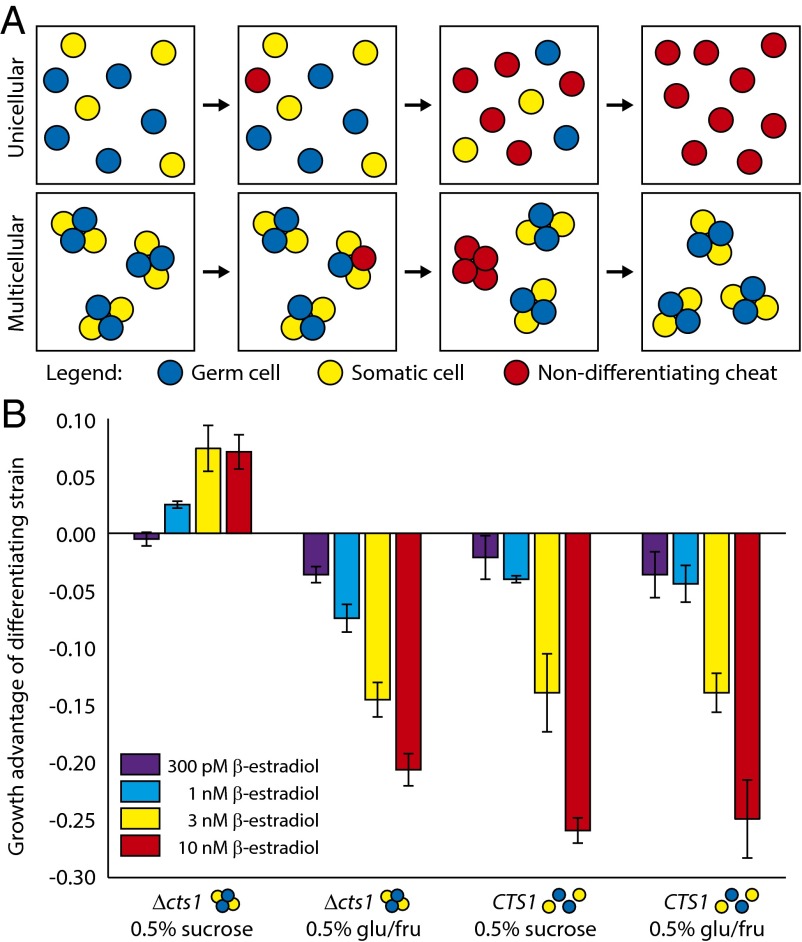
Somatic cells in unicellular strains can only benefit germ cells by secreting useful products into a shared medium; nondifferentiating cheats (e.g., Cre− germ cells) and germ cells in well-mixed media have equal access to somatic cell products, but cheats do not pay the reproductive toll of differentiation. We therefore predicted that cheats would enjoy a fitness advantage over germ cells, allowing them to invade unicellular, differentiating populations (Fig. 4A, Top). In multicellular species, however, significant local accumulation of somatic cell products (in our experiment, monosaccharides) within multicellular groups can give differentiating lineages an advantage over cheats as long as the benefit of better nutrition overcomes the cost of producing slower-replicating, somatic cells (26). This benefit is present even in well-mixed cultures because of the unstirred liquid within and surrounding the clump. In clonally multicellular species, such as our Δcts1 strain, novel cheats arising by mutation will eventually be segregated into cheat-only groups by cell division and group fragmentation. We hypothesized that cheats would then experience reduced access to somatic cell products, potentially negating their growth advantage over germ cells (Fig. 4A, Bottom).
Fig. 4.
Multicellular differentiating strains resist invasion by Cre− cheats. (A) Hypothesized evolutionary outcomes for novel nondifferentiating mutants (red) in unicellular (Top) and multicellular (Bottom) populations. Germ cells are blue and somatic cells are yellow. (B) Growth advantages of differentiating strains (unicellular: yMEW192, multicellular: yMEW208) relative to cheaters (unicellular: yMEW193, multicellular: yMEW209) in minimal media containing 150 nM cycloheximide. Error bars represent 95% CIs of mean growth advantages, determined using data obtained from three biological replicates. Growth advantages of the multicellular strain growing in sucrose minimal medium at 1, 3, and 10 nM β-estradiol are significantly greater than zero (P < 10−3, one-tailed t test); growth advantages of the unicellular strain growing in sucrose are significantly less than zero at all β-estradiol concentrations (P < 10−6, one-tailed t test).
