Animals can evaluate the quality and availability of food in their environment and adjust their development and reproductivity to better accommodate changes. Extensive previous studies have provided valuable insights into the mechanisms by which animals sense amino acids and carbohydrate levels through several signaling systems, including the TORC1 and insulin receptor pathways, respectively.1 However, potential mechanisms underlying the interaction between another major class of nutrients, nucleotides, and developmental programs, remain to be explored. We recently uncovered a cellular system that regulates germline development in response to changes in nucleotide levels, using a model system that employs mutations in both the nematode C. elegans and its food (E. coli).2 Genetic mutations that disrupt 2 cytidine deaminases, CDD-1 and CDD-2, in the pyrimidine salvage pathway rendered the worm hypersensitive to the level of uridine/thymidine in the food source; when fed a uridine/thymidine-poor diet, this double mutant arrests germ cell proliferation and becomes sterile. The cdd-1/2 double mutant enabled us to identify genetically modified E. coli strains that serve as a uridine/thymidine-rich food source. More importantly, we further identified a critical role of the Notch signaling pathway in worms, known to regulate germline stem cell renewal and proliferation,3 in mediating the impact of uridine/thymidine level on germline proliferation. Interestingly, our study did not detect prominent roles of the TORC1 or insulin receptor pathways, implying a mechanistic difference between systems sensing different nutrients.
This nucleotide sensing mechanism likely plays an important protective role for worms that encounter unfavorable environment/food conditions. Cellular nutrient levels in animals are impacted by both the nutrient availability in the environment/food sources and the status of endogenous metabolic pathways that may be impacted by toxins or other stress conditions. In the cdd-1/2 double mutants fed food with low uridine/thymidine levels, the salvage pathway is compromised and the pyrimidines produced through the de novo biogenesis pathway are insufficient to support the growth and development of both somatic tissues and the rapidly proliferating germline. By shutting down germline proliferation through a “check-point” like function of the Notch pathway, animals would avoid irreversible, deleterious damage to both the mother and the progeny caused by exhausting the limited nucleotide pool (Fig. 1).
Figure 1.
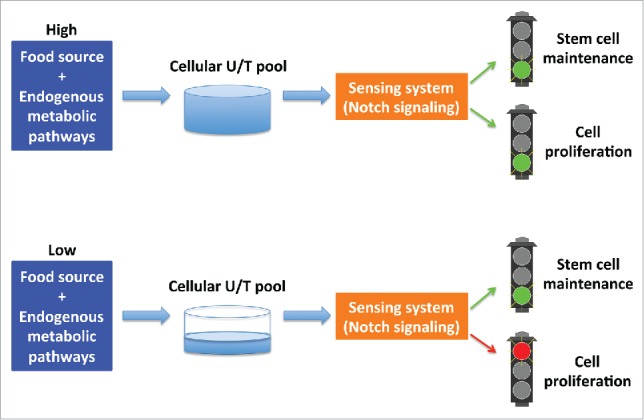
The cellular uridine/thymidine (U/T) pool is impacted by the nucleotides from both food source and endogenous metabolic pathways. The downstream sensing system, including the Notch signaling pathway, senses the nucleotide abundance and regulates the rate of cell proliferation to protect animals/cells from deleterious damage caused by exhausting the nucleotide pool.
Previous studies in Drosophila showed that different types of stem and progenitor cells were able to utilize the insulin signaling pathway to sense nutritional signals (amino acids, glucose and fat) and tune the proliferation rate of these cells to match the available nutrients.4 In C. elegans, it also has been reported that the proliferation of germline stem cells (GSCs) can be regulated by both insulin and TORC1 signaling pathways.5 Similarly, in our study, the decreased Notch activity observed under the low nucleotide condition decreases the GSC proliferation rate, presumably for preservation of the system and restoration of fertility once the environmental condition is improved. Given that these pathways are highly conserved across species, these findings can provide insights for the nutrient sensing mechanism of stem and progenitor cells in higher organisms.
Nucleotide homeostasis is tightly linked to human health and pathological conditions including cancer development. Because the characteristic, uncontrolled proliferation of cancer cells requires a high nucleotide supply, cytotoxic nucleotide analogs have thus been widely used as chemotherapeutic drugs for cancer treatment.6 However, drug resistance has become a major impediment in clinical oncology and one of the major causes of cancer relapse after chemotherapy.7 Worm germ cells and human cancer cells share several key features including robust proliferation, high nutrient demands and possession of a subpopulation of stem cells. The mechanism of nucleotide sensing in the worm may thus provide insight into the drug resistance mechanism in cancer cells. Cancer cells resistant to treatment with nucleotide analogs may have inefficient cellular uptake, impaired nucleotide transporters, or be physically difficult to reach due to their location within the central part of a solid tumor. Due to these characteristics, these cancer cells may experience a reduced level of cytotoxic analog treatment, may be considered “nucleotide starved” under these conditions, and may enter a quiescent state similar to the germline arrest we see in our uridine/thymidine-deficient worms. These surviving resistant cells retain the potential to restore proliferation and generate a new tumor after the drug withdrawal. Therefore, greater understanding of the nucleotide sensing mechanism may guide us to a new direction to eradicate drug resistant cancer cells.
The role of the Notch signaling in modulating germline proliferation in response to changes in nucleotide abundance could be conserved in animals. Future mechanistic study of this sensing mechanism, most interestingly the search for the upstream sensor that bridges the nucleotide levels and Notch activity, will help us not only better understand the molecular detail of the sensing pathway, but also develop new therapeutic approaches to treat diseases linked to nucleotide metabolism.
We thank Minh Than and Aileen Sewell for discussion.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Efeyan A, et al.. Nature 2015; 517:302-10. PMID:25592535; http://dx.doi.org/ 10.1038/nature14190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chi C, et al.. Genes & development 2016; 30:307-20. PMID:26833730; http://dx.doi.org/ 10.1101/gad.275107.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kimble J, et al.. StemBook. Cambridge (MA: ), 2008. PMID:24354021; http://dx.doi.org/ 10.3824/stembook.1.95.1 [DOI] [Google Scholar]
- [4].Shim J, et al.. Development 2013; 140:4647-56. PMID:24255094; http://dx.doi.org/ 10.1242/dev.079087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Korta DZ, et al.. Development 2012; 139:859-70. PMID:22278922; http://dx.doi.org/ 10.1242/dev.074047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Galmarini CM, et al.. Lancet Oncol 2002; 3:415-24. PMID:12142171; http://dx.doi.org/ 10.1016/S1470-2045(02)00788-X [DOI] [PubMed] [Google Scholar]
- [7].Zahreddine H, et al.. Front Pharmacol 2013; 4:28. PMID:23504227; http://dx.doi.org/ 10.3389/fphar.2013.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]


