Abstract
Background
Prior to the advent of tyrosine kinase inhibitor (TKI) therapy, the evaluation of hematologic and cytogenetic responses was sufficient to gauge treatment efficacy in patients with chronic myeloid leukemia. However, with more potent TKI therapies, the majority of patients achieve complete cytogenetic response (CCyR). Furthermore, deeper molecular responses are now commonly achieved, necessitating a reliance on molecular monitoring to assess residual leukemic disease.
Methods/Results
The prognostic significance between molecular responses and duration of CCyR, progression-free survival, and event-free survival is described herein. A discussion of the concept of complete molecular response is also provided and the potential for imatinib treatment discontinuation is evaluated. The implications of rising BCR-ABL1 transcript levels and caveats of molecular monitoring are also described.
Keywords: chronic myeloid leukemia (CML), tyrosine kinase inhibitor (TKI), BCR-ABL, imatinib, molecular response
Introduction
Chronic myeloid leukemia (CML) is a clonal myeloproliferative disease resulting in expansion of hematopoietic cells carrying the oncogenic BCR-ABL1 fusion, which encodes the constitutively active BCR-ABL1 protein tyrosine kinase.1 This fusion, known as the Philadelphia (Ph) chromosome, is the result of a reciprocal translocation between the long arms of chromosomes 9 and 22, t(9;22)(q34;q11) and can be detected by cytogenetic analysis.2 The resulting BCR-ABL1 tyrosine kinase is upstream of numerous signaling pathways and necessary for initiation of leukemogenesis.2–4 Imatinib (Gleevec®/Glivec®; formerly STI571, Novartis Pharmaceuticals Corporation, East Hanover, NJ), nilotinib (Tasigna®; Novartis Pharmaceuticals Corporation, East Hanover, NJ), and dasatinib (Sprycel®; Bristol-Myers Squibb Company, Princeton, NJ) are BCR-ABL1 tyrosine kinase inhibitors (TKIs) designed to inhibit BCR-ABL1 activity and have dramatically improved outcomes for patients with CML.5–7 A recent 8-year follow-up of patients with newly diagnosed CML in chronic phase (CML-CP) treated with imatinib in the phase 3 International Randomized Study of Interferon and STI571 (IRIS) trial reported a cumulative best complete cytogenetic response (CCyR) of 83% and an estimated overall survival (OS) of 93% when only CML-related deaths were considered.8 This review describes the approaches to measuring responses in light of the success of TKI therapy in the treatment of CML and the prognostic significance of those responses.
Definitions and Approaches to Measuring Response in CML
The goals of CML treatment are the return of blood counts to normal values, reduction and elimination of the Ph chromosome, and reduction and elimination of BCR-ABL1 gene expression. Progress toward these goals can be determined by the measurement of hematologic, cytogenetic, and molecular responses, respectively. Before the advent of TKI therapy, the evaluation of hematologic and cytogenetic responses was sufficient to gauge treatment efficacy. However, with more potent TKI therapies, deeper responses are now commonly achieved, necessitating more sensitive methods of disease detection.
Hematologic responses
A complete hematologic response (CHR) is achieved when laboratory values return to normal levels, with a white blood cell count <10,000/mm3, a platelet count <450,000/mm3, the presence of <5% myelocytes plus metamyelocytes, the presence of <20% basophils, the absence of blasts and promyelocytes in peripheral blood, and the absence of extramedullary involvement.5, 9 European LeukemiaNet recommendations state that achievement of CHR within 3 months from the start of therapy is an optimal response.10 Nearly all patients with CML-CP achieve a CHR with TKI therapy.
Cytogenetic responses
Cytogenetic analysis remains the standard for treatment monitoring in CML.10 Conventional cytogenetics requires a bone marrow sample and evaluation of >20 metaphases for the Ph+ chromosome. Categories of cytogenetic response include minimal cytogenetic response, with 36% to 95% Ph+ metaphases; partial cytogenetic response, with 1% to 35% Ph+ metaphases; major cytogenetic response (MCyR), with 0% to 35% Ph+ metaphases; and CCyR, with 0% Ph+ metaphases. Although cytogenetic studies are associated with a wide confidence interval due to the limited number of metaphases evaluated, the association between cytogenetic response and positive outcomes has been well established.11, 12
Fluorescent in situ hybridization (FISH) is an alternative method for assessing cytogenetic response in which approximately 200 interphase cells are analyzed from a peripheral blood sample. While newer FISH techniques use 3 to 4 probes (“double-FISH”, D-FISH) and reduce the number of false-positive results (sensitivity is 1% to 5%), achievement of CCyR cannot be confirmed by FISH; hence, clinicians should be cautious in declaring treatment failure based on low-level FISH positivity.
