Abstract
Purpose
To use multiplane en face Doppler optical coherence tomography (OCT) to measure the change in total retinal blood flow (TRBF) in response to hyperoxia.
Methods
One eye of each healthy human participant (n = 8) was scanned with a commercial high-speed (70-kHz) spectral OCT system. Three repeated scans were captured at baseline and after 10 minutes of oxygen (hyperoxia) by open nasal mask. The procedure was performed twice on day 1 and once more on day 2. Blood flow of each vein was estimated using Doppler OCT at an optimized en face plane. The TRBF was summed from all veins at the optic disc. The TRBF hyperoxic response was calculated as the TRBF percent change from baseline.
Results
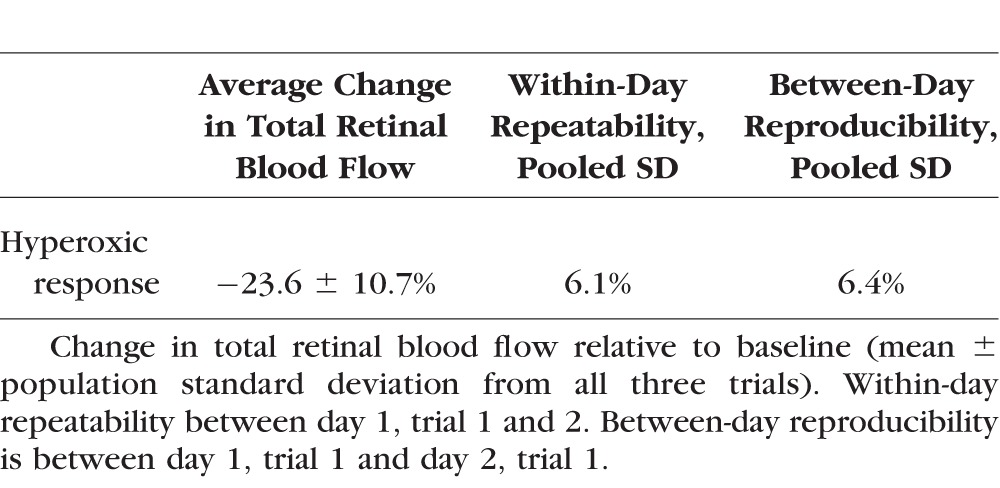
Participants experienced a 23.6% ± 10.7% (mean ± standard deviation [SD]) decrease (P < 0.001, paired t-test) in TRBF during hyperoxia. The within-day repeatability of baseline TRBF was 4.1% and the between-day reproducibility was 10.9% coefficient of variation (CV). Between-grader reproducibility was 3.9% CV. The repeatability and reproducibility (pooled SD) of hyperoxic response were 6.1% and 6.4%, respectively.
Conclusions
The multiplane en face Doppler OCT algorithm was able to detect, in all participants, a decreased TRBF in response to hyperoxia. The response magnitude for each participant varied among repeated trials, and the averaging of multiple trials was helpful in establishing the individual response. This technique shows good potential for the clinical investigation of vascular autoregulation.
Keywords: en face doppler optical coherence tomography, hyperoxia, total retinal blood flow
Retinal tissue has an intrinsic ability to maintain a relatively constant blood supply through a range of hemodynamic conditions.1 This homeostatic mechanism, known as autoregulation, is independent of systemic hormonal or sympathetic control.2,3 Instead, autoregulatory control involves local metabolic, myogenic, and endothelium-related mechanisms.2,4 A deficit in retinal autoregulation is a likely indicator of retinal tissue distress and is thought to have a role in the pathogenesis of such eye diseases as age-related macular degeneration and diabetic retinopathy.5–8 Therefore, measuring the retinal vascular autoregulatory response may be useful diagnostically.
A common approach to induce autoregulatory response is by changing blood gas concentration through alterations in inhaled oxygen level. Since the retina seeks to maintain a constant oxygen tension, blood flow is altered in order to compensate for fluctuations in blood oxygen concentrations. Several methods have been used to study changes in vessel diameter and blood flow during increases of inhaled oxygen concentration (hyperoxia).9 Laser Doppler velocimetry (LDV) showed a decrease in diameter and blood flow among both small and large retinal vessels in response to hyperoxic conditions.10 Laser Doppler flowmetry (LDF) and blue field entoptic technique also detected a decrease in optic disc and macular flow during breathing of increased oxygen.11–13 Dual-beam bidirectional Doppler OCT has also been used to show reduced total retinal blood flow (TRBF) during hyperoxia.14 The application of these methods, however, is limited by the rarity of these instrumentations.
