Abstract
Background
Physicians’ perception may not parallel objective measures of therapeutic targets in patients with diabetes. This is an issue rarely addressed in the medical literature. We aimed to analyze physicians’ perception and characteristics of adequate control of patients with diabetes.
Patients and methods
We studied information on physicians and their patients who participated in the third wave of the International Diabetes Management Practices Study registry in Mexico. This analysis was performed on 2,642 patients, 203 with type 1 diabetes mellitus (T1DM) and 2,439 with type 2 diabetes mellitus (T2DM), treated by 200 physicians.
Results
The patients perceived at target had lower hemoglobin A1c (HbA1c) and fasting blood glucose than those considered not at target. However, overestimation of the frequency of patients with HbA1c <7% was 41.5% in patients with T1DM and 31.7% in patients with T2DM (underestimation: 2.8% and 8.0%, respectively). The agreement between the physicians’ perception and the class of HbA1c was suboptimal (κ: 0.612). Diabetologists and endocrinologists tested HbA1c more frequently than primary care practitioners, internists, or cardiologists; however, no differences were observed in mean HbA1c, for both T1DM (8.4% vs 7.2%, P=0.42) and T2DM (8.03% vs 8.01%, P=0.87) patients. Nevertheless, insulin users perceived at target, who practiced self-monitoring and self-adjustment of insulin, had a lower mean HbA1c than patients without these characteristics (mean HbA1c in T1DM: 6.8% vs 9.6%, respectively; mean HbA1c in T2DM: 7.0% vs 10.1%, respectively).
Conclusion
Although there is a significant physicians’ overestimation about the optimal glycemic control, this global impression and characteristics of patients’ empowerment, such as self-monitoring and self-adjustment of insulin, are associated with the achievement of targets.
Keywords: A1c, care, diabetes, HbA1c, goal, IDMPS, insulin, management, Mexico, opinion, self-monitoring, treat to target
Introduction
According to the National Health and Nutrition Survey 2006 (ENSANUT 2006), 14% of adults live with diabetes in Mexico, with half of the cases being newly diagnosed patients identified during population screening.1 This epidemiological profile represents a doubling in the frequency of diabetes since 1993.2 Hence, diabetes-related late complications are also increasing in Mexico, so that by the year 2014, diabetes was considered the leading cause of general mortality, with more than 80,000 deaths attributed to its related acute and chronic complications.3 This changing paradigm in public health has been explained by the steep rise in the frequency of overweight, in the context of the economical and cultural transition toward a lifestyle of excessive energy intake and low expenditure.4–6
Adequate diabetes care and patients’ knowledge and adherence reduce acute and long-term complications.7,8 However, diabetes is very often diagnosed late and standards of care are not followed as recommended, especially in developing countries.9–11 Physicians may overestimate the effectiveness of the care they provide,12 which may impose a barrier in attaining therapeutic targets in an effective manner. This phenomenon has been demonstrated in the management of chronic diseases such as hypertension and dyslipidemia.12–15 However, patient’s and physician’s perception in relation to diabetes control has been poorly assessed.16–18 Empowerment has been recognized as a patient-centered model of care that enhances adherence and improves aspects of diabetes control and the perception of quality of life.19–25 Empowerment has also been associated with a reduced frequency of chronic complications.26 In fact, lower levels of empowerment are associated with more barriers for an effective management and with poor control.27,28 The objective of the present report is to describe the characteristics of management of Mexican patients with diabetes in relation to their physicians’ perception and characteristics of patients’ empowerment, such as self-monitoring and self-control.
Patients and methods
Study design
The International Diabetes Management Practices Study (IDMPS) was a 5-year multinational survey designed to provide information regarding the clinical practice and care delivered to patients with type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) in developing countries, especially about insulin usage patterns.9,10,29,30 The main objective of this registry is to document changes in clinical practice over a 5-year period, starting in November 2005, and organize recruitment in five waves (every 12 months each). The Internal Committee of Ethics of each participating center reviewed and approved the patients’ enrollment. Approval from a single institutional review board was not obtained as IDMPS was a global multinational registry. Signed informed consent was required for every patient in order to be enrolled in the study.
Participants
The IDMPS was an observational project composed of five registries (the so-called waves) in a 5-year period to assess the current practices in the management of subjects with diabetes. Each wave consisted of two phases: a 2-week cross-sectional registry and a 9-month longitudinal survey. A 3-month interval separated the end of the longitudinal survey and the start of the next wave. Information on the 2007 cross-sectional registry (the third wave) carried on in Mexico is analyzed in the present report. The number of subjects to be recruited in each participating country was determined on a country basis. Based on the assumption that insulin is the least prescribed therapy, the sample was determined in order to establish the frequency of insulin-treated patients. Physicians experienced with insulin management were invited to participate with a maximum of ten patients with T2DM and five with T1DM. Patients of both sexes, aged ≥18 years, who were visiting the physician during the recruitment period of the cross-sectional phase, were selected for the registry. Patients were excluded if they had concomitant participation in another clinical descriptive or interventional study, if they participated in a previous wave of IDMPS, or if they were under current temporary insulin treatment (gestational diabetes, surgery, pancreas cancer, sepsis, and other conditions).
