Abstract
The heart is a very special organ in the body and has a high requirement for metabolism due to its constant workload. As a consequence, to provide a consistent and sufficient energy a high steady-state demand of metabolism is required by the heart. When delicately balanced mechanisms are changed by physiological or pathophysiological conditions, the whole system’s homeostasis will be altered to a new balance, which contributes to the pathologic process. So it is no wonder that almost every heart disease is related to metabolic shift. Furthermore, aging is also found to be related to the reduction in mitochondrial function, insulin resistance, and dysregulated intracellular lipid metabolism. Adenosine monophosphate-activated protein kinase (AMPK) functions as an energy sensor to detect intracellular ATP/AMP ratio and plays a pivotal role in intracellular adaptation to energy stress. During different pathology (like myocardial ischemia and hypertension), the activation of cardiac AMPK appears to be essential for repairing cardiomyocyte’s function by accelerating ATP generation, attenuating ATP depletion, and protecting the myocardium against cardiac dysfunction and apoptosis. In this overview, we will talk about the normal heart’s metabolism, how metabolic shifts during aging and different pathologies, and how AMPK regulates metabolic changes during these conditions.
Introduction
The heart has a high requirement for metabolism due to its constant workload, which is different from other organs in the body. As a consequence, a high-steady demand of metabolism is required by the heart to provide consistent and sufficient energy. In the normal heart, fatty acids provide a major energy supply accounting for 50%–75% (92), whereas glucose oxidation and glycolysis occupy the minor adenosine triphosphate (ATP) production, which is opposite in other muscle cells. Under normal conditions, glucose metabolism and fatty acid metabolism work together to provide ATP; however, during physiological (like aging) and pathophysiological (like chronic ischemia, hypertension, and diabetes) conditions, metabolism between fatty acids and glucose can be dramatically changed to stabilize energy. This metabolic shift has been termed “metabolic remodeling”, which involves the regulation of fatty acids and glucose’s uptake, storage, and oxidation and also expression of genes that encode enzymes which are involved in these regulations (149). Not only physiological or pathological change can alert metabolism: metabolic changes can also lead to pathological changes. Metabolic syndrome is a series of metabolic risk factors and syndromes including hypertension, insulin resistance, and abnormal cholesterol, which may increase the risk of heart disease. Aging is also found to be related to reduction in mitochondrial function, insulin resistance, and deregulated intracellular lipid metabolism.
AMPK’s function as an energy sensor to detect intracellular ATP/AMP ratio stands out as the main regulator of cardiac metabolism, especially during low energy states. There are hundreds of AMPK functions that have been discovered and are still being explored. The main purpose of activated AMPK under energy stress is to accelerate ATP generation (4, 106) and attenuate ATP depletion by increasing glucose transporter type 4 (GLUT-4) gene expression, GLUT-4 translocation, glycolysis (98) and fatty acids oxidation (78), accelerating glucose and fatty acid uptake (130), and inhibiting glycogen and protein synthesis. Since AMPK plays an important role in metabolism regulation, how it regulates metabolism in different physiological and pathophysiological conditions needs to be discussed. For example, AMPK was found to decrease in an aging heart, which may lead to reduced mitochondrial function and dysregulated lipid metabolism. Another example is during hypoxia/ischemia condition; it is well known that AMPK activity is dramatically increased and several downstream regulations are being triggered. Studies also showed that, in mice hearts, activated AMPK was found during pressure overload-induced hypertrophy (155).
In this overview article, the metabolism in physiological and pathophysiological conditions will be discussed, and how the heart’s metabolism adapts to the new workload. AMPK’s role in different conditions will also be revealed.
General Aspects of Metabolism in the Heart
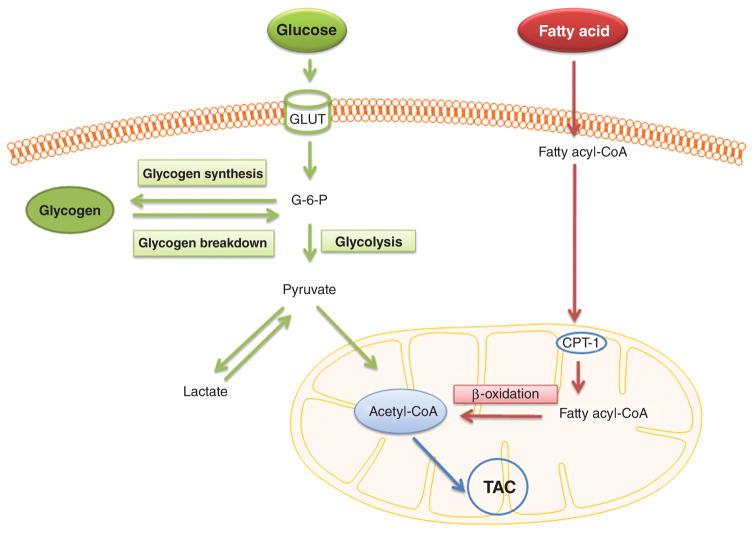
Glucose oxidation, glycolysis and fatty acids oxidation are the main resources of ATP generation for the heart. Glucose in the blood is maintained within a very narrow range, which lead glucose to be a more reliable substrate for heart’s energy production. However, the heart’s preference of uptake fuels partly depends on the energy demand and the fuels’ concentration in the arterial. In a normal heart, fatty acids have been recognized as the preferred ATP resource by both in vivo and in vitro experiments (114). In the ischemia/hypoxia heart induced by coronary artery disease or exercise, oxidation metabolism decreases and glycolysis is stimulated due to the decreased oxygen and nutrient supply. Glycolysis (breakdown of glucose without oxygen demand), although it provides only limited ATP, is a critical energy resource during anaerobic respiration. However, during severe ischemia, the levels of glucose and oxygen decrease as well as glycolysis. Here, the normal heart’s carbohydrate and fatty acid utilization is described to provide a general aspect of metabolism in the heart. A schematic diagram illustrating the metabolism of glucose and fatty acid in cardiomyocyte is shown in Figure 1.
Figure 1.
Schematic diagram illustrates the metabolism of glucose and fatty acid in cardiomyocyte. G-6-P, glucose 6-phosphorylase; CPT-1, carnitine palmitoyl transferae I; TAC, tricarboxylic acid cycle.
Carbohydrate utilization
Glucose is an abundant and important fuel, which is up-took and used by nearly all the organisms by a common processes of metabolic pathways. Glucose for cardiomyocytes is derived from either the bloodstream or intracellular storage of glycogen. In the normal adult heart, fatty acid oxidation contributes to the majority of ATP generation. However, cardiomyocytes still present high and flexible metabolic ability by utilizing glucose, lactate, ketones, and amino acids. This is the reason why the heart can be viewed as an omnivore. There is a broad energy resource in a normal heart; however, glucose contributes as the main fuel for the energy production in the fetal heart or under pathology conditions such as hypertrophy and ischemic heart.
Glycogen synthesis and breakdown
Glycogen is a polysaccharide, and serves as a stored form of carbohydrates. Instead of steady storage, glycogen molecules are in a constant variation of synthesis and degradation. However, these two processes do not happen as a single reversible reaction; indeed, they are occurring at two different pathways and catalyzed by different enzymes. The key enzymes of glycogen synthesis (glycogen synthase) and glycogen breakdown (phosphorylase) are regulated by sympathetic stimulation and the level of energy phosphate as well as metabolic condition changes (47).
Glycogen synthesis occurs at a high rate after a meal and under the influence of insulin, which could stimulate both glucose uptake and glycogen synthesis. After intense heart work or ischemia, glycogen synthesis is also triggered. Furthermore, a low level of glycogen stimulates the synthesis of glycogen, as well. Thus, during fasting, although insulin levels are low, glycogen can still be synthesized. On the other hand, glycogen synthesis is inhibited by the stimulation of the sympathetic nervous system, which activates a cyclic adenosine monophosphate (cAMP) dependent protein kinase. Glycogen synthesis begins with the conversion of glucose 6-phosphate to glucose 1-phosphate by phosphoglucomutase. Then glucose 1-phosphate is polymerized to form glycogen in a two-step reaction: (1) glucose 1-phosphate catalyzed by glucose 1-phosphate uridylyltransferase to form uridine diphosphoglucose. The energy used in this step is from the conversion of uridine triphosphate to uridine diphosphoglucose; (2) releases uridine diphosphate by adding glycogen polymer. The latter step is rate limiting, and glycogen synthase is the key enzyme in these steps.
Glycogen breakdown occurs under two major conditions: the activation of phosphorylase by cAMP and during ischemia. The increased cAMP converts inactivate enzyme phosphorylase b to activate enzyme phosphorylase a by a set of processes. During ischemia, due to the lower energy source influx into the cardiomyocyte, glycogen breakdown into carbohydrate is stimulated. During glycogen breakdown, glycogen is catalyzed by phosphorylase to release glucose 6-phosphorylase. The key enzyme here is phosphorylase a, which is the active form of this enzyme. Phosphorylase b, the inactive form of enzyme, is inhibited by ATP and glucose 6-phosphate and activated by inorganic phosphate (Pi).
Glycolysis
Glycolysis is the process of one mole 6-carbon sugar-glycose, broken down to form two moles of a three-carbon product-pyruvate. Glycolysis, especially, is the metabolic pathway in response to lack of oxygen. Under aerobic conditions, pyruvate is then oxidized in the citrate cycle, whereas, under anaerobic conditions, it is converted to lactate. Thus, this is an important process to produce ATP during oxygen-lacking conditions. Upon the uptake of glucose intracellularly, it is immediately converted by the unidirectional enzyme hexokinase to glucose 6-phosphate, which can be used either for glycogen synthesis or glycolysis. Glycolysis first converts glucose 6-phosphate into fructose 6-phosphase and further to fructose 1, 6-bisphosphate by the enzyme phosphofructokinase (PFK). PFK activity has been proven to be regulated by insulin or increased heart work (62). Thereafter, the 6-carbon hexose biphosphate breaks down into two three-carbon triose phosphates, and then further forms two molecules of pyruvate and four moles of ATP, which is independent of oxygen supply. Two moles of ATP are consumed based on the step transforming glucose to fructose 1, 6-bisphosphate.
In addition to the obvious function to provide ATP in anaerobic heart, glycolysis is also involved in ATP production in an aerobic heart to maintain normal ATP-requiring membrane functions including the sodium pump and the ATP-sensitive potassium channels (164). Glycolysis is also reported to sustain maximal heart work and to promote diastolic relaxation, especially during ischemia (6).
Pyruvate metabolism
Glycolysis-produced pyruvate can be further metabolized by choosing between oxidization to acetyl coenzyme A (acetyl-CoA) or reduction to form lactate. Which pathway to choose is determined by the activities of two enzymes in charge of these two pathways: pyruvate dehydrogenase (PDH), which catalyzes the conversion of pyruvate to acetyl-CoA; and lactate dehydrogenase (LDH), which catalyzes the conversion of pyruvate to lactate.
PDH activity is regulated by two enzymes. Activity is increased after dephosphorylation by PDH phosphatase and is decreased by phosphorylation by PDH kinase. In an oxygen-lacking heart, the activity of PDH kinase increases, followed by inactivation of PDH activity, and decreased pyruvate converts to acetyl-CoA, leading to increased lactate production. Furthermore, lactate can also be controlled to increase the products of the PDH reaction and to decrease by the decreased pyruvate, CoA, and NAD+ levels.
Glucose transport
The uptake of glucose in cardiomyocyte is controlled by the glucose transporters GLUT-1 and GLUT-4. There is no energy consumption when glucose enters into the cardiomyocyte, due to the high concentration of glucose extracellular. The glucose uptake rate is stimulated whenever the glucose transporter is regulated, such as during ischemia or after a meal or exercise. On the other hand, the glucose uptake rate is inhibited when blood fatty acid levels increase, in the fasting state or severe diabetes mellitus. GLUT-1 and GLUT-4 are the main glucose transporters in the heart. GLUT-1 is in charge of insulin-independent glucose transport, whereas GLUT-4 mediates insulin-sensitive glucose transport. Insulin has been recognized as a main regulator of the glucose receptor, especially in its translocation from the cytoplasm to the cell surface. Enhanced glucose uptake contributes to increased glycolysis and the myocardial respiration brought by glucose.
Fatty acid utilization
There are several types of fatty acids which can be used in the heart, such as palmitic, oleic, and linoleic. Among these, the main fatty acid used in the human heart is oleic acid. As we described, fatty acids are the preferred energy source, and this is mainly due to its concentrated energy supply. Compared with carbohydrates and proteins, which can only yield 5 calories/g, fatty acid is able to yield 9 calories/g. Fatty acids are transported into the heart in the form of glycerol esters, such as triacylglycerols or triglycerides, and in the form of free fatty acids (FFAs). Triacylglycerols will not enter the cells until it is being hydrolyzed by an extracellular enzyme-lipoprotein lipase, which is located in the luminal surface of capillary endothelium.
Fatty acid uptake and transfer into mitochondrial matrix
Fatty acid uptake is a passive transport, which is determined by the law of mass action. Because of this, the main factors that determine fatty acid uptake are the concentration of plasma-free fatty acids and the concentration of fatty acids in the cytoplasm. Fatty acid transport proteins and fatty acid translocases facilitate the uptake of fatty acids across the capillary endothelium and cardiac plasma membrane (158).
Before fatty acids get into beta-oxidation (β-oxidation), a series of reactions occur to convert long-chain fatty acid to fatty acyl coenzyme A (acyl-CoA), which is the active form of fatty acid. This reaction is catalyzed by acyl-CoA synthase, located on the outer mitochondrial membrane, and is ATP consuming. β-oxidation occurs inside mitochondria; however, the inner membrane of mitochondria is impermeable to fatty acyl-CoA. Thus, two enzymes, carnitine acyl-transferase I (CPT-I) and carnitine acyl-transferase II (CPT-II), located on the mitochondrial outer membrane and the mitochondrial inner membrane, respectively, facilitate fatty acids’ transport into mitochondria. During this process, CPT-I uses carnitine to replace CoA, and so the substrate changes from fatty acyl-CoA to fatty acyl-carnitine. CPT-II exerts the opposite function to replace carnitine back to CoA, which constructs fatty acyl-CoA, again, in the mitochondrial matrix. β-oxidation is a fast process in the normal heart, so the rate-limiting step of fatty acid metabolism is fatty acid transfer into mitochondria. Several factors determine this traversing. Malonyl-CoA has been proved to be an effective CPT-I inhibitor, which is regulated by acetyl-CoA carboxylase (ACC). ACC has been proven to be a downstream protein of AMP-activated protein kinase (AMPK), an intracellular energy sensor (see below); therefore, fatty acid transport is modulated by the dynamic change of intracellular energy. The concentrations of reactants on each side of the inner membrane can also regulate fatty acid’s transference.
