Abstract
Purpose
The integration of swept-source optical coherence tomography (SS-OCT) into the operating microscope enables real-time, tissue-level three-dimensional (3D) imaging to aid in ophthalmic microsurgery. In this prospective randomized controlled study, we evaluated the impact of SS microscope-integrated OCT (MI-OCT) on ophthalmology residents' performance of ophthalmic microsurgical maneuvers.
Methods
Fourteen ophthalmology residents from a single institution were stratified by year of training and randomized to perform four anterior segment surgical maneuvers on porcine eyes with (MI-OCT+) or without (MI-OCT−) direct intraoperative OCT guidance. Subsequently, both groups repeated the same maneuvers without MI-OCT feedback to test whether initial MI-OCT experience affected subsequent surgical performance. Finally, the MI-OCT− group was crossed over and allowed to repeat the same maneuvers with direct MI-OCT guidance. Each resident completed a survey at the completion of the study.
Results
With direct MI-OCT feedback, residents demonstrated enhanced performance in depth-based anterior segment maneuvers (corneal suture passes at 50% and 90% depth and corneal laceration repair) compared with the residents operating without MI-OCT. Microscope-integrated OCT+ residents continued to outperform the controls when both groups subsequently operated without MI-OCT. For clear corneal wound geometry, there was no statistically significant effect of MI-OCT as applied in this study. Overall, the resident surgeons rated their subjective experience of using MI-OCT very favorably.
Conclusions
Microscope-integrated OCT feedback enhances performance of ophthalmology residents in select anterior segment surgical maneuvers. Microscope-integrated OCT represents a valuable tool in the surgical education of ophthalmology residents.
Keywords: optical coherence tomography, resident education, ophthalmologic surgical procedures
Since the introduction of optical coherence tomography (OCT) in the early 1990s,1 it has permeated nearly all subspecialties of ophthalmology, becoming an essential tool in the diagnosis and treatment of a wide array of ophthalmic diseases. The natural evolution of the applications of OCT technology has followed with its introduction into the operating room. Early attempts with time-domain OCT (TD-OCT) gave way to hand-held spectral-domain OCT (SD-OCT),2 then microscope-mounted SD-OCT (MM-OCT),3 and most recently by microscope-integrated SD-OCT (MI-OCT).4–6 Along with OCT integration into the surgical microscope, the further innovation included intraoperative use of swept-source OCT technology (SS-OCT), which provided increased speed of acquisition and processing of OCT images enabling real-time and three-dimensional (3D) OCT-guided ophthalmic surgery.7 Together, these minimize the intrusion to the surgeon's operative routine. As a result of these advances, intraoperative OCT has gained use in cornea surgery,8–11 vitreoretinal surgery,11,12 and OCT-guided femtosecond laser-assisted cataract surgery.13 However, to date, we could not find any publications regarding the use of intraoperative OCT for ophthalmic resident surgical education (MEDLINE keyword search 6/30/2015: “OCT,” “intraoperative,” “ophthalmology,” “resident,” and “training”).
The Accreditation Council for Graduate Medical Education sets surgical case minimums for ophthalmology residents graduating in the United States. Although a helpful guide, these surgical case minimums are neither a guarantee of competence nor standardization of surgical experience between training programs.14,15 Some authors have suggested the importance of a structured wet laboratory curriculum to teach resident surgery, which can reduce rate of intraoperative complications during resident-performed cataract surgery.16,17 Others have used a surgical simulator, such as EyeSi (VRmagic Holding AG, Mannheim, Germany), to improve efficiency and surgical outcomes.18 However, neither of those approaches allows for tissue-level feedback to the surgeon during simulated training that could potentially be directly transferred to the operating room for use in live human surgery.
In this prospective randomized study performed with ophthalmology residents at a single academic center, we studied and compared the performance of trainee surgeons performing select anterior segment surgical maneuvers with MI-OCT guidance compared with their matched counterparts without MI-OCT. We investigated the effects of direct MI-OCT feedback on the residents' performance, as well as any persistent effect when MI-OCT feedback was removed. Finally, we surveyed the trainee surgeons' subjective feedback on using MI-OCT. To the best of our knowledge, this represents the first report evaluating the impact of intraoperative OCT in training of ophthalmology residents.
Methods
This study was approved by the Institutional Review Board of the Duke University Health System, and all subjects participated with full informed consent. The study adhered to the tenets of the Declaration of Helsinki.
Subjects, Surgical Maneuvers, and Randomization
A group of 14 ophthalmic surgeons-in-training were recruited from Duke University ophthalmology residents. The subjects were randomized into two groups balanced by level of training and surgical experience: with one group assigned to work with the ability to view the MI-OCT (MI-OCT+) and the other without (MI-OCT−) such guidance (Fig. 1A). A total of three first-year, two second-year, and two third-year residents were randomized to each group (N = 14 total). All residents within the same year had equivalent wet laboratory experience prior to the commencement of the study.
