Significance
The biological nitrogen fixation process responsible for the reduction of atmospheric nitrogen to ammonia represents the primary source of nitrogen supporting extant life. We have identified a novel noncoding RNA (ncRNA) in the Pseudomonas stutzeri core genome, called NfiS, that is involved in the stress response and controls the expression of genes located in a genomic nitrogen-fixing (nif) island. NfiS was found to optimize nitrogen fixation by the direct posttranscriptional regulation of nitrogenase gene nifK mRNA. The acquisition of the nif island and the recruitment of NfiS by nifK mRNA are evolutionary events that seem to contribute to fine-tuned regulation of nitrogenase activity in P. stutzeri. This study provides a new regulatory pathway, mediated by an ncRNA for optimal nitrogen fixation, that may operate in other diazotrophs.
Keywords: nitrogen fixation, Pseudomonas stutzeri, regulatory ncRNA, NfiS, nifK mRNA
Abstract
Unlike most Pseudomonas, the root-associated bacterium Pseudomonas stutzeri A1501 fixes nitrogen after the horizontal acquisition of a nitrogen-fixing (nif) island. A genome-wide search for small noncoding RNAs (ncRNAs) in P. stutzeri A1501 identified the novel P. stutzeri-specific ncRNA NfiS in the core genome, whose synthesis was significantly induced under nitrogen fixation or sorbitol stress conditions. The expression of NfiS was RNA chaperone Hfq-dependent and activated by the sigma factor RpoN/global nitrogen activator NtrC/nif-specific activator NifA regulatory cascade. The nfiS-deficient mutant displayed reduced nitrogenase activity, as well as increased sensitivity to multiple stresses, such as osmotic and oxidative stresses. Secondary structure prediction and complementation studies confirmed that a stem-loop structure was essential for NfiS to regulate the nitrogenase gene nifK mRNA synthesis and thus nitrogenase activity. Microscale thermophoresis and physiological analysis showed that NfiS directly pairs with nifK mRNA and ultimately enhances nitrogenase activity by increasing the translation efficiency and the half-life of nifK mRNA. Our data also suggest structural and functional divergence of NfiS evolution in diazotrophic and nondiazotrophic backgrounds. It is proposed that NfiS was recruited by nifK mRNA as a novel regulator to integrate the horizontally acquired nif island into host global networks.
A number of noncoding RNAs (ncRNAs) (also called sRNAs) have been identified in the past years in Escherichia coli and other bacterial species, including members of the Pseudomonas genus (1–4). These RNAs were found to play a role at the posttranscriptional level, being involved in the regulation of diverse physiological processes, such as stress responses, virulence, motility, biofilm formation, quorum sensing, and metabolic control (5–14). In addition to cis-encoded antisense RNAs, many transencoded RNAs exert their regulatory roles through base pairing with the complementary mRNA target, which involves the chaperone protein Hfq to mediate the sRNA–mRNA interaction (4, 14, 15). However, the presence of regulatory ncRNAs has not previously been investigated in Pseudomonas stutzeri.
The Pseudomonas genus constitutes a wide group of species that colonize diverse niches, including plant and animal pathogens as well as nonpathogenic species from water, soil, plant rhizospheres, and marine environments. They play important roles in the nitrogen and carbon cycles (16). However, the biological nitrogen fixation ability, a process by which bacteria reduce molecular N2 gas to ammonia, is rarely encountered in this genus (16–18). It is now established that nitrogen-fixing strains can be found within the P. stutzeri species. Determination of the P. stutzeri A1501, DSM4166, and BAL398 genome nucleotide sequences led to the identification of a conserved, 49-kb nitrogen-fixing island acquired by horizontal gene transfer (HGT) in these three strains (17–20). A recent in silico analysis revealed that the island is also conserved in other available genomes of nitrogen-fixing P. stutzeri (ref. 21 and references therein). The regulation of nif gene transcription in most diazotrophs depends on the sigma factor RpoN/global nitrogen activator NtrC/nif-specific activator NifA regulatory cascade (22). P. stutzeri A1501, which is isolated from the rice rhizosphere in China, is the best studied strain regarding nitrogen fixation. It possesses a general nitrogen regulatory system in the core genome (NtrBC and related genes) (23–25), but, during its evolution, it acquired a nif-specific regulatory system (NifLA) (25) from a diazotrophic ancestor. Thus, the regulatory network controlling nitrogen fixation in A1501 may result from regulatory systems of different evolutionary origins. Colonization of the plant rhizosphere implies that nitrogen-fixing P. stutzeri strains adapt to a range of physiological conditions, such as environmental stresses and nutrient availability (18, 23, 26). Despite the identification of a number of genes potentially involved in rhizosphere competence in the A1501 genome, little is known about the mechanisms of adaptation and survival in the rhizosphere (17). A search for ncRNAs in other nitrogen-fixing organisms was reported (5, 6, 14, 27–29), but none have been described so far as being involved in the regulation of nif gene expression.
A search for ncRNAs in the genome of P. stutzeri A1501 was performed (26), and we now report on the identification of a novel RNA, named NfiS, specific for P. stutzeri strains, involved in the control of nitrogen fixation and the response to oxidative and osmotic stresses. This ncRNA controlled nitrogenase activity directly by the posttranscriptional regulation of nifK mRNA and also indirectly through the induction of the RpoN/NtrC/NifA regulatory cascade via unidentified mechanisms. Further analysis showed that the nfiS genes in diazotrophic and nondiazotrophic P. stutzeri exhibited remarkable differences in secondary structure and regulatory function and evolved in different ways due to different niches and selection pressures. During evolution of P. stutzeri, NfiS was apparently recruited by nifK mRNA as a novel activator to optimize nitrogenase activity in response to specific environmental cues. It is hypothesized that, although not identical to NfiS, the involvement of ncRNAs in the regulation of nitrogen fixation is likely to represent a novel regulatory mechanism that is widely distributed among nitrogen-fixing bacteria.
Results
NfiS Is Hfq-Dependent and Activated by the RpoN/NtrC/NifA Cascade.
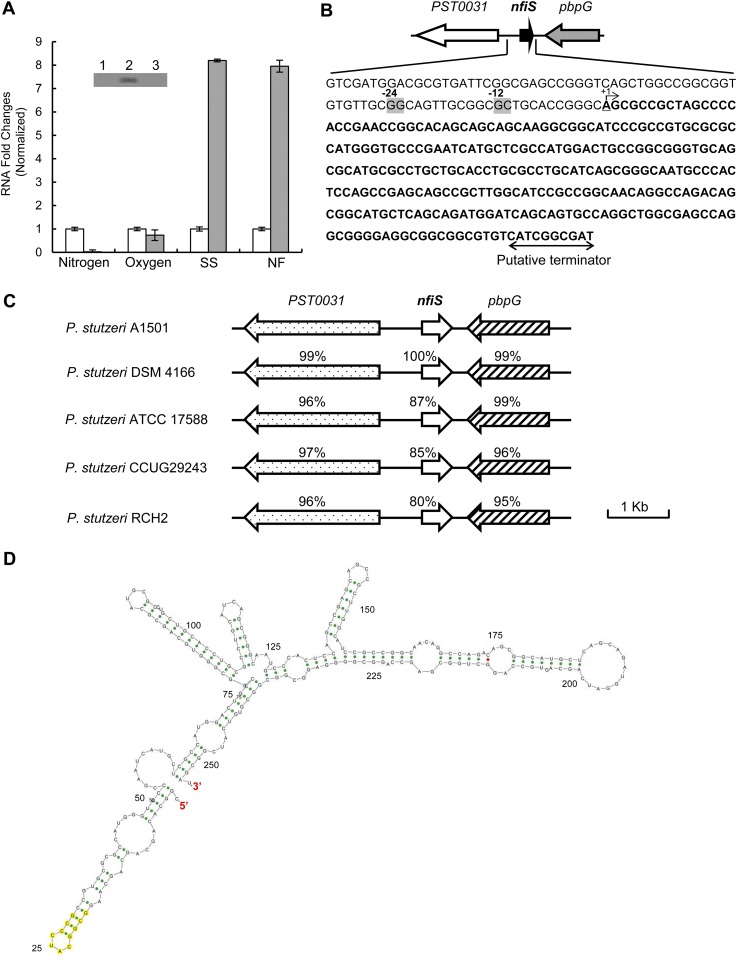
Preliminary experiments revealed that ncRNA01 expression was strongly induced both under conditions of nitrogen fixation and in response to osmotic stress (Fig. S1A). This finding led us to investigate the role of ncRNA01 in the regulation of nitrogen fixation. We therefore renamed this ncRNA the “nitrogen fixation condition-inducible small ncRNA” (NfiS). The nfiS gene (254 nt) is flanked by a gene encoding a low-molecular-weight penicillin-binding protein and a gene encoding a TonB-dependent receptor (Fig. S1B). Furthermore, the gene is conserved in other P. stutzeri strains whose genomes are available and in particular showed 100% identity with the corresponding gene in DSM4166 (19), another nitrogen-fixing strain of P. stutzeri (Fig. S1C). Because we did not identify homologs in any other bacterial species by BLAST search and did not find any match to the RNA families in the Rfam database (rfam.xfam.org), NfiS is likely a novel ncRNA specific to P. stutzeri.
Fig. S1.
Identification and characterization of the P. stutzeri A1501 NfiS. (A) Quantitative RT-PCR analysis of the relative expression level of NfiS under four different physiological conditions: (i) Nitrogen availability (Nitrogen): Bacteria were grown in minimal medium K without added nitrogen under aerobic conditions (gray bar) vs. growth in nitrogen-sufficient conditions (6 mM NH4+; 20% oxygen tension) (open bar); (ii) oxygen level (Oxygen): Microaerobic condition (6 mM NH4+; 0.5% oxygen tension) (gray bar) vs. aerobic conditions (open bar); (iii) sorbitol shock (SS): LB medium supplemented with 0.3 M sorbitol (gray bar) vs. without sorbitol (open bar); (iv) nitrogen fixation (NF): Nitrogen fixation conditions (nitrogen-free; 0.5% oxygen tension) (gray bar) vs. aerobic growth conditions (6 mM ammonium and 20% oxygen tension) (open bar). (Inset) Northern blot detection of the NfiS RNA. Total RNA was extracted from WT strain A1501 under nitrogen excess conditions (lane 1), nitrogen fixation conditions (lane 2), or ammonium-shock conditions (lane 3). (B) Physical map and nucleotide sequence of the nfiS region of P. stutzeri A1501. (gray boxes) Predicted RpoN-binding site. +1, transcription start site mapped by 5′ RACE; arrowheads, putative transcriptional terminator. (C) Genomic organization of the A1501 nfiS gene and comparison with equivalent loci from other Pseudomonas strains. The nfiS sequence is present in all sequenced P. stutzeri strains and shows conservation in both the 5′ and 3′ ends of the genes. The numbers underneath the arrows indicate the percentage of amino acid sequence identity between the A1501 NfiS product and its homologs. (D) Secondary structure of NfiS. The 11-nucleotide sequence predicted to be complementary to nifK mRNA is highlighted in yellow.
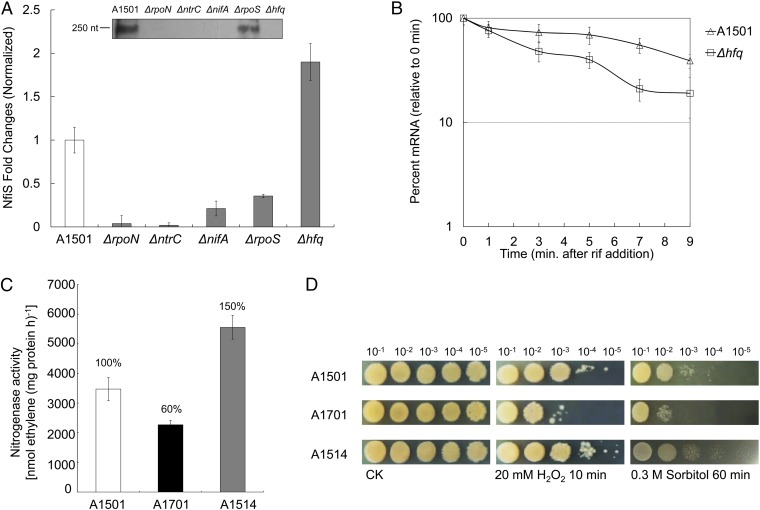
The determination of the nfiS transcription initiation site (Fig. S1B) showed that the transcript starts from an adenine residue, which is the 13th nucleotide downstream from the conserved C residue of the predicted element located at position −12 of the putative RpoN-dependent promoter (GG-N10-GC) (22). No binding site for NtrC or NifA was identified. A band corresponding to the migration of a 250-nt molecule was detected by Northern blot analysis in the WT strain A1501, but it was not detected when the analysis was performed with RNA extracted from deletion mutants for rpoN, ntrC, or nifA under nitrogen fixation conditions (Fig. 1A, Inset, lanes 1–4), which are involved in the regulation of nitrogen fixation (17, 18, 24, 25). The band intensity was decreased when the analysis was performed with RNA extracted from an rpoS deletion mutant, known in other systems as the RNA polymerase sigma factor of the general stress response (30) (Fig. 1A, Inset, lane 5). These results suggested that NfiS transcription is RpoN-dependent and under the control of NtrC, NifA, and RpoS. Quantitative real-time PCR (qRT-PCR) analysis led to similar conclusions (Fig. 1A).