Optical coherence tomography (OCT) angiography was also recently used to show a decrease in flow index and vessel density in the peripapillary retinal microvasculature during hyperoxia.15 However, because OCT angiography primarily measures vascular area and is relatively insensitive to velocity changes, the measured response magnitudes were small.
Quantifying blood flow using Doppler OCT and en face plane has been described previously.16–19 We recently developed an en face flow measurement algorithm with Doppler OCT to improve the measurement of TRBF.20 The advantage of this method is that it provides reliable volumetric measurement of the entire retinal circulation in a scan of only a few seconds using a commonly available commercial instrument. The purpose of this study was to utilize this measurement to investigate the change in retinal blood flow in healthy human participants during hyperoxia.
Methods
Study Population
The study was conducted at Casey Eye Institute at the Oregon Health & Science University (OHSU). The tenets of the Declaration of Helsinki in the treatment of human participants were adhered to in this experiment. The protocol was approved by the Institutional Review Board (IRB). Healthy adult volunteer participants with no history or evidence of ocular disease were recruited at the Casey Eye Institute. Informed consent was obtained from each subject following an explanation of the nature of the study. One eye from each participant was selected and examined throughout the study.
System Setup
The experiment was conducted using a commercial spectral OCT system (RTVue-XR; Optovue, Inc., Fremont, CA, USA). This instrument performs 70,000 A-lines per second and uses a light source centered on 840-nm wavelength. It has a 2.3-mm scan depth with a 5-μm full-width–half-maximum depth resolution in tissue. The time interval between two consecutive axial line scans (A-lines) is 14.3 μs, which corresponds to a phase wrapping velocity limit of 11.1 mm/s. The exposure power at the pupil of 750 μW was within the safety limits set by the American National Standards Institute.21
Each TRBF scan pattern contained five consecutive volumes covering a 2- by 2-mm area centered at the optic disc. Each volume scan contained 80 B-frames with each B-frame containing 500 A-lines. This created a three-dimensional volumetric data set containing 195 en face planes. Each TRBF scan takes 3 seconds. Three consecutive TRBF scans were acquired within approximately 1 minute during each session.
Data Acquisition
Each experimental trial consisted of one baseline and one hyperoxia scan session. In order to establish a baseline, participants were asked to sit and breathe normally for 10 minutes. Baseline blood pressure and heart rate were then recorded. Participants were then fitted with a nasal mask (OxyMask; Southmedic, Barrie, ON, Canada) and given supplemental oxygen for 10 minutes at a rate of 15 L/min. This flow rate should create a mean fraction of inspired oxygen (FiO2) of 80% inhaled oxygen concentration according to Paul et al.22 Following the 10 minutes, hyperoxia was maintained while blood pressure and heart rate were recorded and the scan session was completed.
On day 1, participants completed two trials separated by 10 minutes of breathing normally in order to reestablish baseline. The difference between the two trials was used to established within-day repeatability. On day 2, participants completed a third trial in order to establish between-day reproducibility of baseline TRBF and hyperoxic response.
Data Processing
In this experiment, we used multiplane en face Doppler OCT to detect blood flow, in which the flow is equal to integration of the axial velocity derived from Doppler phase shift in an en face plane. This method eliminates the need to determine the angle between the vessel and OCT beam.16,23 However, a high scan speed is required to obtain three-dimensional volumetric scans and avoid artifacts. In this study, because scans were taken on a relatively slow, 70-kHz spectral OCT system, fringe washout and phase wrapping artifacts made it difficult to find a single en face plane to include all vessels without artifacts. Instead, we determined the flow values of branch retinal veins at different optimized en face planes.20 Since phase wrapping and fringe washout artifacts generally decrease the measured flow, the optimal en face plane was established by searching for the plane of maximum integrated flow in the relevant vein.
