Abstract
Deficiency of methylthioadenosine phosphorylase (MTAP) supports melanoma development and progression through accumulation of its substrate 5’-methylthioadenosine (MTA), which leads amongst others to a constitutive inhibition of protein arginine methyltransferases (PRMTs) and activation of the transcription factor AP-1 via the receptor ADORA2B. Genetic association studies have also suggested that genetic polymorphism in MTAP may modulate the risk of melanoma. Here, we investigated the only globally common non-synonymous single nucleotide polymorphism (SNP) reported to date for MTAP. The SNP rs7023954 is located in exon 3 (c.166G>A), and leads to the conservative substitution of one branched-chain amino acid residue (valine) for another (isoleucine) at position 56 (p.Val56Ile). Whereas genotype frequencies in normal and primary melanoma tissues or cell lines were in Hardy-Weinberg equilibrium based on cDNA amplicon sequencing, a marked (P = 0.00019) deviation was observed in metastatic melanoma tissues and cell lines due to a deficit of heterozygotes. Enzyme assays conducted on the co-dominantly expressed alleles revealed no difference in the conversion rate of MTA to adenine and 5-methylthioribose-1-phosphate, indicating that this known enzymatic activity does not modulate the tumor suppressive function of MTAP.
Introduction
The incidence of malignant melanoma is steadily increasing [1]. Melanoma is a highly invasive cancer characterized by early metastasis and rapid development of resistance to current therapeutic approaches [2]. In the past years, great progress has been made in our understanding of the molecular pathobiology of melanoma. In particular, the identification of driver mutations in genes encoding proteins that play a role in the MAPK pathway, such as BRAF, NRAS, and MEK (MAPK kinase), has spurred the development of novel targeted treatment strategies [3,4]. Another gene that has received increasing interest is methylthioadenosine phosphorylase (MTAP). It catalyzes the first step in the methionine salvage pathway by phosphorylating S-methyl-5'-thioadenosine (MTA), a major byproduct of the polyamine metabolism, to adenine and 5-methylthioribose-1-phosphate [5,6]. Many tumors lack expression of MTAP, due either to its loss at the 9p21 locus or to hypermethylation of its promoter region [5–8]. MTAP deficiency results in the accumulation of MTA [9,10]. High intracellular levels of MTA have been shown to cause deregulation of protein arginine methyltransferases (PRMTs) [8,11]. Further, extracellular MTA affects cellular signaling [8,12] and proliferation [13], not only of cancer cells but also of stromal cells including lymphocytes and fibroblasts, via induction of melanoma-relevant genes such as the growth factors VEGF and bFGF and the metalloproteinases MMP9 and MMP14 [7]. This MTA-based effect could not be observed in melanocytes. Further, it could be shown, that MTA treatment leads to an activation of the transcription factor AP-1 [7], which is highly active in melanoma and influences the expression of a variety of regulators of cell mechanisms involved in melanoma development and metastasis [14]. Activation of AP-1 is modulated by the binding of MTA to the adenosine receptor A2B [12].
In recent years, numerous single nucleotide polymorphisms (SNPs) have been reported for the MTAP locus on chromosome 9p21 to dbSNP, including 120 missense polymorphisms, all but one of which are rare or show high population specificity. The only exception is rs7023954, which is located in exon 3 (c.166G>A) of MTAP and leads to the exchange of valine for isoleucine (p.Val56Ile) [6]. It is found worldwide with a minor allele frequency of 0.3882 and an average observed heterozygosity of 0.475. Spurred by reports of an association of the MTAP locus with melanoma risk [15,16], this study aimed at investigating the frequency of rs7023954 genotypes in normal skin as well as primary and metastatic melanoma tissues and cell lines. Further, we studied allele-specific expression of MTAP at the protein level in trypsin-digested whole-cell extracts using liquid chromatography-mass spectrometry and stable isotope-labeled proteotypic peptides specific for either allele. Finally, we measured enzyme activity of the alleles both in a rabbit reticulocyte lysate translation system as well as in transiently and stably transfected cells.
Material and Methods
Cell lines, culture conditions and tissue samples
Melanoma cell lines Mel Juso, Mel Ho, Mel Ei, Mel Wei, 501Mel, SkMel3, SkMel28, Mel Im, Mel Ju, A375, HMB2, and NHEMs were described in detail previously [6,8,17]. Briefly, cell lines Mel Juso (DSMZ: ACC74), Mel Ho (ATTC: ACC62), Mel Wei, and Mel Ei were derived from primary cutaneous melanomas; Mel Im, Mel Ju, 501Mel, A375 (ATTC: CRL-1619), SkMel3 (ATTC: HTB-69), SkMel28 (ATTC: HTB-72), and HMB2 were derived from metastases of malignant melanomas. Melanoma cell lines WM35, WM793, WM1366, WM3211 (primary cutaneous melanomas) and WM9 and WM293A (metastases of malignant melanoma) were a kind gift by Dr. M. Herlyn (Wistar Institute, Philadelphia, PA, USA) and cultivated as described in Tu2% medium [18]. NHEMs were derived from neonatal skin. Isolation and cultivation of NHEMs were described previously [19]. Generation of stably transfected Mel Juso clones (Mock N, KSNV-01 and KWT-04) was achieved by culturing the cells one day after transfection in selection medium, containing 0.6 mg/mL G418 (Sigma-Aldrich, Taufkirchen, Germany). After 14 days of selection, individual G418-resistant colonies were sub-cloned. Expression of MTAP in these clones was determined by qRT-PCR and Western Blot. NHDF cells were obtained from Promocell (Heidelberg, Germany). Primary human dermal fibroblasts (NHDF1-6) were cultured as described previously [20]. Cells were maintained in DMEM or RPMI1640 (Sigma-Aldrich, Deisenhofen, Germany) supplemented with penicillin (400 U/mL), streptomycin (50 μg/mL), and 10% fetal bovine serum (FBS) (all from Sigma-Aldrich). The cell lines Mel Juso, Mel Ei, Mel Ho, Mel Wei, Mel Im, HMB2, A375, Mel Ju, 501Mel, SKMel3 and SKMel28 were incubated in a humidified atmosphere containing 8% CO2 at 37°C. Cell lines from the Wistar Institute were cultured at 5% CO2 and 37°C. Cells were split at a 1:3 to 1:5 ratio every 4 days.