Measures
The information was collected on standardized case report forms (CRFs) about demographics, medical history, pharmacologic and lifestyle therapy, glycemic control and other therapeutic targets, blood glucose self-monitoring, access to diabetes education, access to specialized care, hospitalizations, and medical complications and work absenteeism, among other variables. CRFs contained the question “Is the patient at target?” at the end of the document, and physicians were instructed to answer this question based on personal opinion about the global status of control of their patients. The possible standardized answers to this question were “yes”, “no”, and “unknown”. This question was not addressed immediately after the section of hemoglobin A1c (HbA1c) and other biochemical measurements in order to avoid perception bias toward a specific metabolic profile. Nevertheless, each investigator filled the CRFs completely, including the laboratory results and the qualitative questions. For the present analysis, our hypothesis was that physicians often overestimate the frequency of the patients at therapeutic target. We regarded HbA1c as pivotal for considering a patient at target, in such a way that if a patient had HbA1c ≥7%, he/she should not be considered under optimal control, even if blood pressure, lipids, body weight, and other variables were at target.
Statistical analyses
Parametric continuous variables were expressed as geometric mean and standard deviation (SD) or minimum and maximum. Categorical variables were expressed as percentages. To compare quantitative variables distributed between the two groups, Student’s t-test or Mann–Whitney U-test was performed in distributions of parametric and nonparametric variables, respectively. Chi-square statistics (ie, Pearson’s chi-squared test or Fisher’s exact test, as corresponded) were used to compare nominal variables in univariate analyses. Kappa test was used to assess the agreement between physicians’ perception and the frequency of patients being at target HbA1c. All P-values were two sided and regarded as significant when P<0.05. Statistical analyses were conducted with the SAS software Version 8.02.
Results
A total of 200 physicians included at least one patient in the third wave of the IDMPS project. Participating physicians were clustered for this analysis in two medical practice groups: 58 endocrinologists/diabetologists and 57 primary care practitioners/internists/cardiologists. The specialty was missing for 85 physicians. Endocrinologists/diabetologists had been practicing medicine for 17.9 years on average and general practitioners/internists/cardiologists for 18.4 years. On average, endocrinologists/diabetologists reported that they usually see 127 patients per month and primary care practitioners/internists/cardiologists declared that they see 103 patients per month. A total of 3,052 patients were recruited, and 2,642 (87%) met the eligibility criteria, among whom 203 patients with T1DM (60.6% women, mean age 31.26 years, median 27 years, 76.8% <40 years) and 2,439 patients with T2DM (60.2% women, mean age 56.7 years, median 57 years, 9.3% <40 years) are described here (Table 1).
Table 1.
Characteristics of Mexican patients with diabetes mellitus included in the 3-week IDMPS study
| Basal characteristics | T1DM patients (n=203) | T2DM patients (n=2,439) |
|---|---|---|
| Age (years), median (IQ range) | 27.0 (22–54) | 57.0 (32–88) |
| Female, % | 60.6 | 60.2 |
| Residency in urban areas, % | 91.0 | 89.0 |
| Illiteracy, % | 2.2 | 5.7 |
| University education, % | 60.6 | 32.1 |
| Private health insurance, % | 11.1 | 9.2 |
| Public health insurance, % | 62.4 | 52.8 |
| Time since diagnosis of diabetes | ||
| <1 year, % | 8.5 | 17.3 |
| 1–4 years, % | 21.1 | 21.8 |
| 5–9 years, % | 23.1 | 25.6 |
| 10–19 years, % | 29.6 | 26.0 |
| ≥20 years, % | 17.6 | 9.2 |
| History of hypertension, % | 23.2 | 54.8 |
| Current smoking status, % | 7.9 | 6.6 |
| Any diabetes-related complication, % | 39.1 | 45.2 |
| BMI, mean (SD) | 24.62 (4.27) | 29.60 (5.78) |
| Waist circumference (cm), mean (SD) | 83.84 (11.87) | 98.81 (13.63) |
| SBP (mmHg), mean (SD) | 114.94 (18.23) | 126.76 (17.46) |
| DBP (mmHg), mean (SD) | 73.60 (10.05) | 77.61 (9.60) |
| Fasting glycemia (mg/dL), mean (SD) | 161.62 (80.90) | 166.42 (78.03) |
| HbA1c, mean (SD) | 8.80 (2.34) | 7.99 (2.14) |
| Total cholesterol (mg/dL), mean (SD) | 179.59 (49.85) | 194.00 (47.49) |
| Triglycerides (mg/dL), mean (SD) | 128.63 (114.74) | 197.09 (118.61) |
Abbreviations: IDMPS, International Diabetes Management Practices Study; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; IQ, interquartile; BMI, body mass index; SD, standard deviation; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c.
Of the patients with T1DM, 91% lived in urban areas and 60.6% had university education, whereas 89% of subjects with T2DM lived in urban conglomerates and only 32% had university education. Most patients received public health insurance (62.4% of subjects with T1DM and 52.8% of subjects with T2DM). Among patients with T1DM, 70.3% had diabetes >5 years since diagnosis. In patients with T2DM, 60.8% had diabetes >5 years since diagnosis. Any late diabetes complication was identified in 39.1% of patients with T1DM and 45.2% of patients with T2DM. The mean age and time since diabetes diagnosis of patients seen by endocrinologists/diabetologists were lower than those patients seen by general practitioners/internists/cardiologists in both T1DM (mean age: 27.6 years vs 36.9 years, respectively; mean diabetes evolution: 10.8 years vs 12.4 years, respectively) and T2DM groups (mean age: 55.4 years vs 57.4 years, respectively; mean diabetes evolution: 8.8 years vs 9.6 years, respectively).
