Abstract
Purpose
To determine the feasibility and accuracy of spectral-domain optical coherence tomography (SD-OCT) based grading of anterior chamber cell, using aqueous sampling as a standard, in a rabbit model of anterior uveitis.
Methods
Adult Dutch-belted rabbits were preimmunized with M. tuberculosis (Tb) H37RA antigen, 1 week prior to induction of anterior uveitis with an intracameral injection of Tb antigen. The anterior chamber was imaged with SD-OCT, followed by a slit lamp examination. Two independent, trained graders recorded their estimate of anterior chamber cell count using the Standardization of Uveitis Nomenclature (SUN) scores for each eye prior to performing an anterior chamber tap to determine the aqueous cell density using a hemocytometer. Using the aqueous cell density as a standard, correlation with SD-OCT counts were compared to those with SUN scores.
Results
Overall, SD-OCT correlated well with the hemocytometer counts (Spearman coefficient = 0.53, P < 0.001) compared with SUN grading and hemocytometer counts (Spearman coefficient = 0.02, P = 0.88). The correlation improved to 0.65 (P < 0.001) when we excluded eyes with corneal thickness ≥ 470 μm. Eyes with corneal thickness ≥ 470 μm exhibited the greatest degree of ocular inflammation and corneal opacity.
Conclusions
In our rabbit model, SD-OCT grading of anterior chamber cell correlated significantly better with aqueous cell counts, compared to traditional slit lamp grading. Spectral-domain optical coherence tomography grading of anterior chamber cell may be a good alternative to SUN grading. Although SUN grading remains the clinical gold standard, alternative quantitative methods to assess ocular inflammation could provide insight into disease mechanism and aid in measuring treatment response.
Keywords: optical coherence tomography, uveitis, anterior chamber cell, SUN grading, anterior chamber sampling
The presence of anterior chamber cell is often used as a measure for disease activity in patients with uveitis. The Standardization of Uveitis Nomenclature (SUN) Working Group established an ordinal grading scale for measuring anterior chamber cell.1 A slit lamp examination using a 1 mm × 1 mm slit beam is used to count the number of cells and the number generated is recorded in a six-step grading system from 0 to 4+. Limitations include moderate to high interobserver variability within the same grade and the lack of precision due to the ordinal based nature of the grading system.2,3 The use of optical coherence tomography (OCT) to identify and quantify anterior chamber cells has been described and has been shown to correlate well with the clinical SUN based grading.3–6 Additionally, spectral-domain OCT (SD-OCT) based identification offers the ability to automate cell quantification and identify differences in cell type by differences in signal reflectivity.3,7
In this study, we compared SD-OCT quantitation of anterior chamber cell to the actual cell count obtained from aqueous sampling in a rabbit model of uveitis. Since SD-OCT grading is based on a continuous scale, we hypothesized that SD-OCT grading may be more accurate and provide a greater dynamic range compared with SUN based grading.
Methods
Institutional Animal Care and Use Committee approval was obtained and adherence to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research was observed. Experimental anterior uveitis was induced in both eyes of six Dutch-belted rabbits utilizing a previously published protocol8 with the following modifications.
Preimmunization
One hundred milligrams of heat-killed M. tuberculosis H37RA (Tb) antigen (DIFCO Laboratories, Detroit, MI, USA) was ultrasonicated in 5 mL mineral oil, USP (Sigma-Aldrich Corp., St. Louis, MO, USA) for 30 seconds or until homogenous. Five hundred microliters was administered subcutaneously via two dorsal injection sites (each site received 250 μL) to minimize local tissue reaction. Injection sites were monitored daily for evidence of local granuloma formation.
Induction of Anterior Uveitis
One hundred milligrams of heat-killed Tb antigen was ultrasonicated in 10 mL balanced salt solution (Alcon, Fort Worth, TX, USA) for 30 to 45 seconds until homogenous. The excess cell debris was separated from the mixture by centrifugation at 2500g for 5 minutes. The supernatant containing the Tb antigen was diluted with additional balanced salt solution (BSS) to obtain concentrations between 1 μg/μL and 0.5 μg/μL, in order to induce a range of inflammatory responses.
