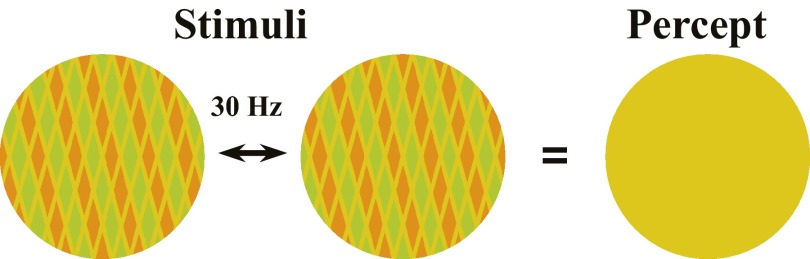
Significance
Here we investigated a key question regarding how the brain resolves conflicting information—specifically, does binocular rivalry competition require conscious awareness of interocular conflicting information? We first showed that a chromatic grating counterphase flickering at 30 Hz became invisible but could induce a significant tilt aftereffect and orientation-selective adaptation. The invisible gratings produced significant BOLD activities in the early visual cortex but not in frontoparietal cortical areas. Further experiments revealed that although the pattern information was not consciously perceived, invisible orientation conflict between the two eyes could induce rivalry competition. Thus, visual competition could occur without conscious representation of the conflicting visual inputs, presumably in the sensory cortex with minimal engagement of high-level cortex and related top-down feedback modulations.
Keywords: binocular rivalry, awareness, visual cortex
Abstract
Binocular rivalry arises when incompatible images are presented to the two eyes. If the two eyes’ conflicting features are invisible, leading to identical perceptual interpretations, does rivalry competition still occur? Here we investigated whether binocular rivalry can be induced from conflicting but invisible spatial patterns. A chromatic grating counterphase flickering at 30 Hz appeared uniform, but produced significant tilt aftereffect and orientation-selective adaptation. The invisible pattern also generated significant BOLD activities in the early visual cortex, with minimal response in the parietal and frontal cortical areas. Compared with perceptually matched uniform stimuli, a monocularly presented invisible chromatic grating enhanced the rivalry competition with a low-contrast visible grating presented to the other eye. Furthermore, switching from a uniform field to a perceptually matched invisible chromatic grating produced interocular suppression at approximately 200 ms after onset of the invisible grating. Experiments using briefly presented monocular probes revealed evidence for sustained rivalry competition between two invisible gratings during continuous dichoptic presentations. These findings indicate that even without visible interocular conflict, and with minimal engagement of frontoparietal cortex and consciousness related top-down feedback, perceptually identical patterns with invisible conflict features produce rivalry competition in the early visual cortex.
When two different images are presented one to each eye, the human visual system resolves the perceptual conflict and engages in binocular rivalry; perception alternates spontaneously between the two eyes’ images (1). Although visual adaptation and noise are known to be factors influencing the dynamics of rivalry (2), the intrinsic factor that initiates rivalry competition remains elusive (3–6). One possibility is that rivalry competition occurs because dichoptic images lead to conflicting perceptual interpretations, and the visual system resolves the perceptual conflict by suppressing one eye’s signal, resulting in a coherent conscious percept. Alternatively, the early stages of the visual system may resolve interocular conflict between dichoptic stimuli regardless of whether or not the perceptual outcomes are compatible. In the first scenario, patterns with invisible conflicting features presumably would not lead to rivalry competition, because there would be no perceptual conflict between eyes; however, the second scenario predicts that rivalry competition should still arise from invisible dichoptic patterns.
In the present study, we investigated whether binocular rivalry could arise from dichoptic stimuli with invisible patterns. The aim was to enhance our understanding of what drives binocular rivalry, including the mechanism that initiates and sustains rivalry competition. If the two eyes’ stimuli, although physically different, yield an identical perceptual outcome, will the brain consider it unnecessary to engage the rivalry mechanism? The results may shed light on the general relationship between visual awareness and resolution of conflicts in visual inputs. It will also help elucidate the role of top-down factors during binocular rivalry (7, 8). When dichoptic patterns are rendered invisible, top-down modulation based on conscious perception of the pattern difference will not be engaged. If binocular rivalry depends on these top-down modulations, then there should be no rivalry competition between invisible patterns; however, if rivalry can be automatically triggered from interocular conflict, then rivalry can still occur with invisible conflicting patterns.
There has long been interest in delineating the relationship between attention and awareness. Previous studies have suggested that visual attention and visual awareness might be two distinct processes in the human brain (9, 10); comparing the effects of attention and awareness on binocular rivalry should shed new light on this important question. Sustained rivalry has been shown to be dependent on focused visual attention. Diverting attention away from the dichoptic stimuli greatly diminishes the rivalrous alternation of two eyes’ signals in the early visual cortex (11) and abolishes behavioral evidence of binocular rivalry (12). In contrast, in the present study we directly tested whether visual awareness of the conflicting spatial patterns is necessary for them to engage in rivalry competition.
In this study, we investigated whether rapidly counterphase-flickering invisible chromatic gratings, which are perceived as an uniform yellow disk, can induce binocular rivalry. A previous psychophysics study showed that flicker adaptation occurs from invisible luminance and chromatic flicker (13), and a functional magnetic resonance imaging (fMRI) study from our group found that the human brain responds to invisible chromatic flicker as far as visual area V4 (14), indicating that the invisible pattern information from chromatic flicker could be processed in retinotopic visual areas. Therefore, such stimuli allow us to ask whether, in the absence of awareness and related high-level cortex, invisible conflicting gratings can induce rivalry competition in the early visual cortex.
Results
To ensure that the orientation information from invisible counterphase-flickered chromatic patterns can reach the primary visual cortex, in experiment 1 we first tested whether such invisible chromatic gratings could activate orientation-selective neurons in the early visual cortex. Prolonged exposure to the invisible grating produced a tilt aftereffect (TAE) and orientation-specific contrast threshold elevation, indicating that the invisible orientation information is effectively represented at least at the primary visual cortex (V1). Experiment 1c showed that invisible gratings produced significant fMRI responses in the early visual cortex, but not in high-level areas in the frontal and parietal cortices. Experiment 2a showed that invisible chromatic gratings presented to one eye prolonged the dominant duration of that eye in competition with a low-contrast orthogonal visible grating in the other eye. Furthermore, using briefly presented probes, experiment 2b directly revealed interocular competition between two invisible gratings, and experiment 2c showed strong evidence for sustained rivalry competition between two invisible gratings during continuous dichoptic presentations.
Experiment 1: Early Visual Cortex Processes Invisible Chromatic Gratings.
It has been shown that an orientation-contingent color aftereffect (McCollough effect) could be induced even when the color alternations are too fast to be consciously perceived (15), suggesting that orientation-color conjunction-selective neurons can track their preferred conjunction much faster than consciousness. Here we sought direct evidence that the chromatic gratings used in our experiments, rendered invisible through fast counterphase modulation, could activate orientation-selective neurons in the early visual cortex, which is a prerequisite for interocular competition. Specifically, we examined whether prolonged exposure to the invisible chromatic gratings could produce a TAE, as well as an orientation-selective contrast threshold elevation.
