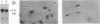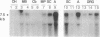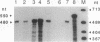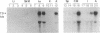Abstract
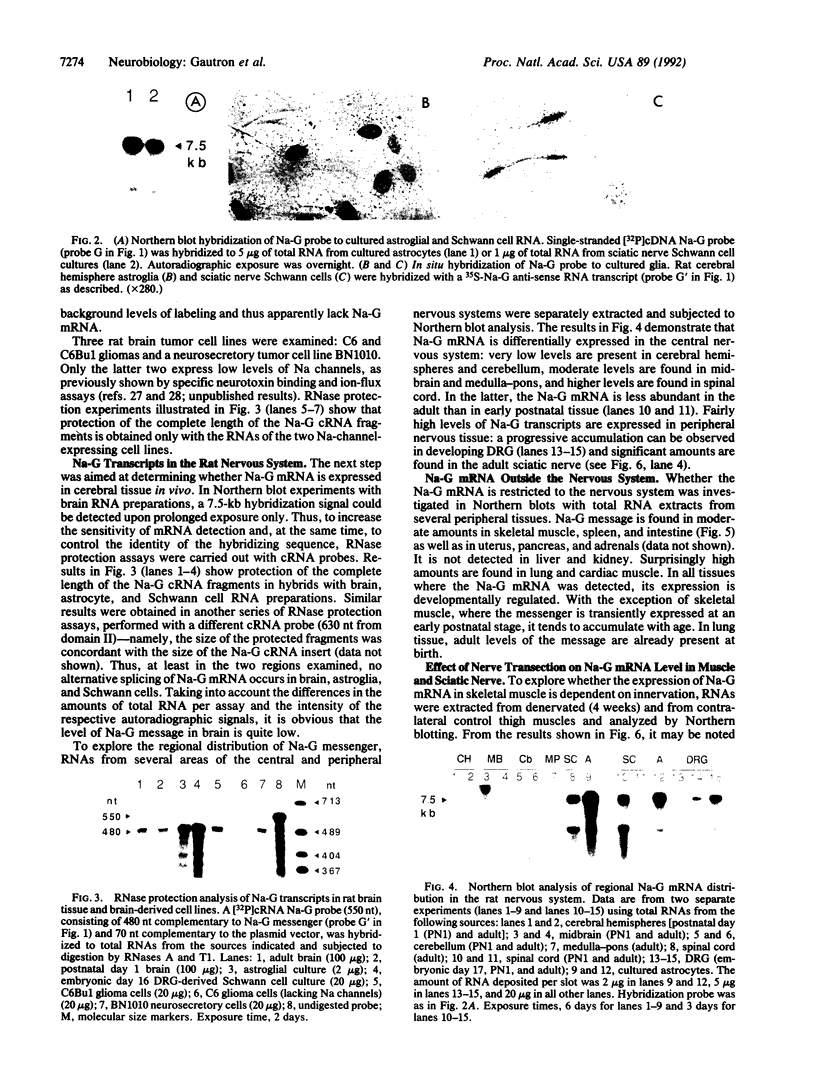
Previous electrophysiological and pharmacological studies on central and peripheral glia revealed the presence of voltage-gated Na channels with properties that are similar but not identical to those of neuronal Na channels. Here we report the isolation and characterization of a cDNA encoding the C-terminal portion of a putative glial Na-channel (Na-G) alpha subunit. The amino acid sequence deduced from this cDNA indicates that the Na-G represents a separate molecular class within the mammalian Na-channel multigene family. By Northern blot, RNase protection, and in situ hybridization assays, we demonstrate that, in addition to brain astroglia, the Na-G mRNA is expressed in cultures of Schwann cells derived from dorsal root ganglia or from sciatic nerve. In vivo, the Na-G mRNA is detected not only in brain, dorsal root ganglia, and sciatic nerve, but also in tissues outside the nervous system including cardiac and skeletal muscle and lung. Its level varies according to the tissue and is developmentally regulated. The sequence and expression data concur in designating Na-G as an distinct type of Na channel, presumably with low sensitivity to tetrodotoxin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnew W. S., Levinson S. R., Brabson J. S., Raftery M. A. Purification of the tetrodotoxin-binding component associated with the voltage-sensitive sodium channel from Electrophorus electricus electroplax membranes. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2606–2610. doi: 10.1073/pnas.75.6.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auld V. J., Goldin A. L., Krafte D. S., Marshall J., Dunn J. M., Catterall W. A., Lester H. A., Davidson N., Dunn R. J. A rat brain Na+ channel alpha subunit with novel gating properties. Neuron. 1988 Aug;1(6):449–461. doi: 10.1016/0896-6273(88)90176-6. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Chun L. L., Corey D. P. Ion channels in vertebrate glia. Annu Rev Neurosci. 1990;13:441–474. doi: 10.1146/annurev.ne.13.030190.002301. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Koroshetz W. J., Chun L. L., Corey D. P. Ion channel expression by white matter glia: the type-1 astrocyte. Neuron. 1990 Oct;5(4):527–544. doi: 10.1016/0896-6273(90)90091-s. [DOI] [PubMed] [Google Scholar]
- Beckh S. Differential expression of sodium channel mRNAs in rat peripheral nervous system and innervated tissues. FEBS Lett. 1990 Mar 26;262(2):317–322. doi: 10.1016/0014-5793(90)80218-8. [DOI] [PubMed] [Google Scholar]
- Beckh S., Noda M., Lübbert H., Numa S. Differential regulation of three sodium channel messenger RNAs in the rat central nervous system during development. EMBO J. 1989 Dec 1;8(12):3611–3616. doi: 10.1002/j.1460-2075.1989.tb08534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan S., Chiu S. Y., Gray P. T., Ritchie J. M. The presence of voltage-gated sodium, potassium and chloride channels in rat cultured astrocytes. Proc R Soc Lond B Biol Sci. 1985 Sep 23;225(1240):299–313. doi: 10.1098/rspb.1985.0063. [DOI] [PubMed] [Google Scholar]
- Brodie C., Sampson S. R. Regulation of the sodium-potassium pump in cultured rat skeletal myotubes by intracellular sodium ions. J Cell Physiol. 1989 Jul;140(1):131–137. doi: 10.1002/jcp.1041400116. [DOI] [PubMed] [Google Scholar]
- Casadei J. M., Gordon R. D., Barchi R. L. Immunoaffinity isolation of Na+ channels from rat skeletal muscle. Analysis of subunits. J Biol Chem. 1986 Mar 25;261(9):4318–4323. [PubMed] [Google Scholar]