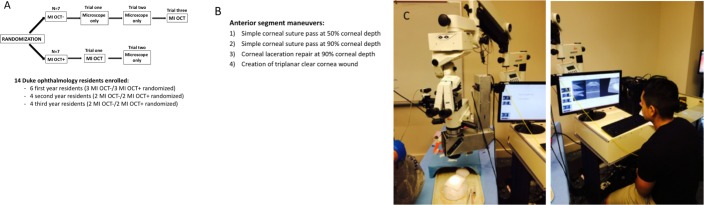
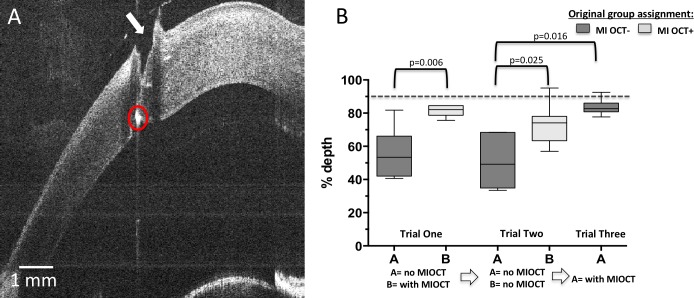
Figure 1.
Fourteen ophthalmology residents were stratified according to the year of training and randomized to either the MI-OCT− or MI-OCT+ group (A). They were asked to perform four surgical maneuvers, including simple corneal pass at 50% and 90% depth, repair corneal laceration at 90% depth, and create triplanar clear-cornea wound on porcine eyes. The MI-OCT− residents initially operated without MI-OCT guidance, whereas the MI-OCT+ residents operated under MI-OCT feedback (trial 1). The same maneuvers were repeated by both groups of residents without MI-OCT feedback (trial 2). Finally, the MI-OCT− group crossed over and repeated all the maneuvers with MI-OCT guidance (trial 3) (A, B). (C) Wet laboratory setup, which included operating microscope with custom-designed, SS–MI-OCT.
The subjects were shown a standardized PowerPoint slide set to introduce the wet laboratory, surgical maneuvers, instrumentation, the operating microscope, and MI-OCT. Freshly enucleated porcine eyes obtained from a local slaughterhouse and stored at 4°C were used for the wet laboratory within 18 hours to minimize corneal edema. The residents were asked to perform a corneal suture pass at 50% thickness, a corneal suture pass at 90% thickness, repair a linear full-thickness corneal laceration with suture at 90% thickness, and construct a triplanar clear-corneal “cataract-type” incision on the porcine eye (Fig. 1). The corneal suture passes and laceration repair were with 10-0 nylon suture on a spatulated needle (Ethicon, Somerville, NJ, USA), whereas the clear-corneal wound was constructed using a standard 2.75-mm disposable keratome for cataract surgery (Alcon, Fort Worth, TX, USA).
In the trials involving MI-OCT feedback, the residents could ask for OCT at any point during the surgical maneuver. The OCT feedback entailed obtaining scans in any orientation anywhere within surgical field as directed by the surgeon. At that point, based on the feedback, the resident could rectify the maneuver (i.e., back out the needle pass and go deeper or shallower) based on the MI-OCT finding for up to three trial attempts. In the trials not involving MI-OCT feedback, the entire procedure was performed using only the operating microscope. For both, however, intraoperative OCT was used to obtain images used for grading the maneuver at the end of the procedure. For depth-based maneuvers, an intraoperative OCT image perpendicular to the needle and when visible along the suture tract was obtained after each completed pass to allow subsequent grading of the depth of the pass (Figs. 1, 2). Each resident performed two independent scored attempts for each maneuver. The depth scores of the individual attempts were averaged and analyzed for statistically significant differences between the MI-OCT+ and MI-OCT− groups. For the clear-corneal incision, a B-scan oriented along the radial axis of the wound was acquired at the conclusion of the maneuver and used for grading.
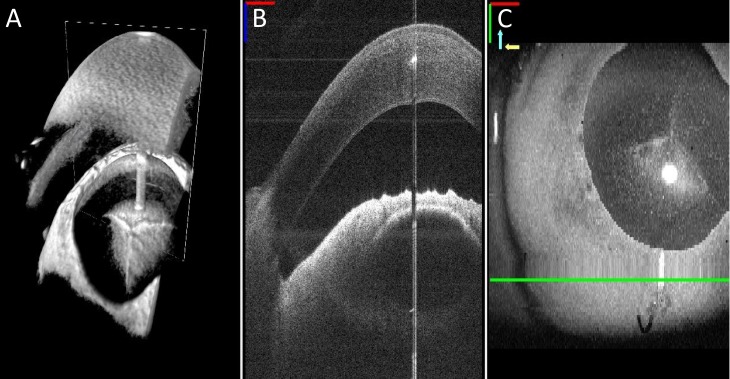
Figure 2.
Close-up view of the MI-OCT display options is demonstrated in A–C. Resident surgeons had a choice to view OCT display data of the surgical field during each maneuver in (A) a 3D view demonstrating the cornea (white arrow) and anterior chamber (red arrow), (B) cross-sectional B-scan view, or (C) as a surface volume projection.
