Abstract
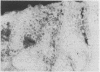
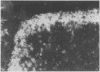
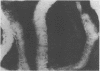
The association between the expression of HLA-DR antigens on cervical epithelium and the local immune state of activation in colposcopically obtained biopsy specimens from patients with histologically documented wart virus infection was investigated. In normal cervical epithelium no HLA-DR staining was detected. No or few IL-2R positive cells were found in the contiguous sections. HLA-DR was expressed by epithelial cells in six out of the 14 samples of wart virus infection. The pattern of fluorescence was focal, but strong and diffuse, to the whole epithelial layer. In the six samples with HLA-DR positive epithelium the numbers of IL-2R positive cells in the lamina propria were strongly increased, ranging between 75 and 90%. HLA-DR expression by cervical epithelium was observed in only two of 12 samples from patients with mixed epithelial non-virus related abnormalities. No increase in the numbers of IL-2R positive cells was observed in this group of patients. Additionally, no significant differences in terms of T lymphocyte infiltrate were found among the three groups. The results indicate that wart virus infection is associated with enhanced HLA-DR epithelial expression and they lend support to the concept that in the human cervix the epithelium actively participates in the local immune response.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albino A. P., Houghton A. N., Eisinger M., Lee J. S., Kantor R. R., Oliff A. I., Old L. J. Class II histocompatibility antigen expression in human melanocytes transformed by Harvey murine sarcoma virus (Ha-MSV) and Kirsten MSV retroviruses. J Exp Med. 1986 Nov 1;164(5):1710–1722. doi: 10.1084/jem.164.5.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud-Battandier F., Cerf-Bensussan N., Amsellem R., Schmitz J. Increased HLA-DR expression by enterocytes in children with celiac disease. Gastroenterology. 1986 Nov;91(5):1206–1212. doi: 10.1016/s0016-5085(86)80018-x. [DOI] [PubMed] [Google Scholar]
- Barclay A. N., Mason D. W. Induction of Ia antigen in rat epidermal cells and gut epithelium by immunological stimuli. J Exp Med. 1982 Dec 1;156(6):1665–1676. doi: 10.1084/jem.156.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland P. W., Warren L. G. Antigen presentation by epithelial cells of the rat small intestine. I. Kinetics, antigen specificity and blocking by anti-Ia antisera. Immunology. 1986 May;58(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Capobianchi M. R., Ameglio F., Tosi R., Dolei A. Differences in the expression and release of DR, BR, and DQ molecules in human cells treated with recombinant interferon gamma: comparison to other interferons. Hum Immunol. 1985 May;13(1):1–11. doi: 10.1016/0198-8859(85)90022-9. [DOI] [PubMed] [Google Scholar]
- Ciampolillo A., Marini V., Mirakian R., Buscema M., Schulz T., Pujol-Borrell R., Bottazzo G. F. Retrovirus-like sequences in Graves' disease: implications for human autoimmunity. Lancet. 1989 May 20;1(8647):1096–1100. doi: 10.1016/s0140-6736(89)92382-9. [DOI] [PubMed] [Google Scholar]
- Fais S., Pallone F. Ability of human colonic epithelium to express the 4F2 antigen, the common acute lymphoblastic leukemia antigen, and the transferrin receptor. Studies in inflammatory bowel disease and after in vitro exposure to different stimuli. Gastroenterology. 1989 Dec;97(6):1435–1441. doi: 10.1016/0016-5085(89)90387-9. [DOI] [PubMed] [Google Scholar]
- Fais S., Pallone F., Squarcia O., Biancone L., Ricci F., Paoluzi P., Boirivant M. HLA-DR antigens on colonic epithelial cells in inflammatory bowel disease: I. Relation to the state of activation of lamina propria lymphocytes and to the epithelial expression of other surface markers. Clin Exp Immunol. 1987 Jun;68(3):605–612. [PMC free article] [PubMed] [Google Scholar]
- Ghosh A. K., Moore M., Street A. J., Howat J. M., Schofield P. F. Expression of HLA-D sub-region products on human colorectal carcinoma. Int J Cancer. 1986 Oct 15;38(4):459–464. doi: 10.1002/ijc.2910380402. [DOI] [PubMed] [Google Scholar]
- Hardcastle J. D., Farrands P. A., Balfour T. W., Chamberlain J., Amar S. S., Sheldon M. G. Controlled trial of faecal occult blood testing in the detection of colorectal cancer. Lancet. 1983 Jul 2;2(8340):1–4. doi: 10.1016/s0140-6736(83)90001-6. [DOI] [PubMed] [Google Scholar]
- Herlyn M., Guerry D., Koprowski H. Recombinant gamma-interferon induces changes in expression and shedding of antigens associated with normal human melanocytes, nevus cells, and primary and metastatic melanoma cells. J Immunol. 1985 Jun;134(6):4226–4230. [PubMed] [Google Scholar]
- Houghton A. N., Thomson T. M., Gross D., Oettgen H. F., Old L. J. Surface antigens of melanoma and melanocytes. Specificity of induction of Ia antigens by human gamma-interferon. J Exp Med. 1984 Jul 1;160(1):255–269. doi: 10.1084/jem.160.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara T., Hooks J. J., Dougherty S. F., Oppenheim J. J. Interleukin 2-mediated immune interferon (IFN-gamma) production by human T cells and T cell subsets. J Immunol. 1983 Apr;130(4):1784–1789. [PubMed] [Google Scholar]
