Abstract
This account describes lanthanide coordination chemistry with a focus on the similarities between lanthanide complexes used in catalysis and those used as contrast agents in magnetic resonance imaging.
Keywords: catalysis, Mukaiyama aldol, lanthanides, contrast agents, magnetic resonance imaging
Graphical Abstract
1 Introduction
The lanthanides compose an alluring region of the periodic table in part because of the wide range of magnetic and luminescence behaviors encompassed by these elements. The properties of these elements make them essential to many aspects of modern life including display screens, computers, automobiles, and medical diagnoses. Additionally, the entire series of elements has a propensity to favor the trivalent oxidation state, which leads to the ability to use one ligand to obtain a series of structurally similar complexes with diverse physicochemical properties. The ready access to structurally related complexes with diverse properties, the Lewis acidic nature of the lanthanide ions, and the ability of these ions to form water-tolerant complexes are some of the major factors that drive my interests and that have guided the research thus far in my career.
This account shares the findings of my research group that have arisen through exploring lanthanide chemistry along the boundary between catalysis and magnetic resonance imaging.
2 Lanthanide Coordination Chemistry
As a graduate student, my first experience with lanthanide chemistry was with GdIII-containing complexes relevant to magnetic resonance imaging.1 At that time, my understanding was that these ions do not participate in covalent bonding, that multidentate ligands were necessary to form complexes with low lability because of purely electrostatic bonding, and that the ions rarely occurred outside of the +3 oxidation state. As I progressed in my research,2 I began to see the beautiful intricacies of the coordination chemistry of these elements. While I refer readers elsewhere for detailed descriptions of the coordination chemistry of the lanthanides,3 some aspects of lanthanide chemistry that specifically influence the research directions in my group include (1) the need for ligands with large denticities (usually >6) to form thermodynamically stable complexes; (2) the observation that even complexes with large conditional thermodynamic stabilities are fluxional; (3) that kinetic and thermodynamic stabilities are influenced by ligand design but differ in their importance depending on application; (4) that there is rich chemistry outside of the +3 oxidation state for lanthanides; and (5) that lanthanides in the +3 oxidation state tend to be hard acids (similar to MnII) but in the +2 oxidation state they become relatively soft (similar to AgI).4
There are several consequences to these five aspects of lanthanide chemistry that permeate the research in my group. The quest for stability in complexes often leads to ligands with large denticities. However, not only is denticity important for stability, but the arrangement of donors about the metal ion is critical for ligand design. For example, if a multidentate ligand blocks seven of nine coordination sites of a metal ion, then mono- or bidentate ligands (such as water or phosphate) can bind to the remaining sites. If the two remaining sites are adjacent to each other, then bidentate ligands tend to preferentially coordinate, blocking water from coordinating (which is of consequence to magnetic resonance imaging).5 If the two binding sites are not adjacent to each other, then water can coordinate to each site with less interference from a bidentate ligand. Studies of water displacement have led to interesting findings with respect to anion sensing with lanthanides.6 Additionally, while the lanthanide ions tend to be most stable in the +3 oxidation state, there is a wealth of chemistry of the +2 and +4 oxidation states for these elements.7 In the other oxidation states, interesting characteristics of both f and d electrons can be observed, and often these characteristics are influenced by environmental factors such as light.8 Furthermore, the trivalent ions tend to be oxophilic, but the divalent ions prefer softer bases.9 These factors all influence ligand design.
Another interesting feature of lanthanides that arises from their large size is unique geometries. Although ligand donor atoms tend to not influence binding in some respects (minimal interactions with 4f orbitals), they can have an effect when linked together in a multidentate ligand or when d-character is involved, for example, with divalent lanthanide ions. Prior to beginning research in this area, I spent little time thinking about geometries larger than octahedrons, but now I am fascinated by larger geometries such as capped square antiprisms, bicapped trigonal antiprisms, muffins, and hula hoops.10
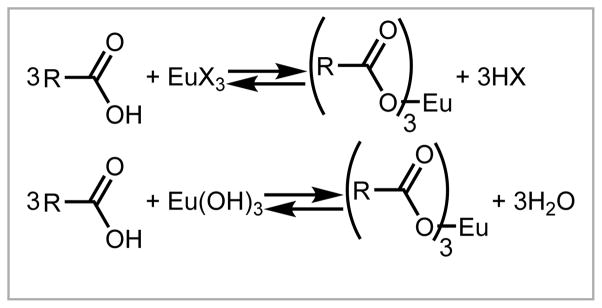
For relevancy to contrast agents for magnetic resonance imaging (molecules that influence contrast in images) or water-tolerant catalysis, lanthanide-containing complexes need to tolerate the presence of water, and coordination chemistry in water comes with a unique set of challenges. For example, when metalating a ligand that contains carboxylic acids with a lanthanide halide salt, a byproduct is HX (Scheme 1).11 The acid lowers the pH of the reaction mixture and, consequently, can reverse the metalation to some extent. This drop in pH can be overcome by adjusting the pH with base or buffer, but the result of such adjustments is a solution of metal complex that contains salts from neutralization of the acid. Lanthanide hydroxides or oxides can be used for the metalation with the byproduct being replaced with water (Scheme 1), but the hydroxide and oxide salts have low aqueous solubility causing the need to heat metalation reactions to increase solubility. Long metalation times at high heat can be potentially damaging to ligands. These factors must be accounted for when planning reactions.
Scheme 1.
Simple balanced reactions between carboxylic acids and lanthanide salts. R and X represent the remainder of the ligand and halides, respectively.
Studying paramagnetic ions in aqueous solutions that might contain other salts is often challenging. Crystallizations from these solutions are often onerous, and even if obtained, solid-state structures are often not representative of the highly fluxional complexes in solution. Depending on the ion, 1H-NMR spectra are at best shifted and at worst (GdIII or EuII) broadened to the point of not being useful. Consequently, other techniques are needed to study these complexes in solution including Job plots, 17O-NMR spectroscopy, cyclic voltammetry, conductivity, luminescence and UV–visible spectroscopies, and electron paramagnetic resonance spectroscopy.12 The challenges associated with studying lanthanide-containing complexes in solution is at times frustrating but is an enticing area of research, in my opinion, because of the great potential for new discoveries.