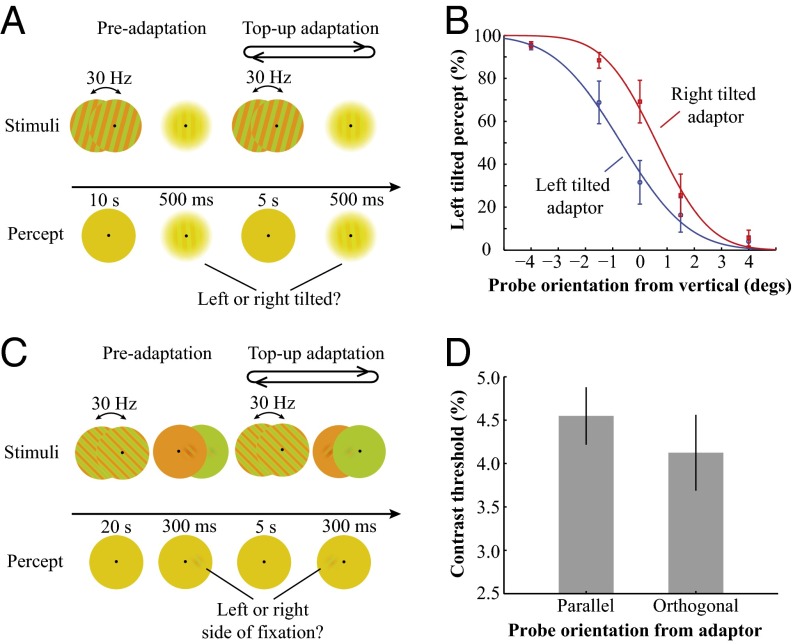
Fig. 1A illustrates the stimuli and procedure for the TAE experiment. Fig. 1B shows that prolonged exposure to the invisible chromatic gratings produced a significant TAE [0.643 degrees, t(4) = 6.935, P = 0.0023]. Vertical test gratings are perceived as tilted away from the adapting orientation after adaptation to an invisible grating tilted 15 degrees from vertical. Fig. S1 shows that subjects' performance to discriminate the orientation of invisible gratings was at chance level in a two-alternative forced choice task (2-AFC). And occasional visible artifacts had minimal influence on the observed TAE (Fig. S2). Fig. 1C illustrates the stimuli and procedure for the orientation-selective adaptation experiment. Fig. 1D shows that after adaptation to the invisible chromatic gratings, the contrast detection threshold for the test Gabor probe parallel with the adapting grating was significantly higher than that for the orthogonal orientation [t(5) = 2.972, P = 0.0311]. These findings are consistent with a previous study showing that orientation-selective adaptation can be induced by prolonged exposure to rapidly contrast-reversed luminance gratings that were subjectively invisible (16). The significant TAE and orientation-selective adaptation effect from invisible chromatic gratings demonstrate that orientation-selective neurons in the early visual cortex (at least V1) were activated by the invisible orientation information.
Fig. 1.
Stimuli, procedure, and results for the behavioral TAE and orientation-selective adaptation experiments. (A) Stimuli and procedure for the TAE experiment. The adaptation procedure started with a 10-s preadaptation period. The adapting stimuli were high-contrast isoluminant R/G square wave gratings tilted 15 degrees clockwise (+) or counterclockwise (−) from the vertical orientation and counterphase flickering at 30 Hz. After the preadaptation period, a low-contrast test grating was briefly presented for 500 ms, and observers made forced-choice responses to indicate whether the test grating appeared top left-tilted or top right-tilted from the vertical orientation. Then 5-s long top-up adaptations were presented, with a 2-AFC probe test following each top-up adaptation period. (B) Psychometric functions of the perceived orientation showing significant TAE. (C) Stimuli and procedure for the orientation-selective adaptation experiment. During the preadaptation period, subjects viewed oblique (+ or −45 degrees) invisible chromatic gratings for 20 s. Then the chromatic pattern was transformed to uniform red/green flickers, and a test Gabor, either parallel or orthogonal to the adapting orientation, was briefly presented. Subjects made forced-choice responses to indicate whether the test stimulus was presented to the left or to the right of fixation. The test Gabor was presented at two oblique orientations with chromatic contrast adaptively adjusted, interleaved with 5-s top-up adaptations to the invisible gratings. (D) The contrast threshold to the probe in the same orientation as the adaptor was significantly higher compared with that in the orthogonal orientation. Error bars indicate SEM.
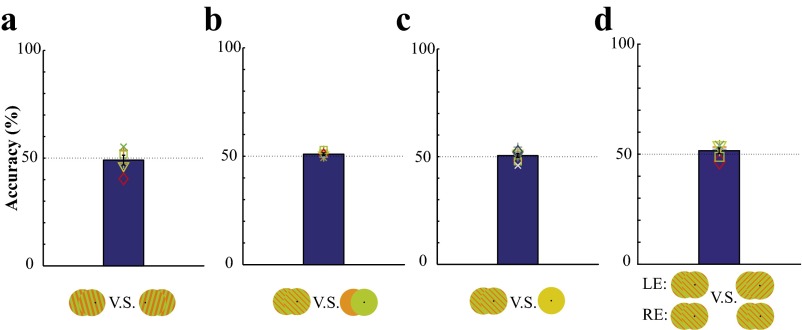
Fig. S1.
(A) The 2-AFC orientation discrimination performance of invisible chromatic gratings (square wave). Invisible chromatic gratings emerged from a solid yellow disk for 2 s at one of the two tilted orientations, accompanied by a pure tone. Subjects made a forced-choice response to indicate whether the invisible grating was tilted clockwise or counterclockwise from the vertical orientation. (B) The 2-AFC detection performance of invisible chromatic gratings (sine wave) from uniform chromatic flickers. Two 600-ms intervals were separated by 500 ms. During one of the intervals, an invisible grating was presented, and during the other, a uniform chromatic flicker was presented. Subjects were asked to identify the interval containing the chromatic grating. (C) The 2-AFC detection performance of invisible chromatic gratings (sine wave) from a static yellow disk. An invisible grating was presented during one of the two intervals, and a static yellow disk was presented during the other interval. Subjects identified the interval containing the chromatic grating. (D) The 2-AFC discrimination performance between dichoptic orthogonal and parallel invisible gratings (sine wave). A reference image with orthogonal or parallel invisible gratings was presented dichoptically for 600 ms, followed by two 600-ms intervals, one with dichoptic orthogonal invisible gratings and the other with dichoptic parallel invisible gratings. Subjects made a forced-choice response to indicate which interval contained the same image as the reference.
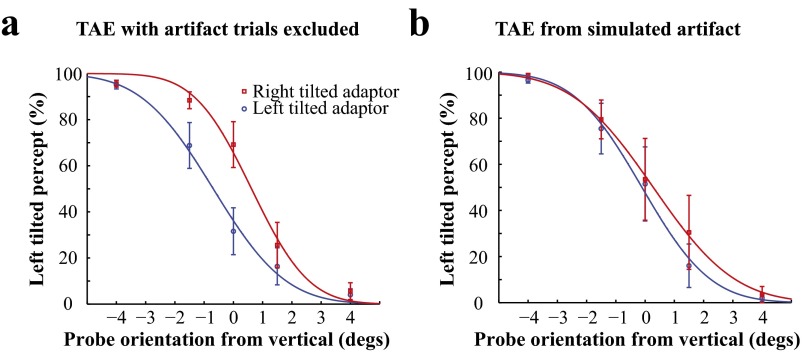
Fig. S2.