In all, 65.3% of patients with T1DM and 47.8% of patients with T2DM received any type of diabetes education. In patients with T2DM, diabetes education was more frequent among insulin users (49% in insulin users and 56.2% in insulin plus oral agents users) than among patients on oral agents or on diet and exercise (46% in oral agents users and 25.6% of patients on diet and exercise exclusively; P<0.001). Among those who had received diabetes education, individual training occurred in 58.9% of patients with T1DM and 69.7% of patients with T2DM. However, 12.5% of patients with T1DM pertained to any patient-centered diabetes association, in contrast with 3.7% of subjects with T2DM.
All patients with T1DM were users of insulin (78.8% insulin treatment alone and 21.2% a combination of therapies), whereas 33.2% of subjects with T2DM were on insulin therapy (10.9% insulin treatment alone and 22.3% a combination of oral agents and insulin; Table 2). Vials plus syringes was the most common means to administer insulin in both T1DM (72.4%) and T2DM (62.3%) groups. Only 10.8% of patients with T1DM and 15.8% of patients with T2DM were users of disposable pens, while 12.3% of patients with T1DM and 20% of patients with T2DM used reusable pens. Self-injection of insulin was usually done by 91.1% of subjects with T1DM and 65.3% of subjects with T2DM.
Table 2.
Characteristics of treatment of Mexican patients with diabetes mellitus included in the 3-week IDMPS study
| Variable | T1DM patients | T2DM patients
|
Total T2DM patients | |||
|---|---|---|---|---|---|---|
| OGLD treatment alone | Insulin treatment alone | OGLD + insulin | Diet and exercise | |||
| Class of OGLD treatment, % | ||||||
| Metformin | 38.5 | 20.6 | 0 | 26.8 | 0 | 22.2 |
| Sulfonylureas | 7.7 | 10.2 | 0 | 8.2 | 0 | 9.6 |
| Metformin + sulfonylureas | 17.9 | 45.3 | 0 | 41.2 | 0 | 44.2 |
| Other | 35.9 | 23.9 | 0 | 23.7 | 0 | 23.9 |
| Current insulin treatment, % | ||||||
| Basal alone | 28.0 | 0 | 65.3 | 71.0 | 0 | 69.1 |
| Basal + prandial | 60.0 | 0 | 13.2 | 11.2 | 0 | 11.9 |
| Others | 1.5 | 0 | 3.4 | 1.8 | 0 | 2.3 |
| Prandial alone | 3.0 | 0 | 1.9 | 1.3 | 0 | 1.5 |
| Premix alone | 7.5 | 0 | 16.2 | 14.7 | 0 | 15.2 |
| Detail of prandial insulin for basal + prandial scheme, % | ||||||
| Basal + aspart | 12.6 | 0 | 11.4 | 5.0 | 0 | 7.4 |
| Basal + lispro | 62.2 | 0 | 48.6 | 48.3 | 0 | 48.4 |
| Basal + regular insulin | 23.5 | 0 | 37.1 | 26.7 | 0 | 30.5 |
| Basal + glulisine | 1.7 | 0 | 2.9 | 20.0 | 0 | 13.7 |
| Detail of basal insulin for basal + prandial scheme, % | ||||||
| Prandial + NPH | 51.3 | 0 | 40.0 | 52.5 | 0 | 47.9 |
| Prandial + glargine | 39.5 | 0 | 51.4 | 33.9 | 0 | 40.4 |
| Prandial + detemir | 8.4 | 0 | 5.7 | 6.8 | 0 | 6.4 |
| Prandial + other basal | 0.8 | 0 | 2.9 | 6.8 | 0 | 5.3 |
Abbreviations: IDMPS, International Diabetes Management Practices Study; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; OGLD, oral glucose lowering drug; NPH, neutral protamine Hagedorn.
The majority of patients, especially insulin users, had a glucometer at home (Table 3). In general, self-monitoring of fasting glycemia occurred on a daily basis in patients with T1DM and every other day in patients with T2DM. In contrast, prandial glucose monitoring was recorded in 57.8% of patients with T1DM and only 26.1% of patients with T2DM. Prandial glucose monitoring occurred less than one time per day in both T1DM and T2DM groups. Surprisingly, either fasting or prandial self-monitoring was more frequent among patients on lifestyle intervention than among insulin users (Table 3).
Table 3.