General anesthesia was induced with intramuscular ketamine (45 mg/kg) and xylazine (6 mg/kg), and subcutaneous buprenorphine (0.03 mg/kg) was given for postoperative analgesia. Once the rabbit was anesthetized, one drop each of 0.5% proparacaine hydrochloride ophthalmic solution, USP (Akorn, Lake Forest, IL, USA) and 1% atropine sulfate ophthalmic solution, USP (Bausch&Lomb, Tampa, FL, USA) were given to each eye for pain control and pupil dilation. Spectral-domain optical coherence tomography and slit-lamp examination were performed on each rabbit to confirm the absence of cells at baseline. Five percent betadine solution was used to sterilize the lids and ocular surface. Twenty microliters of prepared Tb antigen solution was injected into the anterior chamber of each eye. BSS was used to wash away excess betadine after the injection followed by application of bacitracin zinc and polymyxin B sulfate ophthalmic ointment USP (Bausch&Lomb).
SD-OCT Imaging and Slit Lamp Grading
Seven days after the intracameral injections of Tb antigen, the rabbits were ready for OCT imaging. Ketamine (45 mg/kg) and xylazine (6 mg/kg) were administered intramuscularly for anesthesia. The rabbit was positioned upright in a custom holder, and the upper eyelid was temporarily retracted using tape. The eyes were hydrated at all times with polyethylene glycol eye drops (Systane Ultra, Alcon). A custom-built SD-OCT system was used to capture scans of the anterior chamber at 74 kHz line-rate. The spectral-domain optical coherence tomography used a broadband superluminescent diode light source centered at 820 nm with 150 nm bandwidth (Superlum), which provided 1.98 μm axial resolution in air. Lateral resolution was limited by the anterior segment imaging probe optics to approximately 21 μm. The system had a measured −6 dB fall off at 1.1 mm. A 12-mm scan was taken for central cornea thickness measurement, followed by 1 mm × 1 mm scans superior and inferior to the center of the cornea, for determining cell density values (cells/μL). The optical coherence tomography system was calibrated using a ruler prior to collecting data, and the volumes were corrected accordingly. At each location, a 500 (A-scans/B-scan) × 500 (B-scans/volume) scan density was used, with a second scan performed with each horizontal raster in triplicate (three-frames/B-scan). Image frames were manually counted for hyperreflective specks, which were used for cell density calculations. If a speck was detected in the same location in consecutive scans, it was only counted once to prevent oversampling errors.
A Haag-Streit slit-lamp was used to examine and clinically grade each eye immediately after imaging, using the SUN grading scale for cells within a 1 mm × 1 mm slit-beam field of view. The Standardization of Uveitis Nomenclature grading scale is as follows: 0.5+ refers to 1 to 5 cells, 1+ for 6 to 15 cells, 2+ for 16 to 25 cells, 3+ for 26 to 50 cells, and 4+ for more than 50 cells.1 In 54 out of 62 examinations, grading was performed by two trained nonclinicians. In 8 out of 62 examinations, grading was performed by one clinician and one trained nonclinician. The average SUN grade was calculated and used for analysis. Other observations were also noted, including cornea clarity, and the presence of iris inflammatory nodules, fibrin, and cell clumps. The graders were masked to each other and also to the results of the OCT cell counts and aqueous sampling.
Aqueous Sampling
Immediately following slit lamp grading, isoflurane anesthetic (2%–4%) was administered via inhalation to continue sedation. Proparacaine drops were given for analgesia, atropine drops for dilation, and 5% betadine solution was used for sterilization of ocular surface, intended injection site, and surrounding eye-lid tissue. Approximately 0.15 mL fluid was drawn from the anterior chamber through the superior peripheral cornea, using a 0.3-mL syringe and 31-gauge needle (BD, Franklin Lakes, NJ, USA) BD #328438. The needle was passed through the cornea approximately 1 to 2 mm from the limbus with the tip of the needle aiming toward the center of the eye with the bevel down. Care was taken not to disturb the anterior lens capsule and the needle was kept parallel to the iris plane. Fluid was withdrawn slowly into the syringe, the needle removed from the eye, and a sterile swab placed over the wound to stop the egress of fluid. The eye was flushed with BSS then bacitracin/polymyxin B antibiotic ophthalmic ointment was applied. The fluid within the syringe was immediately transferred to a Nageotte (Hausser Scientific, Horsham, PA, USA) or Neubauer (Hausser Scientific) hemocytometer at the time of collection. Manual cell counts were determined after the procedure and the density was calculated.