To test the prediction that cheats could overcome unicellular but not multicellular differentiation, we introduced cheats into unicellular or multicellular differentiating cultures and investigated their fate. We mixed differentiating cultures expressing a third fluorescent protein, Cerulean, with cheats that lack this third color and cannot differentiate because they lack the recombinase whose action gives rise to somatic cells (Cre−). We monitored the relative frequency of the two strains over a series of growth and dilution cycles. In sucrose media, cheats invaded unicellular, differentiating populations but were outcompeted in multicellular, differentiating populations (Fig. 4B). The growth advantage of multicellular, differentiating strains was nullified in monosaccharide-containing media, where somatic cells should confer no fitness advantage to clumps (Fig. 4B). Moreover, the growth advantage of differentiating strains depended strongly on the conversion rate (β-estradiol concentration): higher conversion rates were advantageous only in the multicellular differentiating case (Fig. 4B), where they increased the fraction of somatic cells overall, as well as the fraction of clumps containing at least one somatic cell (Fig. 3C). In unicellular cultures grown on sucrose, or in cultures grown on glucose (unicellular or multicellular), increasing the conversion rate increased the growth advantage of cheats by reducing the growth rate of the germ cell population (Fig. 4B).
Our results show that unicellular, soma-producing strains are evolutionarily unstable to invasion by nondifferentiating cheats. This finding is unlikely to depend on the specific molecular mechanisms that effect differentiation or allow somatic cells to assist germ cells: any form of differentiation in a single-celled species would require that the two cell types exchange resources through a shared medium. From the spontaneous mutation rate in budding yeast [∼ 3 × 10−10 per base pair per cell division (30)] and the size of the recombinase gene, we estimate mutations that inactivate our engineered recombination system would occur in about one out of every 107 cell divisions; this is likely an underestimate of the frequency of inactivating mutations in natural differentiation, which typically requires more loci and thus presents a larger target for mutation (22). Because this frequency is high relative to typical microbial population sizes, cheats would thus likely arise and reach high frequencies shortly after the appearance of unicellular somatic differentiation, explaining the absence of extant species with this life strategy. The short persistence time of unicellular differentiating species makes them an unlikely intermediate in the evolution of development relative to undifferentiated multicellular species, which have persisted for hundreds of millions to billions of years in some clades (1–3). We cannot, however, rule out the possibility that the transient existence of a unicellular differentiating species might suffice for the secondary evolution of multicellularity, which has been observed experimentally in small populations on the timescale of weeks (16, 31). In any case, the differentiating phenotype cannot be stably maintained against cheats until clonal multicellularity evolves.
Our study demonstrates that synthetic biology can directly test hypotheses about evolutionary transitions, complementing retrospective inference through comparison of existing species, experimental evolution, mathematical modeling, and simulation. We note that other major evolutionary transitions, including the appearance of body plans (spatially ordered arrangements of cell types) and life cycles (temporal sequences of growth and dispersion), could be studied through experimental evolution or further engineering of the strains described above. Furthermore, our differentiating strain provides an experimentally tractable version of a common simplifying assumption in population genetics: independent deleterious mutations are modeled as having the same fitness cost (32). In our strains, cyh2r excision is a form of irreversible mutation that always produces the same fitness disadvantage, but the magnitude of the fitness cost can be experimentally varied by altering the cycloheximide concentration. Thus, engineered organisms, including those we have developed, can permit robust experimental testing of a wide variety of outstanding hypotheses in evolutionary biology.
Materials and Methods
Conversion Time Courses and Conversion Rate Assays.
We followed the dynamics of expression of fluorescent proteins during conversion from germ line to soma by adding β-estradiol at the indicated concentration to cultures of pure germ cells (yMEW192), which were pregrown in log phase in YPD [yeast extract, peptone, dextrose (glucose)]. Cultures were maintained in log phase for additional growth while samples were collected for analysis by flow cytometry. At the indicated time point, cultures were washed twice with PBS to remove β-estradiol and resuspended in YPD for additional growth before a final flow cytometry analysis. More details of the design and data from this experiment are shown in Fig. S1.
We measured the rate of germ line to soma conversion by adding β-estradiol to pure cultures of germ cells (yMEW192) pregrown in log phase in YPD (Fig. S2A). Pure cultures of germ cells were maintained in parallel to permit calculation of the number of germ cell generations elapsed through cell density measurement with a Coulter Counter (Beckman Coulter). Samples collected at indicated time points were washed twice in PBS solution to remove β-estradiol and then resuspended in YPD for continued growth, allowing recently converted somatic cells to dilute out remaining mCherry so that cell types could be unambiguously distinguished by flow cytometry. Linear regression of the log fraction of cells that were germ cells vs. the number of germ cell generations elapsed was used to determine a 95% CI of the conversion rate for each experiment using the fit and confint functions in MATLAB (Mathworks). The conversion rates reported in Fig. 1E correspond to the weighted arithmetic mean and corresponding variance calculated with data obtained from three or more independent experiments. More details of the design and data from this experiment are shown in Fig. S2.