β-oxidation
β-oxidation is the process to break down a long-chain acyl-CoA into several two-carbon unit acetyl-CoA. Most long-chain fatty acids contain 16 or 18 carbon atoms. In the procedure of β-oxidation, the oxidation continuously cuts acetyl-CoA from the carboxyl end (-COOH) of the chain through a series of reactions including oxidation, hydration, and thiolysis. The oxidation steps reduce the oxidized form of the nicotinamide adenine dinucleotide coenzyme (NAD+) and flavin adenine dinucleotide (FAD) to form the reduced form of the nicotinamide adenine dinucleotide coenzyme (NADH) and the reduced form of the flavin adenine dinucleotide (FADH2), respectively. During β-oxidation, there is no ATP generation, but the electrons transferred to NADH and FADH2 can contribute to facilitate oxidative phosphorylation.
The citric acid cycle
Both β-oxidation and pyruvate oxidation form acetyl-CoA, which is further oxidized within the mitochondrial matrix by the citric acid cycle. The citric acid cycle, also known as the tricarboxylic acid cycle (TCA cycle) or the Krebs Cycle, is a series of reactions from one mole acetyl-CoA, reduces three moles NAD+ to NADH, one mole FAD to FADH2, and generates two moles carbon dioxide and one mole guanosine-5′-triphosphate (GTP) by substrate-level phosphorylation. The large amounts of ATP are regenerated by oxidizing NADH and FADH2 in the respiratory chain. The details regarding this will not be described here.
Summary
As the two main energy resource in the heart, glucose utilization and fatty acid utilization were described in this part. As we mentioned, fatty acids is the major energy supply to the heart, which occupied 50%–75% (92) of energy. However, glucose oxidation and glycolysis are also playing and important role during different pathophysiological conditions. By understanding how these metabolisms working will help us better understand how they switch between each other in different conditions. In the rest of this article, aging and pathology-affected metabolism will be described.
Adenosine Monophosphate-Activated Protein Kinase
The AMP-activated AMPK activity was first documented in 1973 as a negative regulator of CoA and 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGR) in their biosynthesis of fatty acids and cholesterol, but it was not officially named until 1988 (171). Before 1988, AMPK had appeared in several different studies and reports, but with different aliases, such as HMG-CoA reductase kinase or acetyl-CoA carboxylase kinase. This name was chosen due to one of its physiological activators — AMP. AMPK is a key sensor of cellular energy status, and appears to exist in essentially all eukaryotes. AMPK is expressed in different tissues, such as the liver, brain, and muscle, and there is tissue-specific expression of different AMPK isoforms.
Studies suggested that when a cell is energetically stressed, the increased AMP concentration and decreased ATP concentration causes AMP/ATP ratio change, resulting in an activation of AMPK. Many studies in mammals have shown that AMPK is involved in all levels of energy regulation, from the intracellular level, such as gene transcription, protein synthesis, energy resource metabolism, and mitochondrial biogenesis, to the whole body level, such as food intake (54). It is known that activated AMPKs have several protective functions under energy stress in the heart, such as regulation of glycolysis, glycogen synthesis, glucose oxidation, fatty acid oxidation, protein synthesis, and autophagy. Because of its constant workload, the heart has high requirements for metabolism. Two primary fuels of cardiomyocytes — fatty acids and glucose — are both regulated by AMPK. That is why when talking about metabolism shift in the heart, it is impossible not to mention AMPK. In this part, we will describe the structure, function, and activation of AMPK.
Structure of AMP-activated protein kinase
Gene-encoding AMPK subunits exist universally in essentially all eukaryotes (57). AMPK is a heterotrimeric complex composed of a catalytic α subunit and regulatory β and γ subunits. These three subunits also possess multiple isoforms encoded by distinct genes (α1, α2; β1, β2; γ1, γ2, γ3) in mammals, and up to 12 heterotrimeric combinations can be assembled by those subunits. Specifically, in cardiac muscles, the α2 isoform is predominantly expressed, instead of α1 (85). Each subunit plays a specific role in either AMPK’s activation or stability.
The α and γ subunit are in charge of the activation of AMPK though different mechanisms. The α subunit contains a conserved threonine residue-targeting kinase domain, which is a target for AMPK upstream kinases (AMPKK) that are located within the activation loop, such as liver kinase B1 (LKB1) and Ca2+-activated calmodulin-dependent kinase kinases (CaMKKs). Phosphorylation of Thr172 at the N-terminus of the α subunits is required for AMPKK to activate AMPK in all species from yeast to man, and with the human kinase, causes over 100-fold activation (10, 74).
The regulatory γ subunits have a binding site for both ATP and AMP. Due to its special structure, the binding of AMP and ATP occurs in a mutually exclusive manner. The structure of regulatory γ subunits shows high binding ability, connecting with electrostatic interactions, such as His and Arg residues, and in accord with rearrangements in the interactions depending on whether AMP (activating) or ATP (inhibitory) is bound (74). Specifically, the γ subunit contains four potential nucleotide-binding sites: Cystathionine beta synthase (CBS) domains, of which sites 3 and 4 are recognized to be important for AMP stimulated AMPK allosteric. CBS domain provides AMPK with its ability to sensitively detect the change of AMP:ATP ratio. Because AMPK’s dual effect of both activation by AMP or inhibition by ATP, small changes of AMP levels can induce a dramatic change in AMPK activation (26). The key direct energy sources within the cell are ATP and ADP, which are interconverted by the reaction ATP ↔ ADP + phosphate. The high levels of adenylate kinase expressed in all eukaryotic cells are able to keep the reversible reaction 2ADP ↔ ATP + AMP close to equilibrium. Thus, AMP:ATP ratio showed a square higher variation than ADP:ATP ratio, which makes the AMP:ATP ratio a more sensitive sensor to the cellular energy change (56).
The clear function of β-subunits remains uncertain, but it may be related to supporting the localization of AMPK to some downstream targets located in the glycogen particle, such as glycogen synthase. This is due to the fact that β-subunits contain a carbohydrate-binding module (CBM), which can associate with glycogen particles (61, 123). Furthermore, the β-subunit forms a β-sheet structure, which binds together α and γ subunits. The N-terminal myristylation of the β-subunit is also involved in the phosphorylation of AMP and ADP. Although AMPK’s allosteric activation is only caused by AMP, several studies recently reveal that ADP is also involved in the effects on phosphorylation and dephosphorylation. Although ADP has a similar binding affinity with γ-subunits compared with AMP and ATP, as the formula 2ADP ↔ ATP + AMP shows, ADP is usually present at a higher concentrations than AMP. So, a possibility is that ADP could serve as the main activating signal to activate Thr172’s phosphorylation during energy stress condition. However, AMP’s function of allosteric activation could strengthen AMPK activation in higher levels during a more severe stress. Accordingly, this mechanism makes AMPK a more sensitive mechanism and creates a wider range over stress conditions.
AMP-activated protein kinase’s function in regulation of metabolism in the heart
AMPK as an energy sensor in the heart regulates energy utilization, as well. In general, AMPK activation is beneficial to the heart under energy stress conditions by switching on energy generation pathways and switching off energy consuming pathways to maintain energy homeostasis. AMPK shows its board and effective regulation by controlling different cardiac energy resources. As we described previously, Glucose oxidation, glycolysis and fatty acids oxidation are the main resources of ATP generation for the heart. Here we will talk about AMPK’s regulation of fatty acids utilization, glucose utilization, and protein synthesis. In each regulation, AMPK also showed its ability in regulation metabolism by controlling different levels, such as the enzymes’s activities, gene expression, and/or protein levels. But the main principle to keep in mind is that the purpose of activated AMPK under energy stress is to increase ATP generation and decrease ATP usage.
Fatty acid metabolism
Fatty acids are the main energy fuel in cardiomyocytes. AMPK showed the ability to modulate fatty acids utilization in several levels and processes, including fatty acids uptake, utilization, and transport.
As a well-known downstream target of AMPK, ACC is regarded as a regulator of fatty acid oxidation in heart tissue. As we discussed above, ACC is a key factor of controlling the speed of fatty acid oxidation, therefore, the regulation of ACC is a key issue. As the upstream kinase of ACC, AMPK could phosphorylate ACC and cause a significant decrease of ACC activity (32). Malonyl CoA produced by ACC is an inhibitor of carnitine palmitoyl transferae (CPT-I). CPT-I is the enzyme in charge of importing fatty acyl CoA into the mitochondria. The subsequent increased fatty acyl CoA levels will result in accelerated fatty acid oxidation (78, 79).
Lipoprotein lipase, which may mediate hydrolysis of circulating triglyceride, has been recognized to be the main source of fatty acid for cardiac utilization (7, 152). AMPK’s activation can also regulate cardiac lipoprotein lipase recruit to the coronary lumen, which will further increase fatty acid availability to the heart during increased workload (5).
Moreover, AMPK is also involved in the upregulation of fatty acid transport (95). One of the downstream targets of AMPK is fatty acid translocase (FAT)/CD36. CD36, a fatty acid transport protein, can translocate from intracellular compartments to the plasma membrane after stimulating by activated AMPK. The increased CD36 into cell surface allows the extra incoming fatty acid to be effectively channeled into mitochondrial β-oxidation. AMPK’s mediation also shows at the level of gene expression. In a liver study, AMPK could mediate the suppression of lipogenic gene expression by decreasing the activity of transcriptional factors sterol regulatory element binding protein 1 (SREBP-1) (38) and ChREBP to inhibit fatty acid synthesis. However, no specific study proved this regulation in the heart.
Therefore, AMPK activation could increase fatty acid availability, transport, and oxidation. In light of AMPK’s beneficial effects on fatty acid’s utilization under energy stress or normal condition, AMPK revealed its central mediator function.
Glucose metabolism
Similar to fatty acids, AMPK regulates glucose utilization in myocardium at several levels, such as glucose uptake (130), glycogen synthesis (17), glycolysis, and GLUT-4 translocation. Glucose transporter proteins (GLUTs) regulate glucose uptake in mammalian cells (70). GLUT-4, which facilitates extracellular glucose presented in the cell surface membranes to enter the intracellular, has been found to be stimulated by AMPK, and translocates to cell surface in an insulin-independent manner (130). As a well-known mechanism of insulin-regulating glucose uptake, tether containing a UBX domain, for GLUT-4 (TUG, 60KDa) serves as a regulator of GLUT-4 trafficking. After insulin stimulation or exercising, TUG releases GLUT-4 from the intracellular surface to the cell surface in skeletal muscle and adipocyte. At the same time, the energy sensor AMPK is known to play an important cardioprotective role during myocardial ischemia and reper-fusion (I/R) by regulating GLUT-4 translocation and glucose uptake. Accordingly, in our group, we hypothesized that TUG is one of the downstream targets of AMPK, which can be phosphorylated by hypoxia/ischemia-induced AMPK activation, accordingly accelerating TUG cleavage and increasing GLUT-4 translocation during ischemia/reperfusion injury in the heart. Our preliminary results showed that the ex vivo heart perfusion data demonstrated that by triggering AMPK activation, there is a significant increase in glucose uptake and GLUT-4 translocation during ischemia and reperfusion (p < 0.05) (Ma, Y., Li, J., et al., unpublished data). Intriguingly, preliminary data showed that this increased GLUT-4 translocation to cell surface is caused by dissociation between GLUT-4 and TUG (Ma, Y., Li, J., et al., unpublished data). Moreover, AMPK activation increased TUG cleavage (42KDa) in response to I/R (Ma, Y., Li, J., et al., unpublished data). All of these glucose transporter events are blunted in the AMPK kinase dead (KD) transgenic hearts. In conclusion, cardiac AMPK activation stimulates TUG cleavage and causes the dissociation between TUG and GLUT-4 in the intracellular vesicles. TUG is a critical mediator that modulates cardiac GLUT-4 translocation to cell surface and enhances glucose uptake by AMPK signaling pathway. Other than stimulating GLUT-4 translocation, AMPK also enhances the transcription factor myocyte enhancer factor-2 (MEF-2) to bind with promoters to increase GLUT-4 gene expression (45).
As described above, glycolysis is a critical energy source during anaerobic; and glycogen synthesis happens when the energy demands of the heart are fulfilled. Other than increasing glucose uptake and GLUT-4 translocation, AMPK could also enhance glycolysis during ischemia by activating 6-phosphofructo-2-kinase (PFK-2) at site Ser466 in vitro and intact cells (98). The AMPK-activated PFK-2 increases fructose 2 and 6-bisphosphate concentration, followed by enhanced PFK-1 activation and stimulated glycolysis (98). Furthermore, AMPK has been proven to inhibit glycogen synthase activity and is followed by decreased glycogen synthesis levels in vitro (17, 167). Both enhanced glycolysis and inhibited glycogen synthesis by AMPK aims to stay at ATP level.
Protein metabolism
Besides regulating glucose utilization and fatty acid utilization, another function of AMPK is to compensate for the reduced ATP level by shutting down non-urgent energy-consuming metabolic processes. In an anaerobic heart, the inhibition of protein synthesis is shown in the increased intracellular amino acids level, decreased ribosomal subunits, and increased polysomes compared with an aerobic heart (115). AMPK could either directly inhibit the key enzymes involved in protein synthesis, such as the tuberous sclerosis complex-2 (TSC2) (66), eukaryotic elongation factor-2 kinase (eEF2K) (14), or indirectly regulate several important molecules controlling protein synthesis, such as mammalian target of rapamycin (mTOR) and eukaryotic elongation factor-2 (eEF2) (13, 29). Although regulation of protein synthesis does not play a major role in AMPK’s energy regulation, decreased ATP usage does in fact contribute to cellular ATP conservation.
All in all, AMPK not only functions as a great monitor of energy change, but also regulates energy homeostasis in broad ways. The pathways we discussed above cannot be covered its entire metabolism regulating functions. Nowadays, increasing mechanisms of AMPK’s regulation function are still being researched. Along with AMPK’s metabolism effects, it also plays a role in regulating autophagy (151), mitochondrial biogenesis (16), cell growth (65), and apoptosis (138). Together, these functions support the concept that AMPK regulates metabolism in different pathways and levels.
Aging and Pathology Related Metabolic Shift
Aging
Alterations of metabolism due to senescence happen at different function levels. The aging heart shows affected mitochondrial respiratory functions, especially in oxidative phosphorylation enzymes and principally in the citric acid cycle (68). Aging causes a decline in mitochondrial oxidative metabolism. There is also an increase in free radical production by mitochondria in relation with aging (51, 118, 135). This oxygen radical generation is primarily due to the impairment of energy-linked respiration. The increased membrane damage induced by increased free radical in aging has also been observed (117).