Characteristics of insulin treatment and achievement of therapeutic targets in Mexican patients with diabetes mellitus included in the 3-week IDMPS study
| Variable | T1DM patients | T2DM patients
|
Total T2DM patients | |||
|---|---|---|---|---|---|---|
| OGLD treatment alone | Insulin treatment alone | OGLD + insulin | Diet and exercise | |||
| Patients having glucometer at home, % | 84.2 | 51.7 | 68.4 | 70.8 | 29.3 | 56.9 |
| Patient self-monitoring of fasting capillary blood glucose, % | 80.5 | 50.2 | 62.5 | 70.1 | 28.4 | 55.2 |
| Frequency of fasting self-monitoring per month, mean (SD) | 31.3 (28.2) | 9.4 (10.8) | 15.7 (17.0) | 14.2 (12.4) | 11.6 (20.0) | 11.6 (12.6) |
| Last self-monitoring fasting capillary blood glucose, mean (SD), mg/dL | 141.9 (69.6) | 141.4 (59.9) | 151.0 (56.2) | 154.9 (65.4) | 114.8 (23.4) | 145.9 (61.0) |
| Patient self-monitoring of prandial capillary blood glucose, % | 57.8 | 22.7 | 29.9 | 36.4 | 9.3 | 26.1 |
| Patients at target HbA1c <7%, % | 20.9 | 46.2 | 19.3 | 19.4 | 46.3 | 36.8 |
| Patients at target HbA1c <7% + BP <130/80 mmHg + LDL <100 mg/dL, % | 5.3 | 3.7 | 0.4 | 1.2 | 2.9 | 2.7 |
| Frequency of prandial self-monitoring per month, mean (SD) | 19.7 (22.0) | 7.8 (8.2) | 8.4 (9.5) | 10.3 (11.5) | 17.9 (30.7) | 8.8 (10.1) |
| Last self-monitoring prandial capillary blood glucose, mean (SD), mg/dL | 177.9 (73.9) | 161.5 (64.1) | 173.5 (74.7) | 184.8 (71.9) | 124.8 (24.3) | 169.7 (68.5) |
| Patients perceived at target by their treating physicians, % | 30.5 | 43.2 | 34.6 | 28.4 | 41.3 | 38.9 |
| Perceived reasons for not reaching the target | ||||||
| No compliance to lifestyle recommendations, % | 50 | 50.4 | 43.9 | 46.1 | 37.8 | 48.2 |
| No compliance to drug treatments, % | 8.8 | 13.0 | 11.5 | 12.5 | 27.0 | 13.0 |
| Concomitant illness, % | 5.9 | 5.3 | 16.6 | 11.9 | 2.7 | 8.3 |
| Weight concern, % | 1.5 | 6.7 | 4.5 | 4.1 | 0 | 5.5 |
| Hypoglycemia events, % | 5.1 | 0.3 | 2.5 | 2.2 | 0 | 1.0 |
| Insufficient education, % | 13.2 | 17.1 | 18.5 | 11.9 | 18.9 | 15.9 |
| No self-monitoring of blood glucose, % | 1.5 | 2.0 | 1.9 | 1.6 | 2.7 | 1.9 |
| Insufficient self-monitoring of blood glucose, % | 3.7 | 3.1 | 6.4 | 5.1 | 2.7 | 4.1 |
| Lack of efficacy of current therapy, % | 5.1 | 7.5 | 6.4 | 8.7 | 2.7 | 7.6 |
| Initiation of insulin therapy in basal visit according to results, % | 26.5 | 20.4 | 14.6 | 21.2 | 50.0 | 20.2 |
| Stop of insulin therapy in basal visit according to results, % | 10.0 | 0.9 | 7.6 | 12.2 | 1.1 | 4.1 |
| Change of insulin therapy in basal visit according to results, % | 39.6 | 7.3 | 32.0 | 38.9 | 20.0 | 28.4 |
Abbreviations: IDMPS, International Diabetes Management Practices Study; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; SD, standard deviation; HbA1c; hemoglobin A1c; BP, blood pressure; LDL, low-density lipoprotein; OGLD, oral glucose lowering drug.
The patients perceived at target had a lower mean HbA1c (in patients with T1DM: 7.12% vs 9.56%; in patients with T2DM: 7.26% vs 9.51%; in both comparisons P<0.001) and fasting blood glucose (in patients with T1DM: 128.0 mg/dL vs 178.3 mg/dL; in patients with T2DM: 133.6 mg/dL vs 203.54 mg/dL; in both comparisons P<0.001) than those considered not at target. However, overestimation of the frequency of patients with HbA1c <7% was 41.5% in patients with T1DM and 31.7% in patients with T2DM (underestimation: 2.8% and 8.0%, respectively). The agreement between physician’s perception and class of HbA1c was suboptimal (κ: 0.612). Diabetologists and endocrinologists tested HbA1c more frequently than primary care practitioners, internists, or cardiologists put together (90.3% vs 67.6%, P=0.002); however, no differences were observed in the mean HbA1c for both patients with T1DM (8.4% vs 7.2%, respectively; P=0.42) and patients with T2DM (8.03% vs 8.01%, respectively; P=0.87).
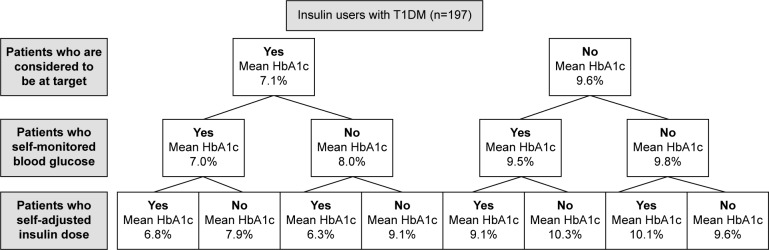
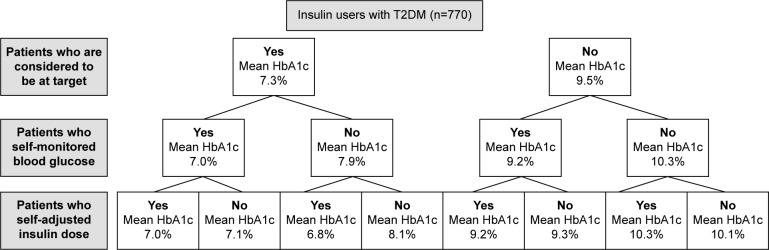
Among insulin users, self-adjustment of insulin dose was practiced by 63.2% of patients with T1DM and by only 29.7% of patients with T2DM. In both T1DM and T2DM groups of insulin users, there was a positive interaction between physicians’ perception, patient’s self-monitoring, and self-adjustment of insulin dose (Figures 1 and 2), so that individuals having all these three characteristics had a mean HbA1c significantly lower than patients who did not have any (in both comparisons, P<0.001).
Figure 1.
Mean last HbA1c levels of insulin-treated patients with T1DM.
Abbreviations: HbA1c, hemoglobin A1c; T1DM, type 1 diabetes mellitus.