Fitness Assays for Relative Growth Difference Between Germ and Somatic Cells.
The growth rate difference between cell types was determined using a fitness assay protocol described previously (33). Briefly, pure cultures of germ (yMEW192) and somatic (yMEW192 convertant) cells pregrown in log phase in YPD containing cycloheximide were combined and maintained in log phase through multiple growth and dilution cycles in the indicated media (Fig. S2B). Samples taken at each dilution step were used to determine the fraction of each cell type by flow cytometry. Pure cultures of germ (yMEW192) cells were maintained in log phase in parallel to determine the number of germ cell generations elapsed through cell density measurements with a Beckman Coulter Counter. The 95% CI for the slope of the linear regression line of log(somatic cells/germ cells) vs. the number of germ cell generations elapsed was determined as described above. The growth rate differences reported in Fig. 1F represent the weighted arithmetic means and corresponding variances of the slopes calculated in three independent experiments.
Steady-State Ratio Assays.
Pure cultures of germ cells (yMEW192) and somatic cells (yMEW192) were mixed to initiate cultures from a range of cell type ratios. Cultures were washed with PBS and resuspended in YPD containing β-estradiol and cycloheximide at the indicated concentrations. Samples were taken at the indicated time points to determine the number of culture doublings since initiation (determined from Coulter Counter measurements of culture density) and the fraction of somatic (mCitrine+ mCherry−) cells in the culture. Cultures typically converged on a steady-state ratio after 30–40 culture doublings.
Growth Rate Assays in Sucrose Minimal Media.
Cultures of converting strains at their steady-state ratios were washed twice with PBS and resuspended at ∼105 cells/mL in minimal media containing 0.5% sucrose and the concentrations of cycloheximide and β-estradiol in which they had been growing previously. Pure cultures of the Cre− strain yMEW193 and the yMEW192 convertant were also used to represent pure cultures of germ and somatic cells of the differentiating strain, respectively. After an acclimation period of approximately 10 h, culture density measurements were taken regularly with a Coulter Counter, and a 95% CI of the slope determined by linear regression of log(culture density) vs. time. The reported growth rates (Fig. 2C) were obtained from the weighted arithmetic means of slopes and their corresponding variances estimated from three or more independent experiments.
Determination of the Distribution of Cell Type Composition in Clumps.
Cultures of Δcts1 strains growing in minimal media containing 0.5% sucrose and the indicated concentrations of cycloheximide and β-estradiol were analyzed by flow cytometry. Clumps in these cultures typically ranged in size from 4 to 30 clumps; forward and side scatter gating could therefore not be used to eliminate events consisting of two or more clumps. Instead, cultures were diluted and vortexed thoroughly before flow cytometry to reduce the probability of multiple clumps being counted as a single event. The efficacy of this strategy was checked by combining pure cultures of germ and somatic cells in the absence of β-estradiol and confirming that the fraction of mCherry+ mCitrine+ events in the mixed culture was less than 1%. Clumps containing only germ cells had negligible fluorescence in the mCitrine channel and thus formed a distinguishable population; likewise, clumps containing only somatic cells had negligible fluorescence in the mCherry channel. Clumps with nonnegligible fluorescence in both channels were considered to contain both cell types.
Competition Assays.
In competition assays, Cerulean+ differentiating strains (unicellular: yMEW192; multicellular: yMEW208) were mixed with Cerulean− Cre− reference strains (unicellular: yMEW193; multicellular: yMEW209) at a 1:1 ratio and passaged through five cycles of growth and dilution in indicated media. Samples taken at each dilution step were analyzed by flow cytometry to determine the ratio between Cerulean+ and Cerulean− events (cells or clumps). The 95% CI of the slope of the linear regression line of log(Cerulean+ events/Cerulean− events) vs. the number of culture doublings elapsed was determined as described above. The growth advantages of the differentiating strain reported in Fig. 4B represent the weighted arithmetic means and corresponding variances of the slopes calculated in three or more independent experiments.
Additional materials and methods are provided as SI Text. Strain genotypes are found in Table S1, plasmid details in Table S2.