Three consecutive trials were performed: trial 1, each resident performed the above surgical maneuvers with (MI-OCT+ group) or without (MI-OCT− group) MI-OCT feedback based on the randomized assignment; trial 2, the same maneuvers were repeated by each surgeon using only the microscope for viewing (no MI-OCT guidance for either group) to test the persistence of effect in the MI-OCT+ group compared with the MI-OCT− group; and trial 3, the same maneuvers were repeated by each surgeon in the MI-OCT− group with intraoperative OCT feedback. This crossover was designed as an internal control to ensure that any observed differences between the group performance in trials 1 and 2 were not due to confounding variables such as innate surgical ability.
Microscope-Integrated OCT Setup
The MI-OCT scanner used was previously published19 and utilized an external display for image viewing. Briefly, a customized mechanical enclosure allowed integration of the MI-OCT into an ophthalmic surgical microscope (Leica Microsystems, Inc., Buffalo Grove, IL, USA). A dichroic mirror folded the OCT beam path onto the surgical microscope path to allow unobstructed surgical viewing with simultaneous (SS-OCT) imaging. The final surgical microscope objective lens was shared between the two systems and ensured that both systems were parfocal. The SS–MI-OCT system used a (SS-OCT) engine utilizing a swept-frequency source centered at 1040 nm with a sweep bandwidth of 100 nm and a sweep rate of 100 kHz (Axsun, Billerca, MA, USA).20 This resulted in a measured system signal-to-noise ratio (SNR) of 99 dB, and axial and lateral resolutions of 7.8 and 15 μm, respectively. The imaging protocol consisted of 500 A-lines/B-scan and 100 B-scans/volume with an average of 5–10 volumes per second. Custom GPU-accelerated software enabled real-time acquisition, processing, and simultaneous display during surgery of a continuously updated and optimized OCT B-scan, top-down retinal view, and volumetric rendering on a computer screen adjacent to the operating microscope. The site of user-specified B-scan of interest in the volume was selected by an assistant using a mouse; at that site, B-scans were averaged 5 times to enhance visualization.
Depth-Based Maneuvers Grading
To grade the suture placement, custom software was created using MATLAB (Mathworks, Natick, MA, USA). On averaged B-scans at the point of maximal depth of the needle and suture passes, the surface of the corneal endothelium and epithelium were marked by a masked trained grader. Two-dimensional correction of the selected B-scan was performed to correct for image distortions due to refraction as previously described.21 The corneal thickness at the suture pass was defined as the shortest distance between the epithelial and endothelial layers that included the position of the surgical needle or suture. The percent depth was calculated as the ratio between the distance from the epithelium to the needle/suture and the distance between the epithelium and endothelium (corneal thickness). In some corneal laceration cases, jagged edges and protrusions occurred in the epithelium at the edge of the laceration. In these B-scans, the epithelial segmentation was fit to a higher-order polynomial to accurately follow these high frequency features. Refraction correction was performed as described previously, except where a significant portion of the corneal tissue was missing in the laceration. In those cases, the refractive index of air (nair) was used to make depth calculations. The best-approximated orthogonal path through the cornea was segmented manually in these instances, and the shortest linear path was found between the endothelium and epithelium.
Corneal Wound Grading
For clear-corneal incision geometry assessment, volume and averaged B-scans parallel to the direction of the keratome path were deidentified, shuffled, and reviewed by two masked expert graders. Graders determined the geometry of the clear corneal incisions on a categorical scale (3 = triplanar wound, 2 = biplanar wound, 1 = monoplanar wound, 0 = unable to determine geometry of the wound) by comparison with standard images for each grade. The scores of the individual graders were averaged for each scan, and the agreement (κ statistic) between the graders was computed.
Surgeon's Survey
All subjects were given an anonymous survey to determine their subjective experience (Supplementary Tables). The MI-OCT+ group was asked to rank their agreement with statements evaluating their comfort in performing simple corneal passes, repairing the corneal laceration, and constructing the clear corneal incision under direct MI-OCT guidance. The MI-OCT− group was asked to rank their agreement with statements evaluating their comfort in performing simple corneal passes, repair of corneal laceration, and construction of clear corneal wound without direct MI-OCT feedback on a 1 to 5 numerical scale. They were then asked to rerank their performance of the same maneuvers with direct MI-OCT feedback at the completion of trial 3 when they were finally provided with MI-OCT guidance. Both groups were asked to rank their overall experience of using MI-OCT, as well as their likelihood of using MI-OCT in their future practice on a 1 to 5 numerical scale. In both groups, the subjective score of 1 represented the lowest confidence score, whereas a score of 5 represented the highest confidence score.
Statistics
The composite scores of the depth-based maneuvers were computed and expressed as box-plots using GraphPad Prism software (GraphPad, La Jolla, CA, USA). The statistical comparisons conducted across the MI-OCT+ and MI-OCT− groups were performed unpaired, using a Wilcoxon rank sum test. Thus, comparisons between MI-OCT− and MI-OCT+ group scores for trial 1 (MI-OCT+ versus MI-OCT−), trial 2 (both groups without MI-OCT), and trial 3 (MI-OCT− group repeating maneuvers using MI-OCT) were performed using the Wilcoxon rank sum test of differences between medians.