(A) Significant TAE after the trials with the artifact were excluded. (B) No significant TAE from occasional exposure to high-contrast chromatic gratings.
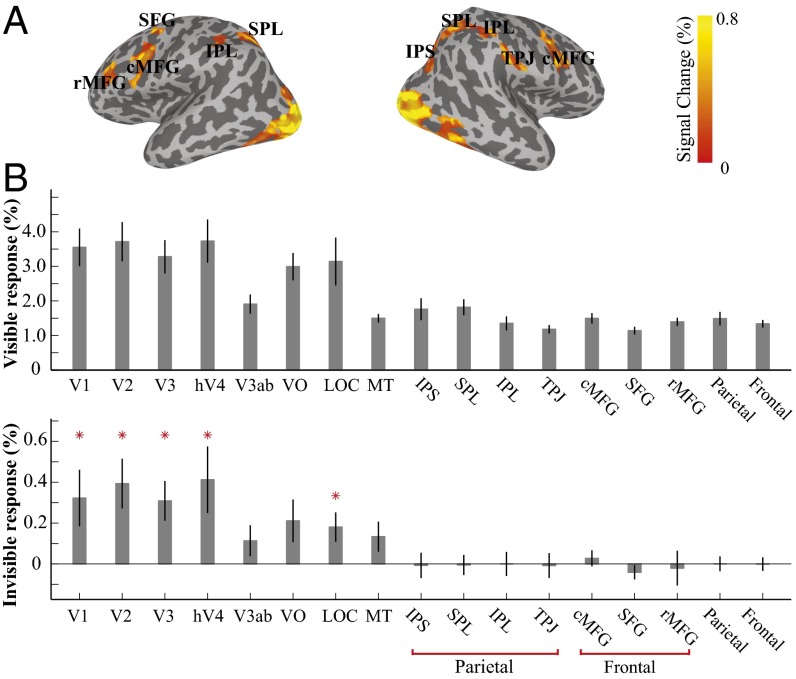
In an fMRI experiment (experiment 1c), we further investigated the neural sites that invisible chromatic gratings could reach in the cerebral cortex. A long spaced event-related design was used to detect BOLD responses to a central stimulus changing from a static solid yellow disk to chromatic gratings flickering at 7.5 Hz (visible) or 30 Hz (invisible) for 2 s, followed by a 14-s interstimulus interval (ISI) of the solid yellow disk. During the experiment, subjects performed a central fixation task to detect occasional luminance changes of the fixation. The results show that invisible gratings produced a significant BOLD response in the early visual areas from V1 to V4, as well as in the lateral occipital complex, but no or minimal response in the parietal and frontal cortices (Fig. 2B). Therefore, the invisible orientation information from fast-flickering chromatic grating reached the early retinotopic visual areas, but remained “invisible” to the frontoparietal brain regions. In experiment 2, we asked whether invisible chromatic gratings, without producing perceptual conflict or activating high-level brain areas, could induce rivalry competition.
Fig. 2.
fMRI BOLD activities to the visible (7.5 Hz) and invisible (30 Hz) chromatic gratings. Each stimulus, centered at the fixation, was provided for 2 s, followed by a 14-s ISI. (A) fMRI BOLD activation from the visible gratings on the cortical surface of a representative subject. Occipital and temporal visual areas, as well as a series of parietal and frontal cortical regions, were activated (threshold: P < 0.05, uncorrected). (B) Mean responses from these areas [regions of interest (ROIs)] were extracted for both the visible and invisible gratings. Mean BOLD activities in different cortical regions were averaged across subjects. Whereas the visible gratings generated significant activities in all ROIs (Upper), the invisible gratings produced significant fMRI responses only in the early and intermediate visual areas, with no or minimal responses in the frontal and parietal cortical areas (Lower). ROI labels: LOC, lateral occipital complex; IPS, intraparietal sulcus; SPL, superior parietal lobe; IPL, inferior parietal lobe; TPJ, temporal parietal junction; SFG, superior frontal gyrus; cMFG, caudal middle frontal gyrus; rMFG, rostral middle frontal gyrus; parietal, average parietal response of IPS, SPL, IPL and TPJ; frontal, average frontal response of SFG, cMFG, and rMFG. Asterisks in the invisible condition indicate significance above 0 (P < 0.05).
Experiment 2: Binocular Rivalry from Invisible Chromatic Gratings.
Because possible alternation between two perceptually identical invisible gratings could not be directly monitored and thus explicitly reported, we used several approaches to assess whether rivalry competition could arise from invisible conflicting patterns. In experiment 2a, we compared the potency of an invisible grating vs. a uniform field when pitted against a visible grating during dichoptic presentation, indexed by the dominance duration of the visible grating. In experiments 2b and 2c, the two dichoptically presented gratings were both rendered invisible, and a monocular probe at the threshold-level contrast was briefly presented to one eye and then the other to gauge each eye’s sensitivity during dichoptic viewing of invisible gratings. In experiment 2b, we adopted the interocular flash suppression paradigm to test whether an invisible grating presented to one eye could be interocularly suppressed by abrupt and unperceived presentation of an orthogonal invisible grating to the other eye. In experiment 2c, to investigate whether two dichoptic invisible gratings could engage in sustained rivalry, we measured the sensitivity to a monocular probe during continuous dichoptic presentation of invisible patterns.
Experiment 2a: Binocular rivalry between invisible and visible chromatic gratings.
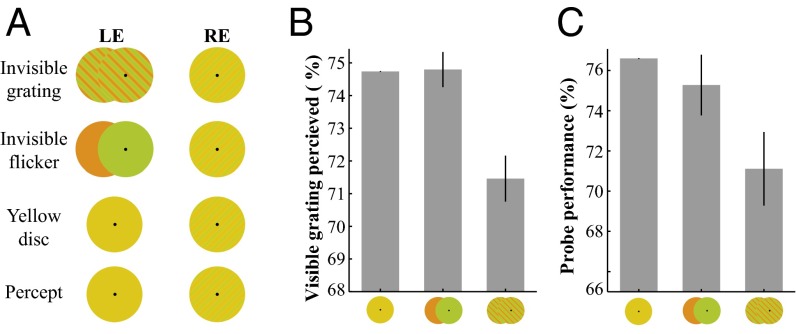
Experiment 2a tested whether invisible chromatic gratings presented to one eye could enhance rivalry competition with an orthogonal visible grating in the other eye. As shown in Fig. 3A, one eye was presented with a low-contrast but clearly visible chromatic grating, and the other eye was presented with one of three types of stimuli: an invisible flickering chromatic grating in the orthogonal orientation, a flickering uniform red/green (R/G) disk, or a static uniform yellow disk. The key here is that all three stimuli were perceived as a static yellow disk; subjects were unable to distinguish among them (Fig. S1 B and C). However, if the spatial pattern in the invisible grating could contribute to rivalry competition, then the visible competing grating would be perceived to disappear more often. During multiple 60-s observation periods, subjects were asked to report whether they perceived the low-contrast visible grating, the static yellow disk, or only parts of the grating (mixed percept). Fig. 3B shows that the total time that subjects perceived the low-contrast grating (all or parts of the grating) was significantly shorter when the stimulus in the fellow eye was the invisible chromatic grating compared with the other two conditions without spatial patterns [vs. R/G disk flicker: t(7) = 4.895, P = 0.0018; vs. static yellow disk: t(7) = 4.663, P = 0.0023]. This finding indicates that the invisible pattern information in the chromatic gratings contributed to and enhanced rivalry competition with an orthogonal visible grating, thereby shortening the dominant duration of the visible grating in sustained rivalry.