Table S1.
Transgenic yeast strains used in this study
| Strain name | Genotype description |
| yMEW17 | W303 MATa BUD4 bar1 leu2-3,112 can1-100 ura3-1 ade2-1 his3-11,15 trp1-1 |
| yMEW192 | yMEW17 TRP1 HIS3 Δho::PSCW11-cre-EBD78::NATMX4 Δleu2::HPHMX4::PACT1-yCerulean-TADH1 |
| ADE2::PENO2-AI-ymCherry-UBQ-cyh2r-TCYH2-AI-ymCitrine-UBQ-SUC2-TSUC2 | |
| yMEW192 | yMEW17 TRP1 HIS3 Δho::PSCW11-cre-EBD78::NATMX4 Δleu2::HPHMX4::PACT1-yCerulean-TADH1 |
| Convertant | ADE2::PENO2-AI-ymCitrine-UBQ-SUC2-TSUC2 |
| yMEW193 | yMEW17 TRP1 HIS3 ADE2::PENO2-AI-ymCherry-UBQ-cyh2r-TCYH2-AI-ymCitrine-UBQ-SUC2-TSUC2 |
| yMEW208 | yMEW17 TRP1 Δho::PSCW11-cre-EBD78::NATMX4 Δleu2::HPHMX4::PACT1-yCerulean-TADH1 |
| ADE2::PENO2-AI-ymCherry-UBQ-cyh2r-TCYH2-AI-ymCitrine-UBQ-SUC2-TSUC2 Δcts1::HIS3 | |
| yMEW209 | yMEW17 TRP1 ADE2::PENO2-AI-ymCherry-UBQ-cyh2r-TCYH2-AI-ymCitrine-UBQ-SUC2-TSUC2 Δcts1::HIS3 |
Table S2.
Plasmids used in the construction of transgenic yeast strains
| Plasmid | Base vector | Insert | Strains with insert integrated by homologous recombination |
| pDL12 | From ref. 17 | PSCW11-cre-EBD78-TCYC1 | — |
| pMEW54 | pRS402 | AI-SUC2-TSUC2 | — |
| pMEW56 | pFA6a-HIS3MX6 | AI-ymCherry-TADH1 | — |
| pMEW61 | pFA6a-HIS3MX6 | PACT1-AI-ymCherry-TADH1 | — |
| pMEW63 | pFA6a-HIS3MX6 | PACT1-AI-ymCherry-TADH1-AI-SUC2-TSUC2 | — |
| pMEW67 | pFA6a-HIS3MX6 | PACT1-AI-cyh2r-TCYH2 | — |
| pMEW71 | pRS402 | 5′SUC2 | — |
| pMEW73 | pRS402 | 5′SUC2 PACT1-AI-ymCherry-TADH1-AI-SUC2-TSUC2 | — |
| pMEW76 | pFA6a-HIS3MX6 | PENO2-AI-cyh2r-TCYH2 | — |
| pMEW81 | pFA6a-HIS3MX6 | AI-ymCherry-UBQ-cyh2r-TCYH2 | — |
| pMEW82 | pRS402 | 5′SUC2 PENO2-AI-ymCherry-UBQ-cyh2r-TCYH2-AI-SUC2-TSUC2 | — |
| pMEW83 | pFA6a-HIS3MX6 | AI-ymCitrine-UBQ-cyh2r-TCYH2 | — |
| pMEW84 | pFA6a-HIS3MX6 | AI-ymCitrine-UBQ-SUC2-TSUC2 | — |
| pMEW90 | pRS402 | 5′SUC2 PENO2-AI-ymCherry-UBQ-cyh2r-TCYH2-AI-ymCitrine-UBQ-SUC2-TSUC2 | yMEW192 |
| yMEW193 | |||
| yMEW208 | |||
| yMEW209 |
SI Text
Plasmid Construction.
A complete list of plasmids used in this study is given in Table S1; plasmid maps and sequences are available on request. Constructs were cloned using standard methods (35, 36) and common base vectors (37, 38). Yeast-optimized sequences encoding the fluorescent proteins Cerulean (39), mCitrine (40), and mCherry (41) (here denoted yCerulean, ymCitrine, and ymCherry) were generously provided by Nicholas Ingolia, Molecular and Cellular Biology, University of California, Berkeley, CA.
loxP, the binding site of Cre recombinase, is 34 bp long and contains inverted repeats (42) that can interfere with PCR-based cloning strategies. We reasoned that inclusion of this inverted repeat within a transcript could also form a hairpin or otherwise interfere with translation. We therefore introduced the loxP sequence into a yeast artificial intron (43) to facilitate cloning. The loxP-containing artificial intron (AI) was then added to the 5′ end of the ymCherry ORF and the ADH1 terminator by fusion PCR and cloned into the base vector pFA6a-HIS3MX6 to generate pMEW56. The constitutive actin promoter PACT1 was then introduced into pMEW56 by ligation-independent cloning, producing pMEW61.