Molecular responses
The majority (83%) of patients with CML treated with TKI therapy achieve a CCyR (elimination of the Ph chromosome in bone marrow metaphases), and therefore more sensitive measurements are necessary to detect minimal residual disease.13 Molecular monitoring accomplishes this by detecting the presence of BCR-ABL1 mRNA using real-time quantitative polymerase chain reaction (RQ-PCR). Molecular monitoring is capable of detecting low levels of disease and is >3 logs more sensitive than conventional cytogenetics.14 In addition, the analysis can be performed on peripheral blood samples, making it more convenient than bone marrow sampling.15 Molecular responses are quantified by measuring the reduction in BCR-ABL1 transcripts relative to a standardized baseline.9 The “International Standardization” process has led to the development of a conversion factor that enables individual laboratories to express BCR-ABL1 transcript levels on an agreed-upon international scale (IS), thus allowing comparison of molecular response between laboratories.16, 17 In the IRIS trial, patients in the imatinib group who had a reduction in the level of BCR-ABL1 transcripts of >3 logs compared to the standardized baseline had a negligible risk of disease progression over the subsequent 12 months.18 As a result, a major molecular response (MMR) was defined as a 3-log reduction or a BCR-ABL1 (IS) = 0.1%.18 A good correlation exists between bone marrow cytogenetics and transcript levels in peripheral blood, with a BCR-ABL1 (IS) ≤10% equivalent to an MCyR and a BCR-ABL1 (IS) ≤1% equivalent to a CCyR. However, unlike cytogenetics, molecular analysis does not provide information about bone marrow morphology or additional chromosomal abnormalities.19
Prognostic Significance of Molecular Responses
There is much evidence that the degree of cytogenetic response at certain time points is well correlated with prognosis. Patients who achieve a CCyR have been shown to have low rates of progression to accelerated phase/blast crisis (AP/BC) and excellent rates of OS.10, 20 The degree of molecular response at certain time points has also been associated with reduced risk of cytogenetic relapse, improved duration of CCyR, progression-free survival (PFS), and event-free-survival (EFS).
MMR is associated with duration of CCyR
A number of studies have demonstrated that achievement of an MMR is associated with improved durations of CCyR compared with patients who did not achieve the same depth of molecular response (Table 1). For example, in a study of 29 patients with CCyR followed for a median of 13 months, none of the 16 patients with BCR-ABL1 (IS) ≤0.1% lost CCyR, while 6 of 13 patients (46%) with BCR-ABL1 (IS) ≥0.1% lost a CCyR (P = .004).21 In another report on 280 patients with CML-CP who achieved a CCyR on imatinib treatment, only 9 of 166 evaluable patients (5%) who also achieved an MMR lost CCyR compared with 25 of 68 patients (37%) who did not achieve an MMR over a median follow-up period of 31 months.22 Likewise, in 97 patients with CML serially treated with imatinib 400 mg/day, those with an MMR at 12 months were less likely to lose CCyR than patients who did not achieve MMR by that time point (Fig. 1).23 Marin et al have reported similar findings in a study of 224 patients in which the probability of losing CCyR by 60 months was 2.6% versus 23.6% for patients who achieved MMR by 12 months compared with patients who did not achieve an MMR.24 At 18 months, the probability of losing CCyR was 0% versus 24.6% for patients with MMR and without MMR, respectively. Additionally, Press et al described 90 patients with CCyR followed for a median of 49 months in which only 15% (12 of 79 patients) with BCR-ABL1 (IS) ≤0.1% lost CCyR compared with 57% (8 of 14 patients) with BCR-ABL1 (IS) >0.1%.25
Table 1.
Duration of Complete Cytogenetic Response by Achievement of Major Molecular Response
| % Losing CCyR | ||||
|---|---|---|---|---|
| Study | N | Pts with MMR at 12 months | Pts without MMR at 12 months | Length of follow-up (months) |
| Paschka et al21 | 29 | 0% | 46% | Median 13 |
| Cortes et al22 | 280 | 5% | 37% | Median 31 |
| Iacobucci et al23 | 97 | 8% (est) | 30% (est) | 36 |
| Marin et al24 | 224 | 2.6% | 23.9% | 60 |
| 0%* | 24.6%* | 60 | ||
| Press et al25 | 90 | 16% | 57% | Median 49 |
CCyR indicates complete cytogenetic response; MMR, major molecular response; Pts, patients; est, estimated.
Patients with and without MMR at 18 months.
Figure 1.
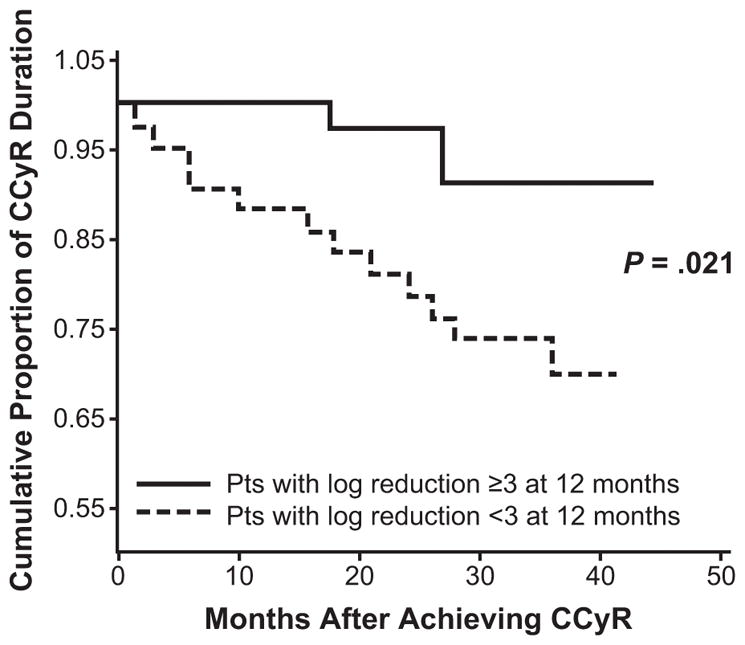
Landmark analysis of duration of complete cytogenetic response (CCyR) by molecular response at 12 months.23
Reproduced and adapted with permission from the American Association for Cancer Research: Iacobucci I, et al. Achieving a major molecular response at the time of a complete cytogenetic response (CCgR) predicts a better duration of CCgR in imatinib-treated chronic myeloid leukemia patients. Clin Cancer Res. 2006;12(10):3037–3042.