Fig. 3.
Binocular rivalry between the dichoptically presented invisible and visible gratings. (A) Stimuli and percepts for the subjective report experiment. One eye was presented with a low-contrast visible grating, and the other eye was presented with one of three stimuli: invisible chromatic grating, flickering R/G disk, or static uniform yellow disk. (B) Duration (in percentage of total presentation time) of the visible grating reported during sustained rivalry. This duration was significantly lower when the competing stimulus was an invisible chromatic grating. (C) Results of the probe detection experiment. The dichoptic stimuli for this experiment were the same as in A. A test probe, generated by brief contrast increment of a small part of the low-contrast visible grating, was presented to the left or to right side of fixation. Subjects made forced-choice responses to indicate whether the probe was presented to the left or the right side of fixation. The contrast increment for the probes was fixed at the threshold level for each individual. The detection performance of monocular probes in the eye with the visible grating was significantly lower when the opposing eye was presented with the orthogonal invisible grating compared with the other two control conditions. Error bars indicate SEM. Data in B and C were normalized relative to the value in the yellow disk control condition. For each subject, data in the yellow disk condition were subtracted from all three conditions, after which the mean value (across subjects) of the yellow disk condition was added back. Such normalization did not affect the within-subject statistics.
To obtain more objective measures, we tested whether sensitivity for the eye with the visible grating could be suppressed (indexed by detection performance of a test probe) by an orthogonal invisible chromatic grating presented to the opposing eye. The dichoptic stimuli were the same as in the preceding experiment, and subjects performed a 2-AFC task to detect a test probe briefly presented to the eye with the visible grating. Fig. 3C shows that the detection performance for the probe was significantly worse when the stimulus in the opposing eye was an invisible chromatic grating compared with the other two control stimuli without a spatial pattern [vs. R/G disk flicker: t(5) = 4.152, P = 0.0089; vs. static yellow disk: t(5) = 3.003, P = 0.0300]. This result clearly shows that invisible chromatic gratings in one eye enhanced interocular suppression, leading to the reduced detection of orthogonal orientation information in the opposing eye, further supporting the idea that invisible patterns contribute to rivalry competition.
Therefore, when presented dichoptically against a low-contrast visible grating, the spatial pattern in the invisible grating prolonged its dominant duration in sustained rivalry and also induced stronger interocular suppression. Given the perceptual difference between the two eyes’ stimuli (one uniform and the other a low-contrast grating), we can conclude that invisible spatial patterns contribute to and enhance interocular competition. The question of whether rivalry competition could be initiated from two perceptually identical invisible patterns remains open, however. We addressed this question in the subsequent experiments.
Experiment 2b: Interocular competition between invisible chromatic gratings.
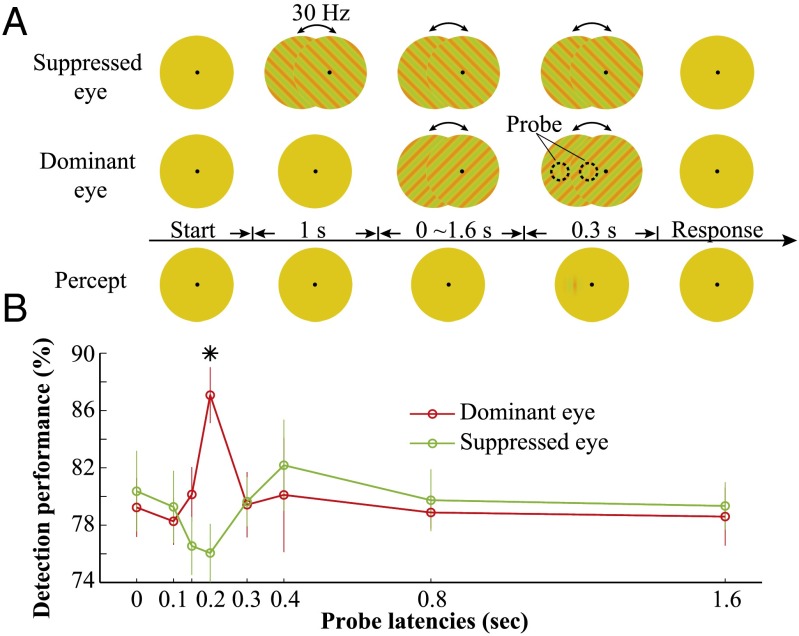
When two visible patterns compete interocularly, a transient change to one eye’s stimulus will enable that eye to gain perceptual dominance almost instantly. This phenomenon, called flash suppression, can be used to test whether interocular competition can be induced from dichoptically presented invisible patterns. In essence, we investigated whether switching from a uniform yellow disk to a perceptually matched invisible grating (i.e., an invisible flash) in one eye could produce interocular flash suppression over an orthogonal invisible grating in the other eye, by measuring the detection performance of a probe presented to the recently flashed (presumably dominant following the flash) eye or to the other eye with the existing invisible grating (presumably suppressed following the flash).
In experiment 2b, one eye was first presented with an invisible chromatic grating, and then in the other eye an initially uniform disk was switched to an orthogonal invisible grating (Fig. 4A). After a variable delay, a test Gabor probe oriented between the two invisible gratings was presented briefly to one eye or the other, and subjects provided 2-AFC responses to the location of the probe. When the probe was presented at approximately 200 ms after the flash, probe detection performance was significantly better when it was presented in the eye with the invisible grating newly switched on compared with the eye with the preexisting invisible grating [t (6) = 4.21, P = 0.0056] (Fig. 4). Fig. S3 shows that the observed interocular competition is not due to rare visual artifact from occasional uncontrolled large eye movements. Visibility tests showed that observers were unable to discriminate the orientation of the invisible gratings in a 2-AFC task (Fig. S1A) or to detect the appearance of invisible gratings in a 2-AFC task (Fig. S1C). These data clearly show that interocular neuronal suppression from the invisible flash occurred approximately 200 ms after onset of the invisible gratings. The onset timing of the flash suppression in the present study is consistent with the timing of initialization of the rivalry between two orthogonal dichoptic visible gratings (17, 18).
Fig. 4.
Interocular competition between two invisible gratings. (A) Stimuli and procedure of the flash suppression experiment. An invisible grating was first presented to the to-be suppressed eye, and then 1 s later the dominant eye was presented with an invisible grating in the orthogonal orientation. After a variable delay of 0–1.6 s, a test Gabor probe in the vertical orientation was briefly presented near the fixation, either to the suppressed eye or to the dominant eye (shown in the dominant eye here). Subjects made forced-choice responses to indicate whether the probe was presented to the left or right side of the fixation. The chromatic contrast of the probe was at the threshold level for each individual subject. (B) Detection performance of the monocular probe at different probe onset latencies. At 200 ms, the detection performance of the probe was significantly higher when presented in the dominant eye compared with in the suppressed eye. *P < 0.01. Error bars indicate SEM.