The statistical comparisons within a group (either MI-OCT− or MI-OCT+) were performed using the Wilcoxon signed rank test of median differences. The Wilcoxon signed rank test was used to ascertain, in a paired fashion, whether there was a statistically significant difference in scores within the MI-OCT− group when they only had the microscope alone (trials 1 and 2) and when they had guidance from MI-OCT (trial 3).
Similarly, the Wilcoxon signed rank test was used to compare, in a paired fashion, within the MI-OCT+ group whether there was a statistically significant difference in scores when they had the MI-OCT guidance first compared with their performance without the MI-OCT afterward.
Last, survey results were analyzed using the Wilcoxon rank sum test. All comparisons of the medians using the Wilcoxon signed rank test and Wilcoxon rank sum test with a P value of <0.05 were deemed statistically significant.
Results
Depth-Based Maneuvers
Corneal Pass at 50% Depth Target.
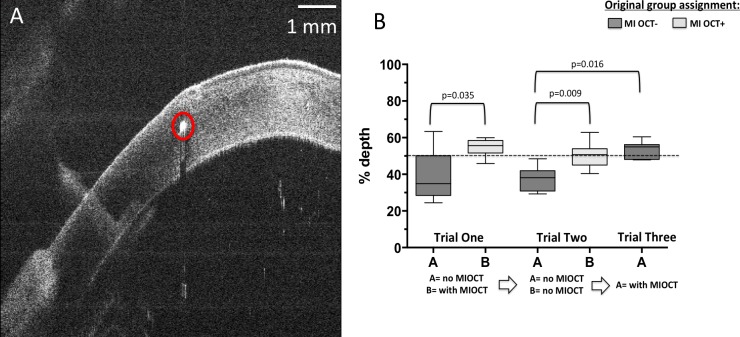
Figure 3A demonstrates a representative orthogonal B-scan of a corneal needle pass at 50% depth (arrow). When asked to perform corneal passes at 50% depth in trial 1, residents operating without MI-OCT feedback (MI-OCT− group) on average placed the pass at 39.4% depth (SD = 13%, median = 35%), whereas residents operating with direct MI-OCT feedback (MI-OCT+ group) on average placed the pass at 54.6% depth (SD = 5%, median = 56%). This difference was statistically significant (P = 0.035; Fig. 3B). When both groups were asked to repeat this corneal pass without MI-OCT in trial 2, the MI-OCT− group on average placed the pass at 37.6% depth (SD = 7%, median = 38%), whereas MI-OCT+ on average placed the pass at 51.0% depth (SD = 7%, median = 51%), which was also significantly different (P = 0.009; Fig. 3B). To ensure that the observed difference was not due to inferior surgical ability in the MI-OCT− group, these subjects were asked to perform the same maneuver with MI-OCT feedback. With MI-OCT feedback, there was a significant difference (P = 0.016) in the performance of the MI-OCT− residents with an average of 53% depth (SD = 5%, median = 55%) compared with their earlier performance without MI-OCT in trial 2 with an average of 37.6 % (SD = 7%, median = 38%).
Figure 3.
Corneal passes with the goal depth of 50% corneal thickness were performed by MI-OCT− and MI-OCT+ group residents. A representative cross-sectional B-scan orthogonal to the direction of corneal needle pass is shown, and the hyperreflective dot (circle) represents an attempted pass at 50% corneal depth (A). The average score of the residents in each group and the median depth scores are compared within the individual groups using paired analysis with the Wilcoxon signed rank test, with relevant comparisons demonstrating statistical significance indicated at P < 0.05 (B).
Corneal Pass at 90% Depth Target.
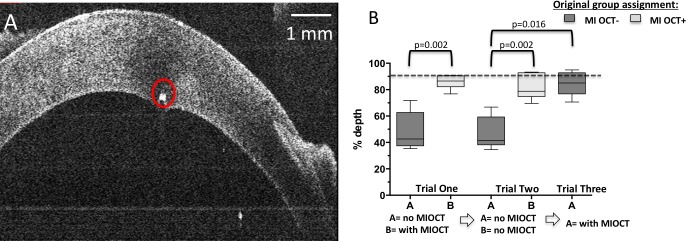
For the corneal pass at 90% depth, a representative B-scan of this maneuver is shown in Figure 4A. When asked to perform this maneuver in trial 1, residents operating without MI-OCT feedback (MI-OCT−) on average placed the pass at 49.1% depth (SD = 14%, median = 43.0), in contrast to 85.5% depth (SD = 5%, median = 87%) for the MI-OCT+ group. This difference was statistically significant (P = 0.002; Fig. 4B). When both groups were asked to repeat this corneal pass without MI-OCT in trial 2, the average was 46.4% depth (SD = 12%, median = 41%) for the MI-OCT− group and 81.5% depth (SD = 9%, median = 79%) for the MI-OCT+ group, which was also a statistically significant difference between the MI-OCT+ and MI-OCT− groups without the use of the microscope (P = 0.002; Fig. 4B). The MI-OCT+ group had no significant difference in depth performance between trials 1 and 2 with and without MI-OCT (median difference = 0.03, P = 0.812). In trial 3, the MI-OCT− group repeated the surgical maneuver with MI-OCT feedback, with the average corneal pass at 84.1% depth (SD = 9%, median = 85%); using the Wilcoxon signed rank test, nonparametric testing for paired data, the MI-OCT− group's performance with and without MI-OCT was compared, and noted to be significantly different (median difference = 0.34, P = 0.016).