Fig. 1.
Transcriptional and functional analysis of NfiS ncRNA in P. stutzeri A1501. (A) nfiS transcription under nitrogen fixation conditions in the WT A1501 and five mutant strains. Total RNA was extracted, and the expression of target genes was measured using qRT-PCR. (Inset) RNA Northern blot assay using RNA extracted from the same strains under the same conditions and hybridized with the nfiS-specific probe. Measurements were normalized to the WT values, and fold differences are plotted. (B) Analysis of the NfiS half-life in the WT A1501 and hfq mutant strains. Rifampicin (400 μg/mL) was added at time 0. At the time indicated, an equal volume of frozen media was added to bring the temperature immediately to 4 °C. RNA was extracted, followed by qRT-PCR. (C) Nitrogenase activity in the WT A1501, ΔnfiS A1701, and NfiS overexpression strain A1514. Error bars represent the SD calculated from three sets of independent experiments. (D) Growth upon oxidative or osmotic stresses. Serial 10-fold dilutions of OD-standardized WT A1501, ΔnfiS A1701, and nfiS overexpression strain A1514 were spotted on LB plates after exposure to 20 mM H2O2 or 0.3 M sorbitol. CK, untreated culture control.
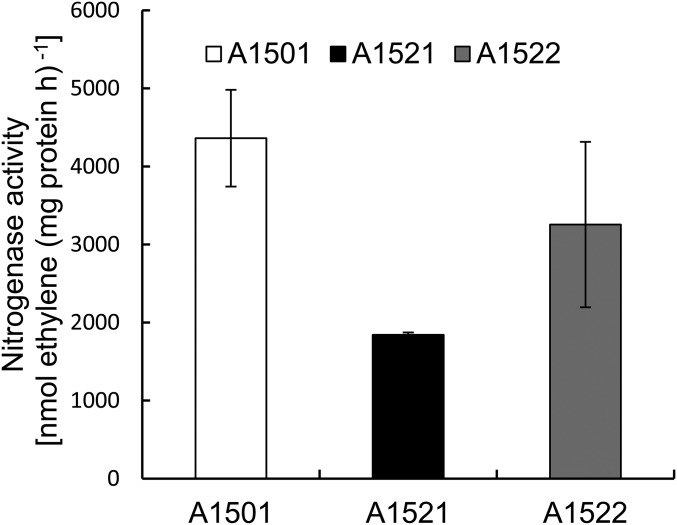
Most base-pairing small RNAs require the RNA chaperone Hfq to form stable sRNA-target-mRNA hybrids or to maintain their own stability (15). An hfq gene was identified in A1501 and found to be involved in the regulation of nitrogen fixation because its inactivation caused a significant decrease in nitrogenase activity (Fig. S2). Although expression of the nfiS gene was up-regulated in the hfq deletion background as determined by qRT-PCR, Northern blot analysis showed that the nfiS transcript was hardly detectable in the absence of Hfq (Fig. 1A, Inset, lane 6), implying that Hfq affected the stability of the NfiS transcript. This possibility was checked by measuring the half-life of the NfiS transcript, which was 7 min for the WT and 3 min for the hfq mutant strain (Fig. 1B), indicating that Hfq had a positive effect on the stability of NfiS.
Fig. S2.
Nitrogenase activity in the WT A1501 and the hfq mutant strains. A1501, WT; A1521, Δhfq; A1522, A1521 containing the complementing plasmid pLAhfq-A1501. Experiments were performed in triplicate with mean values shown.
Inactivation and Overexpression of NfiS Affect both Nitrogenase Activity and Stress Resistance.
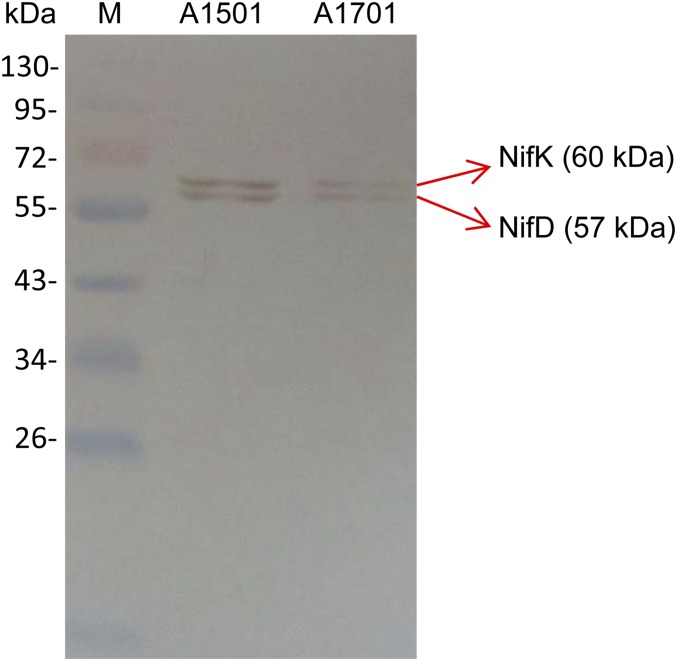
To evaluate the effect of NfiS expression on nitrogenase activity, the nfiS-knockout mutant A1701 (ΔnfiS) was constructed, as well as strain A1514 corresponding to A1501 harboring a plasmid expressing the WT nfiS gene (pLAnfiS-A1501) (SI Materials and Methods and Table S1). Both A1701 and A1514 displayed growth properties similar to that of the WT strain. The nfiS deletion in A1701 resulted in about 40% reduction in nitrogenase activity whereas activity of the NfiS-overexpressing strain A1514 showed an increase to 150% compared with the WT (Fig. 1C). Moreover, it was checked, by Western blot analysis, that the production of the nitrogenase MoFe protein polypeptides (NifK and NifD) was decreased in the ΔnfiS mutant strain (Fig. S3). This finding suggested a correlation between the NfiS level and the nitrogenase synthesis and activity. Therefore, NfiS is a positive regulator required for maximal expression of nitrogenase.
Table S1.
Strains and plasmids used in this study
| Strain/plasmid | Relevant characteristics | Source |
| P. stutzeri strains | ||
| A1501 | WT, Chinese Culture Collection: CGMCC 0351 | (54) |
| ATCC17588 | WT, Chinese Culture Collection: CGMCC 1.1803 | |
| A1550 (ΔrpoN) | rpoN-Cm deletion mutant, Cmr | (24) |
| A1565 (ΔntrC) | ntrC-Cm deletion mutant, Cmr | (32) |
| A1506 (ΔnifA) | nifA-Km deletion mutant, Kmr | (24) |
| A1507 (ΔrpoS) | rpoS-deletion mutant; the rpoS gene was knocked out by homologous suicide plasmid integration using pK18mobsacB as the vector | Lab collection |
| A1521 (Δhfq) | hfq nonpolar insertion mutant; the hfq gene was inactivated by homologous suicide plasmid integration using pK18mob as the vector, Kmr | This study |
| A1522 | hfq nonpolar insertion mutant containing pLAhfq-A1501, Kmr and Tcr | This study |
| A1514 | A1501 containing pLAnfiS-A1501, Tcr | This study |
| A1701 | nfiS-Spc deletion mutant strain, Spcr | This study |
| A1706 | A1701 containing pLAnfiS-A1501, Spcr and Tcr | This study |
| A1707 | A1701 containing pLAnfiS-del9, Spcr and Tcr | This study |
| A1708 | A1701 containing pLAnfiS-ATCC, Spcr and Tcr | This study |
| A1709 | A1701 containing pLAnfiS-mut5, Spcr and Tcr | This study |
| Plasmids | ||
| pK18mob | Mobilizable plasmid containing an Escherichia coli origin of replication, Kmr | (58) |
| pK18mobsacB | Allelic exchange vector, Kmr | (58) |
| pLAFR3 | Mobilizable vector, Tcr | (60) |
| pRK2013 | Helper plasmid for conjugation into P. stutzeri A1501, Kmr | (59) |
| pKatAAD2 | Source of spectinomycin resistance cassette, Spcr | Lab collection |
| pMD18-T | 2.96-kb cloning vector, Ampr | Takara |
| pK18/delS | pK18mobsacB derivative carrying a BamHI/HindIII fragment for homologous recombination, Spcr, Kmr | This study |
| pLAnfiS-A1501 | pLAFR3 derivative carrying the WT nfiS gene under the control of its endogenous promoter, Tcr | This study |
| pLAnfiS-mut5 | pLAFR3 derivative carrying a mutated nfiS gene with a substitution of five nucleotides within the pairing site region, Tcr | This study |
| pLAnfiS-del9 | pLAFR3 derivative carrying a mutated nfiS gene with a complete deletion of the 9-nt sequence downstream of the pairing site, Tcr | This study |
| pLAnfiS-ATCC | pLAFR3 derivative carrying the WT nfiS homolog from P. stutzeri ATCC17588 under the control of its endogenous promoter, Tcr | This study |
| pLAhfq-A1501 | pLAFR3 derivative carrying the WT hfq gene under the control of its endogenous promoter, Tcr | This study |
Fig. S3.
Western blot analysis of the nitrogenase MoFe protein NifD and NifK polypeptides. Lane 1, molecular weight marker (M); lane 2, A1501, WT; lane 3, A1701, ΔnfiS. NifD and NifK polypeptides are indicated by an arrow.
Bacterial ncRNAs are involved in responses to generalized stresses (5, 9, 14, 29). In the presence of 20 mM H2O2 or 0.3 M sorbitol, the nfiS mutant (A1701) was more sensitive than the WT whereas the overexpression of NfiS (A1514) led to enhanced resistance (Fig. 1D). Thus, NfiS plays an important role in the response to oxidative or osmotic stress, possibly contributing to optimal nitrogen fixation, which can be significantly affected by environmental stresses.
NfiS Contributes to the Global and Positive Regulation of Nitrogen Fixation and Stress Resistance-Related Genes.
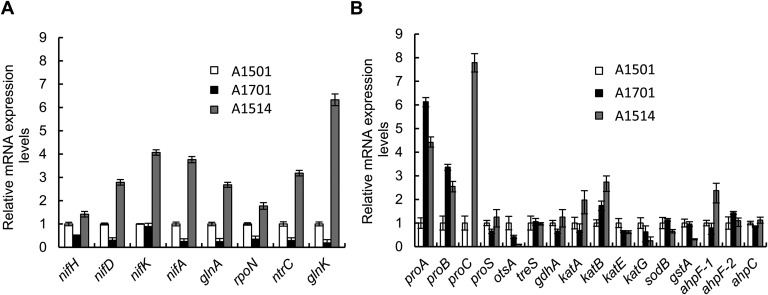
The expression level of a set of nitrogen fixation-related genes was compared in the WT, ΔnfiS (A1701), and NfiS overexpressing (A1514) strains under nitrogen fixation conditions. Deletion of nfiS resulted in decreased expression of the assayed nif and nif-related genes. In contrast, their expression was remarkably enhanced by overproduction of NfiS. In particular, nifA, coding for the transcriptional activator of all nif operons (18, 24, 25), showed a threefold increase, and glnK, coding for a PII family protein (31, 32), showed a sixfold increase (Fig. S4A). In P. stutzeri, GlnK is essential for both NifA synthesis and activity, in particular by preventing the inhibitory effect of NifL on NifA activity (26, 32). These data were in agreement with the observation that inactivation and overexpression of NfiS affected nitrogenase activity (Fig. 1C), suggesting a role in positive regulation of the nif genes. Similarly the expression of the rpoN, glnK, and ntrC genes was decreased in the nfiS mutant strain (A1701) and increased in the NfiS overexpressing strain (A1514), indicating that NfiS could also play a role in the global regulation of nitrogen metabolism. Apparently, this global effect could solely be due to an effect of NfiS on RpoN because all of the genes analyzed here are σ54-dependent. However, the level of NfiS was reduced in mutant strains carrying deletions of nifA or ntrC (Fig. 1A), suggesting a more complex regulatory circuitry.