3 Study of Coordination Environment
The challenges associated with studying lanthanide-containing complexes in aqueous solution have been approached using a variety of innovative analytical methods.12 Much of this innovation exploits luminescence and magnetic properties inherent to the ions and has been driven forward by research related to contrast agents for magnetic resonance imaging. At the start of my independent research career, my research focused on adapting some of these methods to study water-tolerant precatalysts for bond-forming reactions.
I was surprised that I was unable to find reports of the use of the technique to study catalysis; consequently, we began by studying luminescence-decay measurements that are often used to determine the water-coordination number of contrast agents for magnetic resonance imaging. Luminescence-decay measurements have been used to study lanthanide ions in aqueous solution for decades.13 This strategy seemed to be a great starting point because of the relative simplicity of the method and because the data it provided regarding coordination environment in solution was relevant to catalysis.
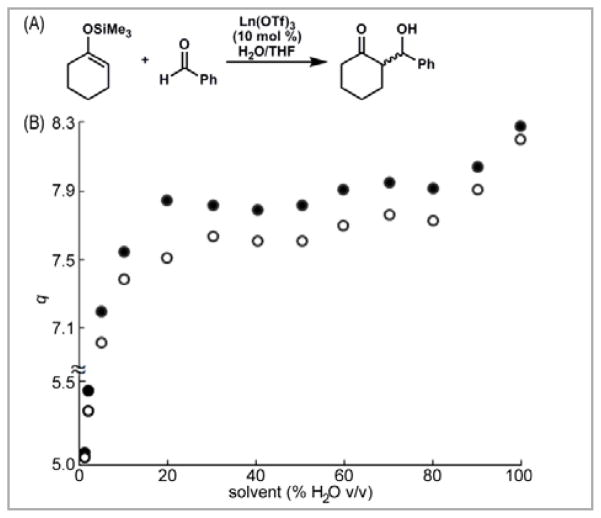
We used this technique to study the water-coordination number of Eu(OTf)3, a precatalyst for carbon–carbon bond-forming reactions in aqueous systems.14 We were able to determine the water-coordination number of this lanthanide salt in the presence and absence of substrates and products of the water-tolerant Mukaiyama aldol reaction (Figure 1).15 Our measurements were fast, water-tolerant, and worked under reaction-relevant conditions. Building on these results, we elucidated structural information about precatalysts that contained multidentate ligands (Scheme 2), and we used this information to propose a mechanism for chiral product formation.16 We also reported the use of this technique, combined with high-performance liquid chromatography, to determine the influence of the water-coordination numbers of precatalysts on steady-state reaction rates.17 From these measurements, correlations were observed between steady state reaction rate and water-coordination number as well as between yield and solvent composition. The methodology can be applied to other LnIII-catalyzed bond-forming reactions in aqueous media to gain a better understanding of the influence of water on the structure–activity relationship between precatalysts and rates of catalysis.
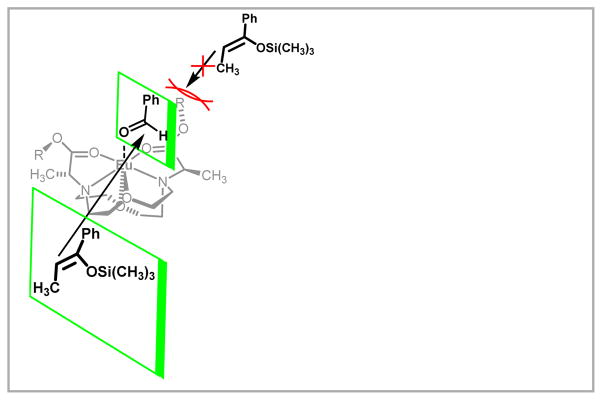
Figure 1.
(A) A Ln(OTf)3-catalyzed Mukaiyama aldol reaction; (B) Water-coordination number, q, of EuIII in THF containing different amounts of H2O before (●) and after (○) the addition of benzaldehyde. The difference in q values between the solid and hollow dots is a measure of the displacement of coordinated water by benzaldehyde. Adapted with permission from Dissanayake, P.; Allen, M. J. J. Am. Chem. Soc. 2009, 6342. Copyright 2009 American Chemical Society
Scheme 2.
Proposed equilibrium leading to activation of benzaldehyde for nucleophilic attack based on q measurements. Adapted with permission from Mei, Y.; Dissanayake, P.; Allen, M. J. J. Am. Chem. Soc. 2010, 12871. Copyright 2010 American Chemical Society
Although we had success with the use of luminescence-decay measurements, our studies were limited to solvents that did not contain OH oscillators. Those oscillators contribute to luminescence quenching in a way that is indistinguishable from water. To include protic solvents, we empirically derived equations relating decay rate to coordination, as had previously been done in water,13 in the presence of co-solvents that are commonly used in organic reactions.18 These derivations resulted in the ability to determine water-coordination numbers in the presence of a wide range of co-solvents, including protic solvents. The results of this research enable the use of luminescence-decay measurements to study lanthanide-based catalytic systems in the presence of any of the seven co-solvents that we studied in any ratio with water.18 The results of these efforts are in a web-based calculator to allow easy access to other researchers.19 This research is important to understanding the dynamics of the inner- and outer-sphere environments of LnIII-based precatalysts in aqueous solvent mixtures. The set of equations that we derived enables the facile acquisition of mechanistic and structural information regarding water-tolerant precatalysts and is a powerful tool for precatalyst design. We expect that our demonstration of the versatility and simplicity of this technique will enable its use by the synthetic community to study new bond-forming reactions.
4 Catalysis with Trivalent Lanthanides
Similar to the application of an analytical technique from contrast agent research to catalysis, we used what we knew about aqueous lanthanide coordination chemistry from contrast agent research to design ligands for asymmetric catalysis.
Carbon–carbon bond-forming reactions are a foundation of synthetic chemistry, and many of these reactions can be catalyzed with water-tolerant lanthanide triflates.14 Previous attempts to use these water-tolerant precatalysts in stereoselective reactions were successful only in a few cases,20 and we thought that viewing precatalysts from the vantage point of contrast agents would enable improvements in stereoselectivity in aqueous solution by enabling rational design of metal–ligand interactions.