Impact of molecular responses on PFS, EFS, and OS
The impact of molecular response rates on PFS and EFS has also been evaluated. Among patients with CCyR at 12 months in the IRIS trial, there was a statistically significant difference in PFS rates between patients with and without MMR at 12 months (100% vs 95%, P = .007).18 However, in a more recent update of the molecular data from the IRIS trial, there was no difference between achievement of an MMR by 12 months compared with lesser rates of molecular response (BCR-ABL1 [IS] >0.1% to 1%) in EFS rates at 7 years (92% vs 91%, P = .25).26 A difference in rates of EFS was observed, however, when molecular responses at the 18-month landmark were considered (95% vs 86%, P = .01). There was little difference in 7-year rates of progression to advanced phase disease between patients with an MMR and those with BCR-ABL1 (IS) >0.1% to 1% at the 18-month landmark (99% vs 96%, P = .054). Importantly, with 8 years of follow-up on the IRIS trial, none of the patients who achieved CCyR and MMR at 12 months on imatinib progressed to AP or BC.8 The degree of molecular response was also found to correlate with the risk of progression in a single-institution study of 85 patients treated with imatinib (400 mg/day in CP patients [n = 72] and 600 mg/day in AP patients [n = 13]).27 Results demonstrated that compared to patients with a ≥3-log reduction in BCR-ABL1 levels, patients with ≥2- to <3-log reductions in BCR-ABL1 transcript levels were at a higher risk for progression (hazard ratio, 3.8; 95% CI: 0.92–16; P = .049), as were patients with a <2-log reduction (hazard ratio, 10; 95% CI: 3.8–28; P < .001) (Fig. 2).
Figure 2.
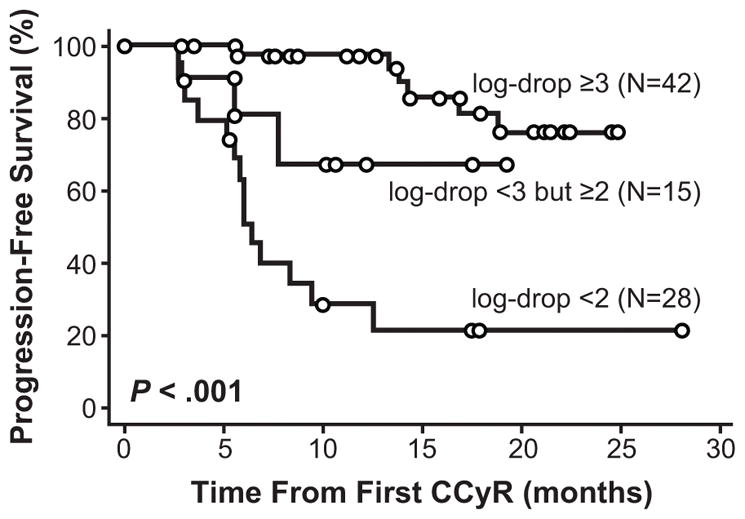
Duration of complete cytogenetic response (CCyR) by molecular response at time of achieving CCyR.27
This research was originally published in Blood. Press RD, Love Z, Tronnes AA, et al. BCR-ABL mRNA levels at and after the time of a complete cytogenetic response (CCR) predict the duration of CCR in imatinib mesylate-treated patients with CML. Blood. 2006;107(11):4250–4256. © the American Society of Hematology.
Other studies, however, have shown that rates of OS and PFS appear independent of molecular response. In an analysis conducted at the M. D. Anderson Cancer Center, 276 patients were analyzed and responses were coded according to best response and response at specific treatment intervals.13 Achievement of molecular response (MMR and < MMR) was not associated with improved OS in patients who achieved a CCyR (Fig. 3). While there was a trend suggesting that higher rates of PFS correlated with better molecular responses, the difference was not clinically relevant (Fig. 3). In a similar single-institution analysis, investigators at the Hammersmith Hospital found no significant difference in PFS or OS rates in patients achieving CCyR at 12 months (n = 121) or at 18 months (n = 106) by whether they had also achieved MMR at those time points (n = 30 at 12 months; n = 38 at 18 months).24, 28 At 12 months, OS and PFS were 96% and 94% versus 93% and 85% for patients with MMR and without MMR, respectively (P = .8, P = .3).24 At 18 months, OS and PFS were 96% and 95% versus 95% and 88% for patients with MMR and without MMR, respectively (P = 1, P = .4).24 One possible explanation for the lack of association between molecular response and long-term outcomes in these studies is that loss of CCyR may (and should) trigger a change in therapy. This early intervention can, successfully prevent progression to AP/BC in most patients, thus masking the adverse consequences of lack of an MMR. In fact, the European LeukemiaNet recommendations suggest that loss of CCyR is a criterion for imatinib failure and support change of therapy in this situation.10
Figure 3.