Tissue specimens
Tissue specimens of snap-frozen melanoma primary tumors (PT60, PT62, PT71, PT87, PT93, PT97, PT104, and TB148) and melanoma metastases (TB43, TB50, TB80, TB90, TB91, TB95, TB101, TB135 and TB147) with clear-cut pathological classification were obtained from the tissue collection of the Institute of Pathology (University of Regensburg, Germany). Sampling and handling of patient material were carried out in accordance with the ethical principles of the Declaration of Helsinki. Use of human tissue material (NL3, NH14, NL146 TB88, TB89, NH118, NH119, NH120, TB121, TB122; TB60, TB62, TB71 TB87, TB93, TB97, TB104, TB148; TB43, TB50, TB80, TB90, TB91, TB95, TB101, TB135, and TB147 obtained from the Department of Dermatology, University Hospital of Regensburg, Germany) had been approved by the local ethics committee of the University of Regensburg (application number 09/11 and 03/151).
RNA isolation and reverse transcription
Total cellular RNA was isolated from cultured cells using the E.Z.N.A® Total RNA Kit I (Omega Bio-Tek/ VWR, Darmstadt, Germany) according to the manufacturer’s instructions. cDNAs were generated from 500 ng total RNA using SuperScript II Reverse Transcriptase Kit (Invitrogen, Groningen, Netherlands) as described previously [21].
Amplification and sequencing of MTAP-Exon 3
For PCR amplification of exon 3 of MTAP, 1 μL cDNA (extracted from tissue or cell lines, or 2 μL genomic DNA (extracted from tissue or cell lines) were added to 0.5 μL of forward and reverse primers (MTAP-for89: 5’-GCCCACTGCAGATTCCTTTC-3’; MTAP-rev416: 5’-GGTCTCATAGTGGTCCTGTC-3’; each 20 μM), 5 μL PCR reaction buffer (10x), 0.5 μL dNTP mix (each 10 mM), and 0.5 μL (2.5 Units) FastStart Taq DNA Polymerase dNTPack (Roche, Mannheim, Germany) in a total reaction volume of 50 μL. The following PCR program was used: 5 min at 95°C (initial denaturation); 60 s at 95°C (denaturation); 30 s at 61°C (annealing), 30 s at 72°C (elongation), repeated 35 times. PCR products were evaluated by gel electrophoresis using a 1.5% agarose gel. Before sequencing, amplicons were purified by precipitation with 30% PEG-8000 (Sigma-Aldrich) to remove unincorporated primers and dNTPs. Purified PCR products were given to GeneArt AG/ Thermo Fisher Scientific Inc. (Regensburg, Germany) as complete mix of 10 ng per 100 bp of PCR-product-length and 10 μM Primer MTAP-Ex3for 5’-CATGCATGCAGCCATCTG-3’ or MTAP-Ex3rev 5’- CGTCAGACCTGTATCTTTAC-3’ for Sanger sequencing.
Analysis of gene expression by qRT-PCR
Quantitative real time-PCR analysis of MTAP was performed using specific primers (MTAPfor2: 5’-GCGAACATCTGGGCTTTG-3’; MTAPrev2: 5’-GCACCGGAGTCCTAGCTTC-3’) and a LightCycler® 480 (Roche, Mannheim, Germany) as described previously [22]. ß-Actin (ß-Actin for: 5′-CTACGTGGCCCTGGACTTCGAGC-3′; ß-Actin rev: 5’-GATGGAGCCGCCGATCCACACGG-3′) was used as a housekeeping gene for normalization. The annealing and melting temperatures were optimized for each primer set. All experiments were repeated at least three times.
Transfection vector
The coding sequence of MTAP was cloned into pCMX-PL1. By specific PCR-reaction using Primer MTAP89for and MTAP987rev the coding sequence of MTAP was amplified [6]. Using the TA Cloning® Kit (Invitrogen, Groningen, Netherlands), the PCR product was cloned into the TOPO-Cloning Vector according to the manufacturer’s instructions. Full-length (CDS) MTAP-cDNA was then cloned into the pCMX-PL1 via KpnI/EcoRI (NEB, Frankfurt, Germany) generating the expression constructs MTAP-56V and MTAP-56I. The respective clones were confirmed by sequencing using MTAPfor89 primer.
Transfection experiments
For re-expression of MTAP, 2x105 Mel Juso cells were seeded into 6-well plates and transfected with 0.5 μg of the expression construct (MTAP-56V or MTAP-56I) or pCMX-PL1 vector using Lipofectamine® LTX reagent (Life Technologies, Darmstadt, Germany) according to the manufacturer’s instructions. Twenty-four hours after transfection the medium was changed and the cells were incubated for another 24 h, before supernatant was collected and stored at -80°C until metabolite analysis. Transfected cells were split for mRNA and protein isolation, as well as for metabolite analysis (1x106 cells). All transfections were repeated at least three times.