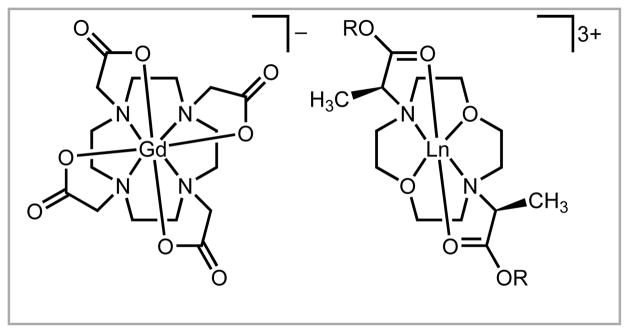
We designed and synthesized a new series of ligands for lanthanides. These precatalysts originated from envisioning modifications to a common contrast agent, GdIII 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate, that is kinetically stable and water soluble: the modifications included space for substrate interactions as well as chiral centers to induce specific stereochemical outcomes (Figure 2).16 We thought that if chiral centers were to induce specific stereochemical outcomes, then the chiral centers would need to be close to the reaction sites on the metal ions. With labile lanthanide ions, multidentate and macrocyclic ligands seemed to be the best way to achieve this goal. Consequently, we synthesized ligands that contained the modifications listed above with the prediction that they would enable efficient chiral induction during catalysis, while maintaining high solubility in water. After synthesizing a series of ligands, we demonstrated the utility of our precatalysts in the water-tolerant Mukaiyama aldol reaction with a wide range of substrates, including the often problematic alkyl aldehydes.16 The new precatalysts yielded β-hydroxy carbonyls from aliphatic and aryl substrates with outstanding syn:anti ratios (up to 49:1) and ee’s (up to 97%). At the time of publication, these were the highest reported values for Lewis acid precatalysts in aqueous medium for the reactions that were studied.16,21 Furthermore, the findings provide insight into the design and synthesis of potent chiral precatalysts for carbon–carbon bond-forming reactions in aqueous media.
Figure 2.
Structures of (left) a gadolinium-containing contrast agent and (right) our LnIII-containing precatalysts.16 Counter ions are omitted for clarity.
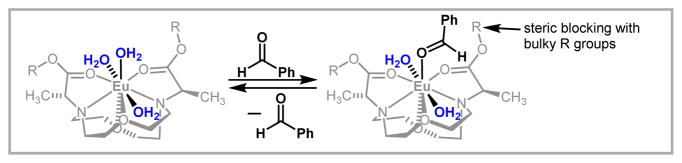
After demonstrating the utility of our system, we used luminescence-decay measurements to infer a mechanism for catalysis and chiral induction (Figure 3). Further study of the ligand system was performed with respect to ligand synthesis, trends in stereoselectivity that result from changing lanthanide ions, and establishing the scope of compatible aldehydes and ketones.22 We observed interesting trends in the relationship between both ionic radius and Lewis acidity to reactivity and selectivity. For example, ligand loading can be decreased when NdIII is used instead of EuIII. Additionally, a broad scope of substrates was compatible with the precatalysts, including aliphatic aldehydes and silyl enol ethers derived from aliphatic ketones. These studies resulted in a new class of LnIII-based chiral precatalysts for aqueous carbon–carbon bond-forming reactions that offers a broad substrate scope and generates products with high enantioselectivity. Furthermore, the stereochemical outcomes of reactions involving our precatalysts have been explored with computational analyses.23
Figure 3.
Proposed TS in the asymmetric Mukaiyama aldol reaction using our new ligands. Reprinted with permission from Mei, Y.; Dissanayake, P.; Allen, M. J. J. Am. Chem. Soc. 2010, 12871. Copyright 2010 American Chemical Society
We sought to understand the structural aspects of the new precatalysts that make them effective. To accomplish this objective, a series of complexes was synthesized that contained different metal-binding functional groups and that placed chiral centers in different positions on the ligand framework.24 We studied these complexes as precatalysts with respect to the relationships among binding affinity, functional group identity, functional group position, reactivity, and selectivity. The complexes were studied using luminescence and NMR measurements to establish the coordination environments of the EuIII-based precatalysts in solution, and these data were compared with yields and selectivities of reactions. The data suggest that an excess of hexadentate ligand leads to complete encapsulation of EuIII and diminished reactivity (Scheme 3). More specifically, the evidence suggests that two-to-one ligand-to-metal binding for hexadentate ligands occurs in the presence of excess ligand. This explanation is corroborated by the low yields of Mukaiyama aldol reactions performed in the presence of excess ligand. A comparison of ligand functional groups, the location of the chiral centers, steric bulk, and type of macrocyclic backbone has given insight into the factors that affect reactivity and selectivity of water-tolerant asymmetric precatalysts.24 These results contribute to the further understanding of LnIII-based precatalysts to be used for asymmetric carbon–carbon bond-forming reactions in aqueous media.
Scheme 3.
Proposed equilibria involving multiple EuIII species with reactivity summarized under the species. The equilibria are based on luminescence-decay, steady-state luminescence measurements, and 1H-NMR data with reactivity and selectivity from Mukaiyama aldol reaction results. Adapted with permission from Averill, D. J.; Allen, M. J. Inorg. Chem. 2014, 6257. Copyright 2014 American Chemical Society
In addition to the results detailed above, we optimized a synthetic route to trans-substituted rings for future synthesis of precatalysts.25 We also studied exchange rates in ionic liquids and in the process observed an interesting reverse of the trend, relative to aqueous systems, between water-exchange rate and ionic radius.26 This change is likely due to steric interactions caused by anion binding to the metal ions in the ionic liquids. These results have important implications to catalysis in ionic liquids in that they demonstrate that the reactivity of ions can be tuned over a wide range using solvent selection.