Fig. S3.
(A) Probe detection performance in the dominant eye and suppressed eye conditions. (B) Performance difference between the two conditions.
Experiment 2c: Sustained rivalry between invisible chromatic gratings.
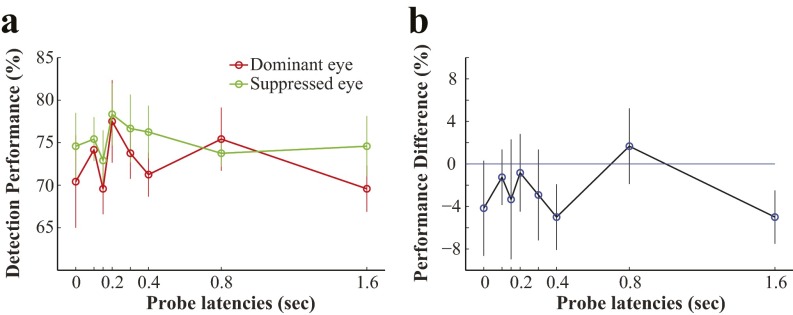
In another series of experiments, we further investigated whether continuously presented dichoptic invisible gratings could lead to sustained rivalry. In the first experiment, we measured sensitivity to a monocular probe when the two eyes’ invisible gratings were either orthogonal or parallel to each other. The orthogonal gratings had the potential to engage in rivalry, whereas the parallel gratings were expected to be fused. The logic for this is that if rivalry competition occurred in the orthogonal condition during the continuous presentation, then we would expect, on average, a lower sensitivity to the monocular probe owing to interocular suppression. Our results show that indeed, sensitivity for detecting the monocular probe was significantly lower in the orthogonal condition than in the parallel condition [t (6) = 3.4968, P = 0.0129] (Fig. 5A). The lower sensitivity in the orthogonal condition supports the existence of sustained alternating interocular suppression between the orthogonal invisible gratings. As shown in Fig. S1D, the dichoptic orthogonal and parallel gratings were perceptually identical; subjects were not aware of interocular conflict during the dichoptic presentation of orthogonal gratings.
Fig. 5.
Sustained rivalry competition between two dichoptic invisible gratings. (A) Orientations of the two eyes’ invisible gratings were either parallel or orthogonal from each other. The detection sensitivity (reciprocal of the detection threshold) of a monocular probe was significantly lower in the orthogonal condition than in the parallel condition. (B) Two orthogonal invisible gratings (one with lower contrast than the other) were presented dichoptically. The detection performance of a monocular probe was significantly lower when it was presented in the eye with the low-contrast grating (probe in the low-contrast eye) than when it was presented in the eye with the high-contrast grating (probe in the high-contrast eye). (C) Two orthogonal gratings were presented dichoptically in full contrast. A monocular probe was presented to one eye every 2 s. The detection performance was higher for probes temporally adjacent to a correctly detected probe (red line) than for probes temporally adjacent to a trial with incorrect responses (green line). *P < 0.05.
In the second experiment, we biased the direction of potential interocular suppression by changing the relative contrast of the two dichoptic invisible gratings, with high contrast in one eye (high-contrast eye) and lower contrast in the opposing eye (low-contrast eye). A threshold-level monocular probe was presented to either eye in random order. The rationale for this approach is that if rivalry competition occurred, then interocular suppression would be stronger from the high-contrast eye to the low-contrast eye than vice versa. Our results show that the probe detection performance was significantly poorer (i.e., exhibited stronger suppression) when the probe was presented to the low-contrast eye than when it was presented to the high-contrast eye [t(6) = 5.4770, P = 0.0015] (Fig. 5B). This sensitivity difference provides further support for the existence of sustained rivalry between two invisible gratings.
In the final experiment, we further investigated the dynamics of the potential sustained rivalry between two dichoptically presented orthogonal invisible gratings. A monocular probe was presented to one eye every 2 s during the 60-s viewing period. If the tested eye underwent alternating dominant and suppressed phases of rivalry, then the detection performance of the probe would be better with the eye in the dominant phase compared with the suppressed phase. In other words, when a correct response is made to the monocular probe, the tested eye would be more likely to be in the dominant phase than in the suppressed phase, and vice versa for an incorrect response. Therefore, probes temporally adjacent to a correctly detected probe (most likely in the dominant phase) should have a greater likelihood of detection than those adjacent to a probe with incorrect responses. Our results show the temporal relationship consistent with this prediction: trials adjacent to the correctly detected probes show significantly better performance than those adjacent to the trials with incorrect responses [t(6) = 3.2430, P = 0.0176] (Fig. 5C). This pattern of result is consistent with predictions based on sustained binocular rivalry alterations during continuous dichoptic presentation of invisible gratings.
To summarize, experiment 2 shows that an invisible grating in one eye can enhance rivalry competition with an orthogonal visible grating in the other eye (experiment 2a). Without any perceived difference in the two eyes’ stimuli, the unperceived onset of an invisible grating can produce interocular flash suppression on another invisible grating (experiment 2b), and there is converging evidence to support the occurrence of sustained rivalry during continuous presentation of conflicting but invisible patterns (experiment 2c).
Discussion
The present study demonstrates that binocular rivalry does not require visual awareness of interocular conflicting information. Even with identical perceptual outcomes, binocular rivalry still occurred between two invisible dichoptic gratings. This result suggests that binocular rivalry transpires when different images are presented to the two eyes, whether or not they lead to conflicting perceptual interpretations. Thus, the neural mechanism responsible for the initiation of rivalry competition operates without consciousness.
The finding that invisible gratings can induce TAE and orientation-selective adaptation, along with direct fMRI measurements, strongly support that the conflicting information from dichoptic invisible gratings was represented in the early visual cortex, but was not effective in activating the frontoparietal cortices. The demonstration of binocular rivalry with invisible gratings suggests that binocular rivalry does not require conscious detection of interocular differences. This observation is consistent with conclusions reached by a recent study showing unreportable rivalry switches between visible motion stimuli (19). Our present findings differ from previously published findings in showing that the rivalry competition can be independent of conscious registration of the conflicting features in the dichoptic stimuli, suggesting that interocular competition is triggered by and resolved by local mechanisms in the early visual cortex.
Previous reports have suggested that binocular rivalry could be independent of awareness. For example, a recent study found evidence of interocular competition from threshold-level motion stimuli (75% accuracy in a 2-AFC task) (20). Although that is an interesting study, given that each eye’s stimulus was at threshold level and often visible, this result by itself could not be taken as clear demonstration of rivalry without awareness. Studies in anesthetized monkeys also showed neural evidence for rivalry-like competition in monkey V1 (4, 21). However, along with the differences between species, there are also important differences between the alert but unaware of the stimulus feature and being in a state of anesthesia; thus, it is difficult to generalize the results from the anesthetized monkeys to alert human observers.
