Abstract
Mosquitoes carrying the endosymbiont bacterium Wolbachia have been deployed in field trials as a biological control intervention due to Wolbachia effects on reducing transmission of arboviruses. We performed mark, release and recapture (MRR) experiments using Wolbachia as an internal marker with daily collections with BG-Traps during the first two weeks of releases in Rio de Janeiro, Brazil. The MRR design allowed us to investigate two critical parameters that determine whether Wolbachia would successful invade a field population: the probability of daily survival (PDS) of Wolbachia-infected Aedes aegypti females, and the wild population density during releases. Released females had a PDS of 0.82 and 0.89 in the first and second weeks, respectively, immediately after releases, which is well within the range of previous estimates of survivorship of wild mosquitoes in Rio de Janeiro. Abundance estimation of wild population varied up to 10-fold higher depending on the estimation method used (634–3565 females on the average-difference model to 6365–16188 females according to Lincoln-Petersen). Wolbachia-released mosquitoes were lower than the density estimation of their wild counterparts, irrespectively of the model used. Individually screening mosquitoes for the presence of Wolbachia reduced uncertainty on abundance estimations due to fluctuation in capturing per week. A successful invasion into local population requires Ae. aegypti fitness is unaffected by Wolbachia presence, but also reliable estimates on the population size of wild mosquitoes.
Introduction
The distribution of diseases such as malaria and dengue frequently overlaps with tropical and subtropical zones in which primary vectors of these diseases are more abundant [1,2]. Considering dengue virus (DENV), about half of the world's population lives at risk of getting infected. In particular, Brazil registered more than one million cases annually in the last three years [3,4]. Since 2010 Brazil has all four DENV serotypes circulating in the country, promoting dengue outbreaks every 4–5 years, often due to the arrival of a new serotype in a susceptible human host population [5–7]. Apart from dengue, two other arboviruses were recently detected in Brazil: chikungunya (CHIKV) [8] and Zika (ZIKV) [9]. The potential association between ZIKV during pregnancy and microcephaly in newborn has raised additional concerns about vector control efforts to mitigate arboviruses transmission. Current evidence shows DENV, CHIKV and ZIKV to be overwhelmingly transmitted by Aedes mosquitoes, especially Aedes aegypti [10,11], but other mosquitoes in the case of ZIKV [12]. The main role of Ae. aegypti as vector of these three arboviruses is probably due to its close association with human dwellings, since females lay eggs in man-made containers, bite preferably human hosts and are more abundant in urbanized landscapes with low vegetation coverage [13–16].
Since there is no vaccine currently available, the best way to reduce arboviruses transmission still relies on vector control, which ultimately aims to maintain Ae. aegypti density below a theoretical threshold to avoid outbreaks [17]. Thus, estimations on mosquito population size, survivorship and spatial distribution in endemic areas becomes critical for improving practices in vector control, e.g., directing the intensification of mechanical and chemical control activities in the districts in which vector population is higher [6, 18, 19].
In Brazil, Ae. aegypti density is often estimated by indexes derived from infestation rates based on larval surveys, in which a sample of around 10% of houses are randomly selected and inspected 4–6 times yearly [20]. These indexes do not provide good estimators on adult mosquito abundance because container productivity and larval mortality are not taken into account [21,22]. Estimates on adult mosquito population density might be achieved through adult sampling using traps [23,24] and mark, release and recapture (MRR) experiments [25–27]. MRR-based estimation has been proposed as a more reliable approach to determine Ae. aegypti population size because it focuses on adult sampling, providing more robust estimates on the mosquito life cycle stage directly responsible for disease transmission [28,29].
One of the most promising strategies designed to reduce arboviruses transmission uses the intracellular endosymbiont, maternally inherited bacterium Wolbachia pipientis, which is naturally present in up to 65% of all insects [30–32]. This approach explores the fact that Wolbachia reduces transmission of key pathogens, including DENV and CHIKV viruses in the Ae. aegypti mosquitoes [33,34]. The wMel strain causes a phenotypic effect called cytoplasmic incompatibility (CI), which is a reproductive incompatibility that prevents females without Wolbachia from producing viable offspring after mating with Wolbachia-infected males. By contrast, Wolbachia-infected females can successfully reproduce after mating with either Wolbachia-infected or wild male [35]. This reproductive advantage increases the frequency of Wolbachia infection in a given population with each subsequent generation. Thus, the control strategy is to release mosquitoes with this bacterium for 10 or more consecutive weeks [36]. The expected outcome is the replacement of wild, vector competent, mosquitoes with Wolbachia-infected mosquitoes, potentially ameliorating the burden of arboviruses.