A truncated version of the artificial intron that lacked the 5′ splice site was added to the 5′ end of the SUC2 ORF and terminator by fusion PCR and cloned into pRS402 to generate pMEW54. The AI-SUC2-TSUC2 amplicon was then cloned into pMEW61 by restriction digestion and ligation to produce pMEW63.
Constructs introduced by conventional plasmid integration are subject to spontaneous loop-out or increase in copy number; our application required the introduction of exactly one copy of the construct, motivating the development of an integration strategy based on gene replacement (44). Both constructs of interest (Fig. 1C) contained sequences homologous to the SUC2 ORF and terminator at their 3′ ends. We therefore designed a base vector containing sequence homologous to the 5′ end of the SUC2 locus so that appropriate restriction digestions would release a fragment that would interact at both ends of the SUC2 locus, replacing the SUC2 gene with the engineered DNA. Three hundred base pairs of the SUC2 promoter region were therefore introduced into the base vector pRS402 5′ to its ADE2 marker by ligation-independent cloning to create pMEW71. The PACT1-AI-ymCherry-TADH1-AI-SUC2-TSUC2 fragment of pMEW63 was then cloned into pMEW71 3′ to the ADE2 marker such that a Bsu36I/BamHI digestion fragment of the resulting plasmid (pMEW73) would contain the ADE2 marker, the construct of interest, and homology to the SUC2 locus at either end to direct homologous recombination.
An intron-free version of the ribosomal protein L28-encoding gene CYH2 containing a previously described (23) mutation conferring cycloheximide resistance, cyh2-N38K (cyh2r), was produced by fusion and site-specific mutagenic PCR. This construct was then introduced in place of ymCherry-TADH1 in pMEW61 to generate pMEW67. To strengthen the cycloheximide resistance conferred by this codominant allele (45), the actin promoter was replaced with the stronger constitute enolase promoter PENO2 (46) by ligation-independent cloning, generating pMEW76.
To express a fluorescent marker and a second protein from a single ORF with minimal disruption of localization or function, a ubiquitin moiety (UBQ) linker was included in both gene fusions (ymCherry-UBQ-cyh2r and ymCitrine-UBQ-SUC2). Cleavage at the C terminus of this linker by native ubiquitin-specific proteases (34) after translation produces two peptides: a fluorescent protein with C-terminal ubiquitin moiety (e.g., ymCherry-Ubq and ymCitrine-Ubq), which does not significantly impact its stability, and the native form of the second protein (e.g., Cyh2r and Suc2 with no N-terminal tags). The first ubiquitin moiety ofUBI4 was amplified and fused to the 3′ end of the AI-mCherry construct and then introduced into pMEW76 by ligation-independent cloning to generate pMEW81. PENO2 was then added to the 5′ end of the AI-ymCherry-UBQ-cyh2r-TCYH2 construct by fusion PCR, and the resulting amplicon was introduced into pMEW73 by restriction digest to create pMEW82.
An AI-ymCitrine-UBQ-SUC2-TSUC2 construct was generated by replacing the ymCherry ORF in pMEW81 with ymCitrine by ligation-independent cloning (producing the intermediate plasmid pMEW83) and then replacing cyh2r-TCYH2 with SUC2-TSUC2 by ligation-independent cloning to generate pMEW84. The AI-ymCitrine-UBQ-SUC2-TSUC2 fragment was then introduced into pMEW82 by restriction digest to create pMEW90, which contains the complete construct used to produce the cyh2r excision strain.
Strain Construction.
A complete list of strains used in this study is given in Table S2. All constructs and markers were introduced via homologous recombination using a standard polyethylene glycol, lithium acetate, and Tris-EDTA yeast transformation protocol (35); integration at the desired loci was confirmed by diagnostic PCR. All strains are constructed in a W303 background in which a loss-of-function mutation in BUD4 has been corrected as previously described (26, 47).