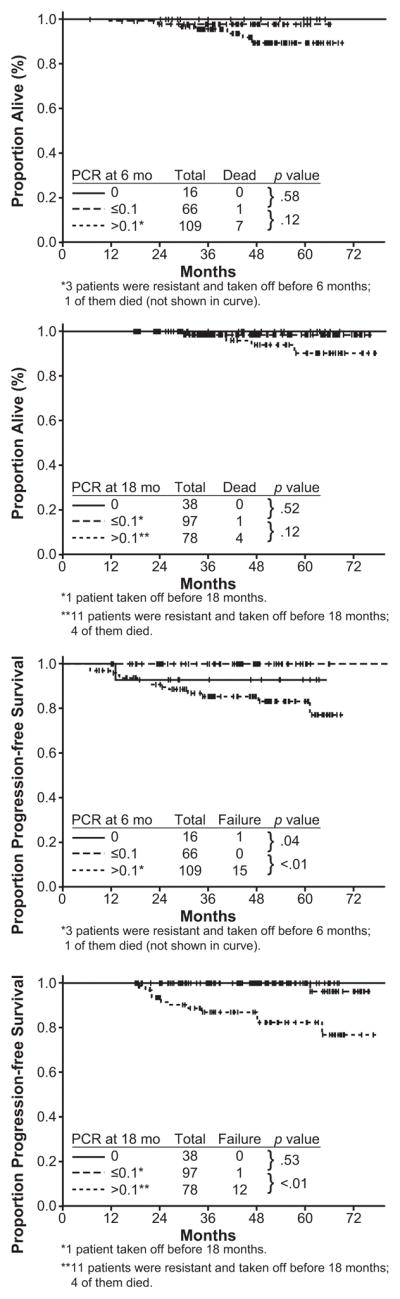
Overall survival and progression-free survival by molecular response at specific time points. 13
Reprinted with permission. Kantarjian H, O’Brien S, Shan J, et al. Cytogenetic and molecular responses and outcome in chronic myelogenous leukemia: need for new response definitions? Cancer. 2008;112(4):837–845.
Influence of time to molecular response
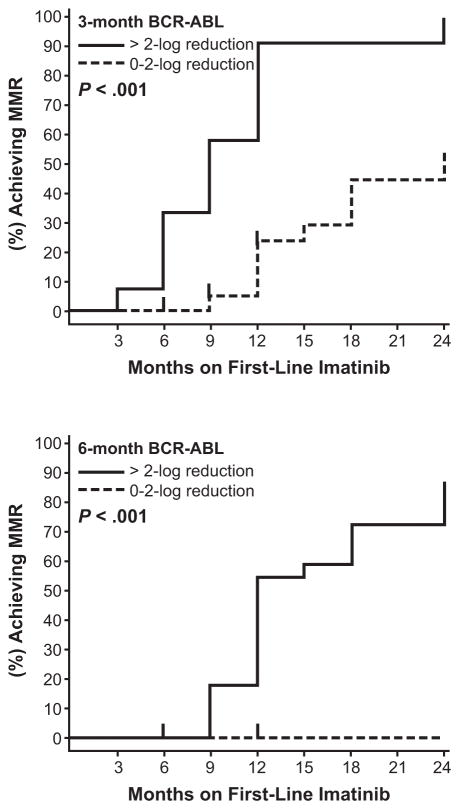
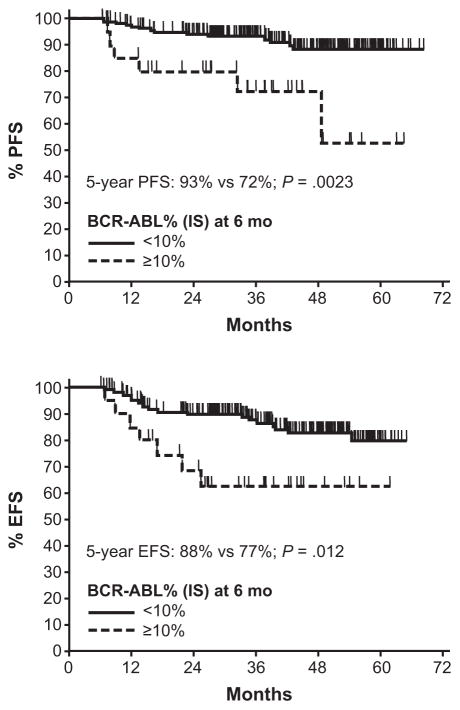
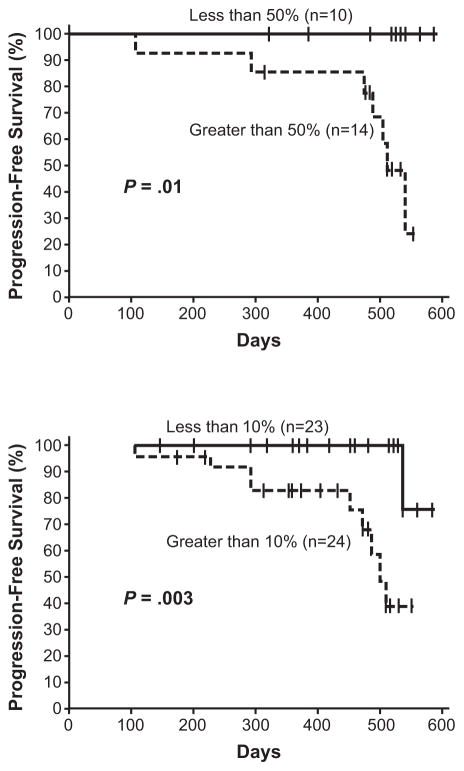
The correlation between outcomes and the time to achieve molecular responses has been investigated. These studies suggest that the degree of molecular response at early time points predicts later achievement of MMR and improved rates of PFS and EFS. For example, in an analysis of 55 patients treated with imatinib 400 mg/day who had peripheral blood collected at more than one time point, patients with a >2-log reduction in BCR-ABL1 transcripts at 3 months had significantly better rates of MMR at 24 months compared with patients who had a ≤2-log reduction in BCR-ABL1 transcripts at 3 months (100% versus 54%, P < .001) (Fig. 4).29 Müller et al have also observed that patients with BCR-ABL1 (IS) >10% at the 6-month landmark had a statistically significantly higher probability of progression and events by 72 months.30 The 5-year PFS rate was 93% versus 72% (P = .0023) and the 5-year EFS rate was 88% versus 77% (P = .012) in patients with BCR-ABL1 (IS) <10% and ≥10%, respectively (Fig. 5). Early molecular responses at 1 and 3 months have also been associated with higher rates of PFS.31 A decrease in BCR-ABL1/ABL1 ratio of 50% at 4 weeks or 10% at 3 months of therapy was associated with a higher probability of PFS (Fig. 6). An alternative analysis by M. D. Anderson Cancer Center investigators focused on the long-term outcomes of patients not achieving CCyR or MMR at specific time points, in an attempt to test the importance of the timing of cytogenetic and molecular responses during imatinib therapy.32 For patients not in CCyR, the probability of achieving a CCyR or MMR with continued imatinib therapy markedly diminished, while the risk of progression increased at 3, 6, and 12 months during the first year of imatinib therapy. Patients exhibiting BCR-ABL1/ABL1 ratios >1% to 10% after 3 months of treatment had a 92% probability of eventually attaining CCyR, which is similar to a 98% probability for patients with BCR-ABL1/ABL1 ≤1%. However, risk of developing an event on therapy was 3-fold higher than that of patients with BCR-ABL1/ABL1 ≤1%, which was quite similar to that of patients with transcript levels >10%. These results underscore the importance of attaining CCyR and MMR at early time points during imatinib therapy (Table 2).
Figure 4.
Achievement of major molecular response by 24 months based on molecular responses at 3 and 6 months.29
Reprinted by permission from Macmillan Publishers Ltd: Leukemia. Branford S, Rudzki Z, Harper A, et al. Imatinib produces significantly superior molecular responses compared to interferon alfa plus cytarabine in patients with newly diagnosed chronic myeloid leukemia in chronic phase. 2003;17(12):2401–2409, © 2003
