Abstract
Objectives
To assess smartphone ownership, use of mobile health (mHealth) applications, and willingness to use this technology to facilitate medication management after kidney transplantation.
Methods
A survey was developed with the use of previously validated questions and administered to stable adult kidney recipients from May to July 2015. Descriptive and comparative statistics were used to assess willingness to utilize mHealth technology as it related to sociodemographics, medication adherence, and medication side effects. Comparisons were also made to a survey administered in 2012. The primary outcome was the incidence of cell phone and smartphone ownership, willingness to use mHealth, immunosuppressant side effects, and self-reported nonadherence.
Results
A total of 142 patients were approached, and 139 (98%) agreed to participate; 96% of respondents indicated mobile phone ownership, 61% owned a smartphone, 30% had prior knowledge of mHealth, and 7% were already using an mHealth app; 78% reported a positive attitude toward the use of mHealth for medication management. Smartphone ownership has nearly doubled since 2012 (61% vs. 35%; P <0.001). Patients <55 years of age were more likely to own smartphones (75% vs. 46%; P <0.001) and to strongly agree with the use of mHealth (62% vs. 36%; P = 0.015). Self-reported nonadherence or severe medication side effects did not appreciably influence a patient's willingness to use mHealth.
Conclusion
Among recipients of kidney transplants, smartphone ownership has dramatically increased, and recipients have a positive attitude toward the use of mHealth for medication management.
Nearly 900,000 individuals living in the United States suffer from end-stage renal disease (ESRD) and approximately 100,000 of these individuals are awaiting kidney transplantation.1, 2 Kidney transplantation is the preferred treatment for ESRD because it affords patients enhanced quality of life and improved life expectancy compared with dialysis.3, 4 Positive health outcomes following kidney transplantation are contingent on strict medication adherence and control of comorbidities.5, 6 In the general population, nonadherence to medication accounts for 33% to 69% of medication-related hospitalizations and $100 billion annually in health care costs.7 Furthermore, it is estimated that 36 out of 100 transplant patients per year are nonadherent to immunosuppressive therapy, based on a review of 147 studies.8
A recent study found the use of mobile health technology (mHealth) to be a promising and sustainable strategy for improving medication adherence and health outcomes for patients with chronic illnesses.9 In only 5 years, mHealth has grown into a billion-dollar industry.10 Smartphone ownership has markedly increased every year since 2008, with the prevalence of smartphone ownership in the general population increasing from 35% to 56% from 2011 to 2013.11 We previously demonstrated that kidney transplant patients thought that mHealth provided a platform for self-efficacy and improved medical management.12 Literature on the clinical application of mHealth is growing, but studies analyzing the use of mHealth as an aid to facilitate medication adherence are limited, particularly in those reporting medication side effects or nonadherence.7
Objectives
The primary aim of this study was to assess smartphone ownership trajectories, use of mHealth apps, and willingness to use such technology to manage medication therapy in kidney transplant recipients, specifically as it relates to medication side effects and nonadherence.
Methods
Study design and setting
This was a cross-sectional survey administered from May to July 2015 to ambulatory kidney transplant recipients being seen for routine follow-up care in the transplant clinic. After the patients' placement into examination rooms, patients were approached by an investigator who assessed their willingness to participate in the survey. The survey assessed demographics, general health information, use of technology, attitudes toward using mHealth for managing and monitoring medication therapy, medication adherence, and side effects.
The survey was built into the Research Electronic Data Capture (REDCap) Web-based system and administered to participants with the use of an iPad. Before the initiation of the survey, mHealth was defined as an app on a smartphone that could assist in medication management and in tracking other health and well-being parameters.
Participants and recruitment
Study participants were recruited from the kidney transplant clinic at the Medical University of South Carolina (MUSC). This was the same population sampled by our research collaborative in 2012. Eligible patients were those who had previously received a kidney transplant, were over 18 years of age, spoke English, and agreed to participate in the survey. There were no incentives to participate in the study. The study was approved by the MUSC Institutional Review Board.
