Abstract
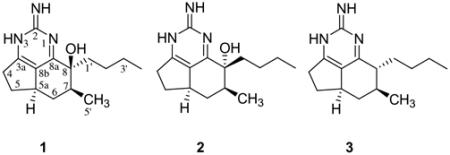
Three tricyclic guanidine alkaloids, including 1,8a;8b,3a-didehydro-8β-hydroxyptilocaulin (1), 1,8a;8b,3a-didehydro-8α-hydroxyptilocaulin (2) and mirabilin B (3), were identified from the marine sponge Monanchora unguifera. 1,8a;8b,3a-Didehydro-8α-hydroxyptilocaulin (2) is a new stereoisomer of 1, the structure of which was elucidated by spectroscopic analysis, comparison of its spectral data with those of 1, and confirmed by X-ray analysis. Compounds 1 and 2 co-crystallized in an unusual perfect order and packed around an approximate inversion center. A mixture of 1 and 2 is active against the malaria parasite Plasmodium falciparum with an IC50 value of 3.8 μg/mL while mirabilin B (3) exhibited antifungal activity against Cryptococcus neoformans with an IC50 value of 7.0 μg/mL and antiprotozoal activity against Leishmania donovani with an IC50 value of 17 μg/mL.
Keywords: Tricyclic guanidine alkaloids, Monanchora unguifera, Sponge, X-ray crystallography, Antimalarial, Antifungal
1. Introduction
Polycyclic guanidine alkaloids are a unique class of sponge-derived metabolites exhibiting a broad range of biological activities.1 Members of this class include the series called ptilocaulins,2–5 crambescins (crambines),6–9 ptilomycalin A, crambescidins,9–15 and batzelladines.15–17 They were isolated separately from marine sponges belonging to the genera Batzella, Monanchora, Arenochalina, and Crambe (Order Poecilosclerida) and Ptilocaulis (Order Axinellida) and starfish Fromia monilis, and Celerina heffernani.18 These metabolites display diverse biological activities including cytotoxicity against P388, L1210, HCT-16, KB cell lines,2,10–12,14 antifungal activity against Candida albicans,10 antiviral activity against Herpes simplex virus type 1 (HSV-1), and Hepatitis-B virus10–13 and potent calcium channel antagonist activity.14 Batzelladine A and B were reported to inhibit the binding of HIV-gp 120 to the CD4 cell-surface receptor protein16 and crambescidins 826, 800, and fromiamycalin inhibited HIV-envelope-mediated fusion and as a result are potential inhibitors of HIV.9 The intriguing molecular structures and their significant biological activities have attracted considerable synthetic interest. The synthesis of a number of guanidine alkaloids have been reported, including ptilocaulin,1,19 crambescins A, B, C1, and C2,1 ptilomycalin A, crambescidins 657, 800,9,20 and batzelladines D,21 E,22 F.23 As part of our continuing efforts to identify biologically active marine natural products for development as antiinfective agents, we evaluated the highly active ethanol extract of the marine sponge Monanchora unguifera (de Laubenfels, 1953) (Order Poecilosclerida: Family Crambeidae) with the guidance of bioassays and obtained 1,8a;8b,3a-didehydro-8β-hydroxyptilocaulin (1) and its stereoisomer, new 1,8a;8b,3a-didehydro-8α-hydroxyptilocaulin (2) and mirabilin B (3). Barrow et al., and Patil et al. reported the isolation of mirabilin B (3) from the sponge Arenochalina mirabilis and Batzella sp. and 1,8a;8b,3a-didehydro-8β-hydroxyptilocaulin (1) from Batzella sp.4,5 In our efforts to identify bioactive leads with activity against HIV and AIDS opportunistic infections, we examined a sponge M. unguifera collected from Discovery Bay, Jamaica. The methanolic extract of M. unguifera exhibited strong antimicrobial activity and was subjected to silica gel column chromatography repeatedly to yield compound 1, 2, and 3.