Statistical Analysis
For each imaging session, a total of four SD-OCT scans were obtained (superior/inferior, high/low density) and the mean was used for total SD-OCT counts. For analysis of superior and inferior scans, the average of the low and high density scan was used. Since the goal was to evaluate associations between systems, and different sessions within each eye reflected different levels of inflammation, each session was treated independently in the statistical analysis. Normality of the quantities was assessed using the Shapiro-Wilk test. Agreement between graders on SUN grades was evaluated using a weighted κ test. Associations between quantities were evaluated using Spearman correlations. Comparisons between regions within animals were made using Wilcoxon signed rank test. Analysis was performed using Microsoft Excel, and a P value less than 0.05 was considered statistically significant.
Results
The Tb model produced exuberant anterior segment inflammation, often with associated corneal thickening, corneal haze, and inflammatory cell clumps on the surface of the iris and anterior lens capsule (Table 1). Over a course of 2 to 3 weeks, inflammatory levels decreased and rabbits were imaged over multiple time points in order to obtain a range of inflammatory grades. A total of six rabbits and 12 eyes were imaged over 62 different sessions (range per eye: four to six sessions). Figure 1 shows examples of hyperreflective specks seen on SD-OCT imaging in inflamed rabbit eyes compared with a control eye. A Shapiro-Wilk test for normality was performed for SD-OCT counts, hemocytometer counts (aqueous sampling), and SUN grades (Table 2). All of the data were found to be nonnormal, and thus Spearman rank correlation was used for our analysis. The average SUN grade was calculated from two independent graders for each sample. The weighted κ between the two graders was 0.76 (95% confidence interval: 0.66–0.87), which shows good agreement between the graders.
Table 1.
Frequency of Severe Ocular Inflammatory Findings
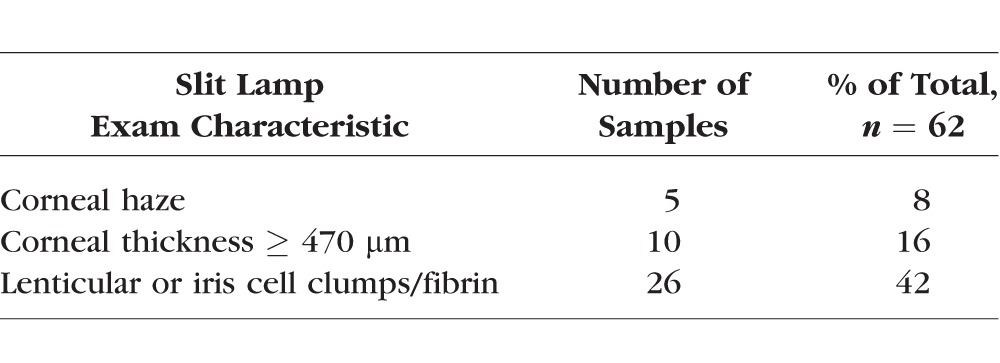
Figure 1.
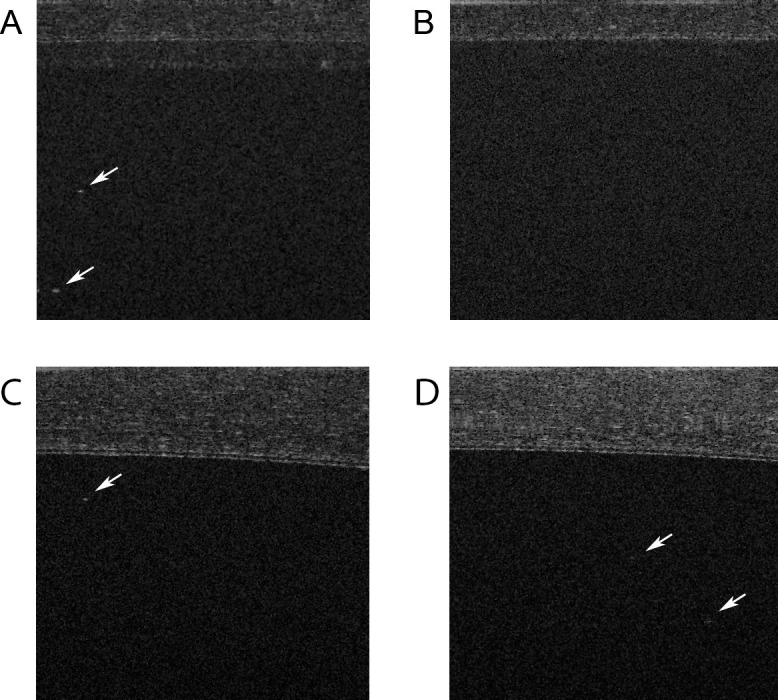
Examples of SD-OCT B-scan image in an inflamed eye (A), control eye (B), and inflamed eyes with thickened corneas (C, D). The arrows point to hyperreflective specks representing cells. The signal toward the top of the image is the cornea. The thick cornea (C, D) attenuates the cell signal compared with A. The signal attenuation is greatest with increasing depth of penetration (D).