Figure 4.
Corneal passes with goal depth of 90% corneal thicknesses were performed by MI-OCT− and MI-OCT+ group residents. A representative cross-sectional B-scan obtained orthogonal to the direction of needle pass demonstrates a hyperreflective dot (circle) at the goal of 90% corneal depth (A). The average score of each resident was plotted, and the median depth scores were compared within individual groups using the Wilcoxon signed rank test, with relevant comparisons demonstrating statistical significance indicated at P < 0.05 (B).
Repair of Corneal Laceration With 90% Depth Target.
Figure 5A demonstrates a representative orthogonal B-scan of a corneal needle pass at 90% depth (arrow) through the laceration. While performing this maneuver in trial 1, residents operating without MI-OCT feedback (MI-OCT−) on average placed the pass at 55.5% depth (SD = 14%, median = 53%), whereas residents operating with direct MI-OCT feedback (MI-OCT+) on average placed the laceration pass at 81.5% depth (SD = 3%, median = 82%). This difference was statistically significant (P = 0.006; Fig. 5B). When both groups were asked to repeat this corneal pass without MI-OCT in trial 2, the MI-OCT− group on average placed the pass at 50.6% depth (SD = 15%, median = 49%), whereas the MI-OCT+ group on average placed the pass at 73.3% depth (SD = 12%, median = 74%), which was also statistically significant (P = 0.025; Fig. 5B). Notably, the MI-OCT+ group, which first operated with direct MI-OCT guidance, continued to perform well in the second trial once MI-OCT guidance was removed. There was no significant difference in their performance (median difference = −0.07, P = 0.11). In trial 3, the MI-OCT− group repeated the surgical maneuvers with MI-OCT feedback, resulting in an average 83.8% suture pass depth (SD = 5%, median = 83%), which was significant compared with their earlier performance without MI-OCT in trial 2 with paired analysis through the Wilcoxon signed ranks test (P = 0.016).
Figure 5.
The maneuvers of corneal laceration repair with goal depth of 90% corneal thickness. Fresh porcine corneas were used by MI-OCT− and MI-OCT+ groups. Fresh porcine corneas were used to construct vertical linear corneal laceration using a 15-blade scalpel. The corneal laceration repair with 10-0 nylon suture was performed by MI-OCT− and MI-OCT+ group residents. A representative cross-sectional B-scan was obtained orthogonal to the direction of needle pass and demonstrates a jagged break in corneal integrity consistent with corneal laceration (arrow) and a hyperreflective dot (circle) at goal depth of 90% corneal thickness (A). The average score of each resident was plotted, and the median depth scores compared across the groups using the Wilcoxon signed rank test, with relevant comparisons demonstrating statistical significance indicated at P < 0.05 (B).
Corneal Incision Geometry
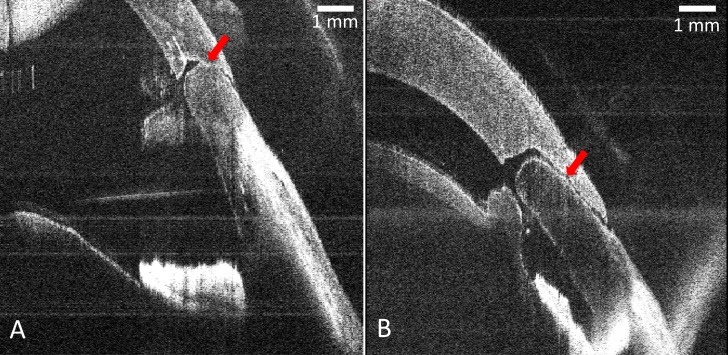
The agreement between the expert graders in scoring corneal incision geometry on a scale of 0 to 3 was determined to not be significantly different (weighted κ = 0.6381). Figures 6A and 6B demonstrate two representative B-scans of the constructed wounds. When asked to create a triplanar incision in trial 1, residents operating without MI-OCT feedback (MI-OCT− group) on average constructed a corneal wound with a mean score of 1.82 planes (SD = 0.67, median = 1.75), whereas residents operating with direct MI-OCT feedback (MI-OCT+ group) had a mean score of 2.14 planes (SD = 0.46, median = 2.00). This difference was not statistically significant (P = 0.244). When both groups were asked to repeat this maneuver without MI-OCT in trial 2, the MI-OCT− group on average constructed wounds with a mean score of 2.41 planes (SD = 0.65, median = 2.75), whereas the MI-OCT+ group had a mean score of 2.38 planes (SD = 0.53, median = 2.50), which was also not statistically significant (P = 0.521). In trial 3, the MI-OCT− group was given an opportunity to repeat the surgical maneuver with MI-OCT feedback and on average received a mean score of 2.21 (SD = 0.86, median = 2.64), which was not significantly different than this group's performance in trial 2 (P = 0.437).
Figure 6.