Figure 2.
Mean last HbA1c levels of insulin-treated patients with T2DM.
Abbreviations: HbA1c, hemoglobin A1c; T2DM, type 2 diabetes mellitus.
Discussion
The primary objective of this study was to assess the therapeutic management of patients with diabetes in the current medical practice in Mexico.29,30 It is confirmed here previous observations on the wide gap prevailing between current recommendations and the actual standards of care delivered to Mexican patients with diabetes.29–32 As previously observed in Mexican and international populations,9,16 physicians tend to overestimate their performance in terms of achieving therapeutic goals. Nonetheless, as we expected, the physician’s good impression on the global achievement of therapeutic targets as well as characteristics of patient’s empowerment (self-monitoring and self-adjustment of insulin dose) interacted positively in attaining the HbA1c goal. Our results suggest that the physician’s perception solely is not a good indicator of the quality of care, but this characteristic of patients who practice self-monitoring and self-adjustment of insulin, provided that they received diabetes education on these characteristics of patients’ empowerment, performed better in terms of HbA1c goals.
Patient empowerment is a multidimensional concept in evolution that was initially defined as “the process whereby patients have the knowledge, skills, attitudes and self-awareness necessary to influence their own behavior and that of others in order to improve the quality of their lives”.33 This concept assumes that changes in self-efficacy are associated with improvements in the quality of diabetes control that ultimately impact on meaningful outcomes. However, this psychosocial approach of the phenomenon based on the evaluation of attitudes and perceptions not always corresponds with better health control.34 When more objective measurements of patients’ participation in self-care are studied, characteristics of a proactive empowerment have been associated with better diabetes outcomes, either in observational or interventional studies.19–28 Thus, the evolving concept of empowerment should ideally include changes and assessments in perceptions, attitudes, level of engagement, adherence, participation in education programs, active and flexible diet changes in response to daily glucose levels, glucose and blood pressure self-monitoring, as well as self-adjustment of medications with a special focus on insulin dosing, among other variables that characterize patients with sufficient knowledge to make rational decisions on their own care.19 Among these characteristics, self-management is the most consistently associated characteristic with better outcomes,35 including resource utilization and glucose endpoints.24,36
Another important observation of the present analysis is that, surprisingly, diabetes specialists performed equal to general practitioners, internists, and cardiologists as a group, in spite of a more frequent laboratory monitoring of patients with T1DM and patients with T2DM by the diabetes-trained physicians. It is important to address that in some institutions, the endocrinologist/diabetologist reviews come as a second-level strategy after the primary care failed to control difficult cases.4 However, although it cannot be ruled out the possibility that diabetes specialists had the most complicated cases, in the present report, we observed that the nonspecialists actually treated older patients and with longer disease durations.
There is a direct relationship between HbA1c and microvascular complications (mainly, neuropathy, nephropathy, and retinopathy), as well as cardiovascular diseases (ie, macrovascular complications, mainly, coronary artery disease, stroke, and peripheral artery disease).37 Also, randomized controlled trials have conclusively demonstrated that the risk of microvascular complications can be reduced by intensive glycemic control in patients with T1DM and patients with T2DM.38–42 Nonetheless, these trials have failed in demonstrating that stringent glycemic control (ie, HbA1c <7%) reduces macrovascular complications.38–42 With a descriptive design, IDMPS has shown comparable findings.17,18,27 More or less stringent goals may be appropriate for certain subgroups of patients.39 However, stringent control of comorbid cardiovascular risk factors may delay a number of complications in most patients.40–44 It is also possible that in order to observe a meaningful risk reduction of macrovascular disease, preventive strategies must reach the patient in the early onset of arterial disease.
In this study, patients with T2DM treated with insulin were more frequently educated about diabetes than patients on oral glucose lowering drug (OGLD) and even more commonly educated than patients managed with diet and exercise only. In a 3-month period, individuals with T2DM on insulin therapy visited a specialist for a follow-up visit as frequently as other patients with T2DM. Unfortunately, insulin-treated patients were not in better glycemic control than those treated with OGLD or lifestyle modifications.
The main limitation of the present report is that most of the physicians who were invited to participate in the IDMPS study had experience with insulin therapy; and hence, the present information may not reflect the real characteristics of the rest of the country. This may also introduce bias by overrepresenting patients with advanced disease. Another limitation is the cross-sectional design and the nonstandardized laboratory assessments. Other possible influencing factors of therapeutic targets achievement were not completely studied, such as physician–patient interaction, the content of the diabetes education sessions, and self-assessments of diabetes complications, among other factors. Nonetheless, this study highlights that some of the characteristics of a proactive patient’s empowerment, by means of self-monitoring and self-adjustment of therapy, constitute an effective approach that should be explicitly tested in clinical trials focusing on therapeutic targets and late complications. Knowledge is necessary for action; hence, these data provide an important basis for impending institutional response toward improvement of the management provided to patients with the most important chronic disease in Mexico.
Conclusion
In the present data set of the IDMPS project, insulin-treated patients received more frequent diabetes education and had more frequent laboratory targets tested by their treating physicians, but they were not in better glycemic control as compared with patients on oral agents or with lifestyle modifications alone. More insulin-treated patients have a glucometer at home, but the self-testing practice is less frequent than patients treated with other alternatives. Although physicians tend to overestimate the global impression of good control, characteristics of patient’s empowerment such as self-monitoring and self-adjustment appear to interact with the global physicians’ impression in identifying patients who achieved glycemic targets.