The β-estradiol–inducible Cre construct previously described by Lindstrom et al. (24) was introduced by homologous recombination at the HO locus. The PACT1-yCerulean construct was amplified by PCR and integrated at the LEU2 locus. The insert of pMEW90 was released by Bsu36I/BamHI digestion before transformation. The CTS1 ORF was deleted by adding 40 bp of CTS1 locus homology to each end of the HIS3MX6 marker [containing the ORF of Schizosaccharomyces pombe gene HIS5, which complements S. cerevisiae his3 mutations (48)] by PCR amplification before transformation. yMEW192 convertants were isolated by diluting and plating cultures of yMEW192, which had previously been grown in media containing β-estradiol to induce conversion.
Media and Growth Conditions.
Minimal media and YPD media were produced as described elsewhere (26). β-estradiol (Sigma-Aldrich E8875) and cycloheximide (Sigma Aldrich C7698) were resuspended at 1 mM in ethanol and stored at −80 °C for less than 1 y; media containing these reagents were stored less than 1 wk at room temperature. Shaken liquid cultures and agar plates were grown at 30 °C. Sucrose was dissolved in solution without heating and stored at 4 °C less than 1 mo to limit spontaneous hydrolysis.
Fluorescence Microscopy.
Still images and time-lapse videos were collected at room temperature using an Eclipse Ti-E inverted microscope (Nikon Instruments) with a 60× Plan Apo VC 1.4NA oil objective, a Photometrics CoolSNAP HQ camera (Roper Scientific), and appropriate filters for yeast-optimized Cerulean (ex = 436/20 nm, em = 480/40 nm), mCitrine (ex = 500/20 nm, em = 535/30 nm), and mCherry (ex = 562/40 nm, em = 641/75 nm) fluorescent proteins. The image processing program Fiji (49) was used to produce composite images and generate video files.
Time-lapse videos of monolayer growth were collected using a CellASICs Y04C ONIX Live Cell Imaging microfluidics flow chamber (EMD Millipore) pretreated by perfusion of Con A solution (1 mg/mL Con A, 1 mM CaCl2, 10 mM NaHPO4 at pH 6) for 5 min at 2 psi, followed by washout with growth media for 5 min at 2 psi. Cells of a late log-phase culture (∼5 × 106 cells/mL) were loaded into the chamber at 5 psi for 10 s. Metamorph 7.7 (Molecular Devices) with the Nikon Perfect Focus System (Nikon Instruments) was used to acquire images at multiple stage positions at exposure times. For videos, fluorescence and bright-field images were collected at multiple stage positions at 15-min intervals.
Flow Cytometry.
An LSR Fortessa (BD Biosciences) with appropriate filters for yeast-optimized Cerulean (ex = 440 nm, em = 470/20 nm), mCitrine (ex = 488 nm, em = 530/30 nm), and mCherry (ex = 561 nm, em = 610/20 nm) was used to perform flow cytometry on well-vortexed samples of culture samples. For unicellular culture samples, appropriate forward and side scatter gating were used to eliminate events corresponding to multiple cells.
Supplementary Material
Acknowledgments
We thank Derek Lindstrom and Dan Gottschling for providing the estradiol-inducible Cre construct PSCW11-cre-EBD78; Alex Schier, Michael Desai, Michael Laub, Cassandra Extavour, David Haig, and members of the A.W.M. and David Nelson laboratories for helpful discussions during manuscript preparation; and Beverly Neugeboren, Linda Kefalas, and Sara Amaral for research support. This work was supported in part by National Science Foundation and Department of Defense National Defense Science and Engineering graduate research fellowships and by NIH/National Institute of General Medical Sciences Grant GM068763.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1608278113/-/DCSupplemental.