- Chelly J., Hamard G., Koulakoff A., Kaplan J. C., Kahn A., Berwald-Netter Y. Dystrophin gene transcribed from different promoters in neuronal and glial cells. Nature. 1990 Mar 1;344(6261):64–65. doi: 10.1038/344064a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chiu S. Y. Changes in excitable membrane properties in Schwann cells of adult rabbit sciatic nerves following nerve transection. J Physiol. 1988 Feb;396:173–188. doi: 10.1113/jphysiol.1988.sp016957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y. Sodium currents in axon-associated Schwann cells from adult rabbits. J Physiol. 1987 May;386:181–203. doi: 10.1113/jphysiol.1987.sp016529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couraud F., Martin-Moutot N., Koulakoff A., Berwald-Netter Y. Neurotoxin-sensitive sodium channels in neurons developing in vivo and in vitro. J Neurosci. 1986 Jan;6(1):192–198. doi: 10.1523/JNEUROSCI.06-01-00192.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. B., Stroobant P. Platelet-derived growth factors and fibroblast growth factors are mitogens for rat Schwann cells. J Cell Biol. 1990 Apr;110(4):1353–1360. doi: 10.1083/jcb.110.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine B., Sassoon D., Buckingham M., Changeux J. P. Detection of the nicotinic acetylcholine receptor alpha-subunit mRNA by in situ hybridization at neuromuscular junctions of 15-day-old chick striated muscles. EMBO J. 1988 Mar;7(3):603–609. doi: 10.1002/j.1460-2075.1988.tb02853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda J., Kameyama M. Tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels in tissue-cultured spinal ganglion neurons from adult mammals. Brain Res. 1980 Jan 20;182(1):191–197. doi: 10.1016/0006-8993(80)90844-6. [DOI] [PubMed] [Google Scholar]
- Gautron S., Daegelen D., Mennecier F., Dubocq D., Kahn A., Dreyfus J. C. Molecular mechanisms of McArdle's disease (muscle glycogen phosphorylase deficiency). RNA and DNA analysis. J Clin Invest. 1987 Jan;79(1):275–281. doi: 10.1172/JCI112794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D., Merrick D., Auld V., Dunn R., Goldin A. L., Davidson N., Catterall W. A. Tissue-specific expression of the RI and RII sodium channel subtypes. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8682–8686. doi: 10.1073/pnas.84.23.8682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Guy H. R., Conti F. Pursuing the structure and function of voltage-gated channels. Trends Neurosci. 1990 Jun;13(6):201–206. doi: 10.1016/0166-2236(90)90160-c. [DOI] [PubMed] [Google Scholar]
- Hartshorne R. P., Catterall W. A. The sodium channel from rat brain. Purification and subunit composition. J Biol Chem. 1984 Feb 10;259(3):1667–1675. [PubMed] [Google Scholar]
- Kallen R. G., Sheng Z. H., Yang J., Chen L. Q., Rogart R. B., Barchi R. L. Primary structure and expression of a sodium channel characteristic of denervated and immature rat skeletal muscle. Neuron. 1990 Feb;4(2):233–242. doi: 10.1016/0896-6273(90)90098-z. [DOI] [PubMed] [Google Scholar]
- Kayano T., Noda M., Flockerzi V., Takahashi H., Numa S. Primary structure of rat brain sodium channel III deduced from the cDNA sequence. FEBS Lett. 1988 Feb 8;228(1):187–194. doi: 10.1016/0014-5793(88)80614-8. [DOI] [PubMed] [Google Scholar]
- Martin-Moutot N., Couraud F., Houzet E., Berwald-Netter Y. High-affinity binding of alpha-scorpion toxin: a neuronal property. Brain Res. 1983 Sep 12;274(2):267–274. doi: 10.1016/0006-8993(83)90704-7. [DOI] [PubMed] [Google Scholar]
- Noda M., Ikeda T., Kayano T., Suzuki H., Takeshima H., Kurasaki M., Takahashi H., Numa S. Existence of distinct sodium channel messenger RNAs in rat brain. Nature. 1986 Mar 13;320(6058):188–192. doi: 10.1038/320188a0. [DOI] [PubMed] [Google Scholar]