In the first two weeks of releases, Wolbachia may be seen as an internal marker, allowing estimates on the probability of daily survival rates of released Wolbachia-infected Ae. aegypti females, but also the population size of wild mosquitoes during releases [37]. These estimates are critical for understanding invasion, because: (1) the daily survival rate of Wolbachia-released mosquitoes is a strong indicator whether Ae. aegypti females are fit to field conditions, (2) the displacement of wild vector populations by refractory Wolbachia-carrying mosquitoes have been shown to have a threshold conditions dictated by Wolbachia frequency in the total population [38]; and (3) to calculate the ideal minimum number of Wolbachia-carrying mosquitoes that have to be released per week to succeed invasion promoting low nuisance in local residents. Therefore, our main objective is to estimate the probability of daily survival rates of Ae. aegypti infected with Wolbachia and population density of wild Ae. aegypti for better characterize Wolbachia invasion.
Materials and Methods
Study area
We conducted MRR studies in the district of Tubiacanga, Rio de Janeiro (area: 8.6 ha; 22°47′08′′S; 43°13′36′′W). Such locality is an isolated middle-class suburban area located on the offshores of Guanabara Bay and distant 2.1Km for the closest community, which discourages mosquito migration. This residential area has paved streets, well-maintained sidewalks and low-moderate vegetation coverage, with around 2902 people living in 867 houses. Most houses have 2–3 bedrooms, large yards, regular water supply and garbage collection.
Mosquitoes release
The strain wMelBr was derived from the backcrossing of Australian Ae. aegypti females wMel-infected with Brazilian males (collected from four districts in Rio de Janeiro) during nine generations [39]. Larvae were reared under optimal rearing conditions, in plastic trays in 3 liters of filtered and dechlorinated water (500 larvae per tray), and fed with 0.45g of Tetramin® Tropical Flakes Fish Food every day. Adult mosquitoes were maintained in a climate controlled insectary, at 27 ± 1°C and 65 ± 5% RH, with a 12:12 hour light:dark cycle, and received constant 10% sucrose solution up to the release day. Before every release, a sample of 100 mosquitoes was screened for Wolbachia to confirm the infection. Releases were conducted outdoors at 05:00AM, once a week, with 5–6 days-old adults at 1:1 sex ratio, with 50 mosquitoes released every four houses. A total of 2,350 Wolbachia-carrying females were released in field each week (2.71 per premise). Releases lasted 20 weeks, but our analyzes considered only the first two weeks, when captured mosquitoes positive for Wolbachia were those we released.
Mosquito collection
Mosquitoes were captured using 30 BG-Sentinel® Traps (BGS, BioGents, Regensburg, Germany) uniformly distributed across Tubiacanga. The kind of trap consists of a collapsible white bucket with white gauze covering its opening. In the middle of the gauze cover, a black tube through which a down flow is created by a fan that causes any mosquito surrounding the trap to be sucked into a catch bag. BGS-Trap uses local energy power and captures predominantly host-seeking Ae. aegypti females, seldom males [40,41]. After starting releases, traps were inspected daily for 14 days, and only female counts were included in the analyses.
Monitoring Wolbachia infection in field-collected mosquitoes
All mosquitoes collected in the BGS-Trap were brought to lab in order to proceed with identification using taxonomic keys [10]. Those classified as Ae. aegypti were screened for the presence of Wolbachia, following the approach taken in Dutra et al 2016 [42].
Estimating the daily survival of Wolbachia-carrying mosquitoes in field
The daily captures of mosquitoes allowed us to estimate the probability of daily survival (PDS) by using the exponential model [43]. Traditionally, the exponential model has been used to describe mortality patterns in MRR experiments with Ae. aegypti procedures [15, 28, 44]. This model assumes that marked, captured individual counts vary over time as an exponential decay with a constant rate, i.e., mosquito mortality is independent of age. This assumption is reasonable given typical mosquito life span.
Estimating the population size of wild mosquitoes using conventional MRR models
The wild population size of Ae. aegypti in Tubiacanga was estimated by conventional models such as the Lincoln-Petersen index, which can be defined as , where M is the number of released females; n is the total number of females captured; and m is the number of marked individuals captured. Moreover, we also used the Fisher-Ford model, which takes into account mortality rate, which was directly estimated in the same MRR experiment, given by the function , where ϕ is the PDS and t is the number of days elapsed after releases [27].
Estimating the population size of wild mosquitoes using average-difference model
The population size of wild Ae. aegypti was estimated using an average-difference model based on Wolbachia releases on North Queensland, Australia. Population size was estimated by the increase of Ae. aegypti captures on BGS-Traps in the district in which Wolbachia was released in comparison with collections on a control site, in 2 weeks before and 2 weeks after releases [37]. We performed similar analysis in Rio, using the district of Jurujuba as a control site, where conditions for mosquito collections was similar to Tubiacanga. For comparing the control site with Tubiacanga we used the expanded data analysis to 4 weeks before and 4 weeks after releases.
Ethics Statement
Mosquito releases in Tubiacanga were authorized by CONEP (CAAE 02524513.0.1001.0008), the National Research Council. The release of mosquitoes does not involve directly endangered or protected species and, from our experience, it does not have any significant impact on endangered or protected species.