In vitro translation in a rabbit reticulocyte lysate translation system
Using the TNT®Quick Coupled Transcription/Translation System (IVT; Promega, Madison, WI, USA) according to the manufacturer’s instructions, pCMX-PL1, MTAP-56V and MTAP-56I were used for recombinant protein expression. The master mix containing all supplements and 1 μg of either vector was incubated at 30°C for 90 min. The translation efficiency of each sample was determined by Western Blot analysis. The recombinant proteins were stored at -20°C until use.
Western Blot
For Western Blot analysis, 5 μL of in vitro translation mix or 40 μg of RIPA-cell lysates were loaded per lane, and performed as described previously using anti-MTAP antibody (0.5 μg/mL ab55517, Abcam, Cambridge, UK) and anti-ß-actin (1:5000; Sigma-Aldrich) [8]. All Western Blots were repeated three times. Protein bands in western blots were quantified using computer based colorimetric quantification analysis (freeware: ImageJ v1.33 downloaded from the NIH website (http://rsb.info.nih.gov/ij)).
Enzyme kinetics
For analyzing MTAP-enzyme-kinetic, the in vitro translation mix with the recombinant proteins or the control, respectively, was diluted ¼ in PBS. Subsequently, 1 μL of the dilution was added to MTA (10 μM) in 20 μL PBS and incubated at 37°C for defined periods of time (30 s to 30 min). The reaction was stopped by adding 600 μL of 80% MeOH, snap-frozen and stored at -80°C until measurement. All samples were prepared in triplicates and the MTAP-enzyme-kinetic was repeated three times.
MTA extraction and analysis by liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS)
For analysis of MTA in cell culture medium, cells were cultured for 24 h. Subsequently, supernatant was collected, centrifuged (1200 rpm), snap-frozen, and stored at -80°C. For determination of intracellular MTA, cells were washed with PBS and harvested by incubation in a solution containing 0.05% (w/v) trypsin and 0.02% (w/v) EDTA. Trypsinization was stopped after 2 min with cell culture medium. After centrifugation (1200 rpm, 4 min), the supernatant was removed, the cell pellet was washed with PBS buffer and the cell number was determined by counting the trypsinized cells. The cell pellet was snap-frozen and stored at -80°C. Samples were further processed as described previously [9].
Analysis of MTAP-56V and MTAP-56I by MS
Cell pellets were lysed by sonification in 300 μL of 100 mM ammonium bicarbonate buffer (pH 7.8) for 10 min. After centrifugation protein concentrations were determined via UV absorption at 280 nm using a NanoDrop2000 system (NanoDrop Instruments, Wilmington, DE, USA). Fifty μg of protein per sample was used for digestion using the RapidACN protocol [23]. Two μg of the resulting peptide mixtures were supplemented with 100 fmol each of the stable isotope-labeled peptides NVDCVLLAR(+10) and NVDCILLAR(+10) and subjected to MRM-HR analysis on a TripleTOF5600+ mass spectrometer (SCIEX GmbH, Darmstadt, Germany) coupled to a Ultimate3000 nano-HPLC (Dionex GmbH, Idstein, Germany). A 28-min-gradient from 4%-40% B (A: 0.1% formic acid; B: 0.1% formic acid in acetonitrile) was used for separation on a 75 μm I.D., 25 cm long C18-column (Acclaim PepMap, 3 μm, 100 Ǻ, flow rate 300 μL/min) with precolumn concentration (Acclaim PepMap, 100 μm I.D., 2 cm, 5 μm, 100 Ǻ, flow rate 5 μL/min). MS/MS-spectra were acquired for 0.5 s each and quantification was accomplished using the Skyline software (MacLean B, et al., Skyline: an open source document editor for creating and analyzing targeted proteomics experiments, Bioinformatics, 2010) based on the transitions from the doubly charged precursors to the singly charged fragment ions y5, y6 and y7.
Statistical analysis
Results are expressed as either mean ± S.E.M. (range) or percent. Comparison between groups was performed using one-way ANOVA with Bonferroni post-hoc test. A p-value <0.05 was considered statistically significant (ns: not significant). All calculations were performed using GraphPad Prism software (GraphPad Software Inc., San Diego, CA, USA). Testing of rs7023954 genotypes for Hardy-Weinberg equilibrium (HWE) was performed at https://www.cog-genomics.org/software/stats using Fisher’s exact test and Lancaster’s mid-P correction for better control over type I error rates [24]. Both genotypic and allelic odds ratios were calculated based on genotype and allele counts, respectively, for rs7023954 in control and primary melanoma samples [25]. The null hypothesis of no association with disease was tested by means of a χ2 test for independence of the rows and columns of 2x2 and 2x3 contingency tables.