Acknowledgments
This registry received funds from Sanofi. The company designed the study; nonetheless, the company did not participate in the selection of patients, data capture, data analysis, manuscript draft, or the decision to summit for publication. The authors are indebted to all IDMPS-3W collaborators (in alphabetic order) as follows:
Acevedo G, Acosta I, Alpízar M, Altamirano E, Álvarez A, Álvarez P, Anaya C, Arellano S, Arreola M, Arroyo A, Arteaga V, Ascensión D, Baeza B, Bancalari C, Baqueiro J, Barón D, Barragán A, Barrientos M, Bastidas M, Bautista J, Beltrán L, Blanco A, Caballero S, Calarco E, Calderón R, Camacho L, Cano R, Caracas N, Cardoso S, Carrillo P, Carvajal M, Casco S, Castañeda R, Castelán F, Castillo O, Cerda I, Cervantes A, Chavira I, Chávez L, Chávez M, Cilia J, Cisneros H, Colín M, Collado E, Colome J, Conrado S, Correa A, Covarrubias M, Cruz E, Dávila O, De la garza N, Del pozo J, Díaz E, Domínguez C, Encinas E, Escalante A, Escalante J, Escalante M, Escudero I, Espinoza J, Estrada A, Estrada K, Fabián M, Fanghanel G, Farjat J, Fernández A, Flores M, Flores V, Franco M, Franco V, Gallardo V, Gamboa F, García P, Garcia H, Garcia J, Garcia L, Garza R, Gomez V, Gonzalez A, Gonzalez G, Gonzalez I, González A, Granillo M, Grover F, Guajardo M, Guerrero J, Gutierrez M, Guzmán A, Guzmán J, Hall J, Hamilton L, Handall V, Hernandez A, Hernandez F, Hernández A, Hernández J, Herrera L, Herrera M, Hinojos L, Ibarra A, Ibarra M, Ibáñez M, Jiménez M, Jurado M, Lavalle F, Lechuga D, Llanas D, Lopez S, López H, López R, Lozano J, Lucio F, Luna R, Macedo N, Macías A, Magallanes F, Maldonado D, Maldonado J, Mancillas L, Mar F, Marquez E, Martínez A, Martínez R, Martínez R, Matildes M, Mauricio G, Mejía A, Mejía J, Mejía L, Mejía M, Mendoza E, Mendoza P, Mercado F, Meza E, Moctezuma J, Moleres J, Monreal R, Montemayor D, Monterrubio N, Montoya J, Mora F, Morales D, Morales F, Morales M, Moreno F, Moreno L, Moreno M, Muñoz A, Muñoz T, Navalles E, Nevares L, Nevarez L, Niño J, Núñez A, Ochoa A, Olmedo V, Ortega A, Osorio D, Ovando R, Parra F, Pascoe S, Pérez C, Pérez H, Pérez N, Quezada M, Radillo P, Rajme V, Ramírez B, Ramírez J, Ramos L, Ramos M, Ríos E, Rivera E, Robles J, Rodriguez H, Rodriguez J, Rodríguez H, Rodríguez R, Romero A, Rosado C, Rosas M, Rosiles S, Rubio Y, Ruiz D, Ruiz E, Saavedra E, Salas R, Salazar H, Salinas S, Sanchez B, Sanchez H, Sanchez L, Sanchez R, Sanchez B, Sanchez S, Sandoval B, Sandoval R, Santibáñez M, Seamanduras L, Secchi N, Solís T, Sosa A, Taméz H, Tapia M, Téllez J, Torres E, Torres J, Torres P, Trasviña K, Trejo M, Triano A, Triano M, Uribe A, Vadillo M, Valdez M, Valdovinos S, Valencia H, Vales M, Valladares Z, Vargas M, Vázquez J, Vázquez P, Vidrio M, Villanueva S, Wakida H, Yamamoto J, Zamora A, and Zayas F.
Footnotes
Author contributions
All authors have contributed to the conception and design of the work and the analysis of the data in a manner substantial enough to take public responsibility for it; each believes the manuscript represents valid work; and each has reviewed the final version of the manuscript and approves it for publication. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
Doctor Fernando J Lavalle-González has received research grants from Sanofi; has served as a research advisor for Sanofi, Novo Nordisk, and Eli Lilly; and has received speaker honoraria from Sanofi, Novo Nordisk, and Eli Lilly.
Doctor Erwin Chiquete has received research grants from Sanofi and Grupo Ferrer; has served as a research advisor for Sanofi, Novartis, and Genzyme; and has received speaker honoraria from Novartis Mexico, Genzyme, and Grupo Ferrer. The authors report no other conflicts of interest in this work.