Fig. S4.
Effect of nfiS deletion or overexpression on gene expression. (A) Expression of nif genes and various global nitrogen regulatory genes. (B) Expression of the oxidative or osmotic-response genes. Relative levels of transcripts are presented as the mean values ± SD, calculated from three sets of independent experiments, and normalized to levels in the WT strain.
The expression levels of oxidative or osmotic stress-related genes were compared in the three strains under sorbitol stress. Deletion and overexpression of nfiS resulted in down- and up-regulation, respectively, of genes such as proC and katA, showing that expression of these genes is NfiS-dependent (Fig. S4B). The proC gene encodes pyrroline carboxylate reductase, a key enzyme in the last step in proline biosynthesis, and katA encodes catalase, both of which play important roles in oxidative or osmotic stress responses (33, 34). Based on these studies, NfiS likely controls nitrogenase activity at the transcriptional level through the regulation of both the RpoN/NtrC/NifA regulatory cascade and certain stress response-related genes via unknown mechanisms.
A Stem-Loop Is Essential for NfiS to Regulate Optimal Nitrogen Fixation.
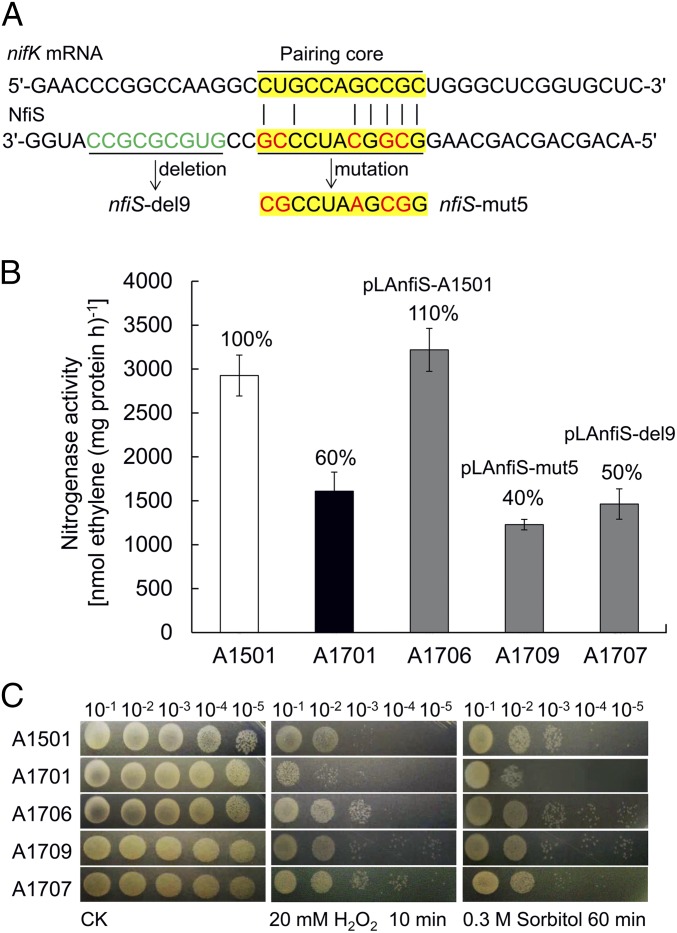
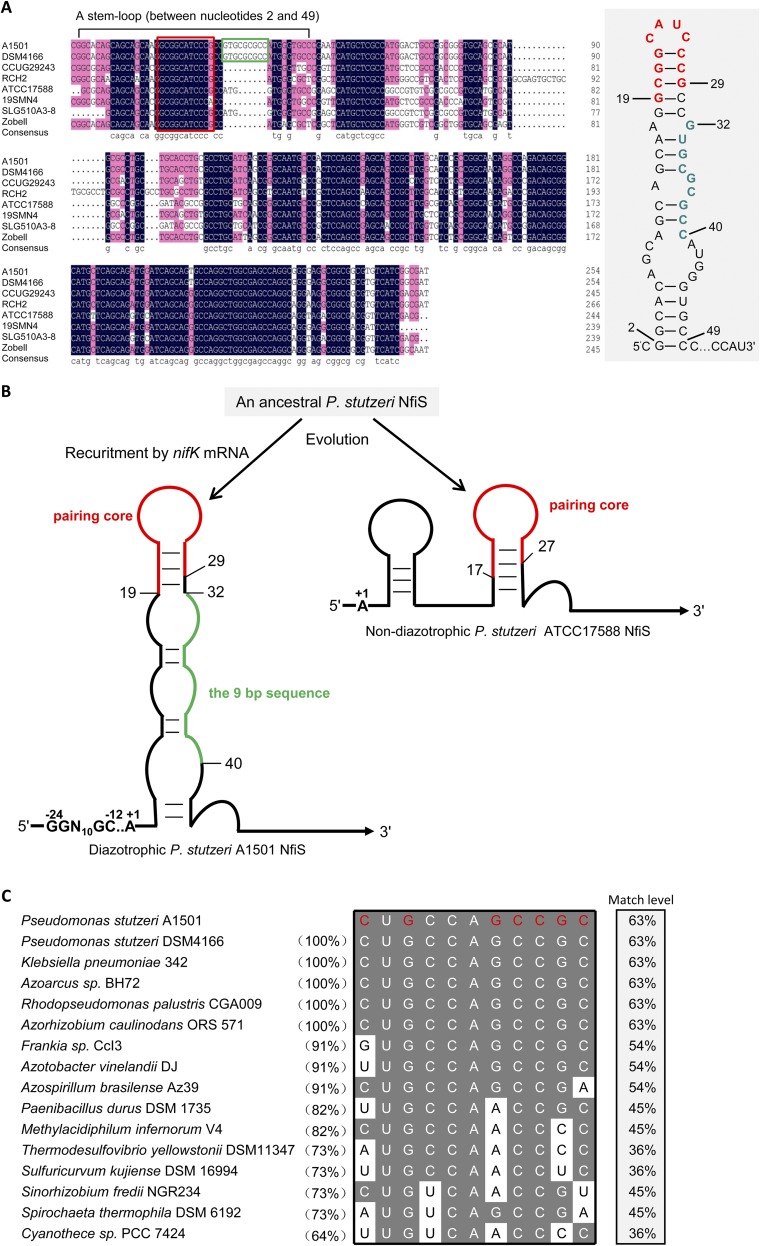
Using bioinformatics tools, we found putative pairing between NfiS and the transcript of the A1501 nifK gene (Fig. 2A) encoding the β-subunit of the MoFe protein of the nitrogenase enzymatic complex (24). The predicted secondary structure of NfiS contains a stem-loop (between nucleotides 2 and 49) with an 11-nt consensus sequence (GCGGCAUCCCG) moderately complementary to the 11 nucleotides (CUGCCAGCCGC) at the 5′ end (nucleotides 207–217) of nifK mRNA (Fig. S1D and Fig. 3A). Furthermore, this stem-loop also contains a 9-nt sequence (GUGCGCGCC) present only in diazotrophic P. stutzeri strains, although absent in nondiazotrophic strains (Fig. S5A).
Fig. 2.
A stem-loop is essential for NfiS regulation of nitrogenase activity. (A) Region of the nifK mRNA showing complementarity to the NfiS stem-loop region and relevant genetic constructs. Yellow, 11-nt pairing region with nifK mRNA; green, 9-nt region. Deletion of the entire 9-nt region and the 5-nt substitution in 11-nt pairing region are underlined, and mutated nucleotides are highlighted in red. (B) Nitrogenase activity in the WT A1501, ΔnfiS A1701, and three complemented strains (A1701 containing pLAnfiS-A1501, pLAnfiS-mut5, and pLAnfiS-del9, respectively). Experiments were performed in triplicate with mean values shown. The percentage of nitrogenase activity compared with WT is indicated. (C) Serial 10-fold dilutions of OD-standardized cultures were spotted on LB plates after exposure to 20 mM H2O2 or 0.3 M sorbitol.
Fig. 3.
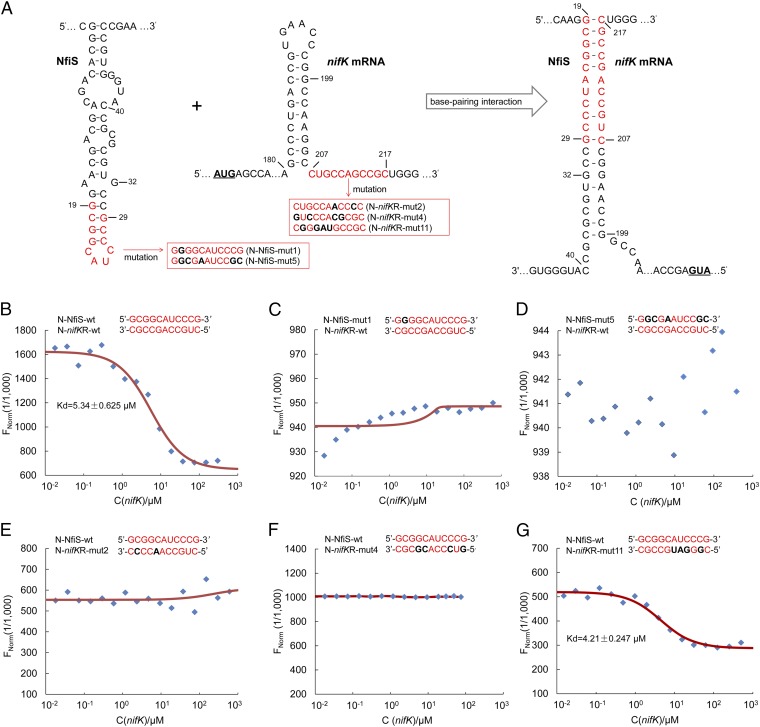
Binding of NfiS to nifK mRNA. (A) Schematic representation of the base-pairing complex formation (Right) between the NfiS stem loop (Left) and the complementary sequence of nifK mRNA (Middle). Pairing nucleotides are shown in red. Point mutations introduced into synthesized oligonucleotide derivatives in red boxes are shown in black and bold. mut, mutation; N, synthesized oligonucleotide; nifKR, nifK mRNA; wt, wild type. (B–G) Determination of the binding affinity of NfiS to nifK mRNA by microscale thermophoresis. The concentration of labeled N-NfiS molecules was constant whereas the concentration of the nonlabeled binding partner N-nifKR molecules varied from 10 nM to 300 μM. See Table S2 and SI Materials and Methods for details.
Fig. S5.
Alignment of nfiS genes identified in P. stutzeri strains and nifK mRNAs in representative diazotrophs. (A) Alignment of nfiS genes identified in different P. stutzeri strains. Shown in a gray box is a stem-loop sequence (from nucleotides 2–49) essential for NfiS regulation of nitrogenase activity. See Figs. 2 and 3 for more details. The 11-nt sequence predicted to be complementary to nifK mRNA and the 9-nt sequence only identified in diazotrophic strains are highlighted in red and green, respectively. Some nucleotides in the stem-loop sequence are numbered. (B) Schematic representation of remarkable secondary structure differences in the NfiS RNAs from diazotrophic or nondiazotrophic P. stutzeri, which may reflect the functional divergences of NfiS evolution under diazotrophic or nondiazotrophic backgrounds. See Structural and Functional Divergences of NfiS Under Diazotrophic and Nondiazotrophic Backgrounds and Discussion for details. (C) Alignment of the P. stutzeri A1501 nifK mRNAs with homologous sequences in the corresponding region of representative diazotrophs. The nifK mRNA complementary sequence nucleotides matching those of NfiS are highlighted in red. Complementary sequence identity (%) between the 11-bp sequence complementary to NfiS identified in the 207 and 217 region of the A1501 nifK mRNA and homologous sequences in the corresponding regions of nifK mRNAs from other diazotrophs is shown in parentheses. Match level (%) of base pairing sequences between nifK mRNA and NfiS is shown in right column.