The raw spectral data were exported from OCT. Custom MatLab (Mathworks, Natick, MA, USA) software was used to calculate the Doppler OCT image, detect vessels, and compute TRBF. A phase-resolved technique was used to calculate Doppler phase shift (D), which is proportional to the axial component of blood flow velocity by
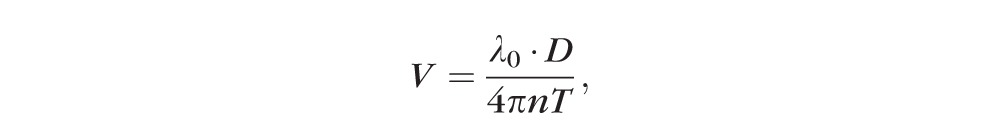 ,
,
|
where λ0 is the central wavelength of the laser, n is the refractive index of water, and T is the time interval between consecutive axial scans.20
The split-spectrum method was used to divide the three-dimensional OCT data into multiple image cubes, corresponding to separate spectral bands. Averaging of the Doppler phase shift in these bands helps to reduce the noise in the Doppler OCT image.20 The averaged Doppler phase shift of static retinal tissue was then used to remove the bulk motion artifact caused by eye movement. An automated phase unwrapping algorithm was applied to correct Doppler phase shift in each en face plane. Then vessel trees were detected as voxels with Doppler phase shift above a threshold set above the noise level. For each vessel segment, the flow is calculated on the en face plane, which maximizes the integration of Doppler phase shift in the vessel area. Veins were distinguished from arteries by the sign of the Doppler shift, based on the knowledge that veins in the optic nerve head flow away from the OCT beam source. Finally, flow of all vein segments was summated for TRBF. The TRBF was estimated only from veins because retinal arteries have more artifacts due to the higher velocity and larger pulsatile variation compared to veins.20
To reduce measurement variability due to pulsatility during the cardiac cycle, the TRBF measurements from all volumes within scans were averaged. Lastly, TRBF was corrected for magnification variations due to axial eye length differences.
Manual Validation
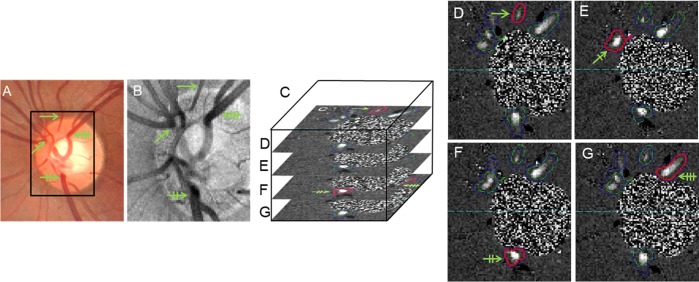
A grader validated the vein decision and optimized en face plane selection generated by the automated algorithm. Volumes were excluded by the grader when motion artifacts interfered with vein identification or flow calculation. Scan session data were included if at least 8 of 15 volumes from the three OCT scans were gradable. Fundus photos (Fig. 1A) and en face structural OCT images (Fig. 1B) were used to confirm retinal veins automatically identified on the en face Doppler phase shift images (Fig. 1C). The grader ensured that each vein was selected once, at either the root or branch (depending on which was larger) but never both. The TRBF is the summation of maximum blood flow for each identified vein (Figs. 1D–G).
Figure 1.
(A) Fundus photo used as a secondary reference to confirm the identification of retinal veins (green arrows with unique tickmark pattern for each vein). Box shows OCT scan area. (B) En face structural OCT image. (C) Illustration showing that the three-dimensional volumetric OCT data were divided into 195 en face planes of 15-μm thickness. (D–G) En face Doppler OCT images. The enclosures outline automatically identified veins. Red enclosures identify the plane of maximum Doppler signal at which flow is measured for the enclosed vein section. Green and blue enclosures outline venous sections that were not used for flow measurement. Each branch vein is measured on only one plane to avoid duplicative flow measurement. Horizontal dotted line separates blood circulation into superior and inferior hemispheres.
Data Analysis
Averages of baseline and hyperoxic measurements for superior, inferior, and TRBF were calculated from all three trials of all participants and compared using a paired t-test. The hyperoxic response was calculated as the TRBF percent change (average ± standard deviation [SD]) from baseline of all participants from all three trials. Analysis of variance (ANOVA) was used to compare the response magnitude between the three trials. Pooled SD was used to measure repeatability and reproducibility of the hyperoxic response. Coefficient of variation (CV) was used to determine within-day repeatability and between-day reproducibility for baseline measurements. Two graders separately graded a randomly selected data subset (33% of data set) in order to determine between-grader reproducibility CV. Within-session repeatability CV was determined for baseline and hyperoxia scan sessions.