Survey design
The survey included 4 domains: demographics, technology, medication side effects, and medication adherence. Patient demographics were assessed with the use of a 13-item questionnaire. Items included information on general demographics, transplant year, health insurance, and general health. The items were adapted from previous research assessing ownership of technology by the Pew Research Center11 and our earlier study.12
Ownership of, use of, and willingness to use technology was assessed with the use of a 26-itemquestionnaire, in which 15 of the 26 items were answered with the use of a 5-level scale ranging from “strongly disagree” to “strongly agree.” Questions included smartphone ownership (yes/no), use of smartphone technology (yes/no), and willingness to use an mHealth app for medication management and safety (5-level scale). The items were based on the previous study by our research collaborative.12
Medication side effects were evaluated with the use of the 8 most-relevant questions derived from the Memphis Survey which is used to assess medication tolerability specific to transplant recipents.13 The items were answered with the use of a 5-level scale ranging from “not at all” to “all the time.” If the response to a side effect was more frequent than “not at all,” the patients were asked how troubling the side effect was for them. Medication adherence was evaluated with the use of an 8-item modified Morisky Medication Adherence Scale.14 The items were answered with the use of a 5-level scale ranging from “never” to “always.”
Statistical analysis
Survey results are reported as percentage for categoric variables and mean ± SD for continuous data. For the overall assessment of the study population, patients who responded “agree” or “strongly agree” with the question regarding technology assessment were grouped together and compared with those who marked any other category. For comparisons based on age, race, income, smartphone ownership, and downloading of apps, we compared those who strongly agreed with using this technology versus all other responses. Within the medication side effects items, patients who reported side effects sometimes, often, or all the time were compared with those who reported very little or not at all. Side effects were defined as when patients reported them as moderately troubling, very troubling, or severely troubling. For medication adherence, those who reported an answer other than “never” were considered to be nonadherent. For inferential statistics, the chi-square test was used to compare proportions by means of contingency tables, and an unpaired t test was used to compare continuous data. All data was imported into SPSS, version 22.0 (IBM Corp., Armonk, NY) to conduct analyses. A two-sided P of <0.05 was used to determine statistical significance.
Results
One-hundred forty-two patients were approached and 139 (98%) agreed to participate. Those who declined did so for lack of time or lack of interest. Transplantation year ranged from 1996 to 2015. The demographics and clinical characteristics of the participants are summarized in Table 1. The results of the survey indicated that nearly all of the respondents, 96% (129/135), owned a mobile phone and 61% (82/135) owned a working smartphone (defined as an Internet-capable cellular device); 67% (91/135), answered that someone in their household had a smartphone, and 87% (117/135) claimed that someone in their household could help them with use of a mobile phone if needed.
Table 1.
Baseline characteristics and technology ownership in study population of kidney transplant recipients
| Characteristic | Proportion or mean ± SD |
|---|---|
| Age group, y | |
| 18–24 | 2.9% (4/138) |
| 25–34 | 10.9% (15/138) |
| 35–44 | 16.7% (23/138) |
| 45–54 | 20.3% (28/138) |
| 55–64 | 29.0% (40/138) |
| ≥65 | 20.3% (28/138) |
| Race | |
| Black | 63.0% (87/138) |
| White | 33.3% (46/138) |
| Household yearly income, $ | |
| <30,000 | 61.3% (84/137) |
| 30,000–49,999 | 14.6% (20/137) |
| 50,000–74,999 | 12.4% (17/137) |
| ≥75,000 | 11.7% (16/137) |
| Education level | |
| Less than high school | 10.9% (15/138) |
| High school | 34.1% (47/138) |
| Some college | 22.5% (31/138) |
| College or more | 32.6% (45/138) |
| Transplantation year | |
| 2015 | 39.9% (55/138) |
| 2014 | 18.8% (26/138) |
| 2013 | 10.9% (15/138) |
| ≤2012 | 30.4% (42/138) |
| Height, inches | 67.6 ± 3.9 |
| Weight, pounds | 186.9 ± 41.8 |
| Body mass index, kg/m2 | 28.8 ± 5.9 |
| History of hypertension | 92.9% (118/127) |
| History of diabetes | 45.7% (58/127) |
| Physical activity/week, min | |
| <30 | 32.6% (45/138) |
| 31–60 | 13.8% (19/138) |
| 61–90 | 5.1% (7/138) |
| 91–120 | 5.8% (8/138) |
| 121–150 | 3.6% (5/138) |
| ≥151 | 39.1% (54/138) |
| Mobile phone ownership (yes) | 95.6% (129/135) |
| Smartphone ownership (yes) | 60.7% (82/135) |
| Computer with Internet at home (yes) | 79.1% (106/134) |
Most respondents had a familiarity with the use of mobile phones. Among these, 74% (100/135) used their mobile phones for text messaging, 61% (82/135) to browse the Internet, 52% (70/135) for e-mail, and 47% (64/135) to download apps. Additionally, a number of patients were tracking health parameters without the use of mHealth: 14% (19/135) used their cellular phones to monitor blood pressure or blood sugar, 28% (38/135) to monitor activity level, and 18% (24/135) to help with tracking medications.