The role of the NfiS stem-loop structure in nitrogen fixation and stress responses was explored further. Two plasmids carrying nfiS mutations with either a substitution of five nucleotides in the putative base-pairing site with nifK mRNA (pLAnfiS-mut5) or a complete deletion of the 9-nt sequence downstream of the pairing site (pLAnfiS-del9) were constructed (Fig. 2A). Complementation of ΔnfiS (A1701) showed that both nitrogenase activity and stress resistance were restored to WT levels by pLAnfiS-1501 (A1706; containing WT nfiS), but pLAnfiS-mut5 (A1709) and pLAnfiS-del9 (A1707) failed to restore nitrogenase activity (Fig. 2B). These two latter plasmids did restore the stress resistance caused by adding 20 mM H2O2 or 0.3 M sorbitol to the medium (Fig. 2C). These results suggested that the stem-loop structure of NfiS plays a role in optimal nitrogen fixation but is not necessary for oxidative or osmotic tolerances.
NfiS Directly Base Pairs with nifK mRNA to Enhance the Translation Efficiency and Half-Life of the Transcript.
Based on the prediction of the NfiS secondary structure and on the complementation data using the WT or mutated nfiS genes, it was hypothesized that NfiS is recruited and paired with nifK mRNA. As shown in Fig. 3A, an 11-nt sequence in the stem-loop structure of NfiS (nucleotides 19–29) pairs with its counterpart at the 5′ end of nifK mRNA (nucleotides 207–217) by seven nucleotides. This 63% match is moderate but is sufficient for the NfiS and nifK mRNA recognition and binding. To assay this hypothesis, a set of 30-nt ssRNA oligonucleotides containing the WT or mutated sequences were synthesized, including an N-NfiS-wt containing the stem-loop structure of NfiS, an N-nifKR-wt containing the complementary region of nifK mRNA, and two mutations (N-NfiS-mut1 and N-NfiS-mut5) containing site-specific mutations in the stem-loop structure of NfiS (Table S2). The interaction of the fluorescently labeled N-NfiS-wt or N-NfiS-mut probe molecules with the nonlabeled competitor molecule N-nifKR-wt was assayed using microscale thermophoresis (MST), allowing sensitive measurement of molecular interactions in solution (35, 36). This analysis indicated that N-NfiS-wt bound to N-nifKR-wt at low micromolar concentrations of the titrant, exhibiting a dissociation constant (Kd) of 5.34 ± 0.63 μM (Fig. 3B), suggesting a relatively strong interaction. In contrast, the two mutated derivatives (N-NfiS-mut1 and N-NfiS-mut5) carrying substitutions of one or more nucleotides in the 11-nt stem-loop motif displayed a complete defect in binding to nifK mRNA (Fig. 3 C and D), indicating that conservation of this motif is critical to the interaction between NfiS and nifK mRNA.
Table S2.
Synthesized ssRNA oligonucleotide derivatives for MST
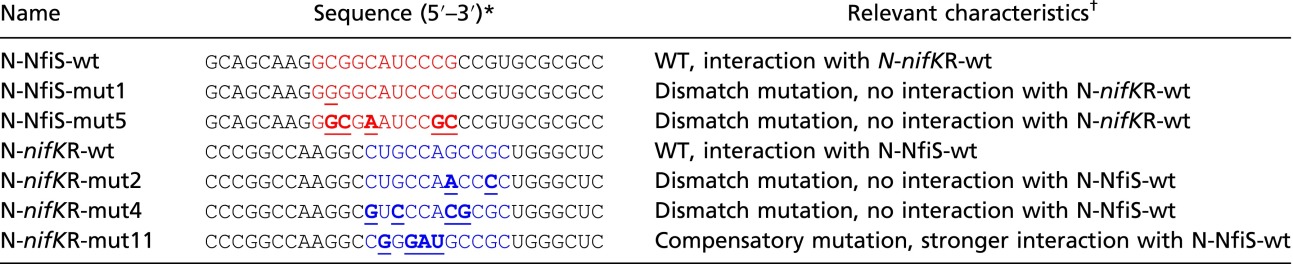 |
mut, mutation; N, 30-nt ssRNA oligonucleotide; nifKR, nifK mRNA; wt, wild type.
The 11-nt sequence of the NfiS stem-loop pairing with nifK mRNA is shown in red. The 11-nt sequence of the complementary region at the 5′ end of nifK mRNA is shown in blue. Point mutations introduced into synthesized oligonucleotide derivatives are underlined.
The WT N-NfiS-wt binds to the WT N-nifKR-wt whereas the compensatory mutated oligonucleotide (N-nifKR-mut11) binds to WT N-NfiS-wt more tightly, as shown in Fig. 3 B and G.
The effect of mutations in the 11-nt sequence at the 5′ end of nifK mRNA (residues 207–217) on base pairing with NfiS was in turn investigated using 30-nt ssRNA oligomers containing mismatch mutations (N-nifKR-mut2 or N-nifKR-mut4) and one compensatory mutation (N-nifKR-mut11). The latter showed a perfect match (100%) with the 11-nt targeting sequence of NfiS (Fig. 3A and Table S2). We further measured the binding affinity of these altered nifK nucleotides with NfiS (N-NfiS-wt). As shown in Fig. 3 E and F, N-nifKR-mut2 or N-nifKR-mut4 exhibited no interaction with N-NfiS-wt. We also measured the binding affinity using N-nifKR-mut11 (100% match level). The compensatory mutation resulted in enhanced binding affinity to N-NfiS-wt compared with the WT situation, showing a Kd of 4.21 ± 0.25 μM (Fig. 3G). These data indicate that nifK mRNA is a direct target of the NfiS ncRNA.
The secondary structure of nifK mRNA contains a putative hairpin structure (nucleotides 181–206) immediately adjacent to the ncRNA binding site, presumably forming an inhibitory structure that affects translation efficiency (Fig. 3A, Middle). In the presence of rifampicin, the half-life of nifK mRNA in the WT strain was 20 min, but it decreased to 15 min for the nfiS mutant (Fig. 4). This transcript stability was restored to WT level by the complementary plasmid (Fig. 4). This result is in good agreement with the results from the complementation assay of nitrogenase activity as shown in Fig. 2B. These results led us to conclude that the regulation of nitrogen fixation by NfiS in A1501 occurs at multiple levels, which, at the posttranscriptional level, is through a direct interaction with target mRNA that increases its translation efficiency and half-life and consequently nitrogenase activity.
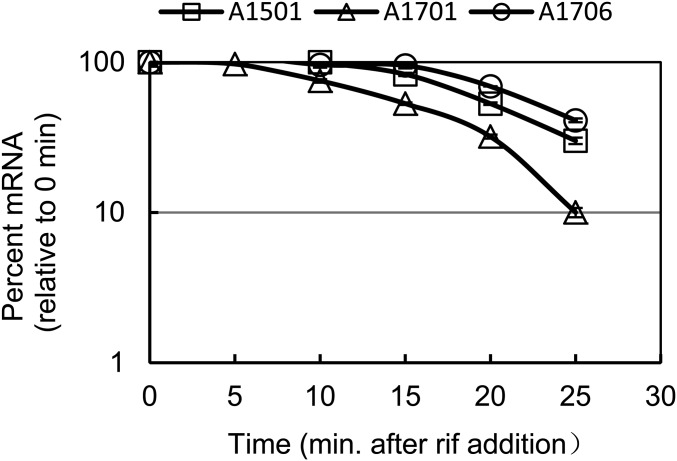
Fig. 4.
Effect of nfiS mutation on the half-life of nifK transcript. Determination of nifK mRNA half-life in the WT A1501, ΔnfiS A1701, and complemented strain A1706 (A1701 containing complementation plasmid pLAnfiS-A1501) in the same conditions as Fig. 1.
Structural and Functional Divergences of NfiS Under Diazotrophic and Nondiazotrophic Backgrounds.
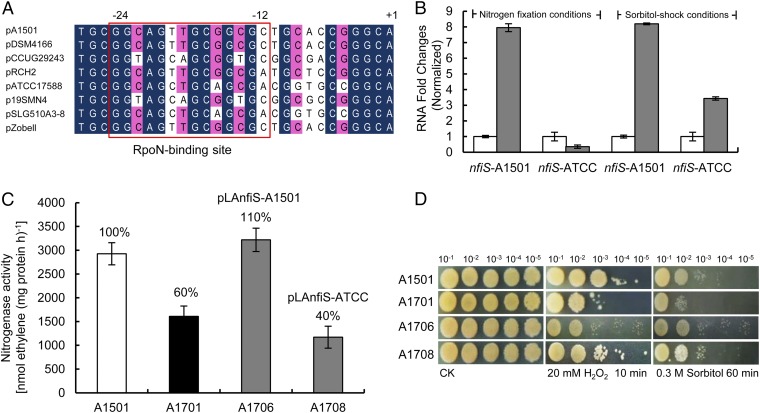
Analysis of the nucleotide sequence of the nfiS gene revealed that the 9-nt sequence identified as a part of a stem-loop required for regulation of nitrogenase synthesis (Fig. 2A) is present only in the nitrogen-fixing strains (e.g., A1501 and DSM4166) (Fig. S5A). The putative RpoN-binding site of the nfiS promoter region is not well-conserved in P. stutzeri ATCC17588, RCH2, and SLG358A3-8, which are not diazotrophic (Fig. 5A). These structural differences may reflect the functional divergences of NfiS evolution in diazotrophic and nondiazotrophic backgrounds.
Fig. 5.
Structural and functional comparison between the diazotrophic and nondiazotrophic P. stutzeri NfiS. (A) Alignment of the P. stutzeri A1501 nfiS promoter region with homologous sequences in P. stutzeri DSM4166 (diazotrophic) and CCUG29243, ATCC17588, 19SMN4, SLG510A3-8, and Zobell. The sequence alignment was performed using CLUSTAL X. Nucleotides conserved at 100% and at >70% are highlighted in black and red, respectively. The nucleotide sequences, which may correspond to the RpoN binding site located at position −12 to −24 from the transcription start, are boxed. Transcriptional start sites are shown. (B) Induction of the nfiS gene in diazotrophic P. stutzeri strain A1501 or nondiazotrophic strain ATCC17588 grown under nitrogen fixation (gray bars) or sorbitol stress conditions (gray bars) vs. control (open bars), as measured by qRT-PCR. Error bars as in Fig. 1. (C) Nitrogenase activity of the WT A1501, ΔnfiS A1701, and two complemented strains (A1701 containing pLAnfiS-A1501 and pLAnfiS-ATCC, respectively). (D) Response to sorbitol and H2O2 stresses in the same strains as in C and the same conditions as in Fig. 2.
The qRT-PCR measurement of NfiS expression in strain ATCC17588 showed a dramatic increase after sorbitol shock but not under nitrogen limitation conditions, in contrast to the observations with A1051 (Fig. 5B). These data suggest the existence of different mechanisms controlling the regulation of NfiS expression in diazotrophic and nondiazotrophic P. stutzeri strains. When a plasmid carrying the nfiS gene of the ATCC17588 strain (pLAnfiS-ATCC) was introduced into the nfiS mutant, restoration of oxidative or osmotic stress-resistant phenotypes was observed whereas there was no complementation for nitrogen fixation ability (Fig. 5 C and D). This result is in agreement with the requirement of the 9-nt region for optimal nitrogenase activity (Fig. 2B), but not for oxidative or osmotic-stress resistances (Fig. 2C).
SI Materials and Methods
Construction of the nfiS-Deletion Mutant Strain.
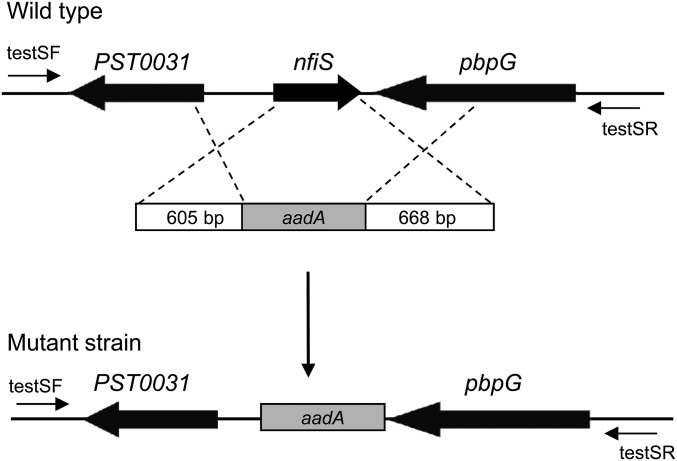
To generate the nfiS mutant strain A1701, an in-frame nfiS deletion (covering residues from −10 to +254) was introduced into the Pseudomonas stutzeri chromosome by homologous recombination as shown in Fig. S6. Amplification of a 605-bp DNA fragment located upstream of nfiS was performed using the primer set upF/upR, and amplification of a 668-bp DNA fragment located downstream of nfiS using the set downF/downR was performed (Table S3). Restriction enzymes sites (BamHI and HindIII) incorporated into the oligonucleotide primers to facilitate vector construction are underlined in the oligonucleotide sequences shown in Table S4. A 915-bp DNA fragment containing the Spc resistance cassette was amplified from the plasmid pKatAAD2 by PCR using primers spcF and spcR. The three amplicons were fused into a 2.2-kb fragment in which the Spc gene is located between the other two amplicons by overlap extension PCR according to the strategy of PCR-based fusions (57). The fusion PCR product was then cloned into the multiple cloning site of the pMD18-T vector (Takara). The resulting plasmid DNA was double-digested with BamHI/HindIII and then cloned into the BamHI/HindIII site of pK18mobsacB (58), generating pK18/delS. The resulting plasmid pK18/delS was incorporated into the genome of P. stutzeri strain A1501 by triparental mating, using pRK2013 (59) as the helper plasmid, and chromosomally integrated upon first cross-over selection for Km and Spc resistances. Excision of the vector by the second cross-over was obtained through enrichment of Km-sensitive cells on 10% (wt/vol) sucrose and 34 μg/mL Spc supplemented plates. Correct recombination was checked using primers testF and testR, followed by nucleotide sequencing of the amplicon obtained. The resulting nfiS deletion mutant, named A1701, was used for further study.