Figure 5.
Progression- and event-free survival rates at 5 years by molecular response at 6 months.30
This research was originally published in Blood. Muller MC, Hanfstein B, Erben P, et al. Molecular Response to First Line Imatinib Therapy Is Predictive for Long Term Event Free Survival in Patients with Chronic Phase Chronic Myelogenous Leukemia - An Interim Analysis of the Randomized German CML Study IV. Blood. 2008;112(11):129. © the American Society of Hematology.
Figure 6.
Rates of progression-free survival by molecular responses at 1 and 3 months.31
The early molecular response to imatinib predicts cytogenetic and clinical outcome in chronic myeloid leukaemia, Vol. 120, No. 6, 2003, 990–999. © 2003 British Journal of Hematology; Reprinted with permission of Wiley-Blackwell, Inc.
Table 2.
Risk of Event Versus Probability of Achieving a Complete Cytogenetic Response According to Molecular Response at Specific Time Points32
| BCR-ABL1/ABL1 Transcript Ratio | Percentage Probability of Outcome According to Transcript Ratio at Specified Time Points (Median Months to Outcome) | |||||
|---|---|---|---|---|---|---|
| MMR (BCR-ABL1/ABL1 <0.05%) | Event | |||||
| 3 mo | 6 mo | 12 mo | 3 mo | 6 mo | 12 mo | |
| ≤0.1% | 100 (3) | 96 (6) | 97 (12) | 4 (13) | 1 (38) | 3 (40) |
| >0.1% to 1% | 84 (6) | 69 (12) | 61 (18) | 3 (46) | 7 (30) | 2 (48) |
| >1% to 10% | 53 (17) | 44 (18) | 20 (33) | 11 (21) | 9 (34) | 8 (47) |
| >10% | 33 (15) | 15 (18) | 7 (46) | 13 (47) | 23 (14) | 50 (19) |
MMR indicates major molecular response; mo, months.
Significance of rising BCR-ABL1 transcript levels
Despite the importance of molecular monitoring in predicting long-term outcomes and evaluating treatment success, minor fluctuations in patients’ BCR-ABL1 transcript levels should not be overinterpreted. For example, an evaluation of 116 patients who achieved durable CCyR (>18 months) with increases in BCR-ABL1 transcript levels verified by 2 consecutive measurements has been conducted.33 Progression was observed in 11 of 116 patients (9.5%) (Table 3). Ten of these were among 44 patients who lost or never achieved an MMR and experienced a >1-log increase in BCR-ABL1 transcript levels. The majority of patients who had achieved CCyR retained the same degree of response despite increasing transcript levels. Therefore, minor fluctuations in BCR-ABL1 (IS) should not necessarily provoke a treatment change. However, patients who lose MMR or never achieved MMR, and have a significant increase in transcripts should be closely monitored. The magnitude of the increase that should be considered significant varies in different reports, from 2-fold to 1-log. Some of this difference depends on the variability of the test at the local laboratory, but for most laboratories an increase of 5-fold or greater should trigger close monitoring and perhaps additional assessments (eg, cytogenetic analysis, mutation analysis).