References
- 1.Grosberg RK, Strathmann RR. The evolution of multicellularity: A minor major transition? Annu Rev Ecol Evol Syst. 2007;38(1):621–654. [Google Scholar]
- 2.Herron MD, Hackett JD, Aylward FO, Michod RE. Triassic origin and early radiation of multicellular volvocine algae. Proc Natl Acad Sci USA. 2009;106(9):3254–3258. doi: 10.1073/pnas.0811205106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schirrmeister BE, Antonelli A, Bagheri HC. The origin of multicellularity in cyanobacteria. BMC Evol Biol. 2011;11:45. doi: 10.1186/1471-2148-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirk DL. A twelve-step program for evolving multicellularity and a division of labor. BioEssays. 2005;27(3):299–310. doi: 10.1002/bies.20197. [DOI] [PubMed] [Google Scholar]
- 5.Bell G, Mooers AO. Size and complexity among multicellular organisms. Biol J Linn Soc Lond. 1997;60(3):345–363. [Google Scholar]
- 6.Bonner JT. On the origin of differentiation. J Biosci. 2003;28(4):523–528. doi: 10.1007/BF02705126. [DOI] [PubMed] [Google Scholar]
- 7.Solari CA, Kessler JO, Goldstein RE. A general allometric and life-history model for cellular differentiation in the transition to multicellularity. Am Nat. 2013;181(3):369–380. doi: 10.1086/669151. [DOI] [PubMed] [Google Scholar]
- 8.Willensdorfer M. On the evolution of differentiated multicellularity. Evolution. 2009;63(2):306–323. doi: 10.1111/j.1558-5646.2008.00541.x. [DOI] [PubMed] [Google Scholar]
- 9.Hom EF, Murray AW. Plant-fungal ecology. Niche engineering demonstrates a latent capacity for fungal-algal mutualism. Science. 2014;345(6192):94–98. doi: 10.1126/science.1253320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hekstra DR, Leibler S. Contingency and statistical laws in replicate microbial closed ecosystems. Cell. 2012;149(5):1164–1173. doi: 10.1016/j.cell.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 11.Bell G. The origin and early evolution of germ cells as illustrated by the volvocales. In: Monroy A, Halvorson HO, editors. The Origin and Evolution of Sex (MBL Lectures in Biology) A. R. Liss; New York: 1985. pp. 221–256. [Google Scholar]
- 12.Buss LW, Mayr Ea. The Evolution of Individuality. Princeton Univ Press; Princeton: 1987. [Google Scholar]
- 13.Goldsby HJ, Knoester DB, Ofria C, Kerr B. The evolutionary origin of somatic cells under the dirty work hypothesis. PLoS Biol. 2014;12(5):e1001858. doi: 10.1371/journal.pbio.1001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith JM, Price GR. The logic of animal conflict. Nature. 1973;246(5427):15–18. [Google Scholar]
- 15.Smith JM. Group selection and kin selection. Nature. 1964;201(4924):1145–1147. [Google Scholar]
- 16.Ratcliff WC, Denison RF, Borrello M, Travisano M. Experimental evolution of multicellularity. Proc Natl Acad Sci USA. 2012;109(5):1595–1600. doi: 10.1073/pnas.1115323109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratcliff WC, et al. Experimental evolution of an alternating uni- and multicellular life cycle in Chlamydomonas reinhardtii. Nat Commun. 2013;4:2742. doi: 10.1038/ncomms3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilbert OM, Foster KR, Mehdiabadi NJ, Strassmann JE, Queller DC. High relatedness maintains multicellular cooperation in a social amoeba by controlling cheater mutants. Proc Natl Acad Sci USA. 2007;104(21):8913–8917. doi: 10.1073/pnas.0702723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Momeni B, Waite AJ, Shou W. Spatial self-organization favors heterotypic cooperation over cheating. eLife. 2013;2:e00960. doi: 10.7554/eLife.00960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greig D, Travisano M. The Prisoner’s Dilemma and polymorphism in yeast SUC genes. Proc Biol Sci. 2004;271(Suppl 3):S25–S26. doi: 10.1098/rsbl.2003.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gore J, Youk H, van Oudenaarden A. Snowdrift game dynamics and facultative cheating in yeast. Nature. 2009;459(7244):253–256. doi: 10.1038/nature07921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davidson EH. The Regulatory Genome: Gene Regulatory Networks in Development and Evolution. Elsevier/Academic Press; Amsterdam: 2006. [Google Scholar]
- 23.Stöcklein W, Piepersberg W, Böck A. Amino acid replacements in ribosomal protein YL24 of Saccharomyces cerevisiae causing resistance to cycloheximide. FEBS Lett. 1981;136(2):265–268. doi: 10.1016/0014-5793(81)80632-1. [DOI] [PubMed] [Google Scholar]