Glucose utilization
Reports have shown that as the age increased, glucose transporters in rat heart muscle decreased (49). However, some other studies showed an opposite result, where the increased myocardial protein content for GLUT-4 indicated an increase in glucose uptake (80, 99, 120). Studies in humans did not clearly show increased myocardial utilization in the aged heart, but when comparing the decline of fatty acid oxidation and utilization, the relative level of glucose metabolism compares with fatty acid metabolism increased in aged hearts (73). It is well established that glucose tolerance becomes worse with aging (46, 168). It has been reported that in the aged heart, energy production from glycolysis was enhanced (81). Furthermore, the activity of PFK, which could estimate the glycolysis activity enzyme, was not significantly affected by age (69). However, LDH was decreased by 32% in the aged heart. LDH catalyzes the conversion of lactate to pyruvate, which is the substrate for mitochondrial metabolism; thus, the decreased LDH in the aging heart activity was correlated with the decreased mitochondrial enzyme activities (69).
PDH contributes to the transformation of pyruvate into acetyl-CoA by pyruvate decarboxylation. Accordingly, PDH is essential for connecting the glycolysis metabolic pathway to the citric acid cycle and releasing energy. Significantly lower PDH activities are found in old rat hearts compared with younger rats. PDH kinase catalyzes inactivation and phosphorylation of the PDH complex (87). However, PDH kinase was not associated with the age-induced decreasing PDH activity, since only a slight alteration of PDH kinase in aging rats was observed (111). Due to the function of the PDH complex, this decreased PDH complex activity may also be associated with decreased blood glucose disposal, increased blood glucose level, and insulin resistance related with aging. As the results showed, from Shimomura’s group, they did not observe any changes in the activity of 3-hydroxyacyl-CoA dehydrogenase — an enzyme involved in the β-oxidation in aged rat hearts. Furthermore, data showed the inactivation of the PDH complex was related to the increased circulating NEFA circulations (18, 40). In light of the decreased PDH complex activity, decreased blood glucose disposal, and increased serum NEFA concentration related with aging, they concluded that the decreased PDH complex may contribute to both inhibiting glucose utilization and keeping the fatty acid oxidation ability.
Fatty acid utilization
Increasing concentrations of serum non-esterified fatty acids (NEFA) were found in rat hearts in an age-related pattern (111). In relation to this, the cardiac fatty acid oxidation is decreased (21). The age-related decline of fatty acid oxidation was also observed in the normal human heart (73). The reason for this change might be due to the decreased CPT-I activity, which is the rate-limiting enzyme for mitochondrial long-chain fatty acid uptake (119). It has also shown decreased lipid fluidity is related to aging. Moreover, when exposed to exogenous iron and H2O2, cardiac mitochondria from elderly hearts showed higher lipid peroxidation and injury (25). Other studies reported that aging promotes lipid peroxidation within the inner mitochondrial membranes (135).
AMPK regulation
The sensitivity of AMPK activation was tested between groups of young and old rats. Results indicated that by activation of AMPK by either 5-aminoimidazole-4-carboxamide-1-β-D-ribonucleotide (AICAR) activating or by exercise, old mice showed blunted activation of AMPK compared with young rats. AMPK has been recognized as a regulator of mitochondrial biogenesis by regulating PGC-1α messenger RNA (mRNA) expression and cytochrome c protein expression levels, mitochondrial DNA content, and mitochondrial density (180). Furthermore, as a downstream effect of activated AMPK, mitochondrial biogenesis was also diminished in old rats (127). In that study, AMPK was stimulated by β-guanidinopropionic acid (β-GPA) feeding (127). The reduced mitochondrial oxidative-phosphorylation activity may further dysregulate lipid oxidation (122). In conclusion, age-related AMPK insensitivity causes reduced mitochondrial biogenesis function and dysregulated lipid metabolism.
Conclusion
There are many changes of other determinants that play various roles in cardiac metabolism. For example, plasma lactate levels are significantly higher in elderly hearts, and this change might be necessary to suppress myocardial fatty acid utilization (30, 137). Overall, myocardial metabolism has a tendency to shift from fatty acid oxidation to glucose oxidation in the aged heart, and this shift allows the heart to depend less on fatty acid metabolism. This shift may also support the myocardium more resistant to ischemia, which is a beneficial effect, especially because coronary artery disease increases with age. On the other hand, this shift may burden the heart in pressure overload-induced left ventricular hypertrophy and in dilated cardiomyopathy (73).
Pathology-related metabolic shift
Coronary heart disease
Rated the top leading cause of death by the World Health Organization, ischemic heart disease is caused by reduced coronary blood supply to the myocardium. The treatment of acute ischemia heart disease is mainly focused on returning blood flow to the ischemic area, through primary angioplasty, coronary artery bypass surgery, anticoagulants, and thrombolytics (37). Although increased blood flow aids in rapid restoration of energy, the irreversible cell damage caused by reperfusion, however, is the main risk of this therapy. The most dangerous risk of this therapy is due to the sudden increased fatty acid and oxygen into cardiomyocytes, which will rapidly enhance fatty acid oxidation and generate reactive oxygen species (ROS). Several pro-survival kinases like ERK, P13K/Akt, and GSK-3β, as an adjunct to reperfusion, have been proposed to be involved in transduction of the protective signals, called the reperfusion injury salvage kinase (RISK) pathway (58). As a consequence, a novel therapeutic strategy has emerged to limit myocardial ischemia/reperfusion injury by activating the RISK pathway.
Fatty acid and glucose utilization
Immediately after the ischemia begins, there will be an energy imbalance. During ischemia, due to the decline of oxygen and energy supply, both glucose oxidation and fatty acid oxidation are shut down. Whereas, during the first stage of ischemia, anaerobic glycolysis begins working to provide limited ATP and the heart switches from lactate uptake to lactate production (113). As the severity of ischemia increases, intracellular glucose and glycogen become depleted and inhibitory metabolites accumulate and inhibit glycolysis. The same process occurs for glucose uptake — in less severe ischemia, glucose uptake increases, but decreases as the duration of ischemia increases (145). As discovered by Young et al., when canine hearts are placed under a low-flow ischemia, GLUT-1 and GLUT-4, which are responsible for glucose transport in the heart, significantly increase the translocate to the cell surface (173). For glucose utilization regulation during ischemia, it is a multiple-site regulation, such as hexokinase activity, the rate of glucose 6-phosphate conversion to glycogen, the activity of phosphofructokinase and pyruvate utilization, and cytosol-derived NADH (93). On the other hand, the inhibited fatty acid oxidation due to mild or moderate ischemia still remains the primary source of acetyl CoA for Krebs cycle. During the recovery stage (reperfusion), fatty acid oxidation recovers immediately and occupies the main source of energy, which significantly inhibits the glucose oxidation rates (92). During this time, high-level glycolysis is not as beneficial due to the uncoupling of increased glycolysis from inhibited glucose oxidation (93). This unbalanced glycolysis and glucose oxidation may produce protons, which are a major contributor of a decrease in the efficiency in reperfusion stage, from glucose metabolism and deterioration of the ischemia and reperfusion (I/R) injury (88, 89). Furthermore, the sudden increase in fatty acids could generate more ROS, which worsens I/R injury. According to the injury during reperfusion stage, several pharmacological agents, such as trimetazidine, ranolzine, L-carnitine, and propionyl L-carnitine, have been designed to decrease fatty acid oxidation and enhance glucose metabolism.
AMP-activated protein kinase regulation
After five minutes of onset ischemia, the phosphorylation of AMPK (p-AMPK) will be increased. This activation of AMPK is due to several intracellular changes, and the increase in AMP/ATP ratio contributes to the majority of AMPK activation. During ischemia, there is a rapid increase of creatine phosphate (CrP), followed by a decreased ATP and increased ADP and AMPK levels. As described above, increased AMP/ATP ratio causes AMPK activation. Furthermore, decreased phosphocreatine/creatine (PCr/Cr) ratio can also activate AMPK (124). In a previous study, it was shown that AMPK can also be activated due to the decreased intracellular PH level. During ischemia, as glycolysis accumulates, intracellular pH drops from 7.2 to less than 6.2, thus AMPK is able to be activated in myocardial ischemia. Most interestingly, when Hue et al. pretreated an anaerobic rat heart with 100nM insulin, the AMPK activation was antagonized. They believe it is through a PI3-K dependent pathway stimulated by insulin, and this is independent with AMP/ATP and PCr/Cr ratio (12).
Nearly all metabolic changes during ischemia or ischemia/reperfusion are related to AMPK regulation. AMPK activated during ischemia is widely in charge of glucose utilization, fatty acid utilization, and many other mechanisms. As for glucose metabolism during ischemia (see review article [134]), AMPK may stimulate either GLUT-1 or GLUT-4 translocation from the intracellular storage pool to the plasma membrane (9, 39, 59, 130), or increase the protein expression of GLUT-1 and GLUT-4 (41). The direct influence of increased glucose receptor moving to sarcolemma membrane is due to increased glucose uptake. Numerous studies have shown the correlation between activated AMPK during ischemia and the increased glucose uptake (39, 59, 155); however, low-flow ischemia with rat hearts showed no relationship between increased p-AMPK and increased glucose uptake (172). AMPK also decreases glycogen synthesis via inhibiting glycogen synthase (175) and increases glycogenolysis via stimulating glycogen phosphorylase (172). AMPK could also activate glycolysis through phosphorylating PFK-2 at Ser466 under mild ischemia. Whereas, under severe ischemia, the accumulated protons and glycolytic by-products would inhibit PFK activation, therefore, glycolysis is inhibited (98).
AMPK’s regulation of fatty acids is mainly through modulating fatty acid oxidation. As we described above, fatty acids are the main energy source, which contribute to over 60% of ATP generation (72). During ischemia stage, fatty acid oxidation was inhibited, whereas, during the reperfusion stage, fatty acid oxidation recovers rapidly and serves as a main source of oxygen consumption and contributes to about 90%–100% of ATP generation (72). Activated AMPK during ischemia plays an important role in this elevation of fatty acid oxidation during reperfusion stage through regulating acetyl-CoA carboxylase (ACC) and malonyl-CoA decarboxylase (MCD), which are two opposite functioning enzymes in charge of converting between malonyl-CoA and acetyl-CoA. Activated AMPK could phosphorylate ACC, which inactivates its function. The inactivated ACC inhibits acetyl-CoA conversion to malonyl-CoA, following the removed inhibition to CPT-I. CPT-I facilitates a control step of fatty acid oxidation-helping fatty acids to be transported across the mitochondrial membrane. Accordingly, increased activity of CPT-I will transport more fatty acids into mitochondrial and TCA cycles. Increased MCD activity modulates fatty acid oxidation in a similar way as ACC, decreasing malonyl-CoA levels. However, it is still controversial regarding MCD’s regulation. During ischemia, MCD showed either unchanged or increased activity (79). Therefore, the role of AMPK regulating MCD is still uncertain. Some studies showed phosphorylated MCD activated by AMPK (121, 133), whereas other studies observed that AMPK is not correlated with MCD (48).
As we described above, sudden increased fatty acid oxidation increases ROS generation. In addition, the uncoupling glycolysis with glucose oxidation increases proton and lactate production (75, 91), which could lead to severe I/R injury. An effective way to minimize I/R injury is to increase the relative proportion of glucose oxidation to fatty acid oxidation. Several pharmacological approaches to increase AMPK during the reperfusion stage showed adjusted glucose and fatty acid oxidation levels. For example, activated protein C (APC), a vitamin K-dependent plasma serine protease that could down-regulate clotting and inflammatory pathways, showed its cardioprotective function through AMPK signaling. This activated AMPK during ischemia shifts metabolism energy source usage from fatty acid oxidation to glucose oxidation (24). Therefore, since we clarified AMPK’s function of increasing fatty acid oxidation, it is important to determine AMPK’s various functions during I/R. More work toward answering this question still needs to be done.
Several AMPK activators showed its cardioprotecitve effects. And some of those AMPK activators’ cardioprotective functions have been proved to be related with AMPK’S metabolic regulation function. Recently, the results in our lab showed the activation of AMPK during reperfusion protects the heart from regulation metabolism (163). Activated protein C (APC) plays a key anticoagulant role in preventing the activation of several procoagulant proteinases (35, 160). Furthermore, studies demonstrated that APC can elicit cytoprotecive responses through its anti-inflammatory signaling effect (108, 128, 153, 154). The cardioprotective function of APC was tested by 2, 3, 5-Triphenyltetrazolium chloride (TTC) staining, which can distinguish the area at risk (ischemic area, shown in red color) and infarct area (shown in white color). Two mutant APC- APC-2Cys (dramatically reduced anticoagulant activity) (8) and APC-E170A (devoid of intracellular signaling activity) (170), was used to determine whether it is the anticoagulant effect or direct cellular effect of APC is in charge of this cardioprotectve function. The result in Figure 2 indicate that APC-2Cys showed cardioprotecion by limiting the infarction, however, APC-E170A did not show any protective function. Accordingly, the results demonstrated that APC has cardioprotective function against I/R injury in the heart, and this effect is mainly through its signaling activity.
Figure 2.
APC reduces myocardial infarct size after ischemia/reperfusion. Hearts were subjected to 20 min ischemia followed by 3 h reperfusion. APC derivatives or saline (control) were administered via the tail vein 5 min before reperfusion. (A) Representative sections of myocardial infarction. (B) The ratio of area at risk (AAR) to myocardial area (left panel) and the ratio of infarct area to AAR (right panel) in mouse hearts of different treatment groups. Values are means ± standard error for four independent experiments. *P < 0.01 versus saline, respectively. Adapted with permission from Wang et al., 2011, ref 162.
Further results proved this cardioprotective function of APC stimulates AMPK phosphorylation during I/R. In this study, mouse hearts were subjected to left anterior descending artery (LAD) occlusion for different time-point ischemia. Result in Figure 3A showed an increasing AMPK phosphorylation in the ischemic area after 2 min of ischemia and this activation of AMPK reached its peak level after 10 min of ischemia, however the activation of AMPK quickly back to basal level after reperfusion started. The result further showed both APC and APC-2Cys can activate AMPK and its downstream protein ACC, but APC-E170A did not show this effect (Fig. 3B). In the sham-operated hearts APC and APC-2Cys activate AMPK and ACC to the same extent. Moreover, after immunoprecipitating AMPKα1 and AMPKα2, they found that, the activation of AMPK by APC and APC-2Cys was contributed by both AMPKα1 and AMPKα2, while APC-E170A has no effect (Fig. 3C, 3D). In conclusion, those results indicated that both APC and APC-2Cys activated AMPK in vivo, and this AMPK activation may contribute to the cardioprotective function of APC and APC-2Cys.