Results
Participant Characteristics
Eight participants, seven male and one female, were recruited. Their age ranged from 23 to 50 years (34 ± 10 years, average ± SD). The participants were emmetropic or myopic, with axial eye lengths of 25.3 ± 0.72 mm as measured by partial coherence biometer.
Quality of Total Retinal Blood Flow Measurements
The multiplane en face Doppler TRBF measurement was applied to each of 15 volumes acquired in three 3-second scans during each scan session. An average of 13 volumes per session were gradable. All of the sessions met the minimum of 8 gradable volumes; therefore usable TRBF averages were obtained for all sessions in all participants. Repeated scans acquired during baseline and hyperoxia sessions showed good within-session TRBF repeatability CV of 6.6% and 8.1%, respectively. Average TRBF measurements from the two baseline sessions on the same day showed a good repeatability CV of 4.1%. Between-day baseline sessions had a reproducibility CV of 10.9%. The between-grader reproducibility CV was 3.9%.
Hyperoxic Response
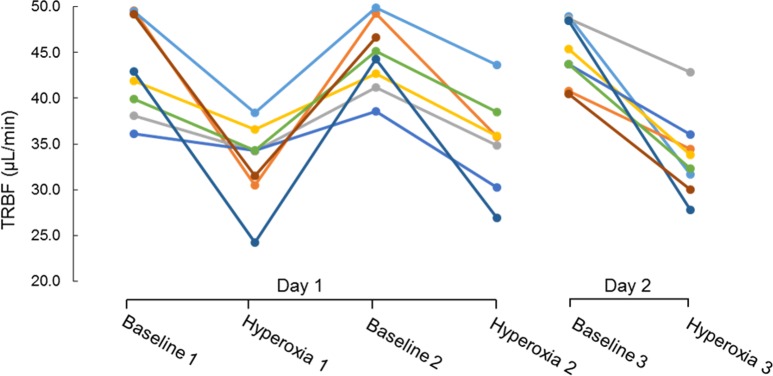
There was no significant decrease in heart rate due to hyperoxia (Table 1). There was a slight reduction in mean arterial pressure of borderline statistical significance. Total and hemispheric retinal blood flow during hyperoxia were significantly reduced compared to baseline (Table 2). Hyperoxic reductions in TRBF were noted in all trials in all participants (Fig. 2). The response magnitude for each participant varied among repeated trials conducted within a day and between days (Table 3). However, there was no systematic difference in response magnitude between any of the three trials (ANOVA, P = 0.69).
Table 1.
Hemodynamic Parameters
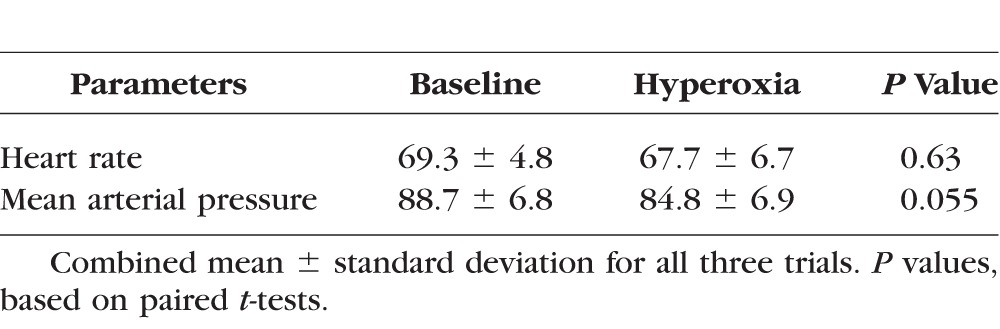
Table 2.
Retinal Blood Flow Measured by Doppler OCT
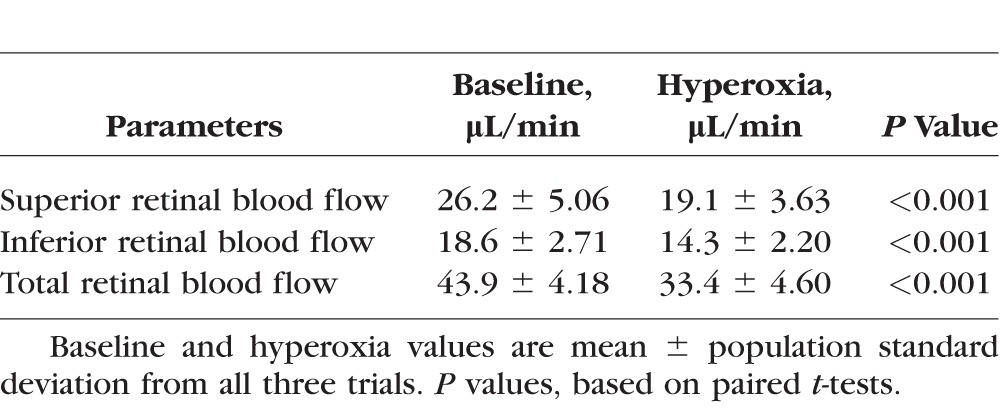
Figure 2.