Results from the present study showing binocular rivalry occurs without visual awareness also provide an interesting and important contrast with recent findings indicating that binocular rivalry depends on attention to the dichoptic stimuli (11, 22). These results support the important view that top-down attention and consciousness are two distinct processes (9, 10). The mechanism responsible for resolving visual conflict between the two eyes is likely in the early visual cortex; its operation requires top-down spatial attention, but not visual awareness to the conflicting information.
In addition to binocular rivalry, there is also a report that the neural dynamics underlying motion-induced blindness (MIB) continue without visual awareness of the MIB stimulus (23). Binocular rivalry and MIB, as well as other paradigms that produce fluctuating subjective experience under constant visual input, have been used to study the neural correlation of consciousness (1, 24–26). Our findings from the present study highlight the important point that fluctuations of neural signals in the brain correlated to the alternating perceptual state are just that, correlations. Some of these neural processes are neither the cause nor the effect of changes in conscious visual perception. Thus, we must be particularly cautious in relating neural fluctuations during bistable perception to the neural correlate of consciousness.
Conclusions
Using chromatic gratings with their spatial patterns rendered invisible by fast flicker, we have demonstrated that binocular rivalry could be induced and sustained from invisible orthogonal gratings that are perceptually identical. In stark contrast to the critical role of focused attention on binocular rivalry (11, 12), visual awareness and the associated frontoparietal representation of the conflicting features are not responsible for the initiation and maintenance of binocular rivalry. Apparently, attention enables the conflict-resolution process, and whereas the engagement of this process is independent of awareness of the conflicting features, the outcome of such a process provides the content of conscious representation.
Materials and Methods
Participants.
Five subjects (three males, three naive subjects) participated in experiment 1a, six subjects (three males, four naive subjects) participated in experiment 1b, and nine subjects (six males, seven naive subjects) participated in experiment 1c. Twelve subjects (five males, 10 naive subjects) participated in experiment 2a, seven subjects (two males, five naive subjects) participated in experiment 2b, and seven subjects (four males, five naive subjects) participated in experiment 2c. The subjects ranged in age from 20 to 34 y, and all had normal or corrected to normal vision. All subjects provided written informed consent before the experiments. The Institutional Review Board of the Institute of Biophysics approved the experimental protocols.
Stimuli and Procedures.
Visual stimuli were generated by MATLAB with the Psychophysics Toolbox extension (27, 28), running on a Mac Pro computer, presented on a 27-inch Apple LED cinema display at a screen resolution of 2,560 × 1,440 at 60 Hz. Rapidly counterphase-flickering (30 Hz) chromatic gratings show a visible artifact in-between the R/G stripes on CRT monitors, but not on high-resolution IPS LED monitors. Before each experiment, the subjective isoluminance of red and green for the chromatic grating was adjusted for each observer with the minimal flicker procedure. The procedure was repeated four times, and the results were averaged.
In experiment 1, the adapting stimuli were 30-Hz flickering R/S square wave gratings. In the TAE experiment, the invisible chromatic gratings were tilted +15 or −15 degrees from vertical, were subtended 1.79 degrees of visual angle, had a spatial frequency of 5 cycles per degree (cpd), and were at 35% color contrast (SI Materials and Methods, Definition of Chromatic Contrast). To minimize the visibility artifacts from small eye movements, solid yellow lines were presented between the red and green stripes of the chromatic grating (Fig. S4). For each grating, the yellow lines were presented at both +15 and −15 degrees. At stimulus onset and offset, the chromatic contrast of the grating was ramped up or down at 500 ms. The test grating was presented at 3% contrast with the same size and spatial frequency as the adapting stimuli, tilted at 0, ±1.5, and ±4 degrees from vertical. Subjects made forced-choice responses to indicate whether the test grating appeared tilted to the left or to the right. Subjects pressed another button if they accidentally perceived any visible artifact during the adaptation period, and that trial was excluded from further analysis.
Fig. S4.
Square wave gratings for the TAE experiment.
In the orientation-selective adaptation experiment, the counterphase flickering chromatic gratings were tilted +45 or −45 degrees from vertical, with 3.35 cpd of spatial frequency, 2.09 degrees in diameter, and at 35% chromatic contrast. Fig. S5 shows the light intensity of the red/green channels for the sine wave chromatic grating. The contrast ramps for stimulus onset and offset were 100 and 200 ms, respectively. The test Gabor patch was 0.27 degrees in diameter, with spatial frequency as the adapting stimuli and adaptive chromatic contrast, either parallel or orthogonal to the adapting orientation. The probe was presented for 100 ms (contrast ramp up and down in Gaussian profile), accompanied by a low-pitched (400 Hz) tone. The distance between the center of probe and the fixation point was 0.42 degrees. At least six 3-down–1-up staircases were collected for each orientation condition. A Weibull function was fitted to the data pooled across all staircases, the detection threshold was defined as the contrast level at 79% accuracy. Subjects pressed a button to skip the current probe detection trial if they encountered any visible artifact.
Fig. S5.
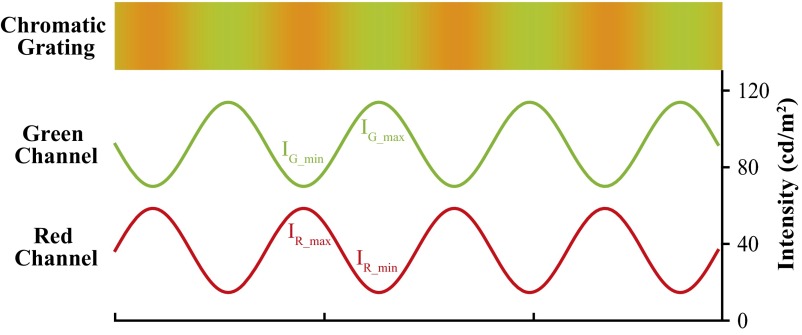
Illustration of green and red channel intensities in the chromatic grating. IG_max and IG_min denote the peak and trough of the green channel intensity; IR_max and IR_min, the peak and trough of the red channel intensity. During isoluminance adjustment, IR_max and IR_min were fixed, whereas the amplitude of the green intensity was adjusted until the flickering percept reached the minimal level.
In the fMRI experiment, chromatic gratings (sine wave pattern) were counterphase flickered at 7.5 Hz (visible) or 30 Hz (invisible) at the central visual field for 2 s, followed by a 14-s ISI. The spatial frequency of the gratings was 2.8 cpd, and the diameter was 4.3 degrees of visual angle. Each functional scan lasted for 256 s. During the scan, subjects performed a central fixation task to detect occasional luminance changes of the fixation. Two scans were collected for the visible grating condition, and four scans were collected for the invisible grating condition. In another two scans, rotating wedge and expanding ring stimuli were used to identify the retinotopic visual areas. More details are provided in SI Materials and Methods, MRI Data Acquisition and Analysis.