Table 3.
Outcomes of Patients in Complete Cytogenetic Response by Increased Level of Detectable BCR-ABL1 Transcripts33
| Follow-up From QPCR Increase (Months) | |||||
|---|---|---|---|---|---|
| QPCR Log Increase | Number of Patients | Imatinib Dose Escalation | CML Progression | Median | Range |
| Persistent MMR | |||||
| Any | 28 | 0 | 0 | 36 | 3–62 |
| Loss of MMR | |||||
| >0.5 to 1 | 12 | 0 | 0 | 34 | 14–59 |
| >1 to 2 | 25 | 0 | 2 | 31 | 6–52 |
| >2 | 11 | 4 | 4 | 45 | 20–57 |
| Not in MMR | |||||
| <1 | 32 | 3 | 1 | 35 | 10–70 |
| >1 | 8 | 1 | 4 | 25 | 12–56 |
QPCR indicates quantitative polymerase chain reaction; CML, chronic myeloid leukemia; MMR, major molecular response.
Complete molecular responses
Elimination of the leukemic clone is the ultimate goal of therapy and the only potential for a CML cure. With TKI therapy, many patients are able to achieve a complete molecular response (CMR), defined as undetectable BCR-ABL1 mRNA transcripts by RQ-PCR and/or nested PCR in 2 consecutive high-quality samples (sensitivity >104).10 The potential use of second-generation TKIs nilotinib and dasatinib in the frontline setting could increase the number of patients achieving this level of response even more.34–36 However, without elimination of the leukemic stem cell population, a cure is not feasible, and currently there is little evidence that achievement of a CMR correlates with improved long-term EFS, PFS, or OS.
Several studies have examined the potential of discontinuing imatinib therapy in patients with a CMR.37–40 The Stop Imatinib (STIM) study evaluated the impact of imatinib discontinuation in patients with long-term (≥2 years) CMR.40 After 12 months of follow-up, molecular relapse (loss of CMR) occurred in 40 of 69 patients (58%). The relapse rate was slightly lower among patients previously treated with interferon compared with those who were not (53% vs 66%, P was not significant) and was more common among women than men (70% vs 42% relapse rate, P = .02). Relapse rates correlated with Sokal risk score: low, 45%; intermediate, 64%; high, 86%; and unknown, 78%. All patients re-achieved CMR after re-initiation of imatinib therapy. As a result of the high relapse rate, discontinuation of TKI therapy in responding patients is not currently recommended outside a clinical study setting.
Discussion
Treatment advances for patients with CML have resulted in excellent long-term outcomes. TKI therapy with imatinib, nilotinib, or dasatinib results in high response rates, many of which can only be measured at a molecular level. As a result, there is an increasing reliance on molecular monitoring as a more sensitive measure to assess treatment efficacy and monitor response. While it has been established that patients who achieve a CCyR also have excellent outcomes, data indicate that achieving high levels of molecular response illustrates treatment efficacy and should be a goal of therapy. The prognostic significance of molecular responses at early time points also provides valuable information and suggests more careful monitoring of patients with suboptimal molecular responses. In patients with MMR (BCR-ABL1 [IS] ≤0.1%), molecular monitoring in peripheral blood may be used in place of cytogenetic analysis to monitor response, thus forgoing the need for bone marrow sampling. However, it must be emphasized that molecular monitoring does not provide information concerning bone marrow morphology or the presence of additional chromosomal abnormalities in Ph+ metaphases. Therefore, occasional cytogenetic analysis is still recommended. One must also keep in mind that low assay sensitivity and sample quality could result in false-negative results and that variations in results of up to 0.5 logs can occur due to differences in assay technique and sample quality. Due to these caveats, changes in treatment should not be based on a single molecular assessment. Instead, fluctuations in transcripts should be monitored and confirmed with follow-up testing and, if necessary, cytogenetic or mutation analysis should be used in conjunction with molecular monitoring when transcript levels rise significantly (≥ 5-fold) or if the patient is in danger of losing an MMR. Various studies also suggest that while deep molecular response should be a goal for all patients and is an indication of treatment success, patients who achieve a CCyR have been shown to have almost as good long-term outcomes as patients with an MMR, thus making CCyR a valid surrogate of long-term survival and the minimal goal to be achieved during TKI therapy.
Footnotes
Financial disclosures and sources of support: Financial support for editorial assistance was provided by Novartis Pharamaceuticals. The authors would like to thank Daniel Hutta, PhD (Articulate Science) for medical editorial assistance. Dr. Cortes received research grant funding from Wyeth, Bristol-Myers Squibb and Novartis Pharmaceuticals Corporation. Dr. Kantarjian received research grant funding from Bristol-Myers Squibb, Novartis Pharmaceuticals Corporation, and Genzyme. Dr. Quintás-Cardama has no relevant financial disclosures.