5 Contrast Agents from Divalent Europium
We have applied knowledge from contrast agents to catalysis, but we have also explored the chemistry of complexes relevant to contrast agents. Magnetic resonance imaging is a powerful, noninvasive technique that can be used to visualize the inside of opaque objects. It can produce images based on the behavior of nuclei, including relaxation times and chemical shifts, in a magnetic field. Consequently, molecules can alter the contrast of images by influencing the behavior of the nuclei of other molecules in a magnetic field. The molecules that alter contrast are referred to as contrast agents, and paramagnetic ions, commonly GdIII, are used to enhance contrast in images.27 Therefore, understanding the coordination chemistry of GdIII and other trivalent lanthanide ions is critical to the design of new contrast agents because the coordination of these ions impacts their ability to alter contrast and also changes their properties with respect to biodistribution and toxicity. We have explored trivalent ions as contrast agents,28 but recently have focused a great deal of effort into divalent europium as a contrast agent.
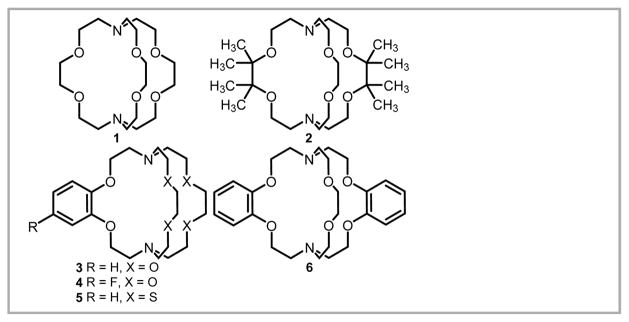
EuII is isoelectronic with GdIII, making it accelerate the relaxation rate of nearby protons in a similar fashion.29 However, the difference in charge density and redox activity between these ions influences their ability to alter contrast as function of field strength and to report redox environments. Despite the favorable properties of EuII, this ion has not been used commonly for imaging because of its propensity to oxidize to EuIII.30 Consequently, we have been extremely interested in stabilizing the electron-rich divalent state of europium to enable access to its electronic properties for imaging. Past research efforts aimed at increasing the stability of aqueous EuII indicated that withdrawing electron density from ligands could increase the oxidative stability of EuII.31,32 Our strategy for favoring EuII over EuIII in aqueous solution involved the synthesis and use of ligands that would preferentially coordinate to large, soft, electron-rich metals like EuII. The template for our ligand design was (2.2.2)-cryptand because at the beginning of my independent career, this ligand produced the most oxidatively-stable, aqueous EuII complex reported.32 This stability is partially due to the better size match between cryptand and the EuII ion relative to the EuIII ion that favors binding of EuII over EuIII.30 We hypothesized that further oxidative stabilization could be achieved by modifying the structure of the cryptand to incorporate electron withdrawing groups and softer donors. To test this hypothesis, we synthesized and studied cryptands 1–6 (Figure 4).33
Figure 4.
Ligands used to observe trends in oxidative stability of aqueous EuII.33
We observed dramatic oxidative stabilization of EuII using cryptands 1–6.33 In fact, our most stable complexes, 4-EuII and 6-EuII, were the most oxidatively stable aqueous EuII complexes reported at the time and were as or more oxidatively stable than FeII in hemoglobin. These results hinted at the possibility of using EuII as a contrast agent in vivo. After exploring the oxidative stability of EuII-containing cryptates, we studied their kinetic stability to transmetallation in vitro and found them to have stability to transmetallation with biologically relevant ions that depended on ligand structure.34 We also explored the ability of our complexes to enhance contrast, and we found that these complexes were more effective contrast agents than conventional GdIII-based agents at ultra-high field strengths (≥7 T),35 and that they did not enhance contrast in T1-weighted images after oxidation.36 Furthermore, we observed interesting behaviors at ultra-high fields: our complexes increased in relaxivity with field strength with a maximum between 7 and 9 T.35,37 This observation is particularly important because ultra-high field strengths offer advantages, in terms of resolution and scan time, over lower field strengths.38 Consequently, ultra-high field strength scanners are of paramount importance in preclinical research.38c Based on the oxidative and kinetic stabilities of EuII-containing cryptates and their relaxivity at 7 T, we were excited to attempt in vivo imaging. To have the best chance of successful imaging in vivo, we chose one of our most oxidatively stable complexes and one of the least oxidizing environments in vivo.39 This combination resulted in the first report of in vivo contrast-enhanced imaging using a divalent lanthanide (Figure 5).39 The divalent species persisted for at least 3 h and selectively enhanced contrast in necrotic tissue. This result is exciting because it suggests the possibility for differentiation of redox environments with a complete lack of contrast enhancement in the oxidizing area.
Figure 5.