Fig. S6.
The schematic representation of the nfiS deletion mutant generated by replacing the nfiS region with the spectinomycin resistance gene aadA (Spcr). Primers testF and testR used to analyze the nfiS deletion are shown by arrows.
Table S3.
The 11-bp sequence complementary to NfiS identified in the corresponding regions of 31 nifK genes from 41 representative diazotrophs of the Cyanobacteria, Proteobacteria, and Actinobacteria (Frankia)
| Taxonomic status | Strain | Homologous sequence | Identity value, %* | |
| 1 | Actinobacteria | Frankia alni ACN14a | CUGCCAGCCGC | 100 |
| 2 | Frankia sp. CcI3 | GUGCCAGCCGC | 91 | |
| 3 | Alphaproteobacteria | Azorhizobium caulinodans ORS 571 | CUGCCAGCCGC | 100 |
| 4 | Gluconacetobacter diazotrophicus PAl 5 | CUGCCAGCCGC | 100 | |
| 5 | Rhodopseudomonas palustrisCGA009 | CUGCCAGCCGC | 100 | |
| 6 | Azospirillum brasilense Az39 | CUGCCAGCCGA | 91 | |
| 7 | Sinorhizobium fredii NGR234 | CUGUCAACCGU | 73 | |
| 8 | Bacteroidetes/Chlorobi | Chlorobaculum parvum NCIB 8327 | CUGCCAGCCGA | 91 |
| 9 | Chlorobium luteolum DSM 273 | CUGCCAGCCGA | 91 | |
| 10 | Betaproteobacteria | Azoarcus sp. BH72 | CUGCCAGCCGC | 100 |
| 11 | Burkholderia phymatum STM815 | GUGCCAGCCGC | 91 | |
| 12 | Burkholderia vietnamiensis G4 | GUGCCAGCCGC | 91 | |
| 13 | Candidatus Accumulibacter phosphatis clade IIA str. UW-1 | CUGCCAGCCGC | 100 | |
| 14 | Dechloromona saromatica RCB | CUGCCAGCCGC | 100 | |
| 15 | Cyanobacteria | Cyanothece sp. PCC 7424 | UUGUCAACCCC | 64 |
| 16 | Nostoc sp. PCC 7120 | UUGCCAACCUG | 64 | |
| 17 | Deltaproteobacteria | Desulfomicrobium baculatum DSM 4028 | CUGUCAGCCCA | 73 |
| 18 | Desulfovibrio magneticus RS-1 | CUGCCAGCCCG | 82 | |
| 19 | Desulfovibrio salexigens DSM 2638 | Not found | — | |
| 20 | Epsilonproteobacteria | Sulfuricurvum kujiense DSM 16994 | UUGCCAACCUC | 73 |
| 21 | Arcobacter nitrofigilis DSM 7299 | Not found | — | |
| 22 | Wolinella succinogenes DSM 1740 | Not found | — | |
| 23 | Euryarchaeota | Methanococcus maripaludis S2 | Not found | — |
| 24 | Methanosarcina acetivoransC2A | Not found | — | |
| 25 | Methanothermobacter thermautotrophicus str. Delta H | Not found | — | |
| 26 | Firmicutes | Paenibacillusdurus DSM 1735 | UUGCCAACCGC | 82 |
| 27 | Clostridium acetobutylicumATCC 824 | Not found | — | |
| 28 | Clostridium beijerinckii NCIMB 8052 | Not found | — | |
| 29 | Fusobacteria | Ilyobacter polytropus DSM 2926 | Not found | — |
| 30 | Gammaproteobacteria | Allochromatium vinosum DSM 180 | CUGUCAGCCGC | 91 |
| 31 | Azotobacter vinelandii DJ | UUGCCAGCCGC | 91 | |
| 32 | Azotobacter chroococcum NCIMB 8003 | UUGCCAGCCGC | 91 | |
| 33 | Klebsiella pneumoniae 342 | CUGCCAGCCGC | 100 | |
| 34 | Methylococcus capsulatus str. Bath | CUGUCAGCCGC | 91 | |
| 35 | Pseudomonas stutzeri DSM4166 | CUGCCAGCCGC | 100 | |
| 36 | Sideroxydans lithotrophicus ES-1 | CUGCCAGCCGC | 100 | |
| 37 | Nitrospirae | Thermodesulfovibrio yellowstonii DSM 11347 | AUGCCAACCCC | 73 |
| 38 | Spirochaetes | Spirochaeta thermophila DSM 6192 | AUGUCAGCCGA | 73 |
| 39 | Spirochaetasmaragdinae DSM 11293 | Not found | — | |
| 40 | Verrucomicrobia | Coraliomargarita akajimensis DSM 45221 | UUGCCAGCCCA | 73 |
| 41 | Methylacidiphilumin fernorum V4 | CUGCCAACCCC | 82 |
The 11-bp sequence complementary to NfiS identified in the 207 and 217 region of the A1501 nifK mRNA vs. homologous sequences in the corresponding regions of nifK mRNAs from other diazotrophs. Em dashes, not applicable.
Table S4.
Primers used in this study
| Primer* | Sequence (5′–3′)† | Amplicon size, bp | Purpose |
| upF | AAAGGATCCGAGCGCAACGTC | 605 | A1701 construct |
| upR | GTCTCCATGCGAGCTCGAATTCGATCATCGGCGATCCGATCAGGC | ||
| spcF | GCCTGATCGGATCGCCGATGATCGAATTCGAGCTCGCATGGAGAC | 915 | |
| spcR | CGCTAGCCCCACCGAACCTTATTTGCCGACTACCTTGGTGATC | ||
| downF | GATCACCAAGGTAGTCGGCAAATAAGGTTCGGTGGGGCTAGCG | 668 | |
| downR | TTTAAGCTTGCTGTTGCTCGGGATG | ||
| a15F | AAAGGATCCATCCAGTTGAGCAAG | 1,592 | pLAnfiS-A1501 construct |
| a15R | TTTAAGCTTGCCCGATGAAGTAGTTC | ||
| hfqF | TTTAAGCTTACCCGACTCGGATTGC | 248 | A1521 construct |
| hfqR | AAAGGATCCTGTCAAAAGGGCATTCG | ||
| a15hfqF | TTTAAGCTTCGAAGAACAAAGAAAC | 358 | pLAhfq-A1501 construct |
| a15hfqR | AAAGGATCCGTCTATTCTCAACAAG | ||
| atccF | AAAGGATCCATCCAGTTGAGCAAG | 1,592 | pLAnfiS-ATCC construct |
| atccR | TTTAAGCTTGCCCGATGAAGTAGTTC | ||
| testSF | TGCTGAGAGTCGTTCCTA | 900 | Validation of A1701 by PCR |
| testSR | TGAAGCCACGAAAGGACA | ||
| testqF | GCCGATTCATTAATGCAGCTGG | 564 | Validation of A1521 by PCR |
| testqR | GAAACATTGACGAAGCCAAC | ||
| GSP1 | CTGGCTCGCCAGCCTGGCACTGC | 5′ RACE | |
| GSP2 | AGCCTGGCACTGCTGATCC | ||
| RTS-A1501F | CCGCTGTCTGGCCTGTT | 143 | qRT-PCR for the nfiS half-life assay |
| RTS-A1501R | CCATGGGTGCCCGAATC | ||
| RTnifKF | TCGAGACCTACCTGGGCAACT | 104 | qRT-PCR for the nifK half-life assay |
| RTnifKR | GGGGTATCGAGCACTTCTTCC | ||
| RTS-ATCCF | ATGGTGGGTGCCGGAG | 200 | qRT-PCR |
| RTS-ATCCR | CCGTCTACCTGCCTGG | ||
| RTnifHF | GAGATGATGGCGATGTATGC | 113 | |
| RTnifHR | GGTCGGTGTTGCGGCTGTTG | ||
| RTnifDF | ACATGATCCACATTTCCCACG | 197 | |
| RTnifDR | GAACAGCGTCTCGATCTCGTC | ||
| RTnifKF | TCGAGACCTACCTGGGCAACT | 104 | |
| RTnifKR | GGGGTATCGAGCACTTCTTCC | ||
| RTnifAF | CGCGAAGACCTCTACTACCG | 139 | |
| RTnifAR | CAGCTTGAGTTTGCGACCCT | ||
| RTglnAF | CGGAGCCGGAGTTCTTCATC | 110 | |
| RTglnAR | GACGTCCTGGTCGGTCATCC | ||
| RTrpoNF | CTTCTTCTCCAGCCACGTCAG | 137 | |
| RTrpoNR | CCAGTAAACCAGCGATCTTGC | ||
| RTntrCF | GATCAATGGCGAATCGGGTAC | 134 | |
| RTntrCR | CAGCTCGGATTCCATCAGGTC | ||
| RTglnKF | AGTCACTGCCATCATCAAGCC | 183 | |
| RTglnKR | GCCACGTCGATCTTCACCTTT | ||
| RTproAF | GCCGCGATTCGTAGATGAT | 168 | |
| RTproAR | CGCTGACGCCAAAGGTGA | ||
| RTproBF | TGGGGTTGATTCGTGCCT | 174 | |
| RTproBR | CCGTGTCGTTCTCGTTGATG | ||
| RTproCF | CCGCCCACCTCGAACAAG | 269 | |
| RTproCR | AACGGCAGTCACCGCATC | ||
| RTproSF | AGGAAATGAACAAGGCAGGC | 257 | |
| RTproSR | GCGGATCTCGTCACGGAAC | ||
| RTotsAF | GGAACAGCCCCATCAGCA | 196 | |
| RTotsAR | CCCAAGCACCGCAACAAC | ||
| RTtreSF | CTTCCGCCTCGCAGAAAT | 124 | |
| RTtreSR | CAGCAGGTTGACCGAATCAC | ||
| RTgdhAF | CTTGCCCGGGGCATAGAG | 217 | |
| RTgdhAR | GCAGTTCGGCGTGACCTTC | ||
| RTkatAF | ATGGACCAATCTGAAGAGCC | 125 | |
| RTkatAR | CGTGCATGAACCGATAACC | ||
| RTkatBF | CTTCTTGCTGAACGAGCGATAC | 288 | |
| RTkatBR | TTCTCCTACGCCGATACCCA | ||
| RTkatEF | GCTGGACCCGACCAAAAT | 250 | |
| RTkatER | CGGACGGTTGATCGGAAT | ||
| RTkatGF | TTCCGCAACTACTACCACGAG | 211 | |
| RTkatGR | TGTCCAGCAGGTTGACGAAG | ||
| RTsodBF | AAGGAAGAGTTCACCAAGACCG | 239 | |
| RTsodBR | ACGAAGTCCCAGTTCACCAG | ||
| RTgstAF | GGAACTGGAGCTGCTGTACG | 136 | |
| RTgstAR | CTCTGCCAGGGTCAGGTCAC | ||
| RTahpF-1F | ACCGGAAGTCATTGAGCAGA | 223 | |
| RTahpF-1R | CTCGCCGTTGAGGTAGATGC | ||
| RTahpF-2F | CAGGACAGCGAGAAGTAGGTT | 295 | |
| RTahpF-2R | GCGAGAAGTCCCAGGAAATG | ||
| RTahpCF | GTCTTCTCGCCCTCTTTCCA | 109 | |
| RTahpCR | AGATCGCTCGTGACGTGTCC | ||
| RT16S-F | CCTACGGGAGGCAGCAG | 160 | |
| RT16S-R | ATTACCGCGGCTGCTGG |
F, forward; R, reverse.