Table 2.
Comparison Between Aqueous Sampling, SD-OCT, and SUN Grading Systems
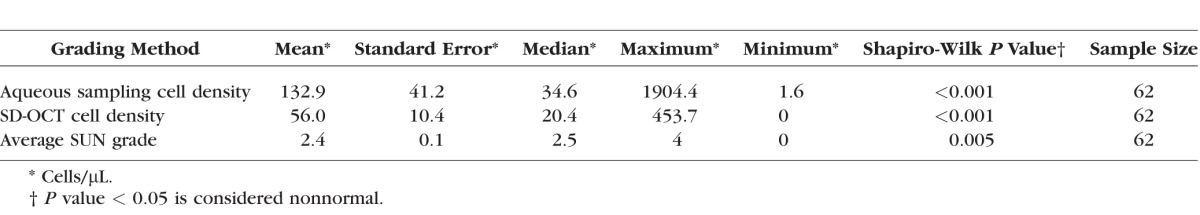
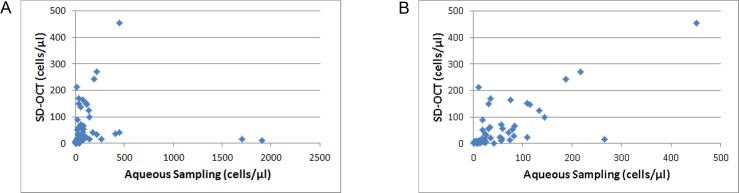
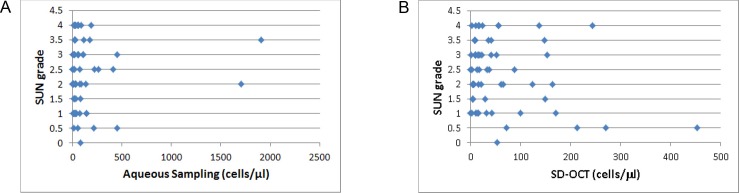
Figure 2A shows a scatterplot of aqueous sampling versus SD-OCT cell counts. We noticed several outliers with very intense inflammatory reactions and very high cell counts. These eyes also had associated central corneal thickness (CCT) of 470 μm or greater. Figure 2B shows a scatterplot excluding eyes with CCT of 470 μm or greater. Fifty-two of the 62 (84%) samples had CCT below 470 μm. The average corneal thickness of our complete data set was 412 ± 57 (mean ± SD) μm, and for samples below 470 μm it was 392 ± 34 μm. Overall, SD-OCT correlated well with aqueous sampling with a Spearman coefficient (ρ) of 0.53 (P < 0.001). This improved to ρ = 0.65 (P < 0.001) when CCT was limited to below 470 μm. Standardization of Uveitis Nomenclature grading of the same eyes correlated poorly with aqueous sampling (Fig. 3A). The overall ρ was 0.02 (P = 0.88) and this did not significantly improve when CCT was below 470 μm (ρ = −0.06, P = 0.65). Similarly, SD-OCT correlated poorly with SUN grading in our study (ρ = −0.13, P = 0.34; Fig. 3B).
Figure 2.
Scatterplots of aqueous cell density compared with SD-OCT density estimates. (A) Several outliers with very high aqueous cell counts are seen toward the bottom right of the plot. (B) When the population is limited to CCT below 470 μm, the outliers are excluded.