The average total retinal blood flow (TRFB) for participants (n = 8) under each breathing condition (mean ± SD). Two-tailed t-tests show statistical significant differences between baseline 1 and hyperoxia 1 (P = 0.05), baseline 2 and hyperoxia 2 (P = 0.001), and baseline 3 and hyperoxia 3 (P = 0.002).
Table 3.
Response of Total Retinal Blood Flow Under Hyperoxia
Discussion
The average baseline TRBF found in this study is within the range of healthy blood flow reported in previous investigations. Bidirectional LDV and Doppler OCT have quantified overall retinal flow profiles ranging from 34 ± 6.3 to 54.7 μL/min.24–27 In a previous publication on multiplane en face Doppler OCT, a TRBF of 45.4 ± 6.7 μL/min was found in a healthy population.20 The within-session TRBF repeatability for baseline and hyperoxia in the current study is slightly better than that in the previous report, 8.6% CV.20 We have further demonstrated that this method of TRBF measurement is reproducible within a day, between days, and between graders.
Although a deficit in retinal vascular autoregulatory response to hyperoxia has been detected in the diseased eye, clinical application requires widely available instrumentation that can detect this deficit with high sensitivity relative to the normal population. Because OCT is widely used in ophthalmology and the new generation of commercial OCT retinal scanners has sufficient speed to utilize the multiplane Doppler technique, our method has the potential to be used for clinical investigation. The remaining obstacle is the relatively large population standard deviation of 10.7% in hyperoxic response, which is nearly half of the average response of 23.6% in our present study. This variability is similar to previous results with dual-beam bidirectional Doppler OCT (13.2%) and LDV (11.9%).14 This variability may be inherent to the complex autoregulatory mechanism,9 which is known to be sensitive to variations in a person's baseline physiological state such as heart rate, blood pressure, and intraocular pressure.23,28–30
If the variability of the autoregulatory response cannot be reduced, another way to increase the contrast would be to enhance the size of the response. Previous studies using the blue field entoptic technique, LDV, and LDF had detected reductions of 37% to 61% in retinal blood flow during exposure to 100% oxygen.11–13,31 The TRBF decrease found in our study (23.6%) fell below this range. Since retinal autoregulation acts to maintain blood gas concentrations, it is likely that the lower inhaled oxygen level that we were able to achieve with nasal mask (estimate 80%) may have induced a weaker response compared to exposure to 100% oxygen.13 Furthermore, the open nasal mask we used (OxyMask) allowed open venting and access to normal atmospheric carbon dioxide. The presence of CO2 has been found to attenuate the vasoconstriction of retinal vessels during hyperoxia, and may further explain the smaller response magnitude observed in this study.32 Thus a potential way to increase the hyperoxic response would be to increase oxygen delivery using a nonrebreather face mask with reservoir bag.
Optical coherence tomography angiography is another technique to measure the retinal autoregulatory response. In a recent study, we used OCT angiography to detect a reduction in peripapillary flow index (8.87 ± 3.09) during the same oxygen exposure method described here.15 Because the OCT angiography signal (decorrelation between consecutive B-frames) reaches a plateau value at low velocity values,33 the flow index is a composite value that mainly measures vascular constriction, but has some sensitivity to velocity reduction in capillaries. Therefore the hyperoxic response measured by OCT angiography was smaller (8.87% mean). Fortunately, the response variability was also smaller (3.09 SD) in the normal population. The ratio between the average response magnitude and population SD was 2.9 for OCT angiography peripapillary flow index and 2.2 for en face Doppler OCT TRBF in the present study. These values cannot be directly compared because the populations were not the same. Both methods may be viable approaches.
In summary, we have demonstrated that multiplane en face Doppler OCT can reproducibly measure TRBF and detect a reduction in blood flow in response to hyperoxia. This method may be useful in an investigation of the retinal vascular autoregulation.