Results
BGS Trap Collections
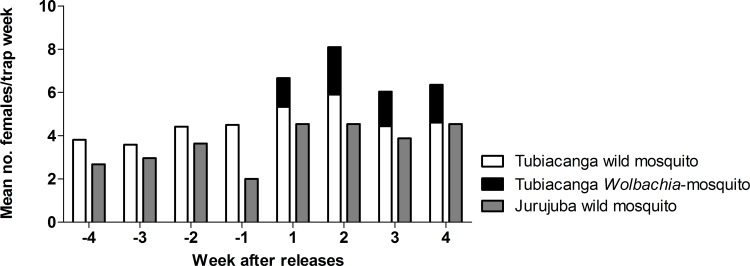
Ae. aegypti density was weekly monitored in Tubiacanga (release area) and Jurujuba (control area) before and after releasing Wolbachia-carrying mosquitoes (Fig 1). After releases started, the number of captured females increased substantially. In the second week of releases, the number of Ae. aegypti females captured was twice the average capture number from the pre-release period.
Fig 1. BG trap collections in field.
Mean captures of female Ae. aegypti per BGS trap/week at the release site Tubiacanga and for the control area Jurujuba, for the periods of four weeks before and four weeks after the releases started. Mosquito captures increased after releases in Tubiacanga.
Daily samples were taken in the first two weeks after starting releases in Tubiacanga (Table 1). The capture rates were 1.7% (n = 40) on week 1 and 2.5% (n = 59) on week 2. The number of wild females was higher than the Wolbachia-carrying mosquitoes in all recapture days. Days 7 and 14 were release days and were removed from the analysis to avoid biases because there were no reliable methods to disentangle those released from the ones released a week earlier. During the releases, males were captured in lower numbers than females in BGS trap: 93 males in week 1 (among them, 23 were Wolbachia-infected) and 94 in week 2 (23 Wolbachia-infected).
Table 1. Daily capture rates after mosquito releases in Tubiacanga.
| Daily captures after release | ||
|---|---|---|
| Week 1 | Wolbachia-carrying mosquito | Wild mosquito |
| Day 1 | 12 | 42 |
| Day 2 | 3 | 21 |
| Day 3 | 8 | 16 |
| Day 4 | 12 | 27 |
| Day 5 | 3 | 28 |
| Day 6 | 2 | 26 |
| Total | 40 | 160 |
| Per BG trap (total/number of traps) | 1.3 | 5.3 |
| Capture rate (total/number of released) | 1.7% | - |
| Week 2 | ||
| Day 8 | 12 | 47 |
| Day 9 | 8 | 29 |
| Day 10 | 17 | 35 |
| Day 11 | 7 | 35 |
| Day 12 | 10 | 33 |
| Day 13 | 5 | 24 |
| Total | 59 | 203 |
| Per BG trap (total/number of traps) | 2.0 | 6.8 |
| Capture rate (total/number of released) | 2.5% | |
Estimation of daily survival of Wolbachia-carrying mosquitoes in field
Considering daily collections in the first two weeks of release, the estimation for the probability of daily survival (PDS) for Wolbachia-carrying mosquitoes in Tubiacanga was 0.82 in week 1, and 0.89 in week 2 (Table 2).
Table 2. Daily survival rate of Wolbachia-carrying females under field conditions.
| Wolbachia-carrying mosquito | Logarithm of counts | ||||
|---|---|---|---|---|---|
| Week 1 | Day 1 | 12 | 2.56 | Slope | -0.20 |
| Day 2 | 3 | 1.39 | exp(slope) | 0.82 | |
| Day 3 | 8 | 2.20 | |||
| Day 4 | 12 | 2.56 | |||
| Day 5 | 3 | 1.39 | |||
| Day 6 | 2 | 1.10 | |||
| Week 2 | Day 8 | 12 | 2.56 | Slope | -0.12 |
| Day 9 | 8 | 2.20 | exp(slope) | 0.89 | |
| Day 10 | 17 | 2.89 | |||
| Day 11 | 7 | 2.08 | |||
| Day 12 | 10 | 2.40 | |||
| Day 13 | 5 | 1.79 |
Estimating the population size of wild mosquitoes based on Wolbachia releases in the field: average-difference model
Using average-difference model we estimate the population size based on changes on BGS capture, 2 weeks before and 2 weeks after the releases (Fig 1). In this method, we calculated the mean number of wild females before releases as 4.5 females/trap. A significant increase was observed after releases started, since this ratio augmented to 6.7 and 8.1 females/trap in weeks 1 and 2, respectively (Table 3). The density of wild population was estimated at 70 and 55 females per trap (wk1 and wk2, respectably).