Figure 3.
APC stimulates AMPK activation. (A) In vivo regional ischemia stimulates phosphorylation of AMPK as shown by immunoblots. Values are means ± S.E., n = 3. *p < 0.05 vs. Sham. (B) Both APC and 2Cys but not E170A trigger AMPK and ACC phosphorylation during reperfusion. (C) and (D) Activation of AMPKα1 and AMPKα2 in the same heart samples as assessed by a kinase assay. Values are means ± standard error, n = 4–6. *p < 0.05 vs. Sham saline; †p < 0.05 vs. I/R saline. Adapted with permission, from Wang et al., 2011, ref 162.
To further determine the metabolic regulation effect of APC, several experiments toward GLUT-4 translocation, glucose uptake, and glucose and fatty acid oxidation were conducted. GLUT4 was first photo labeled by the cell membrane impermeable compound Bio-LC-ATB-BGPA, then was purified by streptavidin-agarose and quantified by immunoblotting against GLUT4-specific antibodies. The result indicated that both APC and APC-2Cys increased GLUT-4 translocation to cell surface during I/R (Fig. 4A). Glucose uptake, measured by metabolized 3H2O using [2]-3H-glucose and labeled bovine serum albumin (BSA) buffer, perfused into the heart. Results in Figure 4B indicate that glucose uptake significantly increases after APC and APC-2Cys treatment during I/R injury compared to the vehicle. The enhanced glucose uptake during I/R with APC could be due to the increased GLUT-4 moved to the cell surface.
Figure 4.
APC increases glucose uptake during ischemia/reperfusion (I/R). (A) APC modulates glucose transporter type 4 (GLUT4) translocation to the membrane. Immunoblotting analysis of cell membrane-bound and total GLUT4 in the heart tissues. (B) C57BL/6 mouse hearts were isolated and perfused with d-[2-3H] glucose-labeled perfusion buffer in the ex vivo working heart perfused system. Isolated hearts were subjected to 10 min of global ischemia followed by 20 min of reperfusion. Values are means ± standard error, n = 6 per group, *P < 0.05 versus control, †P < 0.05 versus I/R vehicle. RLU, relative light units. Adapted with permission from Costa et al., 2011, ref 23.
Glucose oxidation was measured by the amount of [14C] glucose metabolism into 14CO2 in the ex vivo working hearts. Fatty acid oxidation was measured by the incorporation of [9, 10-3H2O] oleate into 3H2O. The results showed that without giving APC, after I/R, glucose oxidation decreases and fatty acid oxidation increases. Yet, after providing APC, the oxidation mechanism was reversed. With the presence of APC, during reperfusion, it was found that there was increased glucose oxidation and decreased fatty acid oxidation when compared with I/R without APC (Fig. 5 A, B). Decreased fatty acid oxidation is supposed to decrease ROS generation. To further explore the oxidative intracellular stress level, we measured the level of GSH/GSSH, which reflects the intracellular oxidative stress status. The results showed that APC administration significantly decreases oxidative stress levels caused by I/R (Fig. 6).
Figure 5.
APC augments glucose oxidation in the heart during ischemia/reperfusion (I/R). Glucose oxidation was analyzed by measuring [14C]glucose incorporation into 14CO2 in ex vivo C57BL/6 mouse hearts subjected to 10 min of ischemia and 20 min of reperfusion. Oleate oxidation was analyzed by measuring the incorporation of [9,10-3H]oleate into 3H2O. Values are means ± standard error, n = 5–6 per group, *P < 0.05 versus control, †P < 0.05 versus I/R vehicle, #P < 0.01 versus I/R APC. PC-2Cys, protein C-2Cys. Adapted with permission, from Costa et al., 2011, ref 23.
Figure 6.

APC-2Cys improves intracellular redox status in the heart during ischemia/reperfusion (I/R). GSH/GSSG ratios were measured with a glutathione detection kit. Values are means ± standard error, n = 11 per group, *P < 0.01 vs. control, †P < 0.01 vs. I/R vehicle. RLU, relative light units. Adapted with permission, from Costa et al., 2011.
Several AMPK activators, such as APC, antithrombin (96), and Jagged1 (Yang H, Li j, unpublished data) have been found to shift metabolism usage from fatty acid oxidation to glucose oxidation during the reperfusion stage to protect the heart from lipid peroxidation and ROS damage. Figure 7 illustrates how AMPK activator administrated during I/R could limit the ROS damage brought by fatty acid oxidation. ROS is mainly generated in the electron transport chain, which can potentially result in increased oxidative stress. NADH and FADH2, which are generated in both β-oxidation and tricarboxylic acid cycle (TAC), are substrates of the electron transport chain. During I/R, increased fatty acid oxidation enhances NADH and FADH2 levels from both β-oxidation and TAC. However, increased glucose oxidation by AMPK activators during I/R would decrease the amount of NADH and FADH2 going into the electron transport chain by omitting β-oxidation. The decreased ROS generation plays an important role in limiting reperfusion damage.
Figure 7.
Schematic diagram illustrates how AMPK activator administrated during I/R could limit the ROS damage brought by fatty acid oxidation. TAC, tricarboxylic acid cycle; ROS, reactive oxygen species.
Conclusion
AMPK showed its critical role in the regulation of metabolism during I/R injury. This can be shown in AMPK kinase-dead mice (AMPK KD). For AMPK KD mice, AMPK α2 cDNA’s lysine 45 was changed to arginine, resulting in a cDNA encoding a kinase-dead protein. And this KD AMPK α2 blocks AMPK phosphorylation and activation (109). AMPK KD mice showed significant decreased left-ventricle developed pressure during ischemia and reperfusion stage compared with wild type (WT). Physiological or pharmacological approaches to increase AMPK have been recognized as a novel therapeutic method, such as leptin (177), adiponectin (140), macrophage migration inhibitory factor (103), activated protein C (163), glitazones (107), and antithrombin. Though the obvious protective function has been shown in these AMPK-activation drugs, it is still far from being used in clinical. More research toward this application in the clinical setting needed to be done in the future.
Hypertrophy
When the heart is under an increased hemodynamic burden, it can adjust to maintain cardiac output. The compensation happens usually: (1) By increasing cross bridge formation; (2) by enhancing muscle mass to bear the increased load; and (3) by secreting neurohormonal to increase contractility. As a long-term and most stable compensate mechanism, increasing mass seemingly plays an important role for hemodynamic overload. However, the number of cardiomyocyte does not change; it is the enlarging of existing cardiomyocytes due to the reason that cardiomyocytes quantity becomes stable and terminally differentiated soon after birth. Thus, during hypertrophy remodeling, some phenomenon occur, such as enlarged myocardium volume, enhanced protein synthesis, changed in gene transcription and translation as well increased myofibrillar assembly (148). There are several reasons that lead to hypertrophy, including exercise, hypertension, and cellular heart disease. As remodeling occurs in the heart, metabolism utilization changes, as well.
Fatty acid and glucose utilization
The main changes of myocardial energy sources in cardiac hypertrophy switch from fatty acid oxidation to glycolysis. It is a series of changes related together to regulate glucose utilization. The major changes are shown in review (76). The increased glucose metabolism in hypertrophied heart is due to the increased glycolysis level (2, 34, 82, 112). This is because glucose oxidation was found to have either no change or decreased change, and this leads to the uncoupling of glycolysis and glucose oxidation (1, 162). Several changes followed from this uncoupling, such as increased activity of LDH and enhanced efflux of lactate from cardiomyocyte (142, 150). LDH is the enzyme in charge of converting pyruvate to lactate to complete the glycolysis process. Consistent with increased glycolysis, glucose uptake showed increasing cardiac hypertrophy (71, 155, 178). However, the expression of enzymes controlling glycolytic capacity are not associated with the change in glycolysis, indicating that the regulation of glycolysis is more likely to happen at a glycolytic level rather than the expression of enzymes (3).
At the same time, fatty acid oxidation and its relative ATP production rate are obviously decreased (174). Furthermore, the expression of CPT-I and medium-chain acyl-CoA dehydrogenase (MCAD) were found to be decreased in the hypertrophic heart (155).
AMPK regulation
We have discussed that the AMP/ATP ratio could cause a direct allosteric activation of AMPK by binding with AMP, as well as indirect facilitation of AMPKK’s phosphorylation to AMPK. The increased AMPK activity was found in chronic pressure overload models (155). Whereas the ratio of phosphocreatine (PCr) to creatine (PCr/Cr) could also regulate AMPK activity via a direct activation or by affecting AMP/ATP ratio though the creatine kinase reaction and the adenylate kinase reaction (42, 55, 124, 156). Actually, in the hypertrophic hearts, AMP/ATP ratio was increased four-fold in the later stage of hypertrophy (155), indicating an energy stress may possibly activate AMPK. In hypertrophy hearts caused by pressure overload, the AMP level is normal, but PCr level decreased. In the study of Tian et al., significant increased AMPK activity was demonstrated in hypertrophied hearts and this activation is an isoform-specific alteration (155). They also showed activated AMPK is concomitant with increased glucose uptake and glucose transporter’s translocation to plasma membrane (155). However, they have also found a decreased fatty acid oxidation rate, which is opposite to AMPK’s function. The mRNA level of CPT-I and MCAD showed a 63% and 72% decrease, respectively, in left ventricular hypertrophy hearts (155). As we described above, CPT-I and MCAD are the rate-limiting enzymes for fatty acid oxidation. Thus, the results indicate that acute AMPK activation increases fatty acid oxidation, whereas long-term AMPK activation induced by hypertrophy does the opposite regulation function (155).
The activated AMPK could also inhibit the mTOR pathway. It has been shown that by using specific inhibitors to decrease mTOR activity, AMPK is able to counteract the development of cardiac hypertrophy (101, 141). Furthermore, studies exhibited that pharmacological activation of AMPK inhibits the mTOR pathway to attenuate the development of hypertrophy (19,20, 84). Interestingly, AMPK and LKB1 also exert its ability to delay the hypertrophy transit to heart failure (HF). Other studies showed that AMPK acts as a negative regulator of cardiac growth, and is inactivated during the development of Akt1-induced hypertrophy in the neonatal rat cardiomyocyte (20, 86). Thus, this result enhances the possibility that AMPK attenuates hypertrophic growth at the beginning stage of hypertrophy (139).
Conclusion
In conclusion, hypertrophy has an obvious metabolic shift, and it has been proven to be correlated to AMPK activation. In review (33), they sum the possibility that AMPK’s activation could have different roles in different stages of hypertrophy. In the early stage of hypertrophy, the pharmacological activation of AMPK could inhibit the growth of hypertrophic cardiomyocyte, whereas in the pathological hypertrophy, AMPK’s activation response to metabolic stress may be able to regulate metabolic shift and delay the deterioration of hypertrophy to HF.
Heart failure
HF is, in fact, a muscular disorder where the heart is unable to pump blood with normal efficacy. A number of causes can lead to HF, such as: (1) coronary artery disease and heart attack, which cause the damage of the heart muscle. (2) the heart overworking such as hypertension (high blood pressure) and valve disease. (3) viral infection or toxin caused primary heart muscle weakness. (4) vitamin deficiency (5) atrial fibrillation. The main goal of therapy toward heart failure is to improve the pumping ability of the heart, thus, the heart can maintain the normal blood volume pumped to the whole body. General treatment for heart failure is to stabilize body fluid and get rid of extra salt by using diuretics, angiotensin receptor blockers, beta blockers and aldosterone antagonists. Furthermore, depending on the cause or the stage of heart failure, a peacemaker implantable device or implantable cardiac defibrillator is also used to improve the heart’s pumping function.
Fatty acid and glucose utilization
One direct abnormality caused by the failing heart is the decreased coronary reserve, which reduces nutrient and oxygen supply to the cardiomyocyte during high workloads. Thus, the heart’s metabolism remodeling appears to adjust to the new energy homeostasis. In HF, which is different from a normal heart’s energy usage, the failing heart’s chief metabolic energy substrates switch from fatty acids to glucose (126, 132). But, it is also dependent on the stage of HF (11, 157). At more advanced stages, fatty acid oxidation is significantly limited; however, this change is not clearly seen at early stages of HF. The inhibited fatty acid oxidation is mainly due to a decreased content of enzymes, including CPT-I and acyl-CoA dehydrogenases. Interestingly, the utilization of these energy substrates switches from the β-oxidation of fatty acid to glycolysis. This switch from fatty acid to carbohydrate could enhance oxidizing efficiency in a failing heart. It has been observed that the overexpression of GLUT-1 could protect against HF induced by pressure overload as well as contractile dysfunction (67). Research showed that in transgenic mice overexpressing GLUT-1, the metabolism changes to inhibiting fatty acid oxidation, increasing glucose uptake, and oxidation. However, when the mice are fed a high-fat diet, their hearts were under an increased oxidative stress and led to cardiac dysfunction (169).
In normal cardiomyocytes, mitochondria occupy around 30% of cardiomyocyte space and provide the heart with more than 90% oxidative capacity during maximal exercise (105), whereas the characteristic feature of HF is the mitochondria dysfunction. The morphological abnormalities of mitochondria include increase in mitochondria number, reduced mitochondria size, and compromised structural integrity (63, 129, 136). The result of deficient oxidative mitochondrial capacity is the uncoupling energy production to utilization (159). It also showed decreased electron transfer chain complexes and oxidative phosphorylation capacity (129, 147, 159), as well as decreased complex I, III, and IV (64, 97) in mitochondria. It has been shown that increased tumor necrosis factor is produced in failing hearts (77, 176), which elevates the production of the second messenger ceramide (102). Ceramide further inhibits complex III, leading to the increased production of ROS (27); accordingly, mitochondria release cytochrome c (83). Studies also suggest that there is a positive correlation between mitochondrial injury severity and indicates heart failure severity, including noradrenaline, ejection fraction, and left-ventricle end-diastolic pressure (131).
AMPK regulation
Both I/R injury and hypertrophy-induced HF can be attenuated by AMPK activation, as we described above. Accordingly, AMPK’s cardioprotective function toward HF cannot be ignored. According to a review (11), several hormones and pharmacological activators of AMPK showed protection against HF. For example, leptin, a hormone that regulates the amount of fat stored in the body, has been found to protect against HF by stimulating STAT-3 and AMPK signaling pathways, which could attenuate cardiac hypertrophy, inflammation, and cardiac dysfunction (100). Another example is metformin, which has shown its cardioprotective function by improving outcomes in patients with HF in clinical studies (36, 60). Since metformin is an AMPK activator, it is possible that its function of improving outcomes in HF patients is through AMPK signaling (53, 179).