Figure 3.
(A) Scatterplot of aqueous cell density compared with SUN grade. (B) Scatterplot of SD-OCT density estimates compared with SUN grade. Standardization of Uveitis Nomenclature grading does not correlate with aqueous sampling or SD-OCT cell density estimates.
Although anterior chamber cells are mobile, they appear to settle with gravity (i.e., formation of a hypopyon). We wanted to test if regional differences could be detected with SD-OCT by imaging each eye in a location superior to the central cornea and also inferior to the central cornea. No significant difference in SD-OCT values between these two locations were detected by Wilcoxon signed rank test (P = 0.50), even after limiting the analysis to CCT below 470 μm (P = 0.44).
Discussion
Anterior chamber inflammation is currently measured and graded by clinical examination using slit-lamp biomicroscopy and the SUN scale. This approach is considered the gold-standard for clinical diagnosis, where aqueous sampling cannot be easily obtained. This study investigates an alternative method for grading inflammatory cells within the anterior chamber using SD-OCT, a technology already being used in clinical settings. Spectral-domain optical coherence tomography allows for the noninvasive capture of cross-sectional image frames where cells can be visualized as reflective spots. Our data show that SD-OCT counts correlated with aqueous cell counts better than the correlation between SUN grading and aqueous cell counts. Spectral-domain optical coherence tomography has known limitations, however. The axial resolution of SD-OCT is approximately 5 μm, and the transverse resolution is approximately 20 μm. Cells smaller than 5 μm may be undetectable and two small cells separated by less than 20 μm may appear as one hyperreflective speck. Fortunately, rabbit lymphocytes, and heterophils are approximately 10 to 15 μm in diameter and monocytes are even larger. The optical coherence tomography signal is also limited by optical penetration depth, as well as signal roll-off, and thus the entire anterior chamber cannot be imaged at once. Signal roll-off limits the ability of SD-OCT to distinguish cells from background with increasing axial depth. If the distribution of cells between anterior and posterior regions is different, then SD-OCT may not provide a completely accurate estimate of anterior chamber cellular density. Our study also suggests corneal thickening attenuates the OCT signal and results in erroneous cellular density estimates. Scans with CCT ≥ 470 μm resulted in SD-OCT cell counts that poorly correlated with aqueous sampling including outliers with very high aqueous sampling cell counts, but low SD-OCT counts (Fig. 2A). This is consistent with previous reports4 where corneal edema resulted in low SD-OCT cell counts compared with eyes with clear corneas and high grades of inflammation.
In contrast to previous reports comparing SUN clinical grading to SD-OCT grading,3–6 we found no correlation between SUN grading and SD-OCT grading in our study. This disparity may be explained by several significant differences. The current study describes a uveitis model in rabbit instead of clinical uveitis in human patients. To our knowledge, SUN grading of rabbit uveitis in the Tb model has yet to be validated. Standardization of Uveitis Nomenclature grading of anterior chamber cell in our model may be less accurate than in humans, resulting in SUN grades that do not accurately reflect anterior segment inflammation. The rabbit model produced severe inflammation including 42% of eyes with inflammatory cell clumps or fibrin in the anterior chamber (Table 1). The average corneal thickness of New Zealand white rabbits is 350 μm9 and 362 ± 15.5 μm for Dutch-belted rabbits.10 In our study, 65% (40 out of 62 samples) were 2 standard deviations above the average CCT (data not shown). This increased corneal thickness may have limited the ability of our graders from accurately assigning SUN grades. Our study also used two nonclinicians to grade the majority of the slit lamp examinations (87%) as opposed to grading by trained uveitis specialists. Our results suggest SUN grading accuracy is highly dependent on observer level of expertise and quality of the sample. Standardization of Uveitis Nomenclature grading requires a dynamic measurement of cells moving in and out of a defined volume through a slit beam, whereas SD-OCT cell counts are taken from static optical sections through a defined volume. This fundamental difference could also help explain why no correlation was found between SUN grading and SD-OCT cell counts or aqueous taps.