Table 3. Population size estimates using average-difference model.
| Variable | Samples calculation (1/2 wk) |
|---|---|
| 1) Mean no. of wild female Ae. aegypti per BGS trap, before release (95% CI) (-1/-2 wk) | 4.5/4.5(1.3–7.5/ 2.7–6.3) |
| 2) Mean no. of female Ae. aegypti per BGS trap after release of Wolbachia- infected mosquitoes (95% CI) | 6.7/8.1(4.3–9.9/ 5.5–11.4) |
| 3) Increase (mean ratio) in BGS collections of female due to released mosquitoes | 2.2/3.6 |
| 4) Ratio of wild to released mosquitoes in BGS collections | 2.0/1.3 |
| 5) Estimated no. of wild mosquitoes per trap = no. of released mosquitoes/trap x ratio of wild to released mosquitoes* | 70/ 55 |
| Estimated no. of wild mosquitoes per premise = no. of released mosquitoes/trap x ratio of wild to released mosquitoes x no. of traps / no. of premises | 2.4/ 1.9 |
| Estimated no. of wild mosquitoes per area (m2) = no. of released mosquitoes/trap x ratio of wild to released mosquitoes x no. of traps / total area | 0.024/ 0.019 |
* The numbers of released mosquitoes/trap was estimated based on a mean of survival mosquitoes in field considering a daily survival of 0.8 (see more details on a S1 Table).
Estimating the population size of wild mosquitoes based on Wolbachia releases in the field: using MRR models
We used MRR models such as the Lincoln-Petersen and Fisher and Ford, to estimate the population size on a daily basis using Wolbachia as a marker (Table 4). The total number estimated by the average-difference method is shown for comparison purposes. The highest numbers were estimated from Lincoln-Petersen model and analysis using the average-difference model resulted in lowest abundance quantities.
Table 4. Wild population size (with confidence intervals) of Ae. aegypti.
| Fisher-Ford | Lincoln-Petersen | Average-difference | ||
|---|---|---|---|---|
| Week 1 | Day 1 | 6,303 | 7,690 | - |
| Day 2 | 8,589 | 12,788 | - | |
| Day 3 | 2,418 | 4,392 | - | |
| Day 4 | 2,259 | 5,008 | - | |
| Day 5 | 6,233 | 16,856 | - | |
| Day 6 | 6,342 | 20,925 | - | |
| Mean | 5,357 (3,355–7,360) | 11,276 (6,365–16,188) | 2,100 (634–3,565) | |
| Per premise | 6.2 (3.9–8.5) | 13.0 (7.3–18.6) | 2.4 (0.7–4.1) | |
| Week 2 | Day 8 | 7,641 | 8,585 | - |
| Day 9 | 6,140 | 7,750 | - | |
| Day 10 | 3,279 | 4,650 | - | |
| Day 11 | 6,567 | 10,463 | - | |
| Day 12 | 4,015 | 7,186 | - | |
| Day 13 | 4,818 | 9,688 | - | |
| Mean | 5,410 (4,086–6,735) | 8,053 (6,406–9,700) | 1,650 (987–2,313) | |
| Per premise | 6.2 (4.7–7.8) | 9.3 (7.3–11.2) | 1.9 (1.1–2.7) |
Discussion
The endosymbiont bacterium Wolbachia has been deployed in field trials as a novel intervention aiming to reduce arboviruses transmission. Wolbachia-infected Ae. aegypti mosquitoes are established in areas of North Queensland, Australia and Vietnam and ongoing releases are taking place in Brazil, Colombia and Indonesia [36, 45, 46]. One of the milestones to obtain a successful invasion of Wolbachia into the local population is to release a sufficient number of mosquitoes which exceeds the threshold invasion [38]. Such threshold for invasion highlights the need for reliable estimates on the population size of wild mosquitoes in the target area. Additionally, invasion is dependent on releasing a mosquito population not only fit to survive in the natural environment but also with a strong cytoplasmic incompatibility, a crucial mechanism to facilitate Wolbachia spread [35]. Using an MRR approach in which mosquitoes are individually screened for Wolbachia presence, we are able to estimate significant aspects of vector biology by performing daily collections of Wolbachia-infected Ae. aegypti mosquitoes released once a week. Herein, we provide estimates of two critical parameters for invasion success: the population size of wild mosquitoes and the probability of daily survival of Wolbachia-carrying mosquitoes in the field.
Ritchie et al. (2013) [37] and Nguyen et al. (2015) [47] took advantage of the Wolbachia releases to estimate wild Ae. aegypti density. However, in their work, the bacteria was not used as an individual marker in the field. Using the average-difference model, they assumed the increase in the capture rates after releases was due to the addition of Wolbachia-infected Ae. aegypti through releases. Using data from the first Wolbachia release in Latin America, we proposed individual Wolbachia screening to use not only the average-difference model but also the Lincoln-Petersen and Fisher-Ford models, and thus compared population size estimates among methods. Different from our results, Ritchie et al. (2013) and Nguyen et al. (2015) showed that released mosquitoes were more abundant than the wild ones, suggesting low vector infestation in release areas. For example, in two sites from North Queensland, a ratio of 1:1.2–1.7 (wild:wMel) was observed during the first two weeks of wMel releases [37]. In Vietnan, a ratio of 1:2–4 was observed during the wMelPop releases. Our results in Rio de Janeiro/Brazil using the same average-difference model showed a ratio of 1: 0.5–0.7, which could hinder Wolbachia invasion due to high density of native mosquitoes. Thus, a successful invasion may require the period for Wolbachia releases to last longer or/and an increase in the number of released mosquitoes.