Besides the metabolic regulation effect of AMPK, it exhibits other protective functions against HF. AMPK is involved in mediating cardiac fibrosis through inhibiting angiotensin II, which is known to be an important mediator of cardiac fibrosis (31). Studies in mesangial cells showed that inhibition of transforming growth factor beta (TGF-β) could induce smad3-dependent transcription and myofibroblast transdifferentiation by AMPK (104). Furthermore, other effects such as anti-inflammation may also contribute to the cardiac protection and remodeling.
Diabetic heart disease
Diabetes is a metabolic disorder disease. Due to the inability to increase insulin level in blood, blood glucose cannot be taken into the cell, which leads to glucose build up in the blood. Diabetic heart disease refers to heart disease that has developed from diabetic patients. Among those people, ones at a younger age exhibit a higher risk for getting heart disease. Diabetic heart disease includes coronary heart disease, heart failure, and/or diabetic cardiomyopathy. The unbalanced myocardial energy metabolism in the diabetes patients might be an important reason for high morbidity of cardiac disease.
Fatty acid and glucose utilization
The diabetic heart, even in normal conditions, has decreased glucose and lactate uptake, whereas the utilization of free fatty acids is increased. For the decreased glucose uptake, glucose transporters (GLUT-1 and GLUT-4) have changed in both protein and mRNA level (15, 44, 143, 144). Furthermore, in a model of the isolated working rat heart, it was discovered that a decreased glycolysis rate (43, 161) was due to glucose 6-phosphate accumulation and fructose 1, 6-bisphosphate and fructose 6-phosphate ratio decline (22, 116).
By pharmacologically decreasing the fatty acid oxidation rate via inhibiting CPT-I or lowering the concentration of plasma free fatty acid, the glucose and lactate oxidation were enhanced to keep the energy homeostasis. Thus, in the diabetic heart, several studies proved that the low-level insulin-induced lipolysis could contribute to impairment of the glucose uptake and oxidation. As we described before, the phosphorylation of PDH is the control step of pyruvate oxidation, and the phosphorylation of PDH is the inactivated status of PDH, which leads to decreased pyruvate oxidation. In diabetic hearts, both decreased insulin levels and high-circulating free fatty acid levels contribute to the decreased PDH activity (166) by enhancing intramitochondrial acetyl CoA levels, then activating PDH kinase to phosphorylate and inactivate PDH (52). Accordingly, the decreased PDH activity was found to correspond with the decreased myocardial glucose uptake and oxidation rate in diabetic rodents and swine (50, 125, 165, 166). In conclusion, the inhibited glucose and lactate oxidation is mainly through elevated fatty acid levels to inhibit PDH activity.
The increased plasma free fatty acid levels (110) and increased myocardial CoA levels (94) in diabetic heart could also contribute to the increased myocardial triacylglycerol levels (28, 110). Another pathway to increase fatty acid oxidation in diabetic heart is via decreased ACC activity, which is highly related with the changes of fatty acid oxidation. Studies indicated that there is no change of ACC in protein expression or mRNA level, however there is a sharp decrease of ACC activity (146). Thus, inhibited malonyl CoA, which is produced by ACC, releases the inhibition to CPT-I, followed by increased fatty acid oxidation (50, 90).
AMP-activated protein kinese regulation
The AMPK activation in diabetic hearts is controversial. Increase, decrease, and no change of AMPK activation have all been reported. In a review article by Stanley et al., the conclusion of increasing AMPK activity in diabetic hearts is mainly because of the evidence of the phosphorylation and inhibition of ACC (146). On the other hand, other articles demonstrated a decrease in AMPK activation in diabetic hearts. Accordingly, the AMPK levels in diabetic hearts and in different types of diabetes still need to be explored.
Conclusion
Although fuels for oxidative metabolism of the human heart shift among glucose-glycogen, lactate, carbohydrates, and free fatty acids during different conditions like fasting, increased heart work, or mild/severe ischemia, fatty acids are still recognized as the major source of energy. In several pathologies, like ischemia/reperfusion and diabetic hearts, to stable ATP generation, metabolism shifts more toward fatty acid metabolism. In other pathologies like cardiac hypertrophy, glucose metabolism is increased. During some pathology (like ischemia/reperfusion) fatty acids are better to capture the residual oxygen, although oxygen is limited. Even in the same pathology, the level of severity of disease can still move the metabolism shift. The facts shown in this overview demonstrate that AMPK plays an important role in different pathologies, especially during ischemia and reperfusion injury. Acute ischemia shows dramatic increased AMPK activation within five minutes, and research shows remaining AMPK activation during reperfusion is an effective way to reduce damage to cardiomyocyte from ischemia and reperfusion injury. Impaired myocardial energetics in cardiac hypertrophy also elicits AMPK activation to stimulate glucose uptake and glycolysis. By physiological or pharmacological activation of AMPK, the cardiomyocyte was protected under energy stress condition. Studies also show the aging mouse heart has deficiency in AMPK activation, which is related with reduced mitochondrial function and dysregulated intracellular lipid metabolism. In summary, AMPK plays an important role in cardiac metabolism regulation. The metabolism shift between glucose oxidation, fatty acid oxidation, and glycolysis and the glucose uptake and AMPK activity in aging and different pathology heart were summarized in Table 1. Although many studies focus on AMPK manipulation for treatment of cardiovascular disease, understanding the role of AMPK in different cardiac diseases to guide pharmacological manipulation for patients still needs more exploring.
Table 1.
Summary of the metabolic changes among glucose oxidation, fatty acid oxidation, glycolysis, the glucose uptake, and AMPK activity in aging and different pathology conditions. References are in parenthesis.
| Glucose Oxidation | Glycolysis | Fatty Acid Oxidation | Glucose Uptake | AMPK | |
|---|---|---|---|---|---|
| Aging | ↑(relative)(73) | ↑(81) | ↓(21,73) | ↑(80,99,120) | low sensitivity (127,180) |
| Ischemia | ↓ | ↑(113) | ↓ | ↑(145) | ↑(163) |
| Severe Ischemia | ↓ | ↓(98,113) | ↓ | ↓(145) | – |
| Ischemia/Reperfusion | ↓(92) | ↑(93) | ↑(92) | ↑(173,23) | −(163) |
| Hypertrophy | ↑(2,34,82,112) | ↑(2,34,82,112) | ↓(174) | ↑(71,155,178) | ↑(155) |
| Heart Failure | ↑(126,132) | ↑ | ↓(126,132) | ↑ | −(11) |
| Diabetic Heart Disease | ↓(50,125,165, 166) | ↓(22,43,116,161) | ↑(28,110) | ↓(15,44,143, 144) | ↑(146) or not sure |
Acknowledgments
The authors thank Ustina Huh for reviewing and editing this manuscript. The work was supported by the American Heart Association 12GRNT 11620029 and 14IRG18290014 and American Diabetes Association Basic Sciences Grant 1-11-BS-131. The authors also give thanks to Wiley publisher for giving us permissions to use the figures from (163) and (23).
References
- 1.Allard MF, Henning SL, Wambolt RB, Granleese SR, English DR, Lopaschuk GD. Glycogen metabolism in the aerobic hypertrophied rat heart. Circulation. 1997;96:676–682. doi: 10.1161/01.cir.96.2.676. [DOI] [PubMed] [Google Scholar]
- 2.Allard MF, Schonekess BO, Henning SL, English DR, Lopaschuk GD. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. The American Journal of Physiology. 1994;267:H742–750. doi: 10.1152/ajpheart.1994.267.2.H742. [DOI] [PubMed] [Google Scholar]
- 3.Allard MF, Wambolt RB, Longnus SL, Grist M, Lydell CP, Parsons HL, Rodrigues B, Hall JL, Stanley WC, Bondy GP. Hypertrophied rat hearts are less responsive to the metabolic and functional effects of insulin. American Journal of Physiology Endocrinology and Metabolism. 2000;279:E487–493. doi: 10.1152/ajpendo.2000.279.3.E487. [DOI] [PubMed] [Google Scholar]
- 4.Amr Moussa JL. AMPK in myocardial infarction and diabetes: the yin/yang effect. Acta Pharmaceutica Sinica B. 2012;2:10. doi: 10.1016/j.apsb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.An D, Kewalramani G, Qi D, Pulinilkunnil T, Ghosh S, Abrahani A, Wambolt R, Allard M, Innis SM, Rodrigues B. beta-Agonist stimulation produces changes in cardiac AMPK and coronary lumen LPL only during increased workload. American Journal of Physiology Endocrinology and Metabolism. 2005;288:E1120–1127. doi: 10.1152/ajpendo.00588.2004. [DOI] [PubMed] [Google Scholar]
- 6.Apstein CS, Gravino FN, Haudenschild CC. Determinants of a protective effect of glucose and insulin on the ischemic myocardium. Effects on contractile function, diastolic compliance, metabolism, and ultrastructure during ischemia and reperfusion. Circulation Research. 1983;52:515–526. doi: 10.1161/01.res.52.5.515. [DOI] [PubMed] [Google Scholar]
- 7.Augustus AS, Kako Y, Yagyu H, Goldberg IJ. Routes of FA delivery to cardiac muscle: Modulation of lipoprotein lipolysis alters uptake of TG-derived FA. American Journal of Physiology Endocrinology and Metabolism. 2003;284:E331–339. doi: 10.1152/ajpendo.00298.2002. [DOI] [PubMed] [Google Scholar]
- 8.Bae JS, Yang LK, Manithody C, Rezaie AR. Engineering a disulfide bond to stabilize the calcium-binding loop of activated protein C eliminates its anticoagulant but not its protective signaling properties. Journal of Biological Chemistry. 2007;282:9251–9259. doi: 10.1074/jbc.M610547200. [DOI] [PubMed] [Google Scholar]
- 9.Barnes K, Ingram JC, Porras OH, Barros LF, Hudson ER, Fryer LG, Foufelle F, Carling D, Hardie DG, Baldwin SA. Activation of GLUT1 by metabolic and osmotic stress: Potential involvement of AMP-activated protein kinase (AMPK) Journal of Cell Science. 2002;115:2433–2442. doi: 10.1242/jcs.115.11.2433. [DOI] [PubMed] [Google Scholar]
- 10.Baron SJ, Li J, Russell RR, Neumann D, Miller EJ, Tuerk R, Wallimann T, Hurley RL, Witters LA, Young LH. Dual mechanisms regulating AMPK kinase action in the ischemic heart. Circulation Research. 2005;96:337–345. doi: 10.1161/01.RES.0000155723.53868.d2. [DOI] [PubMed] [Google Scholar]
- 11.Beauloye C, Bertrand L, Horman S, Hue L. AMPK activation, a preventive therapeutic target in the transition from cardiac injury to heart failure. Cardiovascular Research. 2011;90:224–233. doi: 10.1093/cvr/cvr034. [DOI] [PubMed] [Google Scholar]
- 12.Beauloye C, Marsin AS, Bertrand L, Krause U, Hardie DG, Vanoverschelde JL, Hue L. Insulin antagonizes AMP-activated protein kinase activation by ischemia or anoxia in rat hearts, without affecting total adenine nucleotides. FEBS Letters. 2001;505:348–352. doi: 10.1016/s0014-5793(01)02788-0. [DOI] [PubMed] [Google Scholar]
- 13.Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. Journal of Biological Chemistry. 2002;277:23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- 14.Browne GJ, Finn SG, Proud CG. Stimulation of the AMP-activated protein kinase leads to activation of eukaryotic elongation factor 2 kinase and to its phosphorylation at a novel site, Serine 398. Journal of Biological Chemistry. 2004;279:12220–12231. doi: 10.1074/jbc.M309773200. [DOI] [PubMed] [Google Scholar]
- 15.Camps M, Castello A, Munoz P, Monfar M, Testar X, Palacin M, Zorzano A. Effect of diabetes and fasting on GLUT-4 (muscle/fat) glucose-transporter expression in insulin-sensitive tissues. Heterogeneous response in heart, red and white muscle. The Biochemical Journal. 1992;282(Pt 3):765–772. doi: 10.1042/bj2820765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canto C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Current Opinion in Lipidology. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carling D, Hardie DG. The substrate and sequence specificity of the AMP-activated protein kinase. Phosphorylation of glycogen synthase and phosphorylase kinase. Biochimica et Biophysica Acta. 1989;1012:81–86. doi: 10.1016/0167-4889(89)90014-1. [DOI] [PubMed] [Google Scholar]
- 18.Caterson ID, Fuller SJ, Randle PJ. Effect of the fatty acid oxidation inhibitor 2-tetradecylglycidic acid on pyruvate dehydrogenase complex activity in starved and alloxan-diabetic rats. The Biochemical Journal. 1982;208:53–60. doi: 10.1042/bj2080053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan AY, Dolinsky VW, Soltys CL, Viollet B, Baksh S, Light PE, Dyck JR. Resveratrol inhibits cardiac hypertrophy via AMP-activated protein kinase and Akt. The Journal of Biological Chemistry. 2008;283:24194–24201. doi: 10.1074/jbc.M802869200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan AY, Soltys CL, Young ME, Proud CG, Dyck JR. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. The Journal of Biological Chemistry. 2004;279:32771–32779. doi: 10.1074/jbc.M403528200. [DOI] [PubMed] [Google Scholar]
- 21.Chen JC, Warshaw JB, Sanadi DR. Regulation of mitochondrial respiration in senescence. Journal of Cellular Physiology. 1972;80:141–148. doi: 10.1002/jcp.1040800115. [DOI] [PubMed] [Google Scholar]