References
- 1.Villalpando S, de la Cruz V, Rojas R, et al. Prevalence and distribution of type 2 diabetes mellitus in Mexican adult population: a probabilistic survey. Salud Publica Mex. 2010;52(suppl 1):S19–S26. doi: 10.1590/s0036-36342010000700005. [DOI] [PubMed] [Google Scholar]
- 2.Villalpando S, Shamah-Levy T, Rojas R, Aguilar-Salinas CA. Trends for type 2 diabetes and other cardiovascular risk factors in Mexico from 1993–2006. Salud Publica Mex. 2010;52(suppl 1):S72–S79. doi: 10.1590/s0036-36342010000700011. [DOI] [PubMed] [Google Scholar]
- 3.SALUD [webpage on the Internet] Health Statistics: Main Causes of General Mortality in Mexico. SINAIS; Mexico: [Accessed April 15, 2016]. Available from: http://www.dgis.salud.gob.mx/contenidos/basesdedatos/BD_Cubos.html. [Google Scholar]
- 4.Cantú-Brito C, Chiquete E, Ruiz-Sandoval JL, et al. REACH Latin America Collaborative Group Atherothrombotic disease, traditional risk factors, and 4-year mortality in a Latin American population: the REACH Registry. Clin Cardiol. 2012;35(8):451–457. doi: 10.1002/clc.22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiquete E, Ruiz-Sandoval JL, Murillo-Bonilla L, et al. Central adiposity and mortality after first-ever acute ischemic stroke. Eur Neurol. 2013;70(1–2):117–123. doi: 10.1159/000350762. [DOI] [PubMed] [Google Scholar]
- 6.Basaldúa N, Chiquete E. Common predictors of excessive adiposity in children from a region with high prevalence of overweight. Ann Nutr Metab. 2008;52(3):227–232. doi: 10.1159/000140514. [DOI] [PubMed] [Google Scholar]
- 7.Shichiri M, Kishikawa H, Ohkubo Y, Wake N. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care. 2000;23(suppl 2):B2–B29. [PubMed] [Google Scholar]
- 8.Zullig LL, Gellad WF, Moaddeb J, et al. Improving diabetes medication adherence: successful, scalable interventions. Patient Prefer Adherence. 2015;9:139–149. doi: 10.2147/PPA.S69651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan JC, Gagliardino JJ, Baik SH, et al. Multifaceted determinants for achieving glycemic control: the International Diabetes Management Practice Study (IDMPS) Diabetes Care. 2009;32(2):227–233. doi: 10.2337/dc08-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ringborg A, Cropet C, Jönsson B, Gagliardino JJ, Ramachandran A, Lindgren P. Resource use associated with type 2 diabetes in Asia, Latin America, the Middle East and Africa: results from the International Diabetes Management Practices Study (IDMPS) Int J Clin Pract. 2009;63(7):997–1007. doi: 10.1111/j.1742-1241.2009.02098.x. [DOI] [PubMed] [Google Scholar]
- 11.Jeragh-Alhaddad FB, Waheedi M, Barber ND, Brock TP. Barriers to medication taking among Kuwaiti patients with type 2 diabetes: a qualitative study. Patient Prefer Adherence. 2015;9:1491–1503. doi: 10.2147/PPA.S86719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wexler R, Elton T, Taylor CA, Pleister A, Feldman D. Physician reported perception in the treatment of high blood pressure does not correspond to practice. BMC Fam Pract. 2009;10:23. doi: 10.1186/1471-2296-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McBride P, Schrott HG, Plane MB, Underbakke G, Brown RL. Primary care practice adherence to national cholesterol education program guidelines for patients with coronary heart disease. Arch Intern Med. 1998;158(11):1238–1244. doi: 10.1001/archinte.158.11.1238. [DOI] [PubMed] [Google Scholar]
- 14.Kim SY, Cho IS, Lee JH, et al. Physician factors associated with the blood pressure control among hypertensive patients. J Prev Med Public Health. 2007;40(6):487–494. doi: 10.3961/jpmph.2007.40.6.487. [DOI] [PubMed] [Google Scholar]
- 15.Vargas-Sánchez A, Chiquete E, López-Corrales GE, et al. Evaluation of blood pressure measurements in first ambulatory neurological consultations: a missed part of the physical examination? Arch Cardiol Mex. 2013;83(4):263–266. doi: 10.1016/j.acmx.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki-Saito T, Yokokawa H, Shimada K, Yasumura S. Self-perception of glycemic control among Japanese type 2 diabetic patients: accuracy of patient perception and characteristics of patients with misperception. J Diabetes Investig. 2013;4(2):206–213. doi: 10.1111/jdi.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Souza MS, Karkada SN, Hanrahan NP, Venkatesaperumal R, Amirtharaj A. Do perceptions of empowerment affect glycemic control and self-care among adults with type 2 diabetes? Glob J Health Sci. 2015;7(5):80–90. doi: 10.5539/gjhs.v7n5p80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee YJ, Shin SJ, Wang RH, Lin KD, Lee YL, Wang YH. Pathways of empowerment perceptions, health literacy, self-efficacy, and self-care behaviors to glycemic control in patients with type 2 diabetes mellitus. Patient Educ Couns. 2016;99(2):287–294. doi: 10.1016/j.pec.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Varming AR, Hansen UM, Andrésdóttir G, Husted GR, Willaing I. Empowerment, motivation, and medical adherence (EMMA): the feasibility of a program for patient-centered consultations to support medication adherence and blood glucose control in adults with type 2 diabetes. Patient Prefer Adherence. 2015;9:1243–1253. doi: 10.2147/PPA.S85528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong CK, Wong WC, Wan YF, Chan AK, Chan FW, Lam CL. Patient empowerment programme (PEP) and risk of microvascular diseases among patients with type 2 diabetes in primary care: a population-based propensity-matched cohort study. Diabetes Care. 2015;38(8):e116–e117. doi: 10.2337/dc14-2213. [DOI] [PubMed] [Google Scholar]
- 21.Wang RH, Hsu HC, Lee YJ, Shin SJ, Lin KD, An LW. Patient empowerment interacts with health literacy to associate with subsequent self-management behaviors in patients with type 2 diabetes: a prospective study in Taiwan. Patient Educ Couns. 2016 Apr 4; doi: 10.1016/j.pec.2016.04.001. Epub. [DOI] [PubMed] [Google Scholar]