2. Results and discussion
1,8a;8b,3a-Didehydro-8β-hydroxyptilocaulin (1) and 1,8a;8b,3a-didehydro-8α-hydroxyptilocaulin (2) were isolated as a 1:1 mixture of colorless crystals. The molecular formula C15H23N3O was determined by a pseudomolecular ion at m/z 262.1912 [M+H]+ in the positive high resolution ESI mass spectrum. Two sets of data appeared in the 1H, 13C NMR, and DEPT spectra, indicating this compound was a mixture of two isomers. Also the 13C NMR of 1 and 2 revealed five quaternary signals, two methine signals, six methylene signals, and two methyl signals. The 1H NMR showed two methyl signals at δ 1.07 (d, 3H), 0.83 (t, 3H) for 1 and 1.16 (d, 3H), 0.78 (t, 3H) for 2. These spectral characteristics showed similarities with those of the ptilocaulin alkaloids.2–5 Examination of the 1H and 13C NMR results and comparison with published data revealed a data set assigned to the known compound 1,8a;8b,3a-didehydro-8β-hydroxyptilocaulin.5 Another set of 1H and 13C NMR data were assigned as in Table 1 by analysis of 1H NMR, 13C NMR, DEPT, and HMQC spectra, and by comparison with compound 1. Chemical shifts of C6, C7, and C5′ signals of 2 were downfield to δ 37.4 (+2.2), 42.0 (−5.2), 15.7 (+0.6) compared with corresponding signals in 1, while those signals of C8 and C8a were upfield at δ 74.2 (−1.2) and 163.7 (−0.9). Those data suggested that 2 was the 8α-OH isomer of 1. Fortunately, a single crystal of a 1:1 ratio of 1 and 2 was grown from methanol and X-ray analysis was completed. The crystallographically unique feature is that normally, two diastereomers will either form separate crystals, or if they co-crystallize will occupy the same site in a disordered mixed crystal. This structure is unusual in that the two molecules are perfectly ordered, and pack around an approximate inversion center. The absolute configuration could not be determined, and was arbitrarily assigned as in structures shown in Figure 1.
Table 1.
1H and 13C NMR data for 1,8a;8b,3a-didehydro-8b-hydroxyptilocaulin (1) and its 8a-OH isomer (2) in CDCl3
| Position | 1 |
2 |
||
|---|---|---|---|---|
| 1H NMR | 13C NMR | 1H NMR | 13C NMR | |
| 2 | 163.5 s | 163.2 s | ||
| 3a | 176.4 s | 175.7 s | ||
| 4 | 2.98 (1H, m) | 34.1 t | 2.98 (1H, m) | 33.9 t |
| 2.64 (1H, dd, 16.8, 8.2Hz) | 2.60 (1H, dd, 16.8, 8.2Hz) | |||
| 5 | 2.34 (1H, m) | 33.4 t | 2.34 (1H, m) | 33.2 t |
| 1.55 (1H, m) | 1.55 (1H, m) | |||
| 5a | 2.91 (1H, m) | 38.3 d | 2.88 (1H, m) | 38.0 d |
| 6 | 1.91 (1H, m) | 35.2 t | 1.76 (1H, td, 12.4, 4.8Hz) | 37.4 t |
| 1.20 (1H, m) | 1.38 (1H, td, 12.4, 11.2Hz) | |||
| 7 | 2.01 (1H, m) | 36.8 d | 2.19 (1H, m) | 42.0 d |
| 8 | 75.4 s | 74.2 s | ||
| 8a | 164.6 s | 163.7 s | ||
| 8b | 125.4 s | 125.4 s | ||
| 1′ | 2.12 (1H, td, 12.4, 4.4Hz) | 37.0 t | 1.92 (1H, m) | 36.9 t |
| 1.85 (1H, td, 12.4, 3.6Hz) | 1.85 (1H, td, 12.4, 3.6Hz) | |||
| 2′ | 1.20 (1H, m) | 27.1 t | 1.11 (1H, m) | 27.1 t |
| 0.91 (1H, m) | 0.72 (1H, m) | |||
| 3′ | 1.25 (2H, m) | 23.5 t | 1.14 (2H, m) | 23.1 t |
| 4′ | 0.83 (3H, t, 7.2Hz) | 14.0 q | 0.78 (3H, t, 7.2Hz) | 13.8 q |
| 5′ | 1.07 (3H, d, 6.8Hz) | 15.1 q | 1.16 (3H, d, 7.2Hz) | 15.7 |
Figure 1.