- 24.Lindstrom DL, Gottschling DE. The mother enrichment program: A genetic system for facile replicative life span analysis in Saccharomyces cerevisiae. Genetics. 2009;183(2):413–422. doi: 10.1534/genetics.109.106229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Queller DC. Relatedness and the fraternal major transitions. Philos Trans R Soc Lond B Biol Sci. 2000;355(1403):1647–1655. doi: 10.1098/rstb.2000.0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koschwanez JH, Foster KR, Murray AW. Sucrose utilization in budding yeast as a model for the origin of undifferentiated multicellularity. PLoS Biol. 2011;9(8):e1001122. doi: 10.1371/journal.pbio.1001122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koschwanez JH, Foster KR, Murray AW. Improved use of a public good selects for the evolution of undifferentiated multicellularity. eLife. 2013;2:e00367. doi: 10.7554/eLife.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dohrmann PR, et al. Parallel pathways of gene regulation: Homologous regulators SWI5 and ACE2 differentially control transcription of HO and chitinase. Genes Dev. 1992;6(1):93–104. doi: 10.1101/gad.6.1.93. [DOI] [PubMed] [Google Scholar]
- 29.Kuranda MJ, Robbins PW. Chitinase is required for cell separation during growth of Saccharomyces cerevisiae. J Biol Chem. 1991;266(29):19758–19767. [PubMed] [Google Scholar]
- 30.Lang GI, Murray AW. Estimating the per-base-pair mutation rate in the yeast Saccharomyces cerevisiae. Genetics. 2008;178(1):67–82. doi: 10.1534/genetics.107.071506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boraas M, Seale D, Boxhorn J. Phagotrophy by a flagellate selects for colonial prey: A possible origin of multicellularity. Evol Ecol. 1998;12(2):153–164. [Google Scholar]
- 32.Haldane JBS. The effect of variation of fitness. Am Nat. 1937;71(735):337–349. [Google Scholar]
- 33.Lang GI, Botstein D, Desai MM. Genetic variation and the fate of beneficial mutations in asexual populations. Genetics. 2011;188(3):647–661. doi: 10.1534/genetics.111.128942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bachmair A, Finley D, Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986;234(4773):179–186. doi: 10.1126/science.3018930. [DOI] [PubMed] [Google Scholar]
- 35.Adams A, Cold Spring Harbor L. Methods in Yeast Genetics: a Cold Spring Harbor Laboratory Course Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1997. [Google Scholar]
- 36.Li MZ, Elledge SJ. SLIC: A method for sequence- and ligation-independent cloning. Methods Mol Biol. 2012;852:51–59. doi: 10.1007/978-1-61779-564-0_5. [DOI] [PubMed] [Google Scholar]
- 37.Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Longtine MS, et al. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14(10):953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 39.Rizzo MA, Springer GH, Granada B, Piston DW. An improved cyan fluorescent protein variant useful for FRET. Nat Biotechnol. 2004;22(4):445–449. doi: 10.1038/nbt945. [DOI] [PubMed] [Google Scholar]
- 40.Sheff MA, Thorn KS. Optimized cassettes for fluorescent protein tagging in Saccharomyces cerevisiae. Yeast. 2004;21(8):661–670. doi: 10.1002/yea.1130. [DOI] [PubMed] [Google Scholar]
- 41.Shaner NC, et al. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22(12):1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- 42.Abremski K, Hoess R. Bacteriophage P1 site-specific recombination. Purification and properties of the Cre recombinase protein. J Biol Chem. 1984;259(3):1509–1514. [PubMed] [Google Scholar]
- 43.Yoshimatsu T, Nagawa F. Control of gene expression by artificial introns in Saccharomyces cerevisiae. Science. 1989;244(4910):1346–1348. doi: 10.1126/science.2544026. [DOI] [PubMed] [Google Scholar]
- 44.Orr-Weaver TL, Szostak JW, Rothstein RJ. Yeast transformation: A model system for the study of recombination. Proc Natl Acad Sci USA. 1981;78(10):6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fried HM, Warner JR. Molecular cloning and analysis of yeast gene for cycloheximide resistance and ribosomal protein L29. Nucleic Acids Res. 1982;10(10):3133–3148. doi: 10.1093/nar/10.10.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hauf J, Zimmermann FK, Müller S. Simultaneous genomic overexpression of seven glycolytic enzymes in the yeast Saccharomyces cerevisiae. Enzyme Microb Technol. 2000;26(9-10):688–698. doi: 10.1016/s0141-0229(00)00160-5. [DOI] [PubMed] [Google Scholar]
- 47.Voth WP, Olsen AE, Sbia M, Freedman KH, Stillman DJ. ACE2, CBK1, and BUD4 in budding and cell separation. Eukaryot Cell. 2005;4(6):1018–1028. doi: 10.1128/EC.4.6.1018-1028.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wach A, Brachat A, Alberti-Segui C, Rebischung C, Philippsen P. Heterologous HIS3 marker and GFP reporter modules for PCR-targeting in Saccharomyces cerevisiae. Yeast. 1997;13(11):1065–1075. doi: 10.1002/(SICI)1097-0061(19970915)13:11<1065::AID-YEA159>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 49.Schindelin J, et al. Fiji: An open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.