Results
Genotyping of MTAP rs7023954
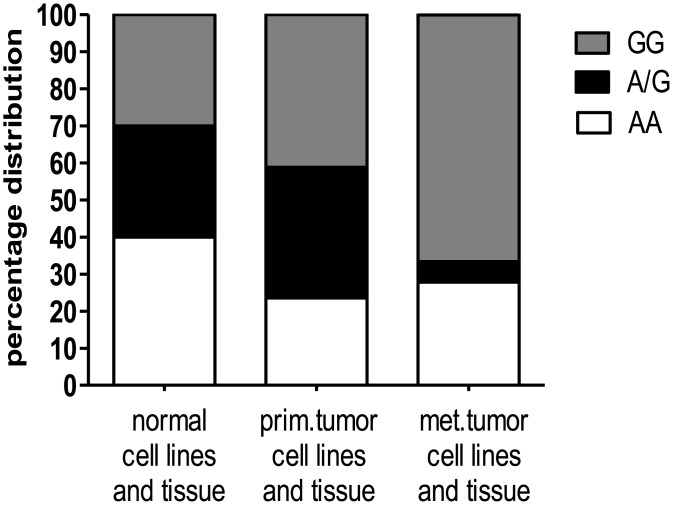
The non-synonymous MTAP SNP rs7023954 (c.G166A, p.Val56Ile, GenBank accession numbers XM 027613 and NM 00D2451) was genotyped by Sanger sequencing of exon 3, which had been PCR amplified from reverse transcribed MTAP transcripts isolated from normal skin as well as primary and metastatic melanoma cell lines and tissues (Fig 1, S1 Table). In both, normal skin and primary melanoma cell lines and tissue, the distribution of genotypes did not deviate significantly from HWE. In contrast, a highly significant (P = 0.00019) deviation from HWE, due to a deficit of heterozygotes, was observed in metastatic tumor tissue and cell lines. However, limiting the analysis to metastatic melanoma tissues only, no significant deviation (P = 0.0719) from HWE could be detected. Finally, testing for a genetic association between rs7023954 and primary melanoma using a χ2 test, no significant association could be found regardless whether the frequency of alleles or genotypes was considered.
Fig 1. Determination of MTAP rs7023954 in cell lines and tissue samples.
The non-synonymous MTAP SNP c.G166A was genotyped by sequencing of cDNA that had been produced from total RNA extracted from both normal as well as primary (prim.) and metastatic (met.) melanoma cell lines and tissues. Distribution of the genotypes AA, AG, and GG is shown. A detailed description of the genotypes of the samples investigated is given in S1 Table.
Quantitation of MTAP rs7023954 allelic expression at the protein level
To confirm expression of the MTAP rs7023954 alleles at the protein level, six cell lines homozygous for either the A- (Mel Im, Mel Ho and A375) or the G-allele (WM793, WM1366 and WM293A) and a heterozygous fibroblast cell line (3F0379) were analyzed by LC-MS/MS using allele-specific stable isotope-labeled proteotypic peptides as internal standards for accurate quantitation (Table 1). We determined isoleucine at position 56 in all samples with the AA genotype and valine in all samples with the GG genotype, respectively, while the heterozygous cell line 3F0379 showed co-dominant expression of both alleles. Expression levels ranged from 20 fmol/mg protein for Mel Ho to 2800 fmol/mg protein for A375. In the other melanoma cell lines, expression differed far less, ranging from 180 to 320 fmol/mg protein. The low level of MTAP protein expression detected in Mel Ho agreed with Western blotting performed previously (Behrmann et al., 2003). In the heterozygous cell line 3F0379, alleles were expressed at 60 fmol/mg protein each.
Table 1. Confirmation of cDNA genotype by liquid chromatography-tandem mass spectrometry (LC-MS/MS) based protein expression analysis.
Sole expression of the 56I-allele was observed in melanoma cell lines Mel Im, Mel Ho, and A375, whereas the 56V-allele was detected exclusively in WM793, WM1366, and WM293A. In the fibroblast cell line 3F0379, both alleles were co-expressed equally.
| Cell lines | Detected variant |
|---|---|
| Mel Im | 56-I |
| Mel Ho | 56-I |
| A375 | 56-I |
| WM793 | 56-V |
| WM1366 | 56-V |
| WM293A | 56-V |
| 3F0379 (NHDF) | 56-I/V |
Enzyme kinetics of in vitro-translated MTAP
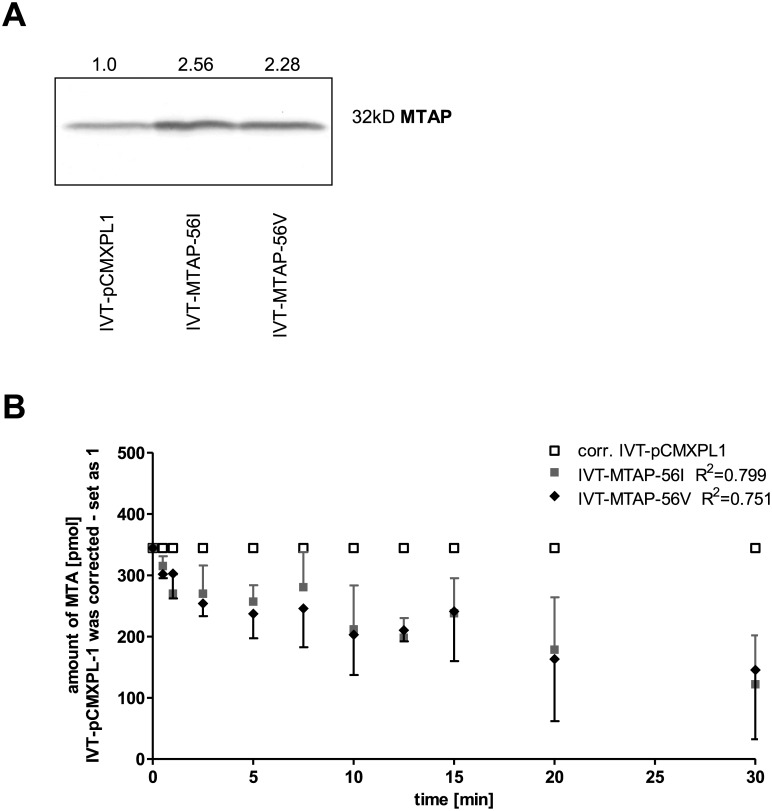
Recombinant MTAP protein with either 56V or 56I was expressed by in vitro transcription/translation (IVT). The translation efficiency of pCMX-PL1 (control vector), MTAP-56V and MTAP-56I was monitored by Western Blot analysis (Fig 2A; S1 Fig). Enzyme kinetic assays showed no difference in the rate of catabolism of MTA between the alleles (Fig 2B).