Our estimates of BGS trapping efficiency for female Wolbachia-carrying mosquito (ranging from 1.7 to 2.5%) were lower when compared to other MRR experiments in Rio de Janeiro. Usually the recapture rates ranged from 7 to 15% [15, 29, 48]. Ritchie et al 2013 [37] in North Queensland had sampling rates of 5 to 10% after releasing Wolbachia-carrying mosquitoes. Our lower recapture rate might be explained by the limited number of BGS traps installed, i.e., roughly, we had one BG every 30 houses. Probably, recapture rates increase if additional collecting methods such as backpack aspirator are used [15].
Mosquito population in Rio de Janeiro presents strong variation over short periods of time, eventually doubling its recapture rate on an interval of few weeks [49]. Under this scenario, estimates on Ae. aegypti population size using the average-difference model may have limited reliability because a fraction of the increase in collections after Wolbachia releases could be due to a natural fluctuation of mosquito population (see Fig 1). In this case, mosquito counts might not be accurate, because the excess numbers are considered to be all Wolbachia-mosquitoes.
We estimated the PDS of Wolbachia-infected mosquitoes under field conditions, which ranged from 0.82 to 0.89 in the first two weeks after releases in Tubiacanga. This is the first estimation of daily survival of Wolbachia Ae. aegypti mosquitoes in the field using methodology from MRR literature. Overall, it seems the wMel strain did not affect significantly the daily survival of Ae. aegypti [37], since the PDS values observed are similar or even higher than PDS values found for wild mosquitoes in previous studies [15, 28, 50]. In particular, Maciel-de-Freitas et al. (2007) [15] found a PDS ranging from 0.71 to 0.75 in Tubiacanga during dry and wet seasons of 2007. Other studies in Rio de Janeiro raised the hypothesis that Ae. aegypti PDS might depend on the urban landscape. For instance, a PDS of ~0.93 was observed in a highly dense typical Brazilian slum, whereas in suburban districts, PDS ranged from 0.73 to 0.89 [48]. Finally, in a sparsely populated high-income neighborhood, we observed the lowest PDS of Rio, varying from 0.61 to 0.70 [15, 39, 48, 51]. Under such scenarios the effect of urban landscape on mosquito survival is potentially due to the availability of human hosts and breeding sites. Maciel-de-Freitas et al. (2007b) [52] observed a tendency towards higher Ae. aegypti survival in areas with a high human density. In crowded districts such as slums, mosquitoes would not present a long flight to find host or breeding sites, reducing the odds of mortality due to harsh environmental factors, insecticide use or even by the defensive host behavior [53].
Given the low capture rates in the field, the exponential model is used extensively for estimating the probability of daily survival. Analysis under the exponential model does not consider the number of individuals captured to be removed from environment due to daily collections, which might impact subsequent collections. A possible approach for estimation under such conditions is to use the method proposed by Buonaccorsi et al (2003) [54].
By individually screening Ae. aegypti females for Wolbachia, we were able to estimate the population size of wild mosquitoes using three different models. MRR models provided higher values of population size than the average-difference model. Probably, the difference is because the average-difference model misdetected some wild mosquitoes as the Wolbachia ones, contributing to underestimate the wild population size (calculated as 2,100 week 1 and 1,650 week 2). This occurs due to large oscillation in number of wild mosquitoes between weeks in Rio de Janeiro. Therefore, MRR models under individual screening by qPCR give us more accurate quantities because in this case, we know precisely which mosquitoes had Wolbachia. Due to high mortality rates, Lincoln-Petersen results possibly overestimated population size (estimated as 11,276 week 1 and 8,053 week 2), since the model does not consider mortality rate in released mosquitoes. Finally, the index from Fisher-Ford model seems to be a reliable estimator of population size (5,357 week 1 and 5,410 week 2), since the model considers mortality rate during the experiment studies.
Our MRR results suggest that fitness of Wolbachia-infected mosquitoes in the field is high enough to promote invasion. By screening mosquitoes individually and using this information to generate quantities to be applied to MRR models, we avoided inaccurate estimations due to fluctuation in the average number of mosquitoes per week. Such technique is deemed highly important to determining in future release sites the minimum number of released individuals, by taking into account the wild population size in order to achieve a sustainable Wolbachia invasion over time.