Thirty percent of respondents (40/135) had heard of mHealth technology, and 25% (10/40) of those were already using an mHealth app for managing and monitoring medication therapy; 86% of respondents (116/135) felt comfortable with a health care provider monitoring their health information by means of mHealth, and 86% (116/135) thought that mHealth would allow their providers to make changes to their medications more quickly. Eighty-seven percent (117/135) were confident that this technology would aid in communication with their providers. Most respondents, 58% (78/135), preferred communicating with providers via text message, with voice mail being the second choice, telephone call the third choice, and the majority of patients, 66% (89/135), listing video conference as their last choice (Table 2).
Table 2.
Understanding of and attitudes toward mHealth in study population of kidney transplant recipients
| Survey item | Proportion |
|---|---|
| Heard of mHealth (yes) | 29.6% (40/135) |
| Already use mHealth (yes) | 7.4% (10/135) |
| Would use mHealth device if free (agree or strongly agree) |
77.8% (105/135) |
| Use mHealth if someone always there to help (agree or strongly agree) |
80.0% (108/135) |
| Comfortable having health monitored with app (agree or strongly agree) |
85.9% (116/135) |
| Comfortable using cellular phone (agree or strongly agree) |
95.5% (129/135) |
| mHealth allows doctor to make changes to meds more quickly (agree or strongly agree) |
85.9% (116/135) |
| Confident privacy would be protected (agree or strongly agree) |
72.6% (98/135) |
| Confident mHealth will help communication with health care team about medical condition (agree or strongly agree) |
86.7% (119/135) |
| Text (frequently or very frequently) | 60.0% (81/135) |
| E-mail (frequently or very frequently) | 35.6% (48/135) |
| Internet (frequently or very frequently) |
49.6% (67/135) |
| Download apps (frequently or very frequently) |
24.4% (33/135) |
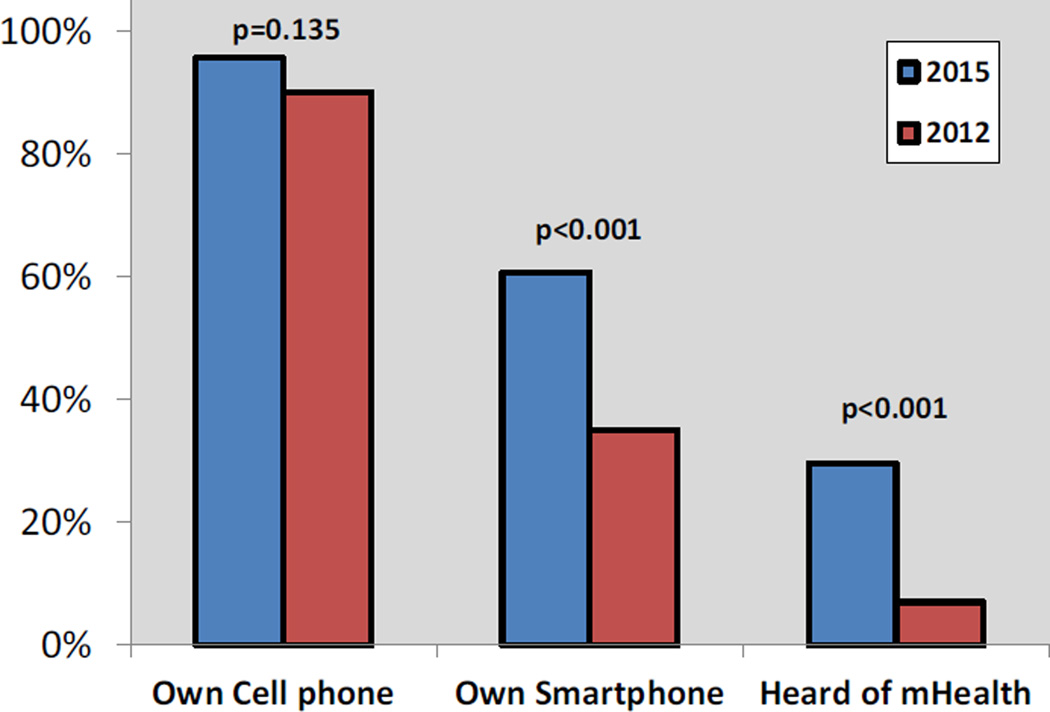
We compared the results of this survey (2015) to our previous survey, conducted in the same kidney transplant clinic in 2012 (Figure 1). Although mobile phone ownership did not significantly increase (90% vs. 96%; P = 0.135), smartphone ownership nearly doubled (35% vs 61%; P <0.001), and the proportion of patients that had heard of mHealth more than quadrupled (7% vs. 30%; P <0.001).
Figure 1.
Comparison of technology ownership and mHealth awareness between 2012 and 2015 survey participants.
Sixty-two percent of patients (86/139) reported at least one medication side effect; the most commonly reported side effects were tremors or jitteriness (40%), diarrhea (26%), and upset stomach or indigestion (21%; Supplemental Figure 1). Forty-two percent (56/133) reported that they sometimes forget to take their medications, 11% (14/133) reported nonadherence in the past 2 weeks, 8% (10/133) reported stopping a medication or decreasing the dose without warning their doctor because it made them feel worse, and 23% (31/133) reported sometimes forgetting their medication when traveling or leaving the house.