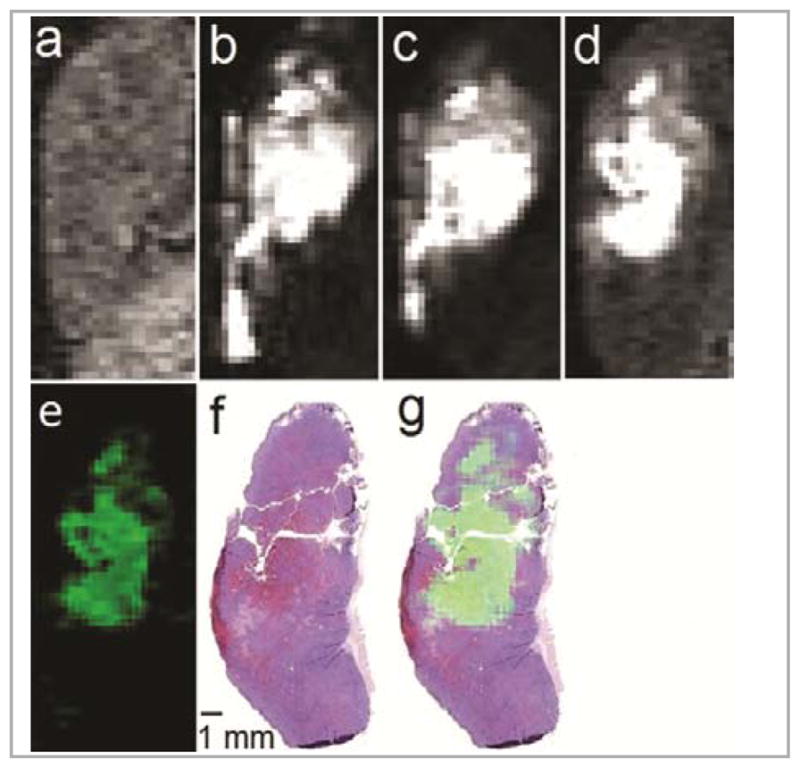
Images of a mouse tumor acquired using 4-EuII. T1-weighted in vivo sagittal plane images of a tumor injected with 4-EuII (a) pre-injection; (b) 3 min, (c) 20 min, and (d) 120 min post-intratumoral injection; (e) difference between the 120 min and pre-injection images (image d minus image a) colored using the ImageJ green lookup table; (f) hematoxylin- and eosin-stained slice of tumor imaged in a–e; and (g) sum of images e and f. All images are on the same scale. Imaging parameters included an echo time of 1.5 ms, repetition time of 11 ms, flip angle of 40°, field of view of 30 mm × 90 mm, and an in-plane resolution of 0.352 × 0.352 mm. Adapted with permission from Ekanger, L. A.; Polin, L. A.; Shen, Y.; Haacke, E. M.; Allen, M. J. Angew. Chem. Int. Ed. 2015, in press. Copyright 2015 John Wiley and Sons
The trends in stability that we observed with cryptates suggest that further stabilization of aqueous EuII and other lanthanide ions is possible.33 In pursuing this further stabilization, we synthesized an aza-cryptate of EuII and serendipitously discovered that it displayed bright yellow luminescence in aqueous solution (Figure 6).40 Solution-phase studies—including a Job plot, 17O-NMR spectroscopy, and conductivity measurements—indicated that a cause of the bright emission was the counter ion not being replaced by water, which quenches luminescence, in solution.40 Interestingly, this divalent project relates back to our initial interest in the luminescence of lanthanides.15,18,24,41
Figure 6.
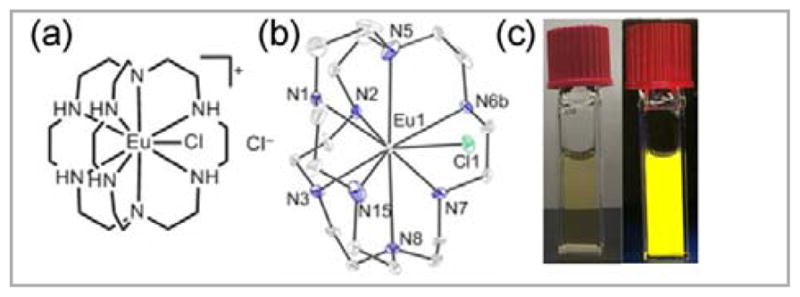
(a) EuII-containing aza-cryptate that has exciting bright yellow luminescence and (b) its crystal structure. Thermal ellipsoids are drawn at 50% probability. Hydrogen atoms, a non-coordinated Cl− counter ion, and a molecule of methanol are not shown for clarity. R-factor = 0.0543. Resolution = 0.54 Å. (c) Pictures of the complex (5.0 mM) in 4 mL cuvettes in a pH 12.0 solution of KOH (left) in ambient light and (right) under a hand held UV lamp (254–400 nm). The 26% quantum yield in aqueous solution is the highest reported value for EuII, with previous values below 1%. Adapted with permission from Kuda-Wedagedara, A. N. W.; Wang, C.; Martin, P. D.; Allen, M. J. J. Am. Chem. Soc. 2015, 4960. Copyright 2015 American Chemical Society
6 Conclusions
Studying the aqueous chemistry of the lanthanides is an exciting and challenging area of research. My research group has been fortunate to have had the opportunity to make interesting discoveries that have blossomed from careful observations and a great deal of effort. Application of the wealth of knowledge reported from the pursuit of contrast agents to catalysis has been a particularly fruitful path for us. I anticipate that similar pursuits based in careful reading of literature that spans a diverse range of topics will lead to the challenging of dogma, provide intellectual stimulation, and lead to important discoveries in the future.
Acknowledgments
I thank my mentors for helping me appreciate a wide range of scientific topics and the importance of communication. I am extremely grateful for the opportunity to learn, explore, and discover new science with all of the talented researchers who have spent time in my laboratory. I am also thankful for the financial support that has made and continues to make our research possible, including generous support from Wayne State University; A. Paul and Carol Schaap; the Elsa U. Pardee Foundation; the American Foundation for Aging Research; the Michigan Initiative for Innovation & Entrepreneurship; Cambridge Isotope Laboratories, Inc.; the National Science Foundation (CAREER CHE-0955000); and the National Institutes of Health (R00EB007129 and R01EB013663).
Biography

Matthew J. Allen is an Associate Professor of chemistry at Wayne State University. After being inspired at Swartz Creek High School to study chemistry by the engaging teacher Phyllis Ziemer, Matt began his research career at Purdue University where he earned his B.S. in chemistry while researching the functionalization of porous silicon surfaces with Jillian Buriak. Throughout his undergraduate career he also had the great opportunity to be an intern at Eli Lilly and Company where he was trained by organic chemists including Tony Zhang and Richard Berglund. He earned his Ph.D. in chemistry from the California Institute of Technology under the mentorship of Thomas Meade in the area of cellular delivery of gadolinium complexes. Matt was an NIH postdoctoral fellow in the laboratories of Laura Kiessling and Ronald Raines at the University of Wisconsin–Madison before joining Wayne State as an Assistant Professor. His laboratory studies the aqueous chemistry of the lanthanides and related areas.