- 22.Chen V, Ianuzzo CD, Fong BC, Spitzer JJ. The effects of acute and chronic diabetes on myocardial metabolism in rats. Diabetes. 1984;33:1078–1084. doi: 10.2337/diab.33.11.1078. [DOI] [PubMed] [Google Scholar]
- 23.Costa R, Morrison A, Wang J, Manithody C, Li J, Rezaie AR. Activated protein C modulates cardiac metabolism and augments autophagy in the ischemic heart. Journal of Thrombosis and Haemostasis: JTH. 2012;10:1736–1744. doi: 10.1111/j.1538-7836.2012.04833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Costa R, Morrison A, Wang J, Manithody C, Li J, Rezaie AR. Activated protein C modulates cardiac metabolism and augments autophagy in the ischemic heart. Journal of Thrombosis and Haemostasis. 2012;10:1736–1744. doi: 10.1111/j.1538-7836.2012.04833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cusack BJ, Mushlin PS, Andrejuk T, Voulelis LD, Olson RD. Aging alters the force-frequency relationship and toxicity of oxidative stress in rabbit heart. Life Sciences. 1991;48:1769–1777. doi: 10.1016/0024-3205(91)90215-w. [DOI] [PubMed] [Google Scholar]
- 26.da Silva Xavier G, Leclerc I, Varadi A, Tsuboi T, Moule SK, Rutter GA. Role for AMP-activated protein kinase in glucose-stimulated insulin secretion and preproinsulin gene expression. The Biochemical Journal. 2003;371:761–774. doi: 10.1042/BJ20021812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Degli Esposti M, McLennan H. Mitochondria and cells produce reactive oxygen species in virtual anaerobiosis: Relevance to ceramide-induced apoptosis. FEBS Letters. 1998;430:338–342. doi: 10.1016/s0014-5793(98)00688-7. [DOI] [PubMed] [Google Scholar]
- 28.Denton RM, Randle PJ. Concentrations of glycerides and phospholipids in rat heart and gastrocnemius muscles. Effects of alloxan-diabetes and perfusion. The Biochemical Journal. 1967;104:416–422. doi: 10.1042/bj1040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dolinsky VW, Dyck JRB. Role of AMP-activated protein kinase in healthy and diseased hearts. Am J Physiol-Heart C. 2006;291:H2557–H2569. doi: 10.1152/ajpheart.00329.2006. [DOI] [PubMed] [Google Scholar]
- 30.Drake AJ, Haines JR, Noble MI. Preferential uptake of lactate by the normal myocardium in dogs. Cardiovascular Research. 1980;14:65–72. doi: 10.1093/cvr/14.2.65. [DOI] [PubMed] [Google Scholar]
- 31.Du JH, Guan TJ, Zhang H, Xia Y, Liu F, Zhang YY. Inhibitory crosstalk between ERK and AMPK in the growth and proliferation of cardiac fibroblasts. Biochemical and Biophysical Research Communications. 2008;368:402–407. doi: 10.1016/j.bbrc.2008.01.099. [DOI] [PubMed] [Google Scholar]
- 32.Dyck JR, Kudo N, Barr AJ, Davies SP, Hardie DG, Lopaschuk GD. Phosphorylation control of cardiac acetyl-CoA carboxylase by cAMP-dependent protein kinase and 5′-AMP activated protein kinase. European Journal of Biochemistry/FEBS. 1999;262:184–190. doi: 10.1046/j.1432-1327.1999.00371.x. [DOI] [PubMed] [Google Scholar]
- 33.Dyck JR, Lopaschuk GD. AMPK alterations in cardiac physiology and pathology: Enemy or ally? The Journal of Physiology. 2006;574:95–112. doi: 10.1113/jphysiol.2006.109389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El Alaoui-Talibi Z, Guendouz A, Moravec M, Moravec J. Control of oxidative metabolism in volume-overloaded rat hearts: Effect of propionyl-L-carnitine. The American Journal of Physiology. 1997;272:H1615–1624. doi: 10.1152/ajpheart.1997.272.4.H1615. [DOI] [PubMed] [Google Scholar]
- 35.Esmon CT. Molecular events that control the protein-C anticoagulant pathway. Thrombosis and Haemostasis. 1993;70:29–35. [PubMed] [Google Scholar]
- 36.Eurich DT, Majumdar SR, McAlister FA, Tsuyuki RT, Johnson JA. Improved clinical outcomes associated with metformin in patients with diabetes and heart failure. Diabetes Care. 2005;28:2345–2351. doi: 10.2337/diacare.28.10.2345. [DOI] [PubMed] [Google Scholar]
- 37.Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacological Reviews. 2007;59:418–458. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- 38.Foretz M, Ancellin N, Andreelli F, Saintillan Y, Grondin P, Kahn A, Thorens B, Vaulont S, Viollet B. Short-term overexpression of a constitutively active form of AMP-activated protein kinase in the liver leads to mild hypoglycemia and fatty liver. Diabetes. 2005;54:1331–1339. doi: 10.2337/diabetes.54.5.1331. [DOI] [PubMed] [Google Scholar]
- 39.Fraser H, Lopaschuk GD, Clanachan AS. Alteration of glycogen and glucose metabolism in ischaemic and post-ischaemic working rat hearts by adenosine A1 receptor stimulation. British Journal of Pharmacology. 1999;128:197–205. doi: 10.1038/sj.bjp.0702765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.French TJ, Holness MJ, MacLennan PA, Sugden MC. Effects of nutritional status and acute variation in substrate supply on cardiac and skeletal-muscle fructose 2,6-bisphosphate concentrations. The Biochemical Journal. 1988;250:773–779. doi: 10.1042/bj2500773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fryer LG, Foufelle F, Barnes K, Baldwin SA, Woods A, Carling D. Characterization of the role of the AMP-activated protein kinase in the stimulation of glucose transport in skeletal muscle cells. The Biochemical Journal. 2002;363:167–174. doi: 10.1042/0264-6021:3630167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujii N, Hayashi T, Hirshman MF, Smith JT, Habinowski SA, Kaijser L, Mu J, Ljungqvist O, Birnbaum MJ, Witters LA, Thorell A, Goodyear LJ. Exercise induces isoform-specific increase in 5′AMP-activated protein kinase activity in human skeletal muscle. Biochemical and Biophysical Research Communications. 2000;273:1150–1155. doi: 10.1006/bbrc.2000.3073. [DOI] [PubMed] [Google Scholar]
- 43.Gamble J, Lopaschuk GD. Glycolysis and glucose-oxidation during reperfusion of ischemic hearts from diabetic rats. Bba-Mol Basis Dis. 1994;1225:191–199. doi: 10.1016/0925-4439(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 44.Garvey WT, Hardin D, Juhaszova M, Dominguez JH. Effects of diabetes on myocardial glucose transport system in rats: Implications for diabetic cardiomyopathy. The American Journal of Physiology. 1993;264:H837–844. doi: 10.1152/ajpheart.1993.264.3.H837. [DOI] [PubMed] [Google Scholar]
- 45.Gong H, Xie J, Zhang N, Yao L, Zhang Y. MEF2A binding to the Glut4 promoter occurs via an AMPKalpha2-dependent mechanism. Medicine and Science in Sports and Exercise. 2011;43:1441–1450. doi: 10.1249/MSS.0b013e31820f6093. [DOI] [PubMed] [Google Scholar]
- 46.Goodman MN, Dluz SM, McElaney MA, Belur E, Ruderman NB. Glucose uptake and insulin sensitivity in rat muscle: Changes during 3–96 weeks of age. The American Journal of Physiology. 1983;244:E93–100. doi: 10.1152/ajpendo.1983.244.1.E93. [DOI] [PubMed] [Google Scholar]
- 47.Greenberg CC, Jurczak MJ, Danos AM, Brady MJ. Glycogen branches out: New perspectives on the role of glycogen metabolism in the integration of metabolic pathways. American Journal of Physiology Endocrinology and Metabolism. 2006;291:E1–8. doi: 10.1152/ajpendo.00652.2005. [DOI] [PubMed] [Google Scholar]
- 48.Habinowski SA, Hirshman M, Sakamoto K, Kemp BE, Gould SJ, Goodyear LJ, Witters LA. Malonyl-CoA decarboxylase is not a substrate of AMP-activated protein kinase in rat fast-twitch skeletal muscle or an islet cell line. Archives of Biochemistry and Biophysics. 2001;396:71–79. doi: 10.1006/abbi.2001.2589. [DOI] [PubMed] [Google Scholar]
- 49.Hall JL, Mazzeo RS, Podolin DA, Cartee GD, Stanley WC. Exercise training does not compensate for age-related decrease in myocardial GLUT-4 content. Journal of Applied Physiology. 1994;76:328–332. doi: 10.1152/jappl.1994.76.1.328. [DOI] [PubMed] [Google Scholar]
- 50.Hall JL, Stanley WC, Lopaschuk GD, Wisneski JA, Pizzurro RD, Hamilton CD, McCormack JG. Impaired pyruvate oxidation but normal glucose uptake in diabetic pig heart during dobutamine-induced work. The American Journal of Physiology. 1996;271:H2320–2329. doi: 10.1152/ajpheart.1996.271.6.H2320. [DOI] [PubMed] [Google Scholar]
- 51.Hansford RG. Bioenergetics in aging. Biochimica et Biophysica Acta. 1983;726:41–80. doi: 10.1016/0304-4173(83)90010-1. [DOI] [PubMed] [Google Scholar]
- 52.Hansford RG, Cohen L. Relative importance of pyruvate dehydrogenase interconversion and feed-back inhibition in the effect of fatty acids on pyruvate oxidation by rat heart mitochondria. Archives of Biochemistry and Biophysics. 1978;191:65–81. doi: 10.1016/0003-9861(78)90068-1. [DOI] [PubMed] [Google Scholar]
- 53.Hardie DG. AMP-activated protein kinase as a drug target. Annu Rev Pharmacol. 2007;47:185–210. doi: 10.1146/annurev.pharmtox.47.120505.105304. [DOI] [PubMed] [Google Scholar]
- 54.Hardie DG. AMPK and SNF1: Snuffing out stress. Cell Metabolism. 2007;6:339–340. doi: 10.1016/j.cmet.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hardie DG, Carling D. The AMP-activated protein kinase–fuel gauge of the mammalian cell? European Journal of Biochemistry/FEBS. 1997;246:259–273. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- 56.Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annual Review of Biochemistry. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- 57.Hardie DG, Ross FA, Hawley SA. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nature Reviews Molecular Cell Biology. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: Targeting the reperfusion injury salvage kinase (RISK)-pathway. Cardiovascular Research. 2004;61:448–460. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 59.Hayashi T, Hirshman MF, Fujii N, Habinowski SA, Witters LA, Goodyear LJ. Metabolic stress and altered glucose transport: Activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes. 2000;49:527–531. doi: 10.2337/diabetes.49.4.527. [DOI] [PubMed] [Google Scholar]
- 60.Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJV, Team RS. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): A multicentre, randomised, open-label trial. Lancet. 2009;373:2125–2135. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 61.Hudson ER, Pan DA, James J, Lucocq JM, Hawley SA, Green KA, Baba O, Terashima T, Hardie DG. A novel domain in AMP-activated protein kinase causes glycogen storage bodies similar to those seen in hereditary cardiac arrhythmias. Current Biology: CB. 2003;13:861–866. doi: 10.1016/s0960-9822(03)00249-5. [DOI] [PubMed] [Google Scholar]
- 62.Hue L, Beauloye C, Marsin AS, Bertrand L, Horman S, Rider MH. Insulin and ischemia stimulate glycolysis by acting on the same targets through different and opposing signaling pathways. Journal of Molecular and Cellular Cardiology. 2002;34:1091–1097. doi: 10.1006/jmcc.2002.2063. [DOI] [PubMed] [Google Scholar]
- 63.Huss JM, Kelly DP. Mitochondrial energy metabolism in heart failure: A question of balance. Journal of Clinical Investigation. 2005;115:547–555. doi: 10.1172/JCI200524405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ide T, Tsutsui H, Kinugawa S, Utsumi H, Kang D, Hattori N, Uchida K, Arimura K, Egashira K, Takeshita A. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circulation Research. 1999;85:357–363. doi: 10.1161/01.res.85.4.357. [DOI] [PubMed] [Google Scholar]
- 65.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 66.Inoki K, Zhu TQ, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 67.Jain M, Liao R, Cui L, D’Agostoni J, Aiello F, Lupak I, Ngoy S, Mortensen RM, Tian R. Cardiac-specific overexpression of GLUT1 improves survival and prevents the development of heart failure secondary to pressure overload in mice. Circulation. 2002;106:306–306. doi: 10.1161/01.cir.0000034049.61181.f3. [DOI] [PubMed] [Google Scholar]
- 68.Janero DR, Hreniuk D, Sharif HM. Hydroperoxide-induced oxidative stress impairs heart muscle cell carbohydrate metabolism. The American Journal of Physiology. 1994;266:C179–188. doi: 10.1152/ajpcell.1994.266.1.C179. [DOI] [PubMed] [Google Scholar]
- 69.Ji LL, Dillon D, Wu E. Myocardial aging: Antioxidant enzyme systems and related biochemical properties. The American Journal of Physiology. 1991;261:R386–392. doi: 10.1152/ajpregu.1991.261.2.R386. [DOI] [PubMed] [Google Scholar]
- 70.Joost HG, Thorens B. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members (review) Molecular Membrane Biology. 2001;18:247–256. doi: 10.1080/09687680110090456. [DOI] [PubMed] [Google Scholar]
- 71.Kagaya Y, Kanno Y, Takeyama D, Ishide N, Maruyama Y, Takahashi T, Ido T, Takishima T. Effects of long-term pressure overload on regional myocardial glucose and free fatty acid uptake in rats. A quantitative autoradiographic study. Circulation. 1990;81:1353–1361. doi: 10.1161/01.cir.81.4.1353. [DOI] [PubMed] [Google Scholar]
- 72.Kantor PF, Dyck JR, Lopaschuk GD. Fatty acid oxidation in the reperfused ischemic heart. The American Journal of the Medical Sciences. 1999;318:3–14. doi: 10.1097/00000441-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 73.Kates AM, Herrero P, Dence C, Soto P, Srinivasan M, Delano DG, Ehsani A, Gropler RJ. Impact of aging on substrate metabolism by the human heart. Journal of the American College of Cardiology. 2003;41:293–299. doi: 10.1016/s0735-1097(02)02714-6. [DOI] [PubMed] [Google Scholar]
- 74.Kemp BE, Akhill JS, Scott JW. AMPK structure and regulation from three angles. Structure. 2007;15:1161–1163. doi: 10.1016/j.str.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 75.King LM, Opie LH. Glucose delivery is a major determinant of glucose utilisation in the ischemic myocardium with a residual coronary flow. Cardiovascular Research. 1998;39:381–392. doi: 10.1016/s0008-6363(98)00100-x. [DOI] [PubMed] [Google Scholar]