Individuals under 55 years of age were significantly more likely to own smartphones (75% vs. 46%; P <0.001). Respondents with annual incomes of less than $30,000 were significantly less likely to own smartphones (54% vs. 72%; P = 0.032). Patients with a college education had similar rates of ownership (68% vs. 58%; P = 0.346). Race was not associated with smartphone ownership (63% vs. 59%; P = 0.710).
Smartphone owners were more likely than non–smartphone owners to strongly agree with the use of mHealth (52% vs. 43%; P = 0.006). Patients under 55 years of age were more likely to strongly agree with the use of mHealth (62% vs. 36%; P = 0.015). Patients who download apps either frequently or very frequently were more likely to strongly agree with the use of mHealth (76% vs. 40%; P <0.001). There was no association between annual income and willingness to use mHealth (49% vs. 47%; P >0.999).
Self-reported nonadherence was higher in Medicaid patients compared with those not receiving Medicaid (43% vs. 25%; P = 0.057), and overall nonadherence to medications was not associated with willingness to use mHealth (50% vs. 46%; P = 0.712). Mean years from transplantation was higher in those who reported severe side effects (4.3 ± 5.4 vs. 2.0 ± 3.3; P = 0.013), but reporting severe side effects was not significantly associated with willingness to use mHealth (46% vs. 50%; P = 0.712; Supplemental Figure 2).
Discussion
The results of this study provide continued evidence that the majority of kidney transplant recipients now own smartphones and are willing to use mHealth apps to improve medication therapy management; 61% owned a smartphone, and 78% were willing to incorporate mHealth into their care if it came at no increased cost.
The trajectory analysis indicates that smartphone ownership had nearly doubled (61% vs. 35%) since 2012, when we previously studied this population. It is reasonable to expect that this high rate of adoption will continue for the near future. Prior knowledge of mHealth technology has markedly increased over the same 3 years (30% vs. 7%), and many patients were already using this technology to assist in the management of medication therapies. These data demonstrate that the trajectory of smartphone ownership and mHealth use is on a steep climb, and it may be reasonable to assume that many transplant recipients may be using this technology to track medication therapies in the near future. Therefore, transplant programs should be considering methods to incorporate this technology into their usual care practices.