Clear corneal incisions were created in porcine eyes by MI-OCT− and MI-OCT+ group residents using 2.75-mm surgical keratomes. Although the MI-OCT+ resident could view the MI-OCT throughout and at the completion of the maneuver, a representative cross-sectional B-scan longitudinal to the length of the wound was obtained at the completion of each maneuver (A, B) for postmaneuver grading of the wound profile visible from two separate representative trials (arrows). All incisions viewed on such B-scans were assigned a grade of 0 to 3 by two expert graders masked to study group.
Subjective Impression of MI-OCT Experience
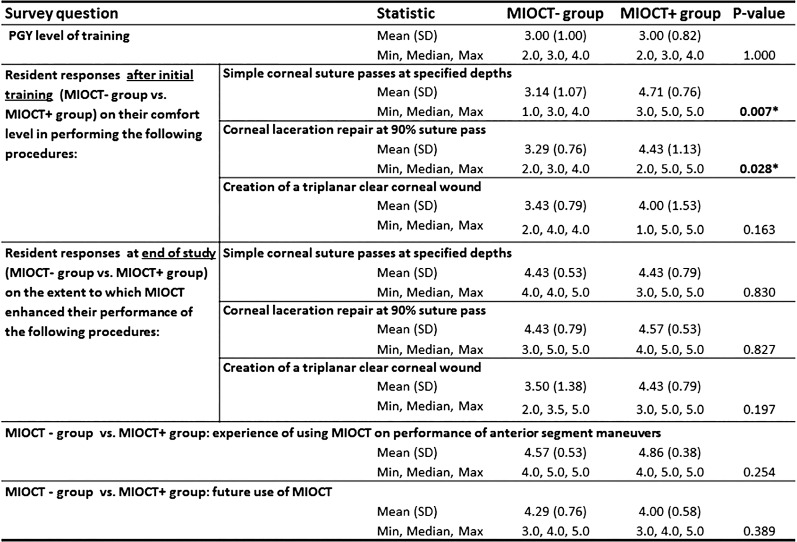
Last, qualitative and quantitative surgeon's subjective feedback was obtained from the residents following completion of the study maneuvers via an administered survey (Fig. 7). After completing the first portion of the study, only the MI-OCT+ group had the direct use of MI-OCT; the MI-OCT+ group reported higher levels of comfort compared with the MI-OCT− group, in performing simple cornea suture passes (P = 0.007) and cornea laceration repair (P = 0.028), but this trend was not observed for creation of triplanar corneal wound. At the end of the training, when both MI-OCT− and MI-OCT+ groups had experienced the direct use of MI-OCT, there was no statistically significant difference between the groups' subjective experience of MI-OCT impact on their ability to perform those same procedures. In total, both MI-OCT− and MI-OCT+ residents on average rated the overall experience of using MI-OCT feedback for anterior segment surgical maneuvers as helpful (score of 4) or very helpful (score of 5), reporting a mean score 4.57 (SD = 0.53) for the MI-OCT− group and 4.86 (SD = 0.38), and a median of 5 for both groups on a 1 to 5 scale. Overall, both groups reported on average as being “more likely” (score 4 on 1–5 scale) to use MI-OCT in their future practice as a result of MI-OCT experience in this study.
Figure 7.
The survey administered to each resident surgeon at the conclusion of initial training and end of the study ranked their subjective experience on a 1 to 5 numerical scale. The mean subjective score with SD and minimum, maximum, and median responses to the survey questions are summarized below. The comparison between the MI-OCT− group and the MI-OCT+ groups was done using the Wilcoxon rank sum test of difference between medians. Asterisk denotes a statistically significant P value.
Discussion
In this randomized prospective study, we demonstrated that MI-OCT feedback results in enhanced surgical performance of ophthalmology residents and improved anatomic outcomes in select anterior segment surgical maneuvers in model eyes. The residents performing depth-based maneuvers (simple corneal passes at 50% and 90% depth, and repair of corneal laceration at 90% depth) with direct MI-OCT feedback outperformed their counterparts who were operating with only the microscope alone. Interestingly, the enhanced performance persisted even when direct MI-OCT assistance was removed, which suggests that MI-OCT can allow for sustained learning of surgical skill even when MI-OCT feedback is not subsequently available. We also demonstrated a favorable subjective experience of the trainee surgeons in using MI-OCT through a surgeon-administered survey. On average, most residents' reported that their comfort in performing study maneuvers increased as a result of using MI-OCT, and residents ranked the MI-OCT experience as either “very helpful” or “helpful,” while reporting on average being more likely to use MI-OCT in their future practice. Overall, the results of this study suggest that MI-OCT feedback can directly impact surgical outcomes, and for the first time, demonstrates its potential utility in surgical training of ophthalmic residents.
Not all surgical maneuvers were amenable to effective MI-OCT feedback under the current study methodology. In our study, we found no effect of MI-OCT feedback on corneal incision geometry. We attributed this to the metal keratome shadowing large portions of the OCT image, which limited feedback while the incision was being created, and the use of an external display of the OCT images, which was adjacent to, but not within the oculars. Both of these limited the surgeon to assessing the wound geometry after, but not readily during the performance of the surgical maneuver. Development of OCT heads-up display through the operating microscope eyepieces and an OCT-compatible keratome may aid in the utility of direct MI-OCT guidance for construction of cataract incisions and other similar maneuvers in the future. The survey questions were used to obtain general feedback and were not provided before and after testing. A more standard validated questionnaire would be useful in future assessment of imaging as a training tool.