Supporting Information
(DOCX)
Acknowledgments
We are grateful to all members of the Mosquitos Vetores: Endossimbiontes e Interação Patógeno Vetor (MV–CPqRR/FIOCRUZ), especially Dr. Luciano A. Moreira and Dr. Fernando Braga Dias, and members of Laboratório de Transmissores de Hematozoários (LATHEMA–FIOCRUZ).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was partially supported by FAPERJ (E-26/110.168/2014), CNPq, the Brazilian Ministry of Health (DECIT/SVS) as well as a grant to Monash University from the Foundation for the National Institutes of Health through the Vector-Based Transmission of Control: Discovery Research (VCTR) program of the Grand Challenges in Global Health Initiatives of the Bill and Melinda Gates Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Schatzmayr HG. Dengue situation in Brazil by year 2000. Mem Inst Oswaldo Cruz. 2000;95 Suppl 1:179–81. [DOI] [PubMed] [Google Scholar]
- 2.Kraemer MU, Sinka ME, Duda KA, Mylne AQ, Shearer FM, Barker CM, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife. 2015. June 30;4:e08347 10.7554/eLife.08347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013. April 25;496(7446):504–7. 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.PAHO/WHO, 2016. Number of reported cases of dengue and severe dengue (SD) in Americas, by country. Avaiable from: http://www.paho.org/hq/index.php?option=com_topics&view=article&id=1&Itemid=40734
- 5.Focks DA, Brenner RJ, Hayes J, Daniels E. Transmission thresholds for dengue in terms of Aedes aegypti pupae per person with discussion of their utility in source reduction efforts. Am J Trop Med Hyg. 2000. January;62(1):11–8. [PubMed] [Google Scholar]
- 6.Maciel-de-Freitas R, Valle D. Challenges encountered using standard vector control measures for dengue in Boa Vista, Brazil. Bull World Health Organ. 2014. September 1;92(9):685–9. 10.2471/BLT.13.119081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fares RC, Souza KP, Añez G, Rios M. Epidemiological scenario of dengue in Brazil. Biomed Res Int. 2015; 2015:321873 10.1155/2015/321873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azevedo RdS, Oliveira CS, Vasconcelos PF. Chikungunya risk for Brazil. Rev Saude Publica. 2015;49:58 10.1590/S0034-8910.2015049006219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanluca C, de Melo VC, Mosimann AL, Dos Santos GI, Dos Santos CN, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz. 2015. June;110(4):569–72. 10.1590/0074-02760150192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Consoli RAGB, Lourenço-de-Oliveira R. Principais Mosquitos de Importância Sanitária no Brasil. 1rst ed. Rio de Janeiro: Fiocruz; 1994. [Google Scholar]
- 11.Chouin-Carneiro T, Vega-Rua A, Vazeille M, Yebakima A, Girod R, Goindin D, et al. Differential Susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika Virus. PLoS Negl Trop Dis. 2016. March 3;10(3):e0004543 10.1371/journal.pntd.0004543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayres CF. Identification of Zika virus vectors and implications for control. Lancet Infect Dis. 2016. March 16(3):278–9. 10.1016/S1473-3099(16)00073-6 [DOI] [PubMed] [Google Scholar]
- 13.Edman JD, Strickman D, Kittayapong P, Scott TW. Female Aedes aegypti (Diptera: Culicidae) in Thailand rarely feed on sugar. J Med Entomol. 1992. November 29(6):1035–8. [DOI] [PubMed] [Google Scholar]
- 14.Braks MA, Lourenço-De-Oliveira R, Juliano SA, Lounibos LP. Convergent habitat segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in southeastern Brazil and Florida. J Med Entomol. 2003. November;40(6):785–94. [DOI] [PubMed] [Google Scholar]
- 15.Maciel-de-Freitas R, Codeço CT, Lourenço-de-Oliveira R. Daily survival rates and dispersal of Aedes aegypti females in Rio de Janeiro, Brazil. Am J Trop Med Hyg. 2007a. April;76(4):659–65. [PubMed] [Google Scholar]
- 16.Castro M, Sánchez L, Pérez D, Sebrango C, Shkedy Z, Van der Stuyft P. The relationship between economic status, knowledge on dengue, risk perceptions and practices. PLoS One. 2013. December 12;8(12):e81875 10.1371/journal.pone.0081875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cattand P, Desjeux P, Guzmán MG, Jannin J, Kroeger A, Medici A, et al. Tropical diseases lacking adequate control measures: dengue, leishmaniasis, and african trypanosomiasis. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, editors. Disease Control Priorities in Developing Countries. 2nd edition. Washington: (DC): World Bank, Chapter 23; 2006. [PubMed] [Google Scholar]