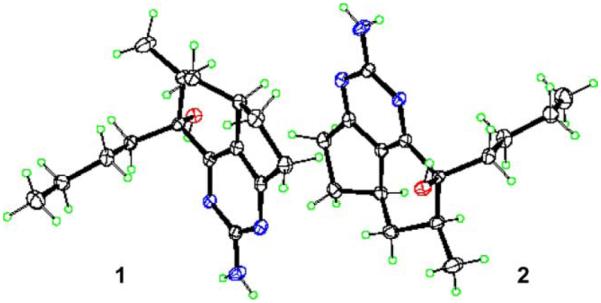
ORTEP drawing of compounds 1 and 2.
The mixture of 1 and 2, and pure compound 3, were evaluated for cytotoxicity, antimicrobial, anti-HIV, and antituberculosis activities. Mirabilin B (3) exhibited antifungal activity against Cryptococcus neoformans with an IC50 value of 7.0 μg/mL and antiprotozoal activity against Leishmania donovani with an IC50 value of 17 μg/mL. The mixture of 1 and 2 is active against the malaria parasite Plasmodium falciparum with an IC50 value of 3.8 μg/mL. Both mirabilin B (3) and the mixture of 1 and 2 did not show significant activity against fourteen tested cancer cell lines including prostate DU-145, ovary IGROV and IGROV-E, mamma SK-BR3, melanoma SK-MEL-28, NSCL A549, leukemia K-562, pancreas PANCL, colon HT-29, HT29-KF, LOVO, LOVO-DOX, cervix HELA, and HELA-APL. Neither of the compounds showed activity against HIV-1 or Mycobacterium tuberculosis.
Since the isolation of ptilocaulin from the Caribbean sponge Ptilocaulis aff. P. spiculifer, 12 ptilocaulin-type tricycle guanidine alkaloids had been reported including isoptilocaulin, (+)-8b-hydroxyptilocaulin, mirabilin A–F, 8a,8b-dehydroptilocaulin, 8a,8b-dehydro-8-hydroxyptilocaulin, and 1,8a;8b,3a-dihydroxy-8-hydroxyptilocaulin from sponges.2–5 Except for the cytotoxicity against L1210 leukemia cells and antimicrobial activity of ptilocaulin and isoptilocaulin, there have been no reports of biological activity for these alkaloids. Our results indicate the bioactivity for this unique class of guanidine alkaloids warrants further investigation and optimization.
3. Experimental
3.1. General experiment procedures
NMR spectra were recorded on Bruker Avance DRX-400 and 500 spectrometers. The ESI-FTMS was acquired on a Bruker–Magnex BioAPEX 30es ion cyclotron Fourier transform mass spectrometer by direct injection into an electrospray interface.
3.2. X-ray analysis
Crystals of the 1:1 mixture of 1 and 2 were colorless, triclinic, space group P1 with cell dimensions a = 7.957 (2) Å, b = 9.400 (2) Å, c = 10.711 (3) Å, α = 106.514, β = 98.163, γ = 104.922, V = 721.9 (3) Å3, Z = 1, Dx = 1.202 Mgm−3. Intensity data for the crystal23 were collected at 102 K on an Nonius KappaCCD (with Oxford Cryostream) diffractometer equipped with Mo Kα(λ = 0.71073 Å) radiation θ range 2.5°–30.5°. Four thousand and three hundred independent reflections were obtained with 3999 reflections having I > 2σ(I). Data reduction was by denzosmn and scalepack.24 The structure was solved by direct methods and refined by full-matrix least-squares using shelxl.25 Hydrogen atoms were constrained in calculated positions. Atomic coordinates, crystal data, final R values, and other details are included in Tables 2–7 of the supporting information.