Acknowledgments
Supported by National Institutes of Health Grants R01EY023285, R01EY024544, DP3 DK104397, P30 EY010572, and an unrestricted grant from Research to Prevent Blindness.
Oregon Health & Science University (OHSU), YJ, OT, and DH have a significant financial interest in Optovue, Inc., a company that may have a commercial interest in the results of this research and technology. These potential conflicts of interest have been reviewed and managed by Oregon Health & Science University. DH and OT receive patent royalties from Carl Zeiss Meditec, Inc. Other authors do not have financial interest in the subject of this article.
Disclosure: A.D. Pechauer, None; O. Tan, Carl Zeiss Meditec, Inc. (R), Optovue, Inc. (I); L. Liu, None; Y. Jia, Optovue, Inc. (I); V. Hou, None; W. Hills, None; D. Huang, Carl Zeiss Meditec, Inc. (R), Optovue, Inc. (I)
References
- 1. Pournaras CJ,, Rungger-Brändle E,, Riva CE,, Hardarson SH,, Stefansson E. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res. 2008; 27: 284–330. [DOI] [PubMed] [Google Scholar]
- 2. Delaey C,, Van De Voorde J. Regulatory mechanisms in the retinal and choroidal circulation. Ophthalmic Res. 2000; 32: 249–256. [DOI] [PubMed] [Google Scholar]
- 3. Laties AM. Central retinal artery innervation. Absence of adrenergic innervation to the intraocular branches. Arch Ophthalmol. 1967; 77: 405–409. [DOI] [PubMed] [Google Scholar]
- 4. Haefliger IO,, Meyer P,, Flammer J,, Luscher TF. The vascular endothelium as a regulator of the ocular circulation: a new concept in ophthalmology? Surv Ophthalmol. 1994; 39: 123–132. [DOI] [PubMed] [Google Scholar]
- 5. Hafez AS,, Bizzarro RL,, Lesk MR. Evaluation of optic nerve head and peripapillary retinal blood flow in glaucoma patients ocular hypertensives, and normal subjects. Am J Ophthalmol. 2003; 136: 1022–1031. [DOI] [PubMed] [Google Scholar]
- 6. Friedman E. A hemodynamic model of the pathogenesis of age-related macular degeneration. Am J Ophthalmol. 1997; 124: 677–682. [DOI] [PubMed] [Google Scholar]
- 7. Schmetterer L,, Wolzt M. Ocular blood flow and associated functional deviations in diabetic retinopathy. Diabetologia. 1999; 42: 387–405. [DOI] [PubMed] [Google Scholar]
- 8. Kohner EM,, Patel V,, Rassam SM. Role of blood flow and impaired autoregulation in the pathogenesis of diabetic retinopathy. Diabetes. 1995; 44: 603–607. [DOI] [PubMed] [Google Scholar]
- 9. Pechauer AD,, Huang D,, Jia Y. Detecting blood flow response to stimulation of the human eye. Biomed Res Int. 2015; 2015: 121973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Riva CE,, Grunwald JE,, Sinclair SH. Laser Doppler Velocimetry study of the effect of pure oxygen breathing on retinal blood flow. Invest Ophthalmol Vis Sci. 1983; 24: 47–51. [PubMed] [Google Scholar]
- 11. Harris A,, Anderson DR,, Pillunat L,, et al. Laser Doppler flowmetry measurement of changes in human optic nerve head blood flow in response to blood gas perturbations. J Glaucoma. 1996; 5: 258–265. [PubMed] [Google Scholar]
- 12. Langhans M,, Michelson G,, Groh MJ. Effect of breathing 100% oxygen on retinal and optic nerve head capillary blood flow in smokers and non-smokers. Br J Ophthalmol. 1997; 81: 365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sponsel WE,, DePaul KL,, Zetlan SR. Retinal hemodynamic effects of carbon dioxide hyperoxia, and mild hypoxia. Invest Ophthalmol Vis Sci. 1992; 33: 1864–1869. [PubMed] [Google Scholar]