In the binocular rivalry experiments (experiment 2), the flickering chromatic gratings were sine wave patterns with the same spatial frequency, size, and contrast as in the orientation-selective adaptation experiment. The sine wave patterns helped further reduce visible artifact to a negligible level (SI Materials and Methods, Visibility Test) compared with the square wave gratings used in experiment 1. In experiment 2a, the contrast for the visible grating was 3%, tilted +45 or −45 degrees from vertical, and orthogonal to the flickering chromatic gratings in the opposing eye. All of the other stimulus parameters were the same as for the TAE experiment. During the 60-s dichoptic presentation period, the contrast for the invisible chromatic gratings was modulated at 1 Hz. For each cycle, the contrast of the chromatic gratings was ramped from 0% to 35% in 250 ms, kept at a plateau for 400 ms, then ramped down to 0% in 250 ms. The next cycle was started after another 100 ms. The test stimulus was defined as a brief contrast increment in a small disk area of the visible grating, which subtended 0.3 degrees in diameter, located 0.42 degrees from the fixation point, and lasted 200 ms. During monocular presentations, the probe detection threshold was estimated with four staircases for each individual. During dichoptic presentations, the probe detection performance at the threshold level contrast was measured in 200 trials for each condition.
In experiment 2b, the contrast ramps for the onset and offset of the invisible chromatic gratings lasted 200 ms. The vertical test Gabor probe was 0.36 cpd in diameter and was presented for 300 ms (contrast modulated in Gaussian profile). The onset latency of the probe was defined as the interval between the onset of the probe and the time when the contrast of the dominant eye’s gratings reached a plateau. The probe was presented at threshold-level contrast, which was measured with 3-down–1-up staircases. Ninety-six trials were tested for each interval.
In experiment 2c, the spatial profiles of stimuli were the same as in experiment 2b. In all three experiments, invisible gratings were presented continuously for 60 s in each trial. In the first experiment, the contrast detection threshold of the probe was estimated using six staircases for each condition. The probe contrast was fixed at the threshold level in the other two experiments. The low-contrast gratings were presented at 10% contrast in the second experiment. The monocular probes were presented every 2 s in the last experiment, and self-paced in the other two experiments. Subjects pressed a button to skip the probe detection test if they perceived any visual artifact.
Subjects from experiments 1 and 2 participated in a series of 2-AFC tests on the visibility of invisible gratings. The results showed that the performance discriminating or detecting the invisible chromatic gratings was at chance level. The stimuli and procedure for the forced choice experiments are described in SI Materials and Methods, Visibility Test.
SI Materials and Methods
Visibility Test.
To verify whether the 30-Hz flickering chromatic gratings were truly invisible, a series of 2-AFC experiments were tested. Six observers from experiment 1 participated in the first 2-AFC experiment. They were asked to discriminate the orientation from flickering chromatic square wave gratings. During each trial, +15- or −15-degree tilted chromatic gratings gradually emerged from a static yellow disk in 500 ms, presented for 2 s, after which the contrast was ramped down to 0 in another 500 ms. Subjects made a forced choice to indicate whether the grating was tilted to the left (−15 degrees) or to the right (+15 degrees). As shown in Fig. S1A, the orientation discrimination performance was at chance level [49.1%, one-sample t test from 50%: t(5) = 0.404, P = 0.703].
In the second experiment, six subjects from experiment 2 (six from experiment 2a and four from experiment 2b) were asked to detect flickering chromatic gratings from R/G disk flickers in a 2-AFC) task. Each trial consisted of two 600-ms intervals separated by a 500-ms time window. The first interval was accompanied by a low-pitched tone, and the second was accompanied by a high-pitched tone. During one of the two intervals, an invisible chromatic grating was presented, and during the other interval and the 500-ms window, uniform invisible flickers were presented. Subjects were asked to indicate which interval contained the chromatic grating. As shown in Fig. S1B, the detection performance was at chance level [51.0%, one-sample t test from 50%: t(5) = 1.508, P = 0.192].
In the third experiment, nine subjects from experiment 2 (six from experiment 2a and seven from experiment 2b) were asked to detect invisible chromatic gratings from a static yellow disk in a 2-AFC task. The procedure was the same as in the second experiment. During one of the two intervals, an invisible chromatic grating was presented, and during the other interval, a static yellow disk was presented. Again, the detection performance was at chance level [50.5%, one-sample t test from 50%: t(8) = 0.511, P = 0.623] (Fig. S1C).
In the final experiment, we tested whether subjects could discriminate between dichoptic orthogonal and parallel invisible gratings. At the beginning of each trial, the fixation turned red, and either orthogonal or parallel invisible gratings were presented dichoptically for 600 ms. Subjects were asked to remember the percept and use it as a reference. The fixation then turned back to black, and the invisible grating turned back to the static yellow disk for 1 s. Subsequently, in two 600-ms intervals, dichoptic orthogonal and parallel gratings were presented in random order, separated by a 500-ms static yellow disk between intervals. Subjects made a forced choice to indicate which interval contained the same stimulus as the reference. Six subjects from experiment 2 participated. Fig. S1D shows that the discrimination performance was at chance level [51.6%, one-sample t test from 50%: t(5) = 1.270, P = 0.260]. During these experiments, if a subject encountered a visible artifact (e.g., owing to large eye movements), he or she pressed another key, and the trial was discarded; 11.33%, 5.90%, 5.67%, and 11.8% of the trials were removed in these experiments, respectively.
Occasional Artifact Produced Minimal TAE.
In the TAE experiment, subjects occasionally encountered a visible artifact owing to large uncontrolled eye movements. Here we evaluated whether this artifact could have any significant influence on the observed TAE. As shown in Fig. S2A, after the trials that contained the artifact were removed, the TAE remained significant [0.650 degrees, t(4) = 9.928, P = 0.00058]. Furthermore, in a control experiment, we simulated the artifact by adding one frame of high-contrast chromatic grating during each adaptation period, during which only the solid yellow disk was presented. This frequency (once per trial) in the simulated condition was much higher than the artifact frequency in experiment 1. Nonetheless, the results for the control experiment (Fig. S2B) show that an occasional high-contrast chromatic grating produced only a minimal TAE [0.262 degrees, t(3) = 2.344, P = 0.101].
Occasional Artifact Produced No Interocular Suppression.
In this control experiment, in which five subjects from experiment 2b participated, we tested whether an occasional visible artifact owing to uncontrolled eye movements (e.g., large saccades) could induce interocular competition as shown in experiment 2b. The visual artifacts were simulated by randomly presenting one frame of full-contrast visible grating in each trial in either the dominant eye or the suppressed eye. Solid yellow discs replaced the invisible chromatic gratings, but all other conditions were the same as in experiment 2b. As shown in Fig. S3, there was no significant difference in performance between the two eyes at all probe latencies. Therefore, the interocular suppression as shown in experiment 2b is not attributed to an occasional visual artifact.
Square Wave Gratings Used in the TAE Experiment.
To minimize visual artifact from small eye movements, solid yellow lines (2 pixels wide) were presented in between the R/G stripes at both −15 and +15 degrees.
Definition of Chromatic Contrast.
Fig. S5 shows the light intensity of the R/G channels to the sine wave chromatic grating. In this study, chromatic contrast was defined by the following formula:
MRI Data Acquisition and Analysis.