Other limitations of our study include the small sample size and a single study site. Validation of the utility of MI-OCT with a larger number of ophthalmology residents and fellows at other programs, and expanding the range of surgical maneuvers for which MI-OCT may be useful in ophthalmic surgical training, such as posterior segment surgery, would be important future steps. Such feedback may also be useful in ophthalmic surgeon self-assessment using a similar wet laboratory setup or with comparable model eye materials (e.g., KITARO kit; FCI Ophthalmics, Pembroke, MA, USA). In addition, a natural future evolution from our study would be for potential integration of MI-OCT feedback for future training of residents and fellows in patient surgical treatment, such as during early experience in repair of corneal lacerations, open globes, and corneal suturing (e.g., penetrating keratoplasty). Determining whether improved anatomic outcomes with MI-OCT feedback in resident-performed and early career surgery will translate into improved visual outcomes, shorter operative time, and faster visual rehabilitation in patients would be important to justify significant cost of acquiring this technology for training or for use during routine surgery.
Clinical assessment from OCT imaging of the eye has revolutionized diagnosis and management of many ophthalmic conditions and is now an indispensable part of practice of almost all ophthalmic subspecialties. Although relatively recently introduced in the operating room, the histology-level feedback that intraoperative imaging provides during ophthalmic surgery holds the potential of a similar disruptive change in the surgical practice of ophthalmology. Multiple studies to date have reported the use of intraoperative OCT in anterior and posterior segment surgery.2,5,9,10,22–26 Recent US Food and Drug Administration approval of microscope-integrated SD-OCT Rescan 700 (Carl Zeiss Meditec, Dublin, CA, USA)4 will allow for access to microscope integrated, heads-up display OCT viewing during live surgery in the United States. Ehlers et al.11 recently published 2-year results of a prospective evaluation of microscope-mounted SD-OCT used at pauses in 275 anterior segment and 256 posterior segment ophthalmic surgeries. In this single center study, the use of MM-OCT (which required moving the microscope to the side) resulted in a mean delay of 4.9 minutes while altering intraoperative surgeon's decision making in more than 40% of surgical cases. The results of our SS–MI-OCT study build on these findings. The current study demonstrates that MI-OCT feedback can not only guide a surgeon's intraoperative decision making, but also has the potential to improve surgical performance, enhance anatomic outcomes, and be used as a training tool that is directly translatable to the operating room for use in live human surgery. As intraoperative OCT technology continues to evolve, so will our understanding of its potential, which will allow for its increasing creative applications in many aspects of ophthalmic surgery.
Supplementary Material
Acknowledgments
Supported by National Institutes of Health Bioengineering Research Partnership Grant R01-EY023039, National Institutes of Health Grant R01-EY024312, and an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology, Duke University School of Medicine.
Disclosure: B. Todorich, None; C. Shieh, None; P.J. DeSouza, None; O.M. Carrasco-Zevallos, None; D.L. Cunefare, None; S.S. Stinnett, None; J.A. Izatt, Bioptigen (I), P; S. Farsiu, None; P. Mruthyunjaya, None; A.N. Kuo, P; C.A. Toth, Alcon (R), Thrombogenics (C), Genentech (F), Bioptigen (F)
References
- 1. Huang D,, Swanson EA,, Lin CP,, et al. Optical coherence tomography. Science. 1991. ; 254: 1178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dayani PN,, Maldonado R,, Farsiu S,, Toth CA. Intraoperative use of handheld spectral domain optical coherence tomography imaging in macular surgery. Retina. 2009. ; 29: 1457–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ehlers JP,, Tao YK,, Farsiu S,, Maldonado R,, Izatt JA,, Toth CA. Visualization of real-time intraoperative maneuvers with a microscope-mounted spectral domain optical coherence tomography system. Retina. 2013. ; 33: 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ehlers JP,, Kaiser PK,, Srivastava SK. Intraoperative optical coherence tomography using the RESCAN 700: preliminary results from the DISCOVER study. Br J Ophthalmol. 2014. ; 98: 1329–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hahn P,, Migacz J,, O'Donnell R,, et al. Preclinical evaluation and intraoperative human retinal imaging with a high-resolution microscope-integrated spectral domain optical coherence tomography device. Retina. 2013. ; 33: 1328–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hahn P,, Migacz J,, O'Connell R,, Izatt JA,, Toth CA. Unprocessed real-time imaging of vitreoretinal surgical maneuvers using a microscope-integrated spectral-domain optical coherence tomography system. Graefe's Arch Clin Exp Ophthalmol. 2013. ; 251: 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nankivil D,, Dhalla AH,, Gahm N,, Shia K,, Farsiu S,, Izatt JA. Coherence revival multiplexed, buffered swept source optical coherence tomography: 400 kHz imaging with a 100 kHz source. Optics Lett. 2014. ; 39: 3740–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cursiefen C,, Steven P,, Roters S,, Heindl LM. Prevention and management of complications in Descemet membrane endothelial keratoplasty (DMEK) and Descemet stripping automated endothelial keratoplasty (DSAEK) [in German]. Ophthalmol Zeitschrift Deutschen Ophthalmologischen Gesellschaft. 2013. ; 110: 614–621. [DOI] [PubMed] [Google Scholar]