Fig 2. Enzyme kinetics of both in vitro-translated MTAP variants.
(A) Western Blot for determination of MTAP expression by in vitro transcription/translation of the pCMX-PL1 (1.0) control plasmid and the expression constructs for MTAP-56I (2.56-fold increased) and MTAP-56V (2.28-fold increased). Densitometric quantification revealed increased MTAP amount. The original Western blot is shown in S1 Fig. (B) Analysis of the metabolic activity of the in vitro-translated products by liquid chromatography–tandem mass spectrometry. Catalytic rates of IVT-MTAP-56I and IVT-MTAP-56V were corrected for the rate of the control, which was set at 1.
Enzyme kinetics of MTAP in transiently and stably transfected cells
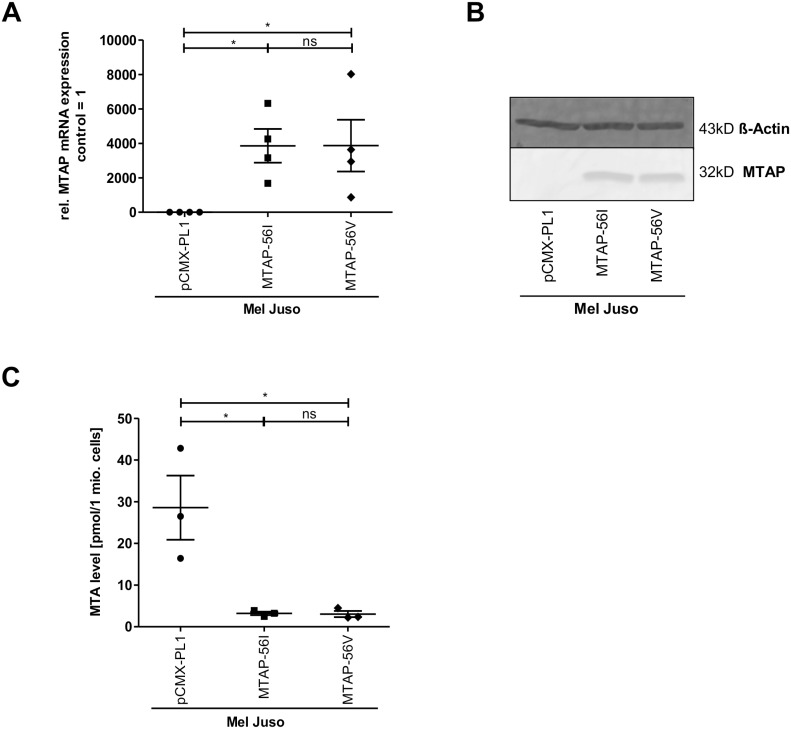
Reaction rates of the two MTAP alleles were also measured in cell culture. To that end, the melanoma cell line Mel Juso, which did not express any MTAP protein detectable by LC-MS/MS, was transfected with control (pCMX-PL1) vector, MTAP- 56I or MTAP-56V expression construct, respectively. Successful transfection was confirmed by qRT-PCR and Western Blot (Fig 3A). Both MTAP-56I and MTAP-56V transfected cells showed a similarly strong reduction in intracellular MTA levels compared to the control (Fig 3B; S2 Fig).
Fig 3. Effect of both MTAP variants in transiently cells.
Quantification of MTAP expression at the mRNA (A) and protein level (B) in the melanoma cell line Mel Juso after transient transfection of expression constructs for MTAP-56I and MTAP-56V or control plasmid (pCMX-PL1). The original Western blot is shown in S2 Fig. (C) Analysis of intracellular MTA levels in the transiently transfected Mel Juso cells.
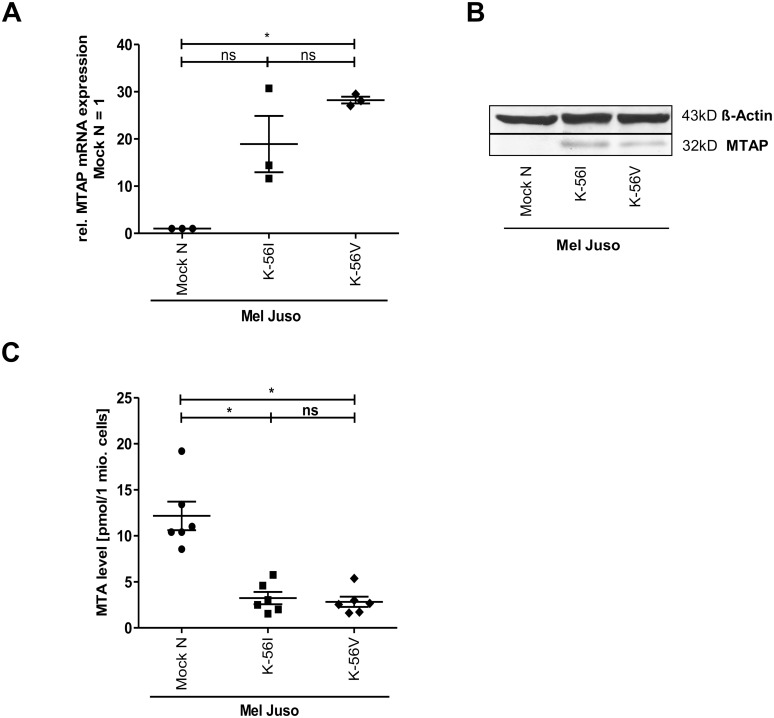
To quantify the differences between MTAP variants in Mel Juso after long-time re-expression of MTAP-56V and MTAP-56I, stably transfected clones were generated and analyzed in the same manner. MTAP expression was confirmed at the RNA and protein level (Fig 4A). The cells were analyzed for intracellular MTA by LC-MS/MS (Fig 4B; S3 Fig). Again, a significant reduction in MTA concentration was detected in MTAP re-expressing cell clones compared to control. The reduction in intracellular MTA concentration was again similar for the alleles.