- 76.Kolwicz SC, Tian R. Glucose metabolism and cardiac hypertrophy. Cardiovascular Research. 2011;90:194–201. doi: 10.1093/cvr/cvr071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kubota T, Miyagishima M, Alvarez RJ, Kormos R, Rosenblum WD, Demetris AJ, Semigran MJ, Dec GW, Holubkov R, McTiernan CF, Mann DL, Feldman AM, McNamara DM. Expression of proinflammatory cytokines in the failing human heart: Comparison of recent-onset and end-stage congestive heart failure. The Journal of Heart and Lung Transplantation: The Official Publication of the International Society for Heart Transplantation. 2000;19:819–824. doi: 10.1016/s1053-2498(00)00173-x. [DOI] [PubMed] [Google Scholar]
- 78.Kudo N, Barr AJ, Barr RL, Desai S, Lopaschuk GD. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5′-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. The Journal of Biological Chemistry. 1995;270:17513–17520. doi: 10.1074/jbc.270.29.17513. [DOI] [PubMed] [Google Scholar]
- 79.Kudo N, Gillespie JG, Kung L, Witters LA, Schulz R, Clanachan AS, Lopaschuk GD. Characterization of 5′AMP-activated protein kinase activity in the heart and its role in inhibiting acetyl-CoA carboxylase during reperfusion following ischemia. Biochimica et Biophysica Acta. 1996;1301:67–75. doi: 10.1016/0005-2760(96)00013-6. [DOI] [PubMed] [Google Scholar]
- 80.Kurokawa T, Ozaki N, Ishibashi S. Difference between senescence-accelerated prone and resistant mice in response to insulin in the heart. Mechanisms of Ageing and Development. 1998;102:25–32. doi: 10.1016/s0047-6374(98)00009-8. [DOI] [PubMed] [Google Scholar]
- 81.Lakatta EG, Yin FC. Myocardial aging: Functional alterations and related cellular mechanisms. The American Journal of Physiology. 1982;242:H927–941. doi: 10.1152/ajpheart.1982.242.6.H927. [DOI] [PubMed] [Google Scholar]
- 82.Leong HS, Grist M, Parsons H, Wambolt RB, Lopaschuk GD, Brownsey R, Allard MF. Accelerated rates of glycolysis in the hypertrophied heart: Are they a methodological artifact? American Journal of Physiology Endocrinology and Metabolism. 2002;282:E1039–1045. doi: 10.1152/ajpendo.00507.2001. [DOI] [PubMed] [Google Scholar]
- 83.Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: Ischemia-reperfusion, aging, and heart failure. Journal of Molecular and Cellular Cardiology. 2001;33:1065–1089. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- 84.Li HL, Yin R, Chen D, Liu D, Wang D, Yang Q, Dong YG. Long-term activation of adenosine monophosphate-activated protein kinase attenuates pressure-overload-induced cardiac hypertrophy. Journal of Cellular Biochemistry. 2007;100:1086–1099. doi: 10.1002/jcb.21197. [DOI] [PubMed] [Google Scholar]
- 85.Li J, Coven DL, Miller EJ, Hu XY, Young ME, Carling D, Sinusas AJ, Young LH. Activation of AMPK alpha- and gamma-isoform complexes in the intact ischemic rat heart. Am J Physiol-Heart C. 2006;291:H1927–H1934. doi: 10.1152/ajpheart.00251.2006. [DOI] [PubMed] [Google Scholar]
- 86.Liao Y, Takashima S, Maeda N, Ouchi N, Komamura K, Shimomura I, Hori M, Matsuzawa Y, Funahashi T, Kitakaze M. Exacerbation of heart failure in adiponectin-deficient mice due to impaired regulation of AMPK and glucose metabolism. Cardiovascular Research. 2005;67:705–713. doi: 10.1016/j.cardiores.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 87.Linn TC, Pettit FH, Hucho F, Reed LJ. Alpha-keto acid dehydrogenase complexes. XI. Comparative studies of regulatory properties of the pyruvate dehydrogenase complexes from kidney, heart, and liver mitochondria. Proceedings of the National Academy of Sciences of the United States of America. 1969;64:227–234. doi: 10.1073/pnas.64.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu B, Clanachan AS, Schulz R, Lopaschuk GD. Cardiac efficiency is improved after ischemia by altering both the source and fate of protons. Circulation Research. 1996;79:940–948. doi: 10.1161/01.res.79.5.940. [DOI] [PubMed] [Google Scholar]
- 89.Liu B, el Alaoui-Talibi Z, Clanachan AS, Schulz R, Lopaschuk GD. Uncoupling of contractile function from mitochondrial TCA cycle activity and MVO2 during reperfusion of ischemic hearts. The American Journal of Physiology. 1996;270:H72–80. doi: 10.1152/ajpheart.1996.270.1.H72. [DOI] [PubMed] [Google Scholar]
- 90.Lopaschuk GD. Abnormal mechanical function in diabetes: Relationship to altered myocardial carbohydrate/lipid metabolism. Coronary Artery Disease. 1996;7:116–123. doi: 10.1097/00019501-199602000-00004. [DOI] [PubMed] [Google Scholar]
- 91.Lopaschuk GD. Optimizing cardiac energy metabolism: A new approach to treating ischaemic heart disease. Eur Heart J Suppl. 1999;1:O32–39. [Google Scholar]
- 92.Lopaschuk GD, Belke DD, Gamble J, Itoi T, Schonekess BO. Regulation of fatty acid oxidation in the mammalian heart in health and disease. Biochimica et Biophysica Acta. 1994;1213:263–276. doi: 10.1016/0005-2760(94)00082-4. [DOI] [PubMed] [Google Scholar]
- 93.Lopaschuk GD, Stanley WC. Glucose metabolism in the ischemic heart. Circulation. 1997;95:313–315. doi: 10.1161/01.cir.95.2.313. [DOI] [PubMed] [Google Scholar]
- 94.Lopaschuk GD, Tsang H. Metabolism of palmitate in isolated working hearts from spontaneously diabetic “BB” Wistar rats. Circulation Research. 1987;61:853–858. doi: 10.1161/01.res.61.6.853. [DOI] [PubMed] [Google Scholar]
- 95.Luiken JJ, Coort SL, Willems J, Coumans WA, Bonen A, van der Vusse GJ, Glatz JF. Contraction-induced fatty acid translocase/CD36 translocation in rat cardiac myocytes is mediated through AMP-activated protein kinase signaling. Diabetes. 2003;52:1627–1634. doi: 10.2337/diabetes.52.7.1627. [DOI] [PubMed] [Google Scholar]
- 96.Ma Y, Wang J, Gao J, Yang H, Wang Y, Manithody C, Li J, Rezaie AR. Antithrombin up-regulates AMP-activated protein kinase signalling during myocardial ischaemia/reperfusion injury. Thrombosis and haemostasis. 2014;113 doi: 10.1160/TH14-04-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Marin-Garcia J, Hu Y, Ananthakrishnan R, Pierpont ME, Pierpont GL, Goldenthal MJ. A point mutation in the cytb gene of cardiac mtDNA associated with complex III deficiency in ischemic cardiomyopathy. Biochemistry and Molecular Biology International. 1996;40:487–495. doi: 10.1080/15216549600201053. [DOI] [PubMed] [Google Scholar]
- 98.Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, Van den Berghe G, Carling D, Hue L. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Current Biology: CB. 2000;10:1247–1255. doi: 10.1016/s0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- 99.Martineau LC, Chadan SG, Parkhouse WS. Age-associated alterations in cardiac and skeletal muscle glucose transporters, insulin and IGF-1 receptors, and PI3-kinase protein contents in the C57BL/6 mouse. Mechanisms of Ageing and Development. 1999;106:217–232. doi: 10.1016/s0047-6374(98)00106-7. [DOI] [PubMed] [Google Scholar]
- 100.McGaffin KR, Witham WG, Yester KA, Romano LC, O’Doherty RM, McTiernan CF, O’Donnell CP. Cardiac-specific leptin receptor deletion exacerbates ischaemic heart failure in mice. Cardiovascular Research. 2011;89:60–71. doi: 10.1093/cvr/cvq288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McMullen JR, Sherwood MC, Tarnavski O, Zhang L, Dorfman AL, Shioi T, Izumo S. Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation. 2004;109:3050–3055. doi: 10.1161/01.CIR.0000130641.08705.45. [DOI] [PubMed] [Google Scholar]
- 102.McTiernan CF, Feldman AM. The role of tumor necrosis factor alpha in the pathophysiology of congestive heart failure. Current Cardiology Reports. 2000;2:189–197. doi: 10.1007/s11886-000-0068-4. [DOI] [PubMed] [Google Scholar]
- 103.Miller EJ, Li J, Leng L, McDonald C, Atsumi T, Bucala R, Young LH. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature. 2008;451:578–582. doi: 10.1038/nature06504. [DOI] [PubMed] [Google Scholar]
- 104.Mishra R, Cool BL, Laderoute KR, Foretz M, Viollet B, Simonson MS. AMP-activated protein kinase inhibits transforming growth factor-beta-induced Smad3-dependent transcription and myofibroblast trans-differentiation. Journal of Biological Chemistry. 2008;283:10461–10469. doi: 10.1074/jbc.M800902200. [DOI] [PubMed] [Google Scholar]
- 105.Mootha VK, Arai A, Kasserra C, Balaban RS. Maximum mitochondrial respiratory capacity of the mammalian heart. Faseb J. 1996;10:1886–1886. [Google Scholar]
- 106.Morrison A, Li J. PPAR-gamma and AMPK–advantageous targets for myocardial ischemia/reperfusion therapy. Biochemical Pharmacology. 2011;82:195–200. doi: 10.1016/j.bcp.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 107.Morrison A, Yan XY, Li J. Acute rosiglitazone treatment is cardioprotective against ischemia/reperfusion injury by modulating AMPK, Akt, and JNK signaling in non-diabetic mice. Circulation. 2011;124 doi: 10.1152/ajpheart.00137.2011. [DOI] [PubMed] [Google Scholar]
- 108.Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109:3161–3172. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 109.Mu J, Brozinick JT, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Molecular Cell. 2001;7:1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- 110.Murthy VK, Shipp JC. Accumulation of myocardial triglycerides ketotic diabetes; evidence for increased biosynthesis. Diabetes. 1977;26:222–229. doi: 10.2337/diab.26.3.222. [DOI] [PubMed] [Google Scholar]
- 111.Nakai N, Sato Y, Oshida Y, Yoshimura A, Fujitsuka N, Sugiyama S, Shimomura Y. Effects of aging on the activities of pyruvate dehydrogenase complex and its kinase in rat heart. Life Sciences. 1997;60:2309–2314. doi: 10.1016/s0024-3205(97)00286-5. [DOI] [PubMed] [Google Scholar]
- 112.Nascimben L, Ingwall JS, Lorell BH, Pinz I, Schultz V, Tornheim K, Tian R. Mechanisms for increased glycolysis in the hypertrophied rat heart. Hypertension. 2004;44:662–667. doi: 10.1161/01.HYP.0000144292.69599.0c. [DOI] [PubMed] [Google Scholar]
- 113.Neely JR, Morgan HE. Relationship between carbohydrate and lipid-metabolism and energy-balance of heart-muscle. Annu Rev Physiol. 1974;36:413–459. doi: 10.1146/annurev.ph.36.030174.002213. [DOI] [PubMed] [Google Scholar]
- 114.Neely JR, Rovetto MJ, Oram JF. Myocardial utilization of carbohydrate and lipids. Progress in Cardiovascular Diseases. 1972;15:289–329. doi: 10.1016/0033-0620(72)90029-1. [DOI] [PubMed] [Google Scholar]
- 115.Neely JR, Whitfiel Cf, Morgan HE. Regulation of glycogenolysis in hearts - Effects of pressure development, glucose, and Ffa. American Journal of Physiology. 1970;219:1083. doi: 10.1152/ajplegacy.1970.219.4.1083. [DOI] [PubMed] [Google Scholar]
- 116.Newsholme EA, Randle PJ. Regulation of glucose uptake by muscle. 7. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, on the concentrations of hexose phosphates, nucleotides and inorganic phosphate in perfused rat heart. The Biochemical Journal. 1964;93:641–651. doi: 10.1042/bj0930641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nohl H. Demonstration of the existence of an organo-specific NADH dehydrogenase in heart mitochondria. European Journal of Biochemistry/FEBS. 1987;169:585–591. doi: 10.1111/j.1432-1033.1987.tb13649.x. [DOI] [PubMed] [Google Scholar]
- 118.Nohl H, Hegner D. The effects of some nutritive antibiotics on the respiration of rat liver mitochondria. Biochemical Pharmacology. 1977;26:433–437. doi: 10.1016/0006-2952(77)90203-9. [DOI] [PubMed] [Google Scholar]
- 119.Odiet JA, Boerrigter ME, Wei JY. Carnitine palmitoyl transferase-I activity in the aging mouse heart. Mechanisms of Ageing and Development. 1995;79:127–136. doi: 10.1016/0047-6374(94)01552-w. [DOI] [PubMed] [Google Scholar]
- 120.Ozaki N, Sato E, Kurokawa T, Ishibashi S. Early changes in the expression of GLUT4 protein in the heart of senescence-accelerated mouse. Mechanisms of Ageing and Development. 1996;88:149–158. doi: 10.1016/0047-6374(96)01733-2. [DOI] [PubMed] [Google Scholar]
- 121.Park H, Kaushik VK, Constant S, Prentki M, Przybytkowski E, Ruderman NB, Saha AK. Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. Journal of Biological Chemistry. 2002;277:32571–32577. doi: 10.1074/jbc.M201692200. [DOI] [PubMed] [Google Scholar]
- 122.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: Possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Polekhina G, Gupta A, Michell BJ, van Denderen B, Murthy S, Feil SC, Jennings IG, Campbell DJ, Witters LA, Parker MW, Kemp BE, Stapleton D. AMPK beta subunit targets metabolic stress sensing to glycogen. Current Biology: CB. 2003;13:867–871. doi: 10.1016/s0960-9822(03)00292-6. [DOI] [PubMed] [Google Scholar]
- 124.Ponticos M, Lu QL, Morgan JE, Hardie DG, Partridge TA, Carling D. Dual regulation of the AMP-activated protein kinase provides a novel mechanism for the control of creatine kinase in skeletal muscle. The EMBO Journal. 1998;17:1688–1699. doi: 10.1093/emboj/17.6.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 126.Razeghi P, Young ME, Alcorn JL, Moravec CS, Frazier OH, Taegtmeyer H. Metabolic gene expression in fetal and failing human heart. Circulation. 2001;104:2923–2931. doi: 10.1161/hc4901.100526. [DOI] [PubMed] [Google Scholar]