Restriction sites are underlined.
Construction of the pLAFR3 Derivatives Carrying WT and Mutated nfiS Genes.
Two plasmids carrying the nfiS gene from two different P. stutzeri WT strains were constructed using the broad host plasmid pLAFR3 as a vector (60) to complement the constructed A1701 deletion strain. A DNA fragment of the WT nfiS gene with its promoter and terminator regions was amplified from genomic DNA of strain A1501, with the primer set a15R/a15F or from the nondiazotrophic strain ATCC17588 with the set atccR/atccF, and ligated into pLAFR3 to yield pLAnfiS-A1501 and pLAnfiS-ATCC, respectively (Table S1). To explore the function of the NfiS stem-loop containing the 11-nt pairing site with nifK mRNA and the 9-nt sequence downstream of the pairing site, two complementation plasmids carrying a mutated nfiS gene, either nfiS-mut5 with the 5-nt substitution of the pairing sequence (GCCCUACGGCG in place of CGCCUAAGCGG) or nfiS-del9 with a complete deletion of the 9-nt sequence, were constructed using pLAFR3. A 585-bp DNA fragment of the nfiS-mut5 gene and a 576-bp DNA fragment of the nfiS-del9 gene, including their promoter and terminator regions, were synthesized from BGI Co. Ltd. Restriction enzymes sites (BamHI and HindIII) were incorporated into two synthesized fragments. The synthesized fragments were digested with BamHI and HindIII and ligated into the corresponding site of pLAFR3, resulting in pLAnfiS-mut5 and pLAnfiS-del9, respectively (Table S1).
Construction of hfq Nonpolar Insertion Mutant and Complementing Plasmid.
The hfq gene was inactivated by homologous suicide plasmid integration using pK18mob as a vector (58). Briefly, a 268-bp internal fragment of the hfq gene was amplified by PCR using oligonucleotide primers designed to generate an amplicon for the creation of the mutation, enabling the transcription of the downstream hflX gene. The amplicon was double-digested with BamHI/HindIII and then cloned into pK18mob. The resulting plasmid was introduced into A1501 by triparental mating, generating hfq nonpolar insertion mutant A1521 (Table S1). Correct recombination was checked by PCR, followed by nucleotide sequencing of the amplicon obtained. To complement the constructed mutant, a 358-bp DNA fragment containing the WT hfq gene with its promoter and terminator regions was amplified by PCR. The amplicon was double-digested with BamHI/HindIII and ligated into pLAFR3 to yield complementing plasmid pLAhfq-A1501 (Table S1).
Quantitative Real-Time PCR.
Total RNA was isolated with an SV Total RNA Isolation System (Promega) and treated with RNase-free DNase I (Promega) to prevent contamination by genomic DNA. qRT-PCR assays were performed using total RNA preparations obtained from three independent cultures (three biological replicates). Primers were designed based on the full genome sequence of P. stutzeri A1501 and are listed in Table S4. The PCR reactions were carried out with Power SYBR Green PCR Master Mix on an ABI Prism 7500 Sequence Detection System (Applied Biosystems) according to the manufacturer’s recommendations. The 16S rRNA gene was used as the endogenous reference control, and relative gene expression was determined using the comparative threshold cycle 2−ΔΔCT method. Data were analyzed using ABI PRISM 7500 Sequence Detection System Software (Applied Biosystems).
The 5′ Rapid Amplification of cDNA Ends Assay.
The transcriptional start site of the nfiS gene was determined using 5′ RACE (Invitrogen) following the manufacturer’s instructions. Briefly, first strand cDNA was generated using the sequence-specific primer GSP1 to the nfiS gene. The original cDNA was purified, and the 3′ end of the cDNA was tailed with dCTP by terminal deoxynucleotidyl transferase (TdT). PCR amplification was performed using the sequence-specific primer GSP2 and the anchor primer AAP provided by the 5′ RACE system. Primers GSP1 and GSP2 specific for the nfiS gene tested here are listed in Table S4. The 5′ RACE products were cloned into the pMD18-T vector (Takara) and sequenced to map the 5′ end of the transcript.
Western Blot Assays for NifDK Expression.
Western blotting was performed on protein extracts of bacterial cells incubated for 5 h under nitrogen fixation conditions (N-free medium; 0.5% oxygen tension). The cell pellet collected from 10-mL cultures at OD600 = 0.1 was dissolved in 200 μL of SDS gel-loading buffer, boiled for 10 min, frozen, and centrifuged for 2 min. Then, 20-μL supernatants were loaded onto the stacking gel. Proteins were separated by SDS polyacrylamide gel electrophoresis (SDS/PAGE) with an acrylamide/bis-acrylamide ratio of 172:1 and transferred to a PVDF membrane (Amersham) by electroblotting. The membrane was incubated with antisera raised against the MoFe-protein (kindly provided by Ying Li, China Agricultural University, Beijing) for 2 h at 37 °C and washed three times in TBS/Tween before incubation with anti-rabbit secondary antibodies (Sangon) for 2 h. Detection was performed using the HRP-DAB Chemistry Kit (Tiangen).
Nitrogenase Activity Assays.
Nitrogenase activity was determined by the acetylene reduction test (61) using a derepression protocol (24), as follows. Cells from an overnight culture in LB medium were centrifuged and resuspended in a 60-mL flask containing 10 mL of N-free minimal medium K, at an OD600 of 0.1. The suspension was incubated at 30 °C, with vigorous shaking, under an argon atmosphere containing 0.5 % oxygen and 10 % acetylene. Gas samples (0.25 mL) were taken at regular intervals to determine the amount of ethylene produced. Samples were analyzed on a polydivinylbenzene porous beads GDX-502 column using a gas chromatograph SP-2100 fitted with a flame ionization detector (Beijing Beifen-Ruili Analytical Instrument Co. Ltd). The content of ethylene in the gas samples was determined by reference to an ethylene standard. Each experiment was repeated at least three times. Protein concentrations were determined by the Bio-Rad protein assay. For expression assays, A1501 was exposed to ammonium shock for 10 min, which involved a sudden change from nitrogen fixation to ammonium shock conditions by addition of 20 mM ammonium. We verified that no discernible loss of ammonium occurred after the shock. The activity of nitrogenase in A1501 was completely repressed by 20 mM ammonium.
Microscale Thermophoresis Measurements.
MST experiments were performed according to ref. 55. A set of 30-nt ssRNA oligonucleotides, containing WT or mutated base-pairing regions of NfiS (N-NfiS probes) or complementary regions of nifK mRNA (N-nifKR competitors), were synthesized by GenePharma Company and are listed in Table S2. The WT and mutated N-NfiS probe molecules were labeled with 6-carboxyfluorescein (FAM). Four microliters of sample containing 500 nM labeled probe and increasing concentrations of a nonlabeled competitor (from 10 nM to 300 μM) were loaded on standard treated silicon capillaries. Thermophoresis in 280 nm was measured using a Monolith NT.115 instrument (NanoTemper Technologies GmbH) at 20 °C in diethylpyrocarbonate (DEPC) water using 20% light-emitting diode (LED) and 40% MST power. The dissociation constants (Kds) were calculated as described (56). Data analyses were performed using Nanotemper Analysis software v.1.2.101 (NanoTemper Technologies).
Stability Measurement of nfiS and nifK Transcripts.
Strains were incubated for 5 h under nitrogen fixation conditions (N-free medium; 0.5% oxygen tension). Two-milliliter samples were collected at different times (0, 5, 10, 15, 20, and 25 min) after the addition of rifampicin (400 μg/mL). Aliquots were added to two volumes of RNA protect (Sigma). After incubating at room temperature for 5 min, samples were centrifuged for 10 min at 4 °C, and pellets were quick frozen in liquid nitrogen and stored at −80 °C until ready for use. Total RNA was isolated using an SV Total RNA Isolation System (Promega) and treated with RNase-free DNase I (Promega). cDNA was synthesized from total RNA using a First Strand cDNA synthesis kit (New England Biolabs) and was used in estimating mRNA levels by qRT-PCR. The primers used to measure the mRNA half-life are listed in Table S4, and qRT-PCR was performed as described above. The relative mRNA concentration was calculated by the comparative threshold cycle (2−ΔΔCT) method with 16S rRNA as the endogenous reference. Gene expression values for each time point, normalized to the endogenous reference, were log-transformed and were imported into GraphPad Prism, version 5. The mRNA half-life was estimated using nonlinear regression analysis with one phase dissociation. Data are presented as the percentage of nfiS or nifK transcript levels relative to the amount of these transcripts at time point zero.
Discussion
This work reports the characterization of NfiS, an ncRNA specific to P. stutzeri, which is dramatically induced under nitrogen fixation conditions. The pattern of nfiS expression in mutant strains for rpoN, ntrC, or nifA impaired in the nitrogen fixation process is in agreement with a role for NfiS in nitrogen fixation. In addition, the mutant strain displayed reduced stress responses, and putative target genes of the kat and pro operons were identified. Structural and functional divergences of NfiS in diazotrophic and nondiazotrophic P. stutzeri are schematically shown in Fig. S5B, reflecting the evolution of NfiS in different ways due to different niches and selection pressures, which is why it is proposed that the primary function of NfiS in P. stutzeri is related to the stress response, and it also implies that NfiS likely contains another nucleotide sequence pairing with the stress resistance target genes, not yet identified.
In the nitrogen-fixing strain P. stutzeri A1501, NfiS, which is located in the core genome, controls the expression of genes located in the horizontally acquired nif island. This finding raises interesting questions concerning the evolution of nitrogen fixation ability within P. stutzeri strains. There is growing evidence that bacteria use gene acquisition to adapt to ecological niches. For example, among nitrogen-fixing systems, the acquisition of symbiotic islands was reported for rhizobia (37); analysis of Azospirillum genomes revealed that a large proportion of the genes were acquired to adapt from marine to terrestrial environments (38) and that the evolution of nitrogenase from anoxic to aerobic environments was made possible due to gene acquisition (39).
However, it has long been established that acquisition of new metabolic properties could decrease host fitness. Small regulatory RNAs together with the chaperone Hfq were reported to play critical roles in the general regulatory pathways operating in the host (4, 14, 40, 41). For example, Skippington and Ragan revealed that more than 80% of genes targeted by Hfq-associated core sRNAs were horizontally acquired within the E. coli–Shigella clade (4). A large number of phenotypic changes were reported in hfq mutant strains of Sinorhizobium meliloti, which were particularly impaired in nodulation of the host plant (42, 43). A recent analysis of Hfq RNA targets in S. meliloti revealed extensive control of symbiotic regulons and of operons involved in the stress response (14). It is proposed that the Hfq chaperone plays a role in the regulation of nitrogenase activity (Fig. S2) and the stability of NfiS (Fig. 1B), which likely results in better integration of laterally acquired nif genes into existing regulatory networks.
Analysis of the secondary structure of NfiS led to the hypothesis that an 11-nt sequence was critical in the control of nitrogen fixation, particularly in the putative pairing with the mRNA of nifK (Fig. S1D and Fig. 2A). Mutational analysis and microscale thermophoresis experiments support the hypothesis that NfiS was recruited for nitrogen fixation and the conclusion that NfiS plays a role in the stabilization of the nifK mRNA resulting in increased nitrogenase activity (Figs. 2B, 3, and 4). What is commonly referred to as the “nitrogenase” (Mo-Nitrogenase) is an enzymatic complex, composed of two proteins: the MoFe protein, a heterotetramer encoded by the nifD and nifK genes, and the Fe protein, a dimer encoded by nifH (44–46). The nitrogenase structural genes (nifHDK) are organized in one operon in most nif systems (22). The nifK gene is located in the distal position, thereby exhibiting relative lower transcriptional and translational activities than proximal genes. In a former work (23), it was shown that, under nitrogen fixation conditions, the relative factors of expression for nifH, nifD, and nifK were of 94-, 54-, and 38- fold, respectively, suggesting that NifK synthesis is a rate-limiting step. Similar decreases in nifHDK transcript abundance were observed in Azotobacter vinelandii, which seem to be affected by RNA secondary structures between three nif structural genes (47). Therefore, the involvement of NfiS in the translation efficiency of the nifK gene, as shown in Fig. 3A, is critical for the synthesis of nitrogenase polypeptides in a correct ratio for optimal nitrogenase activity.