Fig 4. Enzyme kinetics of both MTAP variants in stably transfected cells.
Quantification of MTAP expression at the mRNA (A) and protein level (B) in the melanoma cell line Mel Juso after stable transfection of control plasmid (pCMX-PL1) or expression constructs for MTAP-56I and MTAP-56V. The original Western blot is shown in S3 Fig. (C) Analysis of intracellular MTA levels in the stably transfected Mel Juso cell clones.
Discussion
The chromosomal locus 9p21 has been implicated in the pathogenesis of cutaneous malignant melanoma [26,27]. Several studies have revealed a link between 9p21 and pigmentation [27,28]. Increased pigmentation in metastatic melanoma tissue is associated with both a significant shorter overall survival (OS) and progression free survival (PFS) [29] as well as a poor response to radiotherapy [30]. The locus also harbors, aside from MTAP, the type I interferon (IFN) gene cluster, the two cyclin-dependent kinase inhibitors CDKN2A (encoding p16INK4a and p14ARF) and CDKN2B (encoding p15INK4b), and a long intergenic noncoding RNA, designated antisense noncoding RNA in the INK4 locus (ANRIL), which has been shown to regulate CDKN2A/B by epigenetic mechanisms [31].
Genome-wide association studies (GWAS) have identified a number of loci predicting nevus count, including the 9p21 locus, which is also significantly associated with melanoma risk [15]. Recent studies reported a significant association of the MTAP SNPs rs7023329 (intron 2) and rs10811629 (intron 5) with melanoma incidence and number of melanocytic nevi [28]. Further, the MTAP polymorphism rs10757257*G in intron 1 was found to be significantly associated with melanoma risk in adults and, in particular, with superficial spreading and nodular melanoma subtypes [16].
To date, the non-synonymous SNP rs7023954 in exon 3 of the MTAP gene, which results in a conservative substitution of a branched-chain amino acid residue at position 56 for another, has not been linked to melanoma development. Here, we genotyped rs7023954 in both primary and metastatic melanoma cell lines and tissues and compared the genotype and allele counts observed to those in normal skin tissue and cell lines. We further confirmed in the fibroblast cell line 3F0379, which is heterozygous for rs7023954, the co-dominant expression of the alleles at the protein level by LC-MS/MS using allele-specific stable-isotope labeled peptides as internal standards for accurate quantitation. While the genotype frequencies observed in normal and primary melanoma samples adhered to those expected under the Hardy-Weinberg assumption, a significant deviation from HWE was found in metastatic melanoma samples due to a deficit of heterozygotes. However, considering metastatic melanoma tissue only, no significant deviation from HWE could be detected. This is not an uncommon finding in comparative high-resolution surveys of genome-wide chromosomal abnormalities in established cell lines and primary human tumors [32]. Moreover, in contrast to previous papers that had observed homozygous deletions of the MTAP locus in 3 out of 11 [33] and 1 out 9 investigated melanoma cell lines [6], respectively, no evidence of a diallelic deletion of the MTAP locus was found here. This supports our previous finding, that loss or reduction of MTAP expression in melanoma is more often the result of hypermethylation of the promoter region than chromosomal deletion of the MTAP locus [6].
Tests of genetic association performed on observed allele counts in normal and primary melanoma samples showed a positive, albeit not significant association of MTAP rs7023954*G (OR 1.746, 95% CI = 0.6928–4.4006) with melanoma. It cannot be excluded that the lack of significance is a consequence of the small sample size studied and the resulting lack of statistical power. As a matter of fact, the odds ratio of 1.746 observed for MTAP rs7023954*G is quite similar to that reported for MTAP rs10757257*G and melanoma risk in adults (OR = 1.32, 95% CI = 1.14–1.54). This led us to investigate whether the G allele might differ in enzymatic activity from the A allele. We cloned both alleles and expressed them using both a rabbit reticulocyte lysate translation system as well as transient and stable transfection of an MTAP-null melanoma cell line. In all three systems, enzyme kinetics of the alleles did not differ significantly, indicating that a difference in catabolism of MTA is unlikely to contribute to melanoma development and progression. This concords with a recently published study that found a catalytically inactive version of MTAP, which was introduced into HT1080 fibrosarcoma cells, to be fully capable of reversing various tumor phenotypes such as soft agar colony formation and increased migration and metalloproteinase production [34].
Conclusion
The absence of a difference in the rate of catabolism of MTA to adenine and 5-methylthioribose-1-phosphate between the alleles of the only common amino acid substitution polymorphism described to date for MTAP lends further support to the notion, that the tumor suppressive function of MTAP is independent of its known enzymatic activity.
Supporting Information
Two independent Western blots for the determination of MTAP expression by in vitro transcription/translation.
(EPS)
Two independent Western blots for the determination of MTAP expression after transient transfection.
(EPS)
Two independent Western blots for the determination of MTAP expression after stable transfection.
(EPS)
Distribution of the genotypes AA, AG, and GG of SNP rs7023954 determined by cDNA amplicon sequencing in various normal skin as well as primary (prim.) and metastatic (met.) melanoma cell lines and tissues.