3.3. Sponge collection and taxonomy
The sponge was collected from several locations in Discovery Bay, Jamaica, from about 40m depth, in November 2002. The morphology is shrub-like or bushy, and the texture is very fibrous and tough with some specimens being encrusting. The color in life is bright red and the skeleton consists of straight subtylostyles 200–270 μm long embedded in thick spongin fibers. Microscleres typical of the species were absent. The sponge is M. unguifera (de Laubenfels, 1953) (Order Poecilosclerida: Family Crambeidae). A voucher specimen has been deposited in the Natural History Museum, London (BMNH 2000.7.17.3).
3.4. Extraction and isolation
The freeze-dried sponge M. unguifera (1.8 kg) was extracted four times with 6L of MeOH in a blender. The combined extracts were concentrated in vacuo until dried. The residue (380 g) was extracted with hexane, chloroform, and ethanol, respectively. The chloroform soluble portion (155 g) was subjected to vacuum liquid chromatography using 2kg of silica gel and eluting with chloroform, chloroform/methanol, and methanol to afford 61 fractions (fr. 1–61).
Fractions 8 and 9 (3.2 g) were combined and subjected to repeat silica gel column chromatography eluting with CHCl3–acetone (98:2) and hexane–acetone (9:1) to yield mirabilin B (3, 513mg). Fractions 10 and 11 (19.3g) were combined and rechromatographed on a silica gel column eluted with a gradient of CHCl3–acetone– MeOH from (95:5:0) to (80:20:30) to give 68 sub-fractions (sub-fr. 1–68). Sub-fraction 32–35 were combined and recrystallized with MeOH to afford the mixture of 1 and 2 (24.5mg).
3.4.1. 1,8a;8b,3a-Didehydro-8β-hydroxyptilocaulin (1) and its isomer (2).
Colorless crystal. HRESIMS m/z 262.1912 [M+H]+ (calcd for C15H24N3O 262.1919); 1H and 13C NMR data assigned for 1 and 2 see Table 1.
3.4.2. Mirabilin B (3)
Colorless crystal. HRESIMS m/z 246.1950 [M+H]+ (calcd for C15H24N3 246.1970); 1H and 13C NMR data were in agreement with published data.5
Supplementary Material
Acknowledgements
We acknowledge the China Scholarship Council for financial support (H.-M.H.). This work was supported by NIH (1R01A136596) and PharmaMar. The Natural Resource Conservation Authority, Jamaica and Discovery Bay Marine Laboratory (contribution #681) are gratefully acknowledged for assistance with sample collections. We thank F. T. Wiggers and C. D. Dunbar, the National Center for Natural Products Research for spectral data; B. L. Tekwani, M. Jacob, J. Trott, M. Wright, S. C. Sanders, and B. G. Smiley, from the NCNPR for antiparasitic and antifungal evaluation; PharmaMar for cytotoxicity; R. Schinazi's laboratory at Emory for HIV-1, and S. Franzblau's laboratory at UIC for anti-Mtb studies.
Footnotes
Supplementary data
Supplementary data associated with this article can be found, in the online version, at 10.1016/j.bmc.2004.09. 026.