References
- 1.(a) Allen MJ, Meade TJ. J Biol Inorg Chem. 2003:746. doi: 10.1007/s00775-003-0475-2. [DOI] [PubMed] [Google Scholar]; (b) Allen MJ, Meade TJ. Met Ions Biol Syst. 2004:1. [PubMed] [Google Scholar]; (c) Allen MJ, MacRenaris KW, Venkatasubramanian PN, Meade TJ. Chem Biol. 2004;11:301. doi: 10.1016/j.chembiol.2004.03.003. [DOI] [PubMed] [Google Scholar]; (d) Endres PJ, MacRenaris KW, Vogt S, Allen MJ, Meade TJ. Mol Imaging. 2006:485. [PubMed] [Google Scholar]
- 2.(a) Allen MJ, Wangkanont K, Raines RT, Kiessling LL. Macromolecules. 2009:4023. doi: 10.1021/ma900056b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Allen MJ, Raines RT, Kiessling LL. J Am Chem Soc. 2006:6534. doi: 10.1021/ja061383p. [DOI] [PubMed] [Google Scholar]; (c) Pontrello JK, Allen MJ, Underbakke ES, Kiessling LL. J Am Chem Soc. 2005:14536. doi: 10.1021/ja053931p. [DOI] [PubMed] [Google Scholar]
- 3.(a) Bünzli JCG. Acc Chem Res. 2006:53. doi: 10.1021/ar0400894. [DOI] [PubMed] [Google Scholar]; (b) Evans WJ. Inorg Chem. 2007:3435. doi: 10.1021/ic062011k. [DOI] [PubMed] [Google Scholar]; (c) Sherry AD, Caravan P, Lenkinski RE. J Magn Reson Imaging. 2009:1240. doi: 10.1002/jmri.21966. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Kulyukhin SA. J Alloys Compd. 1998:168. [Google Scholar]
- 4.(a) Pearson RG. J Am Chem Soc. 1963:3533. [Google Scholar]; (b) Parr RG, Pearson RG. J Am Chem Soc. 1983:7512. [Google Scholar]; (c) Lang PF, Smith BC. J Chem Ed. 2010:875. [Google Scholar]
- 5.Supkowski RM, Horrocks WD., Jr Inorg Chem. 1999:5616. doi: 10.1021/ic990597n. [DOI] [PubMed] [Google Scholar]
- 6.(a) Bruce JI, Dickins RS, Govenlock LJ, Gunnlaugsson T, Lopinski S, Lowe MP, Parker D, Peacock RD, Perry JJB, Aime S, Botta M. J Am Chem Soc. 2000:9674. [Google Scholar]; (b) Charbonnière LJ, Ziessel R, Montalti M, Prodi L, Zaccheroni N, Boehme C, Wipff G. J Am Chem Soc. 2002:7779. doi: 10.1021/ja0200847. [DOI] [PubMed] [Google Scholar]; (c) Charbonnière LJ, Schurhammer R, Mameri S, Wipff G, Ziessel RF. Inorg Chem. 2005:7151. doi: 10.1021/ic051033o. [DOI] [PubMed] [Google Scholar]; (d) Hammell J, Buttarazzi L, Huang CH, Morrow JR. Inorg Chem. 2011:4857. doi: 10.1021/ic200075w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Butler SJ, Parker D. Chem Soc Rev. 2013:1652. doi: 10.1039/c2cs35144g. [DOI] [PubMed] [Google Scholar]; (f) Pershagen E, Borbas KE. Coord Chem Rev. 2014:30. [Google Scholar]
- 7.(a) Bogart JA, Lewis AJ, Boreen MA, Lee HB, Medling SA, Carroll PJ, Booth CH, Schelter EJ. Inorg Chem. 2015:2830. doi: 10.1021/ic503000z. [DOI] [PubMed] [Google Scholar]; (b) So YM, Wang GC, Li Y, Sung HHY, Williams ID, Lin Z, Leung WH. Angew Chem Int Ed. 2014:1626. doi: 10.1002/anie.201309764. [DOI] [PubMed] [Google Scholar]; (c) Meyer G. Angew Chem Int Ed. 2014:3550. doi: 10.1002/anie.201311325. [DOI] [PubMed] [Google Scholar]; (d) Jewula P, Berthet JC, Chambron JC, Rousselin Y, Thuéry P, Meyer M. Eur J Inorg Chem. 2015:1529. [Google Scholar]; (e) Han D, Uda T, Nose Y, Okajima T, Murata H, Tanaka I, Shinoda K. Adv Mater. 2012:2051. doi: 10.1002/adma.201200127. [DOI] [PubMed] [Google Scholar]; (f) MacDonald MR, Bates JE, Ziller JW, Furche F, Evans WJ. J Am Chem Soc. 2013:9857. doi: 10.1021/ja403753j. [DOI] [PubMed] [Google Scholar]
- 8.(a) Yin H, Carroll PJ, Anna JM, Schelter EJ. J Am Chem Soc. 2015:9234. doi: 10.1021/jacs.5b05411. [DOI] [PubMed] [Google Scholar]; (b) Ogawa A, Sumino Y, Nanke T, Ohya S, Sonoda N, Hirao T. J Am Chem Soc. 1997:2745. [Google Scholar]; (c) Amiel-Levy M, Hoz S. Chem Eur J. 2010:805. doi: 10.1002/chem.200902198. [DOI] [PubMed] [Google Scholar]; (d) Prasad E, Knettle BW, Flowers RA., II Chem Eur J. 2005:3105. doi: 10.1002/chem.200401163. [DOI] [PubMed] [Google Scholar]