References
- 1.Quintas-Cardama A, Cortes J. Molecular biology of bcr-abl1-positive chronic myeloid leukemia. Blood. 2009;113:1619–1630. doi: 10.1182/blood-2008-03-144790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ren R. Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nat Rev Cancer. 2005;5:172–183. doi: 10.1038/nrc1567. [DOI] [PubMed] [Google Scholar]
- 3.Van Etten RA. Mechanisms of transformation by the BCR-ABL oncogene: New perspectives in the post-imatinib era. Leuk Res. 2004;28(Suppl 1):S21–28. doi: 10.1016/j.leukres.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Steelman LS, Pohnert SC, Shelton JG, Franklin RA, Bertrand FE, McCubrey JA. JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia. 2004;18:189–218. doi: 10.1038/sj.leu.2403241. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian HM, Giles FJ, Bhalla KN, et al. Update on imatinib-resistant chronic myeloid leukemia patients in chronic phase (CML-CP) on nilotinib therapy at 24 months: Clinical response, safety, and long-term outcomes. Blood. 2009;114:464. Abstract 1129. [Google Scholar]
- 7.Shah NP, Kim DW, Kantarjian H, et al. Potent, transient inhibition of BCR-ABL with dasatinib 100 mg daily achieves rapid and durable cytogenetic responses and high transformation-free survival rates in chronic phase chronic myeloid leukemia patients with resistance, suboptimal response or intolerance to imatinib. Haematologica. 2010;95:232–240. doi: 10.3324/haematol.2009.011452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deininger M, O’Brien SG, Guilhot F, et al. International randomized study of interferon vs STI571 (IRIS) 8-year follow up: Sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. Blood. 2009;114:462. Abstract 1126. [Google Scholar]
- 9.Baccarani M, Saglio G, Goldman J, et al. Evolving concepts in the management of chronic myeloid leukemia: Recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108:1809–1820. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- 10.Baccarani M, Cortes J, Pane F, et al. Chronic myeloid leukemia: An update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27:6041–6051. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kantarjian HM, O’Brien S, Cortes JE, et al. Complete cytogenetic and molecular responses to interferon-alpha-based therapy for chronic myelogenous leukemia are associated with excellent long-term prognosis. Cancer. 2003;97:1033–1041. doi: 10.1002/cncr.11223. [DOI] [PubMed] [Google Scholar]
- 12.Kantarjian HM, Smith TL, O’Brien S, Beran M, Pierce S, Talpaz M. Prolonged survival in chronic myelogenous leukemia after cytogenetic response to interferon-alpha therapy. the leukemia service. Ann Intern Med. 1995;122:254–261. doi: 10.7326/0003-4819-122-4-199502150-00003. [DOI] [PubMed] [Google Scholar]
- 13.Kantarjian H, O’Brien S, Shan J, et al. Cytogenetic and molecular responses and outcome in chronic myelogenous leukemia: Need for new response definitions? Cancer. 2008;112:837–845. doi: 10.1002/cncr.23238. [DOI] [PubMed] [Google Scholar]
- 14.Branford S, Hughes TP, Rudzki Z. Monitoring chronic myeloid leukaemia therapy by real-time quantitative PCR in blood is a reliable alternative to bone marrow cytogenetics. Br J Haematol. 1999;107:587–599. doi: 10.1046/j.1365-2141.1999.01749.x. [DOI] [PubMed] [Google Scholar]
- 15.Cross NC, Feng L, Chase A, Bungey J, Hughes TP, Goldman JM. Competitive polymerase chain reaction to estimate the number of BCR-ABL transcripts in chronic myeloid leukemia patients after bone marrow transplantation. Blood. 1993;82:1929–1936. [PubMed] [Google Scholar]
- 16.Branford S, Fletcher L, Cross NC, et al. Desirable performance characteristics for BCR-ABL measurement on an international reporting scale to allow consistent interpretation of individual patient response and comparison of response rates between clinical trials. Blood. 2008;112:3330–3338. doi: 10.1182/blood-2008-04-150680. [DOI] [PubMed] [Google Scholar]
- 17.Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: Review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28–37. doi: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes TP, Kaeda J, Branford S, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349:1423–1432. doi: 10.1056/NEJMoa030513. [DOI] [PubMed] [Google Scholar]
- 19.Ross DM, Branford S, Moore S, Hughes TP. Limited clinical value of regular bone marrow cytogenetic analysis in imatinib-treated chronic phase CML patients monitored by RQ-PCR for BCR-ABL. Leukemia. 2006;20:664–670. doi: 10.1038/sj.leu.2404139. [DOI] [PubMed] [Google Scholar]
- 20.Guilhot F, Larson RA, O’Brien SG, Gathmann I, Druker BJ. Time to complete cytogenetic response (CCyR) does not affect long-term outcomes for patients on imatinib therapy. Blood. 2007;110:16a. Abstract 27. [Google Scholar]
- 21.Paschka P, Müller MC, Merx K, et al. Molecular monitoring of response to imatinib (glivec) in CML patients pretreated with interferon alpha. low levels of residual disease are associated with continuous remission. Leukemia. 2003;17:1687–1694. doi: 10.1038/sj.leu.2403033. [DOI] [PubMed] [Google Scholar]
- 22.Cortes J, Talpaz M, O’Brien S, et al. Molecular responses in patients with chronic myelogenous leukemia in chronic phase treated with imatinib mesylate. Clin Cancer Res. 2005;11:3425–3432. doi: 10.1158/1078-0432.CCR-04-2139. [DOI] [PubMed] [Google Scholar]