- 127.Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, Liu ZX, Dong J, Mustard KJ, Hawley SA, Befroy D, Pypaert M, Hardie DG, Young LH, Shulman GI. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metabolism. 2007;5:151–156. doi: 10.1016/j.cmet.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296:1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 129.Rosca MG, Hoppel CL. Mitochondria in heart failure. Cardiovascular Research. 2010;88:40–50. doi: 10.1093/cvr/cvq240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Russell RR, 3rd, Bergeron R, Shulman GI, Young LH. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. The American Journal of Physiology. 1999;277:H643–649. doi: 10.1152/ajpheart.1999.277.2.H643. [DOI] [PubMed] [Google Scholar]
- 131.Sabbah HN, Sharov V, Riddle JM, Kono T, Lesch M, Goldstein S. Mitochondrial abnormalities in myocardium of dogs with chronic heart-failure. Journal of Molecular and Cellular Cardiology. 1992;24:1333–1347. doi: 10.1016/0022-2828(92)93098-5. [DOI] [PubMed] [Google Scholar]
- 132.Sack MN, Rader TA, Park SH, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation. 1996;94:2837–2842. doi: 10.1161/01.cir.94.11.2837. [DOI] [PubMed] [Google Scholar]
- 133.Saha AK, Schwarsin AJ, Roduit R, Masse F, Kaushik V, Tornheim K, Prentki M, Ruderman NB. Activation of malonyl-CoA decarboxylase in rat skeletal muscle by contraction and the AMP-activated protein kinase activator 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside. Journal of Biological Chemistry. 2000;275:24279–24283. doi: 10.1074/jbc.C000291200. [DOI] [PubMed] [Google Scholar]
- 134.Sambandam N, Lopaschuk GD. AMP-activated protein kinase (AMPK) control of fatty acid and glucose metabolism in the ischemic heart. Progress in Lipid Research. 2003;42:238–256. doi: 10.1016/s0163-7827(02)00065-6. [DOI] [PubMed] [Google Scholar]
- 135.Sawada M, Carlson JC. Changes in superoxide radical and lipid peroxide formation in the brain, heart and liver during the lifetime of the rat. Mechanisms of Ageing and Development. 1987;41:125–137. doi: 10.1016/0047-6374(87)90057-1. [DOI] [PubMed] [Google Scholar]
- 136.Schaper J, Froede R, Hein ST, Buck A, Hashizume H, Speiser B, Friedl A, Bleese N. Impairment of the myocardial ultrastructure and changes of the cytoskeleton in dilated cardiomyopathy. Circulation. 1991;83:504–514. doi: 10.1161/01.cir.83.2.504. [DOI] [PubMed] [Google Scholar]
- 137.Schonekess BO. Competition between lactate and fatty acids as sources of ATP in the isolated working rat heart. Journal of Molecular and Cellular Cardiology. 1997;29:2725–2733. doi: 10.1006/jmcc.1997.0504. [DOI] [PubMed] [Google Scholar]
- 138.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, Kumada M, Sato K, Schiekofer S, Ohashi K, Funahashi T, Colucci WS, Walsh K. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nature Medicine. 2004;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nature Medicine. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shioi T, McMullen JR, Tarnavski O, Converso K, Sherwood MC, Manning WJ, Izumo S. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation. 2003;107:1664–1670. doi: 10.1161/01.CIR.0000057979.36322.88. [DOI] [PubMed] [Google Scholar]
- 142.Smith SH, Kramer MF, Reis I, Bishop SP, Ingwall JS. Regional changes in creatine kinase and myocyte size in hypertensive and nonhypertensive cardiac hypertrophy. Circulation Research. 1990;67:1334–1344. doi: 10.1161/01.res.67.6.1334. [DOI] [PubMed] [Google Scholar]
- 143.Stanley WC, Hall JL, Hacker TA, Hernandez LA, Whitesell LF. Decreased myocardial glucose uptake during ischemia in diabetic swine. Metabolism: Clinical and Experimental. 1997;46:168–172. doi: 10.1016/s0026-0495(97)90297-3. [DOI] [PubMed] [Google Scholar]
- 144.Stanley WC, Hall JL, Smith KR, Cartee GD, Hacker TA, Wisneski JA. Myocardial glucose transporters and glycolytic metabolism during ischemia in hyperglycemic diabetic swine. Metabolism: Clinical and Experimental. 1994;43:61–69. doi: 10.1016/0026-0495(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 145.Stanley WC, Hall JL, Stone CK, Hacker TA, Voss SG, Whitesell LF, Eggleston AM. Acute Myocardial-Ischemia Causes a Transmural Gradient in Glucose Extraction but Not Glucose-Uptake. American Journal of Physiology. 1992;262:H91–H96. doi: 10.1152/ajpheart.1992.262.1.H91. [DOI] [PubMed] [Google Scholar]
- 146.Stanley WC, Lopaschuk GD, McCormack JG. Regulation of energy substrate metabolism in the diabetic heart. Cardiovascular Research. 1997;34:25–33. doi: 10.1016/s0008-6363(97)00047-3. [DOI] [PubMed] [Google Scholar]
- 147.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 148.Sugden PH, Clerk A. Cellular mechanisms of cardiac hypertrophy. Journal of Molecular Medicine. 1998;76:725–746. doi: 10.1007/s001090050275. [DOI] [PubMed] [Google Scholar]
- 149.Taegtmeyer H, Golfman L, Sharma S, Razeghi P, van Arsdall M. Linking gene expression to function: Metabolic flexibility in the normal and diseased heart. Annals of the New York Academy of Sciences. 2004;1015:202–213. doi: 10.1196/annals.1302.017. [DOI] [PubMed] [Google Scholar]
- 150.Taegtmeyer H, Overturf ML. Effects of moderate hypertension on cardiac function and metabolism in the rabbit. Hypertension. 1988;11:416–426. doi: 10.1161/01.hyp.11.5.416. [DOI] [PubMed] [Google Scholar]
- 151.Takagi H, Matsui Y, Hirotani S, Sakoda H, Asano T, Sadoshima J. AMPK mediates autophagy during myocardial ischemia in vivo. Autophagy. 2007;3:405–407. doi: 10.4161/auto.4281. [DOI] [PubMed] [Google Scholar]
- 152.Takahashi S, Suzuki J, Kohno M, Oida K, Tamai T, Miyabo S, Yamamoto T, Nakai T. Enhancement of the binding of triglyceride-rich lipoproteins to the very low density lipoprotein receptor by apolipoprotein E and lipoprotein lipase. The Journal of Biological Chemistry. 1995;270:15747–15754. doi: 10.1074/jbc.270.26.15747. [DOI] [PubMed] [Google Scholar]
- 153.Taylor FB, Chang A, Esmon CT, Dangelo A, Viganodangelo S, Blick KE. Protein-C prevents the coaggulopathic and lethal effects of escherichiacoli infusion in the baboon. Journal of Clinical Investigation. 1987;79:918–925. doi: 10.1172/JCI112902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Taylor FB, Stearns-Kurosawa DJ, Kurosawa S, Ferrell G, Chang ACK, Laszik Z, Kosanke S, Peer G, Esmon CT. The endothelial cell protein C receptor aids in host defense against Escherichia coli sepsis. Blood. 2000;95:1680–1686. [PubMed] [Google Scholar]
- 155.Tian R, Musi N, D’Agostino J, Hirshman MF, Goodyear LJ. Increased adenosine monophosphate-activated protein kinase activity in rat hearts with pressure-overload hypertrophy. Circulation. 2001;104:1664–1669. doi: 10.1161/hc4001.097183. [DOI] [PubMed] [Google Scholar]
- 156.Tian R, Nascimben L, Ingwall JS, Lorell BH. Failure to maintain a low ADP concentration impairs diastolic function in hypertrophied rat hearts. Circulation. 1997;96:1313–1319. doi: 10.1161/01.cir.96.4.1313. [DOI] [PubMed] [Google Scholar]
- 157.Turer AT, Malloy CR, Newgard CB, Podgoreanu MV. Energetics and metabolism in the failing heart: important but poorly understood. Curr Opin Clin Nutr. 2010;13:458–465. doi: 10.1097/MCO.0b013e32833a55a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.van der Vusse GJ, van Bilsen M, Glatz JF. Cardiac fatty acid uptake and transport in health and disease. Cardiovascular Research. 2000;45:279–293. doi: 10.1016/s0008-6363(99)00263-1. [DOI] [PubMed] [Google Scholar]
- 159.Ventura-Clapier R, Garnier A, Veksler V. Energy metabolism in heart failure. J Physiol-London. 2004;555:1–13. doi: 10.1113/jphysiol.2003.055095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Walker FJ, Fay PJ. Regulation of blood-coagulation by the protein-C system. Faseb J. 1992;6:2561–2567. doi: 10.1096/fasebj.6.8.1317308. [DOI] [PubMed] [Google Scholar]
- 161.Wall SR, Lopaschuk GD. Glucose-oxidation rates in fatty acid-perfused isolated working hearts from diabetic rats. Biochimica et Biophysica Acta. 1989;1006:97–103. doi: 10.1016/0005-2760(89)90328-7. [DOI] [PubMed] [Google Scholar]
- 162.Wambolt RB, Henning SL, English DR, Dyachkova Y, Lopaschuk GD, Allard MF. Glucose utilization and glycogen turnover are accelerated in hypertrophied rat hearts during severe low-flow ischemia. Journal of Molecular and Cellular Cardiology. 1999;31:493–502. doi: 10.1006/jmcc.1998.0804. [DOI] [PubMed] [Google Scholar]
- 163.Wang J, Yang L, Rezaie AR, Li J. Activated protein C protects against myocardial ischemic/reperfusion injury through AMP-activated protein kinase signaling. Journal of Thrombosis and Haemostasis: JTH. 2011;9:1308–1317. doi: 10.1111/j.1538-7836.2011.04331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Weiss JN, Lamp ST. Glycolysis preferentially inhibits ATP-sensitive K +channels in isolated guinea pig cardiac myocytes. Science. 1987;238:67–69. doi: 10.1126/science.2443972. [DOI] [PubMed] [Google Scholar]
- 165.Wieland O, Funcke H, Loffler G. Interconversion of pyruvate dehydrogenase in rat heart muscle upon perfusion with fatty acids or ketone bodies. FEBS Letters. 1971;15:295–298. doi: 10.1016/0014-5793(71)80641-5. [DOI] [PubMed] [Google Scholar]
- 166.Wieland O, Siess E, Schulze-Wethmar FH, von Funcke HG, Winton B. Active and inactive forms of pyruvate dehydrogenase in rat heart and kidney: Effect of diabetes, fasting, and refeeding on pyruvate dehydrogenase interconversion. Archives of Biochemistry and Biophysics. 1971;143:593–601. doi: 10.1016/0003-9861(71)90244-x. [DOI] [PubMed] [Google Scholar]
- 167.Wojtaszewski JFP, Jorgensen SB, Hellsten Y, Hardie DG, Richter EA. Glycogen-dependent effects of 5-aminoimidazole-4-carboxamide (AICA)-riboside on AMP-activated protein kinase and glycogen synthase activities in rat skeletal muscle. Diabetes. 2002;51:284–292. doi: 10.2337/diabetes.51.2.284. [DOI] [PubMed] [Google Scholar]
- 168.Yamanouchi K, Nakajima H, Shinozaki T, Chikada K, Kato K, Oshida Y, Osawa I, Sato J, Sato Y, Higuchi M, et al. Effects of daily physical activity on insulin action in the elderly. Journal of Applied Physiology. 1992;73:2241–2245. doi: 10.1152/jappl.1992.73.6.2241. [DOI] [PubMed] [Google Scholar]
- 169.Yan J, Young ME, Cui L, Lopaschuk GD, Liao R, Tian R. Increased glucose uptake and oxidation in mouse hearts prevent high fatty acid oxidation but cause cardiac dysfunction in diet-induced obesity. Circulation. 2009;119:U2818–2131. doi: 10.1161/CIRCULATIONAHA.108.832915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yang LK, Bae JS, Manithody C, Rezaie AR. Identification of a specific exosite on activated protein C for interaction with protease-activated receptor 1. Journal of Biological Chemistry. 2007;282:25493–25500. doi: 10.1074/jbc.M702131200. [DOI] [PubMed] [Google Scholar]
- 171.Yeh LA, Lee KH, Kim KH. Regulation of rat-liver acetyl-Coa carboxylase - Regulation of phosphorylation and inactivation of acetyl-Coa carboxylase by the adenylate energy-charge. Journal of Biological Chemistry. 1980;255:2308–2314. [PubMed] [Google Scholar]
- 172.Young LH, Renfu Y, Russell R, Hu X, Caplan M, Ren J, Shulman GI, Sinusas AJ. Low-flow ischemia leads to translocation of canine heart GLUT-4 and GLUT-1 glucose transporters to the sarcolemma in vivo. Circulation. 1997;95:415–422. doi: 10.1161/01.cir.95.2.415. [DOI] [PubMed] [Google Scholar]
- 173.Young LH, Renfu Y, Russell R, Hu XY, Caplan M, Ren JM, Shulman GI, Sinusas AJ. Low-flow ischemia leads to translocation of canine heart GLUT-4 and GLUT-1 glucose transporters to the sarcolemma in vivo. Circulation. 1997;95:415–422. doi: 10.1161/01.cir.95.2.415. [DOI] [PubMed] [Google Scholar]
- 174.Young ME, Laws FA, Goodwin GW, Taegtmeyer H. Reactivation of peroxisome proliferator-activated receptor alpha is associated with contractile dysfunction in hypertrophied rat heart. The Journal of Biological Chemistry. 2001;276:44390–44395. doi: 10.1074/jbc.M103826200. [DOI] [PubMed] [Google Scholar]
- 175.Young ME, Radda GK, Leighton B. Activation of glycogen phosphorylase and glycogenolysis in rat skeletal muscle by AICAR–an activator of AMP-activated protein kinase. FEBS Letters. 1996;382:43–47. doi: 10.1016/0014-5793(96)00129-9. [DOI] [PubMed] [Google Scholar]
- 176.Yue P, Massie BM, Simpson PC, Long CS. Cytokine expression increases in nonmyocytes from rats with postinfarction heart failure. The American Journal of Physiology. 1998;275:H250–258. doi: 10.1152/ajpheart.1998.275.1.H250. [DOI] [PubMed] [Google Scholar]
- 177.Zelissen PM, Stenlof K, Lean ME, Fogteloo J, Keulen ET, Wilding J, Finer N, Rossner S, Lawrence E, Fletcher C, McCamish M, Author G. Effect of three treatment schedules of recombinant methionyl human leptin on body weight in obese adults: A randomized, placebo-controlled trial. Diabetes, Obesity & Metabolism. 2005;7:755–761. doi: 10.1111/j.1463-1326.2005.00468.x. [DOI] [PubMed] [Google Scholar]
- 178.Zhang J, Duncker DJ, Ya X, Zhang Y, Pavek T, Wei H, Merkle H, Ugurbil K, From AH, Bache RJ. Effect of left ventricular hypertrophy secondary to chronic pressure overload on transmural myocardial 2-deoxyglucose uptake. A 31P NMR spectroscopic study. Circulation. 1995;92:1274–1283. doi: 10.1161/01.cir.92.5.1274. [DOI] [PubMed] [Google Scholar]
- 179.Zhou GC, Myers R, Li Y, Chen YL, Shen XL, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. Journal of Clinical Investigation. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zong HH, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]