- 9.(a) Fieser ME, MacDonald MR, Krull BT, Bates JE, Ziller JW, Furche F, Evans WJ. J Am Chem Soc. 2015:369. doi: 10.1021/ja510831n. [DOI] [PubMed] [Google Scholar]; (b) Bartlett PN, Champion MJD, Light ME, Levason W, Reid G, Richardson PW. Dalton Trans. 2015:2953. doi: 10.1039/c4dt03462g. [DOI] [PubMed] [Google Scholar]; (c) Garcia J, Allen MJ. Eur J Inorg Chem. 2012:4550. doi: 10.1002/ejic.201200159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Ruiz-Martínez A, Casanova D, Alvarez S. Chem Eur J. 2008:1291. doi: 10.1002/chem.200701137. [DOI] [PubMed] [Google Scholar]; (b) Cassanova D, Llunell M, Alemany P, Alvarez S. Chem Eur J. 2005:1479. doi: 10.1002/chem.200400799. [DOI] [PubMed] [Google Scholar]; (c) Burdett JK, Hoffmann R, Fay RC. Inorg Chem. 1978:2553. [Google Scholar]
- 11.Averill DJ, Garcia J, Siriwardena-Mahanama BN, Vithanarachchi SM, Allen MJ. J Vis Exp. 2011:e2844. doi: 10.3791/2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Renny JS, Tomasevich LL, Tallmadge EH, Collum DB. Angew Chem Int Ed. 2013:11998. doi: 10.1002/anie.201304157. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cossy C, Helm L, Merbach AE. Inorg Chem. 1988:1973. [Google Scholar]; (c) Urbanczyk-Pearson LM, Femia FJ, Smith J, Parigi G, Duimstra JA, Eckermann AL, Luchinat C, Meade TJ. Inorg Chem. 2008:56. doi: 10.1021/ic700888w. [DOI] [PubMed] [Google Scholar]; (d) Djanashvili D, Peters JA. Contrast Media Mol Imaging. 2007:67. doi: 10.1002/cmmi.132. [DOI] [PubMed] [Google Scholar]; (e) Averill DJ, Allen MJ. Catal Sci Technol. 2014:4129. [Google Scholar]; (f) Geary WJ. Coord Chem Rev. 1971:81. [Google Scholar]; (g) Southwood-Jones RV, Earl WL, Newman KE, Merbach AE. J Chem Phys. 1980:5909. [Google Scholar]; (h) Buccigross JM, Nelson DJ. Biochem Biophys Res Commun. 1986:1243. doi: 10.1016/s0006-291x(86)80416-8. [DOI] [PubMed] [Google Scholar]; (i) Burai L, Tóth É, Moreau G, Sour A, Scopelliti R, Merbach AE. Chem Eur J. 2003:1394. doi: 10.1002/chem.200390159. [DOI] [PubMed] [Google Scholar]
- 13.(a) Lin Z, Allen MJ. Dyes Pigm. 2014:261. [Google Scholar]; (b) Kropp JL, Windsor MW. J Chem Phys. 1963:2769. [Google Scholar]; (c) Kropp JL, Windsor MW. J Chem Phys. 1965:1599. [Google Scholar]; (d) Horrocks WD, Jr, Sudnick DR. J Am Chem Soc. 1979:334. [Google Scholar]; (e) Beeby A, Clarkson IM, Dickins RS, Faulkner S, Parker D, Royle L, de Sousa AS, Williams JAG, Woods M. J Chem Soc Perkin Trans. 1999;2:493. [Google Scholar]; (f) Supkowski RM, Horrocks WD., Jr Inorg Chim Acta. 2002:44. [Google Scholar]; (g) Kimura T, Kato Y. J Alloys Compd. 1995:284. [Google Scholar]; (h) Kimura T, Kato Y. J Alloys Compd. 1998:867. [Google Scholar]; (i) Barthelemy PP, Choppin GR. Inorg Chem. 1989:3354. [Google Scholar]
- 14.(a) Hamada T, Manabe K, Ishikawa S, Nagayama S, Shiro M, Kobayashi S. J Am Chem Soc. 2003:2989. doi: 10.1021/ja028698z. [DOI] [PubMed] [Google Scholar]; (b) Kobayashi S, Hachiya I. J Org Chem. 1994:3590. [Google Scholar]; (c) Ishitani H, Kobayashi S. Tetrahedron Lett. 1996:7357. [Google Scholar]; (d) Sasai H, Suzuki T, Itoh N, Tanaka K, Date T, Okamura K, Shibasaki M. J Am Chem Soc. 1993:10372. [Google Scholar]; (e) Satoh K, Kamigaito M, Sawamoto M. Macromolecules. 2000:4660. [Google Scholar]; (f) Yu L, Li J, Ramirez J, Chen D, Wang PG. J Org Chem. 1997:903. doi: 10.1021/jo961175n. [DOI] [PubMed] [Google Scholar]; (g) Dzudza A, Marks TJ. J Org Chem. 2008:4004. doi: 10.1021/jo800158k. [DOI] [PubMed] [Google Scholar]; (h) Li HJ, Tian HY, Wu YC, Chen YJ, Liu L, Wang D, Li CJ. Adv Synth Catal. 2005:1247. [Google Scholar]
- 15.Dissanayake P, Allen MJ. J Am Chem Soc. 2009:6342. doi: 10.1021/ja900630d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mei Y, Dissanayake P, Allen MJ. J Am Chem Soc. 2010:12871. doi: 10.1021/ja107197p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Averill DJ, Dissanayake P, Allen MJ. Molecules. 2012:2073. doi: 10.3390/molecules17022073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dissanayake P, Mei Y, Allen MJ. ACS Catal. 2011:1203. [Google Scholar]
- 19. [accessed October 4, 2015];Allen Research Group Home Page. http://chem.wayne.edu/allengroup/teaching.html.