The involvement of NfiS in the posttranslational regulation of nifK raised the question as to whether similar mechanisms could exist in other diazotrophs. Because the complementary sequence of nifK mRNA was identified in the corresponding region of 31 nifK genes from 42 representative diazotrophs (Table S3 and Fig. S5C), it is proposed that similar mechanisms, involving as yet unidentified ncRNA(s), might operate in other diazotrophs. It is also tempting to speculate that other ncRNA targets could be different from nifK mRNA in some systems because NfiS is P. stutzeri-specific. Former reports suggested that an Hfq homolog, NrfA, was required for nifA expression in Azorhizobium caulinodans and Rhodobacter capsulatus although the involvement of an RNA molecule in this regulation was not shown (48–50). The nif islands in P. stutzeri isolated from diverse niches and geographical origin revealed relatively high degrees of conservation (18–21), also implying that this ncRNA-based regulatory role is not limited to A1501 but can be extended to other nitrogen-fixing isolates, including the unique Pseudomonas azotifigens strain DSM17556 that is phylogenetically close to P. stutzeri (18, 21, 51).
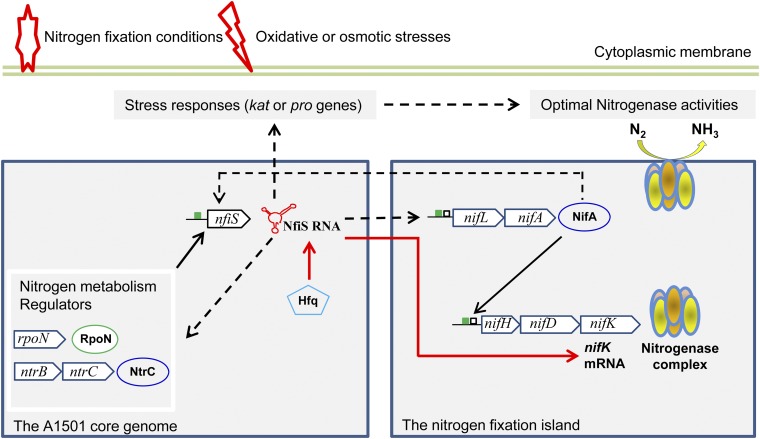
The schematic presented in Fig. 6 tentatively illustrates the role of NfiS in the regulation of nitrogenase activity according to our current knowledge. At the nif-specific level, there are at least two potential mechanisms that may contribute to the NfiS-mediated increase of nitrogenase activity. First, the positive effect of NfiS on the transcription of nif genes is likely mediated via RpoN and NifA, but how NfiS positively affects nifA transcription is not understood at this stage. It is tempting to speculate that this regulation could result from an effect of NfiS on rpoN, ntrC, and glnK, known to be involved in the transcription of nifA (24, 25, 32). Second, NfiS is involved in the posttranscriptional regulation of nifK, increasing the translation efficiency and the half-life of the mRNA. The Hfq protein is likely involved in the stabilization of the NfiS–nifK mRNA complex. Another level of complexity could arise from cross-talk between NfiS and NifA. Data reported in this work suggest that NfiS and NifA positively affect each other. Expression of nfiS was strongly decreased in a nifA mutant (Fig. 1A) whereas expression of nifA was decreased in the nfiS-deleted background (Fig. S4A). In other systems, such as Bradyrhizobium japonicum, targets of NifA include not only the nif and fix genes but also genes that are indirectly related to nitrogen fixation, such as denitrification genes (52, 53). Thus, laterally acquired NifA could affect other core genome-encoded genes via NfiS, establishing a genetic link between nitrogen fixation and other cellular metabolism.
Fig. 6.
Proposed model of NfiS-mediated regulation of optimal nitrogenase activity. The figure shows the interactions between NfiS in the core genome and the horizontally acquired nif island. The green box represents the putative RpoN-dependent promoters, and the black box represents the upstream activator sequences. Solid black and red arrows indicate transcriptional regulation and posttranscriptional regulation, respectively, and dashed lines represent unknown mechanisms. For more details, see the Discussion.
In conclusion, NfiS is, to our knowledge, the first identified Hfq-dependent ncRNA activated by the RpoN/NtrC/NifA cascade in response to environmental signals and is, to our knowledge, the first described case of a bacterial small RNA involved in the regulation of optimal nitrogen fixation via a direct base-paring interaction. At the level of global regulation, NfiS up-regulates various regulators, such as RpoN, NtrC, GlnK, and RpoS, controlling the stress response, nitrogen metabolism, and possibly other functions not yet identified.
Materials and Methods
Bacterial Strains, Plasmids, Oligonucleotides, Media, and Growth Conditions.
Strains and plasmids are listed in Table S1. Details of plasmid and mutant strain construction are in SI Materials and Methods and Fig. S6. P. stutzeri A1501 WT (54) and mutant derivatives were grown in LB or in minimal lactate medium (medium K) at 30 °C (24). Antibiotics were used at the following concentrations: 100 μg/mL ampicillin (Amp), 50 μg/mL kanamycin (Km), 10 μg/mL tetracycline (Tc), and 34 μg/mL spectinomycin (Spc).
Nitrogenase Activity Assays.
Nitrogenase activity was determined with bacterial suspensions incubated at an OD600 of 0.1, in N-free minimal medium [argon atmosphere, containing 0.5% oxygen and 10% (vol/vol) acetylene] at 30 °C, according to the derepression protocol (24). See SI Materials and Methods for more details. The specific activity of nitrogenase was expressed as nmol ethylene per hour per milligram of protein. Protein concentrations were determined using a standard protein assay (Bio-Rad) with BSA as the standard. Each experiment was repeated at least three times.
Abiotic Stress-Resistance Assays.
P. stutzeri WT and mutant derivatives were grown in LB medium at 30 °C to an OD600 of 0.6 and then transferred into fresh LB medium in the presence or absence of 0.3 M sorbitol or 20 mM H2O2. At the time indicated, 10 times serial dilutions were made, and 10 μL of each dilution was spotted onto solid LB plates.
Northern Blot Analyses for NfiS Expression.
Total RNA was isolated from P. stutzeri WT and mutant derivatives grown under nitrogen fixation or non-nitrogen-fixation conditions. RNA samples (10 μg) were separated on 1.5% agarose gels in 1× Mops buffer by electrophoresis and electroblotted onto positively charged nylon membranes (Amersham). Membranes were UV cross-linked and hybridized for 20 h at 65 °C with the suitable digoxigenin-labeled probe. A 30-nt single-strand DNA probe for NfiS was synthesized, and the 5′ end of the synthesized product was labeled with digoxigenin (Sangon Company). Membranes were washed twice in 0.1% SDS/2× SSC buffer and then twice in 0.1% SDS/0.1× SSC buffer before exposure to film (Kodak). Band intensity was analyzed using ImageJ software.
Quantitative Real-Time PCR.
Primer pairs used for qRT-PCR are listed in Table S4. Amplifications were conducted on an ABI PRISM 7500 real time PCR system (Applied Biosystems). See SI Materials and Methods for more details. Data were analyzed using the ABI PRISM 7500 Sequence Detection System Software (Applied Biosystems).
The 5′ Rapid Amplification of cDNA Ends to Determine nfiS Transcriptional Start Site.
The 5′ rapid amplification of cDNA ends (RACE) system (Invitrogen) was used according to the manufacturer’s instructions (SI Materials and Methods). Products were visualized on standard 2% agarose gels stained with ethidium bromide. PCR-generated bands were extracted from the gel, ligated into the pMD18-T vector (Takara), and sequenced.
Western Blot Analysis for NifDK Expression.
Western blotting was performed on protein extracts of bacterial cells incubated for 5 h under nitrogen fixation conditions. Proteins separated by SDS/PAGE were transferred to a PVDF membrane (Amersham) by electroblotting. NifD and NifK polypeptides were revealed with antisera raised against the MoFe-protein. See extra details in SI Materials and Methods.
Microscale Thermophoresis Measurements.
MST experiments were performed according to ref. 55. The dissociation constants (Kds) were calculated as described (56). Data analyses were performed using Nanotemper Analysis software v.1.2.101 (NanoTemper Technologies). See extra details in SI Materials and Methods.
Measurement of NfiS and nifK mRNA Half-Lives.
Two-milliliter samples were collected at different times (0, 5, 10, 15, 20, and 25 min) right after the addition of rifampicin (400 μg/mL), and total RNA was prepared to estimate mRNA levels by qRT-PCR. The mRNA half-lives were estimated using nonlinear regression analysis with one phase dissociation (SI Materials and Methods).
Acknowledgments
We thank Prof. Ray Dixon for helpful suggestions and for critical reading of the typescript and Prof. Ying Li for the gift of MoFe protein antiserum. This work was supported by National Basic Research Program of China Grant 2015CB755700; National Science Foundation of China Grants 31230004, 31470205, and 31470174; and National High-Tech Program Grant 2012AA02A703. This study was also conducted with the support of Institut Pasteur and the One Hundred-Talent Plan of the Chinese Academy of Sciences.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1604514113/-/DCSupplemental.