(DOCX)
Acknowledgments
We are indebted to Nadine Nürnberger (Institute of Functional Genomics, Regensburg) and Elke Perthen (Institute of Functional Genomics, Regensburg) for excellent technical assistance. The authors also acknowledge Prof. Dr. Meenhard Herlyn (Wistar Institute, Philadelphia) for providing cell lines and Prof. Dr. Sigrid Karrer (Department of Dermatology, University Hospital Regensburg) for providing dermal skin tissue specimens.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from the Medical Faculty of the University of Regensburg (ReForM) and the German Research Foundation (DFG BO1573, KFO 262), further by BioSysNet and the German Cancer Aid. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Godar DE. Worldwide increasing incidences of cutaneous malignant melanoma. J Skin Cancer. 2011;2011: 858425 10.1155/2011/858425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villanueva J, Vultur A, Herlyn M. Resistance to BRAF inhibitors: Unraveling mechanisms and future treatment options. Cancer Res. 2011;71: 7137–7140. 10.1158/0008-5472.CAN-11-1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller DM, Flaherty KT. Cyclin-dependent kinases as therapeutic targets in melanoma. Pigment Cell Melanoma Res. 2014;27: 351–365. 10.1111/pcmr.12211 [DOI] [PubMed] [Google Scholar]
- 4.Houslay MD. Hard Times for Oncogenic BRAF-Expressing Melanoma Cells. Cancer Cell. 2011;19: 3–4. 10.1016/j.ccr.2011.01.004 [DOI] [PubMed] [Google Scholar]
- 5.Kirovski G, Stevens AP, Czech B, Dettmer K, Weiss TS, Wild P, et al. Down-Regulation of Methylthioadenosine Phosphorylase (MTAP) Induces Progression of Hepatocellular Carcinoma via Accumulation of 5′-Deoxy-5′-Methylthioadenosine (MTA). Am J Pathol. 2011;178: 1145–1152. 10.1016/j.ajpath.2010.11.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behrmann I, Wallner S, Komyod W, Heinrich PC, Schuierer M, Buettner R, et al. Characterization of Methylthioadenosin Phosphorylase (MTAP) Expression in Malignant Melanoma. Am J Pathol. 2003;163: 683–690. 10.1016/S0002-9440(10)63695-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens AP, Spangler B, Wallner S, Kreutz M, Dettmer K, Oefner PJ, et al. Direct and tumor microenvironment mediated influences of 5’-deoxy-5’-(methylthio)adenosine on tumor progression of malignant melanoma. J Cell Biochem. 2009;106: 210–219. 10.1002/jcb.21984 [DOI] [PubMed] [Google Scholar]
- 8.Limm K, Ott C, Wallner S, Mueller DW, Oefner P, Hellerbrand C, et al. Deregulation of protein methylation in melanoma. Eur J Cancer Oxf Engl 1990. 2013;49: 1305–1313. 10.1016/j.ejca.2012.11.026 [DOI] [PubMed] [Google Scholar]
- 9.Stevens AP, Dettmer K, Wallner S, Bosserhoff AK, Oefner PJ. Quantitative analysis of 5’-deoxy-5’-methylthioadenosine in melanoma cells by liquid chromatography-stable isotope ratio tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;876: 123–128. 10.1016/j.jchromb.2008.10.038 [DOI] [PubMed] [Google Scholar]
- 10.Stevens AP, Dettmer K, Kirovski G, Samejima K, Hellerbrand C, Bosserhoff AK, et al. Quantification of intermediates of the methionine and polyamine metabolism by liquid chromatography-tandem mass spectrometry in cultured tumor cells and liver biopsies. J Chromatogr A. 2010;1217: 3282–3288. 10.1016/j.chroma.2010.01.025 [DOI] [PubMed] [Google Scholar]
- 11.Andreu-Pérez P, Esteve-Puig R, de Torre-Minguela C, López-Fauqued M, Bech-Serra JJ, Tenbaum S, et al. Protein arginine methyltransferase 5 regulates ERK1/2 signal transduction amplitude and cell fate through CRAF. Sci Signal. 2011;4: ra58 10.1126/scisignal.2001936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Limm K, Wallner S, Milenkovic VM, Wetzel CH, Bosserhoff A-K. The metabolite 5′-methylthioadenosine signals through the adenosine receptor A2B in melanoma. Eur J Cancer. 2014;50: 2714–2724. 10.1016/j.ejca.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 13.Andreu-Pérez P, Hernandez-Losa J, Moliné T, Gil R, Grueso J, Pujol A, et al. Methylthioadenosine (MTA) inhibits melanoma cell proliferation and in vivo tumor growth. BMC Cancer. 2010;10: 265 10.1186/1471-2407-10-265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20: 2390–2400. 10.1038/sj.onc.1204383 [DOI] [PubMed] [Google Scholar]
- 15.Newton-Bishop JA, Chang Y-M, Iles MM, Taylor JC, Bakker B, Chan M, et al. Melanocytic nevi, nevus genes and melanoma risk in a large case-control study in the United Kingdom. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2010;19: 2043–2054. 10.1158/1055-9965.EPI-10-0233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kvaskoff M, Whiteman DC, Zhao ZZ, Montgomery GW, Martin NG, Hayward NK, et al. Polymorphisms in nevus-associated genes MTAP, PLA2G6, and IRF4 and the risk of invasive cutaneous melanoma. Twin Res Hum Genet Off J Int Soc Twin Stud. 2011;14: 422–432. 10.1375/twin.14.5.422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacob K, Wach F, Holzapfel U, Hein R, Lengyel E, Buettner R, et al. In vitro modulation of human melanoma cell invasion and proliferation by all-trans-retinoic acid. Melanoma Res. 1998;8: 211–219. [DOI] [PubMed] [Google Scholar]