MRI data were acquired with a 3-T Prisma scanner (Siemens) at the Institute of Biophysics, using a 20-element head coil. A multiband gradient echo sequence was used to acquire functional images [2 mm isotropic voxels; 54 2-mm-thick axial slices; 192 mm field of vision; 96 × 96 matrix; repetition time (TR)/echo time (TE), 2,000/31.2 ms; flip angle, 85°; multiband factor, 2]. High-resolution anatomic volume was obtained with a T1 MPRAGE sequence (1 mm isotropic voxels; 192 1-mm-thick sagittal slices; 256 × 256 matrix with 1-mm in-plane resolution; TR/TE, 2,600/3.02 ms; flip angle, 8°). MRI data were analyzed using AFNI/SUMA (29) and FreeSurfer. The first two volumes of each run were discarded to avoid magnetic saturation effects. Functional data were motion-corrected to remove linear trend, and then converted to percent signal change. BOLD responses to the visible and invisible gratings were extracted by fitting the fMRI data to generalized linear models with the multiple regression approach. Cortical surfaces were inflated with FreeSurfer and transformed to the AFNI/SUMA format. Surface data were smoothed with a Gaussian filter (FWHM, 6 mm). Retinotopic visual areas were defined as described by Wandell and Winawer (30).
Acknowledgments
This work was supported by National Nature Science Foundation of China Grant 31322025, Chinese Academy of Science Grant XDB02050001, and National Institutes of Health Grant R01 EY023101. P.Z. was supported by Hundred-Talent Program of Chinese Academy of Sciences (Y4CBR11001).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 8352.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1604816113/-/DCSupplemental.
References
- 1.Blake R, Logothetis N. Visual competition. Nat Rev Neurosci. 2002;3(1):13–21. doi: 10.1038/nrn701. [DOI] [PubMed] [Google Scholar]
- 2.Kang MS, Blake R. An integrated framework of spatiotemporal dynamics of binocular rivalry. Front Hum Neurosci. 2011;5:88. doi: 10.3389/fnhum.2011.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leopold DA, Logothetis NK. Activity changes in early visual cortex reflect monkeys’ percepts during binocular rivalry. Nature. 1996;379(6565):549–553. doi: 10.1038/379549a0. [DOI] [PubMed] [Google Scholar]
- 4.Bahmani H, Murayama Y, Logothetis NK, Keliris GA. Binocular flash suppression in the primary visual cortex of anesthetized and awake macaques. PLoS One. 2014;9(9):e107628. doi: 10.1371/journal.pone.0107628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tong F, Meng M, Blake R. Neural bases of binocular rivalry. Trends Cogn Sci. 2006;10(11):502–511. doi: 10.1016/j.tics.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Sterzer P, Kleinschmidt A, Rees G. The neural bases of multistable perception. Trends Cogn Sci. 2009;13(7):310–318. doi: 10.1016/j.tics.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Dayan P. A hierarchical model of binocular rivalry. Neural Comput. 1998;10(5):1119–1135. doi: 10.1162/089976698300017377. [DOI] [PubMed] [Google Scholar]
- 8.Hohwy J, Roepstorff A, Friston K. Predictive coding explains binocular rivalry: An epistemological review. Cognition. 2008;108(3):687–701. doi: 10.1016/j.cognition.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe M, et al. Attention but not awareness modulates the BOLD signal in the human V1 during binocular suppression. Science. 2011;334(6057):829–831. doi: 10.1126/science.1203161. [DOI] [PubMed] [Google Scholar]
- 10.Koch C, Tsuchiya N. Attention and consciousness: Two distinct brain processes. Trends Cogn Sci. 2007;11(1):16–22. doi: 10.1016/j.tics.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Zhang P, Jamison K, Engel S, He B, He S. Binocular rivalry requires visual attention. Neuron. 2011;71(2):362–369. doi: 10.1016/j.neuron.2011.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JH, et al. Full-brain coverage and high-resolution imaging capabilities of passband b-SSFP fMRI at 3T. Magn Reson Med. 2008;59(5):1099–1110. doi: 10.1002/mrm.21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shady S, MacLeod DI, Fisher HS. Adaptation from invisible flicker. Proc Natl Acad Sci USA. 2004;101(14):5170–5173. doi: 10.1073/pnas.0303452101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang Y, Zhou K, He S. Human visual cortex responds to invisible chromatic flicker. Nat Neurosci. 2007;10(5):657–662. doi: 10.1038/nn1879. [DOI] [PubMed] [Google Scholar]
- 15.Vul E, MacLeod DI. Contingent aftereffects distinguish conscious and preconscious color processing. Nat Neurosci. 2006;9(7):873–874. doi: 10.1038/nn1723. [DOI] [PubMed] [Google Scholar]
- 16.Falconbridge M, Ware A, MacLeod DI. Imperceptibly rapid contrast modulations processed in cortex: Evidence from psychophysics. J Vis. 2010;10(8):21. doi: 10.1167/10.8.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolfe JM. Influence of spatial frequency, luminance, and duration on binocular rivalry and abnormal fusion of briefly presented dichoptic stimuli. Perception. 1983;12(4):447–456. doi: 10.1068/p120447. [DOI] [PubMed] [Google Scholar]
- 18.Wolfe JM. Afterimages, binocular rivalry, and the temporal properties of dominance and suppression. Perception. 1983;12(4):439–445. doi: 10.1068/p120439. [DOI] [PubMed] [Google Scholar]
- 19.Brascamp J, Blake R, Knapen T. Negligible fronto-parietal BOLD activity accompanying unreportable switches in bistable perception. Nat Neurosci. 2015;18(11):1672–1678. doi: 10.1038/nn.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Platonov A, Goossens J. Eye dominance alternations in binocular rivalry do not require visual awareness. J Vis. 2014;14(11):2. doi: 10.1167/14.11.2. [DOI] [PubMed] [Google Scholar]
- 21.Xu H, et al. Rivalry-like neural activity in primary visual cortex in anesthetized monkeys. J Neurosci. 2016;36(11):3231–3242. doi: 10.1523/JNEUROSCI.3660-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brascamp JW, Blake R. Inattention abolishes binocular rivalry: Perceptual evidence. Psychol Sci. 2012;23(10):1159–1167. doi: 10.1177/0956797612440100. [DOI] [PubMed] [Google Scholar]
- 23.Dieter KC, Tadin D, Pearson J. Motion-induced blindness continues outside visual awareness and without attention. Sci Rep. 2015;5:11841. doi: 10.1038/srep11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haynes JD, Deichmann R, Rees G. Eye-specific effects of binocular rivalry in the human lateral geniculate nucleus. Nature. 2005;438(7067):496–499. doi: 10.1038/nature04169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tong F, Nakayama K, Vaughan JT, Kanwisher N. Binocular rivalry and visual awareness in human extrastriate cortex. Neuron. 1998;21(4):753–759. doi: 10.1016/s0896-6273(00)80592-9. [DOI] [PubMed] [Google Scholar]
- 26.Rees G, Kreiman G, Koch C. Neural correlates of consciousness in humans. Nat Rev Neurosci. 2002;3(4):261–270. doi: 10.1038/nrn783. [DOI] [PubMed] [Google Scholar]
- 27.Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10(4):433–436. [PubMed] [Google Scholar]
- 28.Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spat Vis. 1997;10(4):437–442. [PubMed] [Google Scholar]
- 29.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 30.Wandell BA, Winawer J. Imaging retinotopic maps in the human brain. Vision Res. 2011;51(7):718–737. doi: 10.1016/j.visres.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]