- 18.Olanratmanee P, Kittayapong P, Chansang C, Hoffmann AA, Weeks AR, Endersby NM. Population genetic structure of Aedes (Stegomyia) aegypti (L.) at a micro-spatial scale in Thailand: implications for a dengue suppression strategy. PLOS Negl Trop Dis. 2013;7(1):e1913 10.1371/journal.pntd.0001913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perkins TA, Scott TW, Le Menach A, Smith DL. Heterogeneity, mixing, and the spatial scales of mosquito-borne pathogen transmission. PLoS Comput Biol. 2013. 9(12):e1003327 10.1371/journal.pcbi.1003327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coelho GE, Teixeira Mda G, Coutinho FA, Massad E. Dynamics of the 2006/2007 dengue outbreak in Brazil. Mem Inst Oswaldo Cruz. 2008. September;103(6):535–9. [DOI] [PubMed] [Google Scholar]
- 21.Focks DA, Chadee DD. Pupal survey: an epidemiologically significant surveillance method for Aedes aegypti: an example using data from Trinidad. Am J Trop Med Hyg. 1997. February;56(2):159–67. [DOI] [PubMed] [Google Scholar]
- 22.Morrison AC, Zielinski-Gutierrez E, Scott TW, Rosenberg R. Defining challenges and proposing solutions for control of the virus vector Aedes aegypti PLoS Med. 2008. March 18;5(3):e68 10.1371/journal.pmed.0050068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson PH, Spitzauer V, Ritchie SA. Field sampling rate of BG-sentinel traps for Aedes aegypti (Diptera: Culicidae) in suburban Cairns, Australia. J Med Entomol. 2012. January 49(1): 29–34. [DOI] [PubMed] [Google Scholar]
- 24.Codeço CT, Lima AW, Lima JB, Maciel-de-Freitas R, Honório NA, Galardo AK, et al. Surveillance of Aedes aegypti: comparison of house index with four alternative traps. PLoS Negl Trop Dis. 2015. February 10;9(2):e0003475 10.1371/journal.pntd.0003475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheppard PM, Macdonald WW, Tonn RJ. A new method of measuring the relative prevalence of Aedes aegypti. Bull World Health Organ. 1969;40(3):467–8. [PMC free article] [PubMed] [Google Scholar]
- 26.Trpis M, Hausermann W, Craig GB. Estimates of population size, dispersal, and longevity of domestic Aedes aegypti aegypti (Diptera: Culicidae) by mark-release-recapture in the village of Shauri Moyo in eastern Kenya. J. Med. Entomol. 1995. January;32(1):27–33. [DOI] [PubMed] [Google Scholar]
- 27.Silver JB. Mosquito ecology: field sampling methods 3rd ed. New York: Springer; 2008. [Google Scholar]
- 28.Muir LE, Kay BH. Aedes aegypti survival and dispersal estimated by mark-release-recapture in northern Australia. Am J Trop Med Hyg. 1998. March;58(3):277–82. [DOI] [PubMed] [Google Scholar]
- 29.Villela DA, Figueiredo F, Garcia GA, Maciel-de-Freitas R, Struchiner CJ. A bayesian hierarchical model for estimation of abundance and spatial density of Aedes aegypti. PLoS One. 2015. April 23;10(4):e0123794 10.1371/journal.pone.0123794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH. How many species are infected with Wolbachia? A statistical analysis of current data FEMS Microbiol Lett. 2008. April;281(2):215–20. 10.1111/j.1574-6968.2008.01110.x Epub 2008 Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zug R, Hammerstein P. Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS One. 2012;7(6):e38544 10.1371/journal.pone.0038544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Oliveira CD, Gonçalves DS, Baton LA, Shimabukuro PH, Carvalho FD, Moreira LA. Broader prevalence of Wolbachia in insects including potential human disease vectors. 2015. June;105(3):305–15. 10.1017/S0007485315000085 [DOI] [PubMed] [Google Scholar]
- 33.Moreira LA, Ormaetxe II, Jeffery JA, Lu G, Pyke AT, Hedges LM, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and plasmodium. Cell. 2009. December 24;139(7):1268–78. 10.1016/j.cell.2009.11.042 [DOI] [PubMed] [Google Scholar]
- 34.Ye YH, Carrasco AM, Frentiu FD, Chenoweth SF, Beebe NW, vanden Hurk AF, et al. Wolbachia Reduces the Transmission Potential of Dengue-Infected Aedes aegypti. PLoS Negl Trop Dis. 2015. June 26;9(6):e0003894 10.1371/journal.pntd.0003894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol. 2008. October;6(10):741–51. 10.1038/nrmicro1969 Review [DOI] [PubMed] [Google Scholar]
- 36.Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011. August 24;476(7361):454–7. 10.1038/nature10356 [DOI] [PubMed] [Google Scholar]
- 37.Ritchie SA, Montgomery BL, Hoffmann AA. Novel estimates of Aedes aegypti (Diptera: Culicidae) population size and adult survival based on Wolbachia releases.J Med Entomol. 2013. May;50(3):624–31. [DOI] [PubMed] [Google Scholar]