References and notes
- 1.Heys L, Moore CG, Murphy P. J. Chem. Soc. Rev. 2000;29:57–67. [Google Scholar]
- 2.Harbour GC, Tymiak AA, Rinehart KL, Shaw PD, Hughes RG, Mizsak SA, Coats JH, Zurenko GE, Li LH, Kuentzel SL. J. Am. Chem. Soc. 1981;103:5604–5606. [Google Scholar]
- 3.Tavares R, Daloze D, Braekman JC, Hajdu E, Van Soest RWM. J. Nat. Prod. 1995;58(7):1139–1142. doi: 10.1021/np990403g. [DOI] [PubMed] [Google Scholar]
- 4.Barrow RA, Murray LM, Lim TK, Capon RJ. Aust. J. Chem. 1996;49:767–773. [Google Scholar]
- 5.Patil AD, Freyer AJ, Offen P, Bean MF, Johnson K. J. Nat. Prod. 1997;60:704–707. doi: 10.1021/np960652u. [DOI] [PubMed] [Google Scholar]
- 6.Berlinck RGS, Braekman JC, Daloze D, Hallenga K, Ottinger R, Bruno I, Riccio R. Tetrahedron Lett. 1990;31:6531–6534. [Google Scholar]
- 7.Berlinck RGS, Braekman JC, Daloze D, Bruno I, Riccio R, Rogeau D, Amade P. J. Nat. Prod. 1992;55(4):528–532. doi: 10.1021/np50082a026. [DOI] [PubMed] [Google Scholar]
- 8.Jares-Erijman EA, Ingrum AA, Sun F, Rinehart KL. J. Nat. Prod. 1993;56(12):2186–2188. doi: 10.1021/np50102a025. [DOI] [PubMed] [Google Scholar]
- 9.Chang LC, Whittaker NF, Bewley CA. J. Nat. Prod. 2003;66:1490–1494. doi: 10.1021/np030256t. [DOI] [PubMed] [Google Scholar]
- 10.Kashman Y, Hirsh S, McConnel OJ, Ohtini I, Kusumi T, Kakisawa H. J. Am. Chem. Soc. 1989;111:8925–8926. [Google Scholar]
- 11.Jares-Erijman EA, Sakai R, Rinehart KL. J. Org. Chem. 1991;56:5712–5715. [Google Scholar]
- 12.Jares-Erijman EA, Ingrum AL, Carney JR, Rinehart KL, Sakai R. J. Org. Chem. 1993;58:4805–4808. [Google Scholar]
- 13.Venkateswarlu Y, Reddy MVR, Ramesh P, Rao JV. Indian J. Chem. 1999;38B:254–256. [Google Scholar]
- 14.Berlinck RGS, Braekman JC, Daloze D, Bruno I, Riccio R, Ferri S, Spampinato S, Speroni E. J. Nat. Prod. 1993;56:1007–1015. doi: 10.1021/np50097a004. [DOI] [PubMed] [Google Scholar]
- 15.Braekman JC, Daloze D, Tavares R, Hajdu E, Van Soest RWM. J. Nat. Prod. 2000;63:193–196. doi: 10.1021/np990403g. [DOI] [PubMed] [Google Scholar]
- 16.Patil AD, Kumar NV, Kokke WC, Bean MF, Freyer AJ, Brosse CD, Mai S, Truneh A, Faulkner DJ, Carte B, Breen AL, Hertzberg RP, Johnson RK, Westley JW, Potts BCM. J. Org. Chem. 1995;60:1182–1188. [Google Scholar]
- 17.Patil AD, Freyer AJ, Taylor PB, Carte B, Zuber G, Johnson RK, Faulkner DJ. J. Org. Chem. 1997;62:1814–1819. [Google Scholar]
- 18.Palagiano E, Marino SD, Minale L, Riccio R, Zollo F, Iorizzi M, Carre JB, Debitus C, Lucarain L, Provost J. Tetrahedron. 1995;51:3675–3682. [Google Scholar]
- 19.Cossy J, BouzBouz S. Tetrahedron Lett. 1996;37:5091–5094. [Google Scholar]
- 20.Coffey DS, McDonald AI, Overman LE, Rabinowitz MH, Renhowe PA. J. Am. Chem. Soc. 2000;122:4893–4903. [Google Scholar]
- 21.Ishiwata T, Hino T, Koshino H, Hashimoto Y, Nakata T, Nagasawa K. Org. Lett. 2002;4:2921–2924. doi: 10.1021/ol026303a. [DOI] [PubMed] [Google Scholar]
- 22.Snider BB, Cheng J. Tetrahedron Lett. 1998;39:5697–5700. [Google Scholar]
- 23.Cohen F, Overman LE. J. Am. Chem. Soc. 2001;123:10782–10783. doi: 10.1021/ja017067m. [DOI] [PubMed] [Google Scholar]
- 24.Otwinowski Z, Minor W. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 25.Sheldrick GM. SHELXL97. Program for the Refinement of Crystal Structures. University of Göttingen; Germany: 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