Although there was high receptivity toward the use of mHealth, there was also a group of respondents who were less likely to own or currently use this technology. Those respondents were more likely to have a lower income and be over the age of 55 years. That these respondents were less likely to own a smartphone and far less likely to download apps may indicate a lower level of comfort with this technology. This potential barrier to use of a mHealth app could be addressed by making the system easy to use and by providing skilled assistance and training.
There is a small but growing body of evidence to suggest that this technology may improve outcomes in chronic illnesses. In a randomized controlled pilot study, mHealth significantly increased medication adherence and reduced systolic blood pressure in kidney transplant recipients.9 In a prospective study, the use of text message reminders significantly improved medication adherence and graft outcomes in pediatric liver transplant recipients.15 The occurrence of nonadherence to medications or severe side effects was not associated with decreased willingness to use mHealth. Therefore, by providing accountability through reminders, mHealth could potentially improve medication management and adherence in these high-risk populations.
Limitations
The findings of this study must be evaluated within their context. First, respondents were recruited from a single transplant center, which may limit the generalizability of the findings. However, this center is the sole transplant provider for the state of South Carolina and has a catchment population of more than 4.6 million people with different racial, economic, and educational backgrounds. Second, those who chose to participate might be predisposed to a positive attitude toward mHealth and thereby introduce a positive bias. However, the participation of nearly everyone who was approached suggests that a significant bias toward mHealth is unlikely. One-half of the kidney transplant population, those under 55 years of age, were more likely to “strongly agree” with the use of mHealth, which could limit mHealth's scope in the older population. Despite this, the majority of older patients also responded positively to the use of mHealth and therefore could receive a significant benefit. The majority of kidney transplant patients earn less than $30,000 per year, and these patients were less likely to own smartphones. Because smartphone ownership continues to increase in all income brackets, this population will have increasing access to mHealth. Until then, grants used to eliminate cost could provide these patients with greater opportunity to benefit from mHealth technology. Although a randomized controlled pilot study demonstrated improvement in medication adherence and health outcomes, it cannot be assumed that the respondents' purported interest in mHealth would translate into effective use of this technology.9 This is important, because patients' attitudes towards technology may not translate into behaviors when trying to actually use the technology. Because our sample size was small, there is a risk of type II error. However, this study had a larger sample size and thus a higher level of power than the previous study by our research collaborative, markedly reducing this risk. Another limitation is the potential for selection bias due to those who attend follow-up visits being more likely to be actively involved in their health, be adherent to medication regimens, and use mHealth apps. We did not attempt to survey patients that routinely missed clinic appointments; therefore, these results are not applicable to patients that routinely miss clinic appointments. Finally, it should be noted that the study survey was conducted over a relatively short period of time (3 months) and was cross-sectional. The responses regarding medication adherence and side effects were not validated with the use of any other patient data.
Conclusions
This is our second study assessing the attitudes of transplant recipients toward mHealth, and these new data provide novel information on the significant trajectory of increasing smartphone ownership and use of mHealth technology in this population. These data also demonstrate that medication nonadherence or side effects were not significant obstacles toward willingness to use mHealth technology for medication management. Future studies involving patient-centered use of mHealth to track medications and health parameters are necessary to understand the full impact that this technology may have on high-risk populations such as kidney transplant recipients. As a whole, the majority of the kidney transplant recipients reported here own smartphones and are receptive to mHealth's use to improve medication management.
Supplementary Material
Acknowledgments
Funding: This research was supported with grants from the National Institute of Diabetes and Digestive and Kidney Diseases under award numbers K23DK099440 and T35DK007431. The data collection instrument in the Research Electronic Data Capture system was built with the support of a grant from the National Institutes of Health/National Center of Advancing Translation Sciences under award number L1TR000062.
Footnotes
Disclosure: The authors of this manuscript have no conflicts of interest as it relates to this research.
Previous presentation: Submitted as an abstract to the Academic Surgical Congress, February 2016 (http://www.cvent.com/events/11th-annual-academic-surgical-congress-asc-/event-summary-159e0a28ae8b4228bb5f2d756051dc8d.aspx).