- 18.Hohenauer T, Berking C, Schmidt A, Haferkamp S, Senft D, Kammerbauer C, et al. The neural crest transcription factor Brn3a is expressed in melanoma and required for cell cycle progression and survival. EMBO Mol Med. 2013;5: 919–934. 10.1002/emmm.201201862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spangler B, Kappelmann M, Schittek B, Meierjohann S, Vardimon L, Bosserhoff AK, et al. ETS-1/RhoC signaling regulates the transcription factor c-Jun in melanoma. Int J Cancer. 2012;130: 2801–2811. 10.1002/ijc.26277 [DOI] [PubMed] [Google Scholar]
- 20.Canady J, Arndt S, Karrer S, Bosserhoff AK. Increased KGF expression promotes fibroblast activation in a double paracrine manner resulting in cutaneous fibrosis. J Invest Dermatol. 2013;133: 647–657. 10.1038/jid.2012.389 [DOI] [PubMed] [Google Scholar]
- 21.Braig S, Wallner S, Junglas B, Fuchshofer R, Bosserhoff A-K. CTGF is overexpressed in malignant melanoma and promotes cell invasion and migration. Br J Cancer. 2011;105: 231–238. 10.1038/bjc.2011.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niebler S, Schubert T, Hunziker EB, Bosserhoff AK. Activating enhancer binding protein 2 epsilon (AP-2ε)-deficient mice exhibit increased matrix metalloproteinase 13 expression and progressive osteoarthritis development. Arthritis Res Ther. 2015;17: 119 10.1186/s13075-015-0648-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bluemlein K, Ralser M. Monitoring protein expression in whole-cell extracts by targeted label- and standard-free LC-MS/MS. Nat Protoc. 2011;6: 859–869. 10.1038/nprot.2011.333 [DOI] [PubMed] [Google Scholar]
- 24.Wigginton JE, Cutler DJ, Abecasis GR. A note on exact tests of Hardy-Weinberg equilibrium. Am J Hum Genet. 2005;76: 887–893. 10.1086/429864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clarke GM, Anderson CA, Pettersson FH, Cardon LR, Morris AP, Zondervan KT. Basic statistical analysis in genetic case-control studies. Nat Protoc. 2011;6: 121–133. 10.1038/nprot.2010.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camacho-Vanegas O, Camacho SC, Till J, Miranda-Lorenzo I, Terzo E, Ramirez MC, et al. Primate genome gain and loss: a bone dysplasia, muscular dystrophy, and bone cancer syndrome resulting from mutated retroviral-derived MTAP transcripts. Am J Hum Genet. 2012;90: 614–627. 10.1016/j.ajhg.2012.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang XR, Liang X, Pfeiffer RM, Wheeler W, Maeder D, Burdette L, et al. Associations of 9p21 variants with cutaneous malignant melanoma, nevi, and pigmentation phenotypes in melanoma-prone families with and without CDKN2A mutations. Fam Cancer. 2010;9: 625–633. 10.1007/s10689-010-9356-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maccioni L, Rachakonda PS, Bermejo JL, Planelles D, Requena C, Hemminki K, et al. Variants at the 9p21 locus and melanoma risk. BMC Cancer. 2013;13: 325 10.1186/1471-2407-13-325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brożyna AA, Jóźwicki W, Carlson JA, Slominski AT. Melanogenesis affects overall and disease-free survival in patients with stage III and IV melanoma. Hum Pathol. 2013;44: 2071–2074. 10.1016/j.humpath.2013.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brożyna AA, Jóźwicki W, Roszkowski K, Filipiak J, Slominski AT. Melanin content in melanoma metastases affects the outcome of radiotherapy. Oncotarget. 2016; 17844–17853. 10.18632/oncotarget.7528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Congrains A, Kamide K, Ohishi M, Rakugi H. ANRIL: Molecular Mechanisms and Implications in Human Health. Int J Mol Sci. 2013;14: 1278–1292. 10.3390/ijms14011278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li A, Walling J, Kotliarov Y, Center A, Steed ME, Ahn SJ, et al. Genomic changes and gene expression profiles reveal that established glioma cell lines are poorly representative of primary human gliomas. Mol Cancer Res MCR. 2008;6: 21–30. 10.1158/1541-7786.MCR-07-0280 [DOI] [PubMed] [Google Scholar]
- 33.Ogbah Z, Puig-Butille JA, Simonetta F, Badenas C, Cervera R, Milá J, et al. Molecular characterization of human cutaneous melanoma-derived cell lines. Anticancer Res. 2012;32: 1245–1251. [PubMed] [Google Scholar]
- 34.Tang B, Kadariya Y, Chen Y, Slifker M, Kruger WD. Expression of MTAP inhibits tumor-related phenotypes in HT1080 cells via a mechanism unrelated to its enzymatic function. G3 Bethesda Md. 2014;5: 35–44. 10.1534/g3.114.014555 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two independent Western blots for the determination of MTAP expression by in vitro transcription/translation.
(EPS)
Two independent Western blots for the determination of MTAP expression after transient transfection.
(EPS)
Two independent Western blots for the determination of MTAP expression after stable transfection.
(EPS)
Distribution of the genotypes AA, AG, and GG of SNP rs7023954 determined by cDNA amplicon sequencing in various normal skin as well as primary (prim.) and metastatic (met.) melanoma cell lines and tissues.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.