- Nowak L., Ascher P., Berwald-Netter Y. Ionic channels in mouse astrocytes in culture. J Neurosci. 1987 Jan;7(1):101–109. doi: 10.1523/JNEUROSCI.07-01-00101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Abney E. R., Cohen J., Lindsay R., Noble M. Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface gangliosides, and growth characteristics. J Neurosci. 1983 Jun;3(6):1289–1300. doi: 10.1523/JNEUROSCI.03-06-01289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern P., Thesleff S. Action potential generation in denervated rat skeletal muscle. II. The action of tetrodotoxin. Acta Physiol Scand. 1971 May;82(1):70–78. doi: 10.1111/j.1748-1716.1971.tb04943.x. [DOI] [PubMed] [Google Scholar]
- Reiser G., Hamprecht B. Sodium-channels in non-excitable glioma cells, shown by the influence of veratridine, scorpion toxin, and tetrodotoxin on membrane potential and on ion transport. Pflugers Arch. 1983 Jun 1;397(4):260–264. doi: 10.1007/BF00580258. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M., Rang H. P. Extraneuronal saxitoxin binding sites in rabbit myelinated nerve. Proc Natl Acad Sci U S A. 1983 May;80(9):2803–2807. doi: 10.1073/pnas.80.9.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogart R. B., Cribbs L. L., Muglia L. K., Kephart D. D., Kaiser M. W. Molecular cloning of a putative tetrodotoxin-resistant rat heart Na+ channel isoform. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8170–8174. doi: 10.1073/pnas.86.20.8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrager P., Chiu S. Y., Ritchie J. M. Voltage-dependent sodium and potassium channels in mammalian cultured Schwann cells. Proc Natl Acad Sci U S A. 1985 Feb;82(3):948–952. doi: 10.1073/pnas.82.3.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sills M. N., Xu Y. C., Baracchini E., Goodman R. H., Cooperman S. S., Mandel G., Chien K. R. Expression of diverse Na+ channel messenger RNAs in rat myocardium. Evidence for a cardiac-specific Na+ channel. J Clin Invest. 1989 Jul;84(1):331–336. doi: 10.1172/JCI114158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer H., Ransom B. R., Cornell-Bell A. H., Black J. A., Waxman S. G. Na(+)-current expression in rat hippocampal astrocytes in vitro: alterations during development. J Neurophysiol. 1991 Jan;65(1):3–19. doi: 10.1152/jn.1991.65.1.3. [DOI] [PubMed] [Google Scholar]
- Stühmer W., Conti F., Suzuki H., Wang X. D., Noda M., Yahagi N., Kubo H., Numa S. Structural parts involved in activation and inactivation of the sodium channel. Nature. 1989 Jun 22;339(6226):597–603. doi: 10.1038/339597a0. [DOI] [PubMed] [Google Scholar]
- Terlau H., Heinemann S. H., Stühmer W., Pusch M., Conti F., Imoto K., Numa S. Mapping the site of block by tetrodotoxin and saxitoxin of sodium channel II. FEBS Lett. 1991 Nov 18;293(1-2):93–96. doi: 10.1016/0014-5793(91)81159-6. [DOI] [PubMed] [Google Scholar]
- Trimmer J. S., Cooperman S. S., Tomiko S. A., Zhou J. Y., Crean S. M., Boyle M. B., Kallen R. G., Sheng Z. H., Barchi R. L., Sigworth F. J. Primary structure and functional expression of a mammalian skeletal muscle sodium channel. Neuron. 1989 Jul;3(1):33–49. doi: 10.1016/0896-6273(89)90113-x. [DOI] [PubMed] [Google Scholar]
- Westenbroek R. E., Merrick D. K., Catterall W. A. Differential subcellular localization of the RI and RII Na+ channel subtypes in central neurons. Neuron. 1989 Dec;3(6):695–704. doi: 10.1016/0896-6273(89)90238-9. [DOI] [PubMed] [Google Scholar]
- Yang J. S., Sladky J. T., Kallen R. G., Barchi R. L. TTX-sensitive and TTX-insensitive sodium channel mRNA transcripts are independently regulated in adult skeletal muscle after denervation. Neuron. 1991 Sep;7(3):421–427. doi: 10.1016/0896-6273(91)90294-a. [DOI] [PubMed] [Google Scholar]