- 22.Ebrahimi H, Sadeghi M, Amanpour F, Vahedi H. Evaluation of empowerment model on indicators of metabolic control in patients with type 2 diabetes, a randomized clinical trial study. Prim Care Diabetes. 2016;10(2):129–135. doi: 10.1016/j.pcd.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Traina SB, Mathias SD, Colwell HH, Crosby RD, Abraham C. The Diabetes Intention, Attitude, and Behavior Questionnaire: evaluation of a brief questionnaire to measure physical activity, dietary control, maintenance of a healthy weight, and psychological antecedents. Patient Prefer Adherence. 2016;10:213–222. doi: 10.2147/PPA.S94878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitt A, Reimer A, Hermanns N, et al. Assessing diabetes self-management with the diabetes self-management questionnaire (DSMQ) can help analyse behavioural problems related to reduced glycaemic control. PLoS One. 2016;11(3):e0150774. doi: 10.1371/journal.pone.0150774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong CK, Wong WC, Wan YF, Chan AK, Chan FW, Lam CL. Effect of a structured diabetes education programme in primary care on hospitalizations and emergency department visits among people with Type 2 diabetes mellitus: results from the Patient Empowerment Programme. Diabet Med. 2015 Oct 3; doi: 10.1111/dme.12969. Epub. [DOI] [PubMed] [Google Scholar]
- 26.Wong CK, Wong WC, Wan EY, Chan AK, Chan FW, Lam CL. Macrovascular and microvascular disease in obese patients with type 2 diabetes attending structured diabetes education program: a population-based propensity-matched cohort analysis of Patient Empowerment Programme (PEP) Endocrine. 2016 Jan 19; doi: 10.1007/s12020-015-0843-z. Epub. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki T, Takei R, Inoguchi T, et al. Clinical significance of barriers to blood glucose control in type 2 diabetes patients with insufficient glycemic control. Patient Prefer Adherence. 2015;9:837–845. doi: 10.2147/PPA.S84268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng L, Leung DY, Sit JW, et al. Factors associated with diet barriers in patients with poorly controlled type 2 diabetes. Patient Prefer Adherence. 2016;10:37–44. doi: 10.2147/PPA.S94275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fanghänel Salmón G, Sánchez-Reyes L, Chiquete Anaya E, de la Luz Castro J, Escalante Herrera A. Registro internacional para evaluar la práctica clínica entregada a pacientes con diabetes mellitus tipo 2: Un subanálisis de la experiencia en México. [Multicenter international registry to evaluate the clinical practice delivered to patients with type 2 diabetes mellitus: a sub-analysis of the experience in Mexico] Gac Med Mex. 2011;147(3):226–233. [PubMed] [Google Scholar]
- 30.Lavalle-González FJ, Chiquete E, de la Luz J, et al. IDMPS-3W collaborative group (Mexico) Achievement of therapeutic targets in Mexican patients with diabetes mellitus. Endocrinol Nutr. 2012;59(10):591–598. doi: 10.1016/j.endonu.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Sosa-Rubí SG, Galárraga O, López-Ridaura R. Diabetes treatment and control: the effect of public health insurance for the poor in Mexico. Bull World Health Organ. 2009;87(7):512–519. doi: 10.2471/BLT.08.053256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.González-Villalpando C, López-Ridaura R, Campuzano JC, González-Villalpando ME. The status of diabetes care in Mexican population: are we making a difference? Results of the National Health and Nutrition Survey 2006. Salud Publica Mex. 2010;52(suppl 1):S36–S43. doi: 10.1590/s0036-36342010000700007. [DOI] [PubMed] [Google Scholar]
- 33.Funnell MM, Anderson RM, Arnold MS, et al. Empowerment: an idea whose time has come in diabetes education. Diabetes Educ. 1991;17(1):37–41. doi: 10.1177/014572179101700108. [DOI] [PubMed] [Google Scholar]
- 34.Fitzgerald M, O’Tuathaigh C, Moran J. Investigation of the relationship between patient empowerment and glycaemic control in patients with type 2 diabetes: a cross-sectional analysis. BMJ Open. 2015;5(12):e008422. doi: 10.1136/bmjopen-2015-008422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care. 2002;25(7):1159–1171. doi: 10.2337/diacare.25.7.1159. [DOI] [PubMed] [Google Scholar]
- 36.Wong CK, Wong WC, Lam CL, et al. Effects of Patient Empowerment Programme (PEP) on clinical outcomes and health service utilization in type 2 diabetes mellitus in primary care: an observational matched cohort study. PLoS One. 2014;9(5):e95328. doi: 10.1371/journal.pone.0095328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skyler JS, Bergenstal R, Bonow RO, et al. American Diabetes Association. American College of Cardiology Foundation. American Heart Association Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care. 2009;32(1):187–192. doi: 10.2337/dc08-9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 39.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.ACCORD Study Group Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363(3):233–244. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller ME, Bonds DE, Gerstein HC, et al. ACCORD Investigators The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444. doi: 10.1136/bmj.b5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.ADVANCE Collaborative Group. Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 43.Gagliardino JJ, Aschner P, Baik SH, et al. IDMPS investigators Patients’ education, and its impact on care outcomes, resource consumption and working conditions: data from the International Diabetes Management Practices Study (IDMPS) Diabetes Metab. 2012;38(2):128–134. doi: 10.1016/j.diabet.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Tunceli K, Zhao C, Davies MJ, et al. Factors associated with adherence to oral antihyperglycemic monotherapy in patients with type 2 diabetes. Patient Prefer Adherence. 2015;9:191–197. doi: 10.2147/PPA.S71346. [DOI] [PMC free article] [PubMed] [Google Scholar]