Contributor Information
Robert B. Browning, College of Medicine, Medical University of South Carolina, Charleston, SC.
John W. McGillicuddy, Division of Transplant Surgery, Medical University of South Carolina, Charleston, SC.
Frank A. Treiber, Technology Center to Advance Healthy Lifestyles, College of Nursing, Medical University of South Carolina, Charleston, SC.
David J. Taber, Division of Transplant Surgery, Medical University of South Carolina, Charleston, SC.
References
- 1.United States Renal Data System. 2014 annual data reports. [Accessed July 30, 2015]; Updated 2014. Available at: www.usrds.org/2014/view/Default.aspx. [Google Scholar]
- 2.Scientific Registry of Transplant Recipients. 2012 annual data report. [Accessed July 30, 2015]; Updated 2012. Available at: http://srtr.transplant.hrsa.gov/annual_reports/2012/Default.aspx.
- 3.Neipp M, Karavul B, Jackobs S, Meyer zu Vilsendorf A, Richter N, Becker T, et al. Quality of life in adult transplant recipients more than 15 years after kidney transplantation. Transplantation. 2006;81(12):1640–1644. doi: 10.1097/01.tp.0000226070.74443.fb. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 5.Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003;3(2):178–185. doi: 10.1034/j.1600-6143.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 6.Kasiske BL, Anjum S, Shah R, Skogen J, Kandaswamy C, Danielson B, et al. Hypertension after kidney transplantation. Am J Kidney Dis. 2004;43(6):1071–1081. doi: 10.1053/j.ajkd.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Dayer L, Heldenbrand S, Anderson P, Gubbins PO, Martin BC. Smartphone medication adherence apps: potential benefits to patients and providers. J Am Pharm Assoc. 2013;53(2):172–181. doi: 10.1331/JAPhA.2013.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dew MA, DiMartini AF, de Vito Dabbs A, Myaskovsky L, Steel J, et al. Rates and risk factors for nonadherence to the medical regimen after solid organ transplantation. Transplantation. 2007;83(7):858–873. doi: 10.1097/01.tp.0000258599.65257.a6. [DOI] [PubMed] [Google Scholar]
- 9.McGillicuddy JW, Gregoski MJ, Weiland AK, Rock RA, Brunner-Jackson BM, et al. Mobile health medication adherence and blood pressure control in renal transplant recipients: a proof-of-concept randomized controlled trial. JMIR Res Protoc. 2013;2(2):e32. doi: 10.2196/resprot.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauer AM, Rue T, Keppel GA, Cole AM, Baldwin LM, et al. Use of mobile health (mHealth) tools by primary care patients in the WWAMI region practice and research network (WPRN) J Am Board Fam Pract. 2014;27(6):780–788. doi: 10.3122/jabfm.2014.06.140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith A. Smartphone Ownership 2013. [Accessed January 5, 2015];Pew Research Centers Internet American Life Project. Updated June 2013. Available at: www.pewinternet.org/2013/06/05/smartphone-ownership-2013/ [Google Scholar]
- 12.McGillicuddy JW, Weiland AK, Frenzel RM, Mueller M, Brunner-Jackson BM, et al. Patient attitudes toward mobile phone-based health monitoring: questionnaire study among kidney transplant recipients. J Med Internet Res. 2013;15(1):e6. doi: 10.2196/jmir.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winsett R, Arheat K, Stratta R, Alloway R, Wicks M, et al. Evaluation of an immunosuppressant side effect instrument. Prog Transplant. 2004;14(3):210–216. doi: 10.1177/152692480401400306. [DOI] [PubMed] [Google Scholar]
- 14.Butler JA, Peveler RC, Roderick P, Horne R, Mason JC. Measuring compliance with drug regimens after renal transplantation: comparison of self-report and clinician rating with electronic monitoring. Transplantation. 2004;77(5):786–789. doi: 10.1097/01.tp.0000110412.20050.36. [DOI] [PubMed] [Google Scholar]
- 15.Miloh TR, Arnon AR, Warshaw J, Parkar S, Suchy FJ, et al. Improved adherence and outcomes for pediatric liver transplant recipients by using text messaging. Pediatrics. 2009;124(5):844–850. doi: 10.1542/peds.2009-0415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.