References
- 1.Wassarman KM. Small RNAs in bacteria: Diverse regulators of gene expression in response to environmental changes. Cell. 2002;109(2):141–144. doi: 10.1016/s0092-8674(02)00717-1. [DOI] [PubMed] [Google Scholar]
- 2.Storz G, Vogel J, Wassarman KM. Regulation by small RNAs in bacteria: Expanding frontiers. Mol Cell. 2011;43(6):880–891. doi: 10.1016/j.molcel.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sonnleitner E, Haas D. Small RNAs as regulators of primary and secondary metabolism in Pseudomonas species. Appl Microbiol Biotechnol. 2011;91(1):63–79. doi: 10.1007/s00253-011-3332-1. [DOI] [PubMed] [Google Scholar]
- 4.Skippington E, Ragan MA. Evolutionary dynamics of small RNAs in 27 Escherichia coli and Shigella genomes. Genome Biol Evol. 2012;4(3):330–345. doi: 10.1093/gbe/evs001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamura T, Naito K, Yokota N, Sugita C, Sugita M. A cyanobacterial non-coding RNA, Yfr1, is required for growth under multiple stress conditions. Plant Cell Physiol. 2007;48(9):1309–1318. doi: 10.1093/pcp/pcm098. [DOI] [PubMed] [Google Scholar]
- 6.Jung YS, Kwon YM. Small RNA ArrF regulates the expression of sodB and feSII genes in Azotobacter vinelandii. Curr Microbiol. 2008;57(6):593–597. doi: 10.1007/s00284-008-9248-z. [DOI] [PubMed] [Google Scholar]
- 7.Sonnleitner E, Abdou L, Haas D. Small RNA as global regulator of carbon catabolite repression in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2009;106(51):21866–21871. doi: 10.1073/pnas.pnas.0910308106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sonnleitner E, et al. The small RNA PhrS stimulates synthesis of the Pseudomonas aeruginosa quinolone signal. Mol Microbiol. 2011;80(4):868–885. doi: 10.1111/j.1365-2958.2011.07620.x. [DOI] [PubMed] [Google Scholar]
- 9.Mika F, et al. Targeting of csgD by the small regulatory RNA RprA links stationary phase, biofilm formation and cell envelope stress in Escherichia coli. Mol Microbiol. 2012;84(1):51–65. doi: 10.1111/j.1365-2958.2012.08002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jørgensen MG, Thomason MK, Havelund J, Valentin-Hansen P, Storz G. Dual function of the McaS small RNA in controlling biofilm formation. Genes Dev. 2013;27(10):1132–1145. doi: 10.1101/gad.214734.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wenner N, Maes A, Cotado-Sampayo M, Lapouge K. NrsZ: A novel, processed, nitrogen-dependent, small non-coding RNA that regulates Pseudomonas aeruginosa PAO1 virulence. Environ Microbiol. 2014;16(4):1053–1068. doi: 10.1111/1462-2920.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan X, et al. Cross-talk between a regulatory small RNA, cyclic-di-GMP signalling and flagellar regulator FlhDC for virulence and bacterial behaviours. Environ Microbiol. 2015;17(11):4745–4763. doi: 10.1111/1462-2920.13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papenfort K, Förstner KU, Cong JP, Sharma CM, Bassler BL. Differential RNA-seq of Vibrio cholerae identifies the VqmR small RNA as a regulator of biofilm formation. Proc Natl Acad Sci USA. 2015;112(7):E766–E775. doi: 10.1073/pnas.1500203112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torres-Quesada O, et al. Genome-wide profiling of Hfq-binding RNAs uncovers extensive post-transcriptional rewiring of major stress response and symbiotic regulons in Sinorhizobium meliloti. RNA Biol. 2014;11(5):563–579. doi: 10.4161/rna.28239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soper T, Mandin P, Majdalani N, Gottesman S, Woodson SA. Positive regulation by small RNAs and the role of Hfq. Proc Natl Acad Sci USA. 2010;107(21):9602–9607. doi: 10.1073/pnas.1004435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silby MW, Winstanley C, Godfrey SAC, Levy SB, Jackson RW. Pseudomonas genomes: Diverse and adaptable. FEMS Microbiol Rev. 2011;35(4):652–680. doi: 10.1111/j.1574-6976.2011.00269.x. [DOI] [PubMed] [Google Scholar]
- 17.Yan Y, et al. Nitrogen fixation island and rhizosphere competence traits in the genome of root-associated Pseudomonas stutzeri A1501. Proc Natl Acad Sci USA. 2008;105(21):7564–7569. doi: 10.1073/pnas.0801093105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan YL, et al. In: Genome transcriptome analysis and functional characterization of a nitrogen-fixation island in root-associated Pseudomonas stutzeri. Molecular Microbial Ecology of the Rhizosphere. De Bruijn FJ, editor. Vol 2. Wiley-Blackwell; Hoboken, NJ: 2013. pp. 853–863. [Google Scholar]
- 19.Yu H, et al. Complete genome sequence of the nitrogen-fixing and rhizosphere-associated bacterium Pseudomonas stutzeri strain DSM4166. J Bacteriol. 2011;193(13):3422–3423. doi: 10.1128/JB.05039-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bentzon-Tilia M, Severin I, Hansen LH, Riemann L. Genomics and ecophysiology of heterotrophic nitrogen-fixing bacteria isolated from estuarine surface water. MBio. 2015;6(4):e00929. doi: 10.1128/mBio.00929-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venieraki A, et al. The nitrogen-fixation island insertion site is conserved in diazotrophic Pseudomonas stutzeri and Pseudomonas sp. isolated from distal and close geographical regions. PLoS One. 2014;9(9):e105837. doi: 10.1371/journal.pone.0105837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dixon R, Kahn D. Genetic regulation of biological nitrogen fixation. Nat Rev Microbiol. 2004;2(8):621–631. doi: 10.1038/nrmicro954. [DOI] [PubMed] [Google Scholar]
- 23.Yan Y, et al. Global transcriptional analysis of nitrogen fixation and ammonium repression in root-associated Pseudomonas stutzeri A1501. BMC Genomics. 2010;11:11. doi: 10.1186/1471-2164-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desnoues N, et al. Nitrogen fixation genetics and regulation in a Pseudomonas stutzeri strain associated with rice. Microbiology. 2003;149(Pt 8):2251–2262. doi: 10.1099/mic.0.26270-0. [DOI] [PubMed] [Google Scholar]
- 25.Xie Z, et al. Interaction between NifL and NifA in the nitrogen-fixing Pseudomonas stutzeri A1501. Microbiology. 2006;152(Pt 12):3535–3542. doi: 10.1099/mic.0.29171-0. [DOI] [PubMed] [Google Scholar]
- 26.Lin M, et al. Regulatory coupling of nitrogen and carbon metabolism in nitrogen-fixing Pseudomonas stutzeri A1501. In: De Bruijn FJ, editor. Biological Nitrogen Fixation. Vol 1. Wiley-Blackwell; Hoboken, NJ: 2015. pp. 109–119. [Google Scholar]
- 27.Dühring U, Axmann IM, Hess WR, Wilde A. An internal antisense RNA regulates expression of the photosynthesis gene isiA. Proc Natl Acad Sci USA. 2006;103(18):7054–7058. doi: 10.1073/pnas.0600927103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vercruysse M, et al. Genome-wide detection of predicted non-coding RNAs in Rhizobium etli expressed during free-living and host-associated growth using a high-resolution tiling array. BMC Genomics. 2010;11:53. doi: 10.1186/1471-2164-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huerta JM, Aguilar I, López-Pliego L, Fuentes-Ramírez LE, Castañeda M. The role of the ncRNA RgsA in the oxidative stress response and biofilm formation in Azotobacter vinelandii. Curr Microbiol. 2016;72(6):671–679. doi: 10.1007/s00284-016-1003-2. [DOI] [PubMed] [Google Scholar]
- 30.Weber H, Polen T, Heuveling J, Wendisch VF, Hengge R. Genome-wide analysis of the general stress response network in Escherichia coli: SigmaS-dependent genes, promoters, and sigma factor selectivity. J Bacteriol. 2005;187(5):1591–1603. doi: 10.1128/JB.187.5.1591-1603.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arcondéguy T, Jack R, Merrick M. P(II) signal transduction proteins, pivotal players in microbial nitrogen control. Microbiol Mol Biol Rev. 2001;65(1):80–105. doi: 10.1128/MMBR.65.1.80-105.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He S, et al. Involvement of GlnK, a PII protein, in control of nitrogen fixation and ammonia assimilation in Pseudomonas stutzeri A1501. Arch Microbiol. 2008;190(1):1–10. doi: 10.1007/s00203-008-0354-x. [DOI] [PubMed] [Google Scholar]
- 33.Singh P, Tiwari A, Singh SP, Asthana RK. Proline biosynthesizing enzymes (glutamate 5-kinase and pyrroline-5-carboxylate reductase) from a model cyanobacterium for desiccation tolerance. Physiol Mol Biol Plants. 2013;19(4):521–528. doi: 10.1007/s12298-013-0186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milse J, Petri K, Rückert C, Kalinowski J. Transcriptional response of Corynebacterium glutamicum ATCC 13032 to hydrogen peroxide stress and characterization of the OxyR regulon. J Biotechnol. 2014;190(4):40–54. doi: 10.1016/j.jbiotec.2014.07.452. [DOI] [PubMed] [Google Scholar]
- 35.Wienken CJ, Baaske P, Duhr S, Braun D. Thermophoretic melting curves quantify the conformation and stability of RNA and DNA. Nucleic Acids Res. 2011;39(8):e52. doi: 10.1093/nar/gkr035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baaske P, Wienken CJ, Reineck P, Duhr S, Braun D. Optical thermophoresis for quantifying the buffer dependence of aptamer binding. Angew Chem Int Ed Engl. 2010;49(12):2238–2241. doi: 10.1002/anie.200903998. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan JT, Ronson CW. Evolution of rhizobia by acquisition of a 500-kb symbiosis island that integrates into a phe-tRNA gene. Proc Natl Acad Sci USA. 1998;95(9):5145–5149. doi: 10.1073/pnas.95.9.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wisniewski-Dyé F, et al. Azospirillum genomes reveal transition of bacteria from aquatic to terrestrial environments. PLoS Genet. 2011;7(12):e1002430. doi: 10.1371/journal.pgen.1002430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boyd ES, Costas AMG, Hamilton TL, Mus F, Peters JW. Evolution of molybdenum nitrogenase during the transition from anaerobic to aerobic metabolism. J Bacteriol. 2015;197(9):1690–1699. doi: 10.1128/JB.02611-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madan Babu M, Teichmann SA, Aravind L. Evolutionary dynamics of prokaryotic transcriptional regulatory networks. J Mol Biol. 2006;358(2):614–633. doi: 10.1016/j.jmb.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 41.Navarre WW, McClelland M, Libby SJ, Fang FC. Silencing of xenogeneic DNA by H-NS-facilitation of lateral gene transfer in bacteria by a defense system that recognizes foreign DNA. Genes Dev. 2007;21(12):1456–1471. doi: 10.1101/gad.1543107. [DOI] [PubMed] [Google Scholar]
- 42.Barra-Bily L, et al. Proteomic alterations explain phenotypic changes in Sinorhizobium meliloti lacking the RNA chaperone Hfq. J Bacteriol. 2010;192(6):1719–1729. doi: 10.1128/JB.01429-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao M, Barnett MJ, Long SR, Teplitski M. Role of the Sinorhizobium meliloti global regulator Hfq in gene regulation and symbiosis. Mol Plant Microbe Interact. 2010;23(4):355–365. doi: 10.1094/MPMI-23-4-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rubio LM, Ludden PW. Maturation of nitrogenase: A biochemical puzzle. J Bacteriol. 2005;187(2):405–414. doi: 10.1128/JB.187.2.405-414.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Newton WE. Physiology, biochemistry and molecular biology of nitrogen fixation. In: Hermann B, Stuart JF, Newton WE, editors. Biology of the Nitrogen Cycle. Elsevier; Amsterdam: 2007. pp. 109–129. [Google Scholar]
- 46.Lowe DJ, Thorneley RN. The mechanism of Klebsiella pneumoniae nitrogenase action: Pre-steady-state kinetics of H2 formation. Biochem J. 1984;224(3):877–886. doi: 10.1042/bj2240877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamilton TL, et al. Differential accumulation of nif structural gene mRNA in Azotobacter vinelandii. J Bacteriol. 2011;193(17):4534–4536. doi: 10.1128/JB.05100-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaminski PA, Desnoues N, Elmerich C. The expression of nifA in Azorhizobium caulinodans requires a gene product homologous to Escherichia coli HF-I, an RNA-binding protein involved in the replication of phage Q beta RNA. Proc Natl Acad Sci USA. 1994;91(11):4663–4667. doi: 10.1073/pnas.91.11.4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaminski PA, Elmerich C. The control of Azorhizobium caulinodans nifA expression by oxygen, ammonia and by the HF-I-like protein, NrfA. Mol Microbiol. 1998;28(3):603–613. doi: 10.1046/j.1365-2958.1998.00823.x. [DOI] [PubMed] [Google Scholar]
- 50.Drepper T, et al. The Hfq-like protein NrfA of the phototrophic purple bacterium Rhodobacter capsulatus controls nitrogen fixation via regulation of nifA and anfA expression. FEMS Microbiol Lett. 2002;215(2):221–227. doi: 10.1111/j.1574-6968.2002.tb11394.x. [DOI] [PubMed] [Google Scholar]
- 51.Hatayama K, Kawai S, Shoun H, Ueda Y, Nakamura A. Pseudomonas azotifigens sp. nov., a novel nitrogen-fixing bacterium isolated from a compost pile. Int J Syst Evol Microbiol. 2005;55(Pt 4):1539–1544. doi: 10.1099/ijs.0.63586-0. [DOI] [PubMed] [Google Scholar]
- 52.Bueno E, Mesa S, Sanchez C, Bedmar EJ, Delgado MJ. NifA is required for maximal expression of denitrification genes in Bradyrhizobium japonicum. Environ Microbiol. 2010;12(2):393–400. doi: 10.1111/j.1462-2920.2009.02076.x. [DOI] [PubMed] [Google Scholar]
- 53.Hauser F, et al. Dissection of the Bradyrhizobium japonicum NifA+σ54 regulon, and identification of a ferredoxin gene (fdxN) for symbiotic nitrogen fixation. Mol Genet Genomics. 2007;278(3):255–271. doi: 10.1007/s00438-007-0246-9. [DOI] [PubMed] [Google Scholar]
- 54.Qiu Y, Zhou S, Mo X, You C, Wang D. Investigation of dinitrogen fixation bacteria isolated from rice rhizosphere. Chin Sci Bull. 1981;26(4):383–384. [Google Scholar]
- 55.Jerabek-Willemsen M, Wienken CJ, Braun D, Baaske P, Duhr S. Molecular interaction studies using microscale thermophoresis. Assay Drug Dev Technol. 2011;9(4):342–353. doi: 10.1089/adt.2011.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lippok S, et al. Direct detection of antibody concentration and affinity in human serum using microscale thermophoresis. Anal Chem. 2012;84(8):3523–3530. doi: 10.1021/ac202923j. [DOI] [PubMed] [Google Scholar]
- 57.Shevchuk NA, et al. Construction of long DNA molecules using long PCR-based fusion of several fragments simultaneously. Nucleic Acids Res. 2004;32(2):e19. doi: 10.1093/nar/gnh014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schäfer A, et al. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: Selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene. 1994;145(1):69–73. doi: 10.1016/0378-1119(94)90324-7. [DOI] [PubMed] [Google Scholar]
- 59.Figurski DH, Helinski DR. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Staskawicz B, Dahlbeck D, Keen N, Napoli C. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J Bacteriol. 1987;169(12):5789–5794. doi: 10.1128/jb.169.12.5789-5794.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hardy RFW, Burns RC, Holsten RD. Application of the acetylene-ethylene reduction assay for measurement of nitrogen fixation. Soil Biol Biochem. 1973;5(1):47–81. [Google Scholar]