- Londei M., Bottazzo G. F., Feldmann M. Human T-cell clones from autoimmune thyroid glands: specific recognition of autologous thyroid cells. Science. 1985 Apr 5;228(4695):85–89. doi: 10.1126/science.3871967. [DOI] [PubMed] [Google Scholar]
- Londei M., Lamb J. R., Bottazzo G. F., Feldmann M. Epithelial cells expressing aberrant MHC class II determinants can present antigen to cloned human T cells. Nature. 1984 Dec 13;312(5995):639–641. doi: 10.1038/312639a0. [DOI] [PubMed] [Google Scholar]
- Massa P. T., Dörries R., ter Meulen V. Viral particles induce Ia antigen expression on astrocytes. Nature. 1986 Apr 10;320(6062):543–546. doi: 10.1038/320543a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer L., Shlien R. Evidence for function of Ia molecules on gut epithelial cells in man. J Exp Med. 1987 Nov 1;166(5):1471–1483. doi: 10.1084/jem.166.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisels A., Fortin R. Condylomatous lesions of the cervix and vagina. I. Cytologic patterns. Acta Cytol. 1976 Nov-Dec;20(6):505–509. [PubMed] [Google Scholar]
- Meisels A., Fortin R., Roy M. Condylomatous lesions of the cervix. II. Cytologic, colposcopic and histopathologic study. Acta Cytol. 1977 May-Jun;21(3):379–390. [PubMed] [Google Scholar]
- Michalak T. I., Churchill N. D. Interaction of woodchuck hepatitis virus surface antigen with hepatocyte plasma membrane in woodchuck chronic hepatitis. Hepatology. 1988 May-Jun;8(3):499–506. doi: 10.1002/hep.1840080312. [DOI] [PubMed] [Google Scholar]
- Morris H. B., Gatter K. C., Pulford K., Haynes P., Charnock M., Taylor-Papadimitriou J., Lane E. B., Mason D. Y. Cervical wart virus infection, intraepithelial neoplasia and carcinoma; an immunohistological study using a panel of monoclonal antibodies. Br J Obstet Gynaecol. 1983 Nov;90(11):1069–1081. doi: 10.1111/j.1471-0528.1983.tb06447.x. [DOI] [PubMed] [Google Scholar]
- Natali P. G., De Martino C., Quaranta V., Nicotra M. R., Frezza F., Pellegrino M. A., Ferrone S. Expression of Ia-like antigens in normal human nonlymphoid tissues. Transplantation. 1981 Jan;31(1):75–78. doi: 10.1097/00007890-198101000-00017. [DOI] [PubMed] [Google Scholar]
- Paavonen J. Colposcopic findings associated with human papillomavirus infection of the vagina and the cervix. Obstet Gynecol Surv. 1985 Apr;40(4):185–189. doi: 10.1097/00006254-198504000-00001. [DOI] [PubMed] [Google Scholar]
- Pallone F., Fais S., Capobianchi M. R. HLA-D region antigens on isolated human colonic epithelial cells: enhanced expression in inflammatory bowel disease and in vitro induction by different stimuli. Clin Exp Immunol. 1988 Oct;74(1):75–79. [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Cotran R. S., Reiss C. S., Burakoff S. J., Fiers W., Ault K. A. Ia expression by vascular endothelium is inducible by activated T cells and by human gamma interferon. J Exp Med. 1983 Apr 1;157(4):1339–1353. doi: 10.1084/jem.157.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson B. W., McLemore T. L., Crystal R. G. Gamma interferon is spontaneously released by alveolar macrophages and lung T lymphocytes in patients with pulmonary sarcoidosis. J Clin Invest. 1985 May;75(5):1488–1495. doi: 10.1172/JCI111852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncalli M., Sideri M., Giè P., Servida E. Immunophenotypic analysis of the transformation zone of human cervix. Lab Invest. 1988 Feb;58(2):141–149. [PubMed] [Google Scholar]
- Suciu-Foca N., Rubinstein P., Popovic M., Gallo R. C., King D. W. Reactivity of HTLV-transformed human T-cell lines to MHC class II antigens. Nature. 1984 Nov 15;312(5991):275–277. doi: 10.1038/312275a0. [DOI] [PubMed] [Google Scholar]
- Tabibzadeh S. S., Bettica A., Gerber M. A. Variable expression of Ia antigens in human endometrium and in chronic endometritis. Am J Clin Pathol. 1986 Aug;86(2):153–160. doi: 10.1093/ajcp/86.2.153. [DOI] [PubMed] [Google Scholar]
- Tabibzadeh S. S., Gerber M. A., Satyaswaroop P. G. Induction of HLA-DR antigen expression in human endometrial epithelial cells in vitro by recombinant gamma-interferon. Am J Pathol. 1986 Oct;125(1):90–96. [PMC free article] [PubMed] [Google Scholar]
- Tay S. K., Jenkins D., Maddox P., Singer A. Lymphocyte phenotypes in cervical intraepithelial neoplasia and human papillomavirus infection. Br J Obstet Gynaecol. 1987 Jan;94(1):16–21. doi: 10.1111/j.1471-0528.1987.tb02245.x. [DOI] [PubMed] [Google Scholar]
- Todd I., Pujol-Borrell R., Hammond L. J., Bottazzo G. F., Feldmann M. Interferon-gamma induces HLA-DR expression by thyroid epithelium. Clin Exp Immunol. 1985 Aug;61(2):265–273. [PMC free article] [PubMed] [Google Scholar]
- Tsou K. C., Lo K. W., Rosato E. F., Yuk A., Enterline H., Schwegman C. Evaluation of 5'-nucleotide phosphodiesterase isozyme-V as a predictor for liver metastasis in breast cancer patients. Cancer. 1982 Jul 15;50(2):191–196. doi: 10.1002/1097-0142(19820715)50:2<191::aid-cncr2820500202>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]