- 9. Steven P,, Le Blanc C,, Lankenau E,, et al. Optimising deep anterior lamellar keratoplasty (DALK) using intraoperative online optical coherence tomography (iOCT). Br J Ophthalmol. 2014. ; 98: 900–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Steven P,, Le Blanc C,, Velten K,, et al. Optimizing descemet membrane endothelial keratoplasty using intraoperative optical coherence tomography. JAMA Ophthalmol. 2013. ; 131: 1135–1142. [DOI] [PubMed] [Google Scholar]
- 11. Ehlers JP,, Dupps WJ,, Kaiser PK,, et al. The Prospective Intraoperative and Perioperative Ophthalmic ImagiNg with Optical CoherEncE TomogRaphy (PIONEER) Study: 2-year results. Am J Ophthalmol. 2014. ; 158: 999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Binder S,, Falkner-Radler CI,, Hauger C,, Matz H,, Glittenberg C. Feasibility of intrasurgical spectral-domain optical coherence tomography. Retina. 2011. ; 31: 1332–1336. [DOI] [PubMed] [Google Scholar]
- 13. Nguyen P,, Chopra V. Applications of optical coherence tomography in cataract surgery. Curr Opin Ophthalmol. 2013. ; 24: 47–52. [DOI] [PubMed] [Google Scholar]
- 14. Binenbaum G,, Volpe NJ. Ophthalmology resident surgical competency: a national survey. Ophthalmology. 2006. ; 113: 1237–1244. [DOI] [PubMed] [Google Scholar]
- 15. McDonnell PJ,, Kirwan TJ,, Brinton GS,, et al. Perceptions of recent ophthalmology residency graduates regarding preparation for practice. Ophthalmology. 2007. ; 114: 387–391. [DOI] [PubMed] [Google Scholar]
- 16. Lee AG,, Greenlee E,, Oetting TA,, et al. The Iowa ophthalmology wet laboratory curriculum for teaching and assessing cataract surgical competency. Ophthalmology. 2007. ; 114: e21 –e. [DOI] [PubMed] [Google Scholar]
- 17. Rogers GM,, Oetting TA,, Lee AG,, et al. Impact of a structured surgical curriculum on ophthalmic resident cataract surgery complication rates. J Cataract Refract Surg. 2009. ; 35: 1956–1960. [DOI] [PubMed] [Google Scholar]
- 18. Belyea DA,, Brown SE,, Rajjoub LZ. Influence of surgery simulator training on ophthalmology resident phacoemulsification performance. J Cataract Refract Surg. 2011. ; 37: 1756–1761. [DOI] [PubMed] [Google Scholar]
- 19. Ehlers JP,, Tao YK,, Farsiu S,, Maldonado R,, Izatt JA,, Toth CA. Integration of a spectral domain optical coherence tomography system into a surgical microscope for intraoperative imaging. Invest Ophthalmol Vis Sci. 2011. ; 52: 3153–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carrasco-Zevallos OM, Keller B, Viehland C, et al. 4D microscope-integrated OCT improves accuracy of ophthalmic surgical maneuvers. Proc SPIE. 2016; 9693: 1–7. [Google Scholar]
- 21. Westphal V,, Rollins A,, Radhakrishnan S,, Izatt J. Correction of geometric and refractive image distortions in optical coherence tomography applying Fermat's principle. Optics Express. 2002. ; 10: 397–404. [DOI] [PubMed] [Google Scholar]
- 22. Ehlers JP,, Kernstine K,, Farsiu S,, Sarin N,, Maldonado R,, Toth CA. Analysis of pars plana vitrectomy for optic pit-related maculopathy with intraoperative optical coherence tomography: a possible connection with the vitreous cavity. Arch Ophthalmol. 2011. ; 129: 1483–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ehlers JP,, Ohr MP,, Kaiser PK,, Srivastava SK. Novel microarchitectural dynamics in rhegmatogenous retinal detachments identified with intraoperative optical coherence tomography. Retina. 2013. ; 33: 1428–1434. [DOI] [PubMed] [Google Scholar]
- 24. Ehlers JP,, Tam T,, Kaiser PK,, Martin DF,, Smith GM,, Srivastava SK. Utility of intraoperative optical coherence tomography during vitrectomy surgery for vitreomacular traction syndrome. Retina. 2014. ; 34: 1341–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chavala SH,, Farsiu S,, Maldonado R,, Wallace DK,, Freedman SF,, Toth CA. Insights into advanced retinopathy of prematurity using handheld spectral domain optical coherence tomography imaging. Ophthalmology. 2009. ; 116: 2448–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Knecht PB,, Kaufmann C,, Menke MN,, Watson SL,, Bosch MM. Use of intraoperative fourier-domain anterior segment optical coherence tomography during descemet stripping endothelial keratoplasty. Am J Ophthalmol. 2010. ; 150: 360–365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.