The lack of a 1:1 correlation between SD-OCT counts and aqueous cell counts may be due to several possibilities. Spectral-domain optical coherence tomography may underestimate the actual cell count due to the SD-OCT limitations outlined above. Another possibility, however, is oversampling of the “free floating” aqueous cell population in our experimental model by inadvertently collecting cell clumps stuck to the iris and lens. Although we made every effort not to disturb these clusters of inflammatory cells, it is possible that in some samples, collection errors might have resulted in higher than usual cell counts.
By using an animal model, we were able to determine the aqueous cell density by directly counting the cells withdrawn in an aqueous sample. We found SD-OCT to correlate well with aqueous cell density when corneal thickening was limited. Spectral-domain optical coherence tomography is currently being used in many ophthalmic offices and transition to anterior segment scans could be rapidly and easily adapted. Development of automated cell counting algorithms could further improve the accuracy and precision of SD-OCT cell counts and allow for the rapid evaluation of anterior segment inflammation. Standardization of Uveitis Nomenclature grading in humans is well validated and remains the gold standard for monitoring anterior uveitis. However, the use of SD-OCT may become a useful adjunctive test for the evaluation of patients with anterior uveitis.
Acknowledgments
The authors thank Nihaal Rahman for his assistance in collecting data and calibration of the SD-OCT system.
Supported by NEI 1K08EY023608-03 (AY), Ohio Third Frontier TECH 13-059 (YKT, SKS), and an unrestricted grant from Research to Prevent Blindness.
Disclosure: M. Edmond, None; A. Yuan, None; B.A. Bell, None; A. Sharma, None; R.M. DiCicco, None; L. Tucker, None; J. Bena, None; Y.K. Tao, None; S.K. Srivastava, Allergan (C), Bausch&Lomb (C), Sanofi (C), Optos (C), Carl Zeiss (C), Regeneron (C), Clearside (C), Synergetics (C), Bioptigen (C)
References
- 1. Jabs DA,, Nussenblatt RB,, Rosenbaum JT. Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005. ; 140: 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kempen JH,, Ganesh SK,, Sangwan VS,, Rathinam SR. Interobserver agreement in grading activity and site of inflammation in eyes of patients with uveitis. Am J Ophthalmol. 2008. ; 146: 813–818.e1. [DOI] [PubMed] [Google Scholar]
- 3. Sharma S,, Lowder CY,, Vasanji A,, Baynes K,, Kaiser PK,, Srivastava SK. Automated analysis of anterior chamber inflammation by spectral-domain optical coherence tomography. Ophthalmology. 2015. ; 122: 1464–1470. [DOI] [PubMed] [Google Scholar]
- 4. Agarwal A,, Ashokkumar D,, Jacob S,, Agarwal A,, Saravanan Y. High-speed optical coherence tomography for imaging anterior chamber inflammatory reaction in uveitis: clinical correlation and grading. Am J Ophthalmol. 2009. ; 147: 413–416.e3. [DOI] [PubMed] [Google Scholar]
- 5. Li Y,, Lowder C,, Zhang X,, Huang D. Anterior chamber cell grading by optical coherence tomography. Invest Ophthalmol Vis Sci. 2013. ; 54: 258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Igbre AO,, Rico MC,, Garg SJ. High-speed optical coherence tomography as a reliable adjuvant tool to grade ocular anterior chamber inflammation. Retina. 2014. ; 34: 504–508. [DOI] [PubMed] [Google Scholar]
- 7. Rose-Nussbaumer J,, Li Y,, Lin P,, et al. Aqueous cell differentiation in anterior uveitis using Fourier-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2015. ; 56: 1430–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheng CK,, Berger AS,, Pearson PA,, Ashton P,, Jaffe GJ. Intravitreal sustained-release dexamethasone device in the treatment of experimental uveitis. Invest Ophthalmol Vis Sci. 1995. ; 36: 442–453. [PubMed] [Google Scholar]
- 9. Reichard M,, Hovakimyan M,, Wree A,, et al. Comparative in vivo confocal microscopical study of the cornea anatomy of different laboratory animals. Curr Eye Res. 2010. ; 35: 1072–1080. [DOI] [PubMed] [Google Scholar]
- 10. Thomasy SM,, Raghunathan VK,, Winkler M,, et al. Elastic modulus and collagen organization of the rabbit cornea: epithelium to endothelium. Acta Biomater. 2014. ; 10: 785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]