- 38.Turelli M, Hoffmann AA. Rapid spread of an inherited incompatibility factor in California Drosophila. Nature. 1991. October 3;353(6343):440–2. [DOI] [PubMed] [Google Scholar]
- 39.Dutra HL, Dos Santos LM, Caragata EP, Silva JB, Villela DA, Maciel-de-Freitas R, et al. From lab to field: the influence of urban landscapes on the invasive potential of Wolbachia in brazilian Aedes aegypti Mosquitoes. PLoS Negl Trop Dis. 2015. April 23;9(4):e0003689 10.1371/journal.pntd.0003689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krockel U, Rose A, Eiras AE, Geier M. New tools for surveillance of adult yellow fever mosquitoes: comparison of trap catches with human landing rates in an urban environment. J Am Mosq Control Assoc. 2006. June;22(2):229–38. [DOI] [PubMed] [Google Scholar]
- 41.Maciel-de-Freitas R, Eiras AE, Lourenço-de-Oliveira R. Field evaluation of effectiveness of the BG-Sentinel, a new trap for capturing adult Aedes aegypti (Diptera: Culicidae). Mem Inst Oswaldo Cruz. 2006b. May;101(3):321–5. [DOI] [PubMed] [Google Scholar]
- 42.Dutra HL, Rocha MN, Dias FB, Mansur SB, Caragata EP, Moreira LA. Wolbachia blocks currently circulating Zika virus isolates in brazilian Aedes aegypti mosquitoes. Cell Host Microbe. 2016. June 8;19(6):771–4. 10.1016/j.chom.2016.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gillies MT. Studies on the dispersion and survival of Anopheles gambiae Giles in East Africa, by means of marking and releasing experiments. Bull Entomol Res. 1961. 52: 99–127. [Google Scholar]
- 44.Harrington LC, Françoisevermeylen, Jones JJ, Kitthawee S, Sithiprasasna R, Edman JD, et al. Age-dependent survival of the dengue vector Aedes aegypti (Diptera: Culicidae) demonstrated by simultaneous release-recapture of different age cohorts. J Med Entomol. 2008. March;45(2):307–13. [DOI] [PubMed] [Google Scholar]
- 45.Hoffmann AA, Iturbe-Ormaetxe I, Callahan AG, Phillips BL, Billington K, Axford JK, et al. Stability of the wMel Wolbachia Infection following Invasion into Aedes aegypti Populations. PLoS Negl Trop Dis. 2014. September 11;8(9):e3115 10.1371/journal.pntd.0003115 eCollection 2014 Sep [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frentiu FD, Zakir T, Walker T, Popovici J, Pyke AT, van den Hurk A, et al. Limited dengue virus replication in field-collected Aedes aegypti mosquitoes infected with Wolbachia. PLoS Negl Trop Dis. 2014. February 20;8(2):e2688 10.1371/journal.pntd.0002688 eCollection 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen TH, Nguyen HL, Nguyen TY, Vu SN, Tran ND, Le TN, et al. Field evaluation of the establishment potential of wMelPop Wolbachia in Australia and Vietnam for dengue control. Parasit Vectors. 2015. October 28;8:563 10.1186/s13071-015-1174-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.David MR, Lourenço-de-Oliveira R, Maciel-de-Freitas R. Container productivity, daily survival rates and dispersal of Aedes aegypti mosquitoes in a high income dengue epidemic neighbourhood of Rio de Janeiro: presumed influence of differential urban structure on mosquito biology. Mem Inst Oswaldo Cruz. 2009. September;104(6):927–32. [DOI] [PubMed] [Google Scholar]
- 49.Maciel-de-Freitas R, Lourenço-de Oliveira R 2011. Does targeting key-containers effectively reduce Aedes aegypti. Trop Med Int Health. 2011. August;16(8):965–73. 10.1111/j.1365-3156.2011.02797.x Epub 2011 May 23. [DOI] [PubMed] [Google Scholar]
- 50.Harrington LC, Buonaccorsi JP, Edman JD, Costero A, Kittayapong P, Clark GG, et al. Analysis of survival of young and old Aedes aegypti (Diptera: Culicidac) from Puerto Rico and Thailand. J Med Entomol. 2001. July;38(4):537–47. [DOI] [PubMed] [Google Scholar]
- 51.Maciel-de Freitas R, Eiras AE, Lourenço-de Oliveira R. Calculating the survival rate and estimated population density of gravid Aedes aegypti (Diptera: Culicidae) in Rio de Janeiro, Brazil. Cad Saude Publica. 2008. December;24(12):2747–54. [DOI] [PubMed] [Google Scholar]
- 52.Maciel-de-Freitas R, Marques WA, Peres RC, Cunha SP, de Oliveira RL. Variation in Aedes aegypti (Diptera: Culicidae) container productivity in a slum and a suburban district of Rio de Janeiro during dry and wet seasons. Mem Inst Oswaldo Cruz. 2007b. June;102(4):489–96. [DOI] [PubMed] [Google Scholar]
- 53.Clements AN, Paterson GD. The analysis of mortality and survival rates in wild populations of mosquitoes. J Appl Ecol. 1981. 18:373–399. [Google Scholar]
- 54.Buonaccorsi JP, Harrington LC, Edman JD (2003) Estimation and comparison of mosquito survival rates with release-recapture-removal data. J Med Entomol, 40(1), 6–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.