- 23.Iacobucci I, Saglio G, Rosti G, et al. Achieving a major molecular response at the time of a complete cytogenetic response (CCgR) predicts a better duration of CCgR in imatinib-treated chronic myeloid leukemia patients. Clin Cancer Res. 2006;12:3037–3042. doi: 10.1158/1078-0432.CCR-05-2574. [DOI] [PubMed] [Google Scholar]
- 24.Marin D, Milojkovic D, Olavarria E, et al. European LeukemiaNet criteria for failure or suboptimal response reliably identify patients with CML in early chronic phase treated with imatinib whose eventual outcome is poor. Blood. 2008;112:4437–4444. doi: 10.1182/blood-2008-06-162388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Press RD, Galderisi C, Yang R, et al. A half-log increase in BCR-ABL RNA predicts a higher risk of relapse in patients with chronic myeloid leukemia with an imatinib-induced complete cytogenetic response. Clin Cancer Res. 2007;13:6136–6143. doi: 10.1158/1078-0432.CCR-07-1112. [DOI] [PubMed] [Google Scholar]
- 26.Hughes TP, Hochhaus A, Branford S, et al. Reduction of BCR-ABL transcript levels at 6, 12, and 18 months (mo) correlates with long-term outcomes on imatinib (IM) at 72 mo: An analysis from the international randomized study of interferon versus STI571 (IRIS) in patients (pts) with chronic phase chronic myeloid leukemia (CML-CP) Blood. 2008;112:129–130. Abstract 334. [Google Scholar]
- 27.Press RD, Love Z, Tronnes AA, et al. BCR-ABL mRNA levels at and after the time of a complete cytogenetic response (CCR) predict the duration of CCR in imatinib mesylate-treated patients with CML. Blood. 2006;107:4250–4256. doi: 10.1182/blood-2005-11-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Lavallade H, Apperley JF, Khorashad JS, et al. Imatinib for newly diagnosed patients with chronic myeloid leukemia: Incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol. 2008;26:3358–3363. doi: 10.1200/JCO.2007.15.8154. [DOI] [PubMed] [Google Scholar]
- 29.Branford S, Rudzki Z, Harper A, et al. Imatinib produces significantly superior molecular responses compared to interferon alfa plus cytarabine in patients with newly diagnosed chronic myeloid leukemia in chronic phase. Leukemia. 2003;17:2401–2409. doi: 10.1038/sj.leu.2403158. [DOI] [PubMed] [Google Scholar]
- 30.Müller MC, Hanfstein B, Erben P, et al. Molecular response to first line imatinib therapy is predictive for long term event free survival in patients with chronic phase chronic myelogenous leukemia - an interim analysis of the randomized german CML study IV. Blood. 2008;112:129. Abstract 333. [Google Scholar]
- 31.Wang L, Pearson K, Ferguson JE, Clark RE. The early molecular response to imatinib predicts cytogenetic and clinical outcome in chronic myeloid leukaemia. Br J Haematol. 2003;120:990–999. doi: 10.1046/j.1365-2141.2003.04200.x. [DOI] [PubMed] [Google Scholar]
- 32.Quintas-Cardama A, Kantarjian H, Jones D, et al. Delayed achievement of cytogenetic and molecular response is associated with increased risk of progression among patients with chronic myeloid leukemia in early chronic phase receiving high-dose or standard-dose imatinib therapy. Blood. 2009;113:6315–6321. doi: 10.1182/blood-2008-07-166694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kantarjian HM, Shan J, Jones D, et al. Significance of increasing levels of minimal residual disease in patients with philadelphia chromosome-positive chronic myelogenous leukemia in complete cytogenetic response. J Clin Oncol. 2009;27:3659–3663. doi: 10.1200/JCO.2008.18.6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saglio G, Kim D-W, Issaragrisil S, et al. Nilotinib demonstrates superior efficacy compared with imatinib in patients with newly diagnosed chronic myeloid leukemia in chronic phase: Results from the international randomized phase III ENESTnd trial. Blood. 2009;114 Abstract #LBA-1. [Google Scholar]
- 35.Cortes J, O’Brien S, Jones D, et al. Efficacy of nilotinib in patients (pts) with newly diagnosed, previously untreated philadelphia chromosome (ph)-positive chronic myelogenous leukemia in early chronic phase (CML-CP) Blood. 2009;114:144. Abstract 341. [Google Scholar]
- 36.Cortes J, Borthakur G, O’Brien S, et al. Efficacy of dasatinib in patients (pts) with previously untreated chronic myelogenous leukemia (CML) in early chronic phase (CML-CP) Blood. 2009;114:143. Abstract 338. [Google Scholar]
- 37.Rousselot P, Huguet F, Rea D, et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood. 2007;109:58–60. doi: 10.1182/blood-2006-03-011239. [DOI] [PubMed] [Google Scholar]
- 38.Goh HG, Kim YJ, Kim DW, et al. Previous best responses can be re-achieved by resumption after imatinib discontinuation in patients with chronic myeloid leukemia: Implication for intermittent imatinib therapy. Leuk Lymphoma. 2009;50:944–951. doi: 10.1080/10428190902926973. [DOI] [PubMed] [Google Scholar]
- 39.Cortes J, O’Brien S, Kantarjian H. Discontinuation of imatinib therapy after achieving a molecular response. Blood. 2004;104:2204–2205. doi: 10.1182/blood-2004-04-1335. [DOI] [PubMed] [Google Scholar]
- 40.Mahon FX, Rea D, Guilhot F, et al. Discontinuation of imatinib therapy after achieving a molecular response in chronic myeloid leukemia patients. Blood. 2009;114:353. Abstract 859. [Google Scholar]