- 20.(a) Kobayashi S, Hamada T, Nagayama S, Manabe K. Org Lett. 2001:165. doi: 10.1021/ol006830z. [DOI] [PubMed] [Google Scholar]; (b) Hamada T, Manabe K, Ishikawa S, Nagayama S, Shiro M, Kobayashi S. J Am Chem Soc. 2003:2989. doi: 10.1021/ja028698z. [DOI] [PubMed] [Google Scholar]
- 21.Ollevier T, Plancq B. Chem Commun. 2012:2289. doi: 10.1039/c1cc16409k. [DOI] [PubMed] [Google Scholar]
- 22.Mei Y, Averill DJ, Allen MJ. J Org Chem. 2012:5624. doi: 10.1021/jo300800b. [DOI] [PubMed] [Google Scholar]
- 23.(a) Hatanaka M, Morokuma K. J Am Chem Soc. 2013;135:13972. doi: 10.1021/ja407357c. [DOI] [PubMed] [Google Scholar]; (b) Hatanaka M, Morokuma K. ACS Catal. 2015;5:3731. [Google Scholar]
- 24.Averill DJ, Allen MJ. Inorg Chem. 2014:6257. doi: 10.1021/ic500790q. [DOI] [PubMed] [Google Scholar]
- 25.Hopper LE, Allen MJ. Tetrahedron Lett. 2014:5560. doi: 10.1016/j.tetlet.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin Z, Shelby ML, Hayes D, Fransted KA, Chen LX, Allen MJ. Dalton Trans. 2014:16156. doi: 10.1039/c4dt02492c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.(a) Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Chem Rev. 1999:2293. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]; (b) Ali MM, Liu G, Shah T, Flask CA, Pagel MD. Acc Chem Res. 2009:915. doi: 10.1021/ar8002738. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) De Leon-Rodriguez LM, Lubag AJM, Malloy CR, Martinez GV, Gillies RJ, Sherry AD. Acc Chem Res. 2009:948. doi: 10.1021/ar800237f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Que EL, Chang CJ. Chem Soc Rev. 2010:51. doi: 10.1039/b914348n. [DOI] [PubMed] [Google Scholar]; (e) Tu C, Louie AY. NMR Biomed. 2013:781. doi: 10.1002/nbm.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Heffern MC, Matosziuk LM, Meade TJ. Chem Rev. 2014:4496. doi: 10.1021/cr400477t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Tsitovich PB, Burns PJ, McKay AM, Morrow JR. J Inorg Biochem. 2014:143. doi: 10.1016/j.jinorgbio.2014.01.016. [DOI] [PubMed] [Google Scholar]; (h) Aime S, Botta M, Fasano M, Terreno E. Chem Soc Rev. 1998:19. [Google Scholar]; (i) Hermann P, Kotek J, Kubíćek V, Lukeš I. Dalton Trans. 2008:3027. doi: 10.1039/b719704g. [DOI] [PubMed] [Google Scholar]; (j) Ekanger LA, Allen MJ. Metallomics. 2015:405. doi: 10.1039/c4mt00289j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Pierre VC, Allen MJ, Caravan P. J Biol Inorg Chem. 2014:127. doi: 10.1007/s00775-013-1074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Siriwardena-Mahanama BN, Allen MJ. Molecules. 2013:9352. doi: 10.3390/molecules18089352. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Vithanarachchi SM, Allen MJ. Curr Mol Imaging. 2012:12. doi: 10.2174/2211555211201010012. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Moore JD, Allen MJ. Recent Pat Nanomed. 2011:88. doi: 10.2174/1877912311101020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.(a) Vithanarachchi SM, Allen MJ. Chem Commun. 2013:4148. doi: 10.1039/c2cc36583a. [DOI] [PubMed] [Google Scholar]; (b) Siriwardena-Mahanama BN, Allen MJ. Dalton Trans. 2013:6724. doi: 10.1039/c3dt50885d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wu X, Dawsey AC, Siriwardena-Mahanama BN, Allen MJ, Williams TJ. J Fluorine Chem. 2014:177. doi: 10.1016/j.jfluchem.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Kochi A, Lee HJ, Vithanarachchi SM, Padmini V, Allen MJ, Lim MH. Curr Alzheimer Res. 2015:415. doi: 10.2174/1567205012666150504150125. [DOI] [PubMed] [Google Scholar]; (e) Lutomski CA, El-Baba TJ, Fischer JL, Siriwardena-Mahanama BN, Weidner M, Falkenhagen J, Allen MJ, Trimpin S. J Am Soc Mass Spectrom. 2015 doi: 10.1007/s13361-015-1233-8. in press. [DOI] [PubMed] [Google Scholar]
- 29.(a) Caravan P, Tóth É, Rockenbauer A, Merbach AE. J Am Chem Soc. 1999:10403. [Google Scholar]; (b) Kuda-Wedagedara ANW, Allen MJ. Analyst. 2014:4401. doi: 10.1039/c4an00990h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.(a) Gansow OA, Kausar AR, Triplett KM, Weaver MJ, Yee EL. J Am Chem Soc. 1977:7087. [Google Scholar]; (b) Yee EL, Gansow OA, Weaver MJ. J Am Chem Soc. 1980:2278. [Google Scholar]
- 31.(a) Tóth É, Burai L, Merbach AE. Coord Chem Rev. 2001:363. [Google Scholar]; (b) Burai L, Tóth É, Seibig S, Scopelliti R, Merbach AE. Chem Eur J. 2000:3761. doi: 10.1002/1521-3765(20001016)6:20<3761::aid-chem3761>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 32.Burai L, Scopelliti R, Tóth É. Chem Commun. 2002:2366. doi: 10.1039/b206709a. [DOI] [PubMed] [Google Scholar]
- 33.Gamage N-DH, Mei Y, Garcia J, Allen MJ. Angew Chem, Int Ed. 2010:8923. doi: 10.1002/anie.201002789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia J, Kuda-Wedagedara ANW, Allen MJ. Eur J Inorg Chem. 2012:2135. doi: 10.1002/ejic.201101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia J, Neelavalli J, Haacke EM, Allen MJ. Chem Commun. 2011:12858. doi: 10.1039/c1cc15219j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ekanger LA, Ali MM, Allen MJ. Chem Commun. 2014:14835. doi: 10.1039/c4cc07027e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia J, Allen MJ. Inorg Chim Acta. 2012:324. doi: 10.1016/j.ica.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.(a) Blow N. Nature. 2009:925. doi: 10.1038/458925a. [DOI] [PubMed] [Google Scholar]; (b) Fujii Y, Nakada T. Neurol Med Chir. 2010:833. doi: 10.2176/nmc.50.833. [DOI] [PubMed] [Google Scholar]; (c) Bandettini PA, Bowtell R, Jezzard P, Turner R. Magn Reson Med. 2012:317. doi: 10.1002/mrm.23151. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Moser E. World J Radiol. 2010:37. doi: 10.4329/wjr.v2.i1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ekanger LA, Polin LA, Shen Y, Haacke EM, Allen MJ. Angew Chem Int Ed. 2015 doi: 10.1002/anie.201507227. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuda-Wedagedara ANW, Wang C, Martin PD, Allen MJ. J Am Chem Soc. 2015:4960. doi: 10.1021/jacs.5b02506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore JD, Lord RL, Cisneros GA, Allen MJ. J Am Chem Soc. 2012:17372. doi: 10.1021/ja307098z. [DOI] [PMC free article] [PubMed] [Google Scholar]