- 14. Werkmeister RM,, Palkovits S,, Told R,, et al. Response of retinal blood flow to systemic hyperoxia as measured with dual-beam bidirectional Doppler Fourier-domain optical coherence tomography. PLoS One. 2012; 7: e45876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pechauer AD,, Jia Y,, Liu L,, Gao SS,, Jiang C,, Huang D. Optical coherence tomography angiography of peripapillary retinal blood flow response to hyperoxia. Invest Ophthalmol Vis Sci. 2015; 56: 3287–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Srinivasan VJ,, Sakadzic S,, Gorczynska I,, et al. Quantitative cerebral blood flow with optical coherence tomography. Opt Express. 2010; 18: 2477–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baumann B,, Potsaid B,, Kraus MF,, et al. Total retinal blood flow measurement with ultrahigh speed swept source/Fourier domain OCT. Biomed Opt Express. 2011; 2: 1539–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choi W,, Baumann B,, Liu JJ,, et al. Measurement of pulsatile total blood flow in the human and rat retina with ultrahigh speed spectral/Fourier domain OCT. Biomed Opt Express. 2012; 3: 1047–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee B,, Choi W,, Liu JJ,, et al. Cardiac-gated en face Doppler measurement of retinal blood flow using swept-source optical coherence tomography at 100,000 axial scans per second. Invest Ophthalmol Vis Sci. 2015; 56: 2522–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tan O,, Liu G,, Liang L,, et al. En face Doppler total retinal blood flow measurement with 70 kHz spectral optical coherence tomography. J Biomed Opt. 2015; 20: 066004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. American National Standard for Safe Use of Lasers. New York: American National Standards Institute; 2007: 1–2007. [Google Scholar]
- 22. Paul JE,, Hangan H,, Hajgato J. The OxyMask(™) development and performance in healthy volunteers. Med Devices (Auckl). 2009; 2: 9–17. [PMC free article] [PubMed] [Google Scholar]
- 23. Dumskyj MJ,, Eriksen JE,, Dore CJ,, Kohner EM. Autoregulation in the human retinal circulation: assessment using isometric exercise, laser Doppler velocimetry, and computer-assisted image analysis. Microvasc Res. 1996; 51: 378–392. [DOI] [PubMed] [Google Scholar]
- 24. Riva CE,, Grunwald JE,, Sinclair SH,, Petrig BL. Blood velocity and volumetric flow rate in human retinal vessels. Invest Ophthalmol Vis Sci. 1985; 26: 1124–1132. [PubMed] [Google Scholar]
- 25. Dai C,, Liu X,, Zhang HF,, Puliafito CA,, Jiao S. Absolute retinal blood flow measurement with a dual-beam Doppler optical coherence tomography. Invest Ophthalmol Vis Sci. 2013; 54: 7998–8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang Y,, Fawzi AA,, Varma R,, et al. Pilot study of optical coherence tomography measurement of retinal blood flow in retinal and optic nerve diseases. Invest Ophthalmol Vis Sci. 2011; 52: 840–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y,, Bower BA,, Izatt JA,, Tan O,, Huang D. In vivo total retinal blood flow measurement by Fourier domain Doppler optical coherence tomography. J Biomed Opt. 2007; 12: 041215. [DOI] [PubMed] [Google Scholar]
- 28. Rassam SM,, Patel V,, Kohner EM. The effect of experimental hypertension on retinal vascular autoregulation in humans: a mechanism for the progression of diabetic retinopathy. Exp Physiol. 1995; 80: 53–68. [DOI] [PubMed] [Google Scholar]
- 29. Robinson F,, Riva CE,, Grunwald JE,, Petrig BL,, Sinclair SH. Retinal blood flow autoregulation in response to an acute increase in blood pressure. Invest Ophthalmol Vis Sci. 1986; 27: 722–726. [PubMed] [Google Scholar]
- 30. Riva CE,, Sinclair SH,, Grunwald JE. Autoregulation of retinal circulation in response to decrease of perfusion pressure. Invest Ophthalmol Vis Sci. 1981; 21: 34–38. [PubMed] [Google Scholar]
- 31. Grunwald JE,, Riva CE,, Brucker AJ,, Sinclair SH,, Petrig BL. Altered retinal vascular response to 100% oxygen breathing in diabetes mellitus. Ophthalmology. 1984; 91: 1447–1452. [DOI] [PubMed] [Google Scholar]
- 32. Pakola SJ,, Grunwald JE. Effects of oxygen and carbon dioxide on human retinal circulation. Invest Ophthalmol Vis Sci. 1993; 34: 2866–2870. [PubMed] [Google Scholar]
- 33. Tokayer J,, Jia Y,, Dhalla AH,, Huang D. Blood flow velocity quantification using split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Biomed Opt Express